Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5351
Peer-review started: May 11, 2022
First decision: August 1, 2022
Revised: August 11, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: September 28, 2022
Processing time: 134 Days and 19.8 Hours
Surgical resection is one of the most widely used modalities for the treatment of hepatocellular carcinoma (HCC). Early extrahepatic recurrence (EHR) of HCC after surgical resection is considered to be closely associated with poor prognosis. However, data regarding risk factors and survival outcomes of early EHR after surgical resection remain scarce.
To investigate the clinical features and risk factors of early EHR and elucidate its association with survival outcomes.
From January 2004 to December 2019, we enrolled treatment-naïve patients who were ≥ 18 years and underwent surgical resection for HCC in two tertiary academic centers. After excluding patients with tumor types other than HCC and/or ineligible data, this retrospective study finally included 779 patients. Surgical resection of HCC was performed according to the physicians’ decisions and the EHR was diagnosed based on contrast-enhanced computed tomography or magnetic resonance imaging, and pathologic confirmation was performed in selected patients. Multivariate Cox regression analysis was performed to identify the variables associated with EHR.
Early EHR within 2 years after surgery was diagnosed in 9.5% of patients during a median follow-up period of 4.4 years. The recurrence-free survival period was 5.2 mo, and the median time to EHR was 8.8 mo in patients with early EHR. In 52.7% of patients with early EHR, EHR occurred as the first recurrence of HCC after surgical resection. On multivariate analysis, serum albumin < 4.0 g/dL, serum alkaline phosphatase > 100 U/L, surgical margin involvement, venous and/or lymphatic involvement, satellite nodules, tumor necrosis detected by pathology, tumor size ≥ 7 cm, and macrovascular invasion were determined as risk factors associated with early EHR. After sub-categorizing the patients according to the number of risk factors, the rates of both EHR and survival showed a significant correlation with the risk of early EHR. Furthermore, multivariate analysis revealed that early EHR was associated with substantially worse survival outcomes (Hazard ratio, 6.77; 95% confidence interval, 4.81-9.52; P < 0.001).
Early EHR significantly deteriorates the survival of patients with HCC, and our identified risk factors may predict the clinical outcomes and aid in postoperative strategies for improving survival.
Core Tip: Surgical resection of hepatocellular carcinoma is effective and curative treatment modality. However, early extrahepatic recurrence (EHR) after resection is related to poor prognosis. This study indicates the close correlation between the early EHR and survival outcome (hazard ratio 6.77, 95% confidence interval 4.81-9.52). The time to EHR was 8.8 mo and in 52.7% of early EHR group, EHR occurred as the first recurrence. On multivariate analysis, serum albumin < 4.0 g/dL, serum alkaline phosphatase > 100 U/L, surgical margin involvement, venous and/or lymphatic involvement, satellite nodules, tumor necrosis, tumor size ≥ 7 cm, and macrovascular invasion were associated risk factors with early EHR.
- Citation: Yoon JH, Choi SK, Cho SB, Kim HJ, Ko YS, Jun CH. Early extrahepatic recurrence as a pivotal factor for survival after hepatocellular carcinoma resection: A 15-year observational study. World J Gastroenterol 2022; 28(36): 5351-5363
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5351
Surgical resection remains one of the best options for treating early hepatocellular carcinoma (HCC), along with liver transplantation and radiofrequency ablation[1-3]. With the development of surgical techniques and medical devices, surgical resection of tumors has been performed for a wide spectrum of patients with HCC. Nonetheless, HCC recurrence after surgery leads to an unfavorable prognosis and is still a major issue, with the 2-year recurrence rate ranging from 30.0% to 43.0%[4,5].
HCC recurrence after surgical resection mostly occurs inside the liver (intrahepatic), but recurrence outside the liver (extrahepatic) can also occur[2,6]. Most cases of extrahepatic recurrence (EHR) of HCC generally have an aggressive phenotype, and this contributes to poor survival outcomes[7]. Taketomi et al[6] reported the 3-, 5-, and 10-year cumulative survival rates of EHR as 60.3%, 24.0%, and 6.0%, respectively, which were lower than those in patients with intrahepatic recurrence (IHR) (74.5%, 57.7%, and 23.1%, respectively; P = 0.004). Although IHR of HCC can be managed by various loco-regional treatment modalities and systemic therapies, there are only limited methods for the treatment of EHR. However, despite an expected poor prognosis, favorable outcomes in selected patients with limited EHR have been reported with treatment modalities, such as metastasectomy[8]. Therefore, predicting the risk of EHR is essential, and better outcomes can be accomplished by predicting high-risk patients and monitoring these patients with close follow-up surveillance for early detection of EHR and/or timely postoperative adjuvant therapy.
We have reported simple parameters for predicting EHR after hepatectomy[9], and in that study, approximately 49.2% of the patients with EHR (31 of 63 patients) showed early EHR and demonstrated poorer recurrence-free survival (RFS) and overall survival rates. There have been many reports regarding the risk factors and predictive models for early recurrence mostly concerning IHR of HCC after surgery[10-12]; however, there are limited data regarding the risk factors and survival outcomes related to early EHR after surgical resection of HCC. Moreover, the clinical characteristics and survival outcomes of patients with early EHR in comparison with those with a later onset of EHR have not been examined. Hence, we aimed to investigate the characteristics and potential risk factors of early EHR after curative surgical resection for HCC and explore the relationship between early EHR and survival outcomes.
This study enrolled 890 patients who underwent surgery for HCC from January 2004 to December 2019 at two tertiary hospitals. Patients with tumor types other than HCC, previous treatment experience, and ineligible data were excluded. Finally, 779 patients were enrolled for this study and were analyzed. Surgical resection of HCC was determined by a physician’s decision regarding the tumor stage, liver function, and the patient’s physical status. There had been no changes in the keynote of surgical resection during the study period in both centers. To exclude potential bias regarding surgical technique and concentrate on EHR development and survival rate based on tumor findings, patients who developed EHR prior to the completion of the post-surgical duration of 60 d were excluded.
HCC was diagnosed according to the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center[13]. HCC staging at the time of diagnosis was determined using the modified Union for International Cancer Control (mUICC) staging system[14] and the Barcelona Clinic Liver Cancer (BCLC) classification system[3]. Milan criteria were based on radiologic findings at initial the diagnosis of HCC[15]. Abdominal computed tomography (CT) or magnetic resonance imaging (MRI) and assessment of serological tumor markers were routinely performed 1 mo after surgical resection and at each 3-6-month follow-up visit.
The tumor sizes were measured by radiologic modalities with CT or MRI. The histological differentiation of HCC was graded according to the criteria of Edmondson and Steiner[16]. The presence of macrovascular invasion was defined as vascular invasion of HCC detected on CT imaging or MRI while microvascular invasion was defined as invasion of vascular structures on microscopic analysis of resected tumor specimens. Lymph node metastasis, serosal invasion, bile duct invasion, capsule formation, multicentricity, satellite nodule, intrahepatic metastasis, tumor necrosis, tumor hemorrhage, and fatty changes within tumors were all confirmed from pathological findings of resected specimens. Overall survival was defined as the time interval in days between the date of diagnosis of HCC and the date of death or last follow-up examination. The cause of death of each patient is presented in SupplementaryTable 1.
The diagnosis of EHR was confirmed by contrast-enhanced CT or MRI, and pathological examination was performed only for selected patients as decided by the physician. In addition, chest radiographs, bone scintigraphy, positron emission tomography-CT, and brain MR or CT imaging were used when new signs suggesting tumor metastasis manifested. Early EHR was defined as EHR that developed within 2 years after initial surgical resection. Most cases of EHR were diagnosed during the routine follow-up studies, with a few cases diagnosed during the evaluation of new-onset symptoms or based on significant elevation in alpha-fetoprotein (AFP) or serial AFP elevation without definite intra-hepatic lesions.
This study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. CNUH-2019-203). Because of the retrospective design of our study and the use of de-identified data, the requirement for informed consent was waived under the approval of the Institutional Review Board of Chonnam National University Hospital. The study was performed in compliance with the tenets of the 1975 Helsinki Declaration.
The data are presented as means ± SD or as medians and ranges, as appropriate, for the data type and distribution. Univariate analyses were performed using the chi-squared test or Student’s t-test, as appropriate. Variables with a P value of < 0.05 on univariate analyses were included in a multivariate logistic regression analysis to identify factors predictive of EHR. A multivariate Cox regression model was built using a stepwise backward selection of variables, and variables with a P value of < 0.05 were retained as predictive factors. The risk levels of EHR were defined based on the number of risk factors present, and Kaplan-Meier survival curves were constructed for each risk level. All statistical analyses were performed using SPSS software version 27.0 (IBM Corp., Armonk, NY, United States). The statistical methods of this study were reviewed by Cho Hee Hwang from the Biomedical research institute of Chonnam National University Hospital.
The baseline characteristics of patients with early EHR were assessed (Table 1). These patients were younger, had lower serum albumin levels, and higher initial serum AFP concentration > 400 (IU/mL) than those without early EHR. Additionally, patients with early EHR showed more advanced tumor stage with respect to tumor size, BCLC stage, mUICC stage, and beyond Milan criteria. The patients in the early EHR group presented higher rates of macrovascular invasion and longer duration of hospital stay after liver resection. Furthermore, when we subdivided the patients into those without EHR, those with non-early EHR, and those with early EHR, the following factors exhibited a close relationship to the interval of EHR development: younger age, ALP levels, albumin levels, preoperative serum AFP > 1500 IU/mL, tumor size, tumor numbers, tumor stages, and the presence of macrovascular invasion (Supplementary Table 2).
| Patients without early EHR (n = 705) | Patients with early EHR (n = 74) | P value | |
| Age (yr) | 59.49 ± 10.08 | 56.51 ± 10.37 | 0.020 |
| Male sex (n, %) | 603 (85.5) | 64 (86.5) | 0.824 |
| BMI (kg/m2) | 23.89 ± 3.03 | 24.10 ± 2.98 | 0.790 |
| Etiology of hepatitis, n (%) | 0.569 | ||
| HBV/HCV | 425 (63.2)/55 (8.2) | 53 (73.6)/8 (11.1) | |
| Alcohol/combined | 40 (10.4)/49 (7.0) | 5 (6.9)/2 (2.8) | |
| NASH/unknown | 1 (0.1)/73 (10.8) | 0/4 (5.6) | |
| ALP (U/L) | 89.25 ± 44.04 | 113.23 ± 106.76 | 0.059 |
| Albumin (mg/dL) | 4.36 ± 0.45 | 4.19 ± 0.48 | 0.002 |
| ALBI grade ≥ 2, n (%) | 97 (13.8) | 16 (21.9) | 0.063 |
| ICG R15 | 12.02 ± 7.29 | 12.58 ± 7.62 | 0.566 |
| Preoperative serum AFP > 400 (IU/mL) | 127 (18.6) | 24 (32.9) | 0.004 |
| Sum of tumor size | 4.17 ± 2.44 | 6.06 ± 3.38 | < 0.001 |
| Tumor numbers | 1.19 ± 0.54 | 1.32 ± 0.82 | 0.176 |
| BCLC stage, n (%) | < 0.001 | ||
| 0/A/≥ B | 89 (12.7)/491 (69.9)/122 (17.4) | 3 (4.1)/44 (59.5)/27 (36.5) | |
| Pathological mUICC stage, n (%) | < 0.001 | ||
| I/II/≥ III | 111 (15.8)/431 (61.4)/160 (22.8) | 2 (2.7)/32 (43.2)/40 (54.1) | |
| Radiological mUICC stage, n (%) | < 0.001 | ||
| I/II/≥ III | 112 (15.9%)/463 (66.0%)/127 (18.1%) | 4 (5.4)/43 (58.1)/27 (36.5) | |
| Beyond Milan criteria, n (%) | 179 (25.4) | 40 (54.1) | < 0.001 |
| Macrovascular invasion, n (%) | 32 (4.6) | 11 (14.9) | < 0.001 |
| Hospital stay, days (median, range) | 13.3 ± 7.1 | 15.6 ± 75.6 | 0.049 |
| Follow-up duration, years (median, range) | 4.8 (0.23-15.36) | 1.8 (0.31-1.43) | < 0.001 |
We analyzed the surgical findings of tumor specimens categorized by the presence of early EHR (Table 2). Patients with early EHR had more metastatic lymph nodes, microvascular invasion, and satellite nodules (0.1% vs 4.1%, 16.3% vs 44.6%, and 12.2% vs 23.0%, respectively). Furthermore, the tumor specimens in the early EHR group showed a higher probability of tumor necrosis, hemorrhages, and absence of fatty changes (41.5% vs 82.4%, 43.0% vs 62.2%, and 36.0% vs 20.5%, respectively). On subgroup analysis stratifying patients with EHR into the early EHR and non-early EHR groups, microvascular invasion, serosal invasion, presence of satellite nodules, tumor necrosis, hemorrhages, and absence of fatty changes still showed consistent significance (Supplementary Table 3).
| Patients without early EHR (n = 705) | Patients with early EHR (n = 74) | P value | |
| Margin involvement, n (%) | 16 (2.3%) | 4 (5.5%) | 0.104 |
| Metastatic lymph nodes, n (%) | 1 (0.1) | 3 (4.1) | < 0.001 |
| Microvascular invasion, n (%) | 114 (16.3) | 33 (44.6) | < 0.001 |
| Serosal invasion, n (%) | 13 (1.9) | 4 (5.4) | 0.052 |
| Bile duct invasion, n (%) | 9 (1.3) | 1 (1.4) | 0.975 |
| Capsule formation, n (%) | 456 (66.7) | 53 (71.6) | 0.389 |
| Multicentricity, n (%) | 54 (7.8) | 8 (10.8) | 0.372 |
| Satellite nodule, n (%) | 84 (12.2) | 17 (23.0) | 0.009 |
| Intrahepatic metastasis, n (%) | 8 (1.2) | 2 (2.7) | 0.268 |
| Necrosis, n (%) | 286 (41.5) | 61 (82.4) | < 0.001 |
| Haemorrhage, n (%) | 296 (43.0) | 46 (62.2) | 0.002 |
| Fatty change, n (%) | 246 (36.0) | 15 (20.5) | 0.008 |
| Major Edmondson-Steiner grade, n (%) | 0.243 | ||
| 1/2 | 30 (4.4)/352 (51.8) | 2 (2.7)/30 (41.1) | |
| 3/4 | 274 (40.3)/24 (3.5) | 38 (52.1)/3 (4.1) | |
| Worst Edmondson-Steiner grade, n (%) | 0.094 | ||
| 1/2 | 8 (1.1)/130 (18.9) | 1 (1.4)/5 (6.8) | |
| 3/4 | 357 (51.8)/194 (28.2) | 40 (54.1)/28 (37.8) | |
| Serum AFP > 400 IU/mL on first recurrence | 34 (10.2) | 22 (31.0) | < 0.001 |
| Recurrence-free survival, mo (median, range) | 32.4 (2.24-177.34) | 5.21 (2.04-20.28) | < 0.001 |
| mUICC T stage on first recurrence, n (%) | < 0.001 | ||
| 0/1 | 6 (1.8)/144 (43.6) | 10 (13.7)/23 (31.5) | |
| 2/3 | 131 (39.7)/39 (11.8) | 14 (19.2)/20 (27.4) | |
| 4 | 10 (3.0) | 6 (8.2) | |
| Second treatment modality | 0.070 | ||
| Surgery/RFA | 46 (6.5)/95 (13.5) | 9 (12.2)/10 (13.5) | |
| TACE/RFA + TACE | 156(22.1)/14 (2.0) | 13 (17.6)/6 (8.1) | |
| RT/systemic chemotherapy/BSC | 20 (2.8)/11 (1.5)/20 (2.8) | 3 (4.1)/1 (1.4) |
When comparing the findings on the first recurrence between the early and non-early EHR groups, the proportion of patients with serum AFP > 400 IU/mL was higher (10.2% vs 31.0%, P < 0.001) (Table 2) and the RFS was shorter (32.4 vs 5.21 mo, P < 0.001) in the early EHR group. These trends for serum AFP and RFS also showed consistency when we compared all patients with non-early EHR and early EHR (Supplementary Table 1). Furthermore, the mUICC stage at first recurrence was more advanced in the early EHR group (38.7% vs 75.7% with mUICC stage ≥ 3, P < 0.001), and 52.7% of patients developed EHR as the first recurrence after surgical resection.
We performed Cox regression analysis to assess multiple factors relevant to EHR after surgical resection of HCC (Table 3). Among various risk factors, eight were proven to be closely associated with EHR, including serum albumin < 4.0 g/dL [Hazard ratio (HR), 2.12; P < 0.001], serum ALP > 100 U/L (HR, 1.586; P = 0.018), surgical margin involvement (HR, 2.53; P = 0.032), venous and/or lymphatic involvement (HR, 1.900; P = 0.002), satellite nodules (HR, 1.73; P = 0.011), tumor necrosis (HR, 1.92; P = 0.001), sum of tumor size ≥ 7 cm (HR, 1.84; P = 0.004), and macrovascular invasion (HR, 2.30; P = 0.008).
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Serum albumin < 4.0 g/dL | 2.27 (1.56-3.29) | < 0.001 | 2.19 (1.45-3.31) | < 0.001 |
| Serum ALP > 100 U/L | 1.76 (1.24-2.52) | 0.002 | 1.59 (1.08-2.32) | 0.018 |
| ALBI grade ≥ 2 | 1.71 (1.12-2.59) | 0.012 | ||
| Surgical margin involvement | 2.30 (1.07-4.93) | 0.032 | 2.53 (1.09-5.90) | 0.032 |
| Pathological mUICC stage (III, IVa) | 2.94 (2.09-4.12) | < 0.001 | ||
| Multiple tumors1 | 3.21 (1.57-6.56) | 0.002 | ||
| Venous/lymphatic involvement2 | 2.98 (2.09-4.25) | < 0.001 | 1.90 (1.28-2.83) | 0.002 |
| Serosa invasion | 2.86 (1.33-6.12) | 0.007 | ||
| Bile duct invasion | 3.25 (1.20-8.83) | 0.020 | ||
| Satellite nodule | 2.69 (1.83-3.94) | < 0.001 | 1.73 (1.14-2.63) | 0.011 |
| Intrahepatic metastasis | 2.99 (1.10-8.09) | 0.032 | ||
| Tumor necrosis | 3.18 (2.20-4.61) | <0.001 | 1.92 (1.29-2.79) | 0.001 |
| Tumor hemorrhage | 1.81 (1.28-2.55) | 0.001 | ||
| Sum of tumor size ≥ 7 cm | 3.25 (2.23-4.73) | < 0.001 | 1.84 (1.21-2.77) | 0.004 |
| Macrovascular invasion | 2.48 (1.37-4.50) | 0.003 | 2.30 (1.24-4.26) | 0.008 |
| Serum AFP > 1500 IU/mL | 1.99 (1.27-3.12) | 0.003 | ||
We conducted Cox regression analysis to determine the risk factors associated with survival after surgical resection of HCC and to assess the relationship between early EHR and survival. On multi
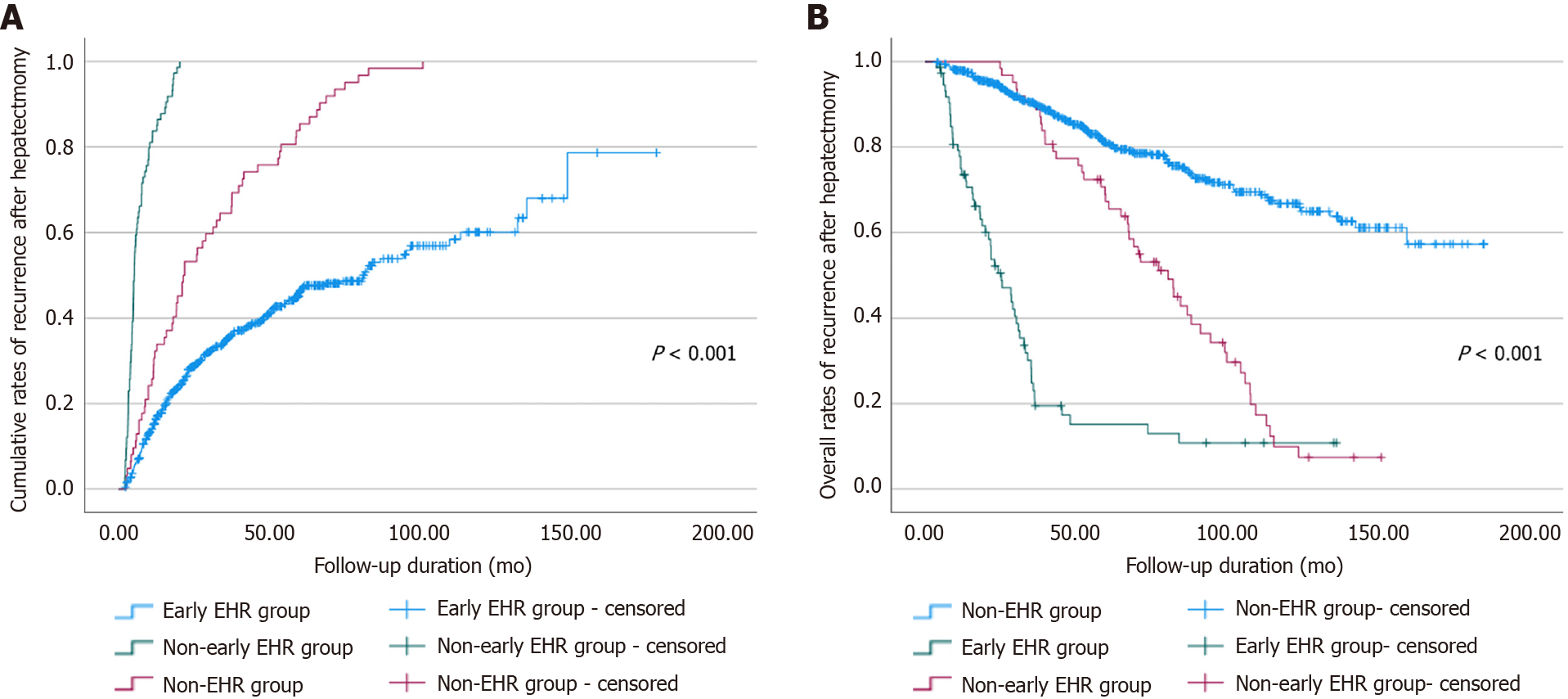
Among the 779 patients enrolled, 136 (17.5%) developed EHR during a median follow-up period of 4.41 years, and 74 (54.4%) exhibited early EHR after surgical resection of HCC. Both the cumulative rates of recurrence and the overall survival rates after surgical resection of HCC showed stepwise correlations with the interval to EHR (Figure 1). The 1-, 3-, 5-, and 10-year cumulative rates of recurrence were 15.8%, 35.4%, 46.5%, and 60.1% in the non-EHR group and were 32.3%, 64.5%, 85.5%, and 100.0% in the non-early EHR group. The early EHR group had a 1-year recurrence rate of 83.8% and 100.0% from the 2nd year after surgical resection (Figure 1A). The 1-, 3-, 5-, and 10-year cumulative rates of overall survival were as follows: 97.8%, 90.2%, 80.9%, and 66.8% for the non-EHR group; 100.0%, 90.3%, 67.2%, and 9.9% for the non-early EHR group; and 74.9%, 23.0%, 15.2%, and 10.8% for the early EHR group (Figure 1B).
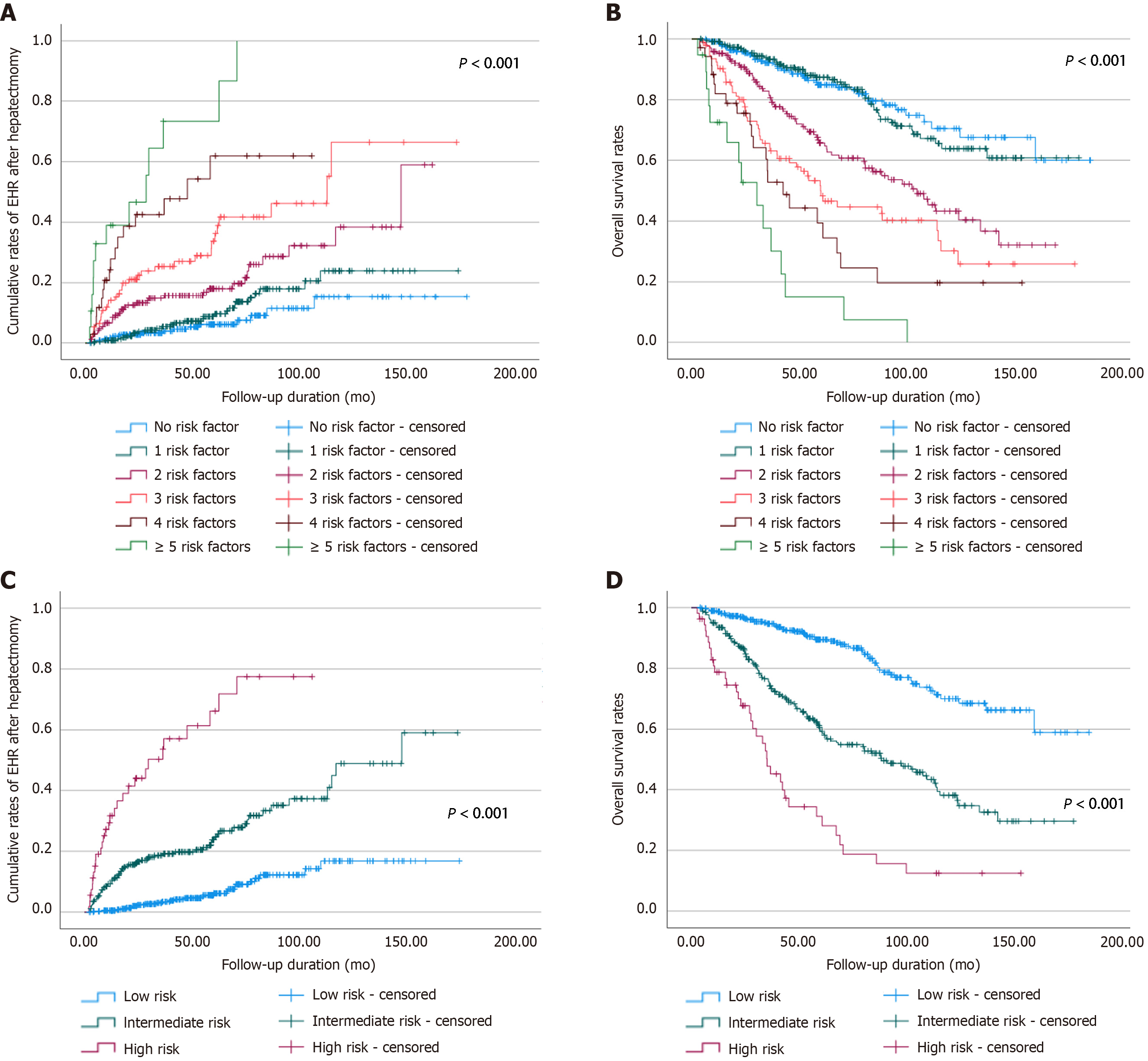
Depending on the eight factors proven to be associated with early EHR, we categorized the patients according to the number of risk factors. The cumulative rates of early EHR onset and survival were analyzed in the sub-categorized patients, and both rates showed stepwise correlations according to the number of risk factors (Figure 2A and B). Consequently, we stratified the patients into three categories: a low-risk group with 0-1 risk factor, an intermediate-risk group with 2-3 risk factors, and a high-risk group with ≥ 4 risk factors. The rates of EHR and survival exhibited a significant correlation with the risk stratification model (Figure 2C and D). The 1-, 2-, 3-, 5-, and 10-year cumulative rates of EHR in each group were as follows: 0.5%, 2.0%, 3.4%, 6.2%, and 16.8% in the low-risk group; 10.3%, 16.1%, 18.6%, 23.8%, and 42.8% in the intermediate-risk group; and 31.7%, 44.0%, 50.2%, 66.2%, and 77.5% in the high-risk group. The 1-, 3-, 5-, and 10-year overall survival rates in each group were as follows: 98.9%, 94.7%, 89.5%, and 70.0% in the low-risk group; 93.9%, 75.7%, 59.3%, and 38.1% in the intermediate-risk group; and 78.8%, 47.7%, 31.2%, and 12.5% in the high-risk group.
EHR of HCC is a well-known predictive marker of poor survival outcomes, and EHR after surgical resection of HCC is deemed to be a pivotal factor for grave prognosis[7,9,17,18]. In this large-scale 15-year observational study conducted at two academic tertiary hospitals, EHR and early EHR occurred in 17.5% and 9.5% of 779 patients with HCC, respectively. Patients with early EHR had a rapid recurrence of HCC after surgical resection and poor survival outcomes. Furthermore, we elucidated the potential risk factors for early EHR after curative surgical resection of HCC.
Since the proportion of IHR cases is higher than that of EHR cases after surgical resection of HCC, previous studies have analyzed various risk factors associated with IHR alone, previous studies have analyzed various risk factors associated with IHR. Portolani et al[19] examined the early and late recurrence of HCC after liver resection and showed that the survival rates in the early recurrence group were significantly lower than those in the late recurrence group (25.7% vs 4.5% at 5 years). Moreover, similar results were demonstrated in a large-scale multicenter study conducted in China by Yan et al[20] comprising 1426 patients, where patients in the early recurrence group showed poor post-recurrence survival (13.5 vs 36.6 mo, P < 0.001). Furthermore, Yang et al[21] stated that recurrent HCC cases with multicentric recurrence may have had greater survival than intrahepatic metastasis cases. In contrast, Byeon et al[22] compared the outcomes of 111 patients with IHR and 41 patients with EHR who had undergone surgical resection for HCC and found that patients in the EHR group showed significantly lower 5-year survival rates than those in the IHR group (21.5% vs 36.3%, P < 0.001). Considering the non-negligible rates of EHR after surgical resection of HCC and the dismal prognosis of patients with EHR, risk stratification and prediction of early EHR may have been of great importance in improving the clinical outcome.
In 52.7% of the patients with early EHR, EHR was the first recurrence of HCC after surgical resection (Supplementary Table 1), and both interval to first recurrence and EHR were significantly shorter in the early EHR group compared with that in the non-early EHR group (5.2 vs 21.6 mo, P < 0.001; and 8.81 vs 58.57 mo, P < 0.001). Moreover, a higher proportion of patients with early EHR showed an advanced stage at first recurrence (mUICC stage ≥ 3, 75.7% vs 38.7%, P < 0.001), and 43.2% of patients had no intrahepatic HCC (mUICC T stage 0) at EHR. This suggests that EHR may occur within a very short interval after surgical resection of HCC with an advanced tumor stage while intrahepatic HCC is not exhibited. Considering the aggressive nature of the tumors in patients with early EHR, adjuvant therapy after surgical resection or meticulous postoperative surveillance may be highly required in a specific group of patients to prompt detection of EHR. In addition, with respect to the high proportion of patients with EHR as their first recurrence (52.7%), pre- and/or peri-operative factors are considered to play critical roles in predicting early EHR.
Among the several factors associated with early EHR, serum albumin < 4.0 g/dL, serum ALP > 100 U/L, surgical margin involvement, venous and/or lymphatic involvement, satellite nodules, tumor necrosis, tumor size ≥ 7 cm, and the presence of macrovascular invasion confirmed on radiologic examination were identified as risk factors related to early EHR, as determined by multivariate Cox regression analysis (Table 3). The concentrations of albumin and/or ALP alone or in combination with other parameters have been proposed as useful markers for predicting recurrence after surgical removal of HCC in previous studies[23-26]. Li et al[27] showed the prognostic value of the albumin-to-ALP ratio in patients with HCC who received liver transplants, whereas Yu et al[26] determined ALP concentration as an independent factor influencing disease-free survival and overall survival that aids in the prediction of recurrence in high-risk patients with HCC. Additionally, involvement of the surgical margins was related to poor clinical outcomes, as shown by a previous study[28] and a meta-analysis conducted by Zhong et al[29], which is consistent with the findings of our study. Chen et al[30] reported a poorer prognosis related to microvascular invasion in solitary small HCC in their meta-analysis, while Hasegawa et al[31] reported the association between lymphatic system involvement and patient survival after surgical resection of HCC, which are consistent with our findings. In a large-scale study of 734 patients who underwent surgery for HCC, satellite nodules, tumor size (> 5 cm), and the presence of macrovascular invasion were closely related to late recurrence of HCC in multivariate analysis (HR, 1.59, 1.49, and 4.63, respectively), and these findings are concordant with our study[32]. A recent study by Wei et al[33] reported that tumor necrosis was associated with the prognosis of patients undergoing surgery for HCC with regard to overall survival and RFS and advanced tumor characteristics, and Ling et al[34] also reported tumor necrosis as a potential parameter of the aggressiveness of HCC. Concordant with these previous study results, our study also demonstrated a significant association between tumor necrosis and early EHR.
In addition, we analyzed multiple factors associated with overall survival after surgical resection of HCC and identified that serum albumin < 4.0 g/dL, serum ALP > 100 U/L, major Edmondson-Steiner grade ≥ 3, pathological mUICC stage III or IVa, satellite nodules, tumor necrosis, and early EHR were related to survival (Table 4). Most risk factors exhibited similar results in the multivariate analysis of factors associated with early EHR (serum albumin < 4.0 g/dL, serum ALP > 100 U/L, satellite nodules, and tumor necrosis). The Edmondson-Steiner grade has been previously reported as a crucial predictor of recurrence and survival by Zhou et al[31] and Martins-Filho et al[32], and our study also showed poor survival outcomes in patients with major Edmondson-Steiner grade ≥ 3. Finally, early EHR showed a significant association with poor survival and increased the risk of death more than six times (HR, 6.77; 95%CI, 4.81-9.52; P < 0.001).
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Serum albumin < 4.0 g/dL | 2.14 (1.61-2.85) | < 0.001 | 2.44 (1.79-3.32) | < 0.001 |
| ALBI grade ≥ 2 | 1.84 (1.36-2.51) | < 0.001 | ||
| Serum ALP > 100 U/L | 1.84 (1.41-2.41) | < 0.001 | 1.62 (1.22-2.16) | 0.001 |
| Single tumor1 | 1.87 (1.21-2.91) | 0.005 | ||
| Major Edmondson-Steiner grade ≥ 3 | 1.64 (1.26-2.13) | < 0.001 | 1.45 (1.10-1.90) | 0.008 |
| Venous/lymphatic involvement2 | 2.44 (1.84-3.22) | < 0.001 | ||
| Pathological mUICC stage (III, IVa) | 2.59 (2.00-3.35) | < 0.001 | 1.63 (1.21-2.22) | 0.002 |
| Bile duct invasion | 2.72 (1.21-6.13) | 0.016 | ||
| Intrahepatic metastasis | 3.66 (1.72-7.77) | 0.001 | ||
| Multicentricity | 1.91 (1.29-2.82) | 0.001 | ||
| Satellite nodule | 2.45 (1.82-3.30) | < 0.001 | 1.92 (1.38-2.69) | < 0.001 |
| Tumor necrosis | 2.12 (1.63-2.77) | < 0.001 | 1.35 (1.030-1.78) | 0.030 |
| Tumor hemorrhage | 1.58 (1.21-2.05) | 0.001 | ||
| Sum of tumor size ≥ 7 cm | 2.21 (1.63-3.00) | < 0.001 | ||
| Beyond Milan criteria | 1.79 (1.38-2.33) | < 0.001 | ||
| Serum AFP > 1500 IU/mL | 1.47 (1.01-2.15) | 0.046 | ||
| Early extrahepatic recurrence | 7.72 (5.67-10.51) | < 0.001 | 6.77 (4.81-9.52) | < 0.001 |
The cumulative rates of first recurrence and overall survival were correlated with the development of EHR and the interval to EHR (Figure 1). In particular, patients with early EHR showed substantially worse survival outcomes (HR, 6.77; 95%CI, 4.81-9.52; P < 0.001). Furthermore, considering the heterogeneity of patient characteristics regarding the tumor nature, degree of liver cirrhosis, and diverse disease courses after surgical resection, predictive markers of early EHR within 2 years after surgical resection with preoperative and/or operative factors may well reflect the initial state of the tumor.
We categorized the patients by the number of eight risk factors associated with early EHR and assessed the probability rates of EHR and overall survival. As demonstrated in Figure 2, there was a significant relationship between the number of the risk factors and the cumulative rates of both EHR and overall survival (P < 0.001). For a simple and intuitive predictive model of EHR, we subgrouped the patients into three categories: a low-risk group with 0-1 risk factor, an intermediate-risk group with 2-3 risk factors, and a high-risk group with ≥ 4 risk factors. The rates of both EHR and survival showed a substantial correlation with these risk stratifications. Hence, the prediction of early EHR after surgical resection of HCC may be regarded as essential, especially for high-risk patients.
Our study had several limitations. First, this study was retrospective and included patients with heterogenous clinical courses and outcomes. The postoperative surveillance methods, schedule, and treatment methods for recurrence of HCC were not fully standardized. To minimize the potential bias owing to the design of this study, we strived to enroll a large number of patients in multiple tertiary academic hospitals. Furthermore, the fact that more than half of the patients (52.7%) developed EHR as their first recurrence highlighted the importance of preoperative and operative factors. Second, although pathological confirmation of EHR was performed for a few patients, most instances of EHR were solely identified at the physician’s discretion using imaging diagnostic modalities. Finally, owing to patient geographic characteristics, the majority of patients had chronic hepatitis B as the etiology of liver cirrhosis and HCC. Further studies are warranted to investigate characteristics and risk factors for early EHR in patients with diverse chronic hepatitis by using a standardized study protocol. Especially, the use of radiomic tumor features for the prediction of recurrence after HCC treatment can be considered as an additional tool for prompt detection of early EHR[35,36]. Using an accurate tool for the prediction of early EHR, the prognosis of patients with a high risk of early EHR may be improved with the utilization of adjunctive post-operative therapy.
Patients with early EHR showed rapid recurrence of HCC after surgical resection and significantly worse survival outcomes. . We derived several risk factors associated with early EHR and the numbers of risk factors correlated with not only with cumulative rates of EHR but also with survival rates. In terms of poor prognosis associated with early EHR, patients who undergo surgical resection for HCC can be stratified by the risk of EHR with our model, and meticulous postoperative surveillance and adjuvant therapies may be required for timely EHR detection and positive clinical outcomes.
Surgical resection is one of the most widely used modalities for the treatment of hepatocellular carcinoma (HCC). Early extrahepatic recurrence (EHR) of HCC after surgical resection is considered to be closely associated with poor prognosis.
Data regarding risk factors and survival outcomes of early EHR after surgical resection remain scarce.
We decided to investigate the clinical features and risk factors of early EHR and elucidate its association with survival outcomes.
From January 2004 to December 2019, we enrolled treatment-naïve patients who were ≥ 18 years and underwent surgical resection for HCC in two tertiary academic centers. After excluding patients with tumor types other than HCC and/or ineligible data, this retrospective study finally included 779 patients. Surgical resection of HCC was performed according to the physicians’ decisions and the EHR was diagnosed based on contrast-enhanced computed tomography or magnetic resonance imaging, and pathologic confirmation was performed in selected patients. Multivariate Cox regression analysis was performed to identify the variables associated with EHR.
Early EHR within 2 years after surgery was diagnosed in 9.5% of patients during a median follow-up period of 4.4 years. The recurrence-free survival period was 5.2 mo, and the median time to EHR was 8.8 mo in patients with early EHR. In 52.7% of patients with early EHR, EHR occurred as the first recurrence of HCC after surgical resection. On multivariate analysis, serum albumin < 4.0 g/dL, serum alkaline phosphatase > 100 U/L, surgical margin involvement, venous and/or lymphatic involvement, satellite nodules, tumor necrosis detected by pathology, tumor size ≥ 7 cm, and macrovascular invasion were determined as risk factors associated with early EHR. After sub-categorizing the patients according to the number of risk factors, the rates of both EHR and survival showed a significant correlation with the risk of early EHR. Furthermore, multivariate analysis revealed that early EHR was associated with substantially worse survival outcomes (hazard ratio, 6.77; 95% confidence interval, 4.81-9.52; P < 0.001).
Early EHR significantly deteriorates the survival of patients with HCC, and our identified risk factors may predict the clinical outcomes and aid in postoperative strategies for improving survival.
Further studies are warranted to investigate characteristics and risk factors for early EHR in patients with diverse chronic hepatitis by using a standardized study protocol. Using an accurate tool for the prediction of early EHR, the prognosis of patients with a high risk of early EHR may be improved with the utilization of adjunctive post-operative therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding J, China; Manrai M, India; Posa A, Italy S-Editor: Wu YXJ L-Editor: A P-Editor: Li X
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3178] [Article Influence: 529.7] [Reference Citation Analysis (37)] |
| 2. | Torimura T, Iwamoto H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin Mol Hepatol. 2021;27:236-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2621] [Article Influence: 873.7] [Reference Citation Analysis (59)] |
| 4. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 5. | Kim J, Kang W, Sinn DH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Substantial risk of recurrence even after 5 recurrence-free years in early-stage hepatocellular carcinoma patients. Clin Mol Hepatol. 2020;26:516-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Taketomi A, Toshima T, Kitagawa D, Motomura T, Takeishi K, Mano Y, Kayashima H, Sugimachi K, Aishima S, Yamashita Y, Ikegami T, Gion T, Uchiyama H, Soejima Y, Maeda T, Shirabe K, Maehara Y. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 8. | Wang L, Ye G, Zhan C, Sun F, Lin Z, Jiang W, Wang Q. Clinical Factors Predictive of a Better Prognosis of Pulmonary Metastasectomy for Hepatocellular Carcinoma. Ann Thorac Surg. 2019;108:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Yoon JH, Lee WJ, Kim SM, Kim KT, Cho SB, Kim HJ, Ko YS, Kook HY, Jun CH, Choi SK, Kim BS, Cho SY, You HS, Lee Y, Son S. Simple parameters predicting extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Sci Rep. 2021;11:12984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Li WF, Yen YH, Liu YW, Wang CC, Yong CC, Lin CC, Cheng YF, Wang JH, Lu SN. Preoperative predictors of early recurrence after resection for hepatocellular carcinoma. Am J Surg. 2022;223:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Beumer BR, Takagi K, Vervoort B, Buettner S, Umeda Y, Yagi T, Fujiwara T, Steyerberg EW, IJzermans JNM. Prediction of Early Recurrence After Surgery for Liver Tumor (ERASL): An International Validation of the ERASL Risk Models. Ann Surg Oncol. 2021;28:8211-8220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Saito A, Toyoda H, Kobayashi M, Koiwa Y, Fujii H, Fujita K, Maeda A, Kaneoka Y, Hazama S, Nagano H, Mirza AH, Graf HP, Cosatto E, Murakami Y, Kuroda M. Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod Pathol. 2021;34:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ; Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1325] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 14. | Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 15. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 16. | EDMONDSON HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 17. | Yoon JH, Goo YJ, Lim CJ, Choi SK, Cho SB, Shin SS, Jun CH. Features of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2020;26:4833-4845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 20. | Yan WT, Li C, Yao LQ, Qiu HB, Wang MD, Xu XF, Zhou YH, Wang H, Chen TH, Gu WM. Predictors and long-term prognosis of early and late recurrence for patients undergoing hepatic resection of hepatocellular carcinoma: a large-scale multicenter study. Hepatobiliary Surg Nutr. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 21. | Yang SL, Luo YY, Chen M, Zhou YP, Lu FR, Deng DF, Wu YR. A systematic review and meta-analysis comparing the prognosis of multicentric occurrence and vs. intrahepatic metastasis in patients with recurrent hepatocellular carcinoma after hepatectomy. HPB (Oxford). 2017;19:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Byeon J, Cho EH, Kim SB, Choi DW. Extrahepatic recurrence of hepatocellular carcinoma after curative hepatic resection. Korean J Hepatobiliary Pancreat Surg. 2012;16:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J, Zou R, Shen J, Li B, Yuan Y. Nomogram to Predict Survival of Patients With Recurrence of Hepatocellular Carcinoma After Surgery. Clin Gastroenterol Hepatol. 2018;16:756-764.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Jeng LB, Li TC, Hsu SC, Chan WL, Teng CF. Association of Low Serum Albumin Level with Higher Hepatocellular Carcinoma Recurrence in Patients with Hepatitis B Virus Pre-S2 Mutant after Curative Surgical Resection. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Ekpanyapong S, Philips N, Loza BL, Abt P, Furth EE, Tondon R, Khungar V, Olthoff K, Shaked A, Hoteit MA, Reddy KR. Predictors, Presentation, and Treatment Outcomes of Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Large Single Center Experience. J Clin Exp Hepatol. 2020;10:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS, Lee WC, Chen MF. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Li H, Wang L, Chen L, Zhao H, Cai J, Yao J, Zheng J, Yang Y, Wang G. Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio in Hepatocellular Carcinoma Patients Treated with Liver Transplantation. J Cancer. 2020;11:2171-2180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of Surgical Margin on the Prognosis of Early Hepatocellular Carcinoma (≤5 cm): A Propensity Score Matching Analysis. Front Med (Lausanne). 2020;7:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Zhong FP, Zhang YJ, Liu Y, Zou SB. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2017;96:e8043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Chen ZH, Zhang XP, Wang H, Chai ZT, Sun JX, Guo WX, Shi J, Cheng SQ. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB (Oxford). 2019;21:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 31. | Hasegawa K, Makuuchi M, Kokudo N, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, Matsuyama Y; Liver Cancer Study Group of Japan. Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2014;259:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 33. | Wei T, Zhang XF, Bagante F, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Grigorie R, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Tumor Necrosis Impacts Prognosis of Patients Undergoing Curative-Intent Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Ling YH, Chen JW, Wen SH, Huang CY, Li P, Lu LH, Mei J, Li SH, Wei W, Cai MY, Guo RP. Tumor necrosis as a poor prognostic predictor on postoperative survival of patients with solitary small hepatocellular carcinoma. BMC Cancer. 2020;20:607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Wang F, Chen Q, Zhang Y, Chen Y, Zhu Y, Zhou W, Liang X, Yang Y, Hu H. CT-Based Radiomics for the Recurrence Prediction of Hepatocellular Carcinoma After Surgical Resection. J Hepatocell Carcinoma. 2022;9:453-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Iezzi R, Casà C, Posa A, Cornacchione P, Carchesio F, Boldrini L, Tanzilli A, Cerrito L, Fionda B, Longo V, Miele L, Lancellotta V, Cellini F, Tran HE, Ponziani FR, Giuliante F, Rapaccini GL, Grieco A, Pompili M, Gasbarrini A, Valentini V, Gambacorta MA, Tagliaferri L, Manfredi R. Project for interventional Oncology LArge-database in liveR Hepatocellular carcinoma - Preliminary CT-based radiomic analysis (POLAR Liver 1.1). Eur Rev Med Pharmacol Sci. 2022;26:2891-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |