Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5250
Peer-review started: March 9, 2022
First decision: April 11, 2022
Revised: April 30, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 28, 2022
Processing time: 197 Days and 22 Hours
Transforming growth factor-beta (TGF-β) is a multifunctional cytokine that performs a dual role as a tumor suppressor and tumor promoter during cancer progression. Among different ligands of the TGF-β family, TGF-β1 modulates most of its biological outcomes. Despite the abundant expression of TGF-β1 in the liver, steatosis to hepatocellular carcinoma (HCC) progression triggers elevated TGF-β1 levels, contributing to poor prognosis and survival. Additionally, elevated TGF-β1 levels in the tumor microenvironment create an immunosuppressive stage via various mechanisms. TGF-β1 has a prime role as a diagnostic and prognostic biomarker in HCC. Moreover, TGF-β1 is widely studied as a therapeutic target either as monotherapy or combined with immune checkpoint inhibitors. This review provides clinical relevance and up-to-date information regarding the potential of TGF-β1 in diagnosis, prognosis, and therapy against HCC.
Core Tip: Transforming growth factor-beta 1 (TGF-β1) exhibits a progressive elevation throughout hepatic dysfunction starting from hepatitis to hepatocellular carcinoma (HCC) as an inflammatory cytokine, pro-fibrogenic marker, immunosuppressive agent and pro-carcinogenic growth factor. Aberrant TGF-β1 activation in HCC is associated with poor prognosis and survival. TGF-β1 mediated immunosuppression disturbs the anticancer surveillance and the efficacy of the immunotherapeutic agent. This pleiotropic effect of TGF-β1 in the context of HCC makes it ideal as a diagnostic, prognostic, and therapeutic candidate in HCC.
- Citation: Devan AR, Pavithran K, Nair B, Murali M, Nath LR. Deciphering the role of transforming growth factor-beta 1 as a diagnostic-prognostic-therapeutic candidate against hepatocellular carcinoma. World J Gastroenterol 2022; 28(36): 5250-5264
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5250
Liver cancer, specifically hepatocellular carcinoma (HCC) is often recognized as an aggressive malignancy, ranking 6th in incidence and 3rd in terms of mortality in 2020, where mortality rates are roughly equivalent to incidence rate[1]. As it develops in the background of chronic inflammation starting from the fatty liver, HCC remains undiagnosed for years until it worsens. The progressive transformation from cirrhosis to HCC also creates longer delays in diagnosis[2]. HCC is commonly diagnosed by liver imaging techniques such as ultrasound, computed tomography and magnetic resonance imaging. Blood biomarkers such as alpha fetoprotein (AFP), protein induced by vitamin K absence or antagonist II levels, and liver biopsy are also used to diagnose HCC[3,4]. Even though the recent guidelines recommend HCC surveillance biannually and antiviral vaccination, the lack of effective surveillance programs significantly contributes to HCC progression to the advanced stage, particularly in high-risk individuals. Once established, HCC cells rapidly proliferate and spread to the extrahepatic site, such as the lungs, portal vein, and lymph nodes. In such cases, in an advanced stage, curative interventions such as liver transplantation, resection, percutaneous ablation, and chemoembolization were not responsive. Systemic drug therapy remains the primary treatment modality[5,6], where immunotherapy and tyrosine kinase inhibitors are the approved treatment options[7]. However, limited response to therapy, the emergence of multidrug resistance, immunosuppressive tumor microenvironment, and lack of validated diagnostic and prognostic biomarkers pose significant obstacles in establishing effective treatment against HCC[8,9].
Transforming growth factor-beta (TGF-β) is a critical homeostasis regulator, which is aberrantly activated during inflammation, fibrosis and carcinogenesis[10]. Among the three isoforms, TGF-β1 is superior in TGF-β signal transduction, especially those related to chronic liver diseases. Since HCC is an inflammation-induced-immunosuppressed malignancy, the role of TGF-β1 signaling in HCC has been extensively evaluated recently[11]. This review presents the potential of the TGF-β superfamily of ligands, specifically TGF-β1, to develop as a therapeutic and prognostic-diagnostic marker candidate against HCC.
TGF-β superfamily of ligands is a dimeric peptide growth factor with more than 30 members in humans, mainly TGF-βs, activins, inhibins, and bone morphogenetic proteins. TGF-βs are categorized into three different isoforms TGF-β1, TGF-β2, and TGF-β3. Of the three isoforms, TGF-β1 is the most evaluated and abundant, found in epithelial, endothelial, hematopoietic, and connective tissue. TGF-β 2 is expressed in epithelial and neuronal cells, while TGF-β3 is found in mesenchymal cells[12]. TGF-βs are implicated in diverse physiological processes, including cell homeostasis and embryonic development[13]. It is a pleiotropic factor that regulates inflammation, fibrogenesis, cell differentiation, proliferation, epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) formation, tumor-suppressive and pro-tumor effect in a cell-context dependent manner[14].
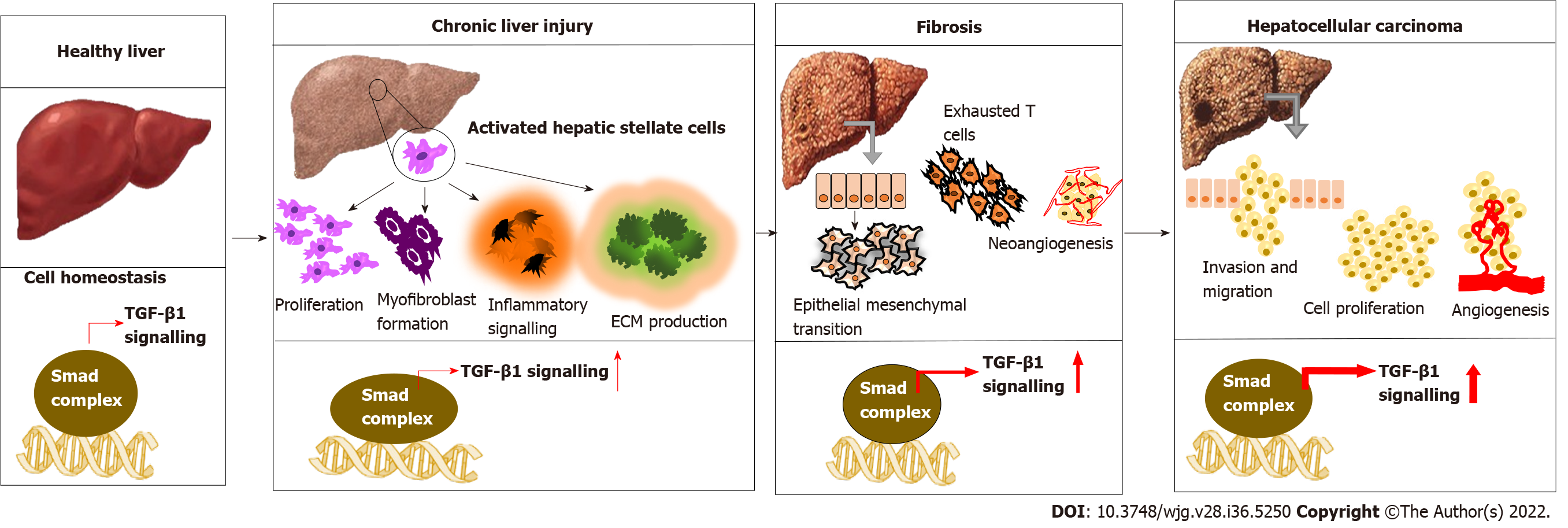
The three isoforms of TGF-β have structural similarity and functional redundancy[15]. However, TGF-β1 is often considered a potent and superior isoform with significant physiological and pathological importance[16]. Importantly, TGF-β1 exerts a cell-context dependent effect in the liver[17,18]. Normal to activated TGF-β1 signature confers a protective effect by inhibiting hepatocyte proliferation and hepatic stellate cells (HSCs) activation, apoptosis induction, preventing fibrosis and improving liver function. While, aberrantly activated TGF-β1 signature manifests as HSC activation and worsening fibrosis to HCC, where tumor cells lose their sensitivity toward the inhibitory effect of TGF-β1[19-21]. Exposure of hepatocytes to various causative factors such as viruses, alcohol, toxicants and other metabolic disorders leads to the release of TGF-β1. Also, other pro-inflammatory cytokines such as tumor necrosis factor-α and growth factors from non-parenchymal liver cells switch on inflammation, production of ECM, and accumulation of fibrous material that eventually progress to cirrhosis[22,23]. A simultaneous increase in integrins, a vital cell adhesion molecule, is observed as the fibrogenesis continues. These integrins interact with TGF-β1 and other ECM proteins, altering signal transduction pathways[24]. Along with the accumulation of genetic mutations, HSC induces TGF-β and β-catenin-dependent EMT, leading to tumor growth in the liver. TGF-β1 continues to increase, promoting neo-angiogenesis by interacting with other pathways and mediating stromal-tumor cell interaction, conferring aggressive phenotype and metastasis[25] (Figure 1). Wang et al[26] demonstrated that both TGF-β1 and TGFβR1 have a crucial role in regulating proliferation, invasion, metastasis and immune response in HCC cells.
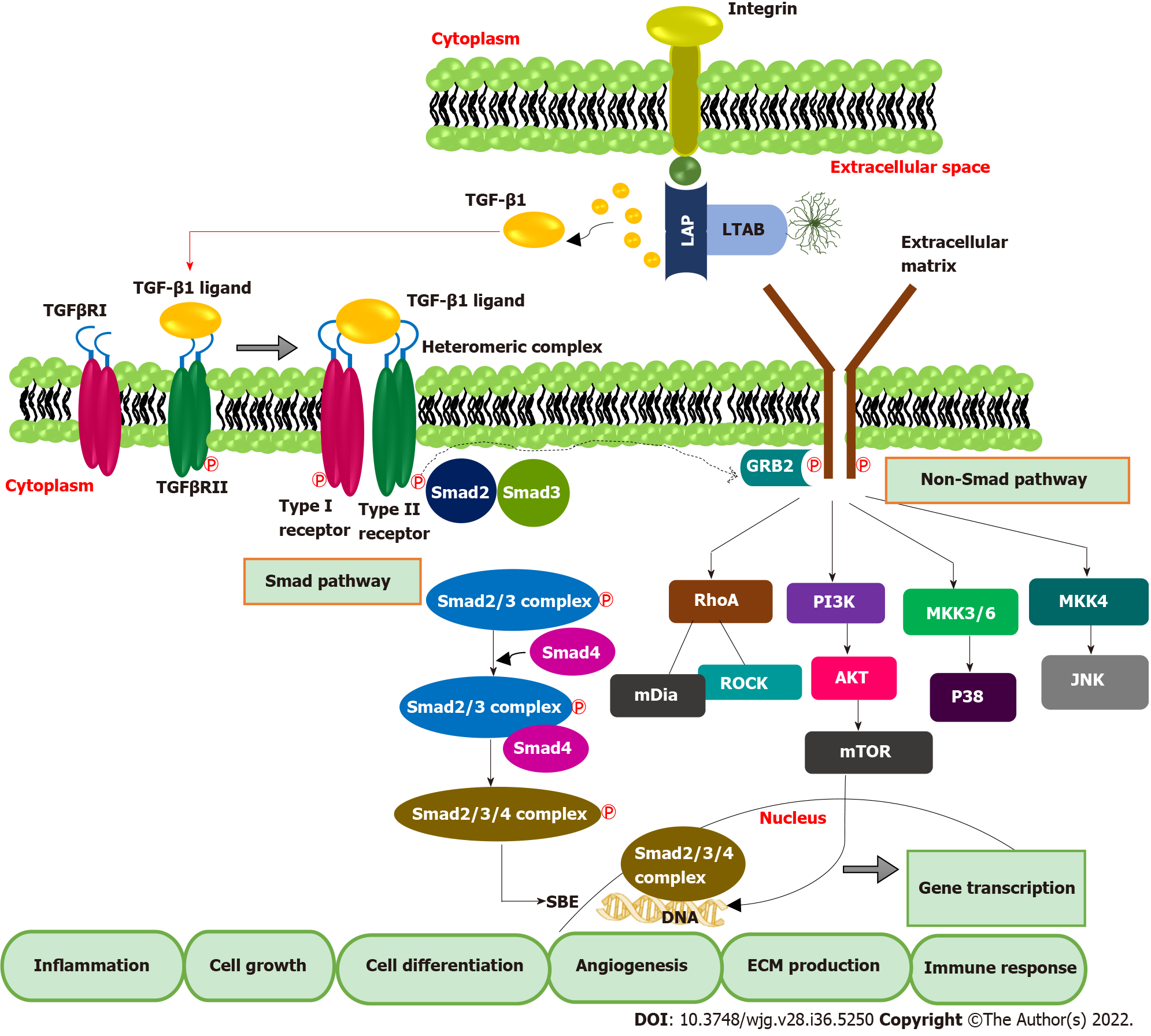
TGF-β1 exerts biological and pathological effects via Smad and non-Smad pathways. TGF-βs are synthesized in the inactivated form and exist as latent TGF-β complex (LTC) by binding with latency-associated protein. Later, LTC is converted to large latent complex (LLC) by interacting with latent TGF-β binding protein in ECM. Integrin signaling plays a significant role in the activation and subsequent release of TGF-β1 from LLC. It is also mediated by other factors such as pH, protease enzyme etc.[27]. In the canonical Smad pathway, activated TGF-β first binds with the extracellular domain of TGF-β receptor type II, which triggers the cross phosphorylation of the kinase domain of TGF-β receptor type I. TGF-β R1 activation leads to the phosphorylation of Smad proteins, Smad 2 and Smad 3. Later Smad-2 and 3 complex bind with the co-Smad, Smad-4 to form a ternary complex. This ternary complex is then translocated into the nucleus, binds to Smad binding elements in DNA, and activates the transcription of TGF-β-dependent genes[28]. Binding of inhibitory Smad, Smad-7 will shut down the activated pathway[29]. In addition to the Canonical Smad pathway, TGF-β can exert biological functions by activating other diverse signalings such as P38, JNK, PI3K/AKT, RAS-ERK, and RHO-ROCK, which constitute the non-Smad pathway of TGF-β signal transduction[30] (Figure 2).
Smad pathway of TGF-β signal transduction also enhances the transcription of FoxP3, predominantly present in T regulatory (Treg) cells[31,32]. A high amount of tumor-infiltrating Treg cells and FoxP3 positive Treg cells in blood is reported in HCC patients, leading to the deterioration of effector T cells such as CD4+ and CD+ cytotoxic T cells lymphocytes, which are pillars of anticancer immunity[33,34]. Together, TGF-β inhibits natural killer (NK) cells, blocks interferon (IFN)-γ secretion, and prevents effector immune cells recruitment to tumor tissue[35,36]. Additionally, TGF-β inhibits IFN-γ secretion by interacting with the activating transcription factor 1[37]. Likewise, TGF-β-RUNX3 transcription factor interaction and co-expression of programmed death ligand 1 (PD-L1) and interleukin-10 promote the transformation of naïve B cells to immunoglobulin A producing B cells, which are crucial in HCC development from non-alcoholic fatty liver[38]. Elevated TGF-β can directly enhance the transcription of PD-1 in HCC. The interaction of PD-1 with PD-L1 causes significant immunosuppression by T cell exhaustion, which manifests as inhibition of T cell activation, proliferation, and cytotoxic action[39,40]. In a recent study, Bao et al[41] reported that TGF-β1 trigger the expression of immune checkpoints such as PD-1 and CTLA4 on HCC cells and attenuates T-cell-mediated anti-tumor immune surveillance. Therefore, up-regulated TGF-β mainly isoforms 1 directly affect immune checkpoint inhibition, and it works as an indicator of T cell exhaustion. This evidence suggests the potential of TGF-β1 targeted immunotherapies against HCC. The pivotal role of the TGF-β1 signature in hepatic dysfunction and HCC extends its potential as a biomarker molecule for diagnosis and prognostic prediction and a therapeutic target.
As an inflammatory-fibrogenic cytokine molecule, the involvement of TGF-β1 in all stages of liver injury, starting from fatty liver, steatosis, fibrosis to cirrhosis, and HCC, is evident. Intergromic analysis of TGF-β gene alterations among the 33 cancer types in the TGCA dataset revealed 39% alterations. Gastrointestinal cancers and HCC exhibited prominent mutation compared with other cancer types[42]. Later, in an HCC-specific transcriptomic analysis, 40% of HCC samples were found with mutations in genes of the TGF-β pathway[43]. Higher TGF-β1 levels in HCC correlate with the high rate of extrahepatic metastasis (EHM), poor prognosis, and low survival rate[44]. After acute/chronic liver injury, liver sinusoidal endothelial cells and HSCs secrete TGF-β1 and up-regulate TGF-β receptors[45,46]. Elevated TGF-β1 level was found in viral and alcohol-induced fibrosis[47]. Thus, TGF-β levels can be used to track the response to therapy so that the decrease in TGF-β 1 level followed by IFN treatment in hepatitis B virus (HBV) patients is associated with improved treatment outcomes. Apart from HSC-triggered TGF-β secretion, hepatitis C virus (HCV) infection can also induce TGF-β1 production in hepatocytes[48]. Likewise, proteomic and phospho-proteomic characterization of 110 tumor and non-tumor tissues of early-stage HBV-associated HCC found an increased expression of TGF-β genes compared with the non-tumor tissue[49]. However, the dichromatic role of TGF-β1 on cancer growth i.e., tumor suppressive in early-stage or oncogenic effect in late-stage, is a matter of concern. Another study indicated a comparative functional genomic approach and illustrated the link between TGF-β expression signature and HCC subtypes. The study shows that TGF-β positive HCC clusters can be categorized into two. HCC with early TGF-β signature exhibit physiological responses while HCC associated with late TGF-β signature showed metastasis and poor survival[50]. N-2-fluorenylacetamide-induced rat hepatoma model is used to investigate the association of TGF-β1 expression with different stages of hepatocarcinogenesis and a progressive elevation of hepatic TGF-β1 and TGF-β1 mRNA was found during the transformation of hepatocytes to malignant cells[51]. Another study indicates that elevated plasma TGF-β1 level was found in 89.5% of HCC patients, and interestingly, among these patients, 93.3% had an AFP level less than 400 μg/L[52]. This suggested that TGF-β1 expression can be a more accurate and sensitive biomarker for early diagnosis of HCC for monitoring the disease progression.
The diagnostic importance of TGF-β1 is established significantly earlier itself. In 1997, Tsai et al[53] investigated the correlation of urine TGF-β1 level with HCC. They found a significant increase in TGF-β1 level in HCC patients compared with other healthy control groups, cirrhotic chronic hepatitis patients. They also reported the association of TGF-β 1 levels with poor prognosis and shorter survival[53]. Later, the same team compared the study with another important tumor marker, AFP, and found that disease progression from cirrhosis to HCC is characterized by a typical elevation in both urinary TGF-β1 and serum AFP with a diagnostic accuracy of about 90%[54,55]. These collective data suggest the potential of TGF-β1 be used along with AFP as a complementary tumor marker to differentiate HCC from cirrhosis correctly. Another study investigated the rationale for parallel determination of TGF-β1 and AFP to diagnose HCC. They found that the TGF-β1 level exhibited a stage-dependent increase in all liver diseases where AFP showed HCC-specific elevation[56]. As TGF-β1 estimation tracks the disease progression pattern, TGF-β can be considered a more sensitive diagnostic marker of HCC. Its specificity is higher when it is analyzed along with AFP. To diagnose and select patients for galunisertib (TGF-β inhibitor) therapy, Cao et al[57] in 2017 performed next-generation sequencing-based analysis in HCC samples and found that m RNA levels of TGF-β1 along with SKIL and PMEPA1 could be better diagnostic markers as well as to select patients who are more likely to respond with galunisertib.
Another study investigated the association of serum TGF-β1 with disease severity in HCC using 180 subjects in different stages of HCC. Group of cirrhotic patients, as well as healthy control, was also maintained. Consistent with the previous reports, this study also found a significant increase in TGF-β1 level in HCC (1687.47 ± 1462.81 pg/mL) as compared with cirrhotic patients (487.98 ± 344.23 pg/mL) and control (250.16 ± 284.16 pg/mL). Additionally, the serum level of TGF-β1 showed exponential elevation as the disease progressed from early to advanced, i.e., during progression from Barcelona Clinic Liver Cancer stage A to D, TGF-β 1 level increased from 652.83-1668.78 pg/mL[56]. The best cut-off value of TGF-β1 detection was determined as 301.9 pg/mL, comparable with the value (370 pg/mL) reported by Shehata et al[58] (Table 1).
| Ref. | Sample size HCC/control | Assay type | TGF-β1 level | Sample type | Outcome of the study |
| [60,61] | 26/20 | ELISA | Control: 1.4 ± 0.8 ng/mL | Plasma | TGF-β1 level showed a progressive elevation from cirrhotic to HCC patients to normal subjects. No significant association was found between plasma TGF-β1 and serum AFP levels |
| HCC: 19.3 ± 1.95 ng/mL (P < 0.05) | |||||
| [62,63] | 70 | ELISA | Control: 2.7 ± 0.7 ng/mL | Plasma | Elevated plasma TGF-β1 levels in HCC patients are associated with increased tumor size, overexpression of tissue inhibitor of metalloproteinase-1 and tumor severity |
| HCC: 7.3 ± 4.3 ng/mL (P < 0.05) | |||||
| [54,55] | 94/50 | 125I-Radio Immuno Assay Kit | Control: 1.5-33.6 μg-1creatinine | Urine | Urinary TGF-β1 and serum AFP levels were higher in HCC than in cirrhotic patients. The study suggested that both TGF-β and AFP can be used as complementary biomarkers to distinguish between HCC and cirrhosis |
| Cirrhotic: 4.3-52.5 μg-1creatinine | |||||
| HCC: 3.5-184 μg-1creatinine (P < 0.0001) | |||||
| [64] | 54/30 | ELISA | TGF-β1 score | Serum | The study team calculated the serum concentration score based on the cut-off limit of 74 pg/mL and 637 pg/mL for TGF-β1 and sFas, respectively. TGF-β1 levels were higher than the cut off value in 23% HCC patients with negative AFP values, suggesting its diagnostic potential in AFP negative HCC |
| Control: 0.6 ± 0.2 | |||||
| HCC: 1.6 ± 0.5 | |||||
| [65] | 38/23 | ELISA | Control: 300 pg/mL | Plasma | Elevated plasma TGF-β1 level can be a useful diagnostic marker in detecting small HCC, with higher sensitivity than AFP |
| HCC: 954.9 pg/mL (P < 0.0001) | |||||
| [66] | 70/32 | ELISA | Control: 2 ng/mL | Plasma | Higher circulating TGF-β1 in HCC patients is associated with suppression of anti-tumor immunity and disease progression |
| HCC: 7.5 ng/mL (P < 0.0001) | |||||
| [52] | 50/30 | ELISA | Control: 0.67 ± 0.1 μg/mL | Serum | Aberrant TGF-β1 expression in HCC is associated with differentiation and worsening of HBV infection |
| HCC: 2.21 ± 1.1 μg/mL (sensitivity = 89.5%, specificity = 94%) | |||||
| RT-PCR | Overexpression TGF-β1 mRNA in HCC patients, P < 0.0001 | Circulating TGF-β1 level and TGF-β1 mRNA expression can be used as sensitive biomarkers for diagnosing HBV induced HCC | |||
| [56] | 23/40 | ELISA | Control: 14.35 ± 8.76 ng/mL | Serum | TGF-β1 is a sensitive diagnostic marker for HCC than AFP. Specificity can be increased with combined evaluation of TGF-β1 and AFP levels |
| HCC: 64.35 ± 33.68 ng/mL (P < 0.05) | |||||
| [67] | 54/30 | ELISA | Control: 39.5 ± 9.8 pg/mL | Serum | The study suggested elevated TGF-β1 and EGFR levels as reliable diagnostic markers for HCC induced, AFP negative HCC |
| HCC: 1194 ± 331 pg/mL (P < 0.0001) | |||||
| [68] | 120/30 | ELISA | Control: 250.16 ± 284.61 pg/mL | Serum | TGF-β1 showed progressive elevation during various stages of liver dysfunction. Higher TGF-β1 level in HCC is associated with tumor grade, pathological stage and invasiveness |
| Cirrhotic: 487.98 ± 344.23 pg/mL | |||||
| HCC: 1687.47 ± 1642 pg/mL (P < 0.0001) | |||||
| [69] | 100/36 | ELISA | Control: 57.29 ± 11.70 ng/mL | Serum | Serum levels of TGF-β were significantly higher in HCC patients than in normal controls |
| HCC: 225.82 ± 48.93 ng/mL (P < 0.0001) |
Background inflammation and indolent transformation are the critical factors that create a waiting time paradox in diagnosing HCC, making the tumor more aggressive and refractory. Since TGF-β, specifically TGF-β1 plays an essential function from the initial hepatic injury to hepatocarcinogenesis, it holds immense potential to validate as a diagnostic marker of HCC. Though the dual functioning of TGF-β1 is still debatable, the diagnostic relevance of TGF-β1 is well evident, and thus, it warrants further investigations and clinical validation.
Poor prognostic characteristics of HCC contribute to late detection, aggressiveness and failure of therapeutic interventions[70]. Molecular pathways of hepato-carcinogenesis are still confusing because of the involvement of diverse molecular pathways, genetic alterations and evolution of malignant cells. Thus, this ultimately results in the worst prognosis within the early stage itself[71]. The expression of TGF-β1 is remarkably increased at the advanced stages of HCC and is involved in initiating EMT, regulating tumor proliferation, and promoting immunosuppressive tumor microenvironment during HCC progression under the challenges like liver cirrhosis, HBV and HCV infections. This warrants screening TGF-β1 levels from the early stages of HCC as a tool for evaluating the clinical outcomes. Depending upon the expression profile of TGF-β1, it is effortless to estimate the clinical impact of therapeutic strategies[72].
A research study conducted by Giannelli et al[73] proposed that TGF-β1 promotes EMT by stimulating homologous proteins like snail and slug. Secretion of TGF-β1 by HCC invasive cell lines, especially cell lines with α3β1-integrin expression, is significantly higher than in non-invasive and cirrhotic cell lines. The patients at the initial and advanced stages of HCC with a higher profile of TGF-β1 possess a poor prognostic ratio with lower overall survival (OS) and disease-free survival rate (DFS)[73,74]. Likewise, another notable experimental study by Lee et al[75] demonstrated that plasma TGF-β1 is positively correlated with critical conditions like EHM, portal vein thrombosis, EHM, and regional lymph node involvement. Statistical studies involving the detailed examination of overall and cumulative survival rates of HCC patients showed that candidates with abundant levels of plasma TGF-β1 manifested remarkably lower survival rates than the candidates with lower expression of TGF-β1. This evidence points to the usefulness of TGF-β1 as a prognostic marker in HCC.
Wang et al[76] elucidated the crucial involvement of TGF-β1 in tumor progression. A total of 180 patients with HCC were selected for the study, and out of 180 HCC patients, 105 patients were found with a solid expression of TGF-β1. This study showed a positive correlation between TGF-β1 and Treg cells. The increased secretion of TGF-β1 at the starting stage of HCC indicates that the tumor may be one of the most critical sources of TGF-β1 in HCC patients. Earlier studies also provided evidence that TGF-β1 promotes the regulatory phenotype and modulates the biological functions of Tregs. By Kaplan-Meier evaluation, HCC patients overexpressing TGF-β1 in neoplastic tissues had a considerably shorter OS and a greater recurrence rate than patients with lower expression[76]. A meta-analysis study conducted by Peng et al[77] reported that TGF-β1 implements an unfavorable prognosis on OS rates of HCC patients with a hazard ratio of about 1.71 and 2.29 from both univariate and multivariate analysis. Additionally, the study indicates the worst prognosis of TGF-β1 upon DFS, relapse-free survival and progression-free survival of 1422 patients via COX univariate analysis with a hazard ratio of about 1.60. In summary, the results from these studies draw out the negative prognostic impact of high TGF-β1 expression on the OS in HCC patients.
TGF-β1 possesses a dual functional role in malignancy; initially, it acts by blocking epidermal growth and promoting tumor suppression, but in later stages, it appears to be involved in the up-gradation of advanced tumors[78]. Embryonic liver fodrin (ELF), a novel form of β-spectrin is involved as a Smad3/4 adaptor in TGF-β mediated tumor suppression signaling pathway. Miscolocalization of Smad3 and Smad4 caused by the dysregulation of ELF resulted in the disruption of TGF- β signaling pathways[79]. A research study conducted by Ji et al[80] investigated the predictive value of both TGF-β1 and ELF in HCC patients after hepatic resection. The expression of TGF-β1 is significantly higher in HCC tissues than in normal liver tissues, while the incidence of ELF is higher in normal liver tissue in contrast with HCC samples. The reports of post-operative survival rates of HCC patients with lower expressions of TGF-β1 showed that DFS and OS rates of HCC patients over 1 (79.4%), 3 (73.5%), and 5 (62.0%) years were significantly higher than the patients with higher expression of TGF-β1 (28.0%, 12.0%, and 12.0%). The study also showed a negative correlation between TGF-β1 and ELF levels. The study also indicates that DFS rates of HCC patients with higher expression of ELF and lower TGF-β1 levels are remarkably more elevated than the HCC patients with low expression of ELF and higher TGF-β1 levels for 1, 3, and 5 years (75.0%, 60.0%, and 57.5% vs 25.0%, 15.9%, and 10.2%, respectively), with P-value less than 0.001. Data from clinicopathological examination exhibited that TGF-β1 positively relates with hepatitis B surface antigen, tumor size, tumor number, TNM, and recurrence, while ELF is negatively correlated with all metastatic characteristics suggesting that ELF is associated with tumor suppressing features. This research study indicates that both TGF-β1and ELF can be included in the category of relevant biomarkers as prognostic agents for evaluating clinical results after hepatic resection[80].
An experimental approach described the correlation and the possibility of Fibroblast growth factor (FGF) receptor 4 (FGFR4) and TGF-β1 as prognostic biomarkers in HCC. FGFR4 is the most predominant isoform of the FGFRs family and is actively involved in various biological activities, including metastasis, differentiation, embryonic development, proliferation, apoptosis and angiogenesis[81]. Multiple studies showed that FGFR4 plays a clear-cut role in the pathogenesis of HCC and the up-regulation of FGFR4 possesses resistance to various targeted therapies[82]. A clinicopathological examination conducted by Chen et al[83] showed that elevated expression of both TGF-β1 and FGFR4 enhances tumors’ invasiveness and metastatic nature. Clinicopathologic characteristics revealed that HCC patients at advanced stages with high TGF-β1 and FGFR4 expression were more likely to be at a higher TNM stage. Statistical data showed that the OS of patients over five-year survival rate is about 8.5%, and the median survival duration is 32.3 mo in case of TGF-β1 positive cases. In contrast, in TGF-β1 negative expression cases, the OS of the patient is about 45.6% and the median survival rate is 50.4 mo. Candidates with high TGF-β1 expression had a short OS rate in contrast to those with negative TGF-β1 expression profiles. Likewise in cases of high levels of FGFR4, the OS rate of patients is very low, that is five-year survival rate is only about 8.3%, and median survival rate is 29.4 mo while in the condition of impeded FGFR4 expression, the five-year survival rate is about 70.1% and the median survival period is 51.2 mo. This study showed a positive correlation between TGF-β1 and FGFR4 as prognostic markers in HCC. Additionally, the results from univariate and multivariate analyses showed that both TGF-β1 and FGFR4 are independent and reliable prognostic factors in HCCs for evaluating the therapeutic response in HCC patients, especially after post-operative procedures[83]. The strong correlation between TGF-β1 expression and survival rates of HCC patients suggests its potential as a prognostic biomarker for HCC (Table 2). In addition to the role of TGF-β1 as an effective prognostic marker, it can also be used for targeted therapeutic strategies.
| Ref. | Sample size HCC/control | Sample and assay type | TGF-β1 level | Survival rate (%), (patients with higher TGF-β1 vs patients with lower TGF-β1) | Outcome of the study | ||
| [75] | 571/551 | Plasma | Control: 3.58 ± 0.17 log10 pg/mL | 1 yr survival (47 vs 60) | 3 yr survival (28 vs 36) P < 0.05 | Plasma TGF-β1 levels showed a positive correlation with tumor size, invasion and extrahepatic metastasis and inversely correlated with survival rates in HCC patients | |
| ELISA | Cirrhotic: 3.20 ± 0.37 log10 pg/mL | ||||||
| HCC: 3.83 ± 0.31 log10 pg/mL | |||||||
| [83] | 126 | Tumor tissue | 84% samples (106/126) showed high intra-tumoral TGF-β1 expression | 5 yr survival (8.5 vs 45.6) | TGF-β1 and FGFR4 were positively correlated in HCC tumor tissues and showed a significant association with shorter survival rates in patients | ||
| Immunohistochemistry | 64.3% samples (81/126) showed high peri-tumoral TGF-β1 expression | ||||||
| [80] | 84/20 | Tumor tissue | TGF-β1 overexpression found in 59.5% samples (50/84) than that of normal liver tissue | 1 yr survival (28 vs 79.4) | 5 yr survival (12 vs 62.6) | TGF-β1 expression was dominant, whereas ELF expression was suppressed in HCC tissues | |
| Immunohistochemistry | Patients with high TGF-β1 and lower ELF expression are associated with poor overall survival and post-operative disease free survival compared with low TGF-β1 and high ELF group | ||||||
| [76] | 184/30 | Plasma and tumor tissue | Elevated plasma TGF-β1 level | 2 yr survival ( 51 vs 77) | 3 yr survival (4 vs 68), P < 0.05 | Higher TGF-β1 expression in tumor tissues triggers Treg cells mediated immunosuppression in tumor microenvironment and contribute to poor prognosis in HCC | |
| ELISA and immunohistochemistry | TGF-β1 was strongly stained in tumor tissue | ||||||
| [84] | 40 | Serum | Before RFA: 63.22 ± 23.61 ng/mL | After RFA: 56.33 ± 24.24 ng/mL | NA | Radiofrequency ablation lowered TGF-β1 and AFP L3% expression in HCC patients | |
| ELISA | Low TGF-β1 and AFP L3% levels were observed in the no recurrence group, suggesting its potential as prognostic markers for HCC | ||||||
Tyrosine kinase inhibitor, Sorafenib was the first approved first-line therapy for advanced HCC. Sorafenib was the first-line therapy for ten years until another tyrosine kinase inhibitor, Levatinib was approved in 2018. Even though tyrosine kinase inhibitors dominated HCC therapy as first-line or second-line options, the efficacy was only modest, with limited treatment outcome, the emergence of drug resistance, and also relapse[85]. Recently treatment strategies adopted a paradigm shift to immunotherapeutic approaches because of the importance of the immune microenvironment in carcinogenesis.
The liver is the most extensive reticuloendothelial system and peripheral immunomodulatory organ in the human body. Immunotherapy is being extensively evaluated in liver cancer[86]. The liver constitutes a vast repository of immune cells including NK cells, kupffer cells, sinusoidal endothelial cells, and innate T cells[87]. Aberrant immune checkpoint activation makes HCC a cold tumor, where anti-tumor immune surveillance is completely abolished[88]. Combination of immune checkpoint inhibitors (ICIs) such as Nivolumab (PD-1 inhibitor) + Ipilimumab (CTLA4 inhibitor) and Atezolizumab (PD-L1 inhibitor) + Bevacizumab [vascular endothelial growth factor (VEGF) inhibitor] got approval as first-line therapy for various cancer such as non-small cell lung cancer (NSCLC), HCC in 2020[89]. Though ICIs exerted a superior effect to tyrosine inhibitors, current immunotherapeutic drugs failed to establish an effective anticancer immunity against HCC. Though the immunotherapeutic approaches modify effector immune cells functions to elicit anti-tumor immune response, the immunosuppressive tumor microenvironment neutralizes the effects of immunotherapy[90].
Analysis of the TGF-β profile of HCC samples in the TGCA data set revealed four categories with typical TGF-β expression[91]. The cluster with a highly activated TGF-β signature, which accounts for 14.5% of samples, exhibited prominent immune exhaustion and poor prognosis. ICIs may not work well in this cluster. Additionally, anti-inflammatory/anti-fibrotic agents targeting TGF-β can improve the immune milieu[92,93]. The majority of HCC samples belong to a cluster of activated TGF-β signature (45%) and showed a low level of an immune response. Hence, combining a TGF-β inhibitor with an immune checkpoint inhibitor can exert a synergistic effect. 30% of HCC samples showed a normal TGF-β signature associated with active immune surveillance; therefore, immunotherapy will be most suitable for this cluster. The fourth cluster is a minor population (9.9%) that exhibits inactivated TGF-β signature with poor immune cell activation and response[94]. With this evidence, it is clear that TGF-β1 signature can be used to decide the suitable therapy or predict the outcome of immunotherapy.
Several specific and non-specific inhibitors of TGF-β, mainly TGF-β1 and 2 inhibitors, are being developed and evaluated against various tumors, including HCC. Regarding non-specific inhibitors, as the primary molecular target is different, its ability to block the TGF-β pathway offers additional benefits as anticancer agents. One such example is Halofuginone, an alkaloid coccidiostat with reported preclinical activity against HCC. In addition to the prominent inhibition of collagen synthesis, Halofuginone also blocks TGF-β1, inhibits ECM formation and fibroblast proliferation, increases IFN-γ and anti-tumor immune response[95,96]. The effect of Halofuginone against advanced progressive solid tumor has been evaluated in phase I clinical trial (NCT00027677), and in 2000, United States Food and Drug Administration gave orphan drug approval status to Halofuginone for treatment of scleroderma[97]. Histone deacetylase exerts epigenetic regulation of TGF-β1 mediated fibrosis and carcinogenesis[98]. Studies indicate that histone deacetylase inhibitors such as Panobinostat have shown effectiveness in HCC animal models and phase I human trials combined with sorafenib (NCT00823290)[99]. Apart from the non-specific inhibitors, recent preclinical interventions combined TGF-β1 targeting antibodies or TGF-βR1 inhibitor with PD-L1 inhibitors and obtained prominent cytotoxic effect and anticancer immune surveillance in various solid tumors[100,101]. Likewise, M7824 is a bifunctional fusion protein with dual targeting of PD-L1 and TGF-β, which has been evaluated in animal models of various cancers either alone or in combination with vaccines[102]. M7824 exerted a significant inhibitory effect on TGF-β1[103]. Among the different TGF-β inhibitors, LY2109761 is an orally bioavailable TGF-β receptor type I inhibitor, which exhibited an antitumor effect in various HCC animal models[104]. LY2109761 inhibited TGF-β1 induced migration, invasion, and anoikis in HCC cells[105,106]. Another study suggested the anti-angiogenic potential of LY2109761, which was superior to the typical VEGF inhibitor, Bevacizumab, and the effect was mediated by suppression of VEGF through inhibition of Smad dependent TGF-β1 signaling[107,108]. In addition to this, anti-TGF-β agents targeting other isoforms are also developed. AP-12009 is a TGF-β2 specific antisense oligonucleotide in a clinical trial to treat glioma and anaplastic astrocytoma[109,110]. Likewise, TGF-β1 directed mRNA was developed as AP-11011, which is evaluated against NSCLC, colon cancer in preclinical models[111]. Lordelimumab is a TGF-β2 specific monoclonal antibody with an anti-fibrotic effect[112]. Many preclinical studies investigated different TGF-β inhibitors, yet, Galunisertib (LY2157299), a kinase inhibitor of TGF-β1, is only the one in current clinical trials[113]. Several clinical trials of Galunisertib are ongoing or completed either alone or combined with Sorafenib, ICIs, and alkylating agents (Table 3).
| Drug | Title of the study | Treatment | Phase | Status | Trial ID |
| Galunisertib | A study of Galunisertib on the immune system in participants with cancer | Monotherapy | Phase I | Completed | NCT02304419 |
| Galunisertib | Galunisertib (LY2157299) and stereotactic body radiotherapy in advanced hepatocellular carcinoma | Combination with radiotherapy | Phase I | Completed | NCT02906397 |
| Galunisertib | A study of LY2157299 in participants with unresectable hepatocellular cancer | Combination with Nivolumab | Phase II | Completed | NCT02423343 |
| Galunisertib | A study of LY2157299 in participants with unresectable hepatocellular cancer | Combination with Sorafenib | Phase I | Completed | NCT02240433 |
| Galunisertib | A study of LY2157299 in participants with advanced hepatocellular carcinoma | Combination with Sorafenib | Phase II | Completed | NCT02178358 |
| Galunisertib | A study of LY2157299 in participants with hepatocellular carcinoma | Combination with Sorafenib/Ramucirumumab | Phase II | Completed | NCT01246986 |
| Galunisertib | Galunisertib and Capecitabine in advanced resistant TGF-beta activated colorectal cancer (EORTC1615) | Combination with Capecitabine | Phase II | Withdrawn | NCT03470350 |
| Galunisertib | A study of LY2157299 in participants with pancreatic cancer (advanced or has spread to another part of the body) | Combination with Gemcitabine | Phase I | Completed | NCT02154646 |
| Galunisertib | A study of Galunisertib (LY2157299) and Durvalumab (MEDI4736) in participants with metastatic pancreatic cancer | Combination with Durvalumab | Phase I | Completed | NCT02734160 |
TGF-β1 exerts a unique regulatory power on inflammation, fibrogenesis, and immune response in HCC. Among other TGF-β isoforms, significant and progressive expression of TGF-β1 during the entire course of HCC pathogenesis, starting from chronic hepatitis to HCC makes it a sensitive and accurate diagnostic marker of HCC. The specificity and sensitivity of TGF-β1 based diagnosis of HCC by parallel estimation of serum AFP. Even after establishing HCC, TGF-β1 continues to elevate as HCC progresses and is associated with poor prognosis and shorter survival. Since TGF-β1 is the master regulator of the immunosuppressive tumor milieu in HCC, TGF-β1 inhibition could sensitize ICI, tyrosine kinase inhibitors, and other systemic or curative interventions. HCC remains the deadliest-refractory tumor predominantly due to its delayed diagnosis. In that context, TGF-β1 is relevant for early diagnosis, prognosis, and therapy. Even though a plethora of supporting evidence is available, still TGF-β1 is not much studied and evaluated compared with other markers such as AFP. Notably, the dichotomic nature of TGF-β signaling in HCC needs to be defined accurately to establish the clinical utility of TGF-β1. Thus, proper and careful determination of the TGF-β1 profile of patients is necessary to choose the suitable patients for TGF-β1 targeted therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Association for Cancer Research, No. EACR26534.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghazy A, Egypt; Ying HQ, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1346] [Article Influence: 336.5] [Reference Citation Analysis (1)] |
| 2. | Patel N, Yopp AC, Singal AG. Diagnostic delays are common among patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2015;13:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford). 2005;7:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Cervello M, McCubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget. 2012;3:236-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Suresh D, Srinivas AN, Kumar DP. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front Oncol. 2020;10:601710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 7. | Kumari R, Sahu MK, Tripathy A, Uthansingh K, Behera M. Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat Oncol. 2018;5:HEP08. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Cuestas ML, Oubina JR, Mathet VL. Hepatocellular carcinoma and multidrug resistance: Past, present and new challenges for therapy improvement. World J Pharmacol. 2015;4:96-116. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Devan AR, Kumar AR, Nair B, Anto NP, Muraleedharan A, Mathew B, Kim H, Nath LR. Insights into an Immunotherapeutic Approach to Combat Multidrug Resistance in Hepatocellular Carcinoma. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Prud'homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87:1077-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P; IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016;283:2219-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 481] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 12. | Katz LH, Likhter M, Jogunoori W, Belkin M, Ohshiro K, Mishra L. TGF-β signaling in liver and gastrointestinal cancers. Cancer Lett. 2016;379:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 898] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 14. | Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 736] [Cited by in RCA: 718] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 15. | Huang J, Qiu M, Wan L, Wang G, Huang T, Chen Z, Jiang S, Li X, Xie L, Cai L. TGF-β1 Promotes Hepatocellular Carcinoma Invasion and Metastasis via ERK Pathway-Mediated FGFR4 Expression. Cell Physiol Biochem. 2018;45:1690-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Yang AT, Hu DD, Wang P, Cong M, Liu TH, Zhang D, Sun YM, Zhao WS, Jia JD, You H. TGF-β1 Induces the Dual Regulation of Hepatic Progenitor Cells with Both Anti- and Proliver Fibrosis. Stem Cells Int. 2016;2016:1492694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Nair B, Nath LR. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduct Res. 2020;40:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1258] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 19. | Caja L, Ortiz C, Bertran E, Murillo MM, Miró-Obradors MJ, Palacios E, Fabregat I. Differential intracellular signalling induced by TGF-beta in rat adult hepatocytes and hepatoma cells: implications in liver carcinogenesis. Cell Signal. 2007;19:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Bedossa P, Peltier E, Terris B, Franco D, Poynard T. Transforming growth factor-beta 1 (TGF-beta 1) and TGF-beta 1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology. 1995;21:760-766. [PubMed] [DOI] [Full Text] |
| 21. | Zhu T, Zhang L, Li C, Tan X, Liu J, Huiqin Li, Fan Q, Zhang Z, Zhan M, Fu L, Luo J, Geng J, Wu Y, Zou X, Liang B. The S100 calcium binding protein A11 promotes liver fibrogenesis by targeting TGF-β signaling. J Genet Genomics. 2022;49:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 23. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2166] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 24. | Levine D, Rockey DC, Milner TA, Breuss JM, Fallon JT, Schnapp LM. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am J Pathol. 2000;156:1927-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Mikula M, Proell V, Fischer AN, Mikulits W. Activated hepatic stellate cells induce tumor progression of neoplastic hepatocytes in a TGF-beta dependent fashion. J Cell Physiol. 2006;209:560-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Wang J, Xiang H, Lu Y, Wu T. Role and clinical significance of TGFβ1 and TGFβR1 in malignant tumors (Review). Int J Mol Med. 2021;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 668] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 28. | Heldin CH, Moustakas A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012;347:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 29. | Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 879] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 30. | Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2613] [Cited by in RCA: 2512] [Article Influence: 193.2] [Reference Citation Analysis (0)] |
| 31. | Johnson LD, Auer R. Commentary on: Jiang B, Zhu F, Cao L, Presley BR, Shen MS, Yang KH. Computational study of fracture characteristics in infant skulls using a simplified finite element model. J Forensic Sci 2017;62(1):39-49. J Forensic Sci. 2018;63:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, Wang S, Chen W. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity. 2017;46:660-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 33. | Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3466] [Cited by in RCA: 3767] [Article Influence: 179.4] [Reference Citation Analysis (0)] |
| 34. | Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 615] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 36. | de Gramont A, Faivre S, Raymond E. Novel TGF-β inhibitors ready for prime time in onco-immunology. Oncoimmunology. 2017;6:e1257453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 37. | Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 753] [Cited by in RCA: 740] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 38. | Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 39. | Park BV, Freeman ZT, Ghasemzadeh A, Chattergoon MA, Rutebemberwa A, Steigner J, Winter ME, Huynh TV, Sebald SM, Lee SJ, Pan F, Pardoll DM, Cox AL. TGFβ1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov. 2016;6:1366-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 220] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 40. | Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342-1356.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1506] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 41. | Bao S, Jiang X, Jin S, Tu P, Lu J. TGF-β1 Induces Immune Escape by Enhancing PD-1 and CTLA-4 Expression on T Lymphocytes in Hepatocellular Carcinoma. Front Oncol. 2021;11:694145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Korkut A, Zaidi S, Kanchi RS, Rao S, Gough NR, Schultz A, Li X, Lorenzi PL, Berger AC, Robertson G, Kwong LN, Datto M, Roszik J, Ling S, Ravikumar V, Manyam G, Rao A, Shelley S, Liu Y, Ju Z, Hansel D, de Velasco G, Pennathur A, Andersen JB, O'Rourke CJ, Ohshiro K, Jogunoori W, Nguyen BN, Li S, Osmanbeyoglu HU, Ajani JA, Mani SA, Houseman A, Wiznerowicz M, Chen J, Gu S, Ma W, Zhang J, Tong P, Cherniack AD, Deng C, Resar L; Cancer Genome Atlas Research Network, Weinstein JN, Mishra L, Akbani R. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGF-β Superfamily. Cell Syst. 2018;7:422-437.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 43. | Chen J, Zaidi S, Rao S, Chen JS, Phan L, Farci P, Su X, Shetty K, White J, Zamboni F, Wu X, Rashid A, Pattabiraman N, Mazumder R, Horvath A, Wu RC, Li S, Xiao C, Deng CX, Wheeler DA, Mishra B, Akbani R, Mishra L. Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway. Gastroenterology. 2018;154:195-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 44. | Reichl P, Haider C, Grubinger M, Mikulits W. TGF-β in epithelial to mesenchymal transition and metastasis of liver carcinoma. Curr Pharm Des. 2012;18:4135-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Tarantino G, Conca P, Riccio A, Tarantino M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F, Capone D. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign? J Transl Med. 2008;6:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Majumdar A, Curley SA, Wu X, Brown P, Hwang JP, Shetty K, Yao ZX, He AR, Li S, Katz L, Farci P, Mishra L. Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Kirmaz C, Terzioglu E, Topalak O, Bayrak P, Yilmaz O, Ersoz G, Sebik F. Serum tumor growth factor‐β1 Levels in patients with cirrhosis, chronic hepatitis B and chronic hepatitis C. Eur Cytokine Netw. 2004;15:112-116. [DOI] [Full Text] |
| 48. | Taniguchi H, Kato N, Otsuka M, Goto T, Yoshida H, Shiratori Y, Omata M. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J Med Virol. 2004;72:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, Xing B, Sun W, Ren L, Hu B, Li C, Zhang L, Qin G, Zhang M, Chen N, Huang Y, Zhou J, Liu M, Zhu X, Qiu Y, Sun Y, Huang C, Yan M, Wang M, Liu W, Tian F, Xu H, Wu Z, Shi T, Zhu W, Qin J, Xie L, Fan J, Qian X, He F; Chinese Human Proteome Project (CNHPP) Consortium. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 624] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 50. | Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008;47:2059-2067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 51. | Dong ZZ, Yao DF, Zou L, Yao M, Qiu LW, Wu XH, Wu W. [An evaluation of transforming growth factor-beta 1 in diagnosing hepatocellular carcinoma and metastasis]. Zhonghua Gan Zang Bing Za Zhi. 2007;15:503-508. [PubMed] |
| 52. | Dong ZZ, Yao DF, Yao M, Qiu LW, Zong L, Wu W, Wu XH, Yao DB, Meng XY. Clinical impact of plasma TGF-beta1 and circulating TGF-beta1 mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:288-295. [PubMed] |
| 53. | Tsai JF, Chuang LY, Jeng JE, Yang ML, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Clinical relevance of transforming growth factor-beta 1 in the urine of patients with hepatocellular carcinoma. Medicine (Baltimore). 1997;76:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Tsai JF, Jeng JE, Chuang LY, Yang ML, Ho MS, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Clinical evaluation of urinary transforming growth factor-beta1 and serum alpha-fetoprotein as tumour markers of hepatocellular carcinoma. Br J Cancer. 1997;75:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Balzarini P, Benetti A, Invernici G, Cristini S, Zicari S, Caruso A, Gatta LB, Berenzi A, Imberti L, Zanotti C, Portolani N, Giulini SM, Ferrari M, Ciusani E, Navone SE, Canazza A, Parati EA, Alessandri G. Transforming growth factor-beta1 induces microvascular abnormalities through a down-modulation of neural cell adhesion molecule in human hepatocellular carcinoma. Lab Invest. 2012;92:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Mohd Azamai ES, Sulaiman S, Mohd Habib SH, Looi ML, Das S, Abdul Hamid NA, Wan Ngah WZ, Mohd Yusof YA. Chlorella vulgaris triggers apoptosis in hepatocarcinogenesis-induced rats. J Zhejiang Univ Sci B. 2009;10:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Cao Y, Agarwal R, Dituri F, Lupo L, Trerotoli P, Mancarella S, Winter P, Giannelli G. NGS-based transcriptome profiling reveals biomarkers for companion diagnostics of the TGF-β receptor blocker galunisertib in HCC. Cell Death Dis. 2017;8:e2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Kohla MA, Attia A, Darwesh N, Obada M, Taha H, Youssef MF. Association of serum levels of transforming growth factor β1 with disease severity in patients with hepatocellular carcinoma. Hepatoma Res. 2017;3:294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Shehata F, Abdel Monem N, Sakr M, Kasem S, Balbaa M. Epidermal growth factor, its receptor and transforming growth factor-β1 in the diagnosis of HCV-induced hepatocellular carcinoma. Med Oncol. 2013;30:673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Shirai Y, Kawata S, Tamura S, Ito N, Tsushima H, Takaishi K, Kiso S, Matsuzawa Y. Plasma transforming growth factor‐β1 in patients with hepatocellular carcinoma. Comparison with chronic liver diseases. Cancer. 1994;73:2275-2279. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Tu S, Huang W, Huang C, Luo Z, Yan X. Contextual Regulation of TGF-β Signaling in Liver Cancer. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Murawaki Y, Ikuta Y, Nishimura Y, Koda M, Kawasaki H. Serum markers for fibrosis and plasma transforming growth factor-beta 1 in patients with hepatocellular carcinoma in comparison with patients with liver cirrhosis. J Gastroenterol Hepatol. 1996;11:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Neuzillet C, de Gramont A, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014;5:78-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 64. | Sacco R, Leuci D, Tortorella C, Fiore G, Marinosci F, Schiraldi O, Antonaci S. Transforming growth factor beta1 and soluble Fas serum levels in hepatocellular carcinoma. Cytokine. 2000;12:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Song BC, Chung YH, Kim JA, Choi WB, Suh DD, Pyo SI, Shin JW, Lee HC, Lee YS, Suh DJ. Transforming growth factor-beta1 as a useful serologic marker of small hepatocellular carcinoma. Cancer. 2002;94:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Okumoto K, Hattori E, Tamura K, Kiso S, Watanabe H, Saito K, Saito T, Togashi H, Kawata S. Possible contribution of circulating transforming growth factor-beta1 to immunity and prognosis in unresectable hepatocellular carcinoma. Liver Int. 2004;24:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Divella R, Daniele A, Gadaleta C, Tufaro A, Venneri MT, Paradiso A, Quaranta M. Circulating transforming growth factor-β and epidermal growth factor receptor as related to virus infection in liver carcinogenesis. Anticancer Res. 2012;32:141-145. [PubMed] |
| 68. | Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, Meyers WC. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Ann Surg. 1995;222:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | An Y, Gao S, Zhao WC, Qiu BA, Xia NX, Zhang PJ, Fan ZP. Transforming growth factor-β and peripheral regulatory cells are negatively correlated with the overall survival of hepatocellular carcinoma. World J Gastroenterol. 2018;24:2733-2740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7-11. [PubMed] |
| 71. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 226] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 72. | Matsuzaki K. Modulation of TGF-beta signaling during progression of chronic liver diseases. Front Biosci (Landmark Ed). 2009;14:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 74. | Giannelli G, Fransvea E, Marinosci F, Bergamini C, Colucci S, Schiraldi O, Antonaci S. Transforming growth factor-beta1 triggers hepatocellular carcinoma invasiveness via alpha3beta1 integrin. Am J Pathol. 2002;161:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Lee D, Chung YH, Kim JA, Lee YS, Lee D, Jang MK, Kim KM, Lim YS, Lee HC. Transforming growth factor beta 1 overexpression is closely related to invasiveness of hepatocellular carcinoma. Oncology. 2012;82:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Liu T, Tang W, Deng B, Chen Y, Zhu J, Shen X. Hepatocellular Carcinoma Cells Induce Regulatory T Cells and Lead to Poor Prognosis via Production of Transforming Growth Factor-β1. Cell Physiol Biochem. 2016;38:306-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 77. | Peng L, Yuan XQ, Zhang CY, Ye F, Zhou HF, Li WL, Liu ZY, Zhang YQ, Pan X, Li GC. High TGF-β1 expression predicts poor disease prognosis in hepatocellular carcinoma patients. Oncotarget. 2017;8:34387-34397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106:djt369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 79. | Wang Z, Liu F, Tu W, Chang Y, Yao J, Wu W, Jiang X, He X, Lin J, Song Y. Embryonic liver fodrin involved in hepatic stellate cell activation and formation of regenerative nodule in liver cirrhosis. J Cell Mol Med. 2012;16:118-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Ji F, Fu SJ, Shen SL, Zhang LJ, Cao QH, Li SQ, Peng BG, Liang LJ, Hua YP. The prognostic value of combined TGF-β1 and ELF in hepatocellular carcinoma. BMC Cancer. 2015;15:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 82. | Lee JJ, Choo SP. The fibroblast growth factor receptor pathway in hepatocellular carcinoma. Hepatoma Res. 2018;4:52. [DOI] [Full Text] |
| 83. | Chen Z, Xie B, Zhu Q, Xia Q, Jiang S, Cao R, Shi L, Qi D, Li X, Cai L. FGFR4 and TGF-β1 expression in hepatocellular carcinoma: correlation with clinicopathological features and prognosis. Int J Med Sci. 2013;10:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | El-Ashram S, Aboelhadid SM, Abdel-Kafy EM, Hashem SA, Mahrous LN, Farghly EM, Kamel AA. Erratum: Saeed, E.-A.; Shawky, M.A.; El-Sayed, M.A.-K.; Shymaa, A.H.; Lilian, N.M.; Eman, M.F.; Asmaa, A.K. Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes. Animals 2020, 10, 537. Animals (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Fan G, Wei X, Xu X. Is the era of sorafenib over? Ther Adv Med Oncol. 2020;12:1758835920927602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Giraud J, Chalopin D, Blanc JF, Saleh M. Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies. Front Immunol. 2021;12:655697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 87. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 775] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 88. | Nishida N, Kudo M. Immune Phenotype and Immune Checkpoint Inhibitors for the Treatment of Human Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, Ji J. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 90. | Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 91. | Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, Losic B, Waxman S, Thung SN, Mazzaferro V, Esteller M, Friedman SL, Schwartz M, Villanueva A, Llovet JM. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology. 2017;153:812-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 663] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 92. | Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 93. | Pinyol R, Sia D, Llovet JM. Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC. Clin Cancer Res. 2019;25:2021-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 94. | Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-β signaling in onset and progression of hepatocellular carcinoma. Dig Dis. 2012;30:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol. 2002;118:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Bièche I, Pines M, Rosenbaum J. Halofuginone suppresses the lung metastasis of chemically induced hepatocellular carcinoma in rats through MMP inhibition. Neoplasia. 2006;8:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Nagler A, Ohana M, Shibolet O, Shapira MY, Alper R, Vlodavsky I, Pines M, Ilan Y. Suppression of hepatocellular carcinoma growth in mice by the alkaloid coccidiostat halofuginone. Eur J Cancer. 2004;40:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007;1773:1572-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 99. | Di Fazio P, Schneider-Stock R, Neureiter D, Okamoto K, Wissniowski T, Gahr S, Quint K, Meissnitzer M, Alinger B, Montalbano R, Sass G, Hohenstein B, Hahn EG, Ocker M. The pan-deacetylase inhibitor panobinostat inhibits growth of hepatocellular carcinoma models by alternative pathways of apoptosis. Cell Oncol. 2010;32:285-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 100. | Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3113] [Cited by in RCA: 3656] [Article Influence: 522.3] [Reference Citation Analysis (1)] |
| 101. | Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1334] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 102. | Knudson KM, Hicks KC, Luo X, Chen JQ, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7:e1426519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 103. | Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, Yu H, Qin G, Sircar A, Hernández VM, Jenkins MH, Fontana RE, Deshpande A, Locke G, Sabzevari H, Radvanyi L, Lo KM. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 104. | Li HY, McMillen WT, Heap CR, McCann DJ, Yan L, Campbell RM, Mundla SR, King CH, Dierks EA, Anderson BD, Britt KS, Huss KL, Voss MD, Wang Y, Clawson DK, Yingling JM, Sawyer JS. Optimization of a dihydropyrrolopyrazole series of transforming growth factor-beta type I receptor kinase domain inhibitors: discovery of an orally bioavailable transforming growth factor-beta receptor type I inhibitor as antitumor agent. J Med Chem. 2008;51:2302-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 107. | Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 108. | Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, Pichler J, Wurm G, Mehdorn M, Strege R, Schuierer G, Villarrubia V, Fellner F, Jansen O, Straube T, Nohria V, Goldbrunner M, Kunst M, Schmaus S, Stauder G, Bogdahn U, Schlingensiepen KH. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | Vallières L. Trabedersen, a TGFbeta2-specific antisense oligonucleotide for the treatment of malignant gliomas and other tumors overexpressing TGFbeta2. IDrugs. 2009;12:445-453. [PubMed] |
| 110. | Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH; Trabedersen Glioma Study Group. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 111. | Schlingensiepen KH, Bischof A, Egger T, Hafner M, Herrmuth H, Jachimczak P, Kielmanowicz M, Niewel M, Zavadova E, Stauder G. The TGF-beta1 antisense oligonucleotide AP 11014 for the treatment of non-small cell lung, colorectal and prostate cancer: Preclinical studies. J Clin Oncol. 2004;22:3132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44:3394-3401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Rodon J, Carducci MA, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |