Published online Sep 21, 2022. doi: 10.3748/wjg.v28.i35.5203
Peer-review started: June 6, 2022
First decision: June 27, 2022
Revised: July 10, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: September 21, 2022
Processing time: 101 Days and 5 Hours
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas with an unpredictable course of illness. A major challenge of AP is the early identification of patients at high-risk for organ failure and death. However, scoring systems are complicated and time consuming, and the predictive values for the clinical course are vague.
To determine whether the dynamic changes in presepsin levels can be used to evaluate the severity of disease and outcome of AP.
In this multicentric cohort study, 133 patients with AP were included. Clinical severity was dynamically evaluated using the 2012 revised Atlanta Classification. Blood presepsin levels were measured at days 1, 3, 5 and 7 after admission by chemiluminescent enzyme immunoassay.
The median concentration of presepsin increased and the clearance rate of presepsin decreased with disease severity and organ failure in AP patients. The presepsin levels on days 3, 5 and 7 were independent predictors of moderately severe and severe AP with time-specific area under the curve (AUC) values of 0.827, 0.848 and 0.867, respectively. The presepsin levels positively correlated with bedside index of severity in AP, Ranson, acute physiology and chronic health evaluation II, computed tomography severity index and Marshall scores. Pre
Blood presepsin levels within 7 d of admission were associated with and may be useful to dynamically predict the severity of disease course and 28-d mortality in AP patients.
Core Tip: Acute pancreatitis has diverse clinical manifestations with an unpredictable clinical course. A major challenge of acute pancreatitis is the early identification of patients at high-risk for organ failure and death. Scoring systems are complicated and time consuming with limited predictive value for the clinical course. In this study, we investigated the association between the dynamic levels of blood presepsin, a new infection biomarker, and the changes of severity in the early course of acute pancreatitis. We found the predictive value of presepsin for 28-d mortality was similar to the bedside index of severity in acute pancreatitis, Ranson and acute physiology and chronic health evaluation II scores.
- Citation: Xiao HL, Wang GX, Wang Y, Tan ZM, Zhou J, Yu H, Xie MR, Li CS. Dynamic blood presepsin levels are associated with severity and outcome of acute pancreatitis: A prospective cohort study. World J Gastroenterol 2022; 28(35): 5203-5216
- URL: https://www.wjgnet.com/1007-9327/full/v28/i35/5203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i35.5203
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas that can lead to systemic inflammatory response syndrome, organ failure and sepsis. AP is one of the most common causes of abdominal emergencies and is associated with mortality rates up to 35%[1,2]. Gallstones and alcohol consumption are the most frequent causes of AP. Irrespective of the etiology, AP has diverse clinical manifestations with an unpredictable clinical course. Various clinical scoring systems such as the bedside index of severity in acute pancreatitis (BISAP) score, Ranson criteria, computed tomography severity index (CTSI) and acute physiology and chronic health evaluation (APACHE) II scores have been developed to predict the illness severity at admission[3]. However, accurate prediction of the clinical course remains difficult[4]. A major challenge of AP is the early identification of patients at high risk for organ failure and death. Moreover, some of these scoring systems are complicated and time consuming with limited predictive value for the clinical course. Therefore, it is necessary to develop simple and convenient biomarkers to dynamically predict the severity of AP in its early course.
Presepsin is a subtype of soluble CD14 formed by a 13 kDa fragment and is an emerging biomarker of infection[5]. CD14 is a glycoprotein receptor for lipopolysaccharide. After lipopolysaccharide binds to CD14, presepsin is released into the blood and cleared by the kidney[6]. Presepsin has been confirmed to predict illness severity[7], number of organs experiencing dysfunction[8] and septic 90-d death rates[9,10]. This study aimed to assess the predictive value of blood presepsin levels for the severity of disease course and outcomes in patients with AP.
This prospective, multicentric, and observational cohort study was conducted at the Emergency Departments of Beijing Friendship Hospital and Beijing Chaoyang Hospital affiliated with Capital Medical University. This study was approved by the Beijing Friendship Hospital Ethics Committee (Approval No. 2017-P2-103-02). Written informed consent was obtained from patients or their relatives. Patients diagnosed with AP as per the 2012 revised Atlanta Classification[11] were screened within 24 h of admission and followed up for 28 d. Patients were classified into one of three groups (mild, moderately severe and severe AP) based on the hospital course. Patients with mild AP had neither local complications nor organ failure. Patients with moderately severe AP had transient (< 48 h) organ failure or local complications or both, whereas patients with severe AP had persistent (> 48 h) organ failure. Organ failure was defined as a Marshall score > 2[11]. Patients were subsequently divided into one of two groups [mild and non-mild (moderately severe and severe) AP] based on their presentation during the first, third, 5th and 7th day of admission. Pregnant women and patients younger than 18 years of age were excluded. All patients received standardized treatment according to the 2012 revised Atlanta Classification[11].
The collected data included age, sex, comorbidities, etiologies, scoring, pancreatic imaging and interventions. Abdominal ultrasonography at admission and abdominal computed tomography scans 72 h after symptom onset were performed. The BISAP score, Ranson score, CTSI, APACHE II score, Marshall score and severity of AP were determined at 1, 3, 5 and 7 d post-admission. BISAP and APACHE II scores were evaluated within 24 h. The Ranson score was calculated within 48 h. Levels of the conventional inflammatory biomarkers procalcitonin and C-reactive protein were obtained from the clinical laboratory data. AP patients were classified into survival and non-survival groups based on their 28-d survival.
Venous blood samples were collected in tubes containing heparin at days 1, 3, 5 and 7 after admission and stored at 4 °C for analysis within 24 h. Presepsin concentration in the blood was measured using a chemiluminescent enzyme immunoassay[12] with a compact automated immunoanalyzer (PATHFAST; Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The lower and upper detection limits of presepsin concentrations were 20 pg/mL and 200000 pg/mL, respectively.
All analyses were performed using SPSS version 25.0 (SPSS, Chicago, IL, United States). With a two-sided α = 0.05, a β = 0.2 and 70% of patients with mild pancreatitis[13], it was determined that 106 patients were required for enrollment, 74 with mild pancreatitis and 32 with non-mild pancreatitis. This study enrolled 137 patients to account for 20% of patients lost to follow-up. The clearance ratio of presepsin was calculated using the following formula: the presepsin level on day 1 minus the presepsin level on days 3, 5 or 7, divided by the presepsin level on day 1 and multiplied by 100%. Data with normal distribution were expressed as mean ± SD and were analyzed by Student's t test or variance analysis. Data with non-normal distribution were expressed as the median with quartiles and were analyzed by the Mann-Whitney U or Kruskal-Wallis tests. The χ2 test or Fisher’s exact test was used for comparison of frequencies. A receiver operating characteristic (ROC) curve was constructed to assess the predictive value of presepsin for moderate/severe AP and 28-d mortality. Prognostic parameters including sensitivity, specificity, positive predictive value and negative predictive value were calculated based on ROC curve analysis. The areas under the ROC curves (AUCs) were compared by MedCalc version 11.4 (MedCalc Software, Ostend, Belgium). The correlation was analyzed by Spearman rank correlation. Binary logistic regression analyses were used to determine the independent predictors for disease severity and 28-d mortality of AP patients. Cox proportional hazards regression model was used to estimate the independent contribution of presepsin for the prediction of 28-d mortality. All statistical tests were two-tailed, and a P value < 0.05 was considered statistically significant.
A total of 137 patients were screened in the emergency departments of the two hospitals from January 2018 to September 2019. Of these, 4 patients were excluded from analysis: 1 patient was diagnosed with pancreatic cancer, and 3 patients were lost to follow-up. Thus, 133 patients were enrolled and classified throughout the course of the disease as mild AP (n = 95 patients), moderately severe AP (n = 21 patients) and severe AP (n = 17 patients) according to the Atlanta 2012 classification. Patient characteristics are described in Table 1. The median age of patients was 65 years. There were 86 males and 47 females. The etiologies of AP included gallstones (63.2%), hypertriglyceridemia (9.0%), post-endoscopic retrograde cholangiopancreatography (11.3%), idiopathic (13.5%) and alcohol (3.0%). The BISAP, Ranson, CTSI and APACHE II scores increased with the severity of disease (all P < 0.01). The incidence of organ failure, mortality and the cost of hospitalization increased with the severity of disease (all P < 0.001).
| Characteristic | Overall, n = 133 | Mild, n = 95 | Moderately severe, n = 21 | Severe, n = 17 | P value |
| Demographics | |||||
| Male, % | 86 (64.7) | 56 (58.9) | 16 (76.2) | 14 (82.4) | 0.086 |
| Age, yr | 65 (54.0-76.0) | 65 (54.0-76.0) | 68 (40.5-72.5) | 59 (43.0-82.0) | 0.860 |
| Comorbidities | |||||
| CHF, % | 19 (17.4) | 16 (18.8) | 3 (18.8) | 0 (0) | 0.402 |
| COPD, % | 3 (2.8) | 2 (2.4) | 0 (0) | 1 (12.5) | 0.188 |
| Diabetes, % | 25 (22.9) | 20 (23.5) | 3 (18.8) | 2 (25.0) | 0.907 |
| Immunosuppression, % | 8 (7.3) | 7 (8.2) | 0 (0) | 1 (12.5) | 0.432 |
| Etiology | 0.003 | ||||
| Gallstones, % | 84 (63.2) | 65 (68.4) | 12 (57.1) | 7 (41.2) | |
| Hypertryglyceridemia, % | 12 (9.0) | 9 (9.5) | 0 (0) | 3 (17.6) | |
| Alcohol, % | 4 (3.0) | 2 (2.1) | 2 (9.5) | 0 (0) | |
| Post-ERCP, % | 15 (11.3) | 13 (13.7) | 1 (4.8) | 1 (5.9) | |
| Idiopathic, % | 18 (13.5) | 6 (6.3) | 6 (28.6) | 6 (35.3) | |
| Scoring | |||||
| BISAP score at admission | 2.0 (1.0-2.0) | 1.0 (1.0-2.0) | 2.0 (2.0-3.0) | 3.0 (2.0-3.0) | < 0.001 |
| Ranson score at 48 h | 2.0 (1.0-4.0) | 2.0 (1.0-3.0) | 3.0 (2.0-5.5) | 4.0 (3.5-6.5) | < 0.001 |
| CTSI score | 4.0 (2.0-4.0) | 2.0 (2.0-4.0) | 4.0 (2.0-6.0) | 4.0 (4.0-5.0) | 0.002 |
| APACHE II score at admission | 8.0 (5.0-12.5) | 7.0 (3.0-10.0) | 9.0 (6.0-15.0) | 18.0 (9.0-19.0) | < 0.001 |
| Imaging | |||||
| Pancreatic necrosis, % | 14 (10.5) | 5 (5.3) | 6 (28.6) | 3 (17.6) | 0.013 |
| Mechanical ventilation, % | 3 (2.3) | 0 (0) | 0 (0) | 3 (17.6) | < 0.001 |
| CRRT, % | 1 (0.8) | 0 (0) | 0 (0) | 1 (5.9) | 0.027 |
| Length of hospital stay in d | 11.74 ± 5.21 | 11.08 ± 3.73 | 13.24 ± 8.40 | 13.59 ± 6.72 | 0.067 |
| Outcomes | |||||
| Single organ failure, % | 30 (22.6) | 0 (0) | 20 (95.2) | 10 (58.8) | < 0.001 |
| Multiple organ failure, % | 8 (6.0) | 0 (0) | 1 (4.8) | 7 (41.2) | < 0.001 |
| 28-d deaths, % | 7 (5.3) | 0 (0) | 0 (0) | 7 (41.2) | < 0.001 |
| Cost in $ | 5276.14 (3265.34-7739.10) | 4482.18 (3127.06-6493.38) | 7242.67 (3356.87-8785.31) | 11222.04 (7457.31-23045.12) | < 0.001 |
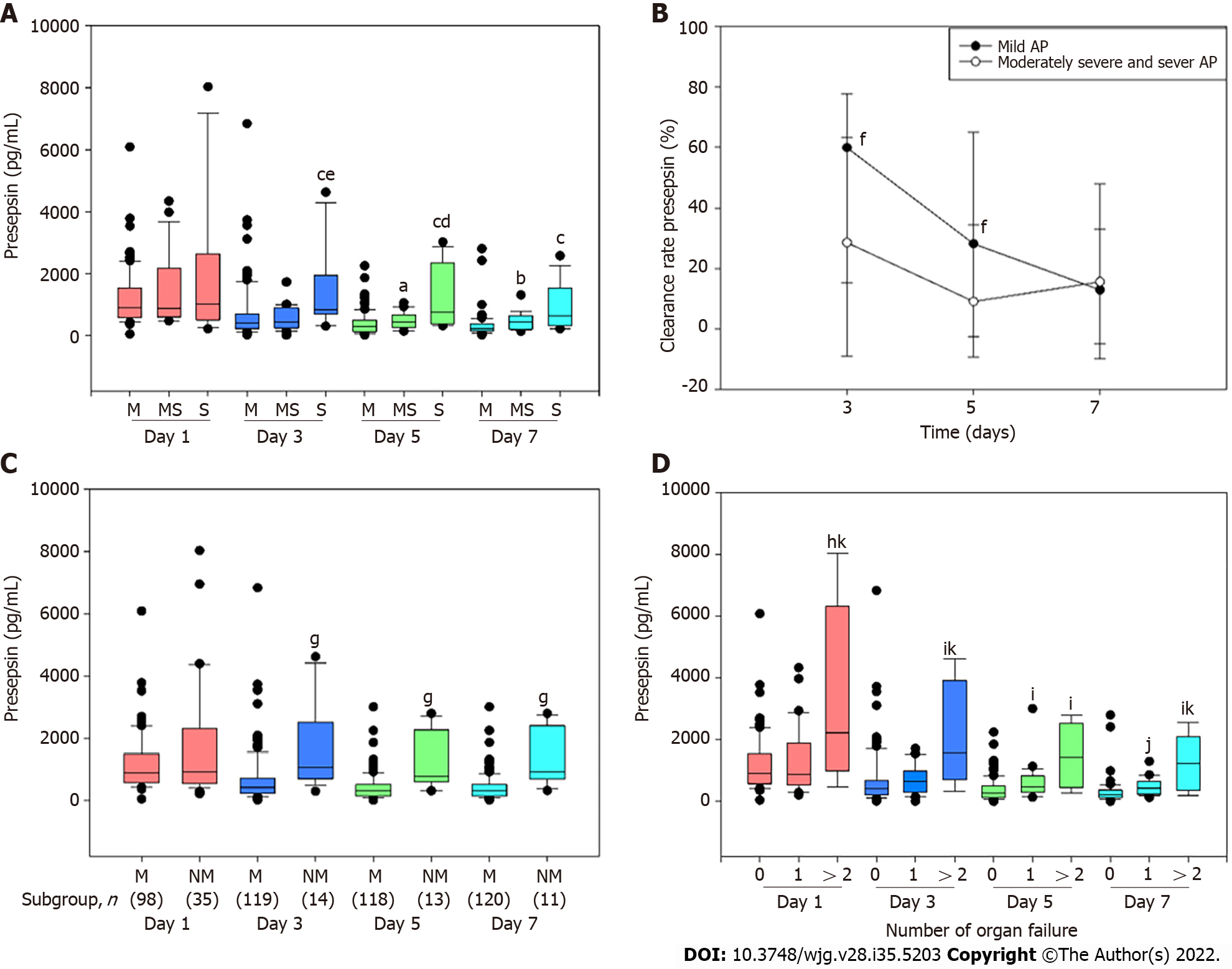
Compared to patients with mild or moderately severe AP, the presepsin concentration at days 3 and 5 were significantly higher in patients with severe AP (Figure 1A). Compared with the mild group, the presepsin concentration in the moderately severe group at days 5 and 7 and in the severe group at day 7 were significantly higher (Figure 1A). The median clearance rate of presepsin on days 3 and 5 in moderately severe and severe patients were reduced compared to patients in the mild group (Figure 1B).
Moreover, presepsin concentration remained significantly higher in the non-mild group compared to the mild group at days 3, 5 and 7 (Figure 1C). The concentrations of presepsin through the 7 d increased progressively with the number of organs failing as defined by the Marshall score (Figure 1D).
Compared to the mild group, the proportion of patients with biliary etiology in the non-mild group was significantly less (69.4% vs 45.7%; P = 0.013) and those with idiopathic AP was significantly higher (6.1% vs 34.3%; P < 0.001). The median presepsin concentration on day 1 in patients with biliary AP was higher compared to those with other etiologies [1154.00 (728.75-2108.50) vs 749.00 (474.00-1174.00); P = 0.001]. The presepsin concentration on day 1 and etiology were independent predictive factors for non-mild AP on day 1 (Table 2). The presepsin concentrations on days 3, 5 and 7, but not etiology, were independent predictive factors for non-mild AP on days 3, 5 and 7, respectively (Table 2). Presepsin concentration showed time-specific AUCs of 0.827, 0.848 and 0.867 on days 3, 5 and 7, respectively, in predicting moderately severe and severe AP (Table 3).
| Variable | β | Standard error | Wald statistic | Degrees of freedom | P value | Odds ratio | 95%CI | |
| Lower limit | Upper limit | |||||||
| Non-mild AP day 1 | ||||||||
| Etiology | 0.456 | 0.133 | 11.707 | 1 | 0.001 | 1.578 | 1.215 | 2.050 |
| Presepsin day 1 | 0.000 | 0.000 | 5.872 | 1 | 0.015 | 1.001 | 1.000 | 1.001 |
| Non-mild AP day 3 | ||||||||
| Etiology | 0.259 | 0.178 | 2.115 | 1 | 0.146 | 1.296 | 0.914 | 1.836 |
| Presepsin day 3 | 0.001 | 0.000 | 8.567 | 1 | 0.003 | 1.001 | 1.000 | 1.001 |
| Non-mild AP day 5 | ||||||||
| Etiology | 0.315 | 0.199 | 2.502 | 1 | 0.114 | 1.371 | 0.927 | 2.026 |
| Presepsin day 5 | 0.002 | 0.000 | 13.141 | 1 | < 0.001 | 1.002 | 1.001 | 1.003 |
| Non-mild AP day 7 | ||||||||
| Etiology | 0.292 | 0.215 | 1.836 | 1 | 0.175 | 1.339 | 0.878 | 2.041 |
| Presepsin day 7 | 0.002 | 0.000 | 13.203 | 1 | < 0.001 | 1.002 | 1.001 | 1.003 |
| Time point | Cutoff (pg/mL) | Sensitivity | Specificity | PPV | NPV | AUROC (95%CI) | P value |
| Day 3 | 657.5 | 92.9% | 71.4% | 76.5% | 90.9% | 0.827 (0.730-0.923) | < 0.001 |
| Day 5 | 593.5 | 84.6% | 80.4% | 81.2% | 83.9% | 0.848 (0.751-0.945) | < 0.001 |
| Day 7 | 601.5 | 72.7% | 89.8% | 87.7% | 76.7% | 0.867 (0.756-0.977) | < 0.001 |
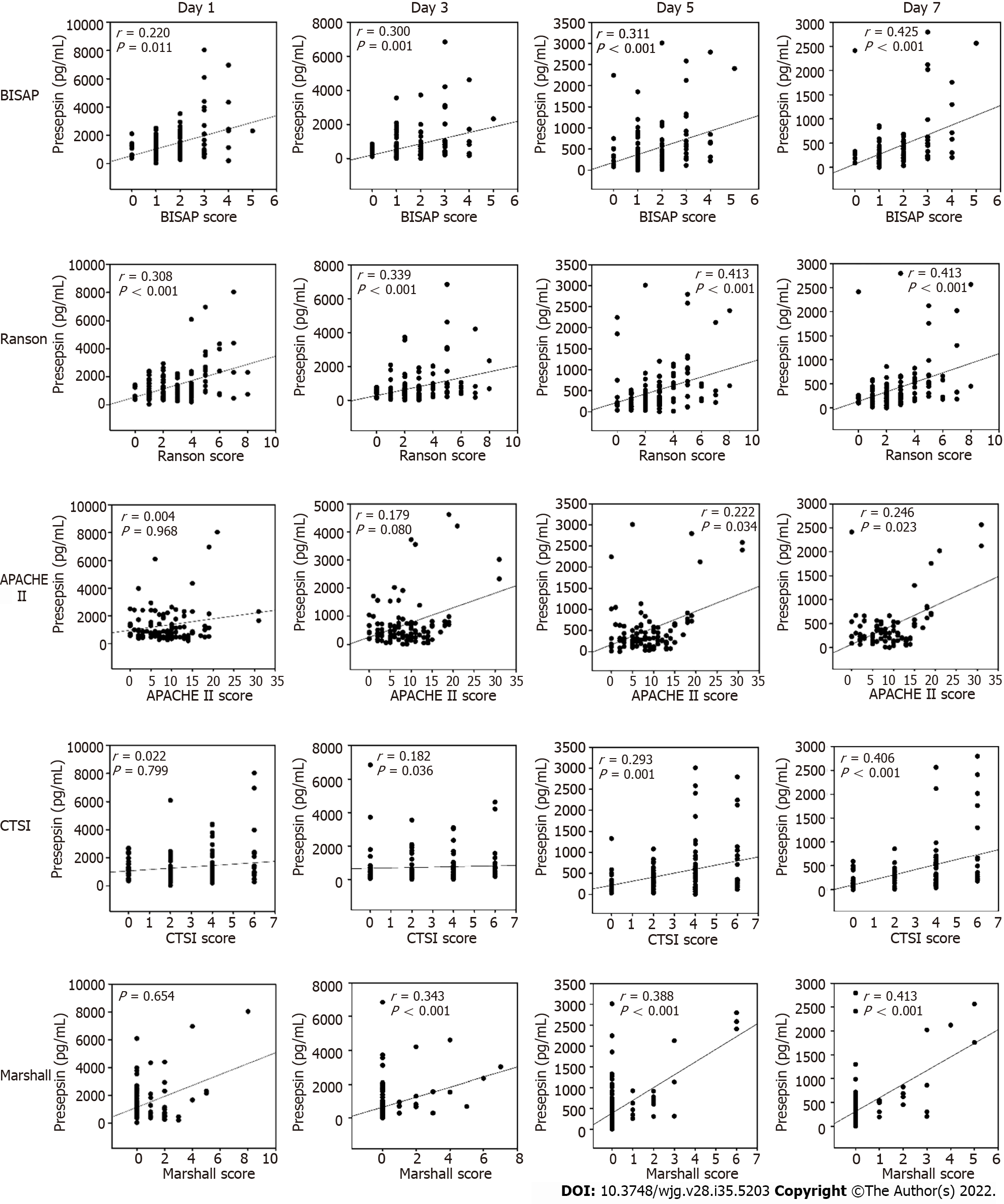
The Spearman correlation analysis revealed that the presepsin levels through day 7 in AP patients positively correlated with BISAP and Ranson scores (all P < 0.05, Figure 2). Positive correlations were also observed between the presepsin concentration on days 5 and 7 with the APACHE II scores. Presepsin levels at days 3, 5 and 7 also positively correlated with CTSI and Marshall scores (all P < 0.05, Figure 2). There was a positive correlation between presepsin and procalcitonin levels on days 1 and 3 and between presepsin and C-reactive protein levels on days 3 and 7 (Table 4).
| Variables | Procalcitonin | C-reactive protein |
| Presepsin day 1 | r = 0.464 | r = 0.179 |
| P < 0.001 | P = 0.080 | |
| Presepsin day 3 | r = 0.318 | r = 0.254 |
| P = 0.002 | P = 0.016 | |
| Presepsin day 5 | r = 0.208 | r = 0.183 |
| P = 0.084 | P = 0.091 | |
| Presepsin day 7 | r = 0.239 | r = 0.312 |
| P = 0.053 | P = 0.007 |
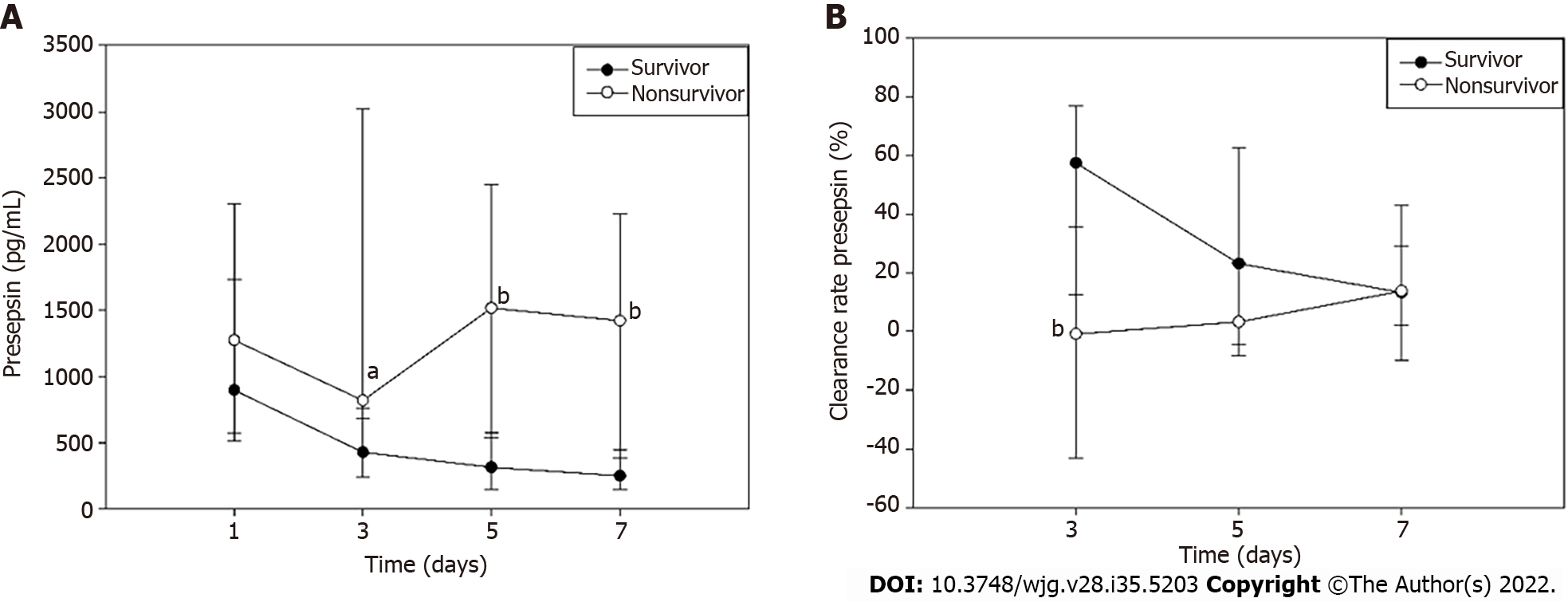
Presepsin levels decreased persistently through the first 7 d after admission in survivors but tended to decrease on day 3 and then increase on day 5 in non-survivors. The presepsin concentration in non-survivors on days 3, 5 and 7 were significantly higher when compared with survivors (Figure 3A). Moreover, the median clearance rate of presepsin on day 3 in the survivors was higher than that in the non-survivors (Figure 3B; P < 0.01).
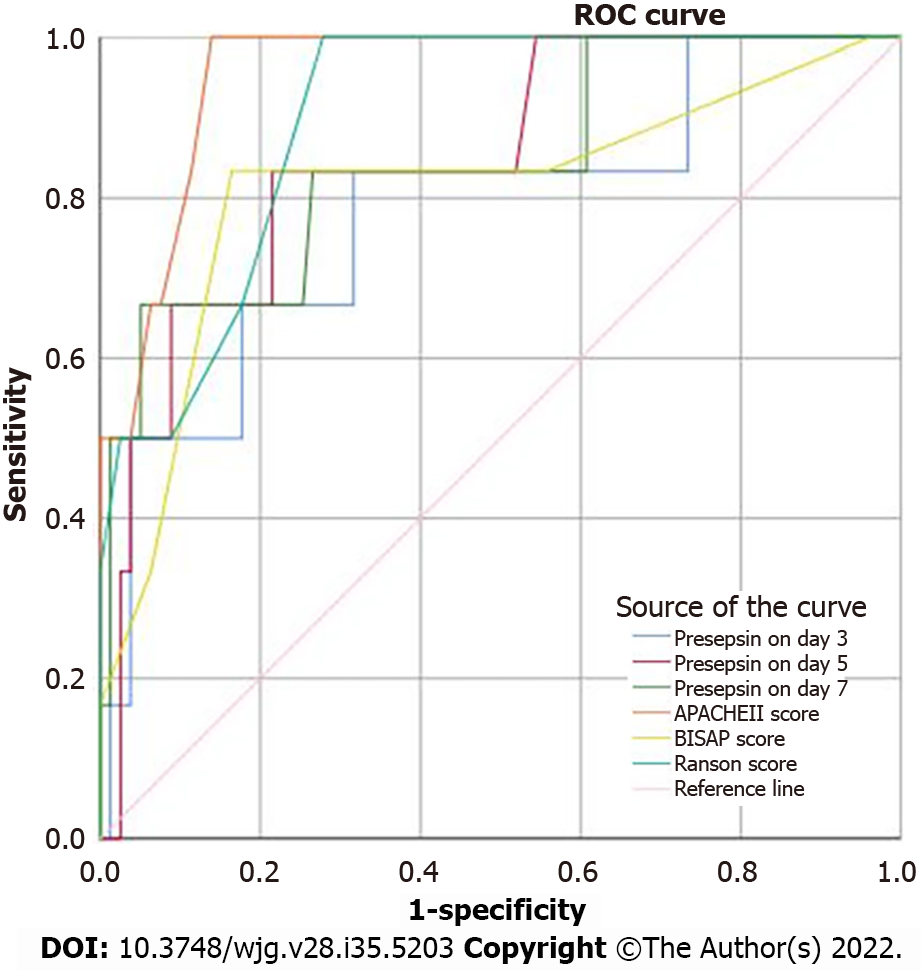
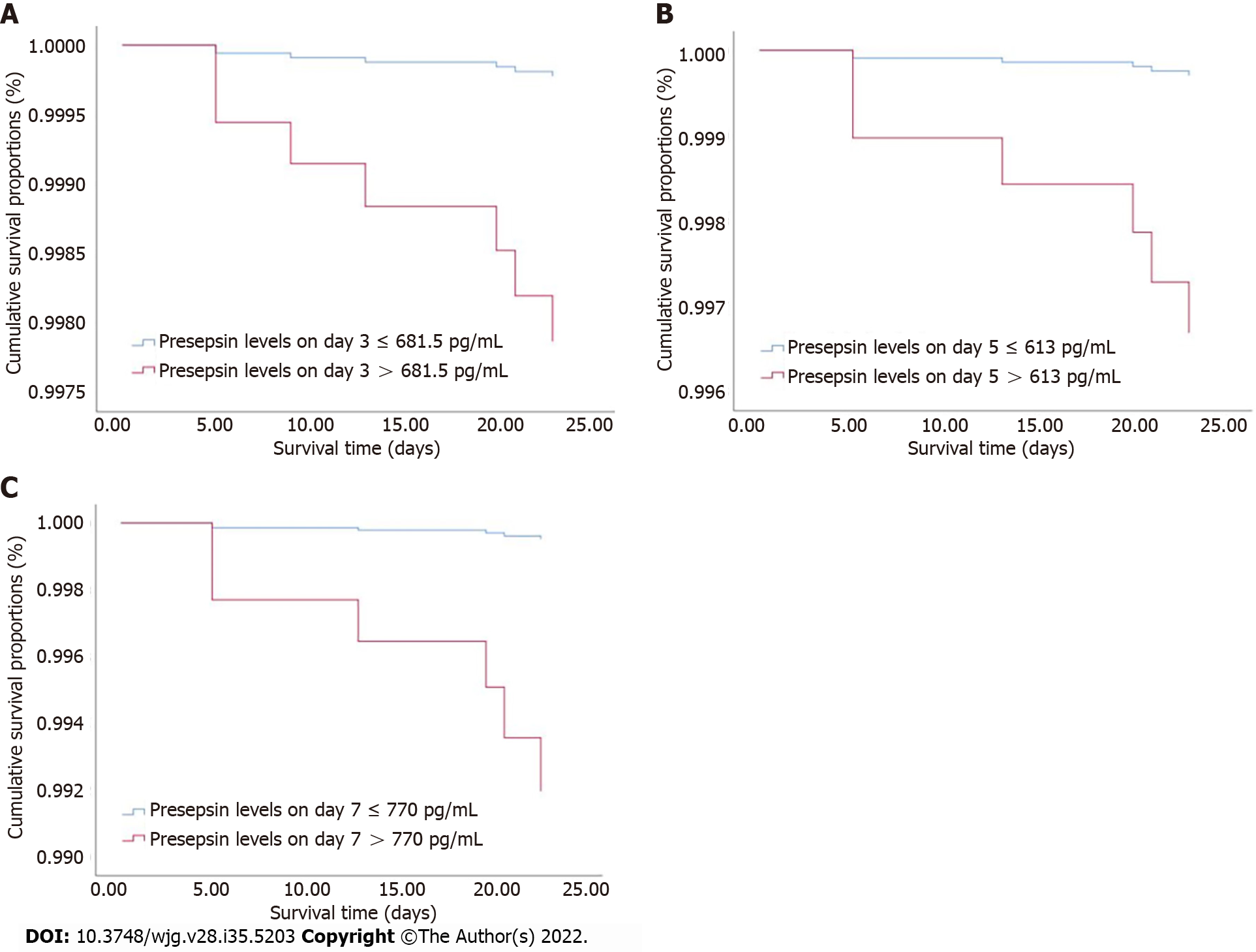
The clinical characteristics between survival and non-survival groups are presented in Table 5. The binary logistic regression analysis showed that the presepsin levels on days 3, 5 and 7 and the BISAP, Ranson and APACHE II scores were independent predictors of 28-d mortality in patients with AP (Table 6). The AUC of presepsin for predicting 28-d mortality in AP patients was 0.781 on day 3, 0.846 on day 5 and 0.843 on day 7, which were slightly lower compared to APACHE II (0.955; all P > 0.05) and Ranson (0.900; all P > 0.05) scores but similar to BISAP (0.811; all P > 0.05) scores (Figure 4). The optimal prognostic cutoff values for predicting 28-d mortality on days 3, 5 and 7 were 681.5 pg/mL, 613 pg/mL and 770 pg/mL of presepsin, respectively.
| Characteristic | 28-d survival outcome | P value | |
| Survival (n = 126) | Non-survival (n = 7) | ||
| Demographics | |||
| Male, % | 79 (62.7) | 7 (100.0) | 0.051 |
| Age, yr | 65 (54-75) | 75 (54-82) | 0.417 |
| Comorbidities | |||
| CHF, % | 19 (18.1) | 0 (0) | 1.000 |
| COPD, % | 2 (1.9) | 1 (25.0) | 0.107 |
| Diabetes, % | 24 (22.9) | 1 (25.0) | 1.000 |
| Immunosuppression, % | 7 (6.7) | 1 (25.0) | 0.266 |
| Etiology | 0.634 | ||
| Gallstones, % | 80 (63.5) | 4 (57.1) | |
| Hypertryglyceridemia, % | 11 (8.7) | 1 (14.3) | |
| Alcohol, % | 4 (3.2) | 0 (0.0) | |
| Post-ERCP, % | 15 (11.9) | 0 (0.0) | |
| Idiopathic, % | 16 (12.7) | 2 (28.6) | |
| Scoring | |||
| BISAP score at admission | 1.0 (1.0-2.0) | 3.0 (2.0-4.0) | 0.003 |
| Ranson score at 48 h | 2.0 (1.0-3.0) | 5.0 (4.0-8.0) | < 0.001 |
| CTSI score | 2.0 (2.0-4.0) | 4.0 (4.0-4.0) | 0.181 |
| APACHE II score at admission | 8.0 (4.8-11.0) | 19.0 (15.0-31.0) | < 0.001 |
| Imaging | |||
| Pancreatic necrosis, % | 13 (10.3) | 1 (14.3) | 0.550 |
| Mechanical ventilation, % | 0 (0.0) | 3 (42.9) | < 0.001 |
| CRRT, % | 0 (0.0) | 1 (14.3) | 0.053 |
| Length of hospital stay in d | 11 (9-14) | 13 (5-21) | 0.568 |
| Outcomes | |||
| Single organ failure, % | 27 (22.1) | 3 (100.0) | 0.013 |
| Multiple organ failure, % | 4 (4.0) | 4 (100.0) | < 0.001 |
| Cost in $ | 4718.99 (3065.47-7104.78) | 22243.29 (10432.63-36309.99) | 0.001 |
| Variable | β | Standard error | Wald statistic | Degrees of freedom | P value | Odds ratio | 95%CI | |
| Lower limit | Upper limit | |||||||
| Presepsin day 3 | 0.001 | 0.000 | 5.013 | 1 | 0.025 | 1.001 | 1.000 | 1.001 |
| Presepsin day 5 | 0.002 | 0.000 | 11.406 | 1 | 0.001 | 1.002 | 1.001 | 1.003 |
| Presepsin day 7 | 0.002 | 0.001 | 11.672 | 1 | 0.001 | 1.002 | 1.001 | 1.003 |
| BISAP score at admission | 1.217 | 0.390 | 9.700 | 1 | 0.002 | 3.376 | 1.571 | 7.255 |
| Ranson score at 48 h | 0.868 | 0.249 | 12.202 | 1 | < 0.001 | 2.383 | 1.464 | 3.880 |
| APACHE II score at admission | 0.477 | 0.166 | 8.251 | 1 | 0.004 | 1.611 | 1.164 | 2.230 |
Using the cutoff values determined by ROC curves, the Cox proportional hazards regression model was adjusted for age, sex and etiology to analyze the 28-d survival curves of patients (Figure 5). The hazard ratio (HR) of presepsin at day 3 was 9.475 (95%CI: 1.133-79.226; P = 0.038), the HR of presepsin at day 5 was 11.191 (95%CI: 1.297-96.518; P = 0.028), and the HR of presepsin at day 7 was 16.495 (95%CI: 2.759-98.615; P = 0.002).
The current findings demonstrated that blood presepsin levels correlated with the severity of AP, and the clearance rate of presepsin was lower in patients with moderately severe or severe AP compared to patients with mild AP. Furthermore, the presepsin levels on days 1, 3, 5 and 7 during the hospital stay independently predicted the severity of AP. High levels of presepsin through the first 7 d after admission were associated with organ failure. It was also found that presepsin positively correlated with the BISAP, Ranson, CTSI, APACHE II, and Marshall scores as well as conventional biomarkers such as procalcitonin and C-reactive protein. Dynamic changes in the concentration of presepsin can be used for ongoing risk stratification of disease course and prediction of 28-d mortality in AP patients. Both presepsin and the clearance rate of presepsin on day 3 may be used as early biomarkers to predict the severity and prognosis of AP.
Recently, the blood presepsin concentration has been shown to be an early biomarker of various infections[14,15] as well as a valuable biomarker for diagnosing the occurrence[16], severity[17] or prognosis[7] of sepsis. Presepsin levels were shown to have good diagnostic and prognostic value for bacterial community-acquired pneumonia (CAP) and intensive care unit (ICU) mortality[18]. Similarly, our previous study showed that presepsin could predict acute respiratory distress syndrome, severe CAP, and 28-d mortality[19]. Recently, Yao et al[20] found that presepsin has a better predictive ability than existing biomarkers for bacterial infection following major hepato-biliary-pancreatic surgery. The study of Hiraki et al[21] showed that a higher concentration of presepsin in the drainage fluid was an independent predictive marker for clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy.
For noninfectious diseases, presepsin correlates with the disease activity of autoimmune diseases, such as systemic lupus erythematosus[22]. Higher presepsin levels are associated with renal and liver dysfunction[10]. Presepsin is a 13 kDa peptide that may be cleared by the kidney[6]. Presepsin levels have been shown to increase as the glomerular filtration rate (GFR) decreases and are markedly high in patients with chronic renal failure or receiving hemodialysis[23]. Presepsin concentrations correlate with serum creatinine and GFR levels in patients with chronic kidney disease[24]. Recently, presepsin was found to be a predictor of acute kidney injury and initiation of renal replacement therapy in sepsis patients[25]. Masson et al[10] found that presepsin levels are significantly higher in septic patients with shock than those without shock. A gender- and age-matched study on patients with severe AP and healthy volunteers showed that presepsin was an independent predictor of local complications, organ failure and in-hospital mortality[26].
In this study, we observed the dynamic changes of plasma presepsin levels and clearance rate of presepsin with time in patients with mild, moderate and severe AP and found that the presepsin concentrations on days 3, 5 and 7 (but not day 1) increased with the severity of AP. A high presepsin value and low clearance rate of presepsin at day 3 were found to be more valuable in early identification of mild or moderate vs severe compared to mild vs moderate disease. Inconsistently, the concentrations of presepsin on day 1 through 7 increased significantly with the number of organs experiencing failure.
In most high-income countries, gallstones (45%) and alcohol abuse (20%) are the most frequent causes of AP[27]. In the current study, gallstones (63.2%), but not alcohol (3.0%), was the most common etiology of AP, which is consistent with the conclusion of an 8-year Chinese study by Zhu et al[28]. Upon multivariate analysis, after adjusting for the confounding factor of etiology, it was found that presepsin levels were independent predictors for the severity of AP on days 1, 3, 5 and 7. In this study, the proportion of patients with biliary etiology were significantly less, and those with idiopathic AP were significantly higher in the moderately severe or severe AP group compared to the mild AP group. It was shown that the presepsin values on day 1, but not on days 3, 5 or 7, were higher in patients with biliary AP than in other etiologies, which is in line with the procalcitonin values of the study of Modrau et al[29]. It is likely that the increase in circulating presepsin found in the present study was associated with organ dysfunction and biliary tract infection, though infectious complications are rare during the early course of severe AP[30].
Procalcitonin is a sensitive biomarker for the detection of pancreatic infection, and C-reactive protein levels ≥ 150 mg/L at day 3 is a prognostic indicator for severe AP[13]. The current study illustrated that presepsin levels were positively correlated with procalcitonin levels on days 1 and 3 and with C-reactive protein levels on days 3 and 7. There was a positive correlation between the presepsin levels and Marshall scores (including respiratory function, cardiovascular system, renal dysfunction) on days 3, 5 and 7. Meanwhile, the current study found a significant positive correlation between the presepsin levels and other AP scores, such as BISAP, Ranson and APACHE II. In this study, increased presepsin levels were found to be an accurate predictor of disease severity. For the prediction of moderately severe or severe AP, the AUCs of presepsin on days 3, 5 and 7 were 0.827, 0.848 and 0.867, respectively, with high sensitivity and specificity.
The dynamic changes in presepsin were different in survivors and non-survivors of this study. In survivors, presepsin levels showed a decreasing trend over time but in non-survivors presepsin levels first decreased and then increased. Similar trends in presepsin levels have been reported in patients with severe sepsis[31]. In addition, clearance rates of presepsin in non-survivors were lower than that of survivors in the early stage of AP (day 3). Potential explanations involve a reduced clearance of presepsin due to reduced kidney function[22-24] and circulatory dysfunction[10]. The most common organ systems to fail in non-survivors were circulatory [71.4% (5/7 patients)], respiratory [85.7% (6/7 patients)] and renal [42.8% (3/7 patients)] systems. Studies have showed that higher presepsin levels were associated with ICU death and mortality in sepsis[7], CAP[18], cardiac arrest patients after return of spontaneous circulation[32] and severe AP[25]. Interestingly, the presepsin levels on days 3, 5,and 7 were found to independently predict 28-d mortality in AP.
This study has certain limitations. First, the patient population was heterogeneous. Second, the study did not compare presepsin with other biomarkers. Third, the relevance of presepsin during the latter course of AP was not studied. Fourth, the association between presepsin levels and hepatorenal function was not evaluated. Future multicentric studies with larger cohorts that consider these factors should be conducted to validate the findings of this study.
This cohort study found that blood presepsin levels in the first 7 d after admission could accurately predict the severity of disease course and 28-d mortality in patients with AP and may be a promising prognostic marker.
Acute pancreatitis (AP) is one of the most common causes of abdominal emergencies and is associated with sepsis, organ failure and high mortality rates (up to 35%). AP has diverse clinical manifestations with an unpredictable clinical course. It is necessary to predict the severity of AP rapidly and accurately.
A major challenge in AP is the early identification of patients at high-risk for organ failure and death. However, scoring systems are complicated and time consuming with limited predictive value for the clinical course. Biomarkers are promising for the dynamic prediction of disease severity.
To determine whether the dynamic levels of an emerging biomarker, presepsin, can be used to evaluate the severity of disease course and outcome of AP.
In this prospective and multicentric cohort study, 133 patients with AP were included from January 2018 to September 2019. Clinical severity (mild, moderately severe and severe AP) was dynamically evaluated using the 2012 revised Atlanta Classification. Blood presepsin levels were measured at days 1, 3, 5 and 7 after admission by chemiluminescent enzyme immunoassay. The patients were followed up for 28 d.
The median concentration of presepsin increased, and the clearance rate of presepsin decreased with disease severity and organ failure in AP patients. The presepsin levels on days 3, 5 and 7 were independent predictors of moderately severe and severe AP with time-specific area under the curve (AUC) values of 0.827, 0.848 and 0.867, respectively. The presepsin levels positively correlated with bedside index of severity in acute pancreatitis, Ranson, acute physiology and chronic health evaluation-II, computed tomography severity index and Marshall scores and conventional biomarkers such as procalcitonin and C-reactive protein. Presepsin levels on days 3, 5 and 7 were independent predictors of 28-d mortality of AP patients with AUC values of 0.781, 0.846 and 0.843, respectively.
The blood presepsin levels within 7 d of admission were associated with and may be useful to dynamically predict the severity of disease course and 28-d mortality in AP patients. Both presepsin and clearance rate of presepsin on day 3 may be used as early biomarkers to predict the severity and prognosis of AP.
Prospective cohort studies report the predictive value of presepsin in the severity of AP. Future research should focus on the guiding significance of presepsin in the treatment of AP, such as antibiotic use.
We thank Drs. Jian-Dong Zhang, Jie Yang, Shuai Xia, Mei-Ying Zhang, Si-Jia Wang and Zheng Wang for collecting cases. We also thank Dr. Shan-Shan Wu for statistical analyses. We also gratefully acknowledge all health care workers on the front line and all patients involved in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balaban DV, Romania; Ferrarese A, Italy S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yu HG
| 1. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 2. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 3. | Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf). 2018;6:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879-13892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (5)] |
| 5. | Shirakawa K, Naitou K, Hirose J, Takahashi T, Furusako S. Presepsin (sCD14-ST): development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clin Chem Lab Med. 2011;49:937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Ackland GL, Prowle JR. Presepsin: solving a soluble (CD14) problem in sepsis? Intensive Care Med. 2015;41:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 2013;17:R244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Nakamura Y, Ishikura H, Nishida T, Kawano Y, Yuge R, Ichiki R, Murai A. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiol. 2014;14:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 10. | Masson S, Caironi P, Fanizza C, Thomae R, Bernasconi R, Noto A, Oggioni R, Pasetti GS, Romero M, Tognoni G, Latini R, Gattinoni L. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015;41:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 11. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |
| 12. | Okamura Y, Yokoi H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta. 2011;412:2157-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 14. | Marazzi MG, Randelli F, Brioschi M, Drago L, Romanò CL, Banfi G, Massaccesi L, Crapanzano C, Morelli F, Corsi Romanelli MM, Galliera E. Presepsin: A potential biomarker of PJI? Int J Immunopathol Pharmacol. 2018;31:394632017749356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Kweon OJ, Choi JH, Park SK, Park AJ. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J Crit Care. 2014;29:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, Borggrefe M, Hoffmann U. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Klouche K, Cristol JP, Devin J, Gilles V, Kuster N, Larcher R, Amigues L, Corne P, Jonquet O, Dupuy AM. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care. 2016;6:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Liu B, Yin Q, Chen YX, Zhao YZ, Li CS. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med. 2014;108:1204-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Yao S, Kaido T, Uozumi R, Hirata M, Iwamura S, Miyachi Y, Macshut M, Sharshar M, Yagi S, Uemoto S. Diagnostic potential of presepsin in bacterial infection following hepato-biliary-pancreatic surgery: A prospective observational study. J Hepatobiliary Pancreat Sci. 2020;27:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Hiraki M, Miyoshi A, Sadashima E, Shinkai Y, Yasunami M, Manabe T, Kitahara K, Noshiro H. The novel early predictive marker presepsin for postoperative pancreatic fistula: A pilot study. Exp Ther Med. 2020;20:2298-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Tanimura S, Fujieda Y, Kono M, Shibata Y, Hisada R, Sugawara E, Nakamura H, Ohmura K, Shimamura S, Mitani A, Shida H, Watanabe T, Kato M, Oku K, Bohgaki T, Amengual O, Yasuda S, Shimizu C, Atsumi T. Clinical significance of plasma presepsin levels in patients with systemic lupus erythematosus. Mod Rheumatol. 2018;28:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Nagata T, Yasuda Y, Ando M, Abe T, Katsuno T, Kato S, Tsuboi N, Matsuo S, Maruyama S. Clinical impact of kidney function on presepsin levels. PLoS One. 2015;10:e0129159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Miyoshi M, Inoue Y, Nishioka M, Ikegame A, Nakao T, Kishi S, Doi T, Nagai K. Clinical evaluation of presepsin considering renal function. PLoS One. 2019;14:e0215791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Shimoyama Y, Umegaki O, Kadono N, Minami T. Presepsin and prognostic nutritional index are predictors of septic acute kidney injury, renal replacement therapy initiation in sepsis patients, and prognosis in septic acute kidney injury patients: a pilot study. BMC Nephrol. 2021;22:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Lin J, Li Z, Zheng Y, Zhang Y, Shao C, Liu G, Li J. Elevated Presepsin Levels are Associated with Severity and Prognosis of Severe Acute Pancreatitis. Clin Lab. 2016;62:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 28. | Zhu Y, Pan X, Zeng H, He W, Xia L, Liu P, Zhu Y, Chen Y, Lv N. A Study on the Etiology, Severity, and Mortality of 3260 Patients With Acute Pancreatitis According to the Revised Atlanta Classification in Jiangxi, China Over an 8-Year Period. Pancreas. 2017;46:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 29. | Modrau IS, Floyd AK, Thorlacius-Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. Am J Gastroenterol. 2005;100:1593-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 527] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Yu H, Qi Z, Hang C, Fang Y, Shao R, Li C. Evaluating the value of dynamic procalcitonin and presepsin measurements for patients with severe sepsis. Am J Emerg Med. 2017;35:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Qi Z, Zhang Q, Liu B, Shao F, Li C. Presepsin As a Biomarker for Evaluating Prognosis and Early Innate Immune Response of Out-of-Hospital Cardiac Arrest Patients After Return of Spontaneous Circulation. Crit Care Med. 2019;47:e538-e546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |