Published online Sep 7, 2022. doi: 10.3748/wjg.v28.i33.4890
Peer-review started: January 12, 2022
First decision: March 8, 2022
Revised: March 16, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 7, 2022
Processing time: 231 Days and 10.3 Hours
Chronic gastritis (CG) is an inflammatory disease of the gastric mucosa. Shen-ling-bai-zhu san (SLBZS), a traditional Chinese medicine formula, is widely used for treating CG. Nevertheless, its effects are currently unclear.
To determine the clinical evidence and potential mechanisms of SLBZS for the treatment of CG.
We systematically searched 3 English (PubMed, Embase, Medline) and 4 Chinese databases (Cochrane Library Central Register of Controlled Trials, China National Knowledge Infrastructure database, Wanfang Data Knowledge Service Platform, and the VIP information resource integration service platform) without language or publication bias restriction. Qualified studies were selected according to pre-set inclusion and exclusion criteria. RevMan 5.3 software was used for meta-analysis and literature quality assessment, Stata 14.0 software was used for sensitivity analysis, GRADE profiler 3.6 was used to evaluate the quality of evidence. And then, network pharmacology analysis was applied to primary research the mechanisms of action of SLBZS on CG.
Fourteen studies were finally included, covering 1335 participants. Meta-analysis indicated that: (1) SLBZS was superior to conventional therapies [risk ratio (RR): 1.29, 95% confidence interval (CI): 1.21 to 1.37, P < 0.00001]; (2) SLBZS was better than conventional therapies [RR: 0.24, 95% confidence interval (95%CI): 0.11 to 0.55, P = 0.0007] in terms of recurrence rate and reversal of Helicobacter pylori positivity (RR: 1.20, 95%CI: 1.11 to 1.30, P < 0.00001); and (3) The safety of SLBZS for CG remains unclear. According to the GRADE method, the quality of evidence was not high. Besides, SNZJS might treat CG by acting on related targets and pathways such as EGFR tyrosine kinase inhibitor resistance, the PI3K-Akt signaling pathway, and others.
SLBZS might be useful in treating CG, but long-term effects and specific clinical mechanisms of it maintain unclear. More samples and high-quality clinical experiments should be assessed and verified in the next step.
Core Tip: A 2012 clinical practice guideline recommended Shenling Baizhu Powder for the Pattern of Spleen and Stomach Deficiency chronic gastritis (CG). The 2020 clinical guideline did not recommended Shen-ling-bai-zhu san (SLBZS), possibly because of inadequate clinical evidence and pharmacological mechanisms. We designed our study to focus on evidence of efficacy and potential mechanisms. Our study showed that SLBZS might be useful in treating CG; however, its long-term effects and mechanisms of action are unclear. Due to the poor quality of the evidence, more samples and high-quality clinical studies should be tested.
- Citation: Jin W, Zhong J, Song Y, Li MF, Song SY, Li CR, Hou WW, Li QJ. Chinese herbal formula shen-ling-bai-zhu-san to treat chronic gastritis: Clinical evidence and potential mechanisms. World J Gastroenterol 2022; 28(33): 4890-4908
- URL: https://www.wjgnet.com/1007-9327/full/v28/i33/4890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i33.4890
Chronic gastritis (CG) is a set of inflammatory diseases of the gastric mucosa[1] and is one of the common diseases of the digestive system. The disease often relapses, accompanied by symptoms that severely affect the quality of life. Chronic atrophic gastritis is associated with intestinal metaplasia and intraepithelial neoplasia, increasing gastric cancer risk. Globally, on average, more than 50% of people may have CG at any given moment[2]. A pathological study of 8892 patients in China found that atrophic gastritis, intestinal metaplasia, and dysplasia were prevalent, occurring in 25.8%, 23.6%, and 7.3% of the population, respectively[3].
The treatment of CG with gastric mucosal repair consists of antacids, antacids, and gastric mucosal protective agents[4-7]. Nevertheless, the efficacy of triple or quadruple therapy is not ideal, and there are frequent side effects[8]. For these reasons, complementary and alternative medicine therapies such as acupuncture[9-12], moxibustion[13,14], and Chinese herbal formulas[15,16] are sought as alternative therapies.
The Chinese herb formula Shenling Baizhu Powder, also known as Shen-ling-bai-zhu-san (SLBZS), is a widely used prescription for digestive tract disease in China derived from the classic herb monograph “Taipinghuiminhejijufang” written in the Song dynasty[17]. Ten commonly used herbs constitute SLBZS; these include Baizhu (Atractylodes macrocephala Koidz), Fuling (Smilax glabra Roxb), Yiyiren [Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf], Renshen (Panax ginseng C.A.Mey), Shanyao (Dioscorea oppositifolia L), Baibiandou (Lablab purpureus subsp. purpureus), Lianzi (Nelumbo nucifera Gaertn), Sharen (Amomum villosum Lour), Jiegeng (Platycodon grandiflorus) and Gancao (Glycyrrhiza uralensis Fisch. ex DC). In China, clinical studies suggested that SLBZS treats CG[18,19] with efficacy. Nevertheless, mechanistic studies based on animal experiments are lacking.
Furthermore, a 2012 Clinical practice guideline[20] recommended Shenling Baizhu Powder for the Pattern of Spleen and Stomach Deficiency CG. The pathogenesis can be summarized as the stomach failing to be nourished because of splenic and gastric qi deficiency and disturbance of qi movement. A 2020 clinical guideline did not recommend SLBZS, possibly because of inadequate clinical evidence and pharmacological mechanisms[21]. Efficacy evidence and potential mechanistic studies are required.
We registered this review and meta-analysis at the PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/#recordDetails), an international prospective system review registration website. The registration number was CRD42020212979. We conducted the study based on the details of this protocol.
Our investigators independently searched PubMed, Embase, Medline, Cochrane Library Central Register of Controlled Trials, China National Knowledge Infrastructure database, Wanfang Data Knowledge Service Platform, and the VIP information resource integration service platform from their inception to November 2021. There were no limitations on language or publication status. They also searched conference articles and clinical registries for possible related trials.
We adopted a search strategy that combined medical subject headings and free words. Two authors (YS, MFL) searched and screened all citations independently. The search strategy was as follows (Table 1): The search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[22].
| Number | Search terms |
| 1 | Mesh descriptor (Medicine, Traditional) explode all trees |
| 2 | (Medicine, Chinese Traditional*): ti,ab,kw |
| 3 | Mesh descriptor(Drugs, Chinese Herbal) explode all trees, |
| 4 | ((Chinese Drugs, Plant*) or (Chinese Herbal Drugs*) or (Herbal Drugs, Chinese*) or (Plant Extracts, Chinese*) or (Chinese Plant Extracts*) or(Extracts, Chinese Plant*)): ti,ab,kw |
| 5 | Mesh descriptor (shen-ling-bai-zhu) explode all trees |
| 6 | ((shen-ling-bai-zhu powder*) or (shen-ling-bai-zhu formula*) or (shen-ling-bai-zhu decoction*) or (shen-ling-bai-zhu decoction*) or (Shen-ling-bai-zhu powder*) or (Shen-ling-bai-zhu formula*) or (Shen-ling-bai-zhu formula*)): ti,ab,kw |
| 7 | Or 1-6 |
| 8 | Mesh descriptor: (Chronic gastritis) explode all trees |
| 9 | ((Chronic gastritis*) or (Digestive System Diseases*) or (Gastrointestinal Diseases*) or (Gastroenteritis*) or (Gastritis*) or (Chronic, gastritis*)): ti, ab, kw |
| 10 | Or 8-9 |
| 11 | Mesh descriptor: (randomized controlled trials) explode all trees |
| 12 | (random*) or (randomly*) or (allocation*) or (random allocation*) or (placebo*) or (double blind*) or (clinical trials*) or (randomized control trial*) or (RCT*) or (controlled clinical trials*): ti, ab, kw |
| 13 | Or: 11-12 |
| 14 | 7 and 10 and 13 |
Randomized controlled trials (RCTs) or quasi-RCTs that reported the effects of SLBZS on CG were included.
Participants: Studies that evaluated patients with a diagnosis of CG were included. For example, we used diagnostic criteria from the standardized consensus on the diagnosis of CG from the Branch of Spleen and Stomach Diseases of the Chinese Society of Traditional Chinese Medicine, China, that depends on endoscopy and pathological examinations[23]. We excluded studies that included CG patients complicated with hypertension, diabetes, heart disease, or severe allergic diseases. There was no restriction on the setting of interest or other population characteristics.
Interventions: SLBZ powder was the primary prescription, regardless of its dosage form, dosage, or course of treatment. If there were other medications, formulas, or traditional Chinese medicine (TCM) therapies (such as acupuncture, moxibustion, and ear-acupressure) in the treatment group, the control groups must also receive these therapies.
Comparisons: Western medicine, active control, and placebo were acceptable. If SLBZ + western medicine was applied in the experimental group, western medicine in the control group must be consistent.
Outcome measures: We considered efficacy outcomes as primary outcome measures, including effectiveness, recurrence rate, symptom score, Helicobacter pylori (H. pylori) eradication, and quality-of-life assessment. Secondary outcome measures were adverse events directly related to CG.
The exclusion criteria were as follows: (1) The study was not an RCT, e.g., retrospective study, cross-sectional study, observational study, case study, animal study, or others; (2) for multiple reports or repeated publications from the same study, we retained the one with a more significant number of details; (3) diagnostic criteria were not reported in trials, disease not CG; and (4) studies or trials used SLBZS as a part of complex interventions; for example, SLBZS decoction plus another herbal medicine formula vs acupuncture therapies. Western medicine is inconsistent in two groups.
According to our study registration protocol, two reviewers (WJ, QJL) independently performed trial searches, study selection, and raw data extraction. A third reviewer (JZ) checked the extracted data. We resolved conflicts through consensus.
According to the Cochrane Handbook details[24], we performed the risk of bias assessment analysis using the Cochrane collaborative bias risk tool in Review Manager 5.3 software. We resolved conflicts by consultation with a third investigator (WWH).
We used Review Manager 5.3 and Stata 14.0 software for statistical analysis. We calculated 95% confidence interval (CI) and mean difference for continuous variables and 95%CI and risk ratio (RR) for dichotomous variables. Differences with P < 0.05 were statistically significant. We determined the heterogeneity of data using Cochrane χ2 and I2 tests. We used a fixed-effect model if there was no significant heterogeneity; otherwise, we used a random-effect model. We conducted subgroup analyses to explore the source of heterogeneity. We determined publication bias by examining funnel plots and Egger’s tests for more than ten trials. We used sensitivity analysis to explore the stability of the results. GRADE profiler 3.6 software was applied to evaluate the quality of evidence.
Collection and screening of pharmacodynamic components in TCM System Pharmacology Database and analysis platform (TCMSP, HTTP://ibts.hkbu.edu.hk/LSP/tcmsp.php) in ginseng, atractylodes, poria cocos, yam, white hyacinth bean, lotus seed, coix seed, amomum fruit, radix platycodi, radix glycyrrhizae as keyword query filter chemical composition. The database contains about 500 drugs listed in the Chinese Pharmacopoeia, providing absorption, distribution, metabolism and excretion, ingredient data, and target and disease information. Oral bioavailability (OB) and drug-like properties (DL) are essential indexes determining whether a compound can be developed into a drug. Based on the relevant literature, OB and DL were set to > 30% and > 0.18, respectively, and the screened compounds were used as candidate ingredients[25,26].
Target prediction of pharmacodynamic components, the simplified molecular Linear Input specification (Simles) number, and Mol structure of each candidate component were retrieved using PubChem. We arranged candidate target genes using PharmMapper online (http://Lilab-ecust.cn/pharmmapper/index.html) and Swiss target prediction (HTTP://www.swisstargetprediction.ch/), and we arranged the standbys in an Excel form.
Prediction of disease Targets Genes associated with CG was identified by searching for “Chronic Gastritis” in GeneCards (http://www.genecards.org/).
SLBZ Powder’s candidate components and target genes were screened and imported into Cytoscape 3.7.2 software using Excel to obtain a component-target network diagram. The predicted disease candidate targets were imported into the online protein interaction (String) database, the species organism was set as human (Homo sapiens), and the PPI map was obtained. The PPI map was imported into Cytoscape 3.7.2 software. The potential targets of Shenlingbaizhu Powder in chronic gastritis can be obtained by merging the component-target network diagram and disease target PPI diagram using Merge software, which can be imported into the online String database the interaction map of potential targets.
Functional mechanism analysis of potential targets GO enrichment analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment annotation analysis of potential target genes were performed using the R package clusterProfiler.
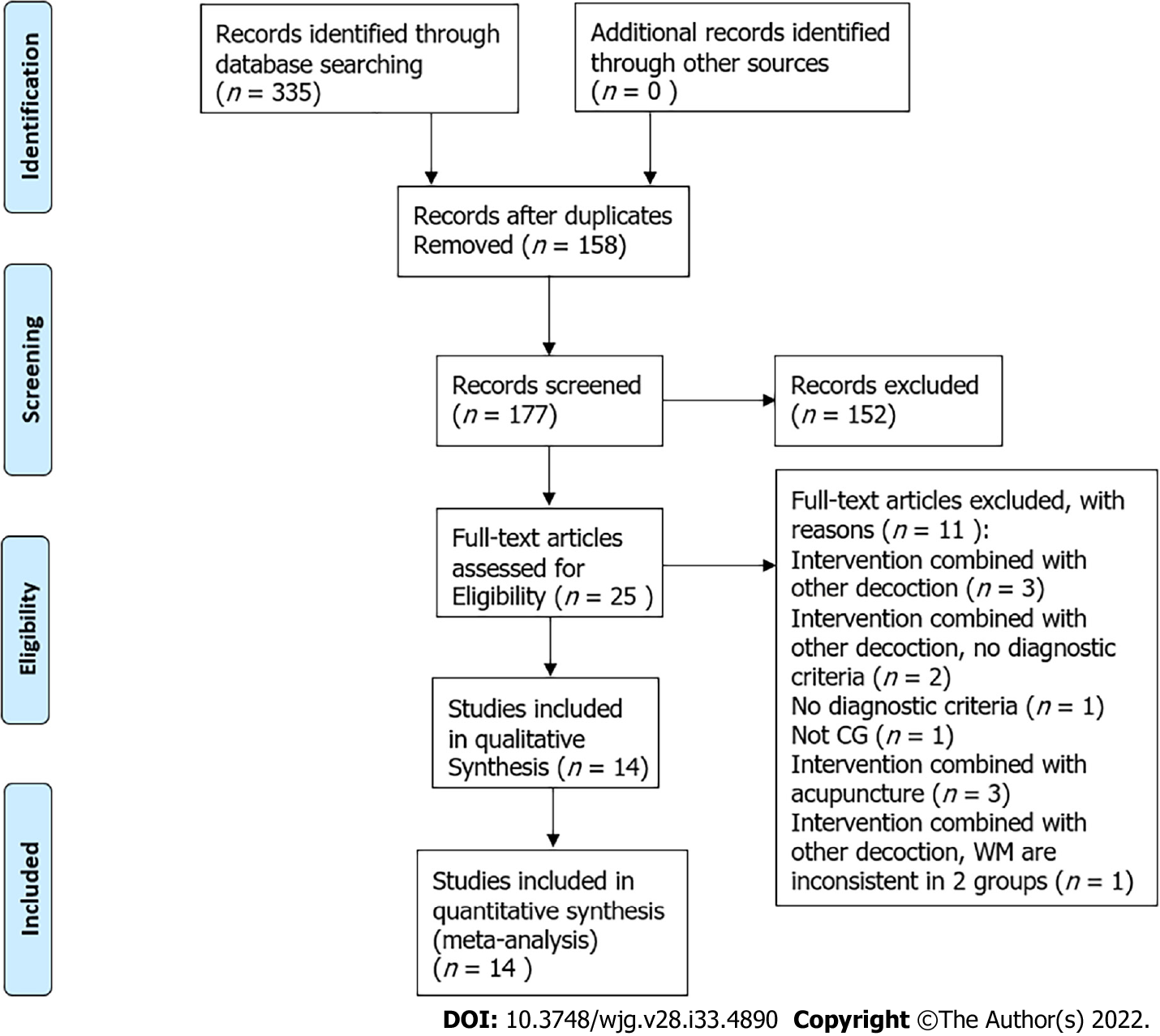
We retrieved 335 trials from 6 databases. When duplicate records were deleted, 177 remained. We excluded 152 studies by reading the title and abstract of the papers, including seven repeatedly published studies, ninety-five reports on the SLBZS experience of experienced TCM doctors, four retrospective studies, seventeen observation studies, one case report, and twenty-eight studies of diseases that were not CG. We read the full texts of the remaining 25 records. We deleted 11 records because of exclusion criteria (Table 2).
| Reason | Ref. (n = 11) |
| Intervention combined with other decoction (n = 3) | Li GS. Observation on the curative effect of Shenling Baizhu Powder and Taohong Siwu Decoction in treating chronic gastritis. Zhongyi Linchuang Zazhi 2007; 19 (10): 260-261 |
| Yang Y. Shenlingbaizhu san and zhaqupingwei powder combined with western medicine in the treatment of chronic gastritis randomized paraller controlled study. Shiyong Zhongyi Neike Zazhi 2013; 27 (10): 40-41 | |
| Yang SX. Clinical study on the treatment of Chronic functional diarrhea with Shenling Baizhu Powder and Lizhong Decoction. Yatai Chuantong Yixue 2017; 30 (13): 145-146 | |
| Intervention combined with other decoction, no diagnostic criteria (n = 2) | Jin JZ. Shenling Baizhu Powder and Zuojin pill to treat chronic gastritis. Shiyong Zhongyi Neike Zazhi 2011; 27 (11): 752 |
| Gao CZ, Yang SM. Observation on curative effect of cefaclor combined with Shenlingbaizhu granule and Muxiang Shunqi pill in treating chronic gastritis. Zhonghua Yixue Chuangxin Zazhi 2012; 9 (22): 127-128 | |
| No diagnostic criteria (n = 1) | Shi ZR. Clinical observation on 8 cases of chronic gastritis treated by Shenling Baizhu Powder. Neimenggu Zhongyi Zazhi 2014 [DOI: 10.16040/j.cnki.cn15-1101.2014.07.024] |
| Not CG(n = 1) | Zhang WW. Clinical observation on 96 cases of spleen deficiency and stomachache treated with Shenling Baizhu Powder. Zhongguo Minzuyixue Yu Minzuyaoxue 2013; 9 (12): 80 |
| Intervention combined with acupuncture (n = 3) | Yang FX. Acupuncture combined with Shenling Baizhu Powder to treat chronic gastritis with spleen deficiency and dampness. Kouqiang Yixue Dianzi Zazhi 2015; 6 (13): 140-143 |
| Wu XR. 30 cases of chronic gastritis with spleen deficiency and dampness treated by acupuncture combined with Shenling Baizhu Powder. Guangming Zhongyi 2015; 30 (5): 1018-1020 | |
| Wu CY. Analysis of curative effect of acupuncture combined with Shenling Baizhu Powder on chronic gastritis with spleen deficiency and dampness. Jixu Yixue Jiaoyu Zazhi 2019; 33 (10): 161-162 | |
| Intervention combined with other decoction, WM are inconsistent in two groups (n = 1) | Yan Z. Clinical study of cefaclor combined with Shenling Baizhu granule and Muxiang Shunqi pill in treating chronic gastritis. Yatai Chuantong Yixue 2015; 11 (18): 106-107 |
Finally, we included 14 studies in our review (flowchart of database search and study identification is shown in Figure 1).
There were 14 Chinese-language RCTs, comprising 1335 participants aged 15-68 years[27-40], published between 2008 and 2020. Interventions in these studies were SLBZS vs conventional medicine or SLBZS + conventional medicine vs conventional medicine. In conventional medicine therapy, there were four methods, including monotherapy in four trials[27,33,34,37], combined therapy in one study[36], triple therapy in seven studies[28,31,32,35,38-40] and two trials of quadruple therapy[29,30]. There were various treatment durations, including 4, 5, 8, and 12 wk.
Total effectiveness was the primary outcome measure in all trials. All trials reported balanced baseline characteristics. Five trials (36%) recorded adverse events[29,31,34,36,38], and three studies reported recurrence rates[34,38,40]. Two studies reported participant withdrawal information[31,34]. No study reported influence on the quality of life as an outcome measure. Characteristics of included studies are shown in Table 3.
| Ref. | Study design | Sample size (E/C) | Gender (E/C) and age (yr) | Duration | Interventions | Period | Outcome measure | Balance report of baseline | |
| Control group | Experimental group | ||||||||
| Yun[27], 2014 | RCT | 48 (24/24) | (13/11) (10/14); (34.96 ± 11. 39)/(34.08 ± 12.82) | Not mentioned | Rabeprazole enteric-coated capsule | Rabeprazole enteric-coated capsule + SLBZD | 4 wk | Effective rate | P > 0.05 |
| Chen et al[28], 2014 | RCT | 79 (40/39) | (24/16) (23/16); (42.6 ± 13.1)/43.5 ± 13.4 | 6-17 mo/6-19 mo | Triple therapy (clarithromycin sustained-release tablets + rabeprazole sodium capsule + metronidazole tablets) | Triple therapy + SLBZD | 4 wk | Effective rate | P > 0.05 |
| Chen et al[29], 2018 | RCT | 60 (30/30) | (14/16) (15/15); (55.45 ± 6.55)/(55.46 ± 6.44) | 3-12 mo | Quadruple therapy (rabeprazole sodium capsule + amoxicillin + clarithromycin sustained-release tablets + biskalcitrate) | Quadruple therapy + SLBZD | 8 wk | Effective rate; H. Pylori eradication; adverse event | P > 0.05 |
| Du[30], 2017 | RCT | 48 (26/22) | (14/12) (12/10); (40.7 ± 6.1)/(41.2 ± 6.6) | 7 mo-9 years/6 mo-8 years | Quadruple therapy (amoxicillin clavulanic potassium chewable tablets + metronidazole + omeprazole + compound bismuth aluminate capsule) | SLBZD | 5 wk | Effective rate | P > 0.05 |
| Gu[31], 2017 | RCT | 98 (49/49) | Not mentioned; 19-58 | Not mentioned | Triple therapy (omeprazole + clarithromycin + amoxicillin) | Triple therapy + SLBZD | 4 wk | Effective rate; H. Pylori eradication rate; adverse event | P > 0.05 |
| Li et al[32], 2020 | RCT | 66 (33/33) | (19/14) (18/15); (58.54 ± 4.65)/(58.62 ± 4.57) | 4-17 years/4-18 years | Triple therapy (mosapride tablet + polyzyme tablets + lansoprazole tablets) | Triple therapy + SLBZD | 12 wk | Effective rate | P > 0.05 |
| Tang[33], 2014 | RCT | 60 (30/30) | (16/14) (17/13); (22-46)/(23-52) | Not mentioned | Omeprazole enteric-coated capsules | Omeprazole Enteric-coated Capsules + SLBZD | 8 wk | Effective rate | P > 0.05 |
| Xia[34], 2015 | RCT | 300 (150/150) | Not mentioned; 18-85 | Not mentioned | Omeprazole enteric-coated capsules | SLBZD | 8 wk | Effective rate; recurrence rate; adverse event | P > 0.05 |
| Xu et al[35], 2018 | RCT | 60 (30/30) | (17/13) (16/14); (55.6 ± 16.4)/(56.8 ± 14.9) | 4-20 years/4-19 years | Triple therapy (mosapride tablet + polyzyme tablets + lansoprazole tablets) | Triple therapy+SLBZD | 12 wk | Effective rate | P > 0.05 |
| Zhang et al[36], 2020 | RCT | 68 (34/34) | (15/19) (17/17); (44.8 ± 5.0)/(45.2 ± 5.4) | 1-12 years/2-14 years | Combination therapy (omeprazole + compound bismuth aluminate granules) | Combination therapy + SLBZD | 8 wk | Effective rate; adverse events | P > 0.05 |
| Zhao and Lin[37], 2010 | RCT | 80 (40/40) | (37/3) (38/2); (46.2 ± 6.7)/(44.2 ± 5.7) | 2-7 years/2-8 years | No alcohol, famotidine | No alcohol, famotidine + SLBZD | 4 wk | Effective rate; | P > 0.05 |
| Zheng[38], 2014 | RCT | 92 (46/46) | (28/18) (30/16); ( 34 ± 5.34)/( 33 ± 5.76) | 5 mo-6 years/7 mo-6 years | Triple therapy (amoxicillin dispersion tablet + omeprazole enteric-coated capsules + clarithromycin tablet) | SLBZD | 4 wk | Effective rate; adverse events; recurrence rate | P > 0.05 |
| Zhuang et al[39], 2019 | RCT | 106 (53/53) | (65/41); (46.20 ± 8.75) | 1-11 years | Triple therapy (omeprazole enteric-coated tablets + clarithromycin dispersible tablets+amoxil capsule) | Triple therapy + SLBZD | 4 wk | Effective rate; H. Pylori’s negative conversion rate | P > 0.05 |
| Zou[40], 2015 | RCT | 170 (85/85) | (86/84); (40.9 ± 11.1) | Not mentioned | Triple therapy (amoxicillin + clarithromycin + omeprazole) | Triple therapy + SLBZD | 8 wk | Effective rate; H. Pylori’s negative conversion rate; recurrence rate | P > 0.05 |
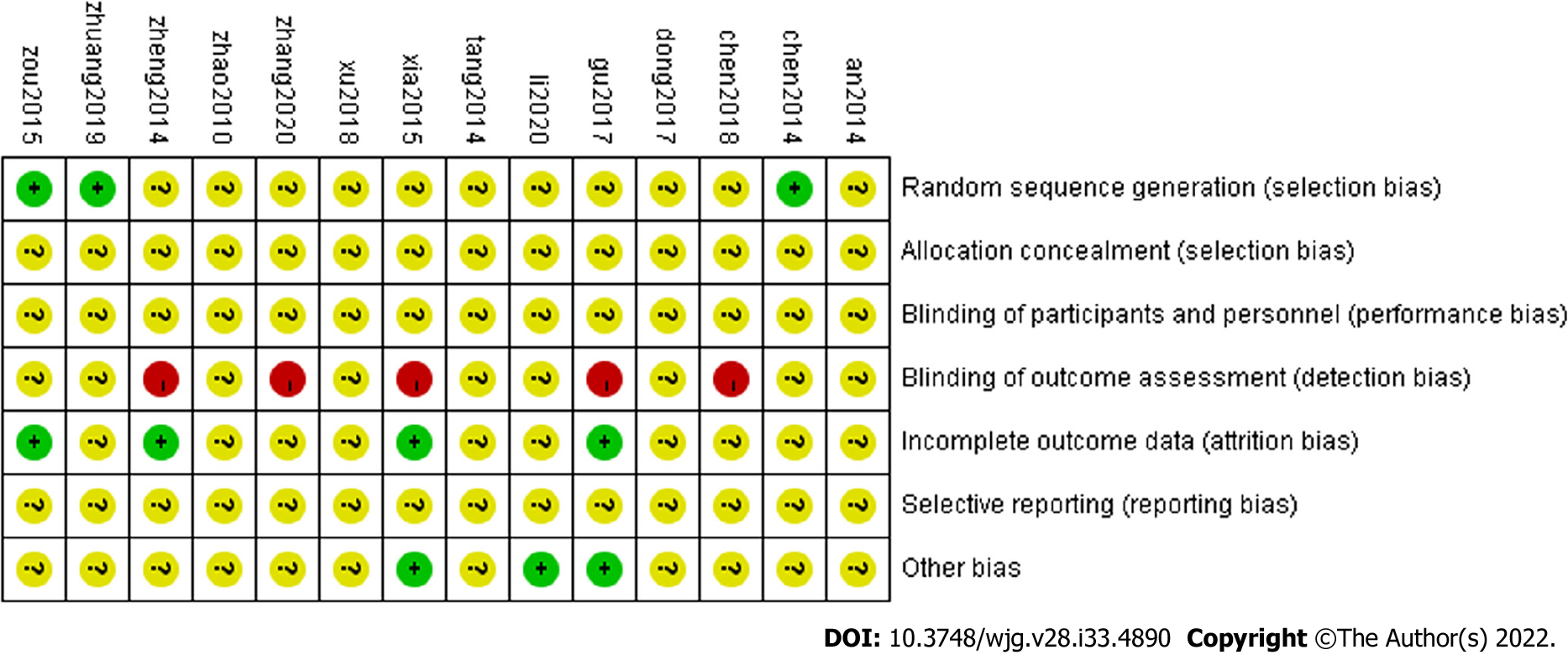
(1) Fourteen trials were consistent at baseline, and all tests referred to RCTs, three studies[28,39,40] mentioned randomization using the “random number table” method; (2) all studies not reported “distribution hidden” method; (3) the “blinding method” was not reported in any study, two studies[31,34] reported “No cases withdrawal and dropped-out,” and three studies[34,38,40] reported “recurrence rate”; (4) selective reporting may come out in studies that there were too few indicators were noted; and (5) we considered some support from pharmaceutical companies that the ethics committee would not approve as other bias. If herbs were offered free by pharmaceutical companies, bias might taint the results. Two studies[34,36] reported that an ethics committee approved the study, suggesting a low bias level. For another 12 studies, we could not determine the effects of other potential sources of bias because there were no reports of herbs’ sources. Details are displayed in Table 4. The included studies were therefore classified as low quality (Figure 2).
| Ref. | Baseline | Randomization | Allocation concealment | Blind method | Withdrawal or dropped-out | Follow up | Protocol and registration | Ethics committee approved |
| Yun[27], 2014 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Chen et al[28], 2014 | Comparability | Random number table | NR | NR | NR | NR | NR | NR |
| Chen et al[29], 2018 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Du[30], 2017 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Gu[31], 2017 | Comparability | Random | NR | NR | No cases withdrawal and dropped-out | NR | NR | Approved |
| Li et al[32], 2020 | Comparability | Random | NR | NR | NR | NR | NR | Approved |
| Tang[33], 2014 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Xia[34], 2015 | Comparability | Random | NR | NR | No cases withdrawal and dropped-out | Recurrence rate | NR | Approved |
| Xu et al[35], 2018 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Zhang et al[36], 2020 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Zhao and Lin[37], 2010 | Comparability | Random | NR | NR | NR | NR | NR | NR |
| Zheng[38], 2014 | Comparability | Random | NR | NR | NR | Recurrence rate | NR | NR |
| Zhuang et al[39], 2019 | Comparability | Random number table | NR | NR | NR | NR | NR | NR |
| Zou[40], 2015 | Comparability | Random number table | NR | NR | NR | Recurrence rate | NR | NR |
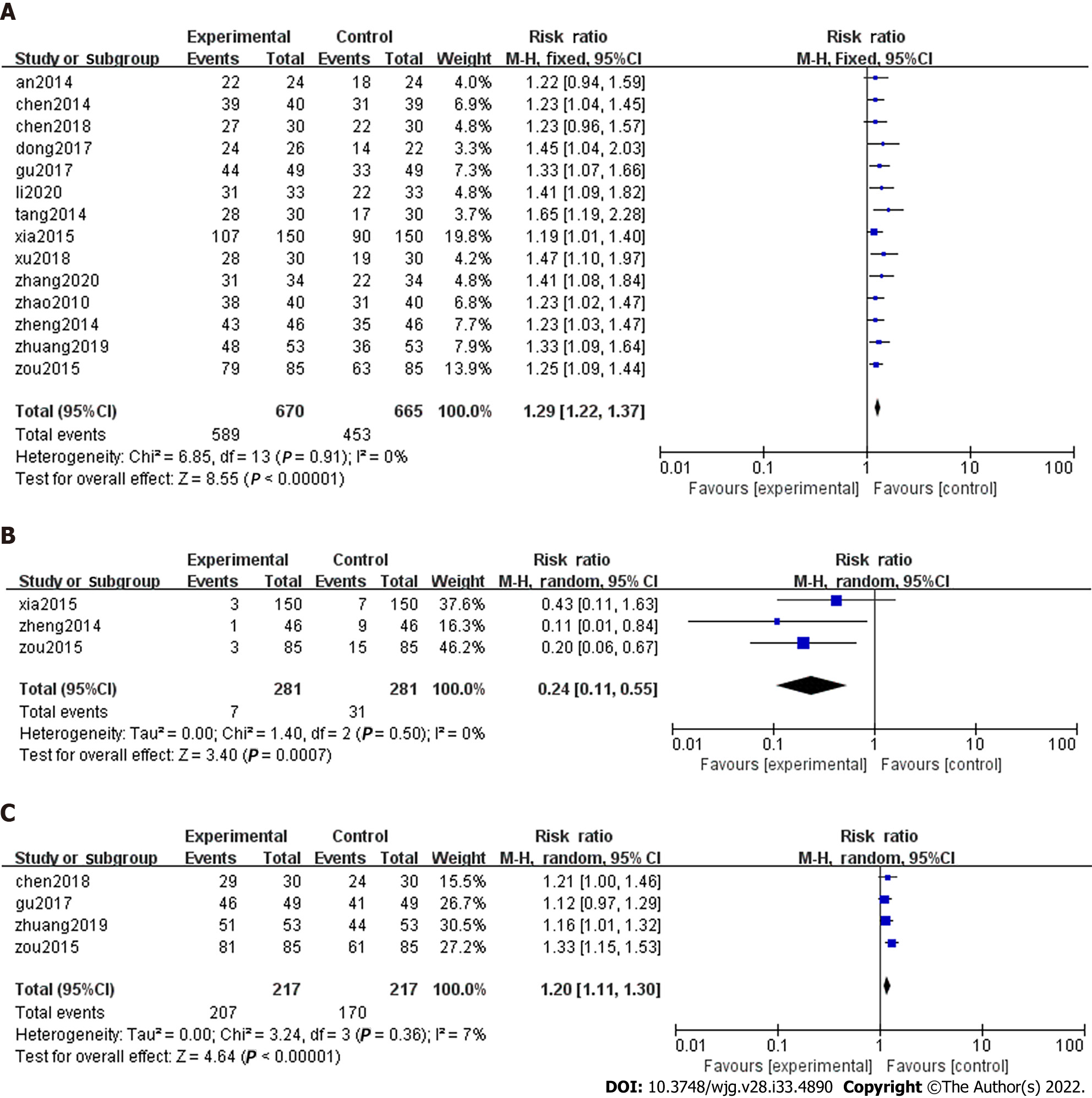
Total effectiveness: Total effectiveness is a composite endpoint composed of improved symptoms and gastroscopy. The results fall into three categories: Obviously effective, effective, and invalid, according to clinical Research on New Chinese Medicines[41]. The details are as follows. Clinical cure: Epigastric pain and symptoms disappeared, gastroscopy returned to normal, i.e., gastric mucosa repair, the disappearance of active inflammation, and mild chronic inflammation; Obviously effective: Epigastric pain and symptoms disappear or diminish. Gastroscopy showed significant improvement; that is, gastric mucosa was nearly normal, active inflammation was gone, and there was less chronic inflammation; Effective: Relief of epigastric pain and other symptoms. Gastroscopy showed reduced gastric mucosal lesions; that is, gastric mucosa was essentially normal, active inflammation was gone, and less chronic inflammation; and Invalid: no improvement or aggravation of clinical symptoms and signs. Gastroscopy showed no change. There were slight differences in this outcome’s composition in various studies due to the non-uniform efficacy assessment criteria. All 14 RCTs compared the total effectiveness rate of SLBZS in patients with CG. SLBZS was superior to conventional therapies (RR: 1.29, 95%CI: 1.22 to 1.37, P < 0.00001) (Figure 3A). Heterogeneity in the total effectiveness was very small (P = 0.91, I2 = 0%).
We created subgroups based on the duration of treatment (4, 5, 8, or 12 wk) (Supplementary Table 1), comparison type (SLBZS vs conventional medicine or SLBZS + conventional medicine vs conventional medicine alone) (Supplementary Table 2), and intervention method (monotherapy, combined therapy, triple therapy, or quadruple therapy) (Supplementary Table 3). These subgroup analyses showed that the effectiveness rate of SLBZS did not differ based on the duration of treatment, combination with other medications, or intervention method (all P > 0.05) (Table 5).
| Subgroup method (total effective rate) | Items | Number of comparisons | Results (risk ratio, 95%CI) | P value for overall effect | I2 | P value for subgroup difference |
| Course of treatment | All comparisons | 14 | 1.29 (1.22,1.37) | < 0.00001 | 0% | |
| Supplementary Table 1 | 4 wk | 5 | 1.27 (1.17,1.37) | < 0.00001 | 0% | |
| 5 wk | 1 | 1.45 (1.04, 2.03) | 0.03 | NA | 0.58 | |
| 8 wk | 5 | 1.28 (1.16, 1.40) | 0.02 | 0% | ||
| 12 wk | 2 | 1.44 (1.19, 1.74) | 0.0002 | 0% | ||
| Comparison type | All comparisons | 14 | 1.23 (1.14, 1.32) | < 0.00001 | 47% | |
| Supplementary Table 2 | SLBZS vs CM | 3 | 1.23 (1.10, 1.38) | 0.0003 | 0% | 0.93 |
| SLBZS + CM vs CM | 11 | 1.23 (1.11, 1.35) | < 0.0001 | 57% | ||
| Intervention method | All comparisons | 14 | 1.29 (1.22, 1.37) | < 0.0001 | 0% | |
| Supplementary Table 3 | Monotherapy | 4 | 1.25 (1.12, 1.40) | < 0.0001 | 5% | |
| Combined therapy | 1 | 1.41 (1.08, 1.84) | 0.01 | NA | 0.82 | |
| Triple therapy | 7 | 1.30 (1.21,1.40) | < 0.0001 | 0% | ||
| Quadruple therapy | 2 | 1.35 (1.11, 1.64) | 0.003 | 0% |
Recurrence rate: Three studies reported recurrence rate[34,38,40]. Pooled raw data showed that SLBZS was better than conventional therapies (RR: 0.24, 95%CI: 0.11 to 0.55, P = 0.0007, Figure 3B).
HP negative conversion rate: Four trials noted the reversal rate for Helicobacter pylori (H. pylori) positivity[29,31,39,40]. Meta-analysis showed that SLBZS was superior to conventional therapies (RR: 1.20, 95%CI: 1.11 to 1.30, P < 0.00001, Figure 3C).
One trial compared the time required for symptom improvement in patients with CG[38]. The experimental group was superior to the control group regarding effects on epigastric stagnation, abdominal distension, belching, acid regurgitation, and nausea (P < 0.05).
There were no reports of significant responses or improvement in the quality-of-life data in these studies. One study reported the Questionnaire for Comprehensive Quality of Life Assessment responses pre- and post-treatment in two groups[36]. After two consecutive months of treatment, scores in all dimensions improved, and the treatment group’s score was significantly higher than that of the treatment group (P < 0.05).
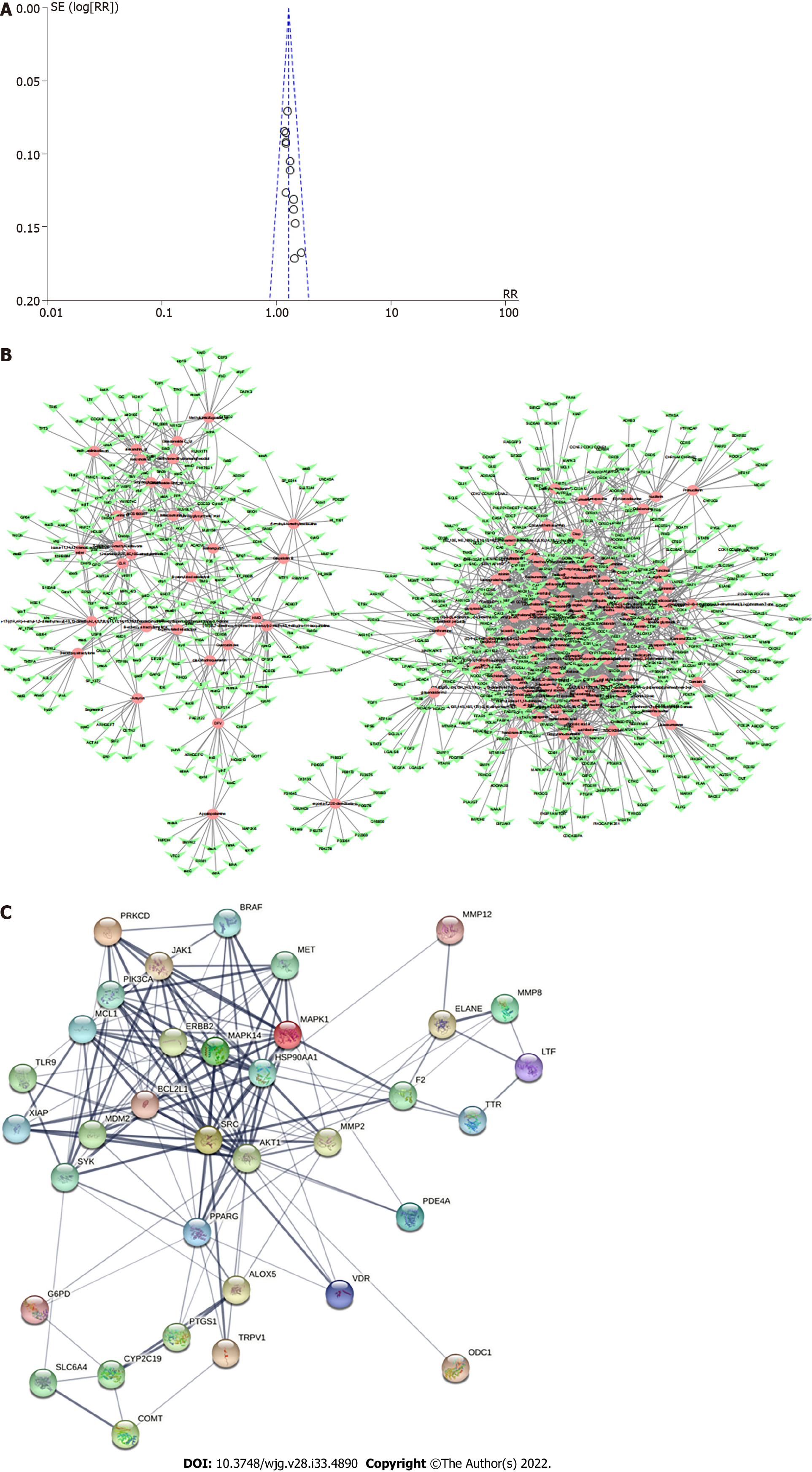
Funnel plots showed the publication bias of the effectiveness rate (Figure 4A). The funnel plot of the effective rate was symmetric, suggesting no significant publication bias. Egger’s test results agreed with the funnel plots (P = 0.005 and 0.000, respectively).
Of the 14 studies, nine RCTs did not mention adverse events[27,28,30,32,33,35,36,39,40]. Two studies mentioned no prominent adverse events[34,29]. Three trials reported adverse events (Table 6); however, no study commented on methods used to manage these events.
| Study | Experiment group | Control group |
| Zhang, 2020 | Diarrhea (2/34) | Dizziness (2/34) and dry mouth (1/34) |
| Chen, 2018 | Headache (1/30), diarrhea (1/30), nausea (1/30) | Headache (2/30), diarrhea (1/30), nausea (2/30), constipation (1/30), rash (1/30) |
| Zheng, 2014 | None | Headache and rash (17.39%) |
GRADE results of SLBZD is shown in (Table 7). However, the quality of evidence was very low or moderate because of the poor methodological quality.
| Quality assessment | Summary of findings | Importance | ||||||||||
| No of patients | Effect | Quality | ||||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | RQLQ | Control | Relative (95%CI) | Absolute | ||
| Effective rate | ||||||||||||
| 14 | Randomized trials | Serious1 | Serious2 | Serious3 | No serious imprecision4 | None | 595/670 (88.8%) | 459/665 (69%) | RR 1.45 (1.22 to 1.37) | 200 more per 1000 (from 152 more to 255 fewer) | Very low | Critical |
| 0.676 | 196 more per 1000 (from 149 fewer to 250 more) | |||||||||||
| Recurrence rate | ||||||||||||
| 3 | Randomized trials | Serious1 | Serious2 | Serious3 | No serious imprecision4 | None | 7/281 (2.5%) | 31/281 (11%) | RR 0.24 (0.11 to 0.55) | 84 fewer per 1000 (from 50 fewer to 98 fewer) | Very low | Important |
| 0.177 | 135 fewer per 1000 (from 58 fewer to 158 fewer) | |||||||||||
| HP negative conversion rate | ||||||||||||
| 4 | Randomized trials | No serious limitations1 | Very serious2 | No serious indirections3 | No serious imprecision4 | None | 207/217 (95.4%) | 170/270 (78.3%) | RR 1.2 (1.11 to 1.3) | 157 more per 1000 (from 86 more to 135 more) | Moderate | Important |
| 0.815 | 163 more per 1000 (from 90 more to 244 more) | |||||||||||
Composition and targets of SCBZS: According to the OB > 30% and DL > 0.18 standard screening, we screened 189 ingredients, including seven in Baizhu (Atractylodes macrocephala Koidz), 15 in Fuling (Smilax glabra Roxb), 9 in Yiyiren [Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf], 22 in Renshen (Panax ginseng C.A.Mey), 15 in Shanyao (Dioscorea oppositifolia L), one in Baibiandou (Lablab purpureus subsp. purpureus), 11 in Lianzi (Nelumbo nucifera Gaertn.), 92 in Gancao (Glycyrrhiza uralensis Fisch. ex DC), ten in Sharen (Amomum villosum Lour.), and seven in Jiegeng (Platycodon grandiflorus). The repeated components and components with no target were deleted, leaving 158 candidate components. Each candidate component’s top 15 target genes were selected, and duplicated genes were identified, with 693 candidate target genes.
PPI network: The component-target network diagram of Shen-ling-bai-zhu Powder visually shows the interaction between pharmacodynamic components and target genes of Shen-ling-bai-zhu Powder (Figure 4B). The network contains 851 nodes with 2445 sides, among which 158 nodes represent candidate ingredients and 693 nodes represent candidate target genes related to drug candidate ingredients. The average number of neighborhood nodes was 5.561. There were 300 nodes and 3325 edges in the disease target interaction network, and the average number of neighborhood nodes in the network was 34.635. A total of 35 potential targets of SLBZS on chronic gastritis can be obtained by analyzing the component-target and disease target interaction networks. Figure 4C visually shows the interaction relationship between potential targets.
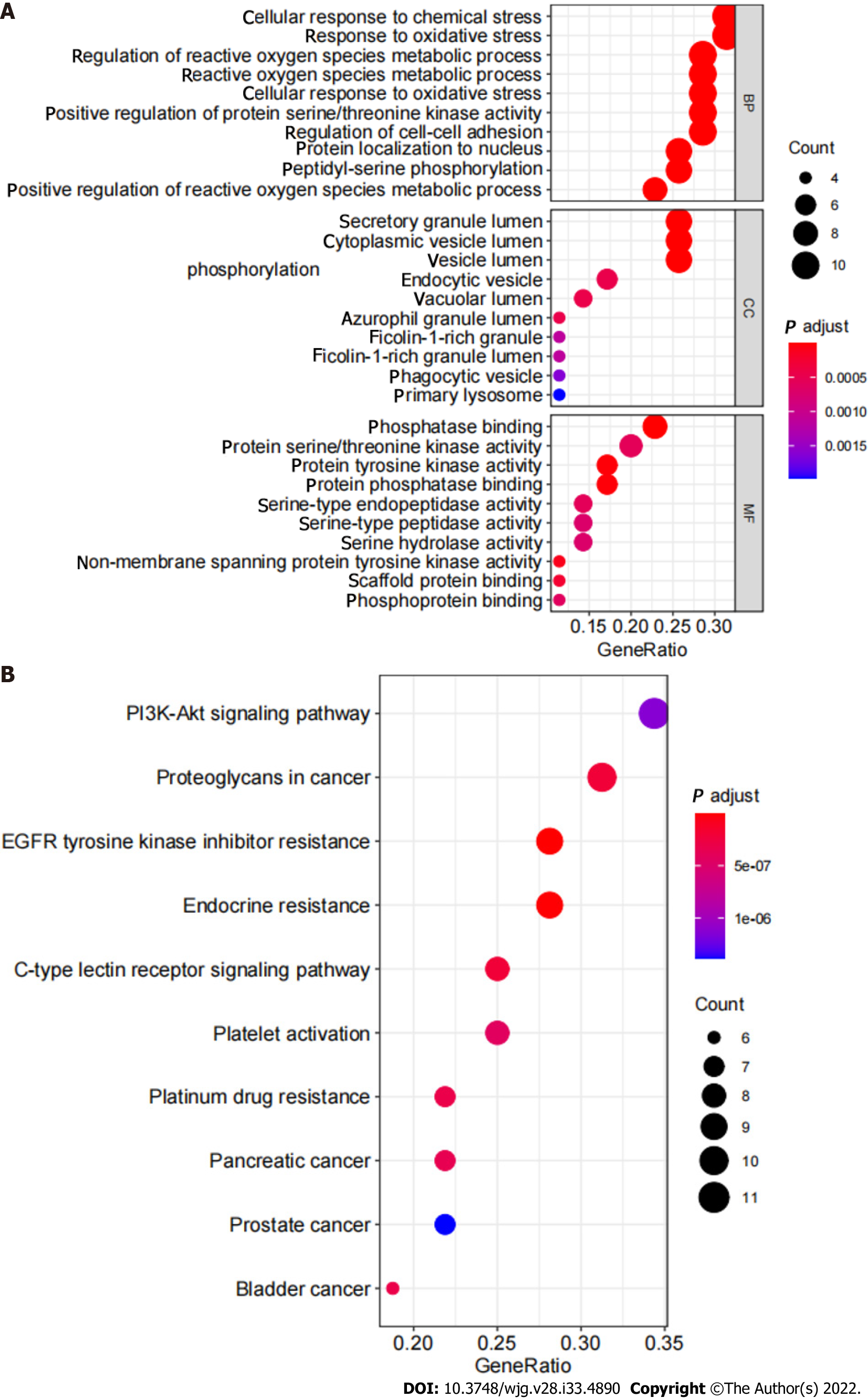
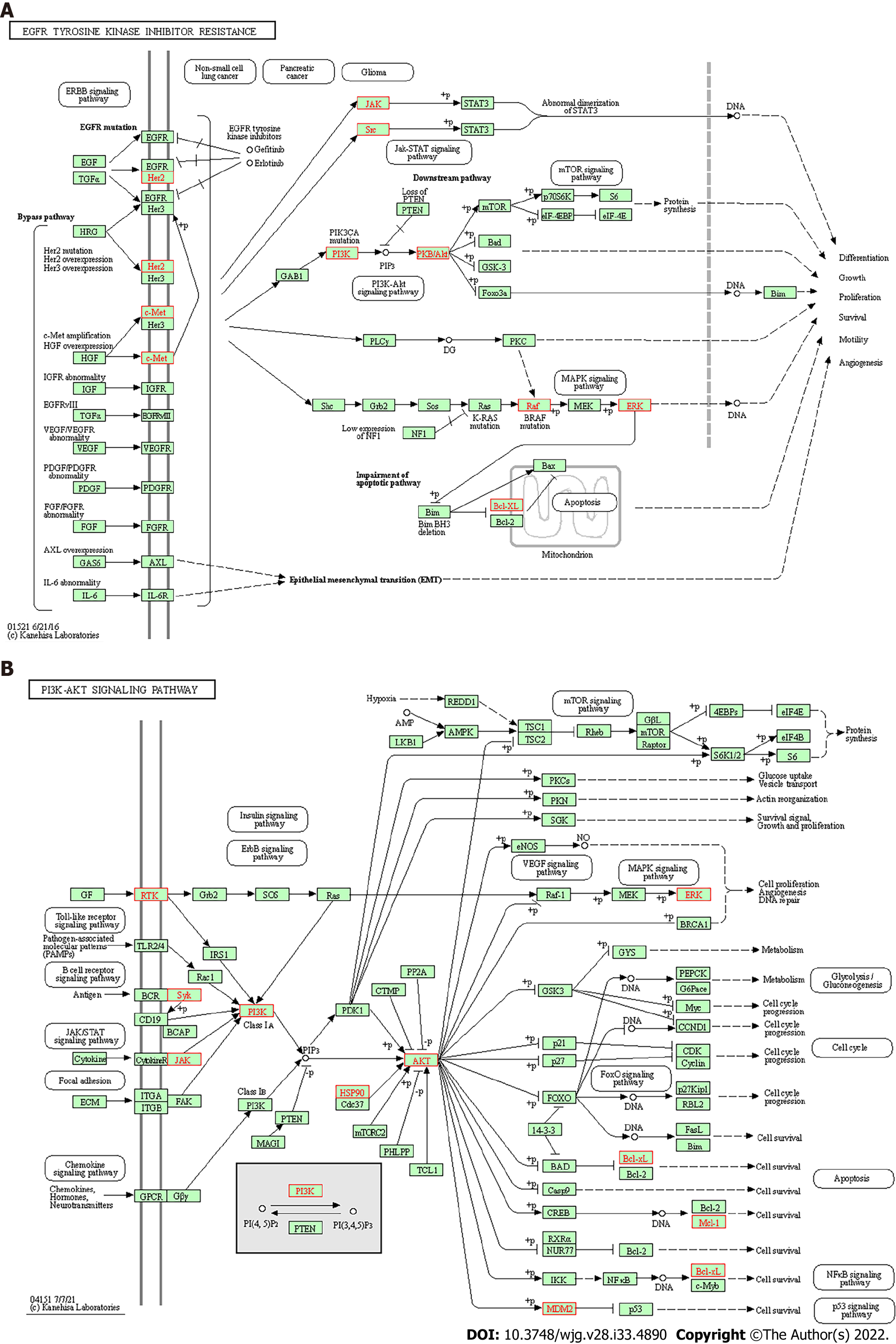
GO enrichment analysis and KEGG pathway enrichment analysis: The results of GO analysis showed that in the BP category, differentially expressed genes were concentrated in the regulation of reactive oxygen species metabolic process, response to oxidative stress, cellular response to chemical stress, and others. Differentially expressed genes are enriched in vesicle lumen, cytoplasmic vesicle lumen, and secretory granule lumen in the CC category. Differentially expressed genes are enriched in tyrosine kinase activity, protein serine/threonine kinase activity, and phosphatase binding (Figure 5A). KEGG pathway analysis results showed that the differentially expressed genes involved EGFR tyrosine kinase inhibitor resistance and the PI3K-Akt signaling pathway (Figure 5B, Figure 6).
Effectiveness and safety of a formula used for CG treatment were evaluated by us. We also summarized the possible pharmacological mechanisms based on collecting as many medical records as possible. Before our study, at least two systematic reviews[42,43] focused on the efficacy of Chinese herbal medicine formulas as CG treatments. However, neither of these reviews included SLBZS as an experimental intervention, and there are no animal studies of SLBZS for CG.
Analysis of the 14 RCTs suggested that SLBZS reverses H. pylori seropositivity and recurrence rates in patients with CG more so than in western medicine. SLBZS formula treats CG based on the current evidence. There were insignificant heterogeneity and publication bias. The safety is not yet established. The study designs were not rigorous, and the GRADE assessment presented moderate and low quality. Therefore, large numbers of rigorously designed RCTs are required to obtain conclusive evidence for the effect and safety of SLBZS for CG.
CG is a common digestive system disorder characterized by an inflammatory condition of the gastric mucosa. CG also leads to mental and psychological disorders like interpersonal sensitivity and depression[44]. On the one hand, studies demonstrated that the link between gut flora and depression is strong[45-47], and gut peptides are essential regulators of microbiota-gut-brain signaling in health and stress-related psychiatric illnesses[45]. On the other hand, intestinal flora can be transformed by TCM compounds[48]. Chinese medicine can regulate the composition and metabolism of intestinal flora and regulate intestinal flora by affecting the secretion of brain-gut peptide and monoamine neurotransmitters, thus improving depression behavior[47-49]. Hence, the anti-inflammatory effect of regulating gut microbiota could represent a complementary and alternative direction for CG with depression symptoms.
According to a study based on Chinese Medicine theory[50], the mechanism of TCM in treating CG is related to neuroprotective mechanisms, immune protective mechanisms, endocrine protective mechanisms, and other factors. A rat study showed that Xiangshaliujunzi decoction improved chronic atrophic gastritis symptoms by activating the TLR2, TLR4/MAPK/NF-κB/iNOS/NO signal pathway[51]. SLBZD reduced intestinal adenoma formation in adenomatous polyposis coli multiple intestinal neoplasia mice by suppressing hypoxia-inducible factor 1α-induced CD4 + CD25 + forkhead box P3 regulatory T cells[19]. Nevertheless, the mechanisms of SLBZS in CG have not been clarified.
In the present study, based on the network pharmacology analysis of drug and disease targets, a collateral relationship revealed the mechanism of SLBZS in the treatment of CG. First, we identified candidate target genes of SLBZS. Then, a protein interaction data network was generated, from which we obtained 36 related protein targets. The most protein targets included SRC, MAPK14, PPARG, and ERBB2. Critical GO entries were included regulation of reactive oxygen species metabolic process, response to oxidative stress, cellular response to chemical stress, protein tyrosine kinase activity, protein serine/threonine kinase activity, phosphatase binding, and others. Key signal pathways were identified in the KEGG enrichment analysis, primarily in EGFR tyrosine kinase inhibitor resistance, the PI3K-Akt signaling pathway, and others.
A study found that alterations in gastric cell stress-adaptive mechanisms due to H. pylori appear crucial during chronic infection[52]; therefore, response to oxidative stress of SLBZS to improve CG symptoms may determine the mechanism. In a future study, we will combine chemical analysis with network pharmacology to study the pharmacological effects of complex formulations comprehensively. The candidate target proteins and the formula’s active ingredients are predicted by analyzing the corresponding networks. The chemical ingredients may be fully identified through experiments to confirm their presence in the formula. Therefore, further animal and clinical experiments are needed for research and exploration.
This study had many limitations: (1) Only small sample sizes Chinese-language RCTs were included, and there were some defects in research design that resulted in the low or moderate quality of evidence; (2) most studies had design flaws like it focused only on results without illustrating a specific implementation of the random method, blind method, and follow-up reporting; (3) despite using validated documents supporting effectiveness assessment criteria, our non-uniform efficacy evaluation approach might influence outcomes and results. It might be challenging to employ the same effectiveness assessment criteria for each trial, as these criteria varied with each update; (4) adverse effects and recurrence rates information is rare reported; (5) the dosage of SLBZS has not been standardized and unified, and therefore the reasonable dosage was difficult to determined; (6) the pharmacology mechanism is unclear, especially the specific analysis of active ingredients and side effects; and (7) conflicts of interest of study investigators or funders may influence the risk of bias due to missing results. None of our included studies clearly reported their Chinese herbal sources, particularly whether pharmaceutical companies provided support. It is difficult to determine whether there were conflicts of interest. Presentation of herb sources in future studies could help determine bias.
This meta-analysis included 14 RCTs and summarized the clinical efficacy and potential mechanisms of the Chinese herbal formula SLBZS in treating CG. However, the methodological quality of the studies was not high, the risk of relapses and adverse reactions was underreported, and related mechanisms lacked validation; therefore, rigorous RCTs and basic science studies should be designed further to determine a definitive association between SLBZS and CG.
The effects and safety of Shen-ling-bai-zhu san (SLBZS) are currently unclear.
A 2012 clinical practice guideline recommended SLBZ Powder for the Pattern of Spleen and Stomach Deficiency CG. The 2020 clinical guideline did not recommend SLBZS, possibly because of inadequate clinical evidence and pharmacological mechanisms. We designed our study to focus on evidence of efficacy and potential mechanisms. This controversy needed clarified.
To determine the clinical evidence and potential mechanisms of SLBZS for the treatment of CG.
Evidence-based meta-analysis and network pharmacology methods.
Fourteen articles were eventually included, covering 1335 participants. SLBZS might treat CG by acting on related targets and pathways such as EGFR tyrosine kinase inhibitor resistance, the PI3K-Akt signaling pathway, and others.
SLBZS might be useful in treating CG, but its long-term effects and specific clinical mechanisms keep unclear.
More samples and high-quality clinical studies should be tested and verified in the next step.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gadour E, United Kingdom; Nassar M, United States S-Editor: Chen YL L-Editor: Filipodia P-Editor: Yu HG
| 1. | Bacha D, Walha M, Ben Slama S, Ben Romdhane H, Bouraoui S, Bellil K, Lahmar A. Chronic gastritis classifications. Tunis Med. 2018;96:405-410. [PubMed] |
| 2. | Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50:657-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, Wang C, Liu Y, Sha W, Wang B, Li Y, Zhang G, Shi R, Xu J, Huang M, Han S, Liu J, Ren X, Wang Z, Cui L, Sheng J, Luo H, Zhao X, Dai N, Nie Y, Zou Y, Xia B, Fan Z, Chen Z, Lin S, Li ZS; Chinese Chronic Gastritis Research group. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Li XY, Zhang JY, Wu TN, Li M. Efficacy and safety evaluation of two regimens including Esomeprazole and amoxicillin for eradication of Helicobacter pylori associated chronic gastritis. Zhongguo Yiyuan Yaoxue Zazhi. 2020;40:427-431. |
| 5. | Liu XM, Deng ZM, Lian YC. Effect of Rebapat combined with triple therapy on gastrointestinal hormone and immune function in patients with chronic atrophic gastritis. Jilin Yixue. 2020;41:2655-2656. |
| 6. | Cheng YX. Effects of quadruple therapy and traditional triple therapy on gastrointestinal function and symptoms in patients with Hp positive chronic gastritis. Zhongguo Shiyong Yiyao. 2020;15:121-123. |
| 7. | Wu L, Du ZQ. The effect of Xiaopi and Weifang combined with quadruple therapy on the efficacy, gastric function and serum inflammatory factors in patients with HP-related chronic atrophic gastritis. Zhongguo Zhongyiyao Zazhi. 2020;22:1-14. |
| 8. | Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, Maldonado-Garza HJ, Garza-González E. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Rev Gastroenterol Hepatol. 2018;12:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Li Z, Zhang H, Wang D, Chen X, Li S. [Research progress on mechanism of acupuncture for chronic atrophic gastritis]. Zhongguo Zhen Jiu. 2016;36:1117-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Wang L, Li G. [Warm acupuncture for chronic atrophic gastritis with spleen-stomach deficiency cold]. Zhongguo Zhen Jiu. 2017;37:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Zhao J, Cheng H, Ding Y. [Research progress of fire needling for chronic gastritis]. Zhongguo Zhen Jiu. 2018;38:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Xu J, Zheng X, Cheng KK, Chang X, Shen G, Liu M, Wang Y, Shen J, Zhang Y, He Q, Dong J, Yang Z. NMR-based metabolomics Reveals Alterations of Electro-acupuncture Stimulations on Chronic Atrophic Gastritis Rats. Sci Rep. 2017;7:45580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Liu CC, Chen JL, Chang XR, He QD, Shen JC, Lian LY, Wang YD, Zhang Y, Ma FQ, Huang HY, Yang ZB. Comparative metabolomics study on therapeutic mechanism of electro-acupuncture and moxibustion on rats with chronic atrophic gastritis (CAG). Sci Rep. 2017;7:14362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Liu X, She C, Zhong H, Liu T, Cao JN, Zhang C, Liu M, Chang XR. [Effect of moxibustion at "Zusanli"(ST36) on metabolites of gastric tissue in rats with chronic atrophic gastritis based on metabonomics]. Zhen Ci Yan Jiu. 2019;44:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Wu XX, Li X, Dang ZQ, Luo WZ, Zhao CP, Yu K. [Clinical therapy of Zisheng decoction recipe for chronic atrophic gastritis with intestinal metaplasia]. Zhongguo Zhong Yao Za Zhi. 2017;42:4882-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Tian G, Wu C, Li J, Liang B, Zhang F, Fan X, Li Z, Wang Y, Liu D, Lai-Han Leung E, Chen J. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res. 2019;144:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Zhang S, Zhou Q, Meng M, Chen W. Efficacy of Shenlingbaizhu formula on irritable bowel syndrome: a systematic review. J Tradit Chin Med. 2020;40:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Cui ZG, Shu MY. Observation on curative effect of cefclone combined with Shenlingbaizhu granules and Muxiang shunqi pills on chronic gastritis. Zhongguo Yixue Chuangxin. 2012;9:2012. |
| 19. | Xu W, Han Q, Liang S, Li L, Sun X, Shao M, Yao X, Xu W. Modified Shenlingbaizhu decoction reduces intestinal adenoma formation in adenomatous polyposis coli multiple intestinal neoplasia mice by suppression of hypoxia-inducible factor 1α-induced CD4+CD25+forkhead box P3 regulatory T cells. J Tradit Chin Med. 2018;38:22-32. [PubMed] |
| 20. | Tang XD, Lu B, Zhou LY, Zhan SY, Li ZH, Li BS, Gao R, Wang FY, Wang P, Yang JQ, Liu G, Zhang YQ, Che GX, Lin M, Bian LQ, Zhao YP; China Academy of Chinese Medical Sciences, Beijing. Clinical practice guideline of Chinese medicine for chronic gastritis. Chin J Integr Med. 2012;18:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Standardization project group of Clinical Application Guide for Dominant Diseases of Chinese Patent Medicine Treatment. Clinical Application Guide of Chinese Patent Medicine in treating Chronic Gastritis (2020). Zhongguo Zhongyixi Jiehe Zazhi. 2021;2:157-163. |
| 22. | Yan Z. Clinical study of cefaclor combined with Shenlingbaizhu granules and Muxiang shunqi pills in treatment of chronic gastritis. Yatai Chuantong Yixue. 2015;11:106-107. |
| 23. | Ai ZZ, Zai YW. Overview of Shenling Baizhu Powder in the Treatment of Digestive System Diseases. Liaoning Zhongyiyao Daxue Xuebao. 2008;10:201-202. |
| 24. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2893] [Article Influence: 482.2] [Reference Citation Analysis (0)] |
| 25. | Wang N, Zheng Y, Gu J, Cai Y, Wang S, Zhang F, Chen J, Situ H, Lin Y, Wang Z. Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Sci Rep. 2017;7:14513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Lee AY, Park W, Kang TW, Cha MH, Chun JM. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J Ethnopharmacol. 2018;221:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Yun A. 48 cases of chronic gastritis were treated with Jiawei Shenling Baizhu Powder. Guangming Zhongyi. 2014;29:305-306. |
| 28. | Chen XL, Zhong RG, Zhang JZ, Xu LZ. Clinical observation on the treatment of chronic erosive gastritis by triple therapy combined with Shenling Baizhu Powder. Xinzhongyi. 2014;46:64-66. |
| 29. | Chen XY, Wang W, Li H. Clinical efficacy and safety of Shenling Baizhu powder as an adjunctive treatment for atrophic gastritis with Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 2018;26:488-493. |
| 30. | Du DG. Experience of treating chronic erosive gastritis, gastric ulcer and Helicobacter pylori infection with Shenling Baizhu Powder. Linchuang Yixue Zazhi. 2017;30:13-14. |
| 31. | Gu XX. Chronic Gastritis Randomized Controlled Study Shenling Baizhu Powder Combined Western Medicin Treatment. Shiyong Zhongyi Neike Zazhi. 2017;31:55-56. |
| 32. | Li L, Zhao L, Feng W, Jiang YL, Yang WX, Wang TG, Xiao GH. Clinical effect of Jiawei Shenling Baizhu Powder on chronic atrophic gastritis with weakness of spleen and stomach. Neimenggu Zhongyiyao. 2020;39:30-31. |
| 33. | Tang YJ. Effective observation of Shenlingbaizhu Decoction for Chronic Ga. Quanmin Jiankang Zazhi. 2014;12:36-37. |
| 34. | Xia CH. Randomized Controlled Study of Chronic Gastritis Shenling Baizhu Powder combined Western Medicine. Shiyong Zhongyi Neike Zazhi. 2015;29:71-72. |
| 35. | Xu MF, Sheng HP, Xu JL. Clinical observation on the treatment of chronic atrophic gastritis with spleen-stomach weakness by Jiawei Shenling Baizhu Powder. Zhongxiyi Jiehe Xinxueguanbing Dianzi Zazhi. 2018;30:160-161. |
| 36. | Zhang L, Huang PY. Clinical Analysis of Shen ling Bai zhu Powder Combined with Western Medicine in Treatment of Hp-negative Erosive Gastritis. Shiyong Zhongyi Neike Zazhi. 2020;34:101-103. [DOI] [Full Text] |
| 37. | Zhao BQ, Lin SQ. Clinical Observation on Therapeutic Effect of ShenLin BaiZhu San in Patients with Chronic Alcoholic Gastritis. Gansu Zhongyiyao Zazhi. 2020;30:23. |
| 38. | Zheng YH. Study of Efficacy of Dialectically Adjusted Dosage of Shenlin Baizhu Powder in Treating Chronic Superficial Gastritis for Patients with Spleen and Stomach Deficiency. Zhonghua Zhongyiyao Xuekan. 2014;31:2533-2535. |
| 39. | Zhuang T, Zhou Y. Observation on the effect of Treating chronic gastritis caused by Hp infection with Shenling Baizhu Powder. Dangdai Yiyao Lintan. 2019;17:207-210. |
| 40. | Zou ZY. Clinical study of dialectically adjusted dosage of Shenlingbaizhu Powder in treatment bof Chronic Superficial Gastritis Patients with Spleen and Stomach Deficiency. Dangdai Yiyao Lintan. 2015;13:18-19. |
| 41. | Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Dai YK, Zhang YZ, Li DY, Ye JT, Zeng LF, Wang Q, Hu L. The efficacy of Jianpi Yiqi therapy for chronic atrophic gastritis: A systematic review and meta-analysis. PLoS One. 2017;12:e0181906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Cao Y, Zheng Y, Niu J, Zhu C, Yang D, Rong F, Liu G. Efficacy of Banxia Xiexin decoction for chronic atrophic gastritis: A systematic review and meta-analysis. PLoS One. 2020;15:e0241202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Zhao X, Wu M, Zhang D, Sun Y, Yang Y, Xie H, Su Y, Jia J, Zhang S. The relationship of interpersonal sensitivity and depression among patients with chronic atrophic gastritis: The mediating role of coping styles. J Clin Nurs. 2018;27:e984-e991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics. 2018;15:36-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 46. | Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97:1223-1241. [PubMed] [DOI] [Full Text] |
| 47. | Hao WZ, Li XJ, Zhang PW, Chen JX. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2020;284:112691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 48. | Ma Y, Guo LN, Liu YL. Advances in the study of intestinal flora and depression. Shiyong Yixue Zazhi. 34:324-327. [DOI] [Full Text] |
| 49. | Meng ZQ, Chen H, Du Y. Research progress of intestinal microflora and Chinese herbal medicine and their combination in the field of depression. Zhongyaoxue. 2020;31:2918-2923. [DOI] [Full Text] |
| 50. | Yan ZM, Bao A, Li HN, Liu SW, Hai XH. Research progress on mechanism of TCM in treating chronic gastritis. Liaoning Zhongyiyao Zazhi. 2019;46:435-438. [DOI] [Full Text] |
| 51. | Lin ZQ, Wang DX, Hong SS, Fu XY. Effects of Xiangsha Liujunzi decoction on TLR signal pathway in gastric mucosa tissues of rats with Helicobacter pylori-induced chronic atrophic gastritis. Zhongguo Zhong Yao Za Zhi. 2016;41:3078-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Díaz P, Valenzuela Valderrama M, Bravo J, Quest AFG. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front Microbiol. 2018;9:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |