Published online Sep 7, 2022. doi: 10.3748/wjg.v28.i33.4846
Peer-review started: June 12, 2022
First decision: July 12, 2022
Revised: July 25, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 7, 2022
Processing time: 80 Days and 6.1 Hours
The frequency of acute hypertriglyceridemic pancreatitis (AHTGP) is increasing worldwide. AHTGP may be associated with a more severe clinical course and greater mortality than pancreatitis caused by other causes. Early identification of patients with severe inclination is essential for clinical decision-making and improving prognosis. Therefore, we first developed and validated a risk predic
To develop and validate a risk prediction score for the severity of AHTGP in Chinese patients.
We performed a retrospective study including 243 patients with AHTGP. Patients were randomly divided into a development cohort (n = 170) and a validation cohort (n = 73). Least absolute shrinkage and selection operator and logistic regression were used to screen 42 potential predictive variables to construct a risk score for the severity of AHTGP. We evaluated the performance of the nomogram and compared it with existing scoring systems. Last, we used the best cutoff value (88.16) for severe acute pancreatitis (SAP) to determine the risk stratification classification.
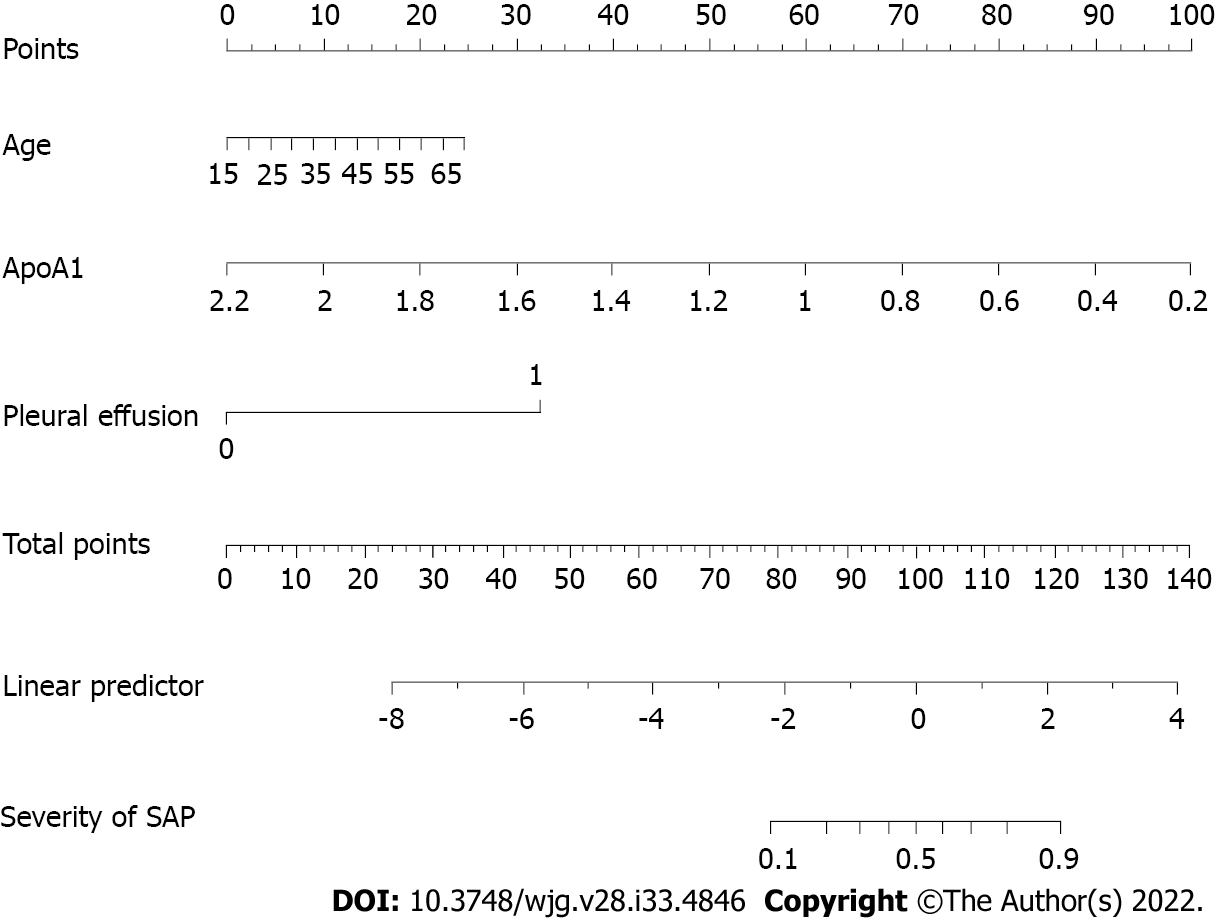
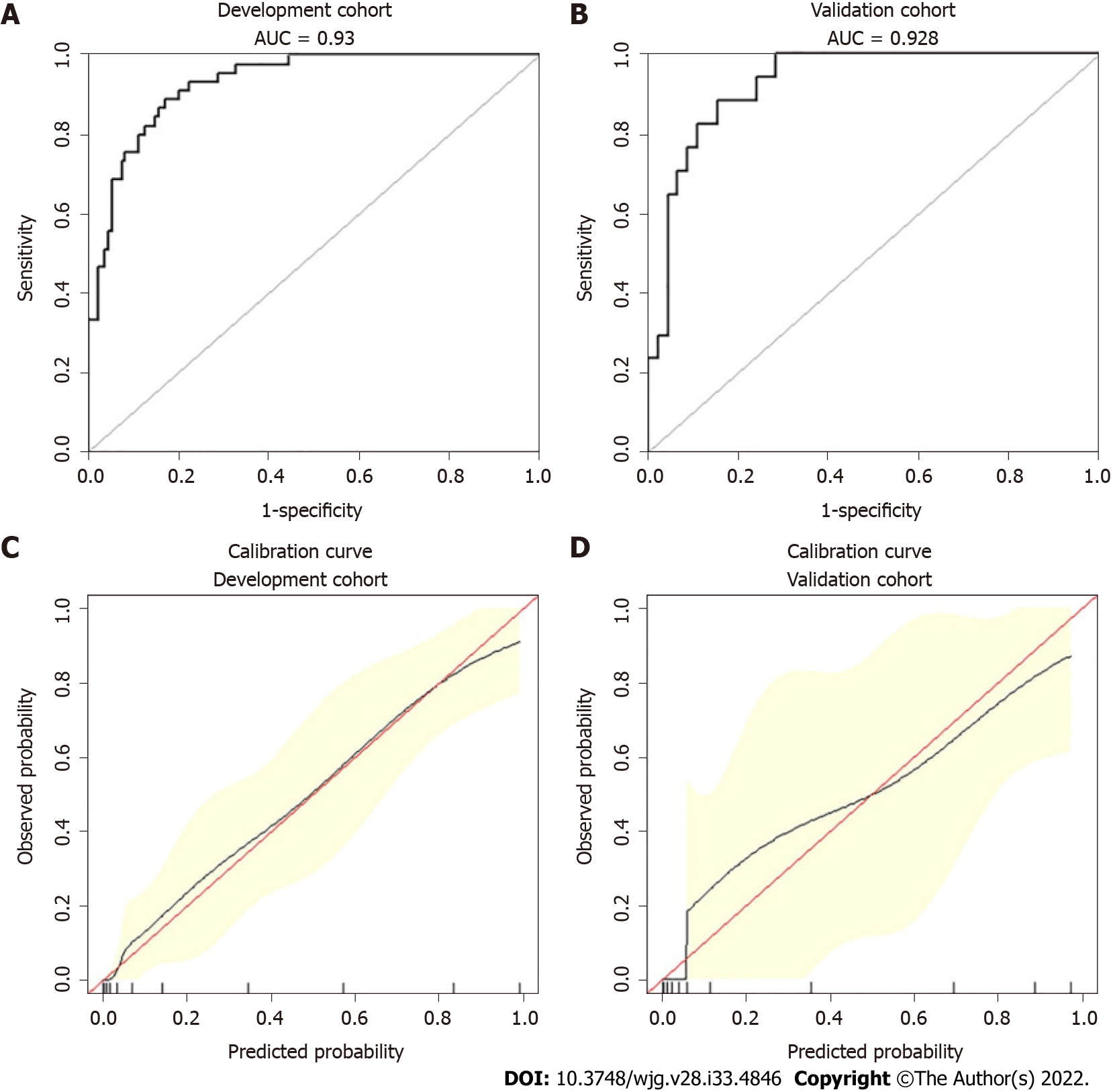
Age, the reduction in apolipoprotein A1 and the presence of pleural effusion were independent risk factors for SAP and were used to construct the nomogram (risk prediction score referred to as AAP). The concordance index of the nomogram in the development and validation groups was 0.930 and 0.928, respectively. Calibration plots demonstrate excellent agreement between the predicted and actual probabilities in SAP patients. The area under the curve of the nomogram (0.929) was better than those of the Bedside Index of Severity in AP (BISAP), Ranson, Acute Physiology and Chronic Health Evaluation (APACHE II), modified computed tomography severity index (MCTSI), and early achievable severity index scores (0.852, 0.825, 0.807, 0.831 and 0.807, respectively). In comparison with these scores, the integrated discrimination improvement and decision curve analysis showed improved accuracy in predicting SAP and better net benefits for clinical decisions. Receiver operating characteristic curve analysis was used to determine risk stratification classification for AHTGP by dividing patients into high-risk and low-risk groups according to the best cutoff value (88.16). The high-risk group (> 88.16) was closely related to the appearance of local and systemic complications, Ranson score ≥ 3, BISAP score ≥ 3, MCTSI score ≥ 4, APACHE II score ≥ 8, C-reactive protein level ≥ 190, and length of hospital stay.
The nomogram could help identify AHTGP patients who are likely to develop SAP at an early stage, which is of great value in guiding clinical decisions.
Core Tip: A risk prediction score (referred to as AAP), including age, the level of apolipoprotein A1 and the presence of pleural effusion, was first built to predict the severity of acute hypertriglyceridemic pancreatitis in Chinese patients. After calibration and verification, this score was shown to have high predictive accuracy and good performance. The risk score could help identify patients who are likely to develop severe acute pancreatitis at an early stage. In comparison with other scores, these scores showed improved accuracy in predicting and better net benefits for clinical decisions. AAP could be of great value in guiding clinical decisions as a convenient and specific tool.
- Citation: Liu ZY, Tian L, Sun XY, Liu ZS, Hao LJ, Shen WW, Gao YQ, Zhai HH. Development and validation of a risk prediction score for the severity of acute hypertriglyceridemic pancreatitis in Chinese patients. World J Gastroenterol 2022; 28(33): 4846-4860
- URL: https://www.wjgnet.com/1007-9327/full/v28/i33/4846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i33.4846
Hypertriglyceridemia (HTG) is the third most common cause of acute pancreatitis (AP)[1]. The fre
At present, four commonly used AP scoring systems are available for the early identification of SAP, including the Acute Physiology and Chronic Health Evaluation (APACHE II), Ranson score, the Bedside Index of Severity in AP (BISAP), and the modified computed tomography (CT) severity index (MCTSI). However, they have limitations[9]. APACHE II collects a large number of parameters, making it complicated and inconvenient to use and poor at predicting disease within 24 h of disease onset. The Ranson score consists of 11 indicators, which must be evaluated upon admission and 48 h following admission. Some of these indicators are not routinely collected during early stages of AP, so early prediction is difficult[10]. BISAP uses five indicators to determine AP severity 24 h after admission. It is easy to use but has low sensitivity for predicting SAP[11]. The MCTSI has outstanding performance in predicting local complications. But it is poor in predicting severity[12]. The AP prediction accuracy could be improved by combining several scoring systems, but it was inconvenient. Without a new scoring system, it was difficult to increase the prediction accuracy[13].
Moreover, many studies have demonstrated that single laboratory indicators can be used to predict the severity of AHTGP, such as the neutrophil-lymphocyte ratio (NLR), white blood cell (WBC) count, red blood cell distribution width (RDW), serum calcium (Ca2+) and C-reactive protein (CRP), which are easy to use in practice but lack high sensitivity or specificity[2,14]. It should be noted that serum triglyceride (TG) levels dose-dependently worsen the outcome of AP[6], but there is still controversy[5]. Recently, several new clinical prediction models have been developed to predict the severity of AP[15,16]. The early achievable severity index (EASY) prediction score as an artificial intelligence model was recently developed based on machine learning for the early and easy prediction of severity in AP[17]. However, almost all of them were developed for all etiologies of pancreatitis and not for HTG-induced pancreatitis separately.
In conclusion, AHTGP may be associated with a more severe clinical course and greater mortality. Early prediction and detection of patients who are likely to develop SAP is of great importance. The purpose of this study is to develop and validate a fast, simple, accurate, and reproducible risk score for predicting severe AHTGP at an early stage.
The study involved a retrospective review of 243 patients diagnosed with AHTGP who were admitted to the Intensive Care Unit of a gastroenterology department of Xuanwu Hospital from November 2012 to January 2022. All patients were diagnosed with AHTGP for the first time, and the possibility of other pancreatic diseases (recurrent AP, chronic pancreatitis, or pancreatic cancer) and cases with missing data were excluded. The following data were recorded: Basic demographics, medical history, vital signs, laboratory tests and X-ray of the chest within 24 h. Pancreatic examinations under CT or magnetic resonance imaging within 72 h. All patients received routine management after admission. This study was approved by the Ethics Committee of the Xuanwu Hospital of Capital Medical University; written informed consent was waived considering the retrospective study design.
The diagnostic criteria for AHTGP were elevated TG level (> 11.30 mmol/L, or 5.65-11.30 mmol/L with lactescent serum) and two or more of the following three symptoms: (1) Abdominal pain consistent with AP; (2) Levels of amylase and/or lipase at least three times above the upper limit of normal; and (3) Abdominal imaging consistent with changes in AP. According to the Atlanta classification revised in 2012, AP severity was divided into three groups based on organ failure status and local and/or systemic complications. The absence of organ failure and local or systemic complications was considered to indicate mild AP (MAP). The presence of transient (within 48 h) organ failure and/or local complications or exacerbation of comorbid disease was regarded as moderately severe AP (MSAP). The presence of persistent (> 48 h) organ failure was considered to indicate SAP. The respiratory, cardiovascular, and renal systems were assessed to identify organ failure, which was defined as a modified Marshall score > 2 for one of these three systems. Acute peripancreatic fluid collection, pancreatic pseudocyst, acute necrotic collection, walled-off necrosis, and infected pancreatic necrosis were defined as local complications[18].
As part of the validation process, five scoring systems were compared to our risk prediction score. Within 24 h of admission, laboratory tests and radiological examinations were conducted to determine APACHE II and BISAP scores. The Ranson score was derived from laboratory tests performed within 48 h of admission. The MCTSI score was determined from CT scans performed within 72 h of admission. The EASY prediction score as an artificial intelligence model was recently developed to predict the severity of AP. It consists of four parts (personal details, anamnestic data, admission data and blood test results) with a total of 23 predictors. We calculated the predicted severity scores for all subjects on a web application (http://easy-app.org/) in the Streamlit Python-based framework.
We selected 42 potential predictive variables based on the literature and previous clinical experience, including demographic variables, medical history, clinical signs, laboratory findings, and imaging results. Demographic variables included continuous and categorical variables: Age, body mass index (BMI), smoking status, and drinking habits. Medical history included categorical variables: Diabetes, hypertension, hyperlipemia, and fatty liver. Clinical signs included continuous variables: Respiratory rate (RR), heart rate (HR), systolic blood pressure, and diastolic blood pressure. Laboratory findings performed within 24 h of admission included WBC, NLR[14], RDW[19], platelet counts (PLT), mean platelet volume (MPV)[20], platelet distribution width (PDW)[21], total cholesterol (TC)[22], TG[6], high-density lipoprotein cholesterol level to low-density lipoprotein cholesterol level ratio (H/L ratio)[23], apolipoprotein A1 (ApoA1)[24], total bilirubin (TBIL), aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin (ALB)[25], blood urea nitrogen (BUN)[24], serum creatinine (Cr)[26], free triiodothyronine (fT3)[27], CRP, procalcitonin (PCT), serum sodium (Na), serum potassium (K), serum Ca2+, prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB)[28], and D-dimer levels[29]. Imaging results included the presence of a pleural effusion according to the chest X-ray within 24 h of admission[30].
We defined the severity of AHTGP according to the revised Atlanta classification given the extensive acceptance of this guideline[18]. SAP was defined as persistent organ failure and was used as the clinical outcome measure.
Statistical comparisons between the non-SAP (MAP + MSAP) and SAP groups were performed with analysis of variance or the Mann–Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. There were 42 variables entered into the selection process, as described here. Least absolute shrinkage and selection operator (LASSO) regression was applied to minimize the potential collinearity of variables measured from the same patient and overfitting of variables. The most predictive covariates were selected by the minimum (λ min). Subsequently, variables identified by LASSO regression analysis and some other important clinical variables were entered into univariable and multivariable logistic regression analyses for further predictor selection. Clinical patient samples were randomly divided (3:1) into development and validation cohorts. We constructed a nomogram and validated the accuracy estimates by using 1000 bootstrap resamples to reduce overfitting. To quantify the discrimination performance of the nomogram for predicting SAP, the concordance index (C-index) was measured in the development set and validation cohort. By plotting the calibration curve, we analyzed the relationship between the observed incidence and predicted probability in the development set and the validation set. Integrated discrimination improvement (IDI) was established to evaluate the improvement of the risk prediction score with other existing scoring systems in the whole set. Decision curve analysis (DCA) was applied to quantify the clinical usefulness of the nomogram in the whole set. All statistical analyses were performed by R software (https://www.r-project.org/, The R Foundation) and Empower-Stats software (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, United States).
To determine the risk stratification classification for AHTGP, the best cutoff value for SAP calculated through the ROC curve was used to divide patients into high-risk and low-risk groups. The differences in clinical manifestations and prognoses between the high- and low-risk groups were also compared to evaluate the efficacy of our risk score.
A total of 243 records from patients diagnosed with AHTGP and discharged from the intensive care units (ICUs) during our study period were included for analysis. The baseline characteristics of the study population are summarized in Table 1. The average age of the entire cohort was 40.12 ± 10.12 years, and there were more males (n = 188, 77.37%) than females (n = 55, 22.63%). We divided our AP patients into two groups, non-SAP (MAP + MSAP) and SAP. The total rate of SAP was 25.51%. There was no significant difference in age, sex or BMI between the two groups. Furthermore, there was no significant difference in medical history between the two groups. In terms of vital signs, patients with SAP had a significantly higher heart rate (92.23 ± 16.29 vs 107.58 ± 18.65, P < 0.001) and respiratory rate (20.47 ± 4.38 vs 25.27 ± 6.21, P < 0.001) index than patients in the non-SAP group. Patients in the two groups showed no significant differences in laboratory findings, including PLT, MPV, H/L ratio, TBIL, ALT, ALP, fT3, Na, K, and TT. However, there were differences in WBC, NLR, RDW, PDW, TC, TG, ApoA1, AST, ALB, BUN, Cr, CRP, PCT, ESR, Ca2+, PT, APTT, FIB, and D-dimer. For imaging results, pleural effusions were significantly more frequently observed among SAP patients (12.71% vs 74.19%, P < 0.001).
| Patient characteristics | All (n = 243) | Non-SAP (n = 181) | SAP (n = 62) | P value |
| Demographic variables | ||||
| Sex, n (%) | 0.297 | |||
| Male | 188 (77.37) | 143 (79.00) | 45 (72.60) | |
| Female | 55 (22.63) | 38 (21.00) | 17 (27.40) | |
| Age (yr) | 40.12 ± 10.12 | 39.60 ± 10.10 | 41.60 ± 10.20 | 0.190 |
| BMI (kg/m2) | 28.27 ± 4.25 | 28.10 ± 4.00 | 28.70 ± 4.90 | 0.367 |
| Medical history | ||||
| Diabetes, n (%) | 0.648 | |||
| Yes | 139 (57.20) | 102 (56.40) | 37 (59.70) | |
| No | 104 (42.80) | 79 (43.65) | 25 (40.30) | |
| Hypertension, n (%) | 0.164 | |||
| Yes | 59 (24.28) | 48 (26.50) | 11 (17.70) | |
| No | 184 (75.72) | 133 (73.50) | 51 (82.30) | |
| Hyperlipidemia, n (%) | 0.250 | |||
| Yes | 152 (62.55) | 64 (35.40) | 27 (43.50) | |
| No | 91 (37.45) | 64 (35.40) | 27 (43.50) | |
| Fatty liver, n (%) | 0.073 | |||
| Yes | 185 (76.13) | 143 (79.00) | 42 (67.70) | |
| No | 58 (23.87) | 38 (21.00) | 20 (32.30) | |
| Drinking, n (%) | 0.483 | |||
| Yes | 120 (49.38) | 87 (48.10) | 33 (53.20) | |
| No | 123 (50.62) | 94 (51.90) | 29 (46.80) | |
| Smoking, n (%) | 0.386 | |||
| Yes | 133 (54.73) | 102 (56.40) | 31 (50.00) | |
| No | 110 (45.27) | 79 (43.60) | 31 (50.00) | |
| Clinical signs | ||||
| HR (bpm) | 96.14 ± 18.17 | 92.23 ± 16.30 | 107.58 ± 18.65 | < 0.001 |
| R (bpm) | 21.66 ± 5.33 | 20.47 ± 4.38 | 25.27 ± 6.21 | < 0.001 |
| SBP (mmHg) | 133.50 ± 17.52 | 133.51 ± 16.56 | 133.48 ± 20.21 | 0.992 |
| DBP (mmHg) | 81.13 ± 12.03 | 80.85 ± 11.28 | 81.92 ± 14.08 | 0.548 |
| Non-SAP (n = 181, 74.49%) | SAP (n = 62, 25.51%) | |||
| Laboratory findings | ||||
| WBC (× 109/L) | 12.53 ± 3.86 | 11.96 ± 3.63 | 14.41 ± 3.97 | < 0.001 |
| NLR | 9.94 ± 7.27 | 8.82 ± 6.98 | 13.19 ± 7.15 | < 0.001 |
| RDW (fL) | 12.87 ± 0.72 | 12.80 ± 0.69 | 13.08 ± 0.78 | 0.009 |
| PLT (× 109/L) | 218.91 ± 61.98 | 216.09 ± 57.99 | 227.15 ± 72.30 | 0.226 |
| MPV (fL) | 10.75 ± 1.05 | 10.68 ± 1.01 | 10.95 ± 1.14 | 0.074 |
| PDW (fL) | 12.66 ± 2.22 | 12.47 ± 2.09 | 13.23 ± 2.47 | 0.018 |
| TC (mmol/L) | 10.15 ± 4.11 | 9.54 ± 3.62 | 11.94 ± 4.92 | < 0.001 |
| TG (mmol/L) | 21.08 ± 7.50 | 20.17 ± 7.31 | 23.75 ± 7.49 | 0.001 |
| H/L ratio | 0.96 ± 1.36 | 0.96 ± 1.36 | 0.97 ± 1.36 | 0.946 |
| ApoA1 (g/L) | 1.02 ± 0.35 | 1.11 ± 0.34 | 0.75 ± 0.23 | < 0.001 |
| TBIL (μmol/L) | 14.16 ± 8.29 | 13.81 ± 7.11 | 15.16 ± 11.03 | 0.987 |
| AST (U/L) | 29.78 ± 17.41 | 27.40 ± 13.03 | 36.76 ± 25.20 | < 0.001 |
| ALT (U/L) | 31.04 ± 28.70 | 30.80 ± 29.94 | 31.73 ± 24.94 | 0.826 |
| ALP (U/L) | 73.91 ± 25.30 | 73.59 ± 22.70 | 74.84 ± 31.88 | 0.737 |
| ALB (g/L) | 41.57 ± 5.91 | 42.22 ± 5.53 | 39.66 ± 6.58 | 0.003 |
| BUN mmol/L | 4.32 ± 2.03 | 4.05 ± 1.39 | 5.10 ± 3.12 | < 0.001 |
| Cr (μmol/L) | 62.62 ± 42.98 | 56.64 ± 15.83 | 80.10 ± 78.56 | < 0.001 |
| fT3 (pmol/L) | 1.97 ± 0.37 | 1.99 ± 0.36 | 1.91 ± 0.41 | 0.134 |
| CRP (mg/L) | 160.20 ± 127.36 | 133.55 ± 112.64 | 237.99 ± 136.59 | < 0.001 |
| PCT (ng/mL) | 0.80 ± 1.93 | 0.59 ± 1.72 | 1.42 ± 2.35 | 0.003 |
| ESR (mm/h) | 42.57 ± 24.76 | 40.71 ± 25.10 | 47.99 ± 23.06 | 0.045 |
| Na (mmol/L) | 133.97 ± 4.29 | 134.28 ± 4.09 | 133.07 ± 4.76 | 0.054 |
| K (mmol/L) | 4.02 ± 0.46 | 3.97 ± 0.35 | 4.19 ± 0.67 | 0.080 |
| Ca2+ (mmol/L) | 2.13 ± 0.26 | 2.19 ± 0.18 | 1.96 ± 0.36 | < 0.001 |
| PT (s) | 13.26 ± 0.98 | 13.12 ± 0.84 | 13.68 ± 1.21 | < 0.001 |
| APTT (s) | 37.82 ± 5.28 | 37.35 ± 5.14 | 39.22 ± 5.45 | 0.016 |
| TT (s) | 15.07 ± 1.10 | 15.08 ± 1.09 | 15.05 ± 1.13 | 0.823 |
| FIB (g/L) | 5.70 ± 2.21 | 5.41 ± 2.10 | 6.56 ± 2.32 | < 0.001 |
| D-dimer (μg/L) | 1.34 ± 1.83 | 0.94 ± 0.98 | 2.51 ± 2.94 | < 0.001 |
| Imaging results | ||||
| Pleural effusion, n (%) | < 0.001 | |||
| Yes | 69 (28.40) | 23 (12.71) | 46 (74.19) | |
| No | 174 (71.61) | 158 (87.30) | 16 (25.81) |
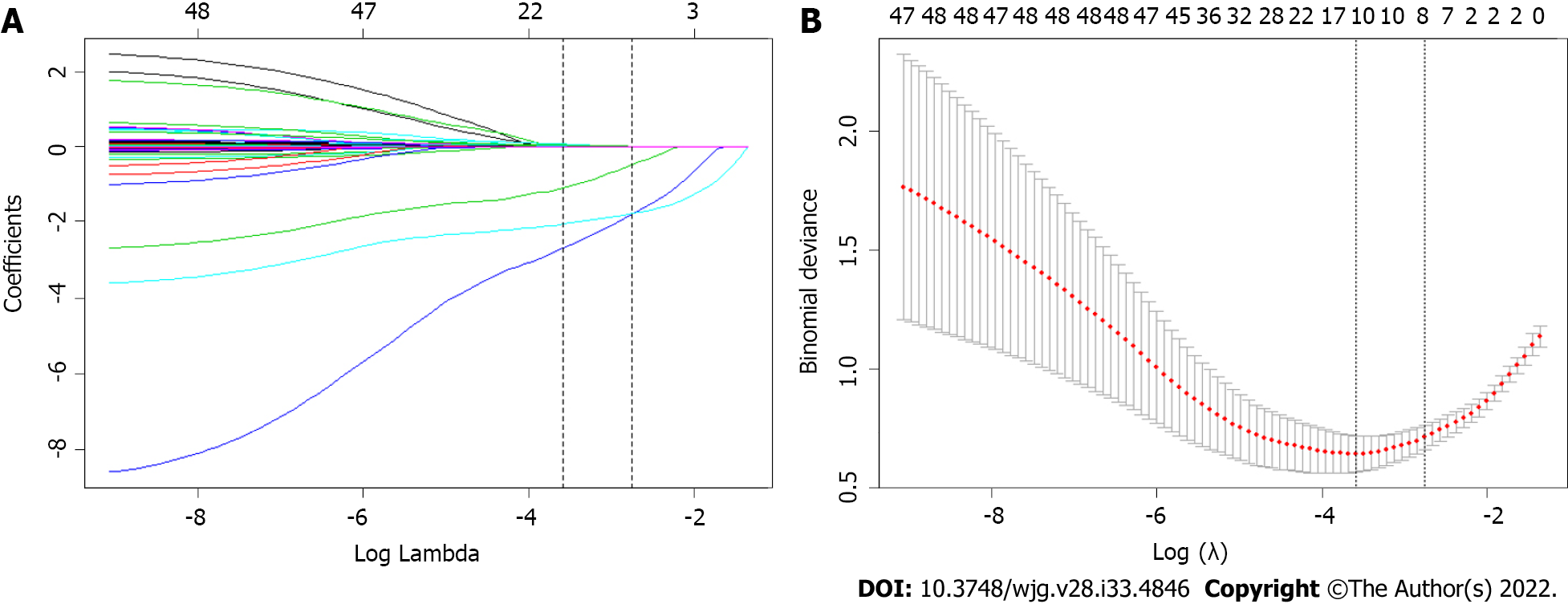
Forty-two variables measured at the hospital within 24 h of admission were included in the LASSO regression. After LASSO regression selection (Figure 1), with a lambda of 0.028, 10 variables remained significant predictors of SAP, including age, HR, RR, ApoA1, WBC, Bun, Cr, Ca2+, D-dimer, and the presence of pleural effusion. According to a previous study, TG levels dose-dependently increase the severity of AP[6]. The presence of metabolic syndrome and its components associated with increasing AP severity were also important factors[31,32]. Therefore, we included TG level, BMI, history of hypertension and diabetes in the regression analysis. Based on the Endocrine Society Clinical Practice Guideline and previously published study, we divided TG levels into three groups as classified by variables (group 1: < 11.3 mmol/L; group 2: 11.3-22.59 mmol/L; group 3: ≥ 22.6 mmol/L) for clinical use[6].
Inclusion of these 14 variables in a logistic regression model resulted in four variables that were independently statistically significant predictors of SAP (Table 2): Age (OR: 1.07; 95%CI: 1.01-1.14; P = 0.033), ApoA1 (OR: 0.02; 95%CI: 0.00-0.12; P < 0.0001), Ca2+ (OR: 0.11; 95%CI: 0.01-0.90; P = 0.040) and the presence of pleural effusion (OR: 15.61; 95%CI: 5.05-48.24; P < 0.0001).
| Variables | Univariable regression | Multivariable regression | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 1.02 (0.99-1.05) | 0.190 | 1.07 (1.01-1.14) | 0.033 |
| BMI (kg/m2) | 1.03 (0.96-1.10) | 0.366 | 1.08 (0.94-1.24) | 0.278 |
| Hypertension | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.60 (0.29-1.24) | 0.167 | 1.02 (0.99-1.06) | 0.201 |
| Diabetes | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.15 (0.64-2.06) | 0.648 | 1.27 (0.38-4.24) | 0.693 |
| HR (bpm) | 1.05 (1.03-1.07) | < 0.0001 | 1.02 (0.99-1.06) | 0.201 |
| RR (bpm) | 1.20 (1.12-1.28) | < 0.0001 | 1.12 (0.99-1.26) | 0.068 |
| TG (mmol/L) | ||||
| < 11.3 | 1.00 | 1.00 | ||
| 11.3-22.6 | 2.06 (0.57-7.49) | 0.273 | 4.20 (0.40-44.25) | 0.232 |
| ≥ 22.6 | 3.43 (0.96-12.20) | 0.057 | 5.95 (0.56-62.75) | 0.138 |
| ApoA1 (g/L) | 0.01 (0.00-0.05) | < 0.0001 | 0.02 (0.00-0.12) | < 0.0001 |
| Cr (μmol/L) | 1.02 (1.01-1.03) | 0.002 | 1.01 (0.99-1.02) | 0.458 |
| Bun (μmol/L) | 1.27 (1.10-1.48) | 0.001 | 1.29 (0.92-1.81) | 0.145 |
| WBC (× 109/L) | 1.18 (1.09-1.28) | < 0.0001 | 1.08 (0.95-1.24) | 0.248 |
| Ca2+ (mmol/L) | 0.02 (0.01-0.09) | < 0.0001 | 0.11 (0.01-1.02) | 0.040 |
| D-dimer (μg/L) | 1.69 (1.35-2.12) | < 0.0001 | 1.24 (0.94-1.63) | 0.122 |
| Pleural effusion | 19.75 (9.64-40.48) | < 0.0001 | 15.62 (5.05-48.24) | < 0.0001 |
Clinical patient samples were randomly divided (3:1) into development and validation cohorts. Due to the small sample size, we used 1000 bootstrap resamples for development and validation to reduce over-the-fit bias. The multivariable analyses demonstrated that age, the reduction in ApoA1 and Ca2+, and the presence of pleural effusion were independent risk factors for SAP. Considering that the level of TG, BMI and history of hypertension and diabetes may also be important predictors, we constructed three predictive models based on different combinations of these factors. The logistic regression function was as follows: Model 1 (including age, ApoA1, and pleural effusion): Log (odds of SAP) =
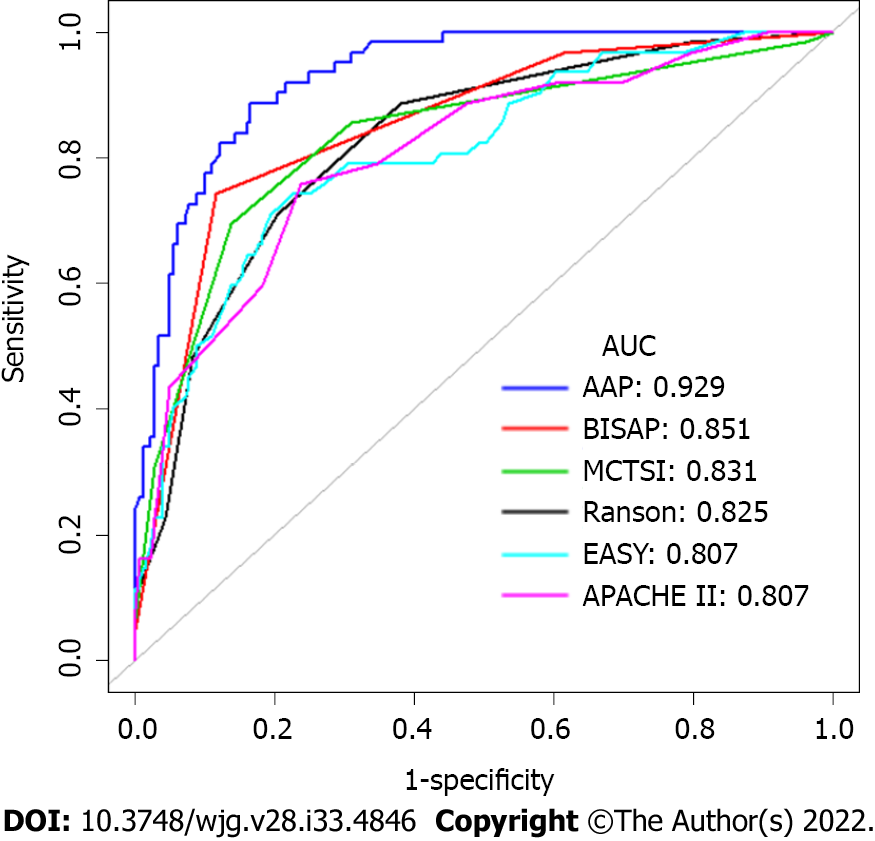
An ROC curve analysis was applied to evaluate the diagnostic efficacy of the new risk prediction score (referred to as AAP) and other clinical scoring systems, including the BISAP, Ranson, APACHE II, MCTSI and EASY. The AUC values of AAP, BISAP, RASON, APACHE II, MCTSI and EASY to predict SAP were 0.929 (95%CI: 0.889-0.958), 0.852 (95%CI: 0.801-0.894), 0.825 (95%CI: 0.771-0.871), 0.807 (95%CI: 0.752-0.855), 0.831 (95%CI: 0.778-0.876), and 0.807 (95%CI: 0.743-0.871), respectively (Figure 4). AAP achieved the highest AUC in predicting SAP among the scoring systems (Table 3). The improvement in the prediction of SAP was evaluated by calculating the IDI. IDIs were employed to compare the discriminative ability between the new model and the other clinical scoring systems. These results demonstrated that our nomogram has a greater potential for accurately predicting SAP than the other four clinical scoring systems (Table 4).
| Variables | ΔAUC | 95%CI | Z statistic | P value |
| AAP-APACHE II | 0.122 | 0.057-0.187 | 3.661 | 0.000 |
| AAP-BISAP | 0.076 | 0.007-0.198 | 3.839 | 0.000 |
| AAP-MCTSI | 0.098 | 0.027-0.305 | 3.111 | 0.002 |
| AAP-Ranson | 0.104 | 0.048-0.159 | 3.670 | 0.000 |
| AAP-EASY | 0.122 | 0.056-0.188 | 3.606 | 0.000 |
| Variables | IDI | SE | 95%CI | Z statistic | P value |
| AAP-APACHE-II | 0.201 | 0.077 | 0.050-0.352 | 2.601 | 0.009 |
| AAP-BISAP | 0.095 | 0.052 | 0.037-0.115 | 1.828 | 0.068 |
| AAP-MCTSI | 0.166 | 0.071 | 0.036-0.160 | 2.339 | 0.019 |
| AAP-Ranson | 0.216 | 0.063 | 0.093-0.338 | 3.444 | 0.001 |
| AAP-EASY | 0.205 | 0.082 | 0.045-0.365 | 2.513 | 0.012 |
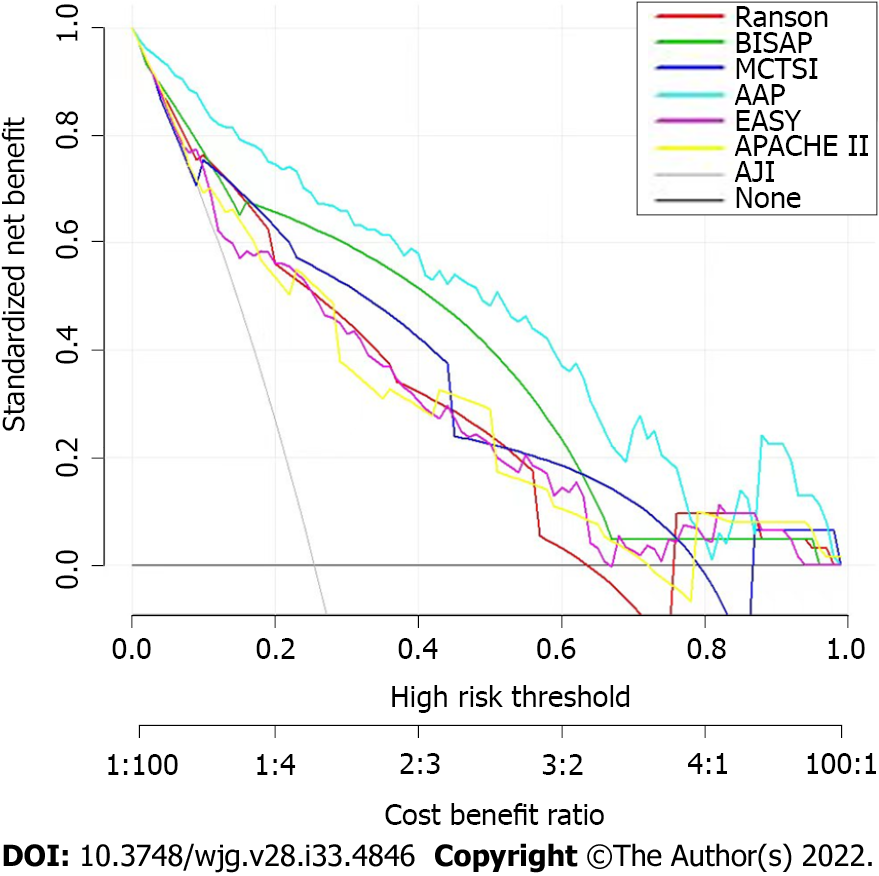
DCA was used to compare the clinical usability and benefits of the nomogram throughout the whole cohort. DCA plots showed that our nomogram had greater net benefits than other system scores for predicting the severity of AP patients, which demonstrated its utility in clinical decision-making (Figure 5).
To determine the risk stratification classification for AP, the best cutoff value (88.16) for SAP calculated through the ROC curve was used to divide patients into high-risk and low-risk groups. We further analyzed the relationship between the AAP cutoff value (88.16) and clinical parameters. The high-risk group was closely related to local and system complications: Ranson ≥ 3, BISAP ≥ 3, MCTSI ≥ 4, APACHE-II ≥ 8, CRP ≥ 190, and the length of hospital stay (Table 5).
| Low-risk group (n = 158) | High-risk group (n = 85) | P value | ||
| Local complications | ||||
| APFC | 43 (27.22%) | 68 (80.00%) | < 0.001 | |
| PPC | 3 (1.90%) | 10 (11.77%) | 0.001 | |
| ANC | 3 (1.90%) | 22 (25.88%) | < 0.001 | |
| WON | 0 (0.00%) | 4 (4.71%) | 0.014 | |
| Systemic complications | ||||
| SIRS | 92 (58.23%) | 72 (84.71%) | < 0.001 | |
| Respiratory failure | 29 (18.35%) | 59 (69.41%) | < 0.001 | |
| Renal failure | 0 (0.00%) | 13 (15.29%) | < 0.001 | |
| Cardiovascular failure | 2 (1.27%) | 5 (5.88%) | 0.040 | |
| Clinical Scoring Systems | ||||
| Ranson ≥ 3 | 28 (17.72%) | 53 (62.35%) | < 0.001 | |
| BISAP ≥ 3 | 0 (0.00%) | 4 (4.71%) | 0.014 | |
| MCTSI ≥ 4 | 15 (9.49%) | 53 (62.35%) | < 0.001 | |
| APACHE-II ≥ 8 | 73 (46.20%) | 68 (80.00%) | < 0.001 | |
| CRP ≥ 190 | 42 (26.58%) | 44 (51.77%) | < 0.001 | |
| Hospital day, days | 13.51 ± 5.45 | 20.04 ± 8.14 | < 0.001 | |
AHTGP has grown in incidence and importance. According to the previously published literature, HTG is the third most common cause of AP[6]. Clinically, AHTGP is similar to other forms of AP, but it is associated with significantly higher complication rates, severity, and mortality[5,6]. Thus, recognizing risk factors for severe AHTGP in the early stages is very important for triaging patients appropriately to ICUs and providing specific treatments. In the present study, we developed a convenient and specific nomogram based on three predictors for predicting the severity of patients with AHTGP. The nomogram showed great calibration and discriminatory abilities in both the development and validation groups. In addition, our nomogram has shown improved prognostic reliability, accuracy and the best net benefit when compared to other clinical scoring systems, such as BISAP, Ranson, APACHE II, CTSI and an artificial intelligence model, the EASY prediction score. Moreover, the model could distinguish patients into low-risk and high-risk groups according to the best cutoff point (88.16). Patients with higher scores had a higher probability of developing SAP than those with lower scores. The cutoff point can help doctors in making medical decisions.
As mentioned in the introduction, four commonly used AP scoring systems, including APACHE II, Ranson, BISAP, and MCTSI, have limitations on their abilities to identify SAP early. According to our results, our prediction score AAP has three easily available parameters lower than other scoring systems, which is easy for clinical application. Meanwhile, it has better performance in diagnostic efficacy and clinical decision-making for patients with AHTGP. It is worth mentioning that an artificial intelligence model-EASY prediction score consisting of 23 parameters was developed recently based on a multicenter, multinational, prospective and observational study[17]. We applied this model to our research population. Although it achieved a high AUC in predicting SAP, it was still the lowest among the AAP and four commonly used AP scoring systems. This may suggest that the prediction ability of the EASY model is limited for pancreatitis caused by HTG.
Notably, we introduced a new predictor, ApoA1, into our risk score compared with other clinical scoring systems. Recent studies have shown a negative correlation between ApoA1 and the severity of AP, and decreased serum levels of ApoA1 have been linked to the occurrence of SAP in patients[24,33]. The mechanism can be explained as follows: In SAP patients, excessive inflammatory cytokines inhibit synthesis. Recent studies showed a negative correlation between ApoA1 and the severity of AP, and decreased serum levels of ApoA1 have been linked to the occurrence of SAP in patients and lead to lipoprotein degradation[34]. Our previous study showed that the best cutoff point of ApoA1 for predicting severe acute hyperlipidemic pancreatitis was 0.8 g/L, whose sensitivity, specificity and Youden index were 0.877, 0.674 and 0.55, respectively[35]. Another study performed by Li et al[33] showed that ApoA1 predicts the optimal critical value of SAP to be 0.8 g/L, whose sensitivity, specificity and Youden index were 0.551, 0.757 and 0.693, respectively[33]. Therefore, ApoA1 has been found to be a reliable and validated indicator of SAP. Therefore, ApoA1 included in our model could increase the specificity and sensitivity of the nomogram.
Pleural effusion is one of the most common thoracic complications in AP patients. According to recent studies, the prevalence ranges from 46.0% to 72.3%[36]. The disruption of the pancreatic duct may result in leakage of pancreatic secretions directly into the peritoneal cavity via the transdiaphragmatic lymphatic channels[37]. According to the up-to-date revised Atlanta criteria, pleural effusion is a strong individual predictor of SAP. Additionally, pleural effusion is a valuable indicator of BISAP score. Yan et al[30] showed that on the basis of PEV, the AUC of 0.816 for predicting severe AP, with a threshold of 69.00 mL, and the sensitivity and specificity were 84.23% and 81.07, respectively. According to this study, pleural effusion volume can serve as a clinical biomarker to predict the severity and outcome of AP[30].
The evidence indicates that patients with HTG present a more severe form of pancreatitis. A previous study confirmed that HTG dose-dependently increases the complications and severity of AP[6]. However, a meta-analysis including 11965 patients from 16 eligible studies found no significant difference in AP severity based on the extent of HTG[5]. This study also explored the association between HTG levels and the severity of AHTGP. Compared to patients with TGs lower than 11.3 mmol/L, we found that patients with TGs above 22.6 mmol/L had a higher OR (OR: 5.95; 95%CI: 0.56-62.75; P = 0.232) than patients with TGs between 11.3 and 22.59 mmol/L (OR: 4.20; 95%CI: 0.40-44.25; P = 0.138). We speculated that there was a trend for HTG to dose-dependently increase the severity of AHTGP. Previous studies showed that the presence of metabolic syndrome and its components, including obesity, diabetes and hypertension, were significantly associated with increasing AP severity[31,32]. Our study showed that there was a trend that the presence of obesity (OR: 1.08; 95%CI: 0.94-1.24; P = 0.278), diabetes (OR: 1.27; 95%CI: 0.38-4.24; P = 0.693) and hypertension (OR: 1.02; 95%CI: 0.99-1.06; P = 0.201) increased the risk of SAP. However, the P values were not significant, probably because of our small sample size. The small sample size of our study makes it difficult to make extensive recommendations. The next step is to conduct a multicenter prospective cohort study with a large sample size to further explore this important issue.
The novelties and strengths of our study include the following: To the best of our knowledge, this is the first study attempting to develop a risk prediction score for HTG-induced pancreatitis. We also compared the risk prediction score with existing scoring systems in a sample of Chinese patients for predicting the severity of AHTGP. Although the established and validated nomogram in our study may provide a convenient and specific tool to assist physicians in clinical decisions, there are some limitations to be taken into account. First, this study was retrospective, so selection and detection bias could exist. Second, it was a single-center study with a small sample size, which lacked multicenter data verification. There is a need to externally validate the risk score prior to clinical use. Furthermore, the data used to develop and validate the score are all from China, which limits their generalizability to other parts of the world.
In this study, we developed a risk score to estimate the prediction of developing SAP among patients with AHTGP based on three variables commonly measured on admission to the hospital. Estimating the risk score could help identify patients who are likely to develop SAP at an early stage. It could be of great value in guiding clinical decisions as a convenient and specific tool and optimizing the use of medical resources by supporting appropriate treatment.
The frequency of acute hypertriglyceridemic pancreatitis (AHTGP) is in-creasing worldwide. AHTGP may be associated with a more severe clinical course and greater mortality than pancreatitis caused by other causes. Early identification of patients with severe inclination is essential for clinical deci-sion-making and improving prognosis. Hence, constructing a risk prediction score with high predictive accuracy and clinical utility for assessing the severity of AHTGP patients is of great importance.
Early prediction and detection of AHTGP patients who are likely to develop severe acute pancreatitis (SAP) is of great importance. Almost of existing clinical scores were developed for all etiologies of pancreatitis and not for hypertriglyceridemia (HTG)-induced pancreatitis separately. To the best of our knowledge, this is the first study attempting to develop a risk prediction score for HTG-induced pancreatitis. This risk score may help guide clinical decisions for these patients.
The purpose of this study was to establish a risk prediction score with easy use and high performance for predicting the severity of AHTGP patients in China, which will help doctors make rational clinical decisions.
We performed a retrospective study of patients with AHTGP. Least absolute shrinkage and selection operator and logistic regression were used to screen predictive variables to construct a nomogram for predicting the severity of AHTGP. The predictive accuracy of the nomogram was estimated using the concordance index. The performance of the nomogram was estimated using a calibration curve. We evaluated the predictive accuracy and net benefit of the risk score and compared it with existing scoring systems via receiver operating characteristic curve analysis and decision curve analysis. We used the best cutoff value for SAP to determine the risk stratification classification.
A risk prediction score consisting of three predictors commonly measured on admission was constructed to predict the severity of SAP. More importantly, our nomogram exhibited high predictive accuracy and good performance. In addition, our nomogram has shown improved prognostic reliability, accuracy and the best net benefit when compared to other clinical scoring systems, such as Bedside Index of Severity in AP, Ranson, Acute Physiology and Chronic Health Evaluation II, modified computed tomography severity index and an artificial intelligence model, the early achievable severity index prediction score. Moreover, the risk prediction score could distinguish patients into low-risk and high-risk groups according to the best cutoff point. The cutoff point can help doctors in making medical decisions.
This risk prediction score have potential usefulness in predicting the presence of SAP at an early stage. It could be of great value in guiding clinical decisions as a convenient and specific tool and optimizing the use of medical resources by supporting appropriate treatment.
To the best of our knowledge, this is the first study attempting to develop a risk prediction score for HTG-induced pancreatitis. But, this was a single-center study with a small sample size, which lacked multi-center data verification. The next step is to conduct a multicenter prospective cohort study with a large sample size to construct specific risk score and externally validate the risk score prior to clinical use.
The authors express special thanks to Gui-Qi Zhu (Department of Liver Surgery, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, 200032, China) for his advice on data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegyi P, Hungary; Tang XB, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Hassanloo J, Béland-Bonenfant S, Paquette M, Baass A, Bernard S. Prevalence, severity and management of hypertriglyceridemia-associated pancreatitis; A 7-year retrospective cohort study at canadian quaternary care hospitals. J Clin Lipidol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Sun YM, Gao F, Chen X, Zhang J. The relationship between triglyceride level and the severity of acute hypertriglyceridemic pancreatitis in Chinese patients. Turk J Gastroenterol. 2020;31:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Zheng Y, Zhou Z, Li H, Li J, Li A, Ma B, Zhang T, Liao Q, Ye Y, Zhang Z, Yang Y, Wang Z, Yang J, Li F. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas. 2015;44:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Tai WP, Lin XC, Liu H, Wang CH, Wu J, Zhang NW, Chen W. A Retrospective Research of the Characteristic of Hypertriglyceridemic Pancreatitis in Beijing, China. Gastroenterol Res Pract. 2016;2016:6263095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kiss L, Fűr G, Mátrai P, Hegyi P, Ivány E, Cazacu IM, Szabó I, Habon T, Alizadeh H, Gyöngyi Z, Vigh É, Erőss B, Erős A, Ottoffy M, Czakó L, Rakonczay Z Jr. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep. 2018;8:14096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Mosztbacher D, Hanák L, Farkas N, Szentesi A, Mikó A, Bajor J, Sarlós P, Czimmer J, Vincze Á, Hegyi PJ, Erőss B, Takács T, Czakó L, Németh BC, Izbéki F, Halász A, Gajdán L, Hamvas J, Papp M, Földi I, Fehér KE, Varga M, Csefkó K, Török I, Farkas HP, Mickevicius A, Maldonado ER, Sallinen V, Novák J, Ince AT, Galeev S, Bod B, Sümegi J, Pencik P, Dubravcsik Z, Illés D, Gódi S, Kui B, Márta K, Pécsi D, Varjú P, Szakács Z, Darvasi E, Párniczky A, Hegyi P; Hungarian Pancreatic Study Group. Hypertriglyceridemia-induced acute pancreatitis: A prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology. 2020;20:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf). 2018;6:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21:2387-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 10. | Wang L, Zeng YB, Chen JY, Luo Q, Wang R, Zhang R, Zheng D, Dong YH, Zou WB, Xie X, Du YQ, Li ZS. A simple new scoring system for predicting the mortality of severe acute pancreatitis: A retrospective clinical study. Medicine (Baltimore). 2020;99:e20646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Yang YX, Li L. Evaluating the Ability of the Bedside Index for Severity of Acute Pancreatitis Score to Predict Severe Acute Pancreatitis: A Meta-Analysis. Med Princ Pract. 2016;25:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Yang L, Liu J, Xing Y, Du L, Chen J, Liu X, Hao J. Comparison of BISAP, Ranson, MCTSI, and APACHE II in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroenterol Res Pract. 2016;2016:1834256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, Singh VK, Slivka A, Whitcomb DC, Yadav D, Banks PA, Papachristou GI. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476-82; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Huang L, Chen C, Yang L, Wan R, Hu G. Neutrophil-to-lymphocyte ratio can specifically predict the severity of hypertriglyceridemia-induced acute pancreatitis compared with white blood cell. J Clin Lab Anal. 2019;33:e22839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hong W, Lillemoe KD, Pan S, Zimmer V, Kontopantelis E, Stock S, Zippi M, Wang C, Zhou M. Development and validation of a risk prediction score for severe acute pancreatitis. J Transl Med. 2019;17:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Qiu Q, Nian YJ, Guo Y, Tang L, Lu N, Wen LZ, Wang B, Chen DF, Liu KJ. Development and validation of three machine-learning models for predicting multiple organ failure in moderately severe and severe acute pancreatitis. BMC Gastroenterol. 2019;19:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, Vincze Á, Bajor J, Gódi S, Czimmer J, Szabó I, Illés A, Sarlós P, Hágendorn R, Pár G, Papp M, Vitális Z, Kovács G, Fehér E, Földi I, Izbéki F, Gajdán L, Fejes R, Németh BC, Török I, Farkas H, Mickevicius A, Sallinen V, Galeev S, Ramírez-Maldonado E, Párniczky A, Erőss B, Hegyi PJ, Márta K, Váncsa S, Sutton R, Szatmary P, Latawiec D, Halloran C, de-Madaria E, Pando E, Alberti P, Gómez-Jurado MJ, Tantau A, Szentesi A, Hegyi P; Hungarian Pancreatic Study Group. EASY-APP: An artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12:e842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4343] [Article Influence: 361.9] [Reference Citation Analysis (45)] |
| 19. | Cheng T, Liu BF, Han TY, Pan P, Liu JZ, Yu H. Efficiency of red cell distribution width in predicting severity and mortality of patients with acute pancreatitis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Lei JJ, Zhou L, Liu Q, Xiong C, Xu CF. Can mean platelet volume play a role in evaluating the severity of acute pancreatitis? World J Gastroenterol. 2017;23:2404-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wang F, Meng Z, Li S, Zhang Y, Wu H. Platelet Distribution Width Levels Can Be a Predictor in the Diagnosis of Persistent Organ Failure in Acute Pancreatitis. Gastroenterol Res Pract. 2017;2017:8374215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Hong W, Zimmer V, Basharat Z, Zippi M, Stock S, Geng W, Bao X, Dong J, Pan J, Zhou M. Association of total cholesterol with severe acute pancreatitis: A U-shaped relationship. Clin Nutr. 2020;39:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Wu Q, Zhong X, Fu M, Yang H, Bo H, Liao X, Hu Z, Wang B, Zhang Z, Jin X, Kang Y. High-density lipoprotein cholesterol to low-density lipoprotein cholesterol ratio in early assessment of disease severity and outcome in patients with acute pancreatitis admitted to the ICU. BMC Gastroenterol. 2020;20:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Zhou CL, Zhang CH, Zhao XY, Chen SH, Liang HJ, Hu CL, Chen NW. Early prediction of persistent organ failure by serum apolipoprotein A-I and high-density lipoprotein cholesterol in patients with acute pancreatitis. Clin Chim Acta. 2018;476:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, Coskun O, Kayacetin E. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2017;16:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Wan J, Shu W, He W, Zhu Y, Zeng H, Liu P, Xia L, Lu N. Serum Creatinine Level and APACHE-II Score within 24 h of Admission Are Effective for Predicting Persistent Organ Failure in Acute Pancreatitis. Gastroenterol Res Pract. 2019;2019:8201096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Yang WQ, Yang Q, Chen WJ, Zhang XB, Xu QQ, Qiao Y, Xu XH, Liu L, Lu XY, Zhu CQ. Low FT3 is a valuable predictor of severe acute pancreatitis in the emergency department. J Dig Dis. 2018;19:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Thong VD, Mong Trinh NT, Phat HT. Factors associated with the severity of hypertriglyceridemia induced acute pancreatitis. Medicine (Baltimore). 2021;100:e25983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Liu C, Zhou X, Ling L, Chen S, Zhou J. Prediction of mortality and organ failure based on coagulation and fibrinolysis markers in patients with acute pancreatitis: A retrospective study. Medicine (Baltimore). 2019;98:e15648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Yan G, Li H, Bhetuwal A, McClure MA, Li Y, Yang G, Zhao L, Fan X. Pleural effusion volume in patients with acute pancreatitis: a retrospective study from three acute pancreatitis centers. Ann Med. 2021;53:2003-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Niknam R, Moradi J, Jahanshahi KA, Mahmoudi L, Ejtehadi F. Association Between Metabolic Syndrome and Its Components with Severity of Acute Pancreatitis. Diabetes Metab Syndr Obes. 2020;13:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Szentesi A, Párniczky A, Vincze Á, Bajor J, Gódi S, Sarlós P, Gede N, Izbéki F, Halász A, Márta K, Dobszai D, Török I, Farkas H, Papp M, Varga M, Hamvas J, Novák J, Mickevicius A, Maldonado ER, Sallinen V, Illés D, Kui B, Erőss B, Czakó L, Takács T, Hegyi P. Multiple Hits in Acute Pancreatitis: Components of Metabolic Syndrome Synergize Each Other's Deteriorating Effects. Front Physiol. 2019;10:1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Li Y, Zheng R, Gao F, Wang L, Feng S, Li J, Huang Z. Association between high-density lipoprotein cholesterol and apolipoprotein A-I and severe acute pancreatitis: a case-control study. Eur J Gastroenterol Hepatol. 2021;33:1517-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Vuilleumier N, Dayer JM, von Eckardstein A, Roux-Lombard P. Pro- or anti-inflammatory role of apolipoprotein A-1 in high-density lipoproteins? Swiss Med Wkly. 2013;143:w13781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Liu Z, Liu Z, Zhang M. Correlation of apolipoprotein levels with the severity of hyperlipidemic acute pancreatitis. Chin J Gen Pract. 2019;1070-1071. |
| 36. | Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT Evaluation of Acute Pancreatitis and its Prognostic Correlation with CT Severity Index. J Clin Diagn Res. 2016;10:TC06-TC11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 37. | Kumar P, Gupta P, Rana S. Thoracic complications of pancreatitis. JGH Open. 2019;3:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |