Published online Sep 7, 2022. doi: 10.3748/wjg.v28.i33.4834
Peer-review started: March 14, 2022
First decision: May 9, 2022
Revised: May 23, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 7, 2022
Processing time: 169 Days and 16.6 Hours
Patients with inflammatory bowel disease (IBD) are prone to several nutritional deficiencies. However, data are lacking on vitamin C deficiency in Crohn’s disease (CD) and ulcerative colitis (UC) patients, as well as the impact of clinical, bio
To determine proportions and factors associated with vitamin C deficiency in CD and UC patients.
In this retrospective study, we obtained clinical, laboratory and endoscopic data from CD and UC patients presenting to the IBD clinic at a single tertiary care center from 2014 to 2019. All patients had an available plasma vitamin C level. Of 353 subjects who met initial search criteria using a cohort discovery tool, 301 ultimately met criteria for inclusion in the study. The primary aim described vitamin C deficiency (≤ 11.4 μmol/L) rates in IBD. Secondary analyses compared proportions with deficiency between active and inactive IBD. Multivariate logistic regression analysis evaluated factors associated with deficiency.
Of 301 IBD patients, 21.6% had deficiency, including 24.4% of CD patients and 16.0% of UC patients. Patients with elevated C-reactive protein (CRP) (39.1% vs 16.9%, P < 0.001) and fecal calprotectin (50.0% vs 20.0%, P = 0.009) had signifi
Vitamin C deficiency was common in IBD. Patients with elevated inflammatory markers and penetrating disease had higher rates of vitamin C deficiency.
Core Tip: This study aimed to determine proportions and factors associated with vitamin C deficiency in inflammatory bowel disease (IBD) patients. In 301 patients, 21.6% had vitamin C deficiency, including 24.4% of Crohn’s disease and 16.0% of ulcerative colitis patients. Patients with elevated C-reactive protein (39.1% vs 16.9%) and fecal calprotectin (50.0% vs 20.0%) had higher proportions of deficiency compared to those without, as did patients with penetrating disease (36.2% vs 20.8%). This study provides the largest data on vitamin C deficiency in IBD, and demonstrates that deficiency is common in this population, particularly those with markers of active luminal or penetrating disease.
- Citation: Gordon BL, Galati JS, Yang S, Longman RS, Lukin D, Scherl EJ, Battat R. Prevalence and factors associated with vitamin C deficiency in inflammatory bowel disease. World J Gastroenterol 2022; 28(33): 4834-4845
- URL: https://www.wjgnet.com/1007-9327/full/v28/i33/4834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i33.4834
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders of the gastrointestinal tract that affect over 1.5 million people in the United States alone[1,2]. Several nutritional deficiencies are well described in patients with IBD, the most common being iron, vitamin B12, vitamin D, zinc, and calcium[3-7]. However, far less literature exists on vitamin C (ascorbic acid) deficiency in this population. While the prevalence of scurvy - the clinical manifestations of vitamin C deficiency - has largely declined in the 21st century, up to 7% of the United States population still possesses vitamin C deficiency. The risk of deficiency is particularly increased in smokers, obese patients, and patients from low-income backgrounds[8]. Among those also at risk are patients with poor vitamin C intake and malabsorptive processes.
Traditionally, in IBD patients, vitamin C deficiency is thought to originate from insufficient con
Vitamin C deficiency can lead to impaired uptake and utilization of iron, poor wound healing, and bleeding[15,16]. The diagnosis of vitamin C deficiency can be all the more challenging to make in patients with IBD, as many nonspecific symptoms of scurvy - fatigue, arthralgias, and cutaneous manifestations - can confound systemic symptoms of CD and UC. Prior studies of patients with IBD have described inadequate vitamin C intake and suboptimal serum vitamin C levels in 22%-70% and 15%-84%, respectively[9,17-20]. Notably, these studies have been small, excluded patients with UC, and occurred prior to the advent of biologic medications. Importantly, cohorts lacked data on clinical, biomarker and endoscopic measures of disease activity to assess for their impact on vitamin C deficiency.
IBD patients are at risk for malnutrition and vitamin C deficiency is an easily reversible condition. Thus, it is essential to understand the prevalence of and factors associated with vitamin C deficiency in this population to better identify those at risk. For this reason, this study aimed to determine rates of vitamin C deficiency in patients with CD and UC and investigate potential factors associated with the development of vitamin C deficiency in this population.
Data were extracted from chart review of patients presenting to the IBD clinic at a single tertiary institution from 2014 to 2019. Patients were identified using a cohort discovery tool (Informatics for Integrating Biology and the Bedside, National Center for Biomedical Computing, Partners HealthCare System, Boston, Massachusetts) at Weill Cornell Medicine, New York. Search criteria included age 18 years and older, diagnosis of CD or UC, and a plasma vitamin C measurement drawn from 2014 to 2019. International Classification of Diseases 10th edition codes were used to identify patients with CD (K50.x) and UC (K51.x). Exclusion criteria included lack of a CD or UC diagnosis, plasma vitamin C level, or IBD-related visit at the time that plasma vitamin C measurement was performed. Three hundred fifty-three subjects matched initial search criteria. In patients with multiple plasma vitamin C levels, the lowest value and associated visit were utilized. This study was conducted retrospectively from data obtained for clinical purposes. The study was approved by the institutional review board at Weill Cornell Medicine, who confirmed that no ethical approval was required.
We extracted covariates readily available in the electronic medical record. Baseline characteristics were collected, including age, sex, race, body mass index (BMI), smoking history, type of IBD (CD or UC), disease duration and prior IBD-related surgeries (i.e., total proctocolectomy, ileocolonic resection, small bowel resection, etc.). Endoscopic scores - within six months of plasma vitamin C level assessment - were collected when available. Disease location and behavior were defined by the Montreal classification for IBD[21]. Patients were evaluated for current and prior IBD medications, including biologic agents such as TNF-α inhibitors (infliximab, adalimumab, golimumab, and certolizumab pegol), vedolizumab, and ustekinumab. Additional laboratory values collected within one week of plasma vitamin C levels were included in the analysis. When available, C-reactive protein (CRP), iron, trans
The primary study outcome was the prevalence of vitamin C deficiency in IBD patients. Vitamin C deficiency was defined as plasma vitamin C level < 11.4 μmol/L[24,25]. Inadequate vitamin C level or marginal deficiency was defined as 11.4-28.0 μmol/L, consistent with prior studies[24,25]. Secondary analyses were performed to assess whether clinical, biomarker or endoscopic disease activity were associated with deficiency. Patients were assessed for clinical disease activity using the Harvey-Bradshaw Index for CD[26] and modified partial Mayo score for UC[27]. Clinically active disease was defined as Harvey-Bradshaw Index > 5 for CD. For UC, clinically active disease was defined as either stool frequency or rectal bleeding > 1 on the modified partial Mayo score. Elevated CRP was defined as > 0.9 mg/dL and elevated fecal calprotectin was defined as > 250 μg/g. Endoscopically severe disease was defined as simple endoscopic score CD > 15 for CD and Mayo endoscopic score ≥ 2 for UC[28-30]. Additional outcomes based on biologic plausibility included the association of vitamin C deficiency with IBD type (CD or UC), obesity (BMI ≥ 30), use of biologic medications, disease location in the small intestine, penetrating disease, IBD-related surgery, elevated CRP, elevated fecal calprotectin, iron deficiency, clinically active disease and endoscopically severe disease.
The primary outcome described the prevalence of vitamin C deficiency in patients with IBD. To address this outcome, based on previous data in 137 CD patients showing a 15% prevalence of vitamin C deficiency[17], in our exploratory cohort of 301 patients, we expected that a two-sided 95% confidence interval (CI) for the prevalence could be constructed to be within ± 4.0% of the observed prevalence proportion. Statistical review was performed by the corresponding author, who has extensive experience performing statistical analyses for clinical research.
For secondary outcomes, variables (listed above) were selected based on biologic plausibility for abnormal vitamin C absorption. To address these secondary outcomes, we performed chi-squared tests or Fisher’s exact tests as appropriate to compare the proportions of vitamin C deficiency between groups. A multivariate logistic regression was performed on covariates selected based on biologic plausibility. These included presence of small bowel disease, penetrating disease, history of IBD-related surgery, obesity, current biologic medication use, elevated CRP, and clinically active disease. Variables with P ≤ 0.2 were selected for inclusion in the final model. CRP was selected as the sole objective inflammatory marker for sample size considerations (i.e., fecal calprotectin and endoscopy data had limited sample size) and to avoid multicollinearity with these other inflammatory assessments. All analyses were performed using Stata Version 16.0 (StataCorp, College Station, TX). Continuous variables were expressed as means ± SD. The multivariate analysis was expressed as odds ratio (OR) with 95%CI.
A total of 301 CD or UC patients with available plasma vitamin C levels were included in the study. Baseline characteristics of the entire cohort are described in Table 1. The mean age of the cohort was 47.6 ± 17.4 years. One hundred ninety (63.1%) subjects were female, and 230 (76.4%) were Caucasian. A total of 201 (66.8%) patients had a diagnosis of CD and 100 (33.2%) had a diagnosis of UC. The mean duration of disease was 17.0 ± 13.6 years. A total of 109 (36.2%) patients had a history of IBD-related surgery and 133 patients (44.2%) were undergoing treatment with a biologic agent at the time of plasma vitamin C level collection. Six patients (2.0%) were active smokers and 42/291 (14.4%) had a BMI ≥ 30. Fifty-nine of 252 patients (23.4%) had iron deficiency, 1/264 (0.4%) had vitamin B12 deficiency, and 38/259 (14.7%) had vitamin D deficiency or insufficiency. Of 292 patients with available disease activity scores, 134 (45.9%) had clinically active disease. Of 113 patients with available endoscopies, 20 (17.7%) had endoscopically severe disease.
| All IBD patients (n = 301) | |
| Female sex | 190 (63.1%) |
| Age (yr) | 47.6 ± 17.4 |
| Ethnicity | |
| Caucasian | 230 (76.4%) |
| Hispanic | 16 (5.3%) |
| African-American | 5 (1.7%) |
| Asian | 14 (4.7%) |
| Not specified | 36 (12.0%) |
| BMI | 24.8 ± 5.2 |
| Obesity (BMI ≥ 30)1 | 42 (14.4%) |
| Active smoking | 6 (2.0%) |
| CD | 201 (66.8%) |
| Disease location | |
| Ileal | 57 (28.4%) |
| Colonic | 34 (16.9%) |
| Ileocolonic | 108 (53.7%) |
| Upper disease | 10 (5.0%) |
| Behavior | |
| Non-stricturing, non-penetrating | 106 (52.7%) |
| Stricturing | 74 (36.8%) |
| Penetrating | 47 (23.4%) |
| UC | 100 (36.8%) |
| Disease location1 | |
| Proctitis | 14 (14.7%) |
| Left-sided colitis | 45 (47.4%) |
| Pancolitis | 36 (37.9%) |
| Disease duration (yr) | 17.0 ± 13.6 |
| IBD related surgery | 109 (36.2%) |
| IBD medications | |
| Current biologic use | 133 (44.2%) |
| Past/ever biologic use | 166 (55.1%) |
| Clinically active disease1 | 134 (45.9%) |
| CD | 96 (49.2%) |
| UC | 38 (39.2%) |
| Endoscopically severe disease1 | 20 (17.7%) |
| CD | 12 (15.6%) |
| UC | 8 (22.2%) |
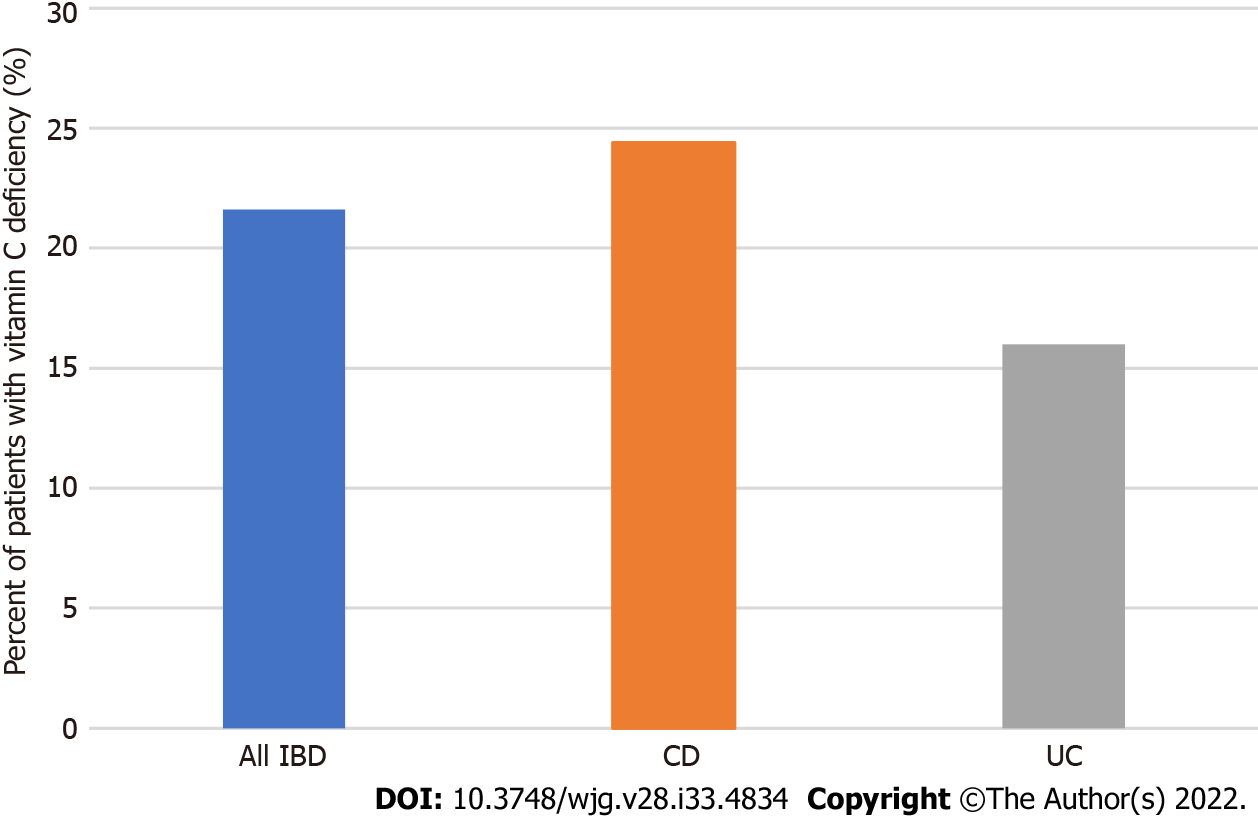
The mean vitamin C level was 35.7 ± 27.8 μmol/L in the entire IBD cohort. For analysis of the primary outcome, 21.6% of IBD patients (65/301) had vitamin C deficiency (< 11.4 μmol/L). An additional 24.6% of IBD patients (74/301) had inadequate vitamin C levels (11.4-28.0 μmol/L). CD patients had numerically higher prevalence of vitamin C deficiency than those with UC, although this result did not reach statistical significance (24.4% vs 16.0%, P = 0.1, Figure 1).
In all IBD patients, those with elevated CRP had higher proportions of vitamin C deficiency (39.1% vs 16.9%, P < 0.001, Table 2) compared to those without elevated CRP. Similarly, patients with elevated fecal calprotectin had higher rates of vitamin C deficiency (50.0% vs 20.0%, P = 0.009) compared to those without fecal calprotectin elevation. In a subgroup with available endoscopic data, those with severe inflammation (n = 20) had numerically higher deficiency rates compared to those without severe inflammation (35.0% vs 22.6% P = 0.2). However, comparable rates of deficiency existed between those with and without clinically active disease (26.1% vs 18.4%, P = 0.1). Obesity (35.7% vs 19.7%, P = 0.02) and current biologic medication use (28.6% vs 15.6%, P = 0.006) were associated with increased rates of vitamin C deficiency on univariate analysis. Among patients on current biologic therapy (n = 133), there were higher proportions of vitamin C deficiency in those using TNF-α inhibitors (17/48) compared with those using non-TNF-α (16/85) biologics (35.4% vs 18.8%, P = 0.03). Iron deficiency (28.8% vs 20.2%, P = 0.2), vitamin D deficiency/insufficiency (28.9% vs 21.3%, P = 0.3), surgery (25.7% vs 19.3%, P = 0.2), and active smoking (50.0% vs 21.0%, P = 0.1) were not associated with higher deficiency rates. On multivariate analysis, elevated CRP was the only factor significantly associated with vitamin C deficiency (OR = 3.1, 95%CI: 1.5-6.6, P = 0.003). Presence of small bowel disease, penetrating disease, history of IBD-related surgery, obesity, use of a biologic agent, and clinically active disease were not.
| Covariates | Prevalence of vitamin C deficiency in patients with covariate | Prevalence of vitamin C deficiency in patients without covariate | P value |
| IBD type | |||
| CD | 49/201 (24.4%) | - | 0.1 |
| UC | 16/100 (16.0%) | - | 0.1 |
| Small bowel disease | 38/165 (23.0%) | 11/36 (30.6%) | 0.3 |
| Penetrating disease | 17/47 (36.2%) | 32/154 (20.8%) | 0.031 |
| IBD related surgery | 28/109 (25.7%) | 37/192 (19.3%) | 0.2 |
| CD | 25/96 (26.0%) | 24/105 (22.9%) | 0.6 |
| UC | 3/13 (23.1%) | 13/87 (14.9%) | 0.4 |
| Obesity (BMI ≥ 30) | 15/42 (35.7%) | 49/249 (19.7%) | 0.021 |
| Active smoking | 3/6 (50.0%) | 62/295 (21.0%) | 0.1 |
| Current biologic use | 38/133 (28.6%) | 26/167 (15.6%) | 0.0061 |
| CRP > 0.9 mg/dL | 25/64 (39.1%) | 36/213 (16.9%) | < 0.0011 |
| CD | 21/51 (41.2%) | 24/134 (17.9%) | 0.0011 |
| UC | 4/13 (30.8%) | 12/79 (15.2%) | 0.2 |
| Fecal calprotectin > 250 ug/g | 10/20 (50.0%) | 12/60 (20.0%) | 0.0091 |
| CD | 9/16 (56.3%) | 10/38 (26.3%) | 0.041 |
| UC | 1/4 (25.0%) | 2/22 (9.1%) | 0.4 |
| Iron deficiency | 17/59 (28.8%) | 39/193 (20.2%) | 0.2 |
| Vitamin D deficiency/insufficiency | 11/38 (28.9%) | 47/221 (21.3%) | 0.3 |
| Clinically active disease | 35/134 (26.1%) | 29/158 (18.4%) | 0.1 |
| CD | 29/96 (30.2%) | 20/99 (20.2%) | 0.1 |
| UC | 6/38 (15.8%) | 9/59 (15.3%) | 0.9 |
| Endoscopically severe disease | 7/20 (35.0%) | 21/93 (22.6%) | 0.2 |
| CD | 5/12 (41.7%) | 18/65 (27.7%) | 0.3 |
| UC | 2/8 (25.0%) | 3/28 (10.7%) | 0.3 |
Among CD patients, patients with penetrating disease had significantly higher rates of vitamin C deficiency compared to patients without penetrating disease (36.2% vs 20.8%, P = 0.03, Table 2). In CD patients, both elevated CRP (41.2% vs 17.9%, P = 0.001) and fecal calprotectin (56.3% vs 26.3%, P = 0.04) were persistently associated with higher proportions of vitamin C deficiency compared to those without elevated biomarkers. In subgroups of CD patients with endoscopic data available, those with endoscopically severe disease (n = 12) had numerically increased prevalence of vitamin C deficiency (41.7% vs 27.7%, P = 0.3). Similarly, CD patients with clinically active disease had numerically higher rates of deficiency (30.2% vs 20.2%, P = 0.1). CD patients with small bowel involvement did not have higher rates of vitamin C deficiency compared to those without small bowel involvement (23.0% vs 30.6%, P = 0.3).
In small subgroups of UC patients with available data, numerical differences persisted between those with elevated CRP (n = 13) and those with elevated fecal calprotectin (n = 4) when compared to those without elevated biomarkers (CRP: 30.8% vs 15.2%, P = 0.2; fecal calprotectin: 25.0% vs 9.1%, P = 0.4). In a small subset of UC patients with available endoscopic data, those with endoscopically severe disease (n = 8) had numerically increased frequency of vitamin C deficiency (25.0% vs 10.7%, P = 0.3). However, clinically active disease was not associated with higher rates of vitamin C deficiency when compared to those with quiescent disease (15.8% vs 15.3%, P = 0.9).
When comparing patients with and without vitamin C deficiency, there was no difference in the presence of one or more clinical features of scurvy (66.2% vs 58.5%, P = 0.3, Table 3). Furthermore, both groups had similar rates of arthritis, cutaneous findings, easy bruising, gingivitis, perifollicular findings and alopecia. Patients with vitamin C deficiency were more likely to report fatigue than those with normal vitamin C levels (43.1% vs 27.5%, P = 0.02). Moreover, vitamin C deficient patients were more likely to report poor wound healing (4.6% vs 0.4%, P = 0.03).
| Vitamin C deficiency (n = 65) | Normal vitamin C level (n = 236) | P value | |
| Presence of ≥ 1 clinical feature(s) of scurvy | 43 (66.2%) | 138 (58.5%) | 0.3 |
| None | 22 (33.8%) | 98 (41.5%) | - |
| Fatigue | 28 (43.1%) | 65 (27.5%) | 0.021 |
| Arthritis/arthralgias | 27 (41.5%) | 96 (40.7%) | 0.9 |
| Skin findings (rash, hyperpigmentation) | 9 (13.8%) | 29 (12.3%) | 0.7 |
| Easy bruising | 6 (9.2%) | 9 (3.8%) | 0.1 |
| Gingivitis | 3 (4.6%) | 6 (2.5%) | 0.4 |
| Poor wound healing | 3 (4.6%) | 1 (0.4%) | 0.031 |
| Perifollicular findings (hemorrhage, folliculitis) | 2 (3.1%) | 1 (0.4%) | 0.1 |
| Alopecia | 1 (1.5%) | 5 (2.1%) | 1.0 |
Though many consider scurvy a historical disease of seafarers, the current study demonstrates that vitamin C deficiency affects a significant minority of IBD patients. In 301 patients, 21.6% of IBD patients had vitamin C deficiency, including 24.4% of CD patients and 16.0% of UC patients. This is approximately three-fold higher than the prevalence of vitamin C deficiency in the overall United States population[8].
Strikingly, in IBD patients with elevated objective markers of inflammation, such as CRP and fecal calprotectin, vitamin C deficiency rates ranged from 39%-50%. Similarly, CD patients with penetrating phenotype had higher deficiency rates. On multivariate analysis, the association between elevated CRP and vitamin C deficiency persisted. To our knowledge, this study uniquely examines the relationship between objectively quantified intestinal inflammation (using endoscopy, n = 113, or fecal calprotectin, n = 80) and vitamin C deficiency in a large cohort. Subgroup analysis in patients with available endoscopic data was concordant with biomarker data, with numerically higher rates of deficiency in those with significant intestinal inflammation. In UC, biomarkers and endoscopic data were more limited, with few patients in groups with elevated CRP, fecal calprotectin and endoscopic inflammation available. Absolute rates of deficiency were non-significantly lower in UC, but numerical differences between UC patients with and without inflammation were similar to these differences in CD. Notably, even in patients without objective evidence of inflammatory disease - based on CRP, calprotectin, and endoscopic score - rates of deficiency ranged from 17%-23%.
This study is the largest to date to report on the prevalence of vitamin C deficiency in IBD. Ad
While previous studies report inadequate vitamin C intake in UC[31], to our knowledge, there are no prior studies describing proportions with vitamin C deficiency in UC. Vitamin C deficiency would be biologically plausible in CD as CD often affects the primary sites of vitamin C absorption in the small bowel. Interestingly, in UC patients (without small bowel disease), 16% had vitamin C deficiency. While dietary data was not available in this study, avoidance of vitamin C rich foods likely contributed to the development of vitamin C deficiency in patients with UC, as has been reported in previous studies[31]. Moreover, patients with UC often have elevated TNF-α, which has been shown to downregulate transporters involved in vitamin C uptake[13,14].
The current study found penetrating disease to be associated with vitamin C deficiency. Previously, development of metabolic bone disease has been associated with a penetrating phenotype[32], although few studies have commented on the development of micronutrient deficiency in CD patients with penetrating disease. Penetrating disease often involves the small bowel and also likely reflects more active, refractory CD. Thus, patients with penetrating phenotypes may be at higher risk of both malabsorption - via inflamed tissue and enteric fistulas - and poor consumption - via dietary avoidance of foods rich in vitamin C that may exacerbate symptoms. These data are consistent with CRP and fecal calprotectin elevations also being associated with deficiency. Interestingly, presence of small bowel disease was not associated with increased risk of vitamin C deficiency in CD, despite the jejunum and ileum being the primary sites of vitamin C absorption. However, historical disease location may have been confounded by patients with inactive small bowel disease. Further studies on patients with active small intestinal disease would be required prior to concluding a lack of association between disease location and deficiency status.
While obesity and biologic medication use have biologic plausibility for deficiency and were associated on univariate analysis[8,13,14,33], the study results in aggregate more strongly support active IBD being associated with vitamin C deficiency. Only CRP was associated with vitamin C deficiency on multivariate analysis. However, it should be noted that in non-IBD populations[8], obesity has been shown to be associated with higher rates of vitamin C deficiency. Prior studies suggest that increased access to low-cost, high-calorie, micronutrient-poor food may explain the association between obesity and multiple vitamin deficiencies[33]. The association of current biologic medication use and vitamin C deficiency is less clear and may be due to this being a marker of a more severe disease course. In a subgroup analysis of patients using biologic therapy, patients on TNF-α inhibitors had higher rates of deficiency compared to those on non-TNF-α agents (i.e., vedolizumab, ustekinumab, etc.), which runs counter to our understanding of TNF-α in vitamin C deficiency. TNF-α is known to downregulate transcription of transporters required for vitamin C uptake[13,14], and thus, one might expect that patients using TNF-α inhibitors would have lower, not higher proportions of deficiency. This further supports the use of anti-TNF agents or biologics as a surrogate for disease severity. Future studies may be warranted to better investigate this mechanism.
This study also highlights the difficulty in making a diagnosis of vitamin C deficiency in patients with IBD. In our cohort, there was no difference in the presence of clinical features of scurvy in patients with vitamin C deficiency compared to those with normal vitamin C levels. Many sequelae of vitamin C deficiency are nonspecific and can mimic or coexist with active IBD, including fatigue, arthralgias, oral lesions, bleeding, poor wound healing, anemia, and iron deficiency[15]. Unfortunately, more specific findings in scurvy, such as perifollicular hemorrhage and follicular hyperkeratosis, occur in only a small minority of vitamin C deficient patients, as our study reiterates. Given the challenge of diagnosing scurvy in this population, providers should have a low threshold to test for vitamin C deficiency and counsel on adequate vitamin C intake. Unlike the relapsing and often refractory nature of IBD in many patients, vitamin C supplementation can lead to rapid resolution of symptoms, including some incorrectly ascribed to IBD. Even in IBD patients with unmeasured vitamin C levels, empiric supplementation is not unreasonable, given vitamin C’s role as an antioxidant, preventing free radical damage and reducing extracellular oxidants[24]. However, future studies demonstrating that vitamin C supplementation can decrease inflammatory burden or improve clinical symptoms would be necessary prior to recommending empiric supplementation as standard of care for this population.
Study limitations include the use of retrospective chart review. This study did not find an association between clinical disease severity and vitamin C deficiency. However, clinical disease indices, particularly in CD, poorly correlate with mucosal disease[34]. Though this study examined the relationship between endoscopic activity and vitamin C deficiency, analyses on this relationship were limited by the small number of patients with significant endoscopic inflammation (n = 20). Yet, numerical differences based on endoscopic inflammation were consistent with CRP and fecal calprotectin data, suggesting that intestinal inflammation impacts vitamin C deficiency. Multivariate analyses utilized a single objective marker of inflammation to avoid multicollinearity. CRP was selected as few patients had elevated fecal calprotectin (n = 20) or significant endoscopic inflammation (n = 20), whereas 277 patients had CRP data available. Additionally, given the retrospective nature of our study, data are restricted to patients who had plasma vitamin C measurements available; these patients were not necessarily being screened for deficiency. Selection bias may exist as such laboratory values may have restricted the population to those more prone to have vitamin C deficiency. Nonetheless, nearly 40% of our study population had no symptoms of scurvy when their vitamin C level was obtained, indicating a sizable component of our cohort were simply being monitored for standard nutritional deficiencies. An additional limitation of this study was that measurements of all micronutrients were not performed. The retrospective nature of our study also limits our examination of whether inadequate consumption was associated with higher rates of deficiency, or whether fasting status at serum collection impacted vitamin C level, as dietary data was not available. The use of chart review to assess for symptoms of vitamin C deficiency is also a limitation that may have led to under-detection of symptoms related to deficiency. Some providers may not routinely screen for symptoms related to scurvy (i.e., gingivitis, alopecia, etc.) and these items may not be reflected in providers’ notes. Thus, reporting bias may exist. Despite this, vitamin C deficiency symptoms were infrequently documented. Lastly, this cohort was comprised of patients at an IBD center affiliated with a tertiary care center. Thus, the subjects in this study may have more severe disease, potentially impacting the generalizability of these data.
The current study demonstrates that vitamin C deficiency exists in a significant portion of patients with IBD, particularly those with objective markers of active luminal or penetrating disease. Clinical features of scurvy did not differ between patients with and without deficiency, reinforcing the challenge of diagnosing scurvy in this population, as symptoms of vitamin C deficiency and IBD may overlap. In summary, vitamin C deficiency exists in a considerable fraction of IBD patients. Thus, identifying and treating this easily reversible condition in these patients is essential.
Patients with inflammatory bowel disease (IBD) are prone to several nutritional deficiencies, including iron, vitamin B12 and vitamin D. However, there is a lack of data on vitamin C deficiency in this population, as well as the impact of clinical, biomarker and endoscopic disease severity on the development of vitamin C deficiency.
As IBD patients are already at risk of malnutrition and as vitamin C deficiency is an easily reversible condition, it would be valuable to understand the prevalence of and factors associated with vitamin C deficiency in this population.
The primary objective assessed the prevalence of vitamin C deficiency in IBD patients. Secondary objectives evaluated proportions with deficiency between active and inactive IBD - using clinical, laboratory and endoscopic data - to better identify those at risk of deficiency.
In this retrospective study, clinical, laboratory and endoscopic data were collected from all Crohn’s disease (CD) and ulcerative colitis (UC) patients who had available plasma vitamin C levels presenting to the IBD clinic at a single tertiary care center from 2014 to 2019. Of 353 subjects who met initial search criteria using a cohort discovery tool, 301 ultimately met criteria for inclusion in the study. The primary aim described vitamin C deficiency (≤ 11.4 μmol/L) rates in IBD, with secondary analyses comparing proportions with deficiency between active and inactive IBD. Multivariate logistic regression analysis evaluated factors associated with deficiency.
In 301 IBD patients, 21.6% had vitamin C deficiency, including 24.4% of CD and 16.0% of UC patients. Patients with elevated C-reactive protein (CRP) (39.1% vs 16.9%, P < 0.001) and fecal calprotectin (50.0% vs 20.0%, P = 0.009) had higher proportions of deficiency compared to those without. Other factors associated with vitamin C deficiency included the presence of penetrating disease (P = 0.03), obesity (P = 0.02) and current biologic medication use (P = 0.006). On multivariable analysis, the objective inflammatory marker utilized for analysis (CRP) was the only factor associated with deficiency (odds ratio = 3.1, 95% confidence interval: 1.5-6.6, P = 0.003).
This study provides the largest data on vitamin C deficiency in patients with IBD, uniquely assesses factors associated with deficiency and provides rigorous assessment of inflammatory status using objective markers. Vitamin C deficiency was common in IBD, particularly those with objective markers of active luminal or penetrating disease. As vitamin C deficiency exists in over one-fifth of IBD patients, it is essential to identify and treat this easily reversible condition in this population.
Future prospective studies with well characterized cohorts, and data on diet, other micronutrient deficiencies, endoscopic assessment, and vitamin C supplementation, may be warranted to further elucidate factors associated with vitamin C deficiency and the impact of supplementation on clinical course in IBD patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira-Duarte M, Portugal; Rathnaswami A, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2154] [Article Influence: 102.6] [Reference Citation Analysis (1)] |
| 2. | Loftus EV Jr. Update on the Incidence and Prevalence of Inflammatory Bowel Disease in the United States. Gastroenterol Hepatol (N Y). 2016;12:704-707. [PubMed] |
| 3. | Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis. 2012;18:1961-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 5. | Jeejeebhoy KN, Duerksen DR. Malnutrition in Gastrointestinal Disorders: Detection and Nutritional Assessment. Gastroenterol Clin North Am. 2018;47:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Battat R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, Bessissow T, Seidman E, Bitton A. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Battat R, Kopylov U, Byer J, Sewitch MJ, Rahme E, Nedjar H, Zelikovic E, Dionne S, Bessissow T, Afif W, Waters PJ, Seidman E, Bitton A. Vitamin B12 deficiency in inflammatory bowel disease: a prospective observational pilot study. Eur J Gastroenterol Hepatol. 2017;29:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Shaghaghi MA, Kloss O, Eck P. Genetic Variation in Human Vitamin C Transporter Genes in Common Complex Diseases. Adv Nutr. 2016;7:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Amir Shaghaghi M, Bernstein CN, Serrano León A, El-Gabalawy H, Eck P. Polymorphisms in the sodium-dependent ascorbate transporter gene SLC23A1 are associated with susceptibility to Crohn disease. Am J Clin Nutr. 2014;99:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Subramanian VS, Sabui S, Subramenium GA, Marchant JS, Said HM. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: a role for NF-κB-mediated signaling. Am J Physiol Gastrointest Liver Physiol. 2018;315:G241-G248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183:E752-E755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Imes S, Dinwoodie A, Walker K, Pinchbeck B, Thomson AB. Vitamin C status in 137 outpatients with Crohn's disease. Effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hodges P, Gee M, Grace M, Thomson AB. Vitamin and iron intake in patients with Crohn's disease. J Am Diet Assoc. 1984;84:52-58. [PubMed] |
| 19. | Hughes RG, Williams N. Leucocyte ascorbic acid in Crohn's disease. Digestion. 1978;17:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Linaker BD. Scurvy and vitamin C deficiency in Crohn's disease. Postgrad Med J. 1979;55:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2354] [Article Influence: 123.9] [Reference Citation Analysis (2)] |
| 22. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 23. | Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, Marcocci C, Rizzoli R, Sempos CT, Bilezikian JP. Controversies in Vitamin D: Summary Statement From an International Conference. J Clin Endocrinol Metab. 2019;104:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 24. | Granger M, Eck P. Dietary Vitamin C in Human Health. Adv Food Nutr Res. 2018;83:281-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Robitaille L, Hoffer LJ. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr J. 2016;15:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2189] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 27. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 712] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 28. | Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1322] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 29. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2252] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 30. | Danese S, Sandborn WJ, Colombel JF, Vermeire S, Glover SC, Rimola J, Siegelman J, Jones S, Bornstein JD, Feagan BG. Endoscopic, Radiologic, and Histologic Healing With Vedolizumab in Patients With Active Crohn's Disease. Gastroenterology. 2019;157:1007-1018.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 31. | Urbano AP, Sassaki LY, Dorna MS, Carvalhaes MA, Martini LA, Ferreira AL. Nutritional intake according to injury extent in ulcerative colitis patients. J Hum Nutr Diet. 2013;26:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Cravo M, Guerreiro CS, dos Santos PM, Brito M, Ferreira P, Fidalgo C, Tavares L, Pereira AD. Risk factors for metabolic bone disease in Crohn's disease patients. Inflamm Bowel Dis. 2010;16:2117-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Via M. The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012;2012:103472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Falvey JD, Hoskin T, Meijer B, Ashcroft A, Walmsley R, Day AS, Gearry RB. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |