Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4716
Peer-review started: April 6, 2022
First decision: June 27, 2022
Revised: July 15, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: August 28, 2022
Processing time: 141 Days and 21.9 Hours
The clinical management and prognosis differ between benign and malignant solid focal liver lesions (FLLs), as well as among different pathological types of malignant FLLs. Accurate diagnosis of the possible types of solid FLLs is important. Our previous study confirmed the value of shear wave elastography (SWE) using maximal elasticity (Emax) as the parameter in the differential diagnosis between benign and malignant FLLs. However, the value of SWE in the differential diagnosis among different pathological types of malignant FLLs has not been proved.
To explore the value of two-dimensional SWE (2D-SWE) using Emax in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs.
All the patients enrolled in this study were diagnosed as benign, malignant or undetermined FLLs by conventional ultrasound. Emax of FLLs and the periphery of FLLs was measured using 2D-SWE and compared between benign and malignant FLLs or among different pathological types of malignant FLLs.
The study included 32 benign FLLs in 31 patients and 100 malignant FLLs in 96 patients, including 16 cholangiocellular carcinomas (CCCs), 72 hepatocellular carcinomas (HCCs) and 12 liver metastases. Thirty-five FLLs were diagnosed as undetermined by conventional ultrasound. There were significant differences between Emax of malignant (2.21 ± 0.57 m/s) and benign (1.59 ± 0.37 m/s) FLLs (P = 0.000), and between Emax of the periphery of malignant (1.52 ± 0.39 m/s) and benign (1.36 ± 0.44 m/s) FLLs (P = 0.040). Emax of liver metastases (2.73 ± 0.99 m/s) was significantly higher than that of CCCs (2.14 ± 0.34 m/s) and HCCs (2.14 ± 0.46 m/s) (P = 0.002). The sensitivity, specificity and accuracy were 71.00%, 84.38% and 74.24% respectively, using Emax > 1.905 m/s (AUC 0.843) to diagnose as malignant and 23 of 35 (65.74%) FLLs with undetermined diagnosis by conventional ultrasound were diagnosed correctly.
Malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
Core Tip: Two-dimensional shear wave elastography (2D-SWE) could address the limitations of point SWE and provide overall elastic information in tumors and locate the stiffest part of the tumors. We used 2D-SWE with maximal elasticity to measure the stiffness of focal liver lesions (FLLs) and to explore the value of 2D-SWE in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs. Our results showed malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was useful complement to conventional ultrasound for the differential diagnosis of FLLs.
- Citation: Guo J, Jiang D, Qian Y, Yu J, Gu YJ, Zhou YQ, Zhang HP. Differential diagnosis of different types of solid focal liver lesions using two-dimensional shear wave elastography. World J Gastroenterol 2022; 28(32): 4716-4725
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4716
The clinical management and prognosis differ between benign and malignant solid focal liver lesions (FLLs), and among different pathological types of malignant FLLs[1-3]. Accurate diagnosis of solid FLLs, not only as benign or malignant FLLs, but also the possible types of malignant FLLs, is important[4-6].
Ultrasound elastography, especially two-dimensional shear wave elastography (2D-SWE) which provides quantitative information about tissue stiffness, is a useful tool to differentiate malignant from benign FLLs[7,8]. Our previous study confirmed the value of SWE in the differential diagnosis between benign and malignant FLLs[9].
However, the value of SWE in the differential diagnosis among different pathological types of malignant FLLs has not been proved. Park et al[10] showed that cholangiocellular carcinoma (CCC) had the lowest shear wave velocity, followed by liver metastases, and hepatocellular carcinoma (HCC) had the highest shear wave velocity; whereas Gerber et al[11] showed that CCC had the highest stiffness and liver metastases had the lowest.
Considering the inhomogeneity of tumor stiffness (especially malignant tumors), we used maximal elasticity (Emax) as the parameter to evaluate the stiffness of FLLs and obtained promising results[9]. In the present study, we used 2D-SWE with Emax as the parameter to measure the stiffness of FLLs and to explore the value of SWE with Emax in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs.
This was a prospective study that was approved by the institutional ethical review board of our hospital. All patients provided written informed consent before ultrasound examination.
Inpatients in the department of Hepatobiliary Surgery between November 2021 and January 2022 who met the following criteria were included: (1) One or more solid FLLs shown on conventional ultrasound with a maximal lesion depth < 8 cm; and (2) The targeted FLL showed as green on quality mode, indicating good quality of SWE. The exclusion criteria were: (1) Patients refused to join the study; and (2) No definite final diagnosis; malignant tumors except for liver metastases had to be diagnosed pathologically after surgery; benign tumors or liver metastases could be diagnosed pathologically or by contrast-enhanced ultrasound (CEUS) together with contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) 1 wk before or after 2D-SWE. We included 132 solid FLLs in 127 patients. The gender, age and history of malignancy were recorded.
An Acuson Sequoia diagnostic ultrasound machine (Siemens Medical Solutions, Mountain View, CA, United States) and a transabdominal 5C1 probe were used for conventional ultrasound examination. Before the examinations, all patients fasted for at least 8 h.
One ultrasound physician with > 15 years’ experience in conventional ultrasound performed the hepatic ultrasound scan to detect FLLs. The length and width of every lesion were measured to calculate the volume according to the formula[12]: V = 0.5 LW2 (V: Volume; L: Length; W: Width). A diagnosis as benign, malignant or undetermined was made for each lesion by the ultrasound physician according to the ultrasound features including the number, size, shape, boundary, echogenicity and blood flow of the lesion and surrounding hepatic echogenicity.
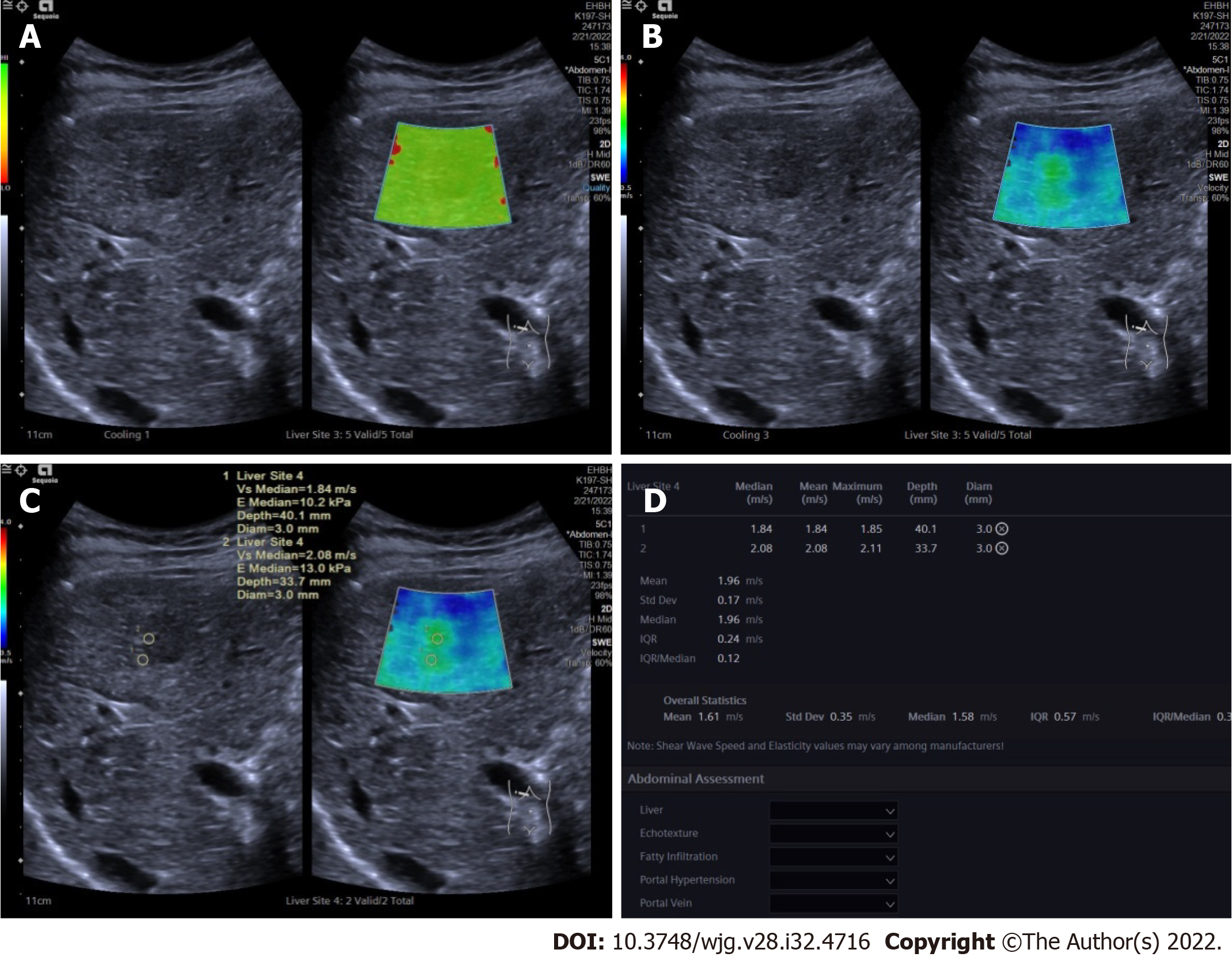
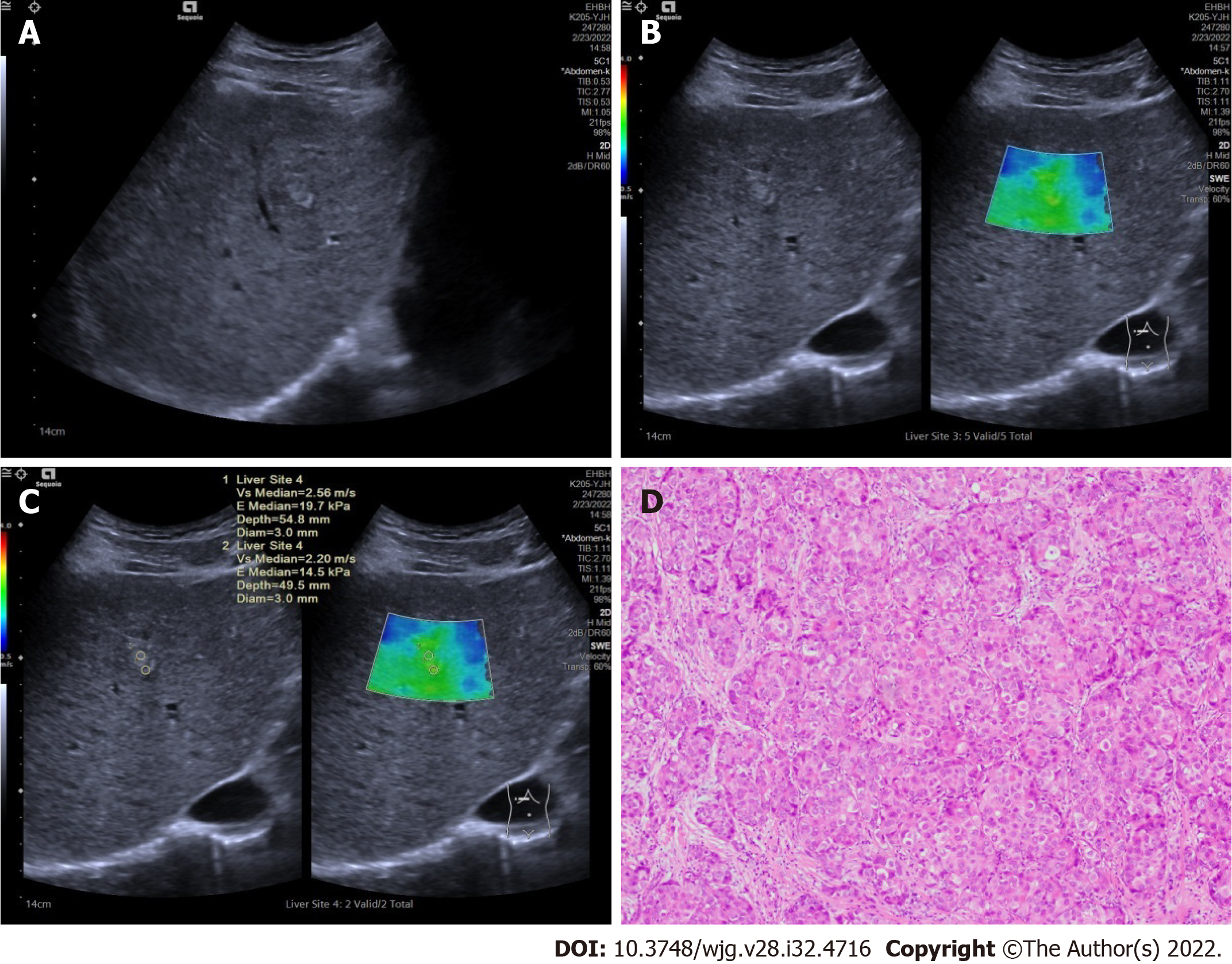
After conventional ultrasound examination, another ultrasound physician who was well trained in ultrasound elastography performed all the 2D-SWE examinations using the same ultrasound equipment and probe, putting no pressure on the skin through the probe. The patients were asked to lie flat on the examination bed. The targeted FLL was shown clearly on the screen and the 2D-SWE mode was activated. A region of interest (ROI) was drawn including the whole or partial tumor and some surrounding liver tissue. If the tumor showed green on quality mode (Figure 1A), it meant that the quality of the 2D-SWE image was up to standard. In velocity mode, we set the speed bar at 0.5-4.0 m/s (Figure 1B); drew two circular ROIs (with 3 mm diameter) to be placed at the stiffest site in the tumor and at the periphery of the tumor (Figure 1C). The maximum values of the two ROIs were recorded (Figure 1D) as Emax for further analysis.
SPSS version 13.0 (IBM Corporation, Chicago, IL, United States) was used for statistical analysis. P < 0.05 was considered statistically significant. Normal distribution using the Kolmogorov-Smirnov test was used first for measurement data. Independent sample t test, one way analysis of variance and least significant difference were used for data with normal distribution and the data were reported as mean ± SD. Otherwise, the Mann-Whitney or Kruskal-Wallis test was used and the data were reported as interquartile range. The cut-off point of Emax was calculated by a receiver operating characteristic curve. The enumerative data were presented as numbers and percentage and Pearson’s chi-square test and Fisher’s exact test were used for statistical analysis.
There were 132 solid FLLs in 127 patients (80 men and 47 women; aged 24-77 years; mean age 54.23 ± 12.04 years) in this study, including 32 benign FLLs in 31 patients (6 focal nodular hyperplasia, 15 hemangioma and 11 diagnosed definitely as benign but without pathological results) and 100 malignant FLLs in 96 patients (16 CCCs, 72 HCCs and 12 liver metastases). The differences in age, gender and history of malignancy between the malignant and benign groups are shown in Table 1. Compared with patients with benign FLLs, patients with malignant FLLs were older and more often male. The history of malignancy had no significant difference between the two groups. The differences in age, gender and history of malignancy among three types of malignancy are shown in Table 2. The age and gender had no significant differences among the three groups, although the age of patients with liver metastases was significantly higher than that of patients with HCC. Patients with liver metastases were more often had a history of malignancy, compared with patients with primary liver cancer, including HCC and CCC.
| Age (yr) | Gender | History of malignancy | |||
| Male | Female | Yes | No | ||
| Malignant (n = 96) | 44.68 ± 12.30 | 73 | 23 | 11 | 85 |
| Benign (n = 31) | 57.31 ± 10.36 | 7 | 24 | 1 | 30 |
| t/χ2 | 5.633 | 28.729 | 1.856 | ||
| P value | 0.000 | 0.000 | 0.291 | ||
Volume of benign FLLs (20.73-113.69 cm3) and malignant FLLs (30.56-79.62 cm3) had no significant difference (Z = 0.263, P = 0.793). Volume of CCCs (39.58-120.22 cm3), HCCs (26.34-75.69 cm3) and liver metastases (32.76-41.67 cm3) had no significant difference (χ2 = 1.869, P = 0.393). For 32 benign FLLs, 12 were diagnosed as benign, 18 as undetermined and two were misdiagnosed as malignant. For 100 malignant FLLs, 81 (12 CCCs, 59 HCCs and 10 metastases) were diagnosed correctly as malignant, 17 were undetermined (4 CCCs, 11 HCCs and 2 liver metastases) and 2 were misdiagnosed as benign (both HCCs). The undetermined rate of conventional ultrasound was 26.52% (35/132). For the FLLs with definite diagnosis by conventional ultrasound, the sensitivity, specificity and accuracy were 85.71%, 97.59% and 95.88%, respectively.
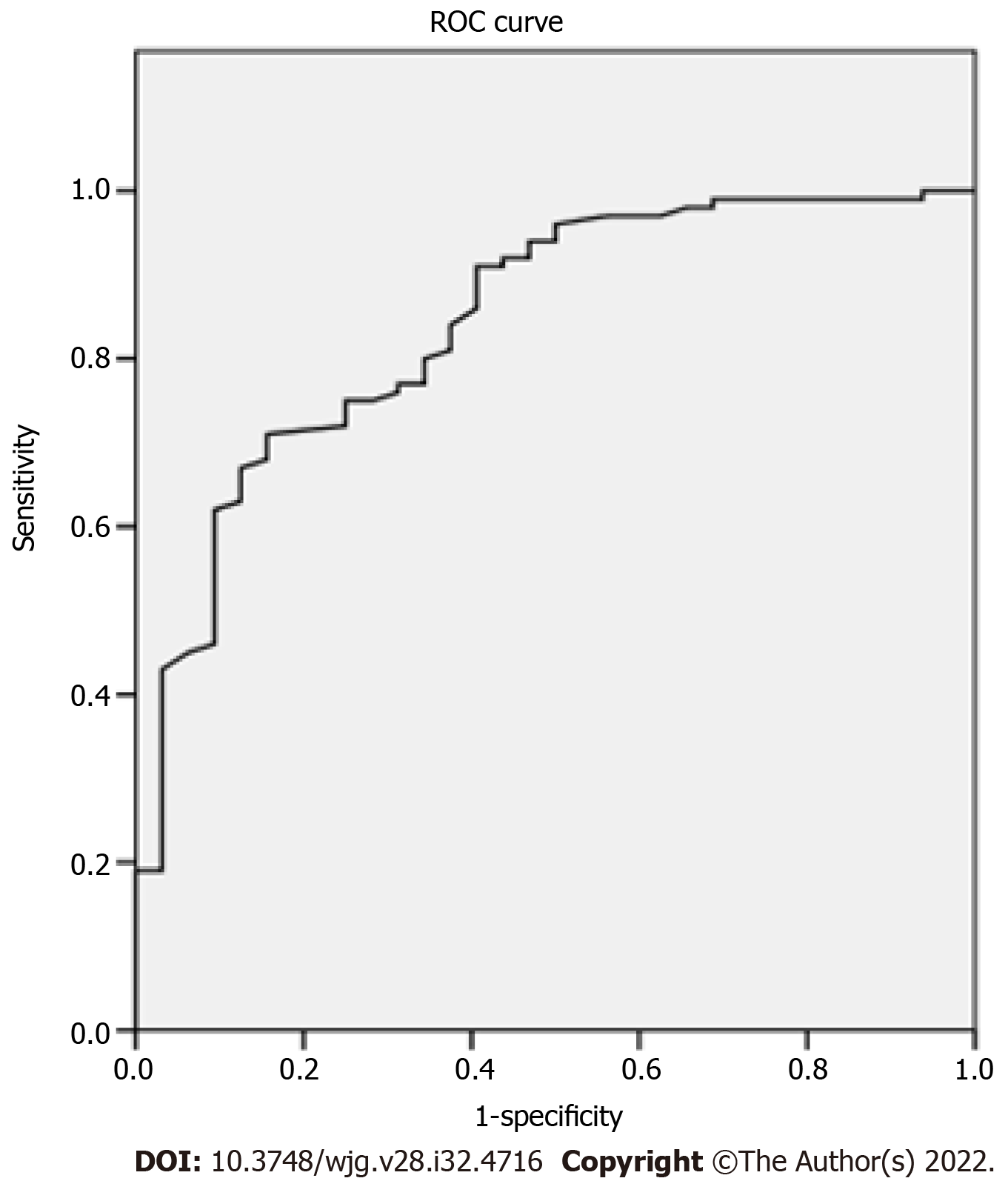
Comparisons between benign and malignant FLLs: The differences in 2D-SWE results between benign and malignant FLLs are shown in Table 3. Emax of malignant FLLs were significantly higher than those of benign FLLs. Emax of the periphery of malignant FLLs were significantly higher than those of benign FLLs. The cut-off point of Emax of the tumors was 1.905 with AUC 0.843 (Figure 2). The sensitivity, specificity and accuracy were 71.00%, 84.38% and 74.24% respectively using Emax > 1.905 m/s for diagnosis as malignant (Figure 3). For 35 FLLs with undetermined diagnosis by conventional ultrasound, 23 (65.71%) FLLs including 14 of 18 benign and nine of 17 malignant FLLs were diagnosed correctly.
| Emax of tumors (m/s) | Emax of periphery of tumors (m/s) | |
| Malignant (n = 100) | 2.21 ± 0.57 | 1.52 ± 0.39 |
| Benign (n = 32) | 1.59 ± 0.37 | 1.36 ± 0.44 |
| t | 5.781 | 2.073 |
| P value | 0.000 | 0.040 |
Comparisons among different types of malignant FLLs: The differences in 2D-SWE results among different types of malignant FLLs are shown in Table 4. There were significant differences among Emax of CCCs, HCCs and liver metastases. Emax of liver metastases was significantly higher than that of CCCs and HCCs. There were no significant differences among Emax values of the periphery of CCCs, HCCs and liver metastases.
In this study, we explored the value of 2D-SWE using Emax as the quantitative parameter in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs. Our results showed that malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
The comparison of patients’ basic information showed that compared with patients with benign FLLs, patients with malignant FLLs tended to be older, male and have a history of malignancy. Among three different types of malignant FLLs, the distribution of patients’ age and gender had no significant difference, while patients with liver metastases were more likely to have a history of malignancy. These results were similar to those in previous studies[13].
Conventional ultrasound is well recognized as the first imaging choice for the screening and diagnosis of FLLs[14]. However, the diagnostic efficiency of conventional ultrasound for the differential diagnosis of benign and malignant FLLs or among different pathological types of malignant FLLs was not good enough. For some FLLs, the diagnosis using conventional ultrasound alone is difficult[15]. That is one of the reasons for the development of new ultrasound technologies, including CEUS and ultrasound elastography. In this study, we diagnosed FLLs as benign, malignant and undetermined by conventional ultrasound and 35 of 132 (26.52%) were diagnosed as undetermined. Further diagnosis using other imaging methods was necessary.
CEUS could provide similar or better diagnostic performance for the differential diagnosis of FLLs without radiation and iodine allergy, compared with contrast enhanced CT[16]. CEUS has particularly high value for the differential diagnosis of complex cystic and cysticlike FLLs[17,18]. In this study, for some benign tumors or liver metastases without pathological diagnosis, the combined results of CEUS together with contrast enhanced CT or contrast enhanced MRI were as final. Compared with CEUS, ultrasound elastography has the advantages of being less expensive and less time-consuming[19].
Our previous study using virtual touch tissue quantification imaging with Emax has proven its value in the differential diagnosis between benign and malignant FLLs[9]. 2D-SWE is one of the most advanced elastography techniques and could provide overall elastic information in tumors and locate the stiffest part[20]. In this study, the cutoff point of Emax was 1.905, which was similar to our previous result[9]. Twenty-three of 35 (65.71%) FLLs with undetermined diagnosis by conventional ultrasound were diagnosed accurately using 2D-SWE with Emax. If FLLs could be diagnosed confidently using conventional ultrasound, no further examination was needed; otherwise, 2D-SWE with Emax could be a useful complement to conventional ultrasound and improve the diagnostic efficiency.
The elastic differences among different types of malignant FLLs are still uncertain. Our results showed that the stiffness of liver metastases was significantly higher than that of primary liver tumors, including CCCs and HCCs, while the stiffness of CCCs and HCCs had no significant differences. This was similar to the study of Park et al[21], which showed that the stiffness of HCCs was significantly lower than that of liver metastases. However, some other studies had the opposite results[10,11]. One study reported that CCCs had the lowest stiffness and HCCs the highest stiffness[10]. Another study found that CCCs had the highest stiffness and liver metastases the lowest[11]. One important reason for the conflicting results is that the sample number in every study was not large enough, and it is necessary to carry out further studies with large samples to confirm our results.
There were some limitations to our study. First, as mentioned above, only a few cases of CCCs and liver metastases were included. Second, no pathological methods were used to confirm the stiffness of the tumors and explain the differences among different pathological types of malignant FLLs.
In conclusion, malignant FLLs were stiffer than benign FLLs and liver metastases were stiffer than primary liver carcinomas. 2D-SWE with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
Conventional ultrasound is the first imaging choice for liver diseases with the diagnostic efficiency not good enough, which promotes the development of new ultrasound technologies, such as contrast enhanced ultrasound and ultrasound elastography. Two dimensional shear wave elastography (2D-SWE) is convenient, less time-consuming and inexpensive. SWE plays an important role in the assessment of liver fibrosis and in the differential diagnosis of benign and malignant focal liver lesions (FLLs). However, its value for the differential diagnosis among different types of malignant FLLs has not been proved.
Though the value of SWE for the differential diagnosis between malignant and benign FLLs was not widely recognized, our previous study showed promising results using maximal elasticity (Emax) as the parameter to differentiate malignant FLLs from benign ones. As the clinical management and prognosis of different pathological types of malignant FLLs are different, it is important to diagnose accurately of the possible pathological types. So, it was necessary to explore the value of 2D-SWE with Emax in differential diagnosis of different pathological types of malignant FLLs.
We aim to explore the value of 2D-SWE using Emax in the differential diagnosis of FLLs, especially among different pathological types of malignant FLLs.
In this study, we diagnosed all FLLs as benign, malignant and undetermined using conventional ultrasound. And the stiffness of FLLs and the periphery of FLLs were evaluated using 2D-SWE and the quantitative parameter Emax. Emax of FLLs and the periphery of FLLs were compared between benign and malignant FLLs or among different pathological types of malignant FLLs.
There were totally 132 FFLs in 127 patients enrolled in the study, including 32 benign FLLs, 16 cholangiocellular carcinomas (CCCs), 72 hepatocellular carcinomas (HCCs) and 12 liver metastases. Thirty-five FLLs were diagnosed as undermined by conventional ultrasound. Emax of malignant FLLs (2.21 ± 0.57 m/s) were significantly higher than those of benign FLLs (1.59 ± 0.37 m/s) (P = 0.000). Emax of the periphery of malignant FLLs (1.52 ± 0.39 m/s) were significantly higher than those of benign FLLs (1.36 ± 0.44 m/s) (P = 0.040). The cut-off point of Emax of the tumors was 1.905 with AUC 0.843. The sensitivity, specificity and accuracy were 71.00%, 84.38% and 74.24% respectively using Emax > 1.905 m/s for diagnosis as malignant and 23 of 35 (65.74%) FLLs with undetermined diagnosis by conventional ultrasound were diagnosed correctly. Emax of liver metastases (2.73 ± 0.99 m/s) was significantly higher than that of primary liver carcinomas, including CCCs (2.14 ± 0.34 m/s) and HCCs (2.14 ± 0.46 m/s) (P = 0.002).
Malignant FLLs were stiffer than benign ones and liver metastases were stiffer than primary liver carcinomas. 2D-SEW with Emax was a useful complement to conventional ultrasound for the differential diagnosis of FLLs.
In this study, we demonstrated the differences of 2D-SWE with Emax between benign and malignant FLLs and among different pathological types of malignant FLLs. Prospective study to explore the value of 2D-SWE with Emax in the evaluatation the different stiffness of liver metastases from different sources or the different stiffness of HCC with different microvascular invasion grade will be necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A, Italy; Giacomelli L, Italy S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Yao J, Liang X, Liu Y, Li S, Zheng M. Trends in Incidence and Prognostic Factors of Two Subtypes of Primary Liver Cancers: A Surveillance, Epidemiology, and End Results-Based Population Study. Cancer Control. 2022;29:10732748211051548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Macias RIR, Banales JM, Sangro B, Muntané J, Avila MA, Lozano E, Perugorria MJ, Padillo FJ, Bujanda L, Marin JJG. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Lang SA, Bednarsch J, Czigany Z, Joechle K, Kroh A, Amygdalos I, Strnad P, Bruns T, Heise D, Ulmer F, Neumann UP. Liver transplantation in malignant disease. World J Clin Oncol. 2021;12:623-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Stollmayer R, Budai BK, Tóth A, Kalina I, Hartmann E, Szoldán P, Bérczi V, Maurovich-Horvat P, Kaposi PN. Diagnosis of focal liver lesions with deep learning-based multi-channel analysis of hepatocyte-specific contrast-enhanced magnetic resonance imaging. World J Gastroenterol. 2021;27:5978-5988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (4)] |
| 5. | Cao SE, Zhang LQ, Kuang SC, Shi WQ, Hu B, Xie SD, Chen YN, Liu H, Chen SM, Jiang T, Ye M, Zhang HX, Wang J. Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrast-enhanced computed tomography. World J Gastroenterol. 2020;26:3660-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 6. | Huf S, Platz Batista da Silva N, Wiesinger I, Hornung M, Scherer MN, Lang S, Stroszczynski C, Fischer T, Jung EM. Analysis of Liver Tumors Using Preoperative and Intraoperative Contrast-Enhanced Ultrasound (CEUS/IOCEUS) by Radiologists in Comparison to Magnetic Resonance Imaging and Histopathology. Rofo. 2017;189:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | da Silva NPB, Hornung M, Beyer LP, Hackl C, Brunner S, Schlitt HJ, Wiggermann P, Jung EM. Intraoperative Shear Wave Elastography vs. Contrast-Enhanced Ultrasound for the Characterization and Differentiation of Focal Liver Lesions to Optimize Liver Tumor Surgery. Ultraschall Med. 2019;40:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wang W, Zhang JC, Tian WS, Chen LD, Zheng Q, Hu HT, Wu SS, Guo Y, Xie XY, Lu MD, Kuang M, Liu LZ, Ruan SM. Shear wave elastography-based ultrasomics: differentiating malignant from benign focal liver lesions. Abdom Radiol (NY). 2021;46:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zhang HP, Gu JY, Bai M, Li F, Zhou YQ, Du LF. Value of shear wave elastography with maximal elasticity in differentiating benign and malignant solid focal liver lesions. World J Gastroenterol. 2020;26:7416-7424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Park H, Park JY, Kim DY, Ahn SH, Chon CY, Han KH, Kim SU. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol. 2013;19:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Gerber L, Fitting D, Srikantharajah K, Weiler N, Kyriakidou G, Bojunga J, Schulze F, Bon D, Zeuzem S, Friedrich-Rust M. Evaluation of 2D- Shear Wave Elastography for Characterisation of Focal Liver Lesions. J Gastrointestin Liver Dis. 2017;26:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1399] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 13. | Lin L, Yan L, Liu Y, Qu C, Ni J, Li H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer. 2020;9:563-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Osho A, Rich NE, Singal AG. Role of imaging in management of hepatocellular carcinoma: surveillance, diagnosis, and treatment response. Hepatoma Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Bartolotta TV, Taibbi A, Midiri M, Matranga D, Solbiati L, Lagalla R. Indeterminate focal liver lesions incidentally discovered at gray-scale US: role of contrast-enhanced sonography. Invest Radiol. 2011;46:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic Performance and Confidence of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Cystic and Cysticlike Liver Lesions. AJR Am J Roentgenol. 2017;209:W119-W127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Corvino A, Catalano O, Setola SV, Sandomenico F, Corvino F, Petrillo A. Contrast-enhanced ultrasound in the characterization of complex cystic focal liver lesions. Ultrasound Med Biol. 2015;41:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Platz Batista da Silva N, Schauer M, Hornung M, Lang S, Beyer LP, Wiesinger I, Stroszczynski C, Jung EM. Intrasurgical dignity assessment of hepatic tumors using semi-quantitative strain elastography and contrast-enhanced ultrasound for optimisation of liver tumor surgery. Clin Hemorheol Microcirc. 2016;64:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kayadibi Y, Ucar N, Kaya MF, Yildirim E, Bektas S. Characterization of Suspicious Microcalcifications on Mammography Using 2D Shear-Wave Elastography. Ultrasound Med Biol. 2021;47:2532-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Shear Wave Elastography of Focal Liver Lesion: Intraobserver Reproducibility and Elasticity Characterization. Ultrasound Q. 2015;31:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |