Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4600
Peer-review started: November 10, 2021
First decision: January 9, 2022
Revised: January 11, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 28, 2022
Processing time: 289 Days and 0.8 Hours
Glycolysis caused by hypoxia-induced abnormal activation of hypoxia inducible factor-1α (HIF-1α) in the immune microenvironment promotes the progression of hepatocellular carcinoma (HCC), leading to enhanced drug resistance in cancer cells. Therefore, altering the immunosuppressive microenvironment by imp-roving the hypoxic state is a new goal in improving cancer treatment.
To analyse the role of HIF-1α, which is closely related to tumour proliferation, invasion, metastasis, and angiogenesis, in the proliferation and invasion of liver cancer, and to explore the HIF-1α pathway-mediated anti-cancer mechanism of sirolimus (SRL) combined with Huai Er.
Previous studies on HCC tissues identified the importance of HIF-1α, glucose transporter 1 (GLUT1), and lactate dehydrogenase A (LDHA) expression. In this study, HepG2 and Huh7 cell lines were treated, under hypoxic and normoxic conditions, with a combination of SRL and Huai Er. The effects on proliferation, invasion, cell cycle, and apoptosis were analysed. Proteomics and genomics techniques were used to analyze the HIF-1α-related signalling pathway during SRL combined with Huai Er treatment and its inhibition of the proliferation of HCC cells.
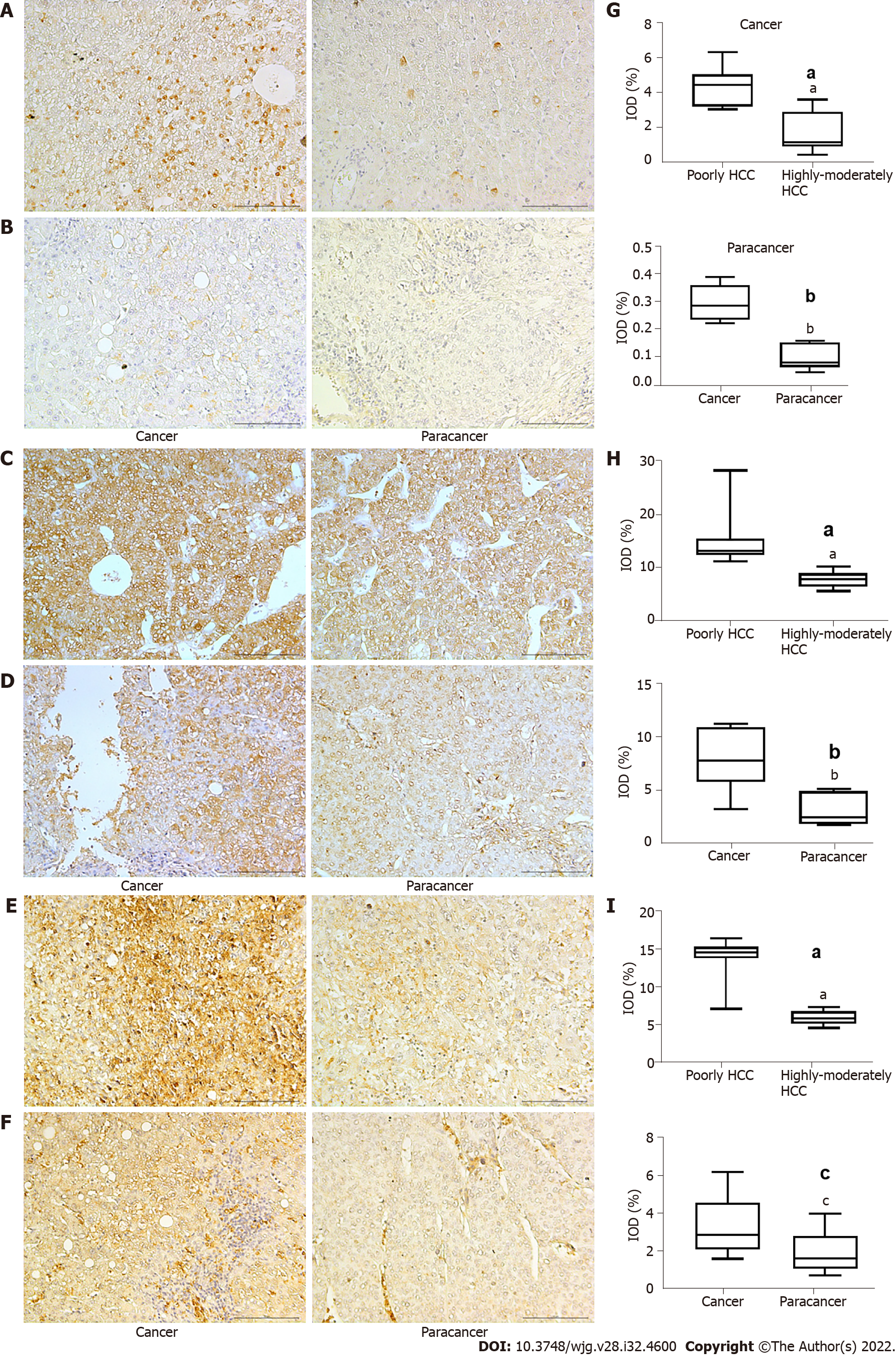
High levels of HIF-1α, LDHA, and GLUT-1 were found in poorly differentiated HCC, with lower patient survival rates. Hypoxia promoted the proliferation of HepG2 and Huh7 cells and weakened the apoptosis and cell cycle blocking effects of the SRL/Huai Er treatment. This was achieved by activation of HIF-1α and glycolysis in HCC, leading to the upregulation of LDHA, GLUT-1, Akt/mammalian target of rapamycin (mTOR), vascular endothelial growth factor (VEGF), and Forkhead box P3 and downregulation of phosphatase and tensin homolog deleted on chromosome ten (PTEN) and p27. The hypoxia-induced activation of HIF-1α showed the greatest attenuation in the SRL/Huai Er (S50 + H8) group compared to the drug treatments alone (P < 0.001). The S50 + H8 treatment significantly downregulated the expression of mTOR and HIF-1α, and significantly reduced the expression of VEGF mRNA. Meanwhile, the combined blocking of mTOR and HIF-1α enhanced the downregulation of Akt/mTOR, HIF-1α, LDHA, and GLUT-1 mRNA and resulted in the downregulation of PTEN, p27, and VEGF mRNA (P < 0.001).
SRL increases the anti-cancer effect of Huai Er, which reduces the promotion of hypoxia-induced HIF-1α on the Warburg effect by inhibition of the PI3K/Akt/mTOR-HIF-1α and HIF-1α-PTEN signalling pathways in HCC.
Core Tip: Hypoxia-mediated glycolysis is associated with poorly differentiated hepatocellular carcinoma (HCC) and a poor prognosis. Hypoxia inducible factor-1α (HIF-1α), induced by hypoxia, promotes the growth of HepG2 and Huh7 cells and weakens the anti-cancer effect of sirolimus (SRL) and Huai Er. SRL increased the anti-cancer effect of Huai Er, which reduced the promotion of hypoxia-induced HIF-1α on the Warburg effect by inhibiting the PI3K/Akt/mammalian target of rapamycin-HIF-1α and HIF-1α-phosphatase and tensin homolog deleted on chromosome ten signalling pathways in HCC.
- Citation: Zhou L, Zhao Y, Pan LC, Wang J, Shi XJ, Du GS, He Q. Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma. World J Gastroenterol 2022; 28(32): 4600-4619
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4600.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4600
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. Its incidence and mortality are increasing annually, particularly in the younger age groups[1]. More than half of the cases are in China, with more than 90% being related to hepatitis B[1]. Therefore, cancer death is the primary cause of death in China, with liver cancer ranking second with 390000 cases[1,2]. Immunosuppressive cells of Forkhead box P3+ (Foxp3+) Tregs, neutrophils, MDSC, and others infiltrate the tumour[3,4]. Hypoxia-induced hypoxia inducible factor-1α (HIF-1α)-mediated glycolysis, infiltration of extracellular matrix, and accumulation of lactic acid in the anoxic microenvironment promote abnormal metabolism of cancer cells. This, combined with abnormal tumour immunity, leads to downregulation of immune surveillance, increased immune escape, and promotion of proliferation, invasion, and metastasis[4].
Hanahan proposed “abnormal energy metabolism“ as the seventh feature of cancer[5], in which the metabolism of tumour cells, mainly represented by abnormal glucose metabolism (Warburg effect) and abnormal lipid metabolism, is closely related to tumour occurrence and metastasis. Recent studies have suggested that abnormal glucose metabolism in cancer is not only related to metabolic enzymes, metabolic pathways, and other related signal transduction pathways, but also to the immune status and local microenvironment of tumour tissues[6-8].
The anoxic environment induces the activation and upregulation of HIF-1α expression which promotes glycolysis, increases glucose transporter 1 (GLUT1) expression, and accelerates tumour cell metabolism[9]. It has been confirmed that HIF-1α can upregulate the activity of glycolytic enzymes by 90%[10] and produce a large amount of lactic acid through metabolism. Lactic acid is transported out of the cells and forms a high lactic acid environment in cancer tissue, leading to metabolic competition with T cells. This further inhibits the function, proliferation, and activation of infiltrated lymphocytes; destroys the killing effect and anti-tumour function; and promotes tumour immune escape[9-12]. However, cancer cells can efficiently use circulating lactic acid to produce glucose by anaerobic glycolysis, and a large number of electrons produced by metabolism adhere to lactic acid to compensate for the voltage instability inside and outside the cell caused by the high-speed electron movement of cancer cells, while ensuring the steady-state demand for energy and microenvironment for rapid growth and proliferation[10-12].
HIF-1α induction by Akt/mammalian target of rapamycin (mTOR)-mediated growth factors only involves the translation of mRNA under normoxia[13]; however, the regulatory mechanism of PI3K/Akt/mTOR on HIF-1α and the activation mechanism of PI3K are not clear under hypoxia. There may be alternative regulated signalling pathways because our results showed that the PI3K/Akt inhibitor, LY294002, cannot completely inhibit the expression of HIF-1α. Inhibiting the expression of HIF-lα and blocking the transmission of oxygen deficiency signals have become a new target for tumour therapy. Huai Er, also known as Trametes robiniophila Murr, is a traditional Chinese medicine. Various studies have demonstrated that Huai Er inhibits cancer progression and improves patient prognosis[14,15]. The purpose of this study was to explore the regulatory mechanism of sirolimus (SRL) and Huai Er on HepG2 cell proliferation caused by abnormal activation of the HIF-1α pathway induced by hypoxia and to provide a theoretical basis for new clinical treatments for liver cancer.
The hepatoma cell lines, HepG2 and Huh 7, were purchased from the Institute of Basic Medicine, Chinese Academy of Medical Sciences. Liver tissue was obtained from the pathological laboratory with written informed consent obtained in accordance with the Declaration of Helsinki of the World Medical Association. The study complied with the Institutional Guidelines for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of Beijing Chaoyang Hospital (No. 2021-1-19-3) and PLA General Hospital (S2108-013-01). The experimental flowchart is shown in Supple
HepG2 and Huh 7 cells were cultured at a density of 1 × 103/well with 100 μL complete medium (10% fetal bovine serum + RMPI-1640, Gibco, United States) overnight for 24 h, then treated with SRL, Huai Er, Ly294002 (Ly), and KC7F2 (KC7) for 24-48 h, and a mixture of 50 μL 1 × PBS and 20 μL MTS (Promega, United States) was added and co-cultured for 2-4 h. The OD value at 490 nm was used to calculate the IC50 values of the drugs and blockers.
HepG2 and Huh7 cells were cultured at a density of 1 × 106/well for 24 h before scratching with a “cross” and culturing with 2 mL of treatment solution. Imaging identified the clear position of scratches, and images were acquired at 0 h, 24 h, and 48 h, the position of the scratch was marked, and the scratch images were analysed using Image J[16]. The treatment groups were as follows: Huai Er (H) (Gaitianli Co.), SRL (S) (Sigma, United States), Ly294002 (Ly) (TargetMol, United States), KC7F2 (KC7) (TargetMol, United States), H + S, H + Ly, H + KC7, S + Ly, S + KC7, Ly + KC7, and S + H + Ly + KC7.
HepG2 and Huh7 cells at a density of 500/well were cultured for 24 h, the culture medium was then changed before the addition of SRL, Huai Er, hypoxia (simulated by 200 μM CoCl2, Sigma, United States), and SRL + Huai Er, and the cells were cultured for 10 d. After washing with 1 × PBS, the cells were fixed with 1% paraformaldehyde, stained with crystal violet, and photographed to observe the size of colonies.
Preparation of single-cell suspension: A single-cell suspension was prepared using 0.25 % non-EDTA trypsin (Gibco, United States) to digest the cultured cells treated with Huai Er, SRL, and Huai Er + SRL.
Apoptosis detection: The prepared single-cell suspension was co-incubated with 5 μL of fluorescein isothiocyanate labelled Annexin V antibody and 2.5 μL of phycoerythrin-labelled PI antibody (BD, United States) for 15 min in a dark room at room temperature, washed with 1 ml precooled 1 × Binding Buffer, and centrifuged at 1200 rpm/min for 5 min, and the supernatant was discarded. The apoptosis rates of HepG2 and Huh 7 cells were determined by flow cytometry.
Cell cycle detection: The prepared single-cell suspension was fixed with 1 mL of 70% precooled ethanol at 4 °C for 12 h. After centrifugation at 1000 g for 5 min, the supernatant was discarded and the cell pellet was washed with 1 mL of precooled 1 × PBS, with 500 μL of PI/RNase staining buffer (BD Biosciences, United States) added for 106-107 cells to re-suspend cells for detection.
Paraffin-embedded tissues were sliced into thin tissue sections of 5 μm and heated at 60 °C for 30 min. The sections then underwent a dewaxing step, 3% hydrogen peroxide inactivation, antigen retrieval in a microwave at 100 °C for 20 min, and blocking for 30 min. Next, the cells were incubated with primary antibodies against HIF-1α, lactate dehydrogenase A (LDHA), and GLUT-1 (1:100) (Proteintech, United States) overnight. The cells were then incubated with the secondary antibody from PV-9000 kits (OriGene Technologies, United States) for 60 min, before staining with the DAB kit (Vector, United States) under a Nikon microscope system.
Cultured cancer cells were lysed by trypsinisation at 4 °C and the total protein was extracted. Protein purity was determined using a bicinchoninic acid assay, according to the manufacturer’s instructions and Western blot analysis was performed.
Total RNA was extracted using the TRIzol reagent (Life Technologies, Carlsbad, CA, United States), and the OD value was determined using a spectrophotometer. Primers were designed using the NCBI online primer design software Primer-BLAST and synthesised by Beijing Jingke Xinye Biotechnology Co., Ltd (China). The names of the primers and the corresponding sequence information are listed in Supplementary Table 1. Then, 24 μL of the reaction system was prepared for cDNA synthesis and stored at -20 °C for later use. After that, a 20 μL reaction system was prepared, and then instantaneous centrifugation was performed in the fluorescence quantitative polymerase chain reaction (PCR) instrument to carry out the reverse transcription (RT)-PCR reaction according to the manufacturer’s protocol (Life Technologies, United States).
Images from an optical microscope were captured and stored, and the results of flow cytometry were analysed using FlowJo10.0 software. The data were analysed using SPSS 22.0. Each index is expressed as the mean ± SD, and the difference between two groups was tested using the t-test. Statistical significance was set at P < 0.05. Single-factor ANOVA was used to determine statistical differences among different experimental groups. Differences were considered significant at P < 0.05.
We identified high expression levels of HIF-1α in patients with poorly differentiated HCC, with expression being higher in the cancer tissue than in para-carcinoma tissue (Figure 1A, B and G) (P < 0.001). Higher levels of GLUT-1 (P < 0.05) and LDHA (P < 0.001) were identified in cancer tissues compared to para-carcinoma tissues, indicating an enhanced glycolytic effect (Warburg effect); these higher levels of expression were also observed in patients with poorly differentiated HCC compared to those with moderately differentiated HCC (Figure 1C-F, H and I) (P < 0.001). The high levels of HIF-1α, GLUT-1, and LDHA were all associated with poorer survival outcomes (P < 0.01). Based on these results and our previous research on the effect of Huai Er and SRL on HCC in a rat model[15], we explored the effect of Huai Er combined with SRL on the glucose metabolism pathway in HepG2 and Huh7 cell lines, to determine the synergistic mechanism of tumour growth inhibition.
The suppression of proliferation of HepG2 and Huh7 cells increased gradually with the administration of SRL at concentrations ranging from 0.1 nM to 1000 nM. A gradual increase was also observed on the administration of Huai Er at concentrations ranging from 4 mg/mL to 8 mg/mL. The optimal inhibitory concentration was identified as 50 nM for SRL (S50) and 8 mg/mL for Huai Er (H8), using IC50 curve calculation. An optimal inhibitory concentration of 25 μM was calculated for Ly294002 (a specific inhibitor of PI3K) (Ly25) and 20 μM for KC7F2 (a selective inhibitor of HIF-1α) (KC7). These optimal concentrations were used in the following study with S50, H8, Ly25, and KC7 groups.
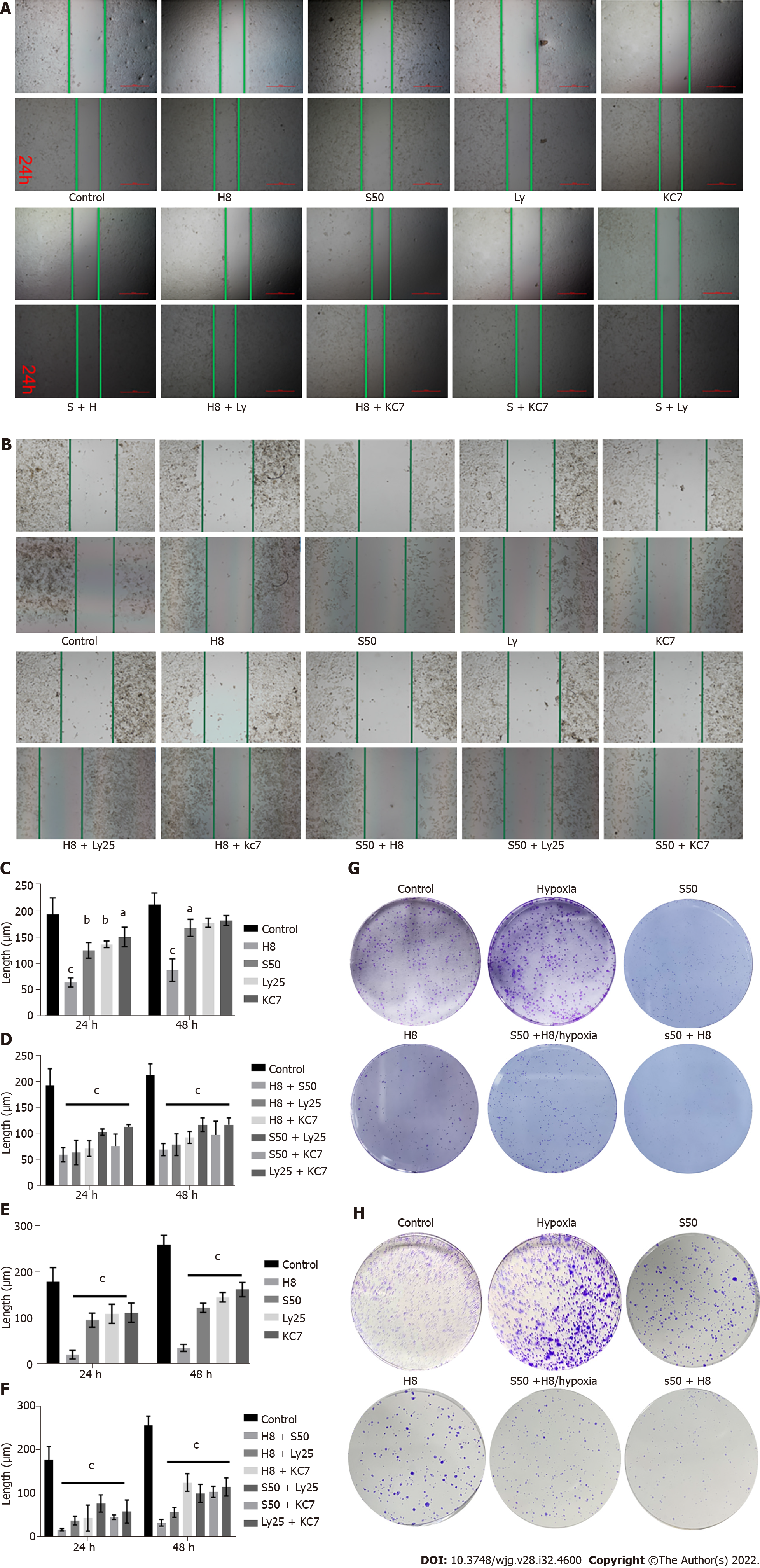
The scratch test results showed that H8 treatment significantly inhibited the invasion of HepG2 and Huh 7 cells compared to S50 treated cells and the control group (Figure 2A and B). The addition of S50 enhanced the inhibitory effect of the H8 treatment, showing a greater inhibitory effect on invasion compared to the monotherapies (Figure 2C and E).
Compared with the control group, the combination of any two drugs, Huai Er, SRL, Ly25, or KC7, significantly inhibited the invasion of cancer cells (P < 0.001) (Figure 2A, B, D and F). Huai Er application combined with S50, Ly25, or KC7 revealed a stronger inhibitory effect on the invasion of HepG2 and Huh 7 cells than other combined applications, with the strongest effect being noted in the H8 + S50 treatment group (P < 0.001) (Figure 2A, B, D and F).
High proliferation of HepG2 and Huh7 cells was observed when maintained in a hypoxic environment at an appropriate concentration of CoCl2, compared to when maintained under normoxic conditions (Figure 2G and H). The inhibitory effect of H8 + S50 on the proliferation of these cells was weakened after culturing under hypoxia (Figure 2G and H).
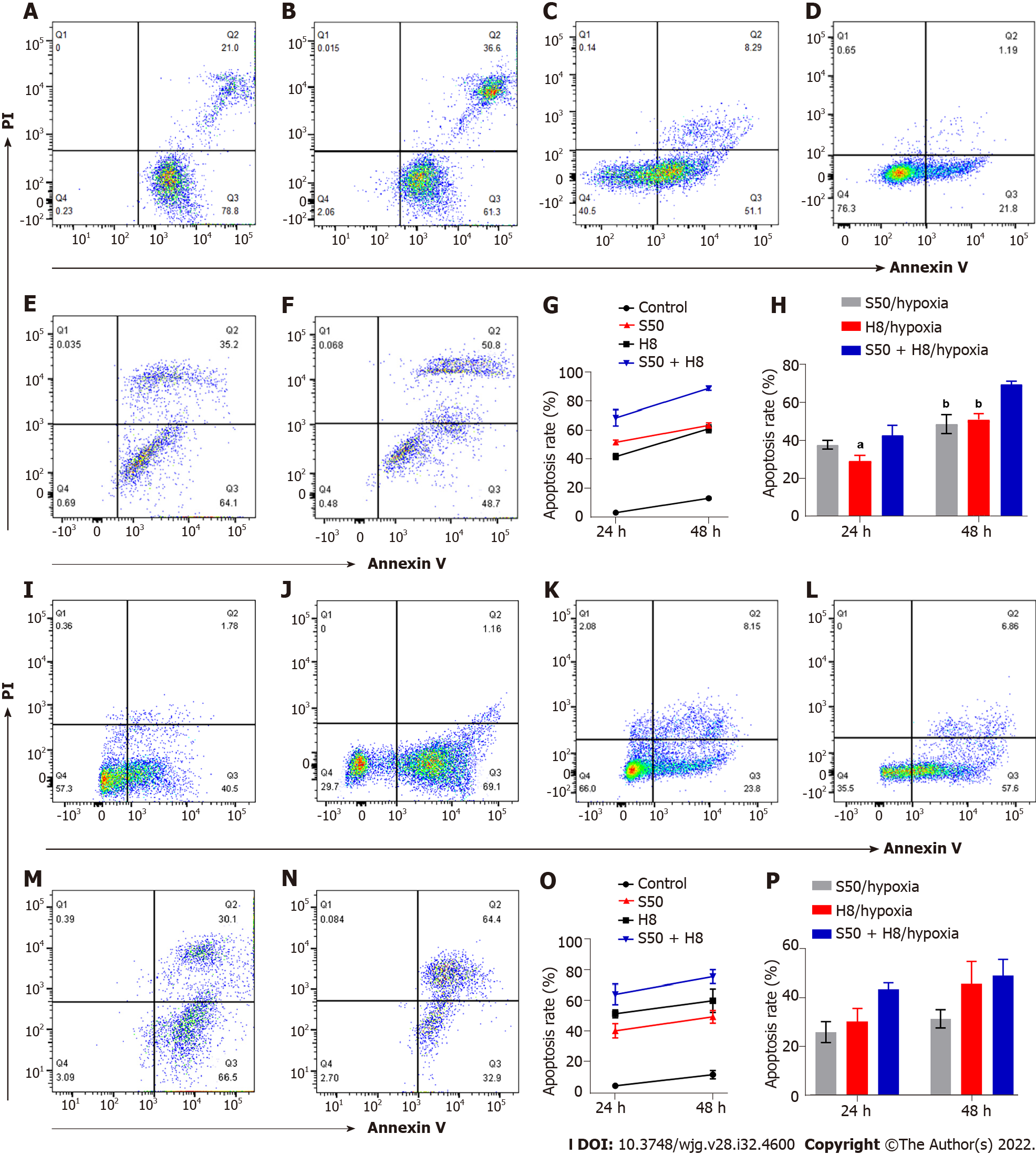
SRL and Huai Er promote the apoptosis of HepG2 and Huh7 cells under normoxia: The apoptosis rate of HepG2 (Figure 3A-H) and Huh7 (Figure 3I-P) cells increased gradually 24 h to 48 h after treatment with S50 and H8 (P < 0.001) (Figure 3A, B, G, I, J and O). The combination of S50 + H8 caused the highest promotion of apoptosis in this time period, compared to the single-drug treatments (P < 0.001) (Figure 3E, G, M and O). These results indicate that Huai Er may enhance the pro-apoptotic effect of S50 on HCC cells.
Hypoxia reduces the apoptosis effect of SRL and Huai Er on HepG2 and Huh7 cells: Despite the longer treatment time under hypoxia, the increased apoptosis rate of HepG2 and Huh7 cells (Figure 3C, D, H, K, L and P), observed with treatment under normoxia, was significantly reduced (P < 0.05, Figure 3). There was also a decrease in apoptosis levels after S50 + H8 treatment under hypoxia (Figure 3E, F, H, M, N and P) in both HepG2 and Huh7 cells.
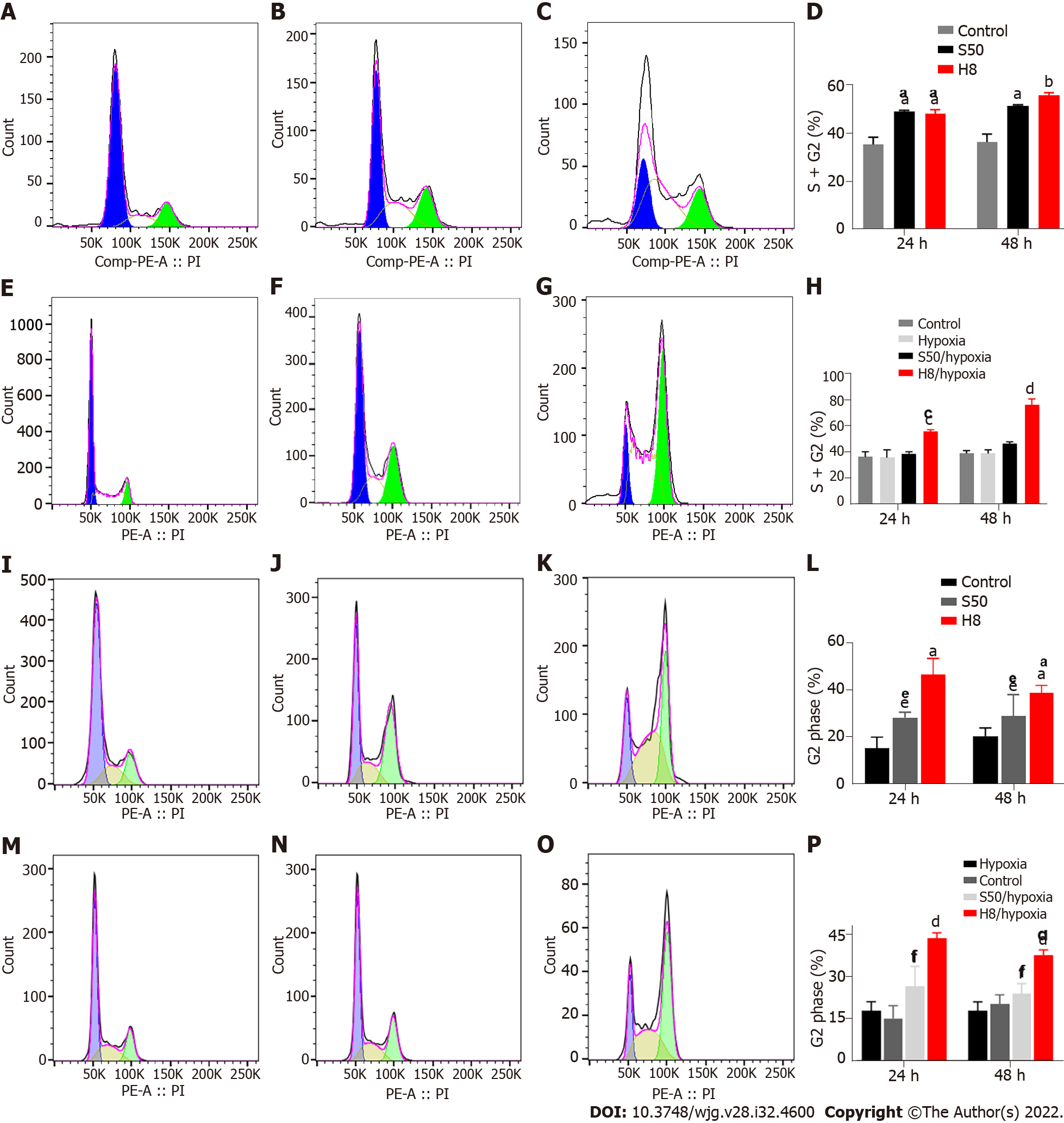
Hypoxia weakens the blocking effect of SRL and Huai Er on the cell cycle: Treatment with S50 and H8 showed a significant blocking effect, in the form of mitotic stagnation in the G2 phase. Increased proportions of S and G2 phase HepG2 cells were observed (Figure 4A-H), while Huh 7 cells showed an increased proportion in the G2 phase (Figure 4I-P). Both cell types had a decreased proportion in the G1 phase (P < 0.01) (Figure 4A-D and I-L). The blocking effect occurred gradually over time (Figure 4A-D and I-L) (P < 0.01). When cultured under hypoxia, the blocking effect of SRL and Huai Er on HepG2 and Huh7 cells was weakened, and there was no significant difference in the S50 treatment group, compared with the hypoxia group after treatment at either time point, (P > 0.05) (Figure 4E, F, H, M, N and P); however, the difference in H8 remained statistically significant (P < 0.001).
SRL application with Huai Er has a synergistic effect in blocking the cell mitotic cycle: S50 + H8 treatment significantly inhibited the cell division of HepG2 cells in the S phase under normoxia, while the ratio of G1 phase to G2 phase cells decreased both at 24 h and 48 h (Supplementary Figure 2A-C) (P < 0.05) (Supplementary Figure 2C). This ratio was significantly higher than that observed for the single drug treatments (P < 0.01) (Supplementary Figure 2E and F), suggesting that SRL and Huai Er may have synergistic inhibitory effects. Hypoxia attenuated the synergistic inhibitory effect of S50 + H8 on the cell cycle of HepG2 (Supplementary Figure 2A-D), decreasing the proportion of S-phase cells (P < 0.01). The ratio of G1 phase to G2 phase cells after S50 + H8 treatment was significantly higher than that of the S50 group (P < 0.01), but not significantly different from that in the H8 group (Supplementary Figure 2G and H), at both timepoints.
Thus, we believe that the combined SRL and Huai Er treatment can significantly arrest the cell cycle of HepG2 and Huh7 cells; therefore, protein and gene-level analyses were mainly focused on HepG2 cells.
To further explore the molecular mechanism of SRL combined with Huai Er, Western blot was used to analyse the expression levels of the key proteins in the PI3K signalling pathway (mTOR) and in hypoxia-induced factor-mediated glycolysis (HIF-1α). Compared with the control and the monotherapy treatments, S50 + H8 significantly reduced the expression levels of mTOR and HIF-1α (Supplemen
Hypoxia can significantly upregulate the expression of HIF-1α mRNA, while increasing the expression of LDHA and GLUT-1 mRNA in anaerobic glycolysis, leading to the promotion of vascular endothelial growth factor (VEGF) mRNA expression (Supplementary Figure 3D and E). Hypoxia also downregulated the mRNA expression of FoxP3, p27, and Phosphatase and tensin homolog deleted on chromosome ten phosphatase and tensin homolog deleted on chromosome ten (PTEN). Although there was an increase in the expression of Akt and mTOR, no significant difference was observed (Supplementary Figure 3D and E). These results suggest that hypoxia-induced HIF-1α upregulation can mediate downstream activation, leading to an enhancement of HepG2 cell proliferation by upregulating the expression of LDHA and GLUT-1.
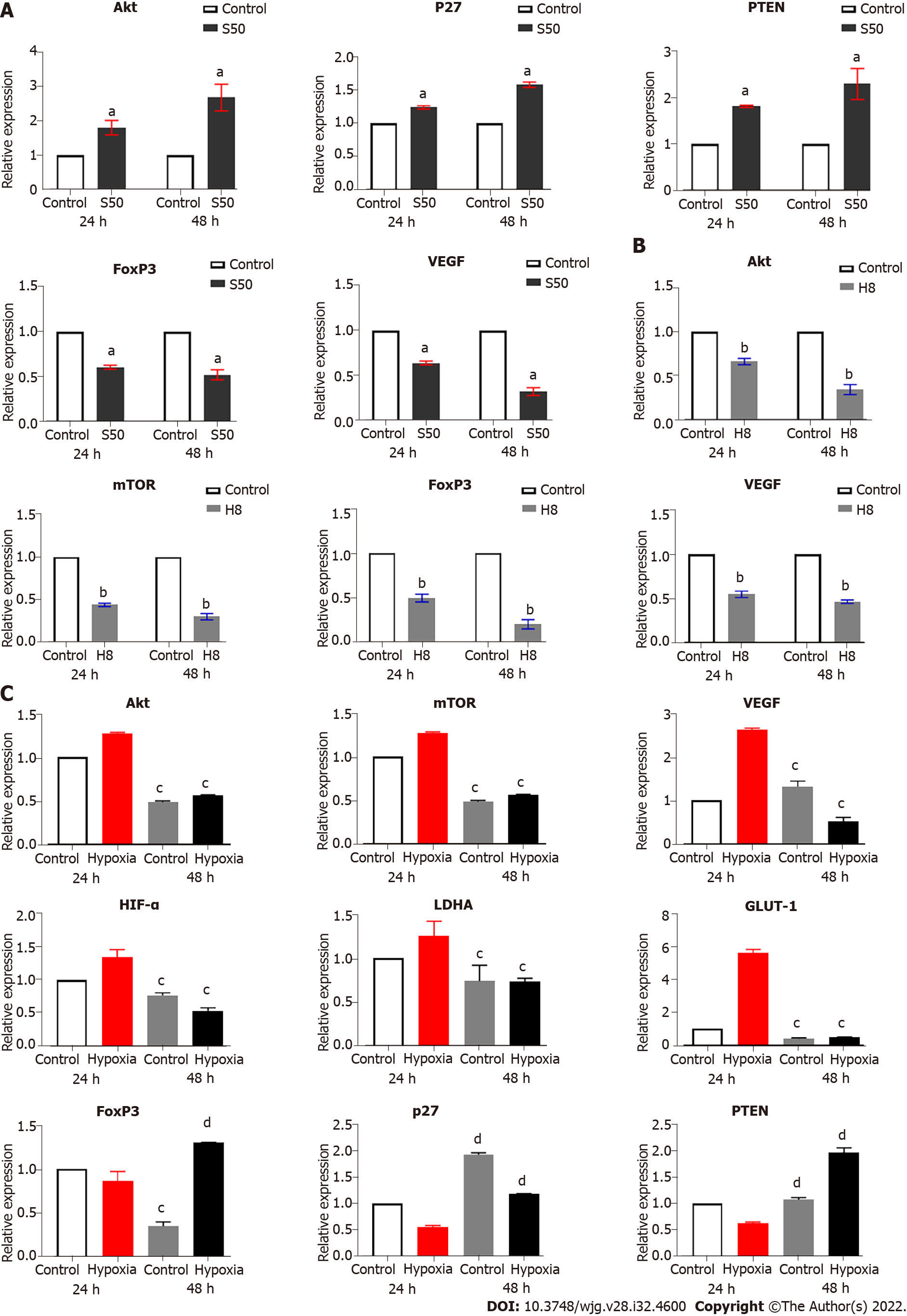
SRL promotes the mRNA expression of Akt, p27, and PTEN, and decreases the mRNA expression of FoxP3 and VEGF: Compared to the control group, the expression of mTOR mRNA showed no significant expression changes. However, SRL significantly upregulated the mRNA expression of Akt, p27, and PTEN; this upregulation changed significantly over time (Figure 5A). In addition, SRL treatment significantly downregulated the mRNA expression of FoxP3 and VEGF over the same timeperiod (Figure 5A).
Effect of Huai Er on the mRNA expression of Akt, mTOR, FoxP3, and VEGF: Compared with the control group, Huai Er treatment significantly downregulated the mRNA expression of Akt, mTOR, FoxP3, and VEGF; this downregulation was significantly increased over time (Figure 5B). The mRNA levels of p27 (P24 h = 0.0066, P48 h = 0.0038) and PTEN (P24 h = 0.003, P48 h = 0.0007) were increased at both time points. Akt and mTOR expression showed the most significant downregulation after treatment, leading to a reduction in FoxP3 and VEGF mRNA levels from 24 h to 48 h.
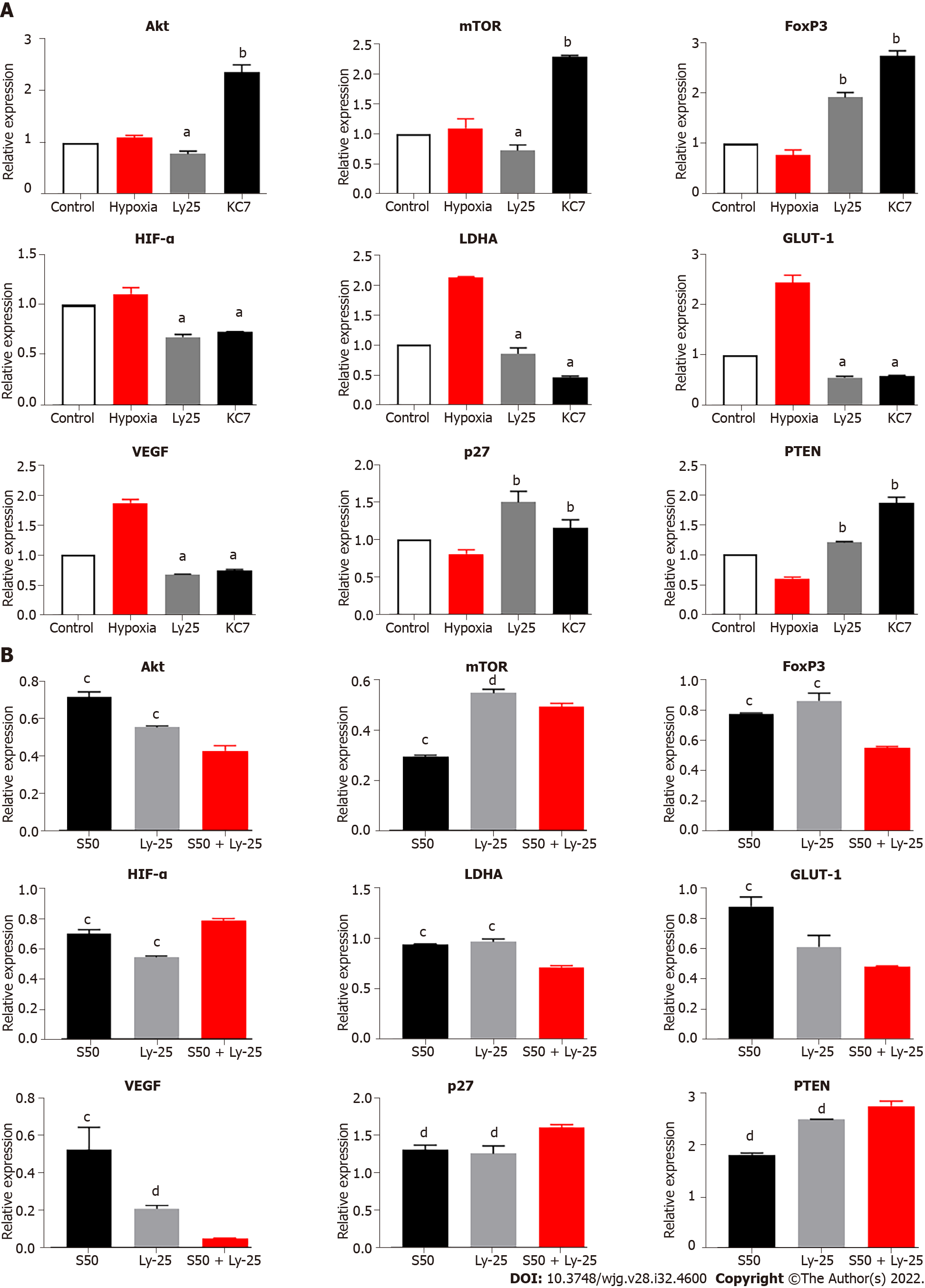
SRL treatment blocked the downstream amplification effect caused by the expression of mTOR mRNA under hypoxia, which significantly downregulated the enhancement effect of hypoxia on HIF-1α mRNA expression (P < 0.01) (Figure 5C). The treatment also decreased the mRNA expression levels of VEGF, LDHA, and GLUT-1 (P < 0.01), and simultaneously upregulated the mRNA expression of p27, PTEN, and FoxP3 (P < 0.001) (Figure 5C). Compared with the hypoxia group, the Huai Er treatment attenuated the hypoxia-induced enhanced mRNA expression of HIF-1α and downregulated the mRNA expression of Akt, mTOR, VEGF, and FoxP3 (P < 0.01) (Figure 5C). It also significantly decreased the mRNA expression levels of LDHA and GLUT-1 (P < 0.001) and upregulated the mRNA expression of p27 and PTEN (P < 0.001) (Figure 5C).
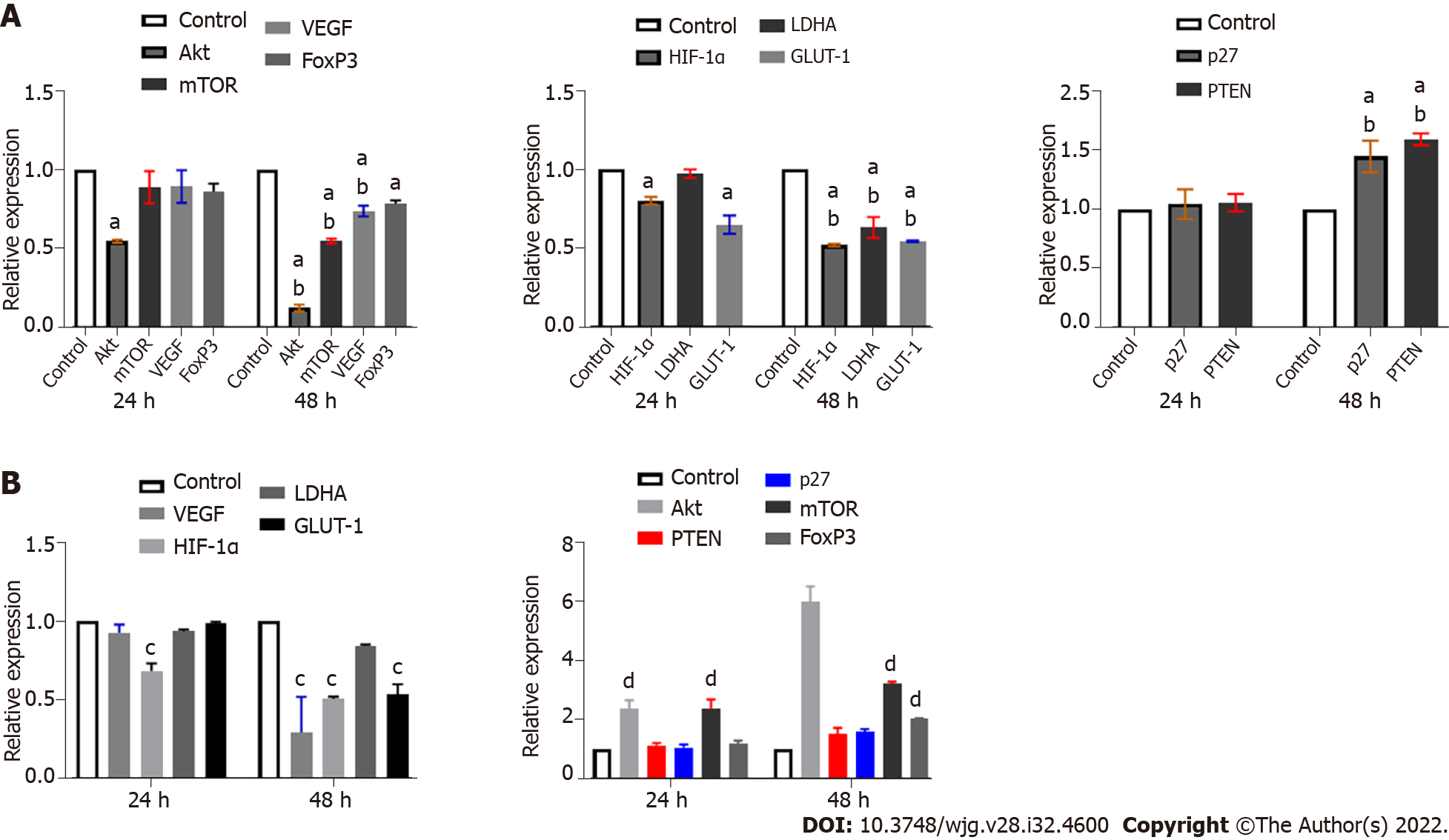
LY294002 specifically inhibits PI3K, leading to a downstream blocking effect. Ly25 treatment significantly downregulated the mRNA expression of Akt and mTOR and caused a reduction in VEGF and FoxP3 mRNA expression. Downregulation of mRNA expression of HIF-1 α, LDHA, and GLUT-1, and upregulation of p27 and PTEN (Figure 6A) were also observed. When compared with the control group, only the expression of Akt mRNA was significantly downregulated at 24 h (P < 0.05) (Figure 6); significant differences among the other groups only became apparent after 48 h (P < 0.05) (Figure 6A). Except for FoxP3, there were significant differences between 48 h and 24 h in the other groups (P < 0.05) (Figure 6A).
KC7F2 blocks HIF-1α and results in decreased mRNA expression of LDHA and GLUT-1
Under normoxia, HIF-1α in tissues was quickly degraded by the intracellular, oxygen-dependent, ubiquitin protease degradation pathway, and its stable expression was weak. Therefore, we used the KC7F2-specific blockade of HIF-1α as a reference. Upon treatment with KC7F2, the mRNA expression of Akt, mTOR, PTEN, and p27 was upregulated, while the mRNA expression of VEGF, HIF-1α, LDHA, and GLUT-1 was downregulated (Figure 6B).
The mRNA expression of Akt, mTOR, VEGF, HIF-1α, LDHA, and GLUT-1 was decreased after treatment with Ly25 (P < 0.05) (Figure 7A), while the mRNA expression of p27, PTEN, and FoxP3 was upregulated (P < 0.05) (Figure 7A). KC7F2 specifically inhibited the expression of HIF-1α, which downregulated the hypoxia-induced HIF-1α mRNA expression (P < 0.05). There was also a significant downregulation of LDHA, GLUT-1, and VEGF mRNA expression (P < 0.05) (Figure 7A), while upregulation of p27 and PTEN mRNA expression was observed. However, blocking with KC7 did not inhibit the Akt/mTOR signalling pathway (P < 0.05) (Figure 7A), indicating that other regulatory pathways may be involved.
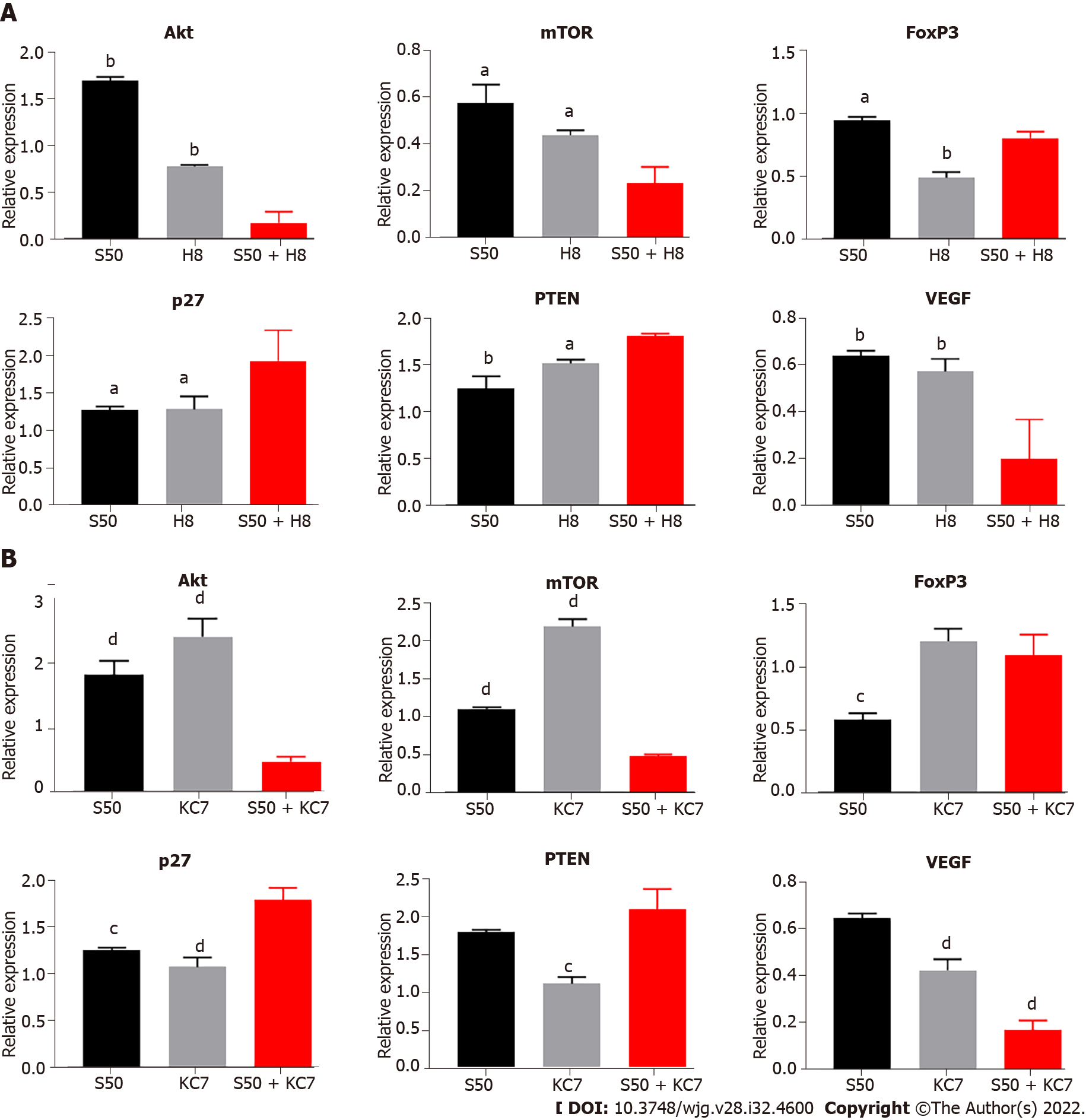
The S50 + H8 combined treatment significantly downregulated the mRNA levels of Akt and mTOR to a greater extent than the downregulation observed with the H8 treatment alone. In contrast, individual S50 treatment resulted in an upregulation (Figure 8A). The downregulation of Akt and mTOR mRNA expression resulted in a significant downregulation of VEGF (P < 0.01) and FoxP3 mRNA expression (P < 0.05) (Figure 8A), and an upregulation of p27 (P < 0.01) and PTEN mRNA expression (P < 0.05) (Figure 8A).
Compared with S50 and Ly25 individual treatments, the S50 + Ly25 combination significantly reduced the mRNA expression of VEGF and FoxP3 (P < 0.05) (Figure 7B), and significantly upregulated the mRNA expression of PTEN and p27; there was a significant difference in p27 mRNA between the two groups (P < 0.05). PTEN expression only showed a significant difference between the S50 and S50 + Ly25 groups (P < 0.05) (Figure 7B). Downregulation of Akt, LDHA, and GLUT-1 mRNA expression was observed in the S50 + Ly25 group and was more significant than that in the hypoxia group; the difference between groups was significant (P < 0.01) (Figure 7B).
Compared with the S50 and KC7F2 groups, the combined treatment of S50 + KC7F2 significantly downregulated the mRNA expression of VEGF (P < 0.01) (Figure 8B) and upregulated the mRNA expression of p27 (P < 0.05) and PTEN (P < 0.01) (Figure 8B). S50 + KC7F2 treatment also significantly downregulated the mRNA levels of Akt and mTOR (P < 0.01) (Figure 8B), and neutralised the downregulation of FoxP3 mRNA expression by S50 and upregulation of FoxP3 mRNA expression by KC7F2 (Figure 8B).
A large number of infiltrated lymphocytes, fibroblasts, macrophages, neutrophils, myeloid-derived suppressor cells (MDSCs), and other immune cells result in local immunosuppression and a hypoxic environment which activates HIF-1α[4]. The highly acidic microenvironment caused by the abnormal metabolism of cancer cells and local microvascular infiltration plays an important role in tumor recurrence, proliferation, and invasion[4]. In this study, we indirectly confirmed that high expression of HIF-1α, LHDA, and GLUT-1 in poorly differentiated HCC indicates active glycolysis.
The anoxic and hyperacidic environment of local cancer tissue leads to the inhibition of immune cell function and downregulation of immune cell activity. Cancer cells can constantly adapt to this microenvironment, absorb circulating lactic acid for energy supply, and thus compete with infiltrating immune cell metabolism. This results in a lack of nutrition for T cells in local tissues, low anti-tumour effect, and resultant immune escape of tumour cells[7,12].
It has been confirmed that highly infiltrated regulatory T cells and neutrophils in the local microenvironment are negatively correlated with tumour pathological differentiation and prognosis. In addition, the increase in FoxP3+ Tregs and neutrophil-to-lymphocyte ratio in peripheral blood before surgery is related to a poor prognosis after surgery[17,18]. We confirmed the relationship between FoxP3+ Tregs and recurrence of liver cancer and its inhibitory effect on T cells[15,17]. We found that SRL combined with Huai Er and Thymalfaxin could reduce the level of FoxP3+ Tregs in peripheral blood, delaying the time to tumour recurrence after liver transplantation[19]. However, the specific mechanism of action of FoxP3+ Tregs remains unclear. The present study aimed to explore whether SRL application in combination with Huai Er can achieve antitumour effects by affecting the glycolytic pathway.
Due to the immunosuppressive microenvironment of hypoxia and lactic acid accumulation, most of the CD8+ T lymphocytes infiltrating HCC were in a non-functional state and had a low immune response[10,20]. The cytokine TGF-β and chemokines CXCR4 and CXCR7 produced during tumour proliferation tend to recruit circulating neutrophils and Tregs to the local microenvironment; tumour-related neutrophils can recruit Tregs in cancer tissues through the role of chemokines[3,21]. This interaction between neutrophils and Tregs may enhance the inhibitory properties of the tumour microenvironment. Our previous studies confirmed that FoxP3+ Tregs and PD-L1+ neutrophils are highly expressed in HCC patients, with or without recurrence[17]. The levels of IL-10 and TGF-β in peripheral blood are significantly higher in patients with cancer than in normal individuals, which is consistent with published results[15,17]. FoxP3+ Tregs inhibit CD8+ T cells to promote tumour growth by secreting IL-10 and TGF-β, which may be a target for the anticancer mechanism of Huai Er. In this study, the cell proliferation assay confirmed that SRL and Huai Er inhibited the growth and proliferation of HepG2 and Huh 7 cell lines in a time- and dose-dependent manner. Treatment with SRL at 50 nM and Huai Er at 8 mg/mL showed the greatest inhibition.
The inhibitory effect of SRL combined with Huai Er was significantly better than that of either of them alone and that of SRL or Huai Er combined with LY294002 and KC7F2 when observing cell invasion by cell scratch assay. This suggested a synergistic effect in inhibiting the invasion of HepG2 and Huh 7 cells. Furthermore, SRL combined with Huai Er displayed high inhibition of the proliferation of cancer cell colonies. These results indicate that Huai Er and SRL inhibited the proliferation and invasion of HepG2 and Huh 7 cells in a concentration- and time-dependent manner.
We confirmed that the apoptosis-promoting effects of SRL and HuaiEr on HepG2 and Huh 7 cells affected mainly early apoptosis, with the combined treatment having a greater effect. Furthermore, SRL and Huai Er significantly increased the proportion of cells in the S + G2 or G2 phase, and this proportion was higher in the combined treatment group compared to the monotherapy groups.
We believe that the inhibitory effect of SRL combined with Huai Er on HepG2 and Huh 7 cell proliferation is achieved mainly by inducing cell cycle arrest in the G2 phase and promoting apoptosis.
Hypoxia is a common pathophysiological change during the development of most solid tumours[22]. The expression of HIF-1α is first activated by hypoxia in tissue cells, which induces CD4+ T cells to differentiate into FoxP3+ Tregs[23] and plays a role in the regulation of FoxP3 expression[24,25]. Hypoxia can induce the expression of PD-L1 (CD274) on the surface of immune cells (macrophages, neutrophils, dendritic cells, MDSCs, etc.) and cancer cells[26], which competitively binds to PD-1 receptors on the surface of T cells, resulting in impaired T cell activation function[27,28]. In this study, hypoxia promoted the formation and growth of cancer cell colonies, resulting in a significant increase in the number of cancer cell colonies in the S50 + H8 group. In addition, hypoxia increased the survival rate of HepG2 and Huh 7 cells, whereas Huai Er and SRL promoted their apoptosis.
Although the apoptotic effect increased gradually over time, the maximum apoptotic effect under normoxic conditions was significantly lower than that under hypoxic conditions in both the single drug and S50 + H8 groups. Although hypoxia could significantly downregulate the blocking effect of SRL on the S + G2 phase of HepG2 cells and G2 phase of Huh7 cells, it had less effect on the cell cycle stagnation caused by H8 treatment. Under hypoxic conditions, the S50 + H8 group still had an increased proportion of cells in the S + G2 phase although this proportion was lower than that observed in normoxia. This may be because Huai Er is involved in multiple signalling pathways rather than acts within a single cell signalling pathway which is blocked during hypoxia.
Under normoxic conditions, SRL and Huai Er can act synergistically to inhibit tumour proliferation, but this effect is weakened under hypoxic conditions, possibly due to downstream effects activated by HIF-1α. This indicates that removing the hypoxic environment of tumours may be the key to increasing the efficacy of anti-cancer drugs.
When cultured under hypoxia, the promotion of HepG2 and Huh 7 cell proliferation and the decrease in apoptosis and cell cycle progression by S50 + H8 treatment indicated that hypoxia plays an important role in HCC. Under hypoxic conditions, HIF-1α and mTOR in HCC cell lines were shown to be upregulated; in addition, LDHA, GLUT-1, and VEGF mRNA expression was upregulated and PTEN and p27 mRNA expression was inhibited. The mRNA expression levels of Akt and mTOR were upregulated in the hypoxic environment, although this was not statistically significant. The relative decrease in Akt and mTOR mRNA expression over time may be related to the activation of the HIF-1α-PTEN-Akt pathway by hypoxia, thereby increasing the glucose metabolism mediated by HIF-1α.
The decrease in HIF-1α and mTOR levels in the S50, H8, and S50 + H8 treatment groups may indicate a potential target pathway for S50 + H8 application in the treatment of HCC. With the intervention of SRL, the mRNA expression of VEGF and FoxP3 was downregulated, while the mRNA expression of Akt, PTEN, and p27 was upregulated. With the intervention of Huai Er, the mRNA expression of Akt, mTOR, VEGF, and FoxP3 was downregulated, while the mRNA expression of PTEN and p27 was upregulated. The combined effects of downregulation of VEGF, Akt, and mTOR mRNA expression and upregulation of p27 and PTEN were observed more significantly in the S50 + H8 group than in the individual treatment groups. The downregulation of FoxP3 mRNA was lower than that in the H8 treatment group; this may be associated with the immunomodulatory function of SRL.
Under hypoxic conditions, the accumulation of HIF-1α mRNA after treatment with SRL and Huai Er was downregulated. In addition, the mRNA expression of Akt, mTOR, VEGF, LDHA, and GLUT-1 was downregulated, and PTEN and P27 were upregulated. However, the effect of Huai Er combined with SRL was enhanced compared with that of monotherapies, which further confirmed that SRL combined with Huai Er exerted anti-tumour effects through the PI3K-Akt-mTOR-HIF-1α pathway. Previous studies have reported that hypoxia-induced cumulative activation of HIF-1 α can downregulate the mRNA expression of PTEN and P27, enhance the glycolytic function of tumour cells, and promote the growth and proliferation of tumour cells by upregulating LDHA and GLUT-1 mRNA expression[29]. There have been debates in the literature regarding the mechanism by which[30] hypoxia decreases the expression of FoxP3, with some saying that this is not HIF-1 dependent[31]. In our study, the expression changes of FoxP3 were not consistent with HIF-1 α under hypoxic conditions; this requires further research on co-culture with T cells. The final effect of a significant reduction in VEGF mRNA expression was observed, and therefore, we believe that there exists a co-effect of the HIF-1α-PTEN-Akt /mTOR pathway.
Most anticancer drugs decrease the downstream activation effect by reducing the activity of the PI3K-Akt-mTOR pathway[32,33]. PTEN negatively regulates PI3K/Akt signalling and is a well-known tumour suppressor[34]. Studies have demonstrated a strong correlation between alterations in the PTEN/PI3K/Akt cascades and the carcinogenesis of human tumours, including HCC, therefore making it a promising therapeutic target for HCC[34-36]. It has been proved that an increase in PTEN can inhibit the expression of PI3K/pAkt[37]. The upregulation of PTEN can indirectly increase p27 by secreting signal factors[38,39], while the increased PTEN acts on the PI3K-Akt-mTOR pathway and downregulates the expression of pAkt[40,41], further weakening the downstream activation effect. We believe that in this study, the intervention of SRL and Huai Er played a role by downregulating the activity of the Akt/mTOR pathway and increasing the expression of PTEN. SRL can increase the sensitivity of HepG2 cells to Huai Er and exert an anticancer effect through the PI3K-Akt-mTOR-PTEN and PTEN-Akt/mTOR-FoxP3 regulation pathways.
To further investigate the regulatory effect of the above-mentioned effector drugs on HIF-1α, LY294002 and KC7F2 blockers were selected to interfere with the expression of Akt and HIF-1α in the cell pathway. LY294002 inhibits downstream activation and the results showed that LY294002 could significantly reduce the hypoxia-induced upregulation of HIF-1α mRNA and attenuate the hypoxia-mediated upregulation of LDHA, GLUT-1, and VEGF mRNA expression. We observed that the mRNA expression of PTEN and p27 was significantly upregulated, while Akt and mTOR were downregulated, further confirming the existence of the HIF-1α-PTEN-Akt/mTOR intervention pathway. In addition, the expression of FoxP3 was significantly upregulated, confirming that the regulation of FoxP3 by hypoxia did not completely depend on changes in HIF-1α. We also blocked HIF-1α, using KC7F2 inhibitor, and found that the expression of HIF-1α, LDHA, GLUT-1, and VEGF was significantly downregulated, especially LHDA. The enhancement effect on PTEN and FoxP3 was higher than that with LY294002 treatment, which further confirmed that the effect of hypoxia on FoxP3 was independent of the change in HIF-1α and the existence of the HIF-1α-PTEN effect. The combined treatment of SRL + Ly and SRL + KC7 showed that the regulation of PTEN, p27, and the mRNA expression of VEGF and FoxP3 was consistent with the trend seen during single drug intervention. Based on the above discussion, we further summarize the functional pathways of this study (Supplementary Figure 4).
Thus, we believe that hypoxia can induce the accumulation of HIF-1α and enhance its mediated downstream promoting effect, thereby promoting the growth of tumour cells. SRL can enhance the hypoxia-induced HIF-1α downregulation by Huai Er and attenuate the enhanced effect of glucose metabolism, mediated by HIF-1α, thus exerting an antitumour effect through the PI3K-Akt-mTOR-HIF-1α and HIF-1α-PTEN-Akt/mTOR pathways.
The overexpression of HIF-1α, the role of FoxP3 in regulating transcription, and the combined intervention of both on the expression of post-transcriptional proteins require further study. In addition, the co-culture of cells affected by T-cell killing function to determine changes in gene and protein levels will be the next research direction of this study.
SRL increases the anti-cancer effect of Huai Er, which reduces the promotion of hypoxia-induced HIF-1α on the Warburg effect by inhibiting the PI3K/Akt/mTOR-HIF-1α and HIF-1α-PTEN signalling pathways in HCC.
Hypoxic and high lactate environment further aggravates the aerobic glycolytic effect of cancer and promotes the proliferation and metastasis of liver cancer. Hypoxia-inducible factor 1α plays an important role in the Warburg effect.
We found in clinical practice that the combination of sirolimus (SRL) and Huai Er granules can prolong the survival time of liver transplant patients and delay tumor recurrence. The mechanism of such combination therapy is unclear.
To clarify the regulatory mechanism of SRL combined with Huaier intervention on the Warburg effect.
In order to solve the scientific problems raised in this study, immunohistochemistry, cell culture, cell scratch and clone formation assays, and flow cytometry were used to analyze the changes of cell levels.
Hypoxia-mediated glycolysis was associated with poorly differentiation HCC and a lower prognosis. Hypoxic-induced HIF-1α promoted the growth of HepG2 and Huh7 cells, which was weakened with the treatment of SRL and Huai Er. SRL increased the anti-cancer effect of Huai Er, which reduced the promotion of hypoxia-induced HIF-1α on the Warburg effect by inhibiting the PI3K/Akt/mammalian target of rapamycin (mTOR)-HIF-1α and HIF-1α-phosphatase and tensin homolog deleted on chromosome ten (PTEN) signaling pathways in HCC.
SRL increases the anti-cancer effect of Huai Er, which reduces the promotion of hypoxia-induced HIF-1α on the Warburg effect by inhibiting the PI3K/Akt/mTOR-HIF-1α and HIF-1α-PTEN signaling pathways in HCC.
This study confirmed that SRL combined with Huai Er can downregulate the Warburg effect mediated by HIF-1α, laying a foundation for the combined treatment of HCC with traditional Chinese and western medicine.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lashen SA, Egypt; Zaghloul MS, Egypt S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15305] [Article Influence: 3061.0] [Reference Citation Analysis (4)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 3. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646-1658.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 617] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 4. | Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1568] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 5. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 6. | Amann T, Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets. 2009;13:1411-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich J, Oefner PJ, Kreutz M, Bosserhoff AK, Hellerbrand C. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 8. | He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1α on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 9. | Kakudo N, Morimoto N, Ogawa T, Taketani S, Kusumoto K. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1α Activation. PLoS One. 2015;10:e0139890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Weljie AM, Jirik FR. Hypoxia-induced metabolic shifts in cancer cells: moving beyond the Warburg effect. Int J Biochem Cell Biol. 2011;43:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, White E, Rabinowitz JD. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 1207] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 12. | Chen YJ, Mahieu NG, Huang X, Singh M, Crawford PA, Johnson SL, Gross RW, Schaefer J, Patti GJ. Lactate metabolism is associated with mammalian mitochondria. Nat Chem Biol. 2016;12:937-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 13. | Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1342] [Cited by in RCA: 1396] [Article Influence: 139.6] [Reference Citation Analysis (1)] |
| 14. | Chen Q, Shu C, Laurence AD, Chen Y, Peng BG, Zhen ZJ, Cai JQ, Ding YT, Li LQ, Zhang YB, Zheng QC, Xu GL, Li B, Zhou WP, Cai SW, Wang XY, Wen H, Peng XY, Zhang XW, Dai CL, Bie P, Xing BC, Fu ZR, Liu LX, Mu Y, Zhang L, Zhang QS, Jiang B, Qian HX, Wang YJ, Liu JF, Qin XH, Li Q, Yin P, Zhang ZW, Chen XP. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67:2006-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Zhou L, Pan LC, Zheng YG, Zhang XX, Liu ZJ, Meng X, Shi HD, Du GS, He Q. Reduction of FoxP3 + Tregs by an immunosuppressive protocol of rapamycin plus Thymalfasin and Huaier extract predicts positive survival benefits in a rat model of hepatocellular carcinoma. Ann Transl Med. 2020;8:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34536] [Cited by in RCA: 38001] [Article Influence: 2923.2] [Reference Citation Analysis (0)] |
| 17. | Zhou L, Wang J, Lyu SC, Pan LC, Shi XJ, Du GS, He Q. PD-L1 + NEUT, Foxp3 + Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma. Technol Cancer Res Treat. 2021;20:15330338211045820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Zhou L, Wang J, Zhang XX, Lyu SC, Pan LC, Du GS, Lang R, He Q. Prognostic Value of Preoperative NLR and Vascular Reconstructive Technology in Patients With Pancreatic Cancer of Portal System Invasion: A Real World Study. Front Oncol. 2021;11:682928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Zhou L, Pan LC, Zheng YG, Du GS, Fu XQ, Zhu ZD, Song JY, Liu ZJ, Su XZ, Chen W, Zheng DH, Suo LL, Yang SZ. Novel strategy of sirolimus plus thymalfasin and huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: A single center experience. Oncol Lett. 2018;16:4407-4417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Vaupel P, Mayer A. Hypoxia-Driven Adenosine Accumulation: A Crucial Microenvironmental Factor Promoting Tumor Progression. Adv Exp Med Biol. 2016;876:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Hayashi Y, Yokota A, Harada H, Huang G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019;110:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1α to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Lequeux A, Noman MZ, Xiao M, Van Moer K, Hasmim M, Benoit A, Bosseler M, Viry E, Arakelian T, Berchem G, Chouaib S, Janji B. Targeting HIF-1 alpha transcriptional activity drives cytotoxic immune effector cells into melanoma and improves combination immunotherapy. Oncogene. 2021;40:4725-4735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1642] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 26. | Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M. Erratum: Inflammation-induced IgA + cells dismantle anti-liver cancer immunity. Nature. 2017;552:430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064-5074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1966] [Article Influence: 178.7] [Reference Citation Analysis (0)] |
| 28. | Schultz L, Chaux A, Albadine R, Hicks J, Kim JJ, De Marzo AM, Allaf ME, Carducci MA, Rodriguez R, Hammers HJ, Argani P, Reuter VE, Netto GJ. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 2011;35:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Hsu TS, Lai MZ. Hypoxia-inducible factor 1α plays a predominantly negative role in regulatory T cell functions. J Leukoc Biol. 2018;104:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Wang XD, Liu CX, Wu YL, Chen J. [Hypoxia and its simulant CoCl(2) down-regulates Foxp3 expression independent from HIF-1alpha]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:533-536. [PubMed] |
| 31. | Tang H, Li RP, Liang P, Zhou YL, Wang GW. miR-125a inhibits the migration and invasion of liver cancer cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2015;10:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Yulyana Y, Ho IA, Sia KC, Newman JP, Toh XY, Endaya BB, Chan JK, Gnecchi M, Huynh H, Chung AY, Lim KH, Leong HS, Iyer NG, Hui KM, Lam PY. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015;23:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375-13378. [PubMed] [DOI] [Full Text] |
| 34. | Liu F, He Y, Liang Y, Wen L, Zhu Y, Wu Y, Zhao L, Li Y, Mao X, Liu H. PI3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer Cell Int. 2013;13:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 1787] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 36. | Zhu L, Sun Y, Zhang S, Wang L. Rap2B knockdown suppresses malignant progression of hepatocellular carcinoma by inactivating the PTEN/PI3K/Akt and ERK1/2 pathways. Mol Cell Biochem. 2020;466:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Wang DD, Yang SJ, Chen X, Shen HY, Luo LJ, Zhang XH, Zhong SL, Zhao JH, Tang JH. miR-222 induces Adriamycin resistance in breast cancer through PTEN/Akt/p27kip1 pathway. Tumour Biol. 2016;37:15315-15324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Hackenbeck T, Knaup KX, Schietke R, Schödel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009;8:1386-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, Nan K, Yao Y, Tian T. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017;108:620-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 40. | Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1014] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 41. | Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 786] [Article Influence: 87.3] [Reference Citation Analysis (0)] |