Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4442
Peer-review started: January 13, 2022
First decision: April 16, 2022
Revised: April 26, 2022
Accepted: July 24, 2022
Article in press: July 24, 2022
Published online: August 21, 2022
Processing time: 215 Days and 4.4 Hours
Health utility assessments have been developed for various conditions, including chronic liver disease. Health utility scores are required for socio-economic evaluations, which can aid the distribution of national budgets. However, the standard health utility assessment scores for specific health conditions are largely unknown.
To summarize the health utility scores, including the EuroQOL 5-dimensions 5-levels (EQ-5D-5L), EuroQol-visual analogue scale, short from-36 (SF-36), RAND-36, and Health Utilities Index (HUI)-Mark2/Mark3 scores, for the normal population and chronic liver disease patients.
A systematic literature search of PubMed and MEDLINE, including the Cochrane Library, was performed. Meta-analysis was performed using the RevMan software. Multiple means and standard deviations were combined using the StatsToDo online web program.
The EQ-5D-5L and SF-36 can be used for health utility evaluations during antiviral therapy for hepatitis C. HUI-Mark2/Mark3 indicated that the health utility scores of hepatitis B patients are roughly 30% better than those of hepatitis C patients.
The EQ-5D-5L is the most popular questionnaire for health utility assessments. Health assessments that allow free registration would be useful for evaluating health utility in patients with liver disease.
Core Tip: This study summarized current knowledge about health utility assessments, including the EuroQOL 5-dimensions 5-levels (EQ-5D-5L), EuroQol-visual analogue scale, short from-36, RAND-36, and Health Utilities Index-Mark2/Mark3. The EQ-5D-5L is the most popular questionnaire for health utility assessments. Health utility assessments need to be used widely and routinely.
- Citation: Ishinuki T, Ota S, Harada K, Kawamoto M, Meguro M, Kutomi G, Tatsumi H, Harada K, Miyanishi K, Kato T, Ohyanagi T, Hui TT, Mizuguchi T. Current standard values of health utility scores for evaluating cost-effectiveness in liver disease: A meta-analysis. World J Gastroenterol 2022; 28(31): 4442-4455
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4442
The quality of health is an important factor when assessing medical management rather than simple survival periods[1,2]. Health utility is an important factor in medical assessments and socio-economic politics[3]. National health budgets have risen steadily in various countries, and governments need to deeply consider the need to maintain a socio-economic balance[4]. Therefore, health benefits should be compared with social costs to avoid national financial collapse.
It is difficult to quantify health quality at regular intervals[5]. We are developing wearable devices that can automatically obtain health data, including data regarding mental health. Some health utility assessments require the use of questionnaires, which are associated with low compliance and involve bothersome calculations[2,6,7]. Before launching our novel health utility assessment tool, we performed this meta-analysis in order to summarize the currently available health utility assessment tools. The most useful questionnaire for evaluating health status depending on liver disease status or sex is unclear. In addition, no universal health utility assessment values for specific liver diseases or the normal population have been reported. Therefore, we conducted a meta-analysis to estimate health utility assessment values for specific populations.
The EuroQOL 5-dimensions 5-levels (EQ-5D-5L) is the simplest instrument for evaluating health utility and has been widely translated into various languages with high reliability and validity[6,8-10]. It only involves five questions and five answering levels. The health utility scores produced by the EQ-5D-5L can be used to calculate quality-adjusted life year (QALY) values[8]. The Health Utilities Index Mark 2/Mark 3 is another instrument for evaluating health utility scores and can also be used to obtain QALY values[11]. However, the Health Utilities Index is complicated, as it involves 45 questions, which take a long time to answer. The short-form 36-item (SF-36) is also widely used to evaluate health quality, although it does not directly involve QALY evaluations[9,12,13].
There are two types of SF-36, and the copyrights to these tools belong to The RAND Corporation (Santa Monica, CA, United States)[14] and QualityMetric (Johnston, RI, United States), respectively[15]. However, most researchers do not actively consider which version they use[12]. Therefore, the exact method and results of such assessments are not always described in the literature (Table 1).
| Questionnaire | Total | Permission | Company/Organization |
| EQ-5D-5L | Five questions | Registration required | The EuroQol Research Foundation. |
| Health Utilities Index Mark 2 or 3 | 45 questions | Purchase required | Health Utilities Inc. |
| 36-Item Short Form Survey | 36 questions | Purchase required | QualityMetric |
| 36 questions | Free | The RAND Corporation |
In this meta-analysis, we describe the scores obtained with various health utility indexes (HUIs) in normal healthy populations or patients with different types of liver disease (Table 2)[16-32].
| Ref. | Subjects and countries | EQ-5D-5L | EQ-VAS | HUI-mark | SF-36 | Type of SF-36 | Others |
| Jenkinson et al[16] | Normal population from United Kingdom | O | RAND® | ||||
| Ratcliffe et al[17] | Normal population/Liver transplantation patients from United Kingdom | Δ | Δ | O | Not described1 | ||
| Chong et al[18] | Normal population from Canada | O | Δ | Δ | Δ1 | ||
| Grieve et al[19] | Population from United Kingdom | O | |||||
| Bondini et al[20] | Population from United States | O | Δ1 | CLDQ | |||
| Dan et al[21] | Population from United States | O | SF-6D | ||||
| Björnsson et al[22] | Population from Sweden | O | O | Not described1 | |||
| Hsu et al[23] | Population from Vancouver | O | v2 | HQLQv2 | |||
| McDonald et al[24] | Population from United Kingdom | O | |||||
| Scalone et al[25] | Population from United Kingdom | O | Δ | ||||
| Vahidnia et al[26] | Population from United States | Δ | O | ||||
| Kaishima et al[27] | Population from Japan | O | |||||
| Blanco et al[28] | Population from Spain | Δ | O | ||||
| Kesen et al[29] | HCV patients from Turkey | O | Not described1 | HADS | |||
| Cortesi et al[30] | Population from Italy | O | O | ||||
| Karimi Sari et al[31] | HCV patients from Iran | O | Not described1 | ||||
| Zanone et al[32] | HCV patients from Italy | O |
The PICOS scheme was used to set appropriate inclusion criteria. A systematic literature search of PubMed and MEDLINE, including the Cochrane Library, was performed independently by two authors (Ishunuki T and Ota S). The search was limited to human studies whose findings were reported in English. No restrictions were placed on the type of publication, the publication date, or publication status. The search strategy was based on different combinations of words for each database. For the PubMed database, the following combination was used: (("liver"[MeSH Terms] OR "liver"[All Fields] OR "livers"[All Fields] OR "liver s"[All Fields]) AND "qol"[All Fields]) AND (1990/1/1: 3000/12/12[pdat]). For the MEDLINE database, the following combination was used: [quality of life (QOL) and Liver].
The two independent authors screened the titles and abstracts of the primary studies identified in the database search. Duplicate studies were excluded. The following inclusion criteria were employed for the meta-analysis: (1) Studies that compared QOL in patients who had liver disease; (2) Studies that compared QOL between male and female patients with liver disease; (3) Studies that reported at least one QOL outcome; and (4) If the same institute reported more than one study, only the most recent or the highest-level study was included.
The same two authors extracted the following primary data: (1) The questionnaires used for each QOL evaluation; (2) The first author, year of publication, and type of study; (3) The etiology of the disease and the number of times each intervention was performed; and (4) The timing of the evaluations.
Meta-analyses were performed using the RevMan software (version 5.3.; The Cochrane Collaboration). The mean differences (MD) between groups were calculated for continuous variables. The interquartile ranges of the data were transformed by dividing them by 1.35 to produce alternative standard deviation values. Multiple means and standard deviations were combined using the StatsToDo online web program (https://www.statstodo.com/index.php).
The chi-square test was used to evaluate heterogeneity, and the Cochran Q and I2 statistics were reported. The I2 value describes the percentage variation between studies in degrees of freedom. P values of <0.05 were considered significant.
The EQ-5D-5L has been widely investigated as a tool for evaluating general health in normal populations and patients with different stages of liver disease (Table 3)[17,18,22,25-27,30,32]. Health utility indices should be affected by age, sex, ethics, religion, and geography. However, the EQ-5D-5L produced similar utility indices for groups with different health statuses (Table 3), such as normal healthy individuals (0.8413 ± 0.1905) and hepatitis C virus (HCV)-infected patients with compensated or decompensated cirrhosis (0.8113 ± 0.2261 and 0.7903 ± 0.2182), HCV-infected patients exhibiting a sustained virologic response (SVR) (0.846 ± 0.1816), and patients with hepatocellular carcinoma 0.8127 ± 0.2084).
| Ref. | Total | Mean | SD |
| Normal healthy individuals | |||
| Ratcliffe et al[17] | 3386 | 0.85 | 0.03 |
| Chong et al[18] | 1518 | 0.821 | 0.011 |
| Björnsson et al[22] | 29353 | 0.819 | 0.217 |
| Vahidnia et al[26] | 1565 | 0.94 | 0.1 |
| Cortesi et al[30] | 6800 | 0.915 | 0.107 |
| Total | 42622 | 0.8413 | 0.1905 |
| Compensated cirrhosis with hepatitis C | |||
| Chong et al[18] | 24 | 0.74 | 0.085 |
| Grieve et al[19] | 40 | 0.55 | 0.34 |
| Björnsson et al[22] | 76 | 0.749 | 0.212 |
| Scalone et al[25] | 222 | 0.736 | 0.259 |
| Kaishima et al[27] | 20 | 0.824 | 0.106 |
| Cortesi et al[30] | 574 | 0.891 | 0.119 |
| Zanone et al[32] | 94 | 0.68 | 0.37 |
| Total | 1050 | 0.8113 | 0.2261 |
| Decompensated cirrhosis with hepatitis C | |||
| Chong et al[18] | 9 | 0.66 | 0.2 |
| Grieve et al[19] | 64 | 0.45 | 0.24 |
| Björnsson et al[22] | 53 | 0.565 | 0.266 |
| Kaishima et al[27] | 4 | 0.524 | 0.25 |
| Cortesi et al[30] | 523 | 0.859 | 0.14 |
| Total | 653 | 0.7903 | 0.2182 |
| Sustained virologic response | |||
| Chong et al[18] | 36 | 0.83 | 0.065 |
| Grieve et al[19] | 24 | 0.82 | 0.21 |
| Björnsson et al[22] | 52 | 0.792 | 0.209 |
| Zanone et al[32] | 91 | 0.89 | 0.18 |
| Total | 203 | 0.846 | 0.1816 |
| Hepatocellular carcinoma | |||
| Chong et al[18] | 15 | 0.65 | 0.21 |
| Grieve et al[19] | 64 | 0.45 | 0.24 |
| Scalone et al[25] | 85 | 0.777 | 0.241 |
| Kaishima et al[27] | 14 | 0.75 | 0.057 |
| Cortesi et al[30] | 545 | 0.867 | 0.146 |
| Total | 723 | 0.8127 | 0.2084 |
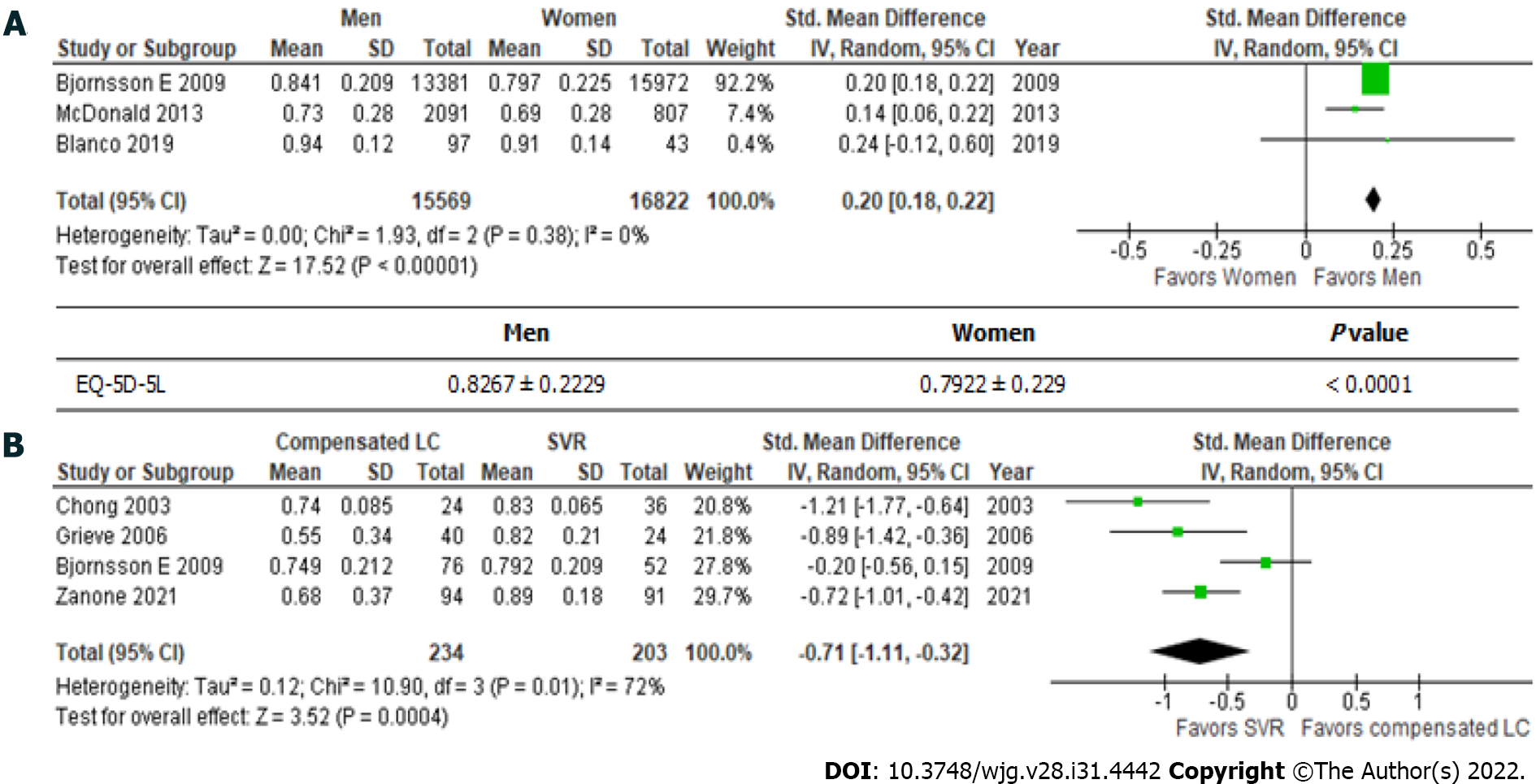
In general, the EQ-5D-5L produces significantly higher scores in males than in females (Figure 1A) (0.8267 ± 0.229 vs 0.7922 ± 0.239; P < 0.001). The mean total EuroQol-visual analogue scale score for the general population was found to be 79.796 ± 17.614 in two independent studies (Table 4)[26,30].
The SF-36 consists of eight scales, including physical functioning (85.07 ± 15.40); role limitations due to physical health problems (RP)(82.50 ± 25.15); bodily pain (BP) (77.62 ± 17.55); general health perceptions (GH) (63.37 ± 14.16); vitality, energy, or fatigue (VT) (63.37 ± 14.16); social functioning (SF) (86.97 ± 15.13); role limitations due to emotional problems (RE) (83.94 ± 23.57); and general mental health (63.37 ± 14.16). Although the eligible healthy controls differed among countries and age groups, the health utility scores produced by each scale were similar (Table 5)[16,17,22,23].
| Ref. | Total | Mean | SD |
| Physical function | |||
| Björnsson et al[22] | 339 | 87 | 19 |
| Jenkinson et al[16] M 60 | 681 | 80 | 22.1 |
| Jenkinson et al[16] W 60 | 684 | 74.8 | 23.5 |
| Ratcliffe et al[17] | 8883 | 85.4 | 2.55 |
| Hsu et al[23] | 9367 | 85.8 | 20 |
| Total | 19954 | 85.07 | 15.40 |
| Role physical | |||
| Björnsson et al[22] | 339 | 82 | 32 |
| Jenkinson et al[16] M 60 | 717 | 78.8 | 36.1 |
| Jenkinson et al[16] W 60 | 757 | 76.8 | 36.9 |
| Ratcliffe et al[17] | 9151 | 83.7 | 4.4 |
| Hsu et al[23] | 9367 | 82.1 | 33.2 |
| Total | 20331 | 82.50 | 25.15 |
| Body pain | |||
| Björnsson et al[22] | 339 | 72 | 27 |
| Jenkinson et al[16] M 60 | 724 | 78.8 | 23.6 |
| Jenkinson et al[16] W 60 | 779 | 75 | 25.1 |
| Ratcliffe et al[17] | 9214 | 80 | 3.05 |
| Hsu et al[23] | 9367 | 75.6 | 23 |
| Total | 20423 | 77.62 | 17.55 |
| General health | |||
| Björnsson et al[22] | 339 | 68 | 24 |
| Jenkinson et al[16] M 60 | 707 | 62.9 | 20.3 |
| Jenkinson et al[16] W 60 | 763 | 59 | 21.4 |
| Ratcliffe et al[17] | 9089 | 61.1 | 2.75 |
| Hsu et al[23] | 9367 | 65.8 | 18 |
| Total | 20265 | 63.37 | 14.16 |
| Vitality, energy, fatigue | |||
| Björnsson et al[22] | 339 | 68 | 24 |
| Jenkinson et al[16] M 60 | 707 | 62.9 | 20.3 |
| Jenkinson et al[16] W 60 | 763 | 59 | 21.4 |
| Ratcliffe et al[17] | 9089 | 61.1 | 2.75 |
| Hsu et al[23] | 9367 | 65.8 | 18 |
| Total | 20265 | 63.37 | 14.16 |
| Social function | |||
| Björnsson et al[22] | 339 | 88 | 21 |
| Jenkinson et al[16] M 60 | 729 | 86.9 | 22.6 |
| Jenkinson et al[16] W 60 | 783 | 85.9 | 22.6 |
| Ratcliffe et al[17] | 9219 | 87.8 | 2.8 |
| Hsu et al[23] | 9367 | 86.2 | 19.8 |
| Total | 20437 | 86.97 | 15.13 |
| Role emotional | |||
| Björnsson et al[22] | 339 | 86 | 29 |
| Jenkinson et al[16] M 60 | 714 | 85.8 | 29.5 |
| Jenkinson et al[16] W 60 | 756 | 83.3 | 32.5 |
| Ratcliffe et al[17] | 9159 | 83.7 | 4.4 |
| Hsu et al[23] | 9367 | 84 | 31.7 |
| Total | 20335 | 83.94 | 23.57 |
| Mental health, emotional, well-being | |||
| Björnsson et al[22] | 339 | 50 | 10 |
| Jenkinson et al[16] M 60 | 697 | 78 | 17.5 |
| Jenkinson et al[16] W 60 | 742 | 74.4 | 18.5 |
| Ratcliffe et al[17] | 9014 | 74.6 | 2.35 |
| Hsu et al[23] | 9367 | 77.5 | 15.3 |
| Total | 20159 | 75.64 | 12.23 |
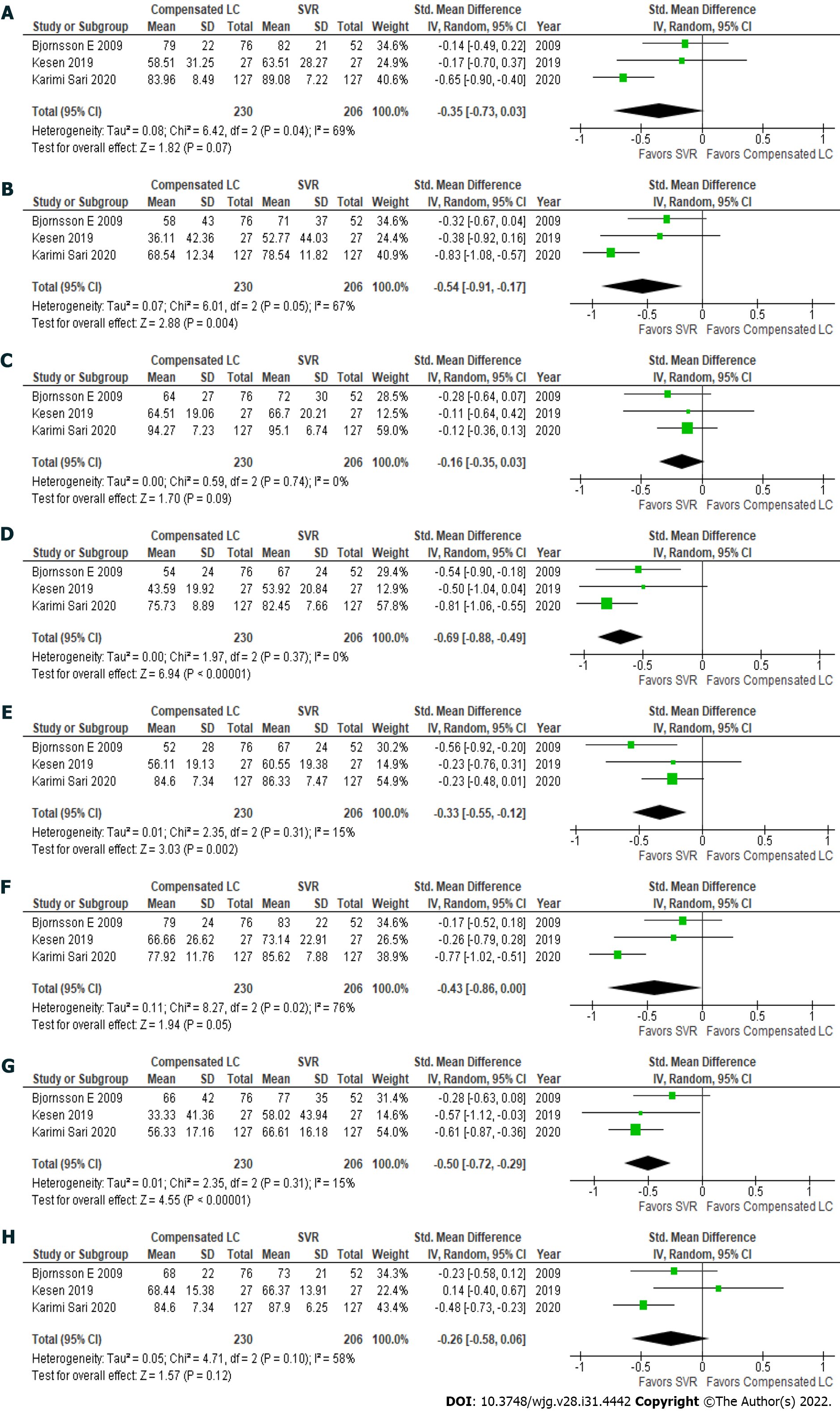
Patients with hepatitis C had achieved an SVR exhibited significantly better health utility scores for each SF-36 scale (Figure 2)[22,29,31] and the EQ-5D-5L (Figure 1B)[18,19,22,32] than those with compensated liver cirrhosis (Table 6)[18,19,22,29,31,32]. In particular, significant differences in the scores for RP (61.5 ± 31.6 vs 73.3 ± 27.3), GH (64.8 ± 20.9 vs 74.8 ± 18.5), VT (70.5 ± 24.0 vs 78.1 ± 18.4), RE (56.8 ± 32.0 vs 68.1 ± 27.3), and the EQ-5D-5L (0.6863 ± 0.3065 vs 0.846 ± 0.1816) were seen between these groups. These results indicate that health utility indices improve by 10%-20% after patients with hepatitis C achieve an SVR.
| Questionnare | Compensated LC | SVR | P value | % improvement |
| SF-36: Physical function | 79.3 ± 19.3 | 83.9 ± 17.8 | 0.07 | 105.8 |
| SF-36: Role physical | 61.5 ± 31.6 | 73.3 ± 27.3 | 0.004 | 119.2 |
| SF-36: Body pain | 80.8 ± 23.1 | 85.4 ± 21.3 | 0.09 | 105.7 |
| SF-36: General health | 64.8 ± 20.9 | 74.8 ± 18.5 | < 0.001 | 115.4 |
| SF-36: Vitality | 70.5 ± 24.0 | 78.1 ± 18.4 | 0.002 | 110.8 |
| SF-36: Social function | 77.0 ± 19.0 | 83.3 ± 15.6 | 0.05 | 108.2 |
| SF-36: Role emotional | 56.8 ± 32.0 | 68.1 ± 27.3 | < 0.001 | 119.9 |
| SF-36: Mental health | 77.2 ± 16.8 | 81.3 ± 15.2 | 0.12 | 105.3 |
| EQ-5D-5L | 0.6863 ± 0.3065 | 0.846 ± 0.1816 | < 0.001 | 123.3 |
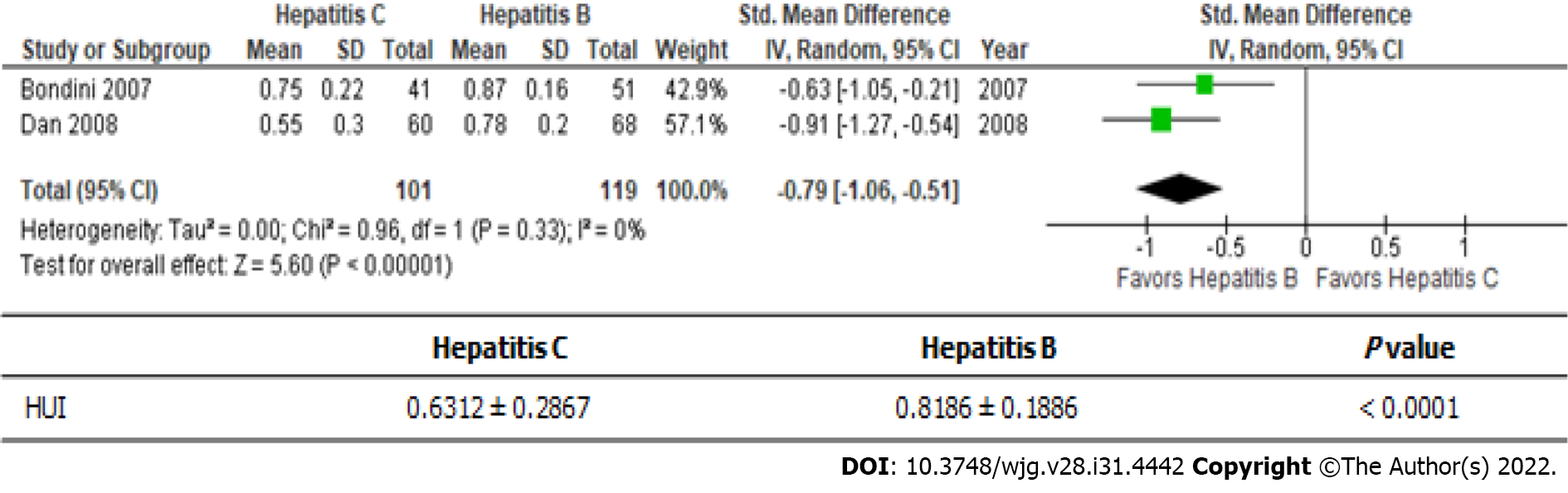
Hepatitis B and C are the main causes of viral-associated chronic liver disease (Figure 3)[20,21]. The health utility scores of hepatitis B patients were significantly better than those of hepatitis C patients (0.6312 ± 0.2867 vs 0.8186 ± 0.1886); i.e., there was a roughly 30% difference between the scores of these patients.
In this meta-analysis, we summarized the findings of previous studies examining health utility evaluations in patients with chronic liver disease. Various questionnaires have been used to evaluate health utility in different populations/at different times. The EQ-5D-5L is the most popular of the questionnaires used to examine health utility scores internationally[17].
One of the concerns regarding the application of health utility scores is their sensitivity[33]. For example, the health utility scores produced by the EQ-5D-5L for patients with compensated cirrhosis and decompensated cirrhosis did not differ significantly (Table 3). On the other hand, the health utility scores for hepatitis C patients with compensated liver cirrhosis and those who achieved an SVR differed significantly according to both the SF-36 and EQ-5D-5L (Table 6). This indicated that both questionnaires are suitable for evaluating health utility in hepatitis C patients after viral elimination. Although the health utility scores derived from the EQ-5D-5L were calculated from 5 questions, the score range of the EQ-5D-5L (123.3%) was greater than that of the SF-36 (105.8%-119.2%). Therefore, the EQ-5D-5L could be suitable for evaluating health utility scores in this specific disease state. On the other hand, EQ-5D-5L-derived health utility scores are based on only five personal factors, mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Therefore, their sensitivity and any ceiling effects should be validated in each language and ethnic group.
It is well known that the prevailing subtype of viral hepatitis differs depending on the geographic region[34]. Hepatitis B is the prevailing subtype in East Asia[13], whereas hepatitis C is the most common in Western countries[35]. Both types of hepatitis can be controlled by nucleic acid analogs[36]. In this meta-analysis, the HUI scores of hepatitis C patients were roughly 30% lower than those of hepatitis B patients. The differences between hepatitis B and hepatitis C need to be investigated using the EQ-5D-5L and SF-36 in future.
The second concern regarding the use of questionnaires for health assessments relates to the number of questions in each questionnaire. The EQ-5D-5L consists of only five questions[8], whereas the other tools consist of 36[14-16] or 45[11] questions. The number of questions affects study compliance, especially in the elderly[37]. If possible, the number of questions should be minimized.
The last concern is about gaining permission to use such questionnaires for health utility assessments. It takes great effort to develop a questionnaire. However, health utility assessments need to be repeated continuously. In certain human health emergencies, the use of some vaccines has been allowed without patent royalties having to be paid[38]. Commercial companies that own the rights to health assessments should reconsider their policies regarding their use.
Health assessments that allow free registration would be useful for evaluating health utility in patients with liver disease. Alternatively, a portable QOL tracker could be used to perform QOL evaluations of any patient-reported outcome, and we are currently developing such a tracker.
The most useful questionnaire for evaluating health status depending on liver disease status or sex is unclear.
No universal health utility assessment values for specific liver diseases or the normal population have been reported.
The objective of this study was to conduct a meta-analysis to estimate health utility assessment values for specific populations in the liver disease.
A systematic literature search was performed using PubMed and MEDLINE, including the Cochrane Library.
The short from-36 and EuroQOL 5-dimensions 5-levels (EQ-5D-5L) can be used for health utility evaluations during antiviral therapy for hepatitis C.
The EQ-5D-5L is the most popular questionnaire for health utility assessments. Health assessments that allow free registration would be useful for evaluating health utility in patients with liver disease.
Alternatively, a portable quality of life (QOL) tracker could be used to perform QOL evaluations of any patient-reported outcome in future.
We thank Sandy Tan and Miyako Nara for their valuable discussions and help in preparing this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jin X, China; Jing X, China; Yeo W, China A-Editor: Yao QG, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83:691-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1453] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 2. | Hazawa Y, Kutomi G, Shima H, Honma T, Ohmura T, Wada A, Mikami T, Hotta M, Narumi M, Ishinuki T, Kuno Y, Meguro M, Takemasa I, Okazaki M, Masuoka H, Asaishi K, Ohyanagi T, Hui TT, Mizuguchi T. The Unique Mental Impacts of Breast-Conserving Surgery and Mastectomy According to a Multi-Centered Cross Sectional Survey Conducted in Japan. Arch Breast Cancer. 2020;7:119-126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 732] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 4. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1750] [Article Influence: 291.7] [Reference Citation Analysis (0)] |
| 5. | Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience? BMJ. 2001;322:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 516] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Koide R, Kikuchi A, Miyajima M, Mishina T, Takahashi Y, Okawa M, Sawada I, Nakajima J, Watanabe A, Mizuguchi T. Quality assessment using EQ-5D-5L after lung surgery for non-small cell lung cancer (NSCLC) patients. Gen Thorac Cardiovasc Surg. 2019;67:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kikuchi A, Koide R, Iwasaki M, Teramoto M, Satohisa S, Tamate M, Horiguchi M, Niwa N, Saito T, Mizuguchi T. Assessing quality of life using the brief cancer-related worry inventory for gynecological surgery. World J Obstet Gynecol. 2019;8:1-7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3307] [Cited by in RCA: 4095] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 9. | Ishinuki T, Ota S, Harada K, Tatsumi H, Miyanishi K, Nagayama M, Takemasa I, Ohyanagi T, Hui TT, Mizuguchi T. Health-related quality of life in patients that have undergone liver resection: A systematic review and meta-analysis. World J Meta-Anal. 2021;9:88-100. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Shiroiwa T, Fukuda T, Ikeda S, Igarashi A, Noto S, Saito S, Shimozuma K. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25:707-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Whitehurst DG, Engel L, Bryan S. Short Form health surveys and related variants in spinal cord injury research: a systematic review. J Spinal Cord Med. 2014;37:128-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | de Medeiros MMD, Carletti TM, Magno MB, Maia LC, Cavalcanti YW, Rodrigues-Garcia RCM. Does the institutionalization influence elderly's quality of life? BMC Geriatr. 2020;20:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1804] [Cited by in RCA: 2008] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 15. | Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 16. | Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1197] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 17. | Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M; Cost-Effectiveness of Liver Transplantation Team. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, Bassendine M, Main J, Thomas H. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55:1332-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Bondini S, Kallman J, Dan A, Younoszai Z, Ramsey L, Nader F, Younossi ZM. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Dan AA, Kallman JB, Srivastava R, Younoszai Z, Kim A, Younossi ZM. Impact of chronic liver disease and cirrhosis on health utilities using SF-6D and the health utility index. Liver Transpl. 2008;14:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Björnsson E, Verbaan H, Oksanen A, Frydén A, Johansson J, Friberg S, Dalgård O, Kalaitzakis E. Health-related quality of life in patients with different stages of liver disease induced by hepatitis C. Scand J Gastroenterol. 2009;44:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Hsu PC, Krajden M, Yoshida EM, Anderson FH, Tomlinson GA, Krahn MD. Does cirrhosis affect quality of life in hepatitis C virus-infected patients? Liver Int. 2009;29:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | McDonald SA, Hutchinson SJ, Palmateer NE, Allen E, Cameron SO, Goldberg DJ, Taylor A. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. J Hepatol. 2013;58:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Scalone L, Ciampichini R, Fagiuoli S, Gardini I, Fusco F, Gaeta L, Del Prete A, Cesana G, Mantovani LG. Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Qual Life Res. 2013;22:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Vahidnia F, Stramer SL, Kessler D, Shaz B, Leparc G, Krysztof DE, Glynn SA, Custer B. Recent viral infection in US blood donors and health-related quality of life (HRQOL). Qual Life Res. 2017;26:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Kaishima T, Akita T, Ohisa M, Sakamune K, Kurisu A, Sugiyama A, Aikata H, Chayama K, Tanaka J. Cost-effectiveness analyses of anti-hepatitis C virus treatments using quality of life scoring among patients with chronic liver disease in Hiroshima prefecture, Japan. Hepatol Res. 2018;48:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Blanco JR, Barrio I, Ramalle-Gómara E, Beltran MI, Ibarra V, Metola L, Sanz M, Oteo JA, Melús E, Antón L. Gender differences for frailty in HIV-infected patients on stable antiretroviral therapy and with an undetectable viral load. PLoS One. 2019;14:e0215764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Kesen O, Kani HT, Yanartaş Ö, Aykut UE, Gök B, Gündüz F, Yılmaz Y, Özdoğan OC, Özen Alahdab Y. Evaluation of depression, anxiety and quality of life in hepatitis C patients who treated with direct acting antiviral agents. Turk J Gastroenterol. 2019;30:801-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Cortesi PA, Conti S, Scalone L, Jaffe A, Ciaccio A, Okolicsanyi S, Rota M, Fabris L, Colledan M, Fagiuoli S, Belli LS, Cesana G, Strazzabosco M, Mantovani LG. Health related quality of life in chronic liver diseases. Liver Int. 2020;40:2630-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Karimi-Sari H, Hosseini MA, Nikjoo N, Bagheri Baghdasht MS, Alavian SM. Patient-reported outcomes of sleep, mood and quality of life after treatment of chronic hepatitis C infection using direct-acting antiviral agents. Clin Microbiol Infect. 2020;26:1093.e5-1093.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Zanone MM, Marinucci C, Ciancio A, Cocito D, Zardo F, Spagone E, Ferrero B, Cerruti C, Charrier L, Cavallo F, Saracco GM, Porta M. Peripheral neuropathy after viral eradication with direct-acting antivirals in chronic HCV hepatitis: A prospective study. Liver Int. 2021;41:2611-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 465] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 34. | Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (20)] |
| 35. | Nainan OV, Alter MJ, Kruszon-Moran D, Gao FX, Xia G, McQuillan G, Margolis HS. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology. 2006;131:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Holmes JA, Rutledge SM, Chung RT. Direct-acting antiviral treatment for hepatitis C. Lancet. 2019;393:1392-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Uchmanowicz B, Chudiak A, Mazur G. The influence of quality of life on the level of adherence to therapeutic recommendations among elderly hypertensive patients. Patient Prefer Adherence. 2018;12:2593-2603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Davis BG. Could You Patent the Sun? ACS Cent Sci. 2021;7:508-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |