Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4431
Peer-review started: April 21, 2022
First decision: May 30, 2022
Revised: June 12, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: August 21, 2022
Processing time: 117 Days and 3 Hours
T1b gallbladder carcinoma (GBC) is defined as a tumor that invades the perimuscular connective tissue without extension beyond the serosa or into the liver. However, controversy still exists over whether patients with T1b GBC should undergo cholecystectomy alone or radical GBC resection.
To explore the optimal surgical approach in patients with T1b gallbladder cancer of different pathological grades.
Patients with T1bN0M0 GBC who underwent surgical treatment between 2000 and 2017 were included in the Surveillance, Epidemiology, and End Results database. The Kaplan-Meier method and log-rank test were used to analyze the overall survival (OS) and disease-specific survival (DSS) of patients with T1b GBC of different pathological grades. Cox regression analysis was used to identify independent predictors of mortality and explore the selection of surgical methods in patients with T1b GBC of different pathological grades and their relationship with prognosis.
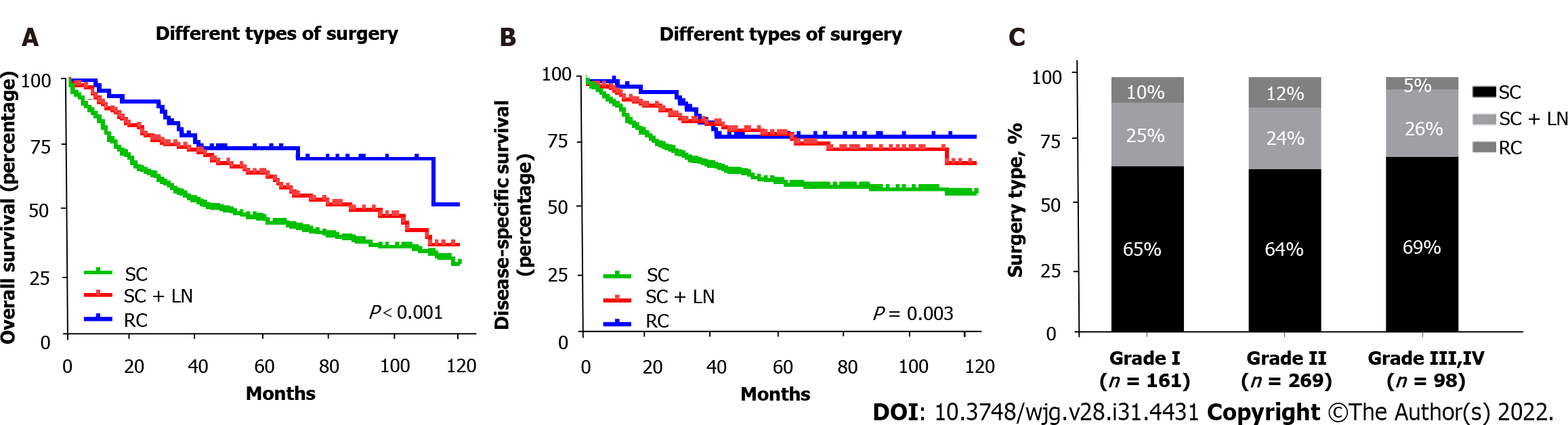
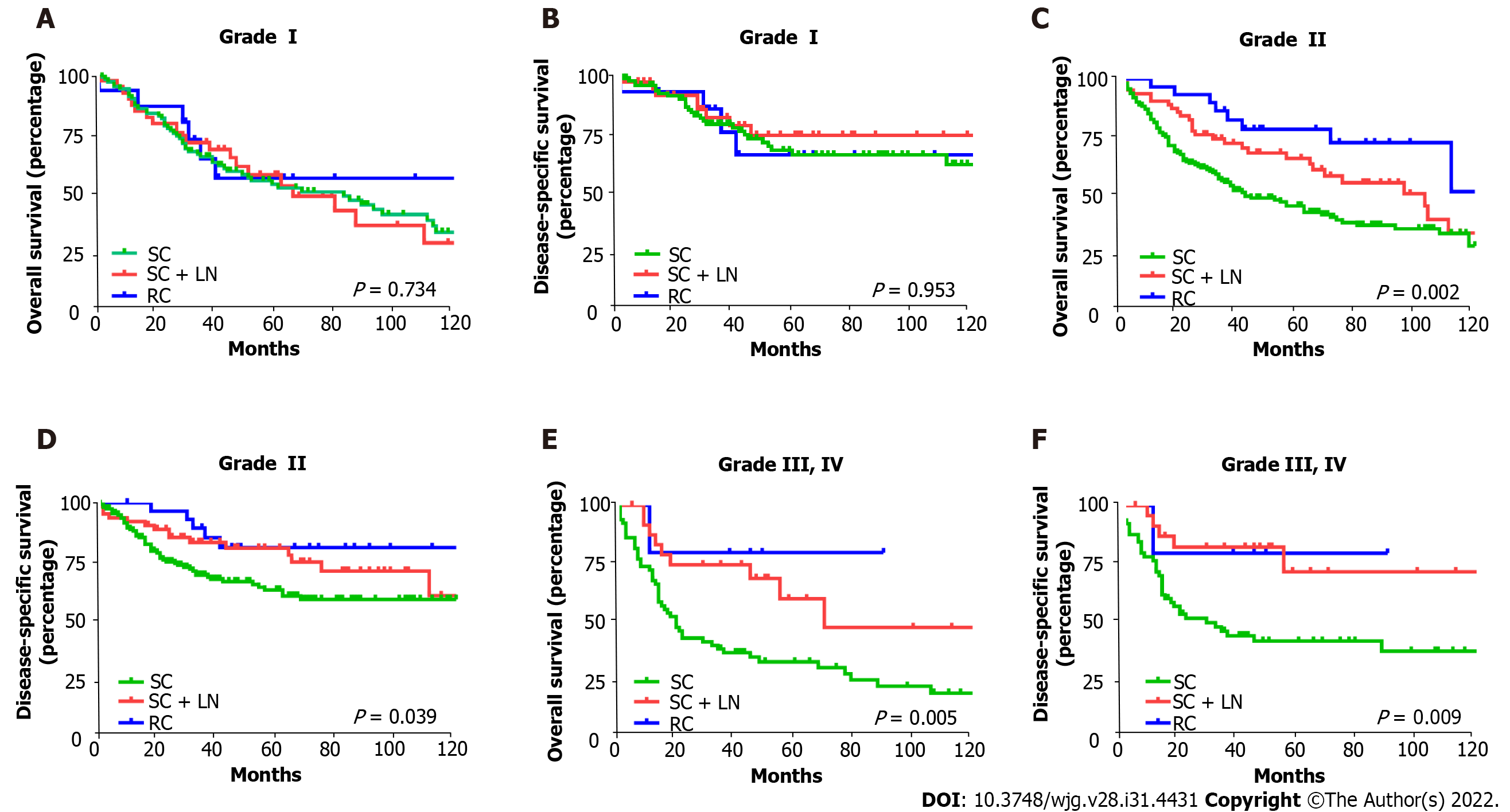
Of the 528 patients diagnosed with T1bN0M0 GBC, 346 underwent simple cholecystectomy (SC) (65.5%), 131 underwent SC with lymph node resection (SC + LN) (24.8%), and 51 underwent radical cholecystectomy (RC) (9.7%). Without considering the pathological grade, both the OS (P < 0.001) and DSS (P = 0.003) of T1b GBC patients who underwent SC (10-year OS: 27.8%, 10-year DSS: 55.1%) alone were significantly lower than those of patients who underwent SC + LN (10-year OS: 35.5%, 10-year DSS: 66.3%) or RC (10-year OS: 50.3%, 10-year DSS: 75.9%). Analysis of T1b GBC according to pathological classification revealed no significant difference in OS and DSS between different types of procedures in patients with grade I T1b GBC. In patients with grade II T1b GBC, obvious survival improvement was observed in the OS (P = 0.002) and DSS (P = 0.039) of those who underwent SC + LN (10-year OS: 34.6%, 10-year DSS: 61.3%) or RC (10-year OS: 50.5%, 10-year DSS: 78.8%) compared with those who received SC (10-year OS: 28.1%, 10-year DSS: 58.3%). Among patients with grade III or IV T1b GBC, SC + LN (10-year OS: 48.5%, 10-year DSS: 72.2%), and RC (10-year OS: 80%, 10-year DSS: 80%) benefited OS (P = 0.005) and DSS (P = 0.009) far more than SC (10-year OS: 20.1%, 10-year DSS: 38.1%) alone.
Simple cholecystectomy may be an adequate treatment for grade I T1b GBC, whereas more extensive surgery is optimal for grades II-IV T1b GBC.
Core Tip: T1b gallbladder carcinoma (GBC) is defined as a tumor that invades the perimuscular connective tissue without extension beyond the serosa or into the liver. However, controversy still exists over whether patients with T1b GBC should undergo cholecystectomy alone or radical GBC resection. In this study, we included patients with different histological grades of T1b GBC and compared the survival time of patients who underwent simple cholecystectomy, cholecystectomy with lymph node resection, or radical cholecystectomy to explore the optimal surgical approach for these patients.
- Citation: Shao J, Lu HC, Wu LQ, Lei J, Yuan RF, Shao JH. Simple cholecystectomy is an adequate treatment for grade I T1bN0M0 gallbladder carcinoma: Evidence from 528 patients. World J Gastroenterol 2022; 28(31): 4431-4441
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4431.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4431
Gallbladder carcinoma (GBC) is the most common malignant tumor of the biliary tract, accounting for 80%-95% of malignant tumors of the biliary tract. The overall average survival of patients with GBC is only 6 mo, and the 5-year survival rate is < 5%[1,2]. According to global cancer statistics from 2020, 115949 people have been diagnosed with GBC worldwide and 84965 have died from this condition[3]. The treatment effect of adjuvant therapy, chemotherapy, radiotherapy, and targeted therapy on GBC remains unsatisfactory despite recent improvements in diagnosis and treatment methodology, and surgical resection remains the first choice for the treatment of GBC.
Different surgical resection methods are used to treat GBC, based on staging. According to the current tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) guidelines, simple cholecystectomy (SC, gallbladder removal alone) is the appropriate treatment for patients with Tis or T1a GBC, radical cholecystectomy (RC, including cholecystectomy, lymph node (LN) dissection, and liver wedge resection) or expanded radical resection of GBC is recommended for patients with T1b-T3 GBC, and surgery is not recommended for T4 GBC[4-6]. However, whether patients with T1b GBC undergo SC or RC had remained controversial for a long time. A previous study found that the long-term survival rate of patients with T1b GBC after SC was equivalent to that after RC[7]. Some studies have also found that the prognosis of patients with T1b who underwent RC of GBC is significantly improved compared to that of patients who underwent cholecystectomy alone[8,9]. Therefore, whether patients with T1b GBC undergo SC or RC remains a clinical problem that surgeons must address.
Recent studies have found that, in addition to TNM staging, tumor pathological grading plays an important role in tumor prognosis and surgical selection. Studies have pointed out that low-grade tumors in the tongue[10], breast[11], and thyroid[12] have a significantly worse prognosis than high-grade tumors in the same locations. Furthermore, studies have pointed out that the median survival of patients with grade I GBC is significantly better than that of patients with grade II-IV GBC, indicating that tumor grade is also an extremely important indicator of the prognosis of GBC[13]. However, a question worthy of discussion is whether pathological classification can be used as the basis for the selection of surgery (SC or RC) in T1b patients.
In the present study, we obtained the treatment and survival data of patients with T1b GBC from the Surveillance, Epidemiology, and End Results (SEER) database[14], analyzed the survival of patients with different histological grades of T1b GBC, and compared the survival of patients who underwent SC, cholecystectomy with LN resection, and RC to assess the optimal surgical approach.
The SEER database was established by the National Cancer Institute and contains follow-up information from patients with cancer. We used the SEER-18 database, derived from 18 regional registries representing approximately one-third of the US population, to collect data on patients with GBC between 2000 and 2017, including patient age, sex, histological codes, tumor histology, TNM stage (6th AJCC TNM staging system), tumor grade, surgical information, and patient survival.
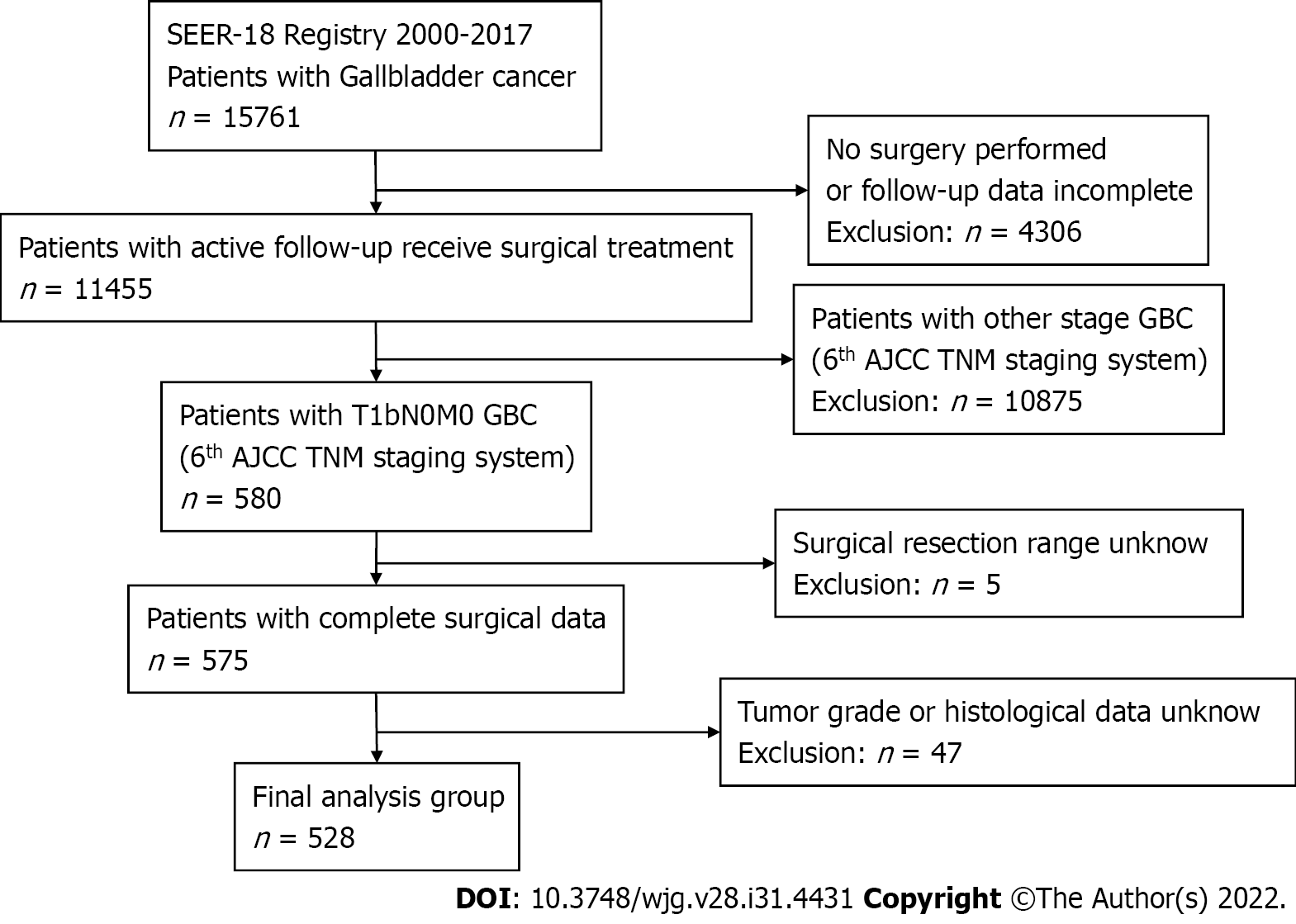
Tumor histology and site codes were used to identify patients in the SEER database with GBC between 2000 and 2017. A total of 15671 patients were included in this study. Patients were excluded from our study for the following reasons: patients did not undergo surgery, patients with GBC other than T1bN0M0, incomplete follow-up data, unknown surgical resection range, tumor grade, or histological data. According to the 6th edition of the AJCC staging system, all T1b patients were staged according to clinical classification based on physical examination, imaging, endoscopy, biopsy, surgical exploration, and other relevant examinations; 528 patients with T1bN0M0 GBC were included (Figure 1). According to the surgical treatment information in the SEER database, patients who underwent SC without LN resection in our study were categorized as SC, those who underwent cholecystectomy with LN resection were categorized as SC + LN, and patients who underwent cholecystectomy and any type of liver resection with extensive LN dissection were categorized as RC. The major outcomes of this study were overall survival (OS) and disease-specific survival (DSS).
Both continuous and categorical variables (such as age and sex, tumor grade, tumor histological type, surgical approach, etc.) are presented as numbers (n) and percentages (%). Kaplan-Meier survival curves were generated to analyze the OS and DSS between the different groups, and the P values for the survival curves were determined using the log-rank test. A multivariate Cox proportional hazards model was built to verify the independent role of prognostic factors, and variables with a P value of < 0.1 on the log-rank test were incorporated into the model. The final model was built using a stepwise selection method, and the results were presented as adjusted hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) and P values. All P values were two-sided, and values of P < 0.05 were considered statistically significant. Statistical analyses were performed using the statistical software Statistical Product and Service Solutions (SPSS) IBM (version 19.0).
In this retrospective study, 528 patients had pathologically confirmed T1bN0M0 GBC between 2000 and 2017. Of these, 385 (72.9%) were women, and 143 (27.1%) were men. The histological types were adenocarcinoma (73.3%), papillary adenocarcinoma (15%), and other tissue types (11.7%). The tumor pathological classification was grade I (30.5%), grade II (50.9%), and grades III and IV (18.6%). Therefore, grade III and grade IV GBC were combined into the same subgroup for analysis in the present study as few patients had grade IV GBC. Among the 528 patients with GBC, 346 underwent SC (65.5%), 131 underwent SC + LN (24.8%), and 51 underwent RC (9.7%) (Table 1).
| Characteristics | n = 528, n (%) | 10-year survival | |
| OS, % | DSS, % | ||
| Age, yr | |||
| < 70 | 220 (41.7) | 46.4% | 62.7% |
| ≥ 70 | 308 (58.3) | 21.9% | 58.9% |
| P < 0.001 | P = 0.023 | ||
| Gender | |||
| Male | 143 (27.1) | 13.7 | 47.5 |
| Female | 385 (72.9) | 37.8 | 64 |
| P = 0.007 | P = 0.016 | ||
| Grade | |||
| Grade I | 161 (30.5) | 33.1 | 64.7 |
| Grade II | 269 (50.9) | 32.6 | 62.1 |
| Grade III, IV | 98 (18.6) | 28.6 | 48.9 |
| P = 0.056 | P = 0.002 | ||
| Histological type | |||
| Adenocarcinoma | 387 (73.3) | 30.5 | 61.6 |
| Papillary | 79 (15.0) | 40.2 | 61.8 |
| Other | 62 (11.7) | 29 | 51 |
| P = 0.059 | P = 0.058 | ||
| Surgery | |||
| SC | 346 (65.5) | 27.8 | 55.1 |
| SC + LN | 131 (24.8) | 35.5 | 66.3 |
| RC | 51 (9.7) | 50.3 | 75.9 |
| P < 0.001 | P = 0.002 | ||
Univariate analysis performed using the log-rank test revealed that the histological type (P = 0.059) and tumor grade (P = 0.056) did not significantly affect the 10-year OS of patients with T1b GBC. Younger age (P < 0.001) and female sex (P = 0.007) were associated with better OS, and patients who underwent RC (50.3%) or SC + LN (35.5%) achieved better OS than those who underwent SC (27.8%) (P < 0.001). The 10-year DSS was not significantly affected by histological type (P = 0.058), similar to the OS, and DSS rates were significantly higher in younger (P = 0.023) and female patients (P = 0.016). The DSS was also significantly affected by the extent of surgery, and the 10-year DSS of patients who underwent RC (75.9%) or SC + LN (66.3%) was higher than that of patients who underwent SC (55.1%) (P = 0.002). Although tumor grade had no significant effect on the 10-year OS of patients with T1b GBC, we found that the 10-year DSS of patients with grade III and IV tumors (48.9%) was significantly lower than that of patients with grade I (64.7%) or II tumors (62.1%) (P = 0.002) (Table 1).
Kaplan-Meier survival curves were generated for different types of surgery in patients with T1b GBC. The 10-year OS of patients who underwent more extensive surgery, including SC + LN (35.5%) and RC (50.3%), was significantly better than that of patients who underwent SC alone (27.8%) (P < 0.001) (Figure 2A). Consistent with OS, we found that the 10-year DSS of patients who underwent SC + LN (66.3%) or RC (75.9%) was significantly higher than that of patients who underwent SC (55.1%) (P = 0.003) (Figure 2B).
Using multivariate Cox regression analysis incorporating age, sex, tumor grade, tumor histological type, and surgery type, we confirmed that age, sex, and surgery type were independently associated with OS in patients with T1b GBC. Patients who underwent SC + LN (HR: 0.71, 95%CI: 0.53-0.95, P = 0.020) or RC (HR: 0.54, 95%CI: 0.32-0.89, P = 0.015) experienced a significant OS benefit compared to patients who underwent SC alone. Independent factors affecting DSS were sex, tumor grade, and surgery type, consistent with OS, and patients who underwent SC + LN (HR: 0.56, 95%CI: 0.37-0.83, P = 0.020) or RC (HR: 0.49, 95%CI: 0.27-0.92, P = 0.015) had improved DSS compared with patients who underwent SC. Table 2 shows the results of the multivariate Cox regression analysis of patients with T1b GBC based on the 10-year OS and DSS. Based on the above results, we concluded that patients with T1b GBC who underwent RC or SC + LN treatment had better OS and DSS than those who underwent SC, regardless of the pathological grade (Table 2).
| Variable | 10-year overall survival | 10-year disease-specific survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | ||||
| < 70 | Referent | Referent | ||
| ≥ 70 | 2.03 (1.57-2.63) | < 0.001 | NA | NA |
| Gender | ||||
| Male | Referent | Referent | ||
| Female | 0.71 (0.55-0.91) | 0.007 | 0.62 (0.45-0.87) | 0.005 |
| Grade | ||||
| Grade I | Referent | Referent | ||
| Grade II | NA | NA | 1.21 (0.83-1.76) | 0.321 |
| Grade III, IV | NA | NA | 2.20 (1.43-3.38) | < 0.001 |
| Histological type | ||||
| Adenocarcinoma | Referent | Referent | ||
| Papillary | NA | NA | NA | NA |
| Other | NA | NA | NA | NA |
| Surgery | ||||
| SC | Referent | Referent | ||
| SC + LN | 0.71 (0.53-0.95) | 0.020 | 0.56 (0.37-0.83) | 0.004 |
| RC | 0.54 (0.32-0.89) | 0.015 | 0.49 (0.27-0.92) | 0.025 |
To verify the role of tumor grading in choosing the surgical approach for patients with T1b GBC, we divided the 528 patients with GBC into three subgroups based on tumor grade and analyzed the impact of different surgical approaches in each subgroup on 10-year OS and DSS. The type of surgery varied slightly between the different tumor grades, and Figure 2C shows the proportion of different surgical approaches in each subgroup. Of the 161 patients with grade I T1b GBC, 105 underwent SC (65%), 40 underwent SC + LN (25%), and 16 underwent RC (10%); of the 269 patients with grade II T1b GBC, 173 underwent SC (64%), 64 underwent SC + LN (24%), and 32 underwent RC (12%). Of the 98 patients with grade III or IV T1b GBC, 68 underwent SC (69%), 25 underwent SC + LN (26%), and 5 underwent RC (5%) (Figure 2C). Interestingly, no statistically significant differences were observed in OS (P = 0.734) and DSS (P = 0.953) between the different surgical types in patients with grade I T1b GBC (Figure 3A and B). However, an obvious improvement in the OS of patients with grade II T1b GBC who underwent SC + LN (34.6%) or RC (50.5%) was observed compared to that of patients who underwent SC (28.1%) (P = 0.002). The DSS of patients who underwent SC + LN (61.3%) or RC (78.8%) was also much higher than that of patients who underwent SC (58.3%) (P = 0.039) (Figure 3C and D). Moreover, the OS and DSS of patients with grade III and IV T1b GBC were both significantly affected by the type of surgery, and SC + LN (48.5%) or RC (80%) had a far more beneficial effect on OS than SC (20.1%; P = 0.005). Similar to OS, the DSS in patients who underwent SC + LN (72.2%) or RC (80%) was also much higher than that of patients who underwent SC (38.1%) (P = 0.009) (Figure 3E and F). These results show that patients with grade I T1b GBC who undergo SC can attain a survival benefit equivalent to that associated with SC + LN or RC.
To further verify that surgery type did not significantly affect OS and DSS in patients with grade I T1b GBC, we conducted a univariate analysis of 161 patients with grade I T1b GBC. Using the log-rank test, we found that age (P = 0.022) and sex (P = 0.030) significantly affected the OS of patients with grade I T1b GBC, whereas the histological type of the tumor (P = 0.799) and surgical method (P = 0.734) had no significant effect on OS. Age (P = 0.431), sex (P = 0.071), tumor histological type (P = 0.562), and surgical method (P = 0.953) did not significantly affect DSS in patients with grade I T1b GBC (Table 3).
| Characteristics | n = 161, n (%) | 10-year survival | |
| OS, % | DSS, % | ||
| Age, yr | |||
| < 70 | 72 (41.7) | 65.8 | 70.6 |
| ≥ 70 | 89 (58.3) | 58.0 | 79.1 |
| P = 0.0221 | P = 0.431 | ||
| Gender | |||
| Male | 49 (27.1) | 54.6 | 72.6 |
| Female | 112 (72.9) | 63.6 | 75.9 |
| P = 0.0301 | P = 0.071 | ||
| Histological type | |||
| Adenocarcinoma | 116 (73.3) | 62.8 | 76.2 |
| Papillary | 32 (15.0) | 60.1 | 74.6 |
| Other | 13 (11.7) | 46.2 | 60.6 |
| P = 0.799 | P = 0.562 | ||
| Surgery | |||
| SC | 105 (65.5) | 60.1 | 76.1 |
| SC + LN | 41 (24.8) | 65.2 | 75.3 |
| RC | 15 (9.7) | 56.6 | 67.0 |
| P = 0.734 | P = 0.953 | ||
Subsequently, we performed multivariate Cox regression analysis based on age, sex, tumor grade, tumor histological type, and surgery type and found that age and sex were independent influencing factors for OS in patients with grade I T1b GBC; older age was associated with poor OS (HR: 1.67, 95%CI: 1.07-2.59, P = 0.023), and women had better OS (HR: 0.62, 95%CI: 0.40-0.96, P = 0.031). The histological type of the tumor and surgical method were not independent factors for OS in patients with grade I T1b GBC. Age, sex, histological tumor type, and surgical method were not independent risk factors for DSS in patients with grade I T1b GBC. Lastly, we verified that the type of surgery did not affect the OS and DSS of 161 patients with grade I T1b GBC (Table 4).
| Variable | 10-year overall survival | 10-year disease-specific survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | ||||
| < 70 | Referent | Referent | ||
| ≥ 70 | 1.67 (1.07-2.59) | 0.023 | NA | NA |
| Gender | ||||
| Male | Referent | Referent | ||
| Female | 0.62 (0.40-0.96) | 0.031 | NA | NA |
| Histological type | ||||
| Adenocarcinoma | Referent | Referent | ||
| Papillary | NA | NA | NA | NA |
| Other | NA | NA | NA | NA |
| Surgery | ||||
| SC | Referent | Referent | ||
| SC + LN | NA | NA | NA | NA |
| RC | NA | NA | NA | NA |
The current staging of GBC follows the TNM staging system of the AJCC guidelines, in which primary tumor invasion (T) is a crucial factor in the AJCC staging criteria that determines the surgical approach for GBC[15]. The goal of surgical intervention for GBC is to achieve R0 resection, which is the most important factor in predicting long-term survival. According to the staging system of the AJCC guidelines, Tis refers to the tumor in situ, T1a lesions invade the lamina propria, T1b lesions invade the muscular layer, T2 Lesions invade the connective tissue around the gallbladder muscle without extending to the serosal membrane or liver, T3 tumors perforate the gallbladder serosa or penetrate the liver or one other adjacent organ, and T4 tumors are defined as those that invade the main portal vein, hepatic artery, or two or more adjacent organs[16]. According to the recommendations of the National Comprehensive Cancer Network (NCCN) diagnosis and treatment guidelines, surgical treatment for Tis and T1a GBC should be SC, and RC is the first choice for the treatment of GBC with T1b-T3, whereas surgery is not recommended for T4[17]. Nevertheless, previous studies have also pointed out the controversy in the treatment of T1b, and some studies still reported long-term survival after SC for patients with T1b GBC that is comparable to that after radical resection and did not recommend extended cholecystectomy or radical resection for T1b GBC[18,19]. Therefore, controversy about the surgical treatment of T1b GBC has existed in clinical practice for many years. For example: (1) Should patients with GBC diagnosed as T1b by pathology undergo radical resection; (2) incidental CBC (IGBC) is defined as cholecystectomy for benign gallbladder lesions, but the postoperative pathological diagnosis is GBC[20,21]; and (3) should patients with T1b IGBC undergo another surgical procedure so that RC can be performed?
Tumor grade was categorized as well-differentiated (grade I), moderately differentiated (grade II), poorly differentiated (grade III), or undifferentiated (grade IV), depending on the pathological morphology of the tumor. As an important tumor index, pathological grading plays a crucial role in the prognosis and treatment of many tumors. For example, patients with differentiated T1N0M0 thyroid cancer should undergo total thyroidectomy, whereas patients with undifferentiated T1N0M0 thyroid cancer require total thyroidectomy and LN dissection[22]. However, to our knowledge, the role of tumor grade in the selection of surgical treatment for T1b GBC has not yet been explored. In our study, 528 cases of T1bN0M0 GBC were grouped according to the pathological grade. By analyzing the survival of patients with various pathological grades of GBC following different surgical methods, we found that both DSS and OS of patients with grade II-IV T1b GBC who underwent extensive surgery improved markedly compared to those who underwent SC. However, SC had a comparable survival benefit for both OS and DSS in patients with grade I T1b GBC compared to patients who underwent SC + LN or RC. Using Cox regression analysis, we also found that surgery type was not an independent factor associated with survival in patients with grade I T1b GBC. These results indicate that the surgery type does not significantly affect OS or DSS in patients with grade I T1b GBC. Patients with grade I T1b GBC who undergo SC alone could obtain a similar survival benefit compared with those treated with SC + LN or RC, and more extensive surgery is the optimal treatment for T1b patients with grade II-IV tumors.
Thus, according to the NCCN guidelines and our research results, we propose the following answers to the two questions given above: (1) For GBC found during the operation, if perioperative frozen pathological examination reveals the TNM stage to be T1b, tumor histopathology should continue to be graded. If the pathological grade is confirmed to be grade I T1b GBC, SC should be performed, and patients with grade II-IV T1b GBC should undergo RC; and (2) for patients with T1b IGBC, the finding should be combined with the pathological grade to decide whether to perform surgery again; patients with grade II-IV T1b IGBC should undergo a second operation and radical resection of GBC to obtain survival benefits. Patients with grade I T1b IGBC do not need to undergo reoperation, which prevents the pain caused by the second operation and saves the patient money in terms of medical expenses.
To the best of our knowledge, this study is the first to use the SEER database, one of the largest population databases, to evaluate the role of tumor grading in choosing surgical approaches for patients with T1b GBC. However, our findings have some unavoidable limitations. First, this study is a retrospective analysis, and the SEER database lists only initial surgical treatment, so patients who underwent subsequent treatment may be included in our study. Furthermore, information on tumor recurrence, metastasis, or progression was also not available in the SEER database. Hence, prospective and multi-center studies of patients with T1b GBC are needed to further verify the impact of surgical methods on the prognosis of T1b patients with different pathological grades to provide a more appropriate, evidence-based rationale for determining the optimal surgical method for patients with T1b GBC of different pathological grades, and patients with grade I T1b GBC who underwent SC should be monitored for tumor recurrence, metastasis, or progression to validate the findings of our study.
We demonstrated a comparable survival benefit for patients with grade I T1b GBC who underwent SC, SC + LN or RC, whereas patients with grade II-IV T1b GBC benefit from SC + LN or RC, suggesting that SC may be a suitable treatment for patients with grade I T1b GBC, whereas RC or expanded radical resection is more suitable for those with grade II-IV T1b GBC.
The surgical treatment of T1b gallbladder cancer has been controversial.
To solve the problem of choosing the surgical treatment for T1b gallbladder cancer.
To explore the optimal surgical approach in patients with T1b gallbladder cancer of different pathological grades.
Analysis of survival differences in patients with different pathological grades of T1b gallbladder cancer who received different surgical treatment methods.
Patients with grade I T1b gallbladder cancer who underwent simple cholecystectomy attained a survival benefit equivalent to that of simple cholecystectomy with lymph node resection or radical cholecystectomy.
Simple cholecystectomy is an adequate treatment for grade I T1b gallbladder carcinoma (GBC), whereas expanded radical resection is more suitable for grade II-IV T1b GBC.
Simple cholecystectomy is a suitable treatment for patients with grade I T1b GBC.
The authors acknowledge the efforts of the SEER Program tumor registries in providing high-quality open resources for research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra TS, India; Sahin TT, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon. 2008;6:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 4. | Misra MC, Guleria S. Management of cancer gallbladder found as a surprise on a resected gallbladder specimen. J Surg Oncol. 2006;93:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica MI, Davila R, Ensminger WD, Gibbs JF, Laheru D, Malafa MP, Marrero J, Meranze SG, Mulvihill SJ, Park JO, Posey JA, Sachdev J, Salem R, Sigurdson ER, Sofocleous C, Vauthey JN, Venook AP, Goff LW, Yen Y, Zhu AX. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 8. | Wagholikar GD, Behari A, Krishnani N, Kumar A, Sikora SS, Saxena R, Kapoor VK. Early gallbladder cancer. J Am Coll Surg. 2002;194:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cucinotta E, Lorenzini C, Melita G, Iapichino G, Currò G. Incidental gall bladder carcinoma: does the surgical approach influence the outcome? ANZ J Surg. 2005;75:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Chuang ST, Chen CC, Yang SF, Chan LP, Kao YH, Huang MY, Tang JY, Huang CM, Huang CJ. Tumor histologic grade as a risk factor for neck recurrence in patients with T1-2N0 early tongue cancer. Oral Oncol. 2020;106:104706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Metzger-Filho O, Ferreira AR, Jeselsohn R, Barry WT, Dillon DA, Brock JE, Vaz-Luis I, Hughes ME, Winer EP, Lin NU. Mixed Invasive Ductal and Lobular Carcinoma of the Breast: Prognosis and the Importance of Histologic Grade. Oncologist. 2019;24:e441-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Ho AS, Luu M, Barrios L, Balzer BL, Bose S, Fan X, Walgama E, Mallen-St Clair J, Alam U, Shafqat I, Lin DC, Chen Y, Van Eyk JE, Maghami EG, Braunstein GD, Sacks WL, Zumsteg ZS. Prognostic Impact of Histologic Grade for Papillary Thyroid Carcinoma. Ann Surg Oncol. 2021;28:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Shibata K, Uchida H, Iwaki K, Kai S, Ohta M, Kitano S. Lymphatic invasion: an important prognostic factor for stages T1b-T3 gallbladder cancer and an indication for additional radical resection of incidental gallbladder cancer. World J Surg. 2009;33:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg. 2018;153:588-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 15. | Giannis D, Cerullo M, Moris D, Shah KN, Herbert G, Zani S, Blazer DG 3rd, Allen PJ, Lidsky ME. Validation of the 8th Edition American Joint Commission on Cancer (AJCC) Gallbladder Cancer Staging System: Prognostic Discrimination and Identification of Key Predictive Factors. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Liao X, Zhang D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch Pathol Lab Med. 2021;145:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Benson AB 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 18. | Lee H, Kwon W, Han Y, Kim JR, Kim SW, Jang JY. Optimal extent of surgery for early gallbladder cancer with regard to long-term survival: a meta-analysis. J Hepatobiliary Pancreat Sci. 2018;25:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 19. | Kim HS, Park JW, Kim H, Han Y, Kwon W, Kim SW, Hwang YJ, Kim SG, Kwon HJ, Vinuela E, Járufe N, Roa JC, Han IW, Heo JS, Choi SH, Choi DW, Ahn KS, Kang KJ, Lee W, Jeong CY, Hong SC, Troncoso A, Losada H, Han SS, Park SJ, Yanagimoto H, Endo I, Kubota K, Wakai T, Ajiki T, Adsay NV, Jang JY. Optimal surgical treatment in patients with T1b gallbladder cancer: An international multicenter study. J Hepatobiliary Pancreat Sci. 2018;25:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Varshney S, Butturini G, Gupta R. Incidental carcinoma of the gallbladder. Eur J Surg Oncol. 2002;28:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 22. | Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018;68:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |