Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4376
Peer-review started: April 20, 2022
First decision: June 2, 2022
Revised: June 14, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 21, 2022
Processing time: 118 Days and 2.8 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy with a rising incidence worldwide. The prognosis of HCC patients after radical resection remains poor. Radiomics is a novel machine learning method that extracts quantitative features from medical images and provides predictive information of cancer, which can assist with cancer diagnosis, therapeutic decision-making and prognosis improvement.
To develop and validate a contrast-enhanced computed tomography-based radio
A total of 150 HCC patients were randomly divided into a training cohort (n = 107) and a validation cohort (n = 43). Radiomics features were extracted from the entire tumour lesion. The least absolute shrinkage and selection operator algorithm was applied for the selection of radiomics features and the construction of the radiomics signature. Univariate and multivariate Cox regression analyses were used to identify the independent prognostic factors and develop the pred
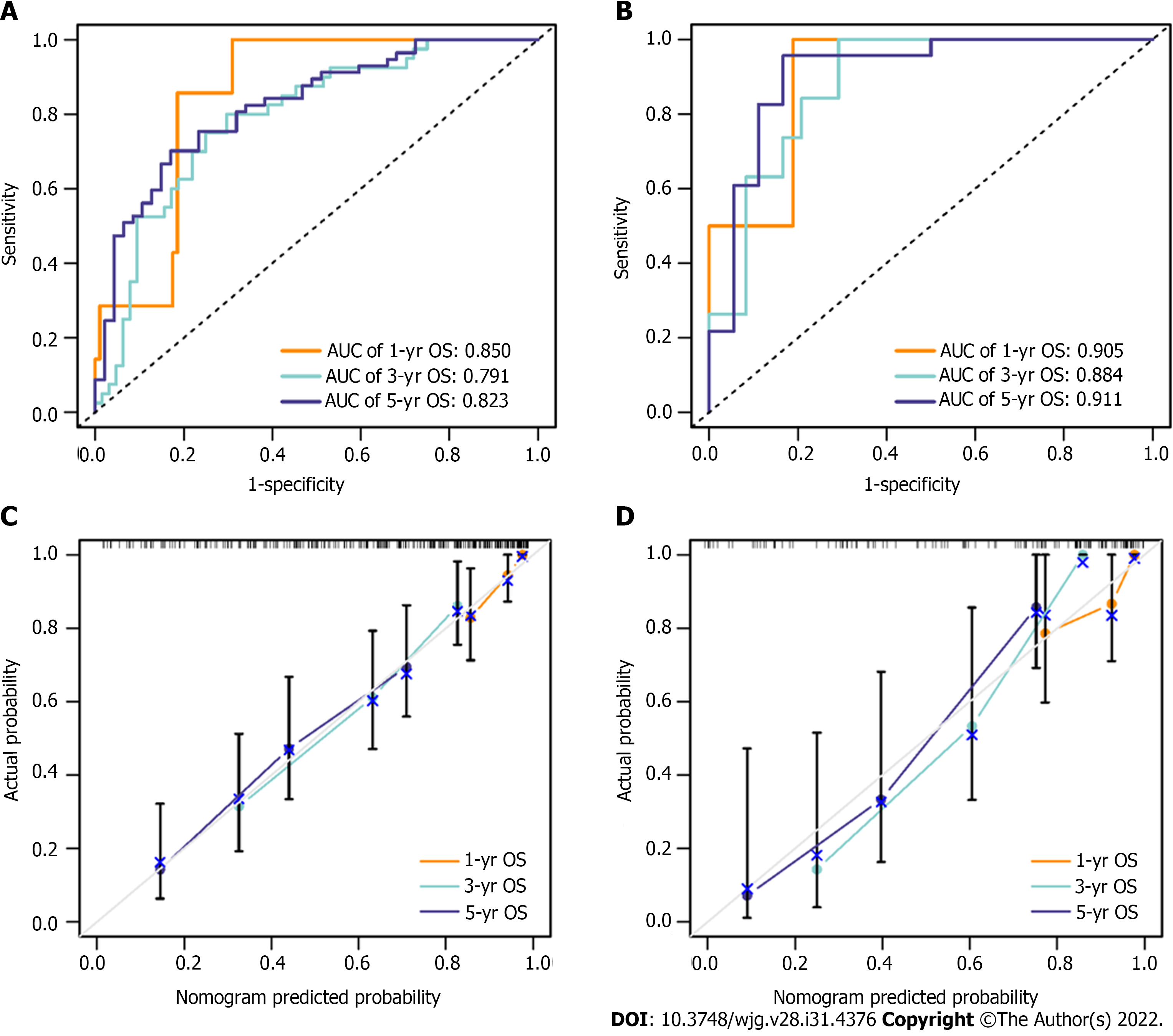
In total, seven radiomics features were selected to construct the radiomics signature. According to the results of univariate and multivariate Cox regression analyses, alpha-fetoprotein (AFP), neutrophil-to-lymphocyte ratio (NLR) and radiomics signature were included to build the nomogram. The C-indices of the nomogram in the training and validation cohorts were 0.736 and 0.774, respectively. ROC curve analysis for predicting 1-, 3-, and 5-year OS confirmed satisfactory accuracy [training cohort, area under the curve (AUC) = 0.850, 0.791 and 0.823, respectively; validation cohort, AUC = 0.905, 0.884 and 0.911, respectively]. The calibration curve analysis indicated a good agreement between the nomogram-prediction and actual survival. DCA curves suggested that the nomogram had more benefit than traditional staging system models. Kaplan–Meier survival analysis indicated that patients in the low-risk group had longer OS and disease-free survival (all P < 0.0001).
The nomogram containing the radiomics signature, NLR and AFP is a reliable tool for predicting the OS of HCC patients.
Core Tip: The prognosis of hepatocellular carcinoma (HCC) patients remains poor even after radical resection. Therefore, a precise and reliable tool to predict the prognosis of HCC patients is urgently needed. We established a predictive model incorporating radiomics features extracted from preoperative contrast-enhanced computed tomography images, alpha-fetoprotein and neutrophil-to-lymphocyte ratio to predict the overall survival of patients with HCC, and the model was visualized via a nomogram. The nomogram showed good accuracy for survival prediction.
- Citation: Deng PZ, Zhao BG, Huang XH, Xu TF, Chen ZJ, Wei QF, Liu XY, Guo YQ, Yuan SG, Liao WJ. Preoperative contrast-enhanced computed tomography-based radiomics model for overall survival prediction in hepatocellular carcinoma. World J Gastroenterol 2022; 28(31): 4376-4389
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4376.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4376
Primary liver cancer is the sixth most common malignancy and the third leading cause of cancer-related mortality in the world. Hepatocellular carcinoma (HCC) accounts for 75%-85% of primary liver cancers[1]. Presently, the main therapies for HCC include surgical resection, local ablation, interventional embolization and liver transplantation. For HCC patients with early-stage disease, hepatectomy and liver transplantation are the mainstay curative treatments. Due to the insidious onset and lack of evident clinical symptoms in the early stage, patients with HCC are often diagnosed at an advanced stage. Even after surgical resection, the prognosis of HCC patients remains poor due to postoperative recurrence and metastasis. It has been reported that the recurrence rate within 5 years reaches 60%[2].
Alpha-fetoprotein (AFP) has been widely applied as a biomarker of HCC for diagnosis, monitoring treatment response, assessing prognosis and detecting early recurrence. However, the specificity of AFP for diagnosing HCC is 99%-100%, but the sensitivity is only 20% to 45%[3]. Moreover, nearly 31% of patients with HCC are AFP negative[4]. Therefore, AFP still has limitations as a biomarker of HCC. It has been reported that the tumour microenvironment is closely related to the initiation and progression of HCC[5]. Recent studies have shown that a high density of tumour-infiltrating lymphocytes is associated with favourable outcomes[6]. The neutrophil-to-lymphocyte ratio (NLR) was reported to be an independent prognostic factor for patients with HCC[7,8].
Radiomics is a new method of medical image analysis that uses a series of data-mining algorithms or statistical analysis tools for the high-throughput extraction of quantitative metric features[9,10] to obtain prognostic and predictive information for clinical decision support. It has been recognized that intratumor heterogeneity is often associated with tumour subtyping and can significantly impact prognosis and response to treatment[11,12]. Traditional radiological analysis is mainly based on naked-eye observation, which primarily focuses on tumour size and anatomical location but ignores intratumor heterogeneity. Radiomics features are able to provide a comprehensive overview of intratumor heterogeneity in a noninvasive manner[13]. Several recent studies indicate that radiomics features may potentially be a useful diagnostic and prognostic biomarker in liver cancer and other tumour types[14-16].
Due to the insufficiency of accurateness and objectiveness for prognostic markers in the prognostic evaluation of HCC patients, a precise and reliable tool to predict the prognosis of HCC patients is urgently needed. In the present study, we aimed to develop and validate a model based on contrast-enhanced computed tomography (CECT) images and clinical-pathologic characteristics to predict the overall survival (OS) of HCC patients.
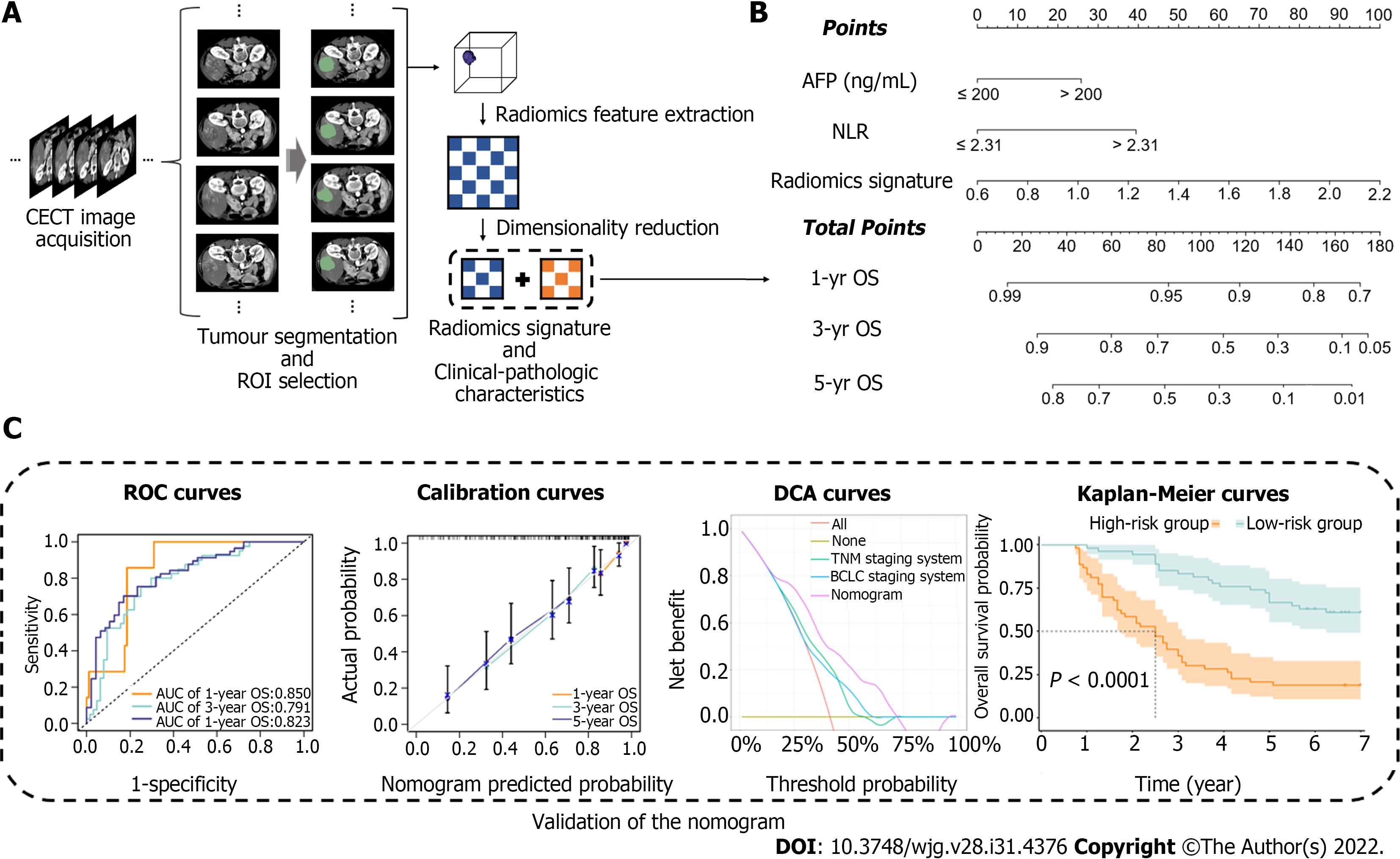
This study was composed of the following steps: (1) Patient recruitment and data collection; (2) CECT image acquisition, tumour segmentation, region of interest (ROI) selection, radiomics feature extraction and radiomics signature construction; (3) The radiomics signature and clinical-pathologic characteristics were combined to build a predictive model and visualized via a nomogram; and (4) Evaluation of the predictive model using receiver operating characteristic (ROC) curves, calibration curves, decision curve analysis (DCA) and Kaplan–Meier curves.
This retrospective study was approved by the research ethics committee of the Affiliated Hospital of Guilin Medical University and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
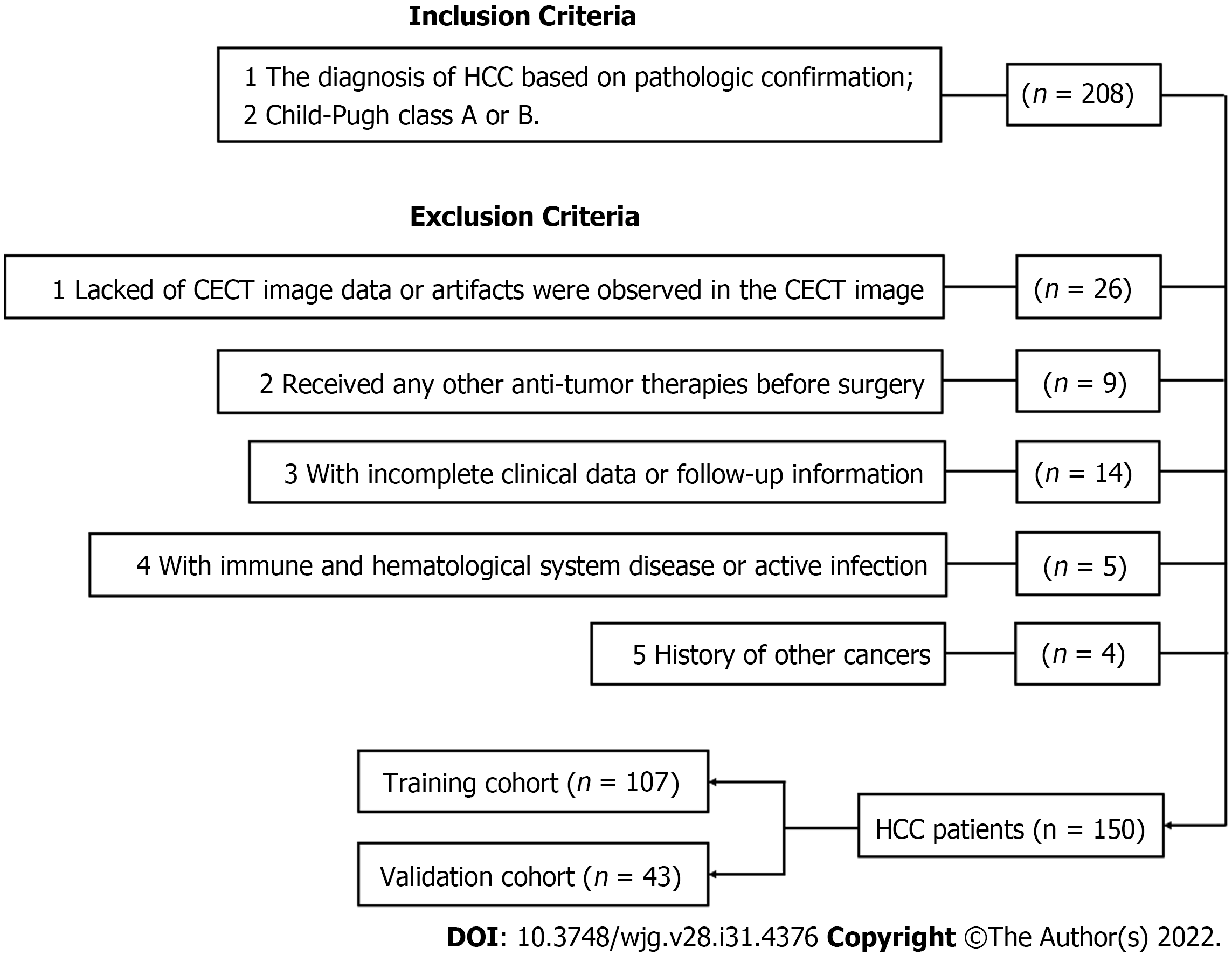
A total of 208 HCC patients who underwent radical resection at the Affiliated Hospital of Guilin Medical University with pathologically confirmed HCC were recruited from January 2014 to September 2017. Among them, 150 individuals fulfilled the inclusion and exclusion criteria (Figure 1). Radical resection was defined as a completed resection of the tumour mass with pathologically confirmed negative margins and no residual tumour or new lesion observed in two observations at an interval of no less than 4 wk. All tumour tissue samples were diagnosed by at least two experienced pathologists independently. All patients underwent CECT scans and haematological examinations before surgery. The 150 enrolled HCC patients were randomly divided into a training cohort (n = 107) and a validation cohort (n = 43) at a ratio of 2.5:1. Demographic and clinical-pathologic data were collected from medical records, including age, sex, alanine aminotransferase, AFP, American Joint Committee on Cancer tumor, node and metastasis (TNM) stage, Barcelona clinic liver cancer (BCLC) stage, hepatitis B surface antigen (HBsAg) and NLR.
Each patient was followed up via outpatient review. Routine postoperative examinations, including routine blood tests, liver function tests, renal function tests, serum AFP levels and abdominal ultrasonography, were performed every 2 mo after surgery within 2 years and then every 6 mo thereafter. A CECT scan was recommended if the examination results were abnormal or tumour recurrence was suspected. For patients who did not attend the follow-up visit, follow-up information was obtained by phone. OS was defined as the time from surgery to death or the last follow-up date, while disease-free survival (DFS) was defined as the time from surgery to tumour first intrahepatic and/or extrahepatic recurrence, death, or the last follow-up date.
Abdominal CECT scans were performed with two scanners: A Lightspeed VCT XT (GE Healthcare, United States) and an Optima CT660 (GE Healthcare, United States). The scanner was operated in cine mode, and the parameters were as follows: tube voltage of 120 kV, automatic tube current modulation with noise index of 8, tube rotation speed of 600 ms, pitch of 0.985:1, collimator of 0.625 mm. Iopromide (Ultravist 300, Bayer-Schering Pharma, Germany) was given intravenously in a volume of 1.5 mL/kg at a rate of 4 mL/s via the antecubital vein. The slice thickness and interval of the arterial and portal venous phase images was 5 mm. All images were reconstructed into images with a 1.25 mm slice thickness and 1.25 mm interval. All data were transferred to an advanced workstation (AW 4.7).
All CECT images in this study met the criteria delineated by the American Association for the Study of Liver Disease guidelines[17]. CECT images were exported in digital imaging and communication in medicine (DICOM) format from the picture archiving and communication system database. All DICOM images were converted to neuroimaging informatics technology initiative format by the SimpleITK package (version 1.2.0) of Python software (version 3.7).
Tumour segmentation was performed by 3D Slicer software (version 4.11.20210226). ROIs were drawn on each layer of the tumour in the horizontal plane from the upper boundary to the lower boundary. Tumour lesions were semiautomatically outlined on all arterial phase and portal venous phase images, and manual corrections were implemented whenever necessary. The images were reviewed independently by two blinded radiologists with 7 and 8 years of experience, and a third radiologist resolved any discrepancies. For patients with multiple tumours, only the largest tumour was selected.
CECT image normalization and radiomics feature extraction were conducted by the Pyradiomics package (version 3.0.3) of Python software. The radiomics features extracted from ROIs included first order features, shape features (2D and 3D), gray level co-occurrence matrix features, gray level size zone matrix features, gray level run length matrix features, gray level dependence matrix features and neighbouring gray tone difference matrix features. Due to the large number of features, dimensionality reduction was essential. The least absolute shrinkage and selection operator (LASSO) algorithm with a 10-fold cross-validation approach was used to reduce the data dimension in the training cohort. Afterwards, the radiomics score, which was defined as the radiomics signature, was generated by linearly combining the selected radiomics features and their weighted coefficients. The workflow of radiomics analysis and radiomics signature construction is shown in Figure 2A. Afterwards, the concordance index (C-index) and ROC curve analysis were used to estimate the predictive value of the radiomics signature for 1-, 3- and 5-year OS.
According to our previous study[18], the optimal cut-off value for NLR in predicting the prognosis of HCC patients after curative resection was 2.31, which had both maximum sensitivity and specificity [area under the curve (AUC) = 0.723, 95%CI: 0.664–0.777].
Normality of distributions was tested by the Shapiro–Wilk test. Clinical-pathologic characteristics were compared by Student’s t test and are presented as the mean ± SD for continuous variables conforming to a normal distribution. Nonnormally distributed continuous variables were compared using the Wilcoxon signed rank test and are presented as the median with interquartile range. Categorical variables were compared using the Pearson χ2 test. Univariate and multivariate regression analyses were performed in the training cohort using the Cox proportional hazards model to identify the independent predictors for nomogram construction. The rms and regplot packages were used to establish a nomogram and calibration curve. The ROC curve analysis was performed using the timeROC package. The C-index, ROC curve and calibration curve were used to assess the accuracy of the nomogram. The Kaplan–Meier method and log-rank test were conducted to compare the different survival rates between the low- and high-risk subgroups in different cohorts. We built two predictive models based on BCLC and TNM staging systems. Patients were categorized into stage 0, stage A, stage B and stage C according to BCLC staging system; grade I, grade II, grade III and grade IV according to TNM staging system. DCA was conducted to compare the abovementioned two traditional staging systems with the nomogram. All statistical analyses were performed using SPSS software (version 24.0) or R software (version 4.1.2) and accepted as significant at P < 0.05.
A total of 150 HCC patients were enrolled in this study according to inclusion and exclusion criteria, with an average age of 49.9 years (range, 20-75 years), and 130 patients were male. A total of 136 patients were diagnosed with cirrhosis, while 137 patients were classified as Child–Pugh class A. A total of 124 patients were positive for HBsAg. Patients were randomly divided into a training cohort (n = 107) and a validation cohort (n = 43). The demographic and clinical-pathologic characteristics are summarized in Table 1. There were no significant differences in variables between the two cohorts.
| Variables | Training cohort (n = 107) | Validation cohort (n = 43) | P value |
| Age, yr (mean ± SD) | 49.77 ± 10.57 | 50.35 ± 11.43 | 0.766 |
| Sex (male/female) | 94/13 | 36/7 | 0.501 |
| Alcohol abuse (present/absent) | 44/63 | 19/24 | 0.731 |
| Tumor number (multiple/single) | 22/85 | 10/33 | 0.716 |
| Tumor diameter, cm (> 5/≤ 5) | 69/38 | 24/19 | 0.322 |
| MVI (present/absent) | 46/61 | 17/26 | 0.698 |
| Cirrhosis (present/absent) | 100/7 | 36/7 | 0.123 |
| TNM stage (I-II/III-IV) | 54/53 | 18/25 | 0.340 |
| BCLC stage (0-A/B-C) | 61/46 | 20/23 | 0.243 |
| WBC, × 109/L (median, IQR) | 6.05 (5.00-7.3) | 6.11 (5.04-8.04) | 0.290 |
| Platelets, × 109/L (mean ± SD) | 190.75 ± 71.91 | 202.02 ± 79.32 | 0.401 |
| LYMPH, × 109/L (median, IQR) | 1.63 (1.19-1.94) | 1.74 (1.35-2.04) | 0.229 |
| NEUT, × 109/L (median, IQR) | 3.75 (2.68-4.07) | 3.90 (2.85-5.17) | 0.385 |
| DB, μmol/L (median, IQR) | 5.20 (4.20-5.81) | 4.85 (3.68-5.56) | 0.077 |
| TB, μmol/L (median, IQR) | 12.50 (9.43-15.91) | 13.38 (8.93-17.11) | 0.840 |
| ALB, g/L (mean ± SD) | 38.42 ± 4.30 | 36.87 ± 5.49 | 0.069 |
| GLB, g/L (median, IQR) | 31.80 (28.80-35.28) | 32.22 (29.80-35.44) | 0.480 |
| GGT, U/L (mean ± SD) | 115.26 ± 136.68 | 105.49 ± 82.44 | 0.662 |
| ALT, U/L (median, IQR) | 31.00 (21.24-44.14) | 27.60 (17.46-42.88) | 0.306 |
| AST, U/L (median, IQR) | 34.50 (26.55-51.51) | 35.40 (25.55-46.02) | 0.891 |
| AFP, ng/mL (> 200/≤ 200) | 50/57 | 23/20 | 0.454 |
| HBsAg (positive/negative) | 91/16 | 33/10 | 0.225 |
| NLR (> 2.31/≤ 2.31) | 50/57 | 21/22 | 0.815 |
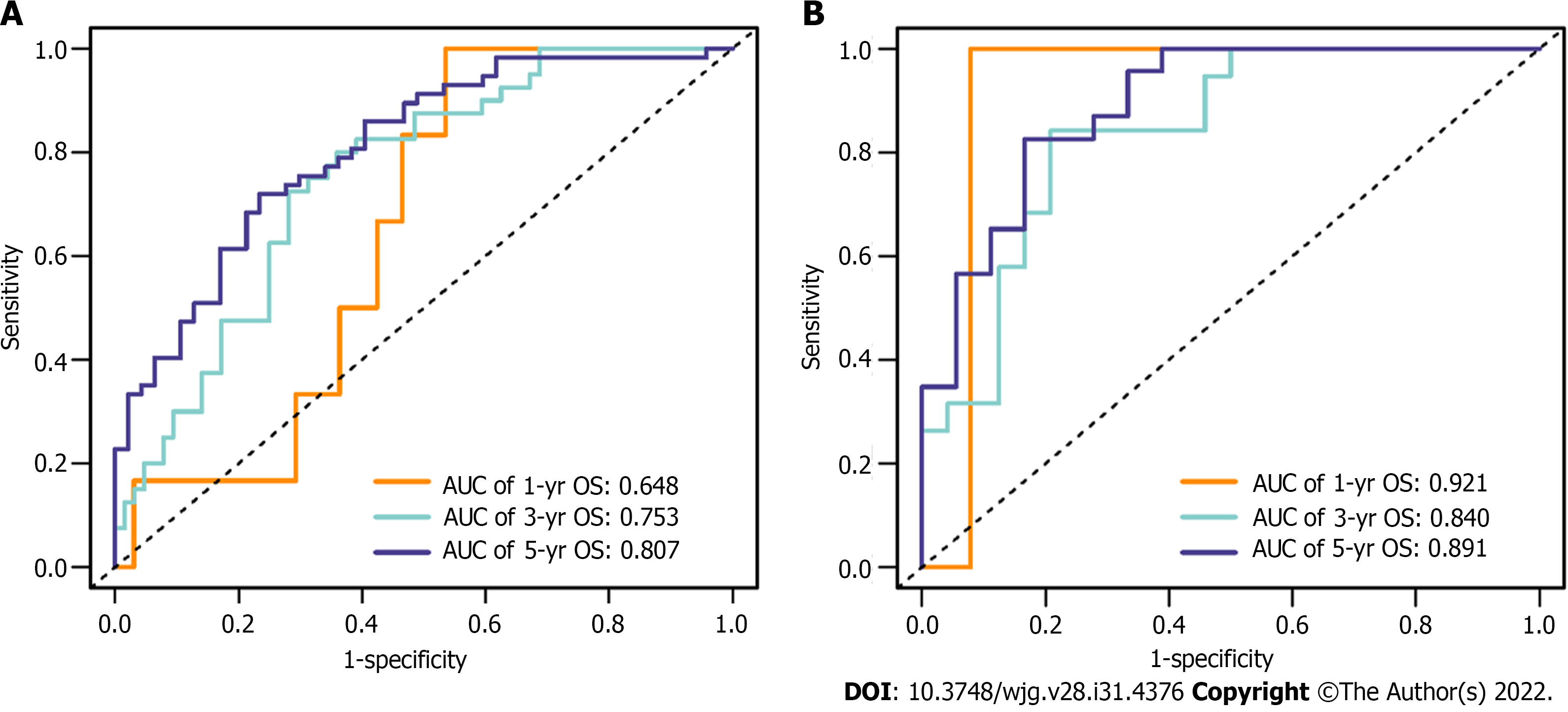
In total, 1926 radiomics features were extracted from the ROIs. Based on the training cohort, radiomics features were reduced to 7 survival-related features by using the LASSO algorithm. The name of the selected features and the formula of the radiomics score are shown in Supplementary Table 1. The C-indices of the radiomics signature for predicting OS in the training and validation cohorts were 0.689 (95%CI: 0.626–0.751) and 0.746 (95%CI: 0.650–0.842), respectively. The ROC curves of the radiomics signature for predicting 1-, 3- and 5-year OS are shown in Figure 3 (AUC = 0.648, 0.753 and 0.807, respectively, in the training cohort; AUC = 0.921, 0.840 and 0.891, respectively, in the validation cohort).
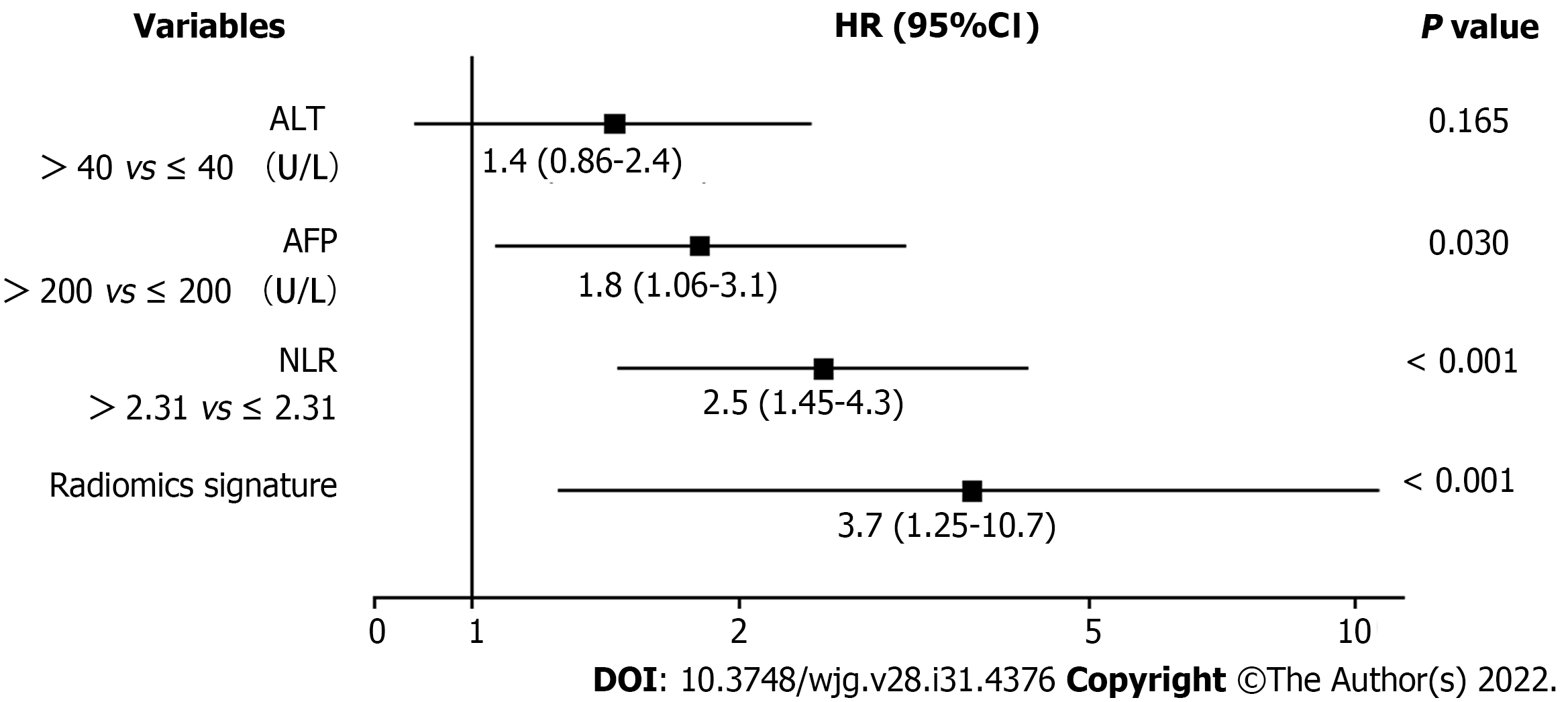
Based on the univariate regression analysis (Table 2), four variables with P < 0.05 were enrolled in the multivariate regression analysis. The results of multivariate regression analysis are displayed as forest plots (Figure 4). AFP [hazard ratio (HR), 1.8; 95%CI: 1.06–3.1, P = 0.03], NLR (HR, 2.5; 95%CI: 1.45–4.3, P < 0.001) and radiomics signature (HR, 3.7; 95%CI: 1.25–10.7, P = 0.018) were identified as independent predictors of OS. The abovementioned three variables were included to develop the predictive model and visualized via a nomogram (Figure 2B). The C-indices of the nomogram in the training and validation cohorts were 0.736 (95%CI: 0.681–0.791) and 0.774 (95%CI: 0.697–0.851), respectively. The AUC values of 1-, 3-, and 5-year OS were 0.850, 0.791 and 0.823, respectively, in the training cohort (Figure 5A) and 0.905, 0.884 and 0.911, respectively, in the validation cohort (Figure 5B). The calibration curves of the nomogram demonstrated good agreement between the predicted and actual survival probabilities (Figure 5C and D).
| Variables | Univariate Cox regression analysis | ||
| HR | 95%CI | P value | |
| Age, yr | 0.993 | 0.971-1.016 | 0.545 |
| Sex (male vs female) | 0.789 | 0.376-1.657 | 0.532 |
| Alcohol abuse (present vs absent) | 1.133 | 0.692-1.854 | 0.620 |
| Tumor number (multiple vs single) | 1.060 | 0.594-1.892 | 0.843 |
| Tumor diameter, cm (> 5 vs ≤ 5) | 1.147 | 0.681-1.934 | 0.606 |
| MVI (present vs absent) | 1.506 | 0.921-2.461 | 0.102 |
| Cirrhosis (present vs absent) | 1.112 | 0.404-3.063 | 0.837 |
| DB, μmol/L (> 6.8 vs ≤ 6.8) | 1.551 | 0.766-3.140 | 0.223 |
| ALB, g/L (> 35 vs ≤ 35) | 0.677 | 0.388-1.181 | 0.169 |
| GGT, U/L (> 50 vs ≤ 50) | 1.482 | 0.879-2.499 | 0.140 |
| ALT, U/L (> 40 vs ≤ 40) | 1.671 | 1.002-2.787 | 0.049 |
| AFP, ng/mL (> 200 vs ≤ 200) | 2.202 | 1.340-3.619 | 0.002 |
| HBsAg (positive vs negative) | 1.013 | 0.516-1.991 | 0.969 |
| NLR (> 2.31 vs ≤ 2.31) | 3.160 | 1.901-5.251 | < 0.001 |
| Radiomics signature | 10.315 | 3.829-27.786 | < 0.001 |
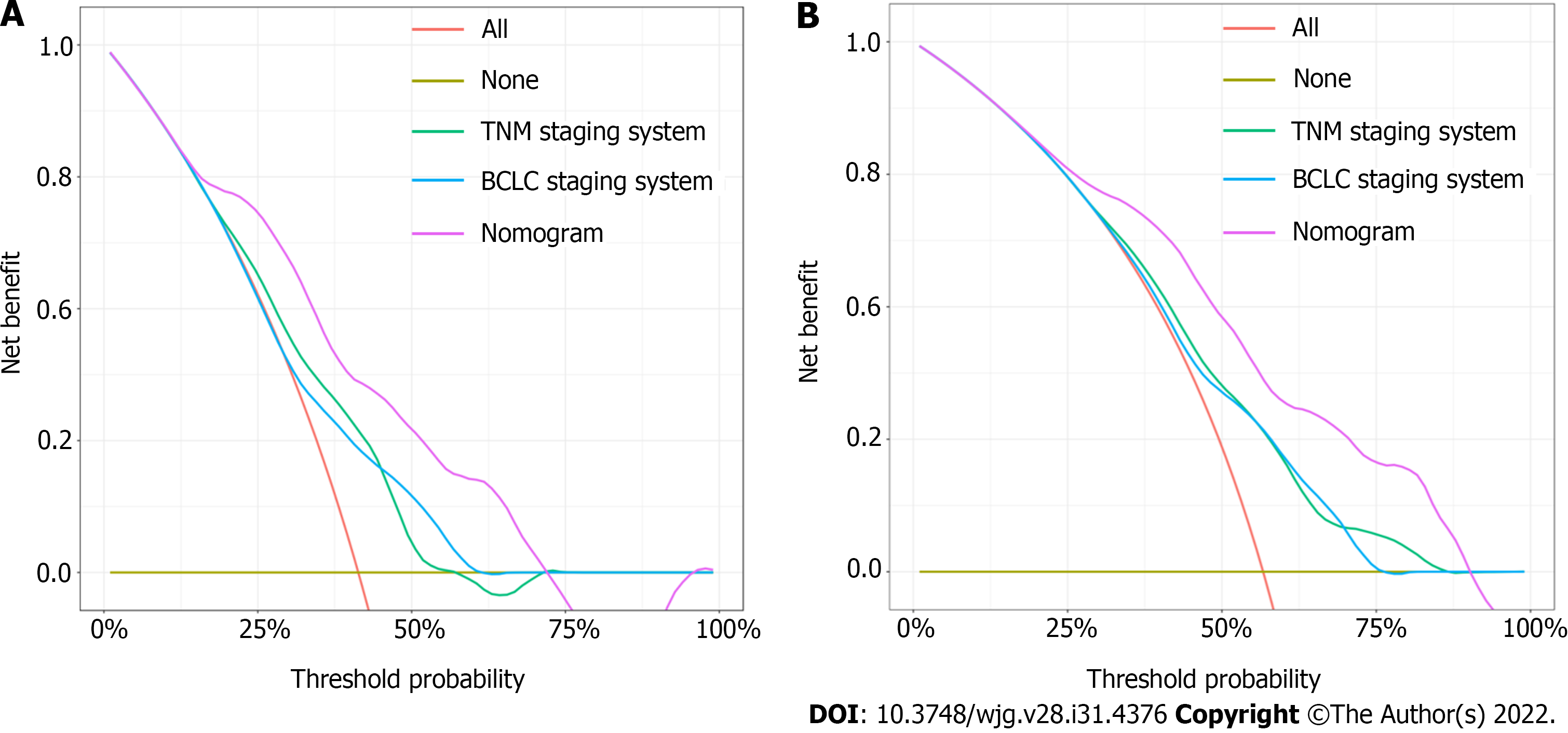
DCA curves showed that the nomogram received more net benefit than the BCLC staging system model and TNM staging system model in predicting 3- and 5-year OS at reasonable threshold probability (Figure 6).
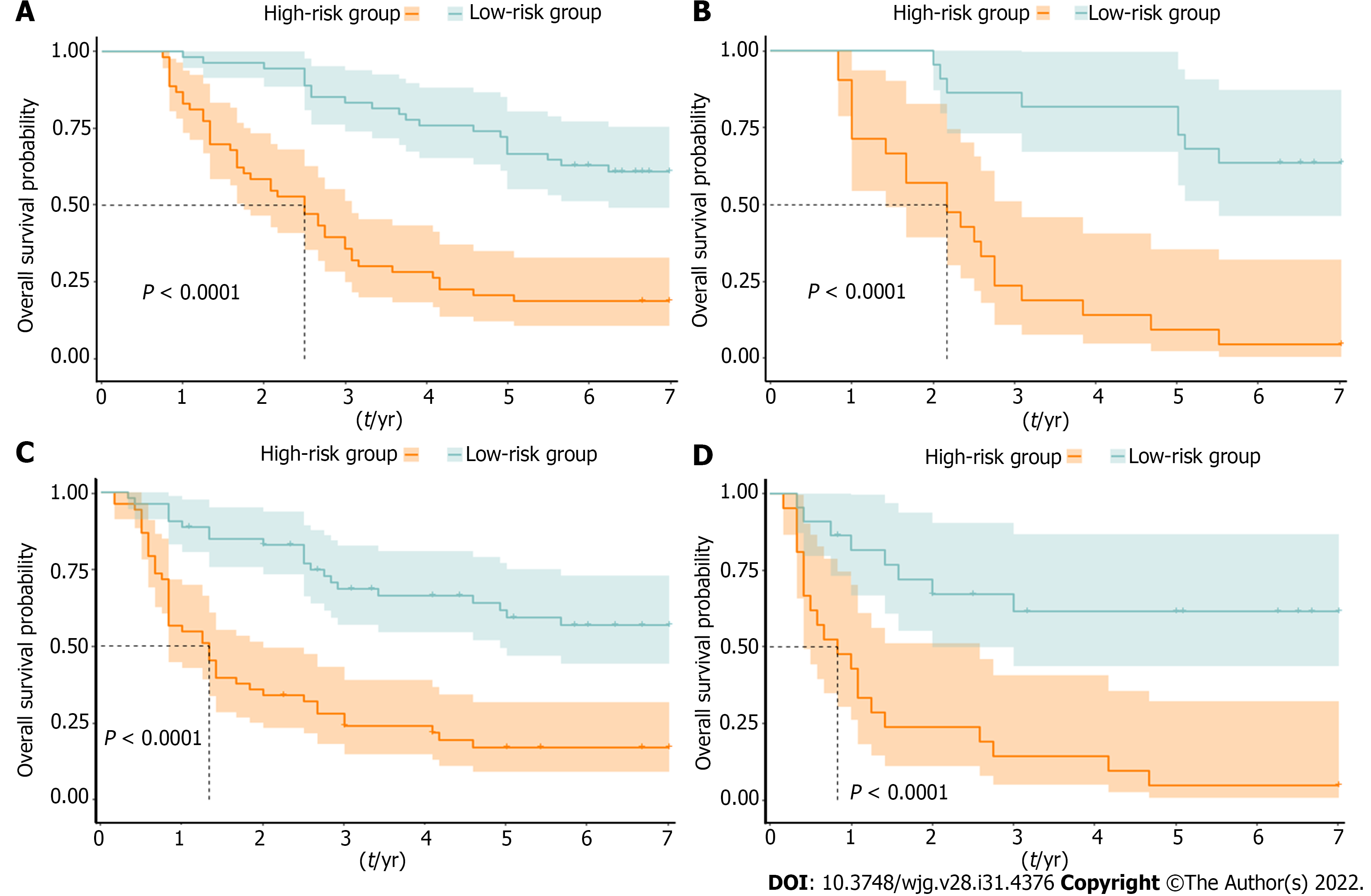
To further explore the risk stratification ability of the nomogram, we calculated the total points of the nomogram for each patient. The total points conformed to a normal distribution in the training and validation cohorts. Patients were categorized into low- and high-risk subgroups based on whether the total points of the patient were lower (training cohort ≤ 89.8; validation cohort ≤ 66.0) or higher (training cohort > 89.8; validation cohort > 66.0) than the median points of each cohort. Kaplan–Meier curves indicated that patients in the low-risk subgroup had significantly longer OS and DFS, with all P < 0.001 (Figure 7).
In this study, we developed a nomogram for predicting the survival of HCC patients after radical hepatectomy. Seven radiomics features were selected from 1926 radiomics features, and then integrated into a single radiomics signature to comprehensively estimate CECT images. We included AFP, NLR and radiomics signature to build the predictive nomogram. The C-indices of the nomogram in the training cohort and validation cohort were 0.736 and 0.774, respectively. The AUCs and the calibration curve indicated satisfactory accuracy in both the training and validation cohorts (AUC of 1-, 3- and 5-year survival = 0.850, 0.791, 0.823 and 0.905, 0.884, 0.911, respectively). The high AUCs of the nomogram indicate a high accuracy in predicting OS. In DCA, comparing the traditional staging system with our nomogram with respect to predictive ability and clinical practicality, the predictive nomogram was superior to the traditional staging system (BCLC and TNM staging system) in predicting 3- and 5-year survival.
To our knowledge, our radiomics-based model that contains NLR and AFP to predict survival in patients with HCC is entirely novel. A few previous studies[19,20] have investigated the capability of radiomics analysis in predicting the prognosis of HCC patients, and these studies analysed the largest cross-sectional area, whereas entire-tumour analysis was conducted in this study, which can provide more comprehensive information and more effective evaluation of the tumour. Thus, compared with previous studies, radiomics analysis in this study may achieve better performance. Various models combining radiomics features with clinical-pathologic factors to predict prognosis have been developed. Nevertheless, very few studies have enrolled radiomics features and NLR as variables to build a prognostic prediction model for HCC patients. Wang et al[21] established an magnetic resonance imaging-based radiomics model incorporating a few clinical factors for predicting the 5-year survival of patients with HCC after radical surgery. The mean AUC in the validation cohort was 0.7578 (95%CI: 0.7056–0.8100).
As precision medicine has developed, accurate prediction of patient prognosis is the principal component of individualized therapy and improving patient prognosis. It has been reported that intratumor heterogeneity is common in a variety of tumours and correlated with clinical outcomes[22]. However, information about intratumor heterogeneity obtained from routine clinical examinations is limited. Radiomics analysis refers to computer-aided data mining of quantitative high-throughput imaging features extracted from medical images[9,10], and it has been reported that radiomics features have potential prognostic value in liver cancer[14], lung cancer[15] and breast cancer[16]. Compared with the interpretation of traditional radiology, radiomics could provide comprehensive information regarding intratumor heterogeneity that may be unable to be obtained by the naked eye of radiologists. To a certain extent, radiomics overcomes these limitations of traditional radiology. As a biomarker associated with HCC, AFP has been widely used for early diagnosis and monitoring of HCC. AFP was considered an independent risk factor for postoperative survival; moreover, patients with low serum AFP levels had longer OS after radical resection. However, AFP still has some limitations in the prognosis prediction of HCC. Prior studies have reported that AFP has no ability to predict prognosis in small HCC (diameter ≤ 3 cm)[23]. Immune cells such as neutrophils, macrophages and lymphocytes within the tumour microenvironment have been confirmed to affect tumour development and progression[24]. There is evidence suggesting that lymphocytes can inhibit tumour proliferation, invasion, and metastasis by enhancing immune surveillance[25]. Moreover, tumour-infiltrating lymphocytes were associated with better outcomes in a variety of tumours, probably linked to tumour infiltration, antitumor activity, lymphocyte induction and inhibition of angiogenesis[26]. One study indicated that neutrophils promote tumour invasion[27]. Neutrophils can inhibit the proliferation of lymphocytes and induce lymphocyte apoptosis[28]. Patients with an elevated NLR level have relative neutrophilia and lymphocytopenia. Therefore, a high NLR indicates a poor prognosis, and this has been confirmed in numerous cancers, including gastric cancer[29], colorectal cancer[30] and pancreatic cancer[31]. To date, there is growing attention to the potential of NLR to be a prognostic biomarker in patients with HCC[32,33]. The NLR is a modality of measuring systemic inflammation that is relatively inexpensive and conveniently obtained from routine preoperative blood tests. In our previous study, the optimal cut-off value of NLR was determined to be 2.31 for predicting the prognosis of patients with HCC, and this was confirmed by not only our previous retrospective trial but also other prospective clinical trials[32]. In summary, NLR is a potential independent predictor for HCC.
As a standardized and noninvasive imaging modality, CECT is widely utilized for diagnosis, staging, clinical decision-making and treatment response monitoring across numerous cancer types. An advantage of radiomics analysis is that it was performed on existing CECT images as a routine preoperative examination for patients with malignant tumours. Moreover, the variables included in this nomogram were easily acquired from routine blood examinations.
All CECT images were reconstructed with a slice thickness of 1.25 mm, and a previous report showed that slice thickness does not considerably influence the stability of the parameters[34].
The current study has several limitations. First, this study was a single-centre retrospective study. Insufficient data heterogeneity could be a major limitation of single-centre studies. Therefore, more patients from other centres are needed to further validate the reliability and clinical applicability of this prognostic model. Second, HCC is considered to be related to various aetiological factors, including alcohol, aflatoxin, hepatitis B virus and hepatitis C virus. Different aetiologies might result in different outcomes in HCC. Hepatitis B virus was the main aetiology of HCC in the present study. Thus, future studies are required to validate the efficacy and accuracy of this predictive model in HCC with different aetiologies. Third, the different contrast agents or the different injection speeds may affect the quantification of radiomic features[35] and thus affect the accuracy of the model. To overcome this limitation, multi-institutional validation and cross-validation are required in the future. Fourth, regions of interest were outlined semiautomatically and corrected manually when necessary. Hence, a certain degree of selection bias of the ROIs might be created by the different observers. This is a challenging problem for radiomics analysis to eliminate or reduce biases. Fifth, the correlation between radiomics features and biological behaviour remains unknown. Recently, radio-genomics has become an emerging area that integrates radiomics and genomics. Radio-genomics is the discipline that studies the relationship between image phenotypes and genomics, which may contribute to precision medicine development[36]. Further research is still required to determine the potential correlation between radiomics and genomics in HCC. In future studies, we will incorporate genomic characteristics associated with HCC prognosis to construct a more comprehensive radio-genomics predictive model.
In conclusion, the nomogram combining the radiomics signature, NLR and AFP may contribute to postoperative outcome prediction and clinical treatment decision-making for HCC patients.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. The prognosis of HCC patients remains poor. Radiomics is an artificial intelligent-based method for obtaining prognostic and predictive information which may contribute to clinical outcomes improvement.
Currently, a few studies have analysed the largest cross-sectional area of HCC tumour. In this study, we have analysed the entire-tumour to build a more comprehensive prognostic prediction model with clinical characteristics. We aimed to develop a radiomics model for predicting the overall survival of HCC patients after hepatectomy.
In this study, we aimed to develop a radiomics model based on contrast-enhanced computed tomo
A total of 150 HCC patients were enrolled and randomly divided into a training cohort (n = 107) and a validation cohort (n = 43) at ratio 2.5:1. Radiomics features were extracted from the CECT images. In training cohort, the least absolute shrinkage and selection operator algorithm was applied for radiomics features selection and radiomics signature construction. Univariate and multivariate Cox regression analyses were used to develop the predictive model. The accuracy of the model was assessed with the concordance index, receiver operating characteristic curve and calibration curve. The clinical practicality was evaluated by decision curve analysis. The survival between the low- and high-risk subgroups was compared using Kaplan–Meier methodology.
In total, seven radiomics features were selected to construct the radiomics signature. Alpha-fetoprotein, neutrophil-to-lymphocyte ratio and radiomics signature were identified as independent risk predictors to build the predictive model. The C-indices of the model in the training and validation cohorts were 0.736 and 0.774, respectively. In receiver operating characteristic curve for predicting 1-, 3-, and 5-year overall survival, area under the curve (AUC) = 0.850, 0.791 and 0.823, respectively in training cohort; AUC = 0.905, 0.884 and 0.911, respectively in validation cohort. The calibration curve analysis indicated a good agreement between the model-prediction and actual survival. Decision curve analysis suggested that the predictive model had more benefit than traditional staging system models. In Kaplan–Meier survival analysis, patients in the low-risk group had longer overall survival and disease-free survival.
The predictive model is a reliable tool for predicting the overall survival of HCC patients after radical hepatectomy.
More precise and reliable tool to predict the prognosis of HCC patients is urgently needed. Radiomics is a new method for obtaining prognostic and predictive information. In this study, we aimed to develop a predictive model based on CECT images and clinical-pathologic characteristics to predict the overall survival of HCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Hu X, China S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 2. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 686] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Zong J, Fan Z, Zhang Y. Serum Tumor Markers for Early Diagnosis of Primary Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2020;7:413-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40:1733-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Nakagawa S, Umezaki N, Yamao T, Kaida T, Okabe H, Mima K, Imai K, Hashimoto D, Yamashita YI, Ishiko T, Chikamoto A, Baba H. Survival impact of lymphocyte infiltration into the tumor of hepatocellular carcinoma in hepatitis B virus-positive or non-B non-C patients who underwent curative resection. Hepatol Res. 2018;48:E126-E132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Liao R, Tang ZW, Li DW, Luo SQ, Huang P, Du CY. Preoperative neutrophil-to-lymphocyte ratio predicts recurrence of patients with single-nodule small hepatocellular carcinoma following curative resection: a retrospective report. World J Surg Oncol. 2015;13:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, Soejima Y, Maehara Y. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 9. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5551] [Article Influence: 616.8] [Reference Citation Analysis (3)] |
| 10. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 3853] [Article Influence: 296.4] [Reference Citation Analysis (2)] |
| 11. | Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6102] [Cited by in RCA: 5960] [Article Influence: 458.5] [Reference Citation Analysis (0)] |
| 12. | Tu SM, Bilen MA, Hess KR, Broaddus RR, Kopetz S, Wei C, Pagliaro LC, Karam JA, Ward JF, Wood CG, Rao P, Tu ZH, General R, Chen AH, Nieto YL, Yeung SC, Lin SH, Logothetis CJ, Pisters LL. Intratumoral heterogeneity: Role of differentiation in a potentially lethal phenotype of testicular cancer. Cancer. 2016;122:1836-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2262] [Cited by in RCA: 3249] [Article Influence: 295.4] [Reference Citation Analysis (0)] |
| 14. | Zheng BH, Liu LZ, Zhang ZZ, Shi JY, Dong LQ, Tian LY, Ding ZB, Ji Y, Rao SX, Zhou J, Fan J, Wang XY, Gao Q. Radiomics score: a potential prognostic imaging feature for postoperative survival of solitary HCC patients. BMC Cancer. 2018;18:1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, Liang C, Tian J. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology. 2016;281:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 16. | Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, Conzen SD, Whitman GJ, Sutton EJ, Net JM, Ganott M, Huang E, Morris EA, Perou CM, Ji Y, Giger ML. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology. 2016;281:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 17. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 18. | Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, Shi W, Yuan S, Tahir SA, Jin J, He S. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl Oncol. 2014;7:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). 2019;44:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 2017;42:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 21. | Wang XH, Long LH, Cui Y, Jia AY, Zhu XG, Wang HZ, Wang Z, Zhan CM, Wang ZH, Wang WH. MRI-based radiomics model for preoperative prediction of 5-year survival in patients with hepatocellular carcinoma. Br J Cancer. 2020;122:978-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 22. | O'Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 23. | Giannini EG, Marenco S, Borgonovo G, Savarino V, Farinati F, Del Poggio P, Rapaccini GL, Anna Di Nolfo M, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) group. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2767] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 25. | Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2014] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 26. | Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678-2683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 614] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 27. | Imai Y, Kubota Y, Yamamoto S, Tsuji K, Shimatani M, Shibatani N, Takamido S, Matsushita M, Okazaki K. Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: an in vitro study. J Gastroenterol Hepatol. 2005;20:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PubMed] |
| 29. | Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol. 2014;110:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 844] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 31. | Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 32. | Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Shiraki T, Ishizuka M, Kubota K, Kato M, Matsumoto T, Mori S, Shimizu T, Aoki T. An elevated neutrophil-to-lymphocyte ratio predicts a poor postoperative survival in primary hepatocellular carcinoma patients with a normal preoperative serum level of alpha-fetoprotein. Surg Today. 2019;49:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Duda D, Kretowski M, Bezy-Wendling J. Effect of Slice Thickness on Texture-Based Classification of Liver Dynamic CT Scans. In: Saeed K, Chaki R, Cortesi A, Wierzchoń S, editors. Computer Information Systems and Industrial Management. CISIM 2013: Proceedings of the 12th IFIP TC8 International Conference on Computer Information Systems and Industrial Management. Berlin: Springer, 2013: 96-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Kakino R, Nakamura M, Mitsuyoshi T, Shintani T, Hirashima H, Matsuo Y, Mizowaki T. Comparison of radiomic features in diagnostic CT images with and without contrast enhancement in the delayed phase for NSCLC patients. Phys Med. 2020;69:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Gopal N, Yazdian Anari P, Turkbey E, Jones EC, Malayeri AA. The Next Paradigm Shift in the Management of Clear Cell Renal Cancer: Radiogenomics-Definition, Current Advances, and Future Directions. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |