Published online Jan 21, 2022. doi: 10.3748/wjg.v28.i3.348
Peer-review started: May 5, 2021
First decision: June 12, 2021
Revised: June 24, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: January 21, 2022
Processing time: 252 Days and 20.5 Hours
Cirrhosis is an important health problem characterized by a significant change in liver parenchyma. In animals, this can be reproduced by an experimental model of bile duct ligation (BDL). Melatonin (MLT) is a physiological hormone synthesized from serotonin that has been studied for its beneficial properties, including its antioxidant potential.
To evaluate MLT’s effects on oxidative stress, the inflammatory process, and DNA damage in an experimental model of secondary biliary cirrhosis.
Male Wistar rats were divided into 4 groups: Control (CO), CO + MLT, BDL, and BDL + MLT. MLT was administered (20 mg/kg) daily beginning on day 15 after biliary obstruction. On day 29 the animals were killed. Blood samples, liver tissue, and bone marrow were collected for further analysis.
BDL caused changes in biochemical and histological parameters and markers of inflammatory process. Thiobarbituric acid (0.46 ± 0.01) reactive substance levels, superoxide dismutase activity (2.30 ± 0.07) and nitric oxide levels (2.48 ± 0.36) were significantly lower (P < 0.001) n the groups that received MLT. DNA damage was also lower (P < 0.001) in MLT-treated groups (171.6 ± 32.9) than the BDL-only group (295.5 ± 34.8). Tissue damage and the expression of nuclear factor kappa B, interleukin-1β, Nrf2, NQO1 and Hsp70 were significantly lower in animals treated with MLT (P < 0.001).
When administered to rats with BDL-induced secondary biliary cirrhosis, MLT effectively restored the evaluated parameters.
Core Tip: The experimental model of secondary biliary cirrhosis, by ligation of the main bile duct, mimics the clinical situation, with biochemical, enzymatic, histological changes, and similar biological, inflammatory, genotoxic markers and oxidative stress triggers. Melatonin, used as an antioxidant therapeutic agent, has been shown to be effective in reversing the changes caused at different levels due to its antioxidant, anti-inflammatory, cytoprotective action, including a reduction in DNA damage, with significant improvement and future therapeutic potential.
- Citation: Colares JR, Hartmann RM, Schemitt EG, Fonseca SRB, Brasil MS, Picada JN, Dias AS, Bueno AF, Marroni CA, Marroni NP. Melatonin prevents oxidative stress, inflammatory activity, and DNA damage in cirrhotic rats. World J Gastroenterol 2022; 28(3): 348-364
- URL: https://www.wjgnet.com/1007-9327/full/v28/i3/348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i3.348
Secondary biliary cirrhosis, a late complication of prolonged obstruction of the extrahepatic bile duct, causes cholestasis[1]. Cholestatic liver damage, defined according to histopathological and biochemical criteria as an accumulation of toxic bile acids, plays a fundamental role in liver necrosis and fibrosis[2-4].
Prolonged common bile duct obstruction in rats is an experimental model that induces secondary biliary cirrhosis in 28 d and mimics human liver disease in clinical, laboratory and histological parameters[2,3,5].
Oxidative stress plays a determinant role in the pathophysiology of liver diseases due to the accumulation of reactive oxygen species, protein oxidation, lipid per
Oxidative stress, due to the accumulation of reactive oxygen species, destabilizes cell homeostasis. Nrf2, which regulates cellular response to oxidative damage and the expression of most antioxidant enzymes under normal conditions, is kept inactive by the protein Keap1. In stressful situations for the cell, Nrf2 dissociates and translocates to the nucleus, where it binds to the promoter sequence as an antioxidant response element and activates genes, initiating the transcription of new antioxidant enzymes[8-10]. The Keap1/Nrf2 pathway is responsible for regulating both cytoprotective genes and defense antioxidants, including superoxide dismutase (SOD), catalase, and glutathione peroxidase, as well as glutathione reductase, gamma glutamylcysteine ligase, xenobiotic detoxification, NAD (P) H: Quinone oxidoreductase 1 (NQO1), and genes from the glutathione S-transferase family[11,12].
Increased production of reactive oxygen species, the presence of inflammatory mediators such as interleukins (IL-1β and IL-6), tumor necrosis factor alpha (TNF-α), nuclear factor kappa B (NF-kB), and increased nitric oxide levels may be related to the development of fibrosis in liver cirrhosis[2,3].
Hsp70 is an endogenous protein that plays a protective role in cell function, assisting in protein synthesis. Studies have shown that Hsp70 induction occurs in response to various stimuli, such as exposure to toxins, glucose deprivation, and reactive oxygen species formation, as well as liver cirrhosis[13,14].
Melatonin (MLT), N-acetyl-5-methoxytryptamine, is a hormone synthesized by the pineal gland, which is produced rhythmically and is inhibited by light[15,16]. The antioxidant effect of MLT is related to its amphiphilic chemical structure, which facilitates its crossing through biological barriers and allows activity in both aqueous and lipid environments[15,17]. The numerous attributes of MLT include antioxidant capacity and anti-inflammatory and immunomodulatory effects[18]. Exogenous MLT has protective effects on hepatic ischemia-reperfusion injury[19]. The inadequate expression of MLT predisposes liver cells to immune- and oxidative stress-related damage. MLT, via epigenetic modulation, was able to suppress NF-κB signaling activation and protecting against apoptotic signaling induced by either oxidative stress or high concentrations of bile[20]. MLT, participates in regulating multiple physiological functions, including sleep, circadian rhythms, and neuroendocrine processes.
Current evidence shows that MT protects against liver injury by inhibiting oxidation, inflammation, haematopoietic stem cell (HSC) proliferation, and hepatocyte apoptosis, thereby inhibiting the progression of liver cirrhosis[17].
Inflammation and oxidative stress play an important role in the pathophysiology of cirrhosis and other liver diseases, which is why pharmacological interventions can change the evolution of the disease. Non-alcoholic fatty liver disease (NAFLD) patients who underwent treatment with Essentiale Forte and tryptophan or MT for 14 mo had reduced expression of GGTP, triglycerides, low-density lipoprotein cholesterol and proinflammatory cytokines including IL-1, IL-6 and TNF-α, although there was no significant difference in alanine aminotransferase level or other biochemical parameters[21]. NAFLD patients treated with MT were found to have significantly lower aspartate aminotransferase (AST) and high-sensitivity C-reactive protein levels and a better liver grade than those who received placebo[22]. MLT seems safe and effective in the short term as a sedative in patients with CTP classes A and B cirrhosis and SD. This finding may have clinical applications in the holistic management of patients with cirrhosis[23]. There is a positive association between high serum MLT levels prior to LT, one-year survival after LT, and total antioxidant capacity[24].
The aim of the present study was to evaluate MLT’s effects on oxidative stress, the inflammatory process, and DNA damage in an experimental model of secondary biliary cirrhosis.
This study was conducted at the Animal Experimentation Unit and the Second Laboratory of Experimental and Inflammatory Pneumological Sciences of the Hospital de Clínicas de Porto Alegre after approval by the Institutional Commission for the Treatment and Use of Animals (protocol 2016-0373).
Animal handling was carried out according to Brazilian federal legislation (Law 11794/2008), Brazilian Council for the Control of Animal Experimentation (CONCEA) rules, the State Code for the Protection of Animals, and local legislation regarding the care and use of animals in experimental research.
Twenty-four male Wistar rats (mean weight 300 g) were divided into four experimental groups: Control (CO), control treated with MLT (CO + MLT), bile duct ligation (BDL) and BDL treated with MLT (BDL + MLT). During the experiment, the animals were maintained in cages (47 cm × 34 cm × 18 cm) lined with wood shavings, under a 12 h light/dark cycle and controlled temperature (18-22 °C), with free access to food and water.
On the first day of the experiment, BDL surgery was performed, as well as simulated surgery in the CO and CO + MLT groups according to Kountouras et al (1984)[25]. On the 15th day of the experiment, the animals began receiving MLT in daily doses of 20 mg/kg of body weight. The treatment continued until the 28th day.
On the 29th day, the animals were weighed and anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (95 mg/kg) and 2% xylazine hydrochloride (8 mg/kg). Blood was then collected from the retro-orbital plexus with a glass capillary tube and placed in a test tube with heparin to prevent coagulation.
After blood collection, the animals were sacrificed by anesthetic overdose (three times the therapeutic dose, according to the CONCEA guidelines. Upon confirmation of death, a ventral midline laparotomy was performed, after abdominal trichotomy and disinfection. The liver was removed, sectioned, and stored for subsequent analysis. One liver fragment was submerged in a 10% formaldehyde solution for 24 h for histological examination, one fragment was stored in a fixative containing glutaraldehyde for subsequent analysis by scanning electron microscopy, a third fragment was frozen at -80 °C for further analysis, and bone marrow samples were collected for the micronucleus test.
After dissection, the liver was placed in 10% buffered formalin and later embedded in paraffin blocks. The paraffin blocks were then attached to a microtome (Leitz-1512 Microtome, Leitz, Wetzlar, Germany) for cutting. The slides were then stained with hematoxylin-eosin and washed in running water. In the dehydration phase, the structures went through a series of three baths: One in absolute alcohol and two in xylol. The cover slip was then fixed into place using Canada balsam or Entellan, which completed the preparation process. The slides were analyzed with a Nikon Labophot binocular microscope equipped with a digital camera. Using the Image-Plus software (Media Cybernetics, Bethesda, MD, United States), images were captured at different magnifications.
Tissue samples were fixed in 10% formalin and embedded in paraffin. The paraffin blocks were then attached to a microtome (Leitz® 1512) and cut in 3 μm sections. The slides were stained in hematoxylin-eosin for 5 min each and then washed in running water. In the dehydration phase, the structures went through a series of three baths: One in absolute alcohol and 2 in xylol. The cover slip was fixed into place using Canada Balsam. The slides were analyzed with a microscope equipped with a digital camera. Using Image-Plus software, images were captured at 200× magnification.
Nine ml of phosphate buffer were added per gram of tissue, which was then homogenized in an Ultra-Turrax homogenizer (IKA-Werk, Staufen, Germany) for approximately 40 s and kept on ice, followed by centrifugation in a SORVALL RC-5B refrigerated Superspeed Centrifuge (Du Pont Instruments, Miami, FL, United States) for 10 min at 4000 rpm[26]. The precipitate was discarded and the supernatant was used to quantify the proteins.
Liver samples were collected for scanning electron microscopy. After collection, the samples were immersed in a fixative solution containing glutaraldehyde. The samples were then washed, dehydrated, desiccated and metallized, followed by analysis in an electron microscope (Jeol JSM-T330, Tokyo, Japan) at 5000× magnification.
Liver integrity was assessed by measuring liver enzymes AST, alanine aminotransferase, and alkaline phosphatase in plasma with a Liquiform Labtest® kit (a kinetic spectrophotometric assay).
The protein content in liver homogenate was determined using the Bradford method[27]. LPO was investigated with a thiobarbituric acid reactive substances (TBARS) assay, with the concentration expressed in nmol/mg of protein[28]. SOD activity was measured in a plate reader, evaluating its ability to inhibit the superoxide radical from reacting with adrenaline. The results were expressed in SOD units per milligram of protein[29]. The production of nitric oxide metabolites [nitrites (NO2)/nitrates(NO3)] was measured indirectly with the Griess reaction. This assay is based on the enzymatic reduction of NO3 to NO2 in the presence of nitrate reductase and NQO1, with subsequent colorimetric determination of NO2 using the Griess reagent (a mixture of sulfanilamide and NO2-specific N-[1-Naphthyl]ethylenediamine). The results were expressed in mmol/L[30].
To assess DNA damage, we used the alkaline version of the comet assay described by Tice et al (2000)[31]. Aliquots of 10 ml of liver cell suspension were mixed with 0.75% low-melting agarose and placed 1.5% agarose-coated slides; these slides were immersed in a lysis solution, which allowed the migration of DNA fragments by electrophoresis. The results were expressed in a damage index, obtained by visual assessment of damage classes (from 0 to 4), and damage frequency, calculated from the number of cells with vs without tails[32].
As described by Mavournin et al (1990)[33], bone marrow samples were collected from both femurs for the micronucleus test. To collect the samples, the proximal end of each femur was cut to expose the spinal canal, allowing extraction. To count normochromatic erythrocytes, polychromatic erythrocytes (PCE), and micronuclei in the PCE, an optical microscope with an immersion objective was used, and at least 2000 PCE were analyzed per animal. The polychromatic/normochromatic erythrocyte ratio was also determined by assessing the frequency of PCE in 1000 erythrocytes from each animal[34].
IL-1β cytokine levels were assessed using a microsphere-based multiplex assay (MILLIPLEX Map Kit, Rat Cytokine/Chemokine Magnetic Bead Panel, Cat. No. RECYTMAG-65K; Millipore Corporation, Billerica, MA, United States). Cytokine detection was performed by adding specific fluorescence conjugated antibodies.
Quantification was based on a standard curve with known dilutions, and the results were expressed in pg/mg. The samples were analyzed in a Luminex 200TM reader (Luminex, Austin, TX, United States) according to manufacturer instructions.
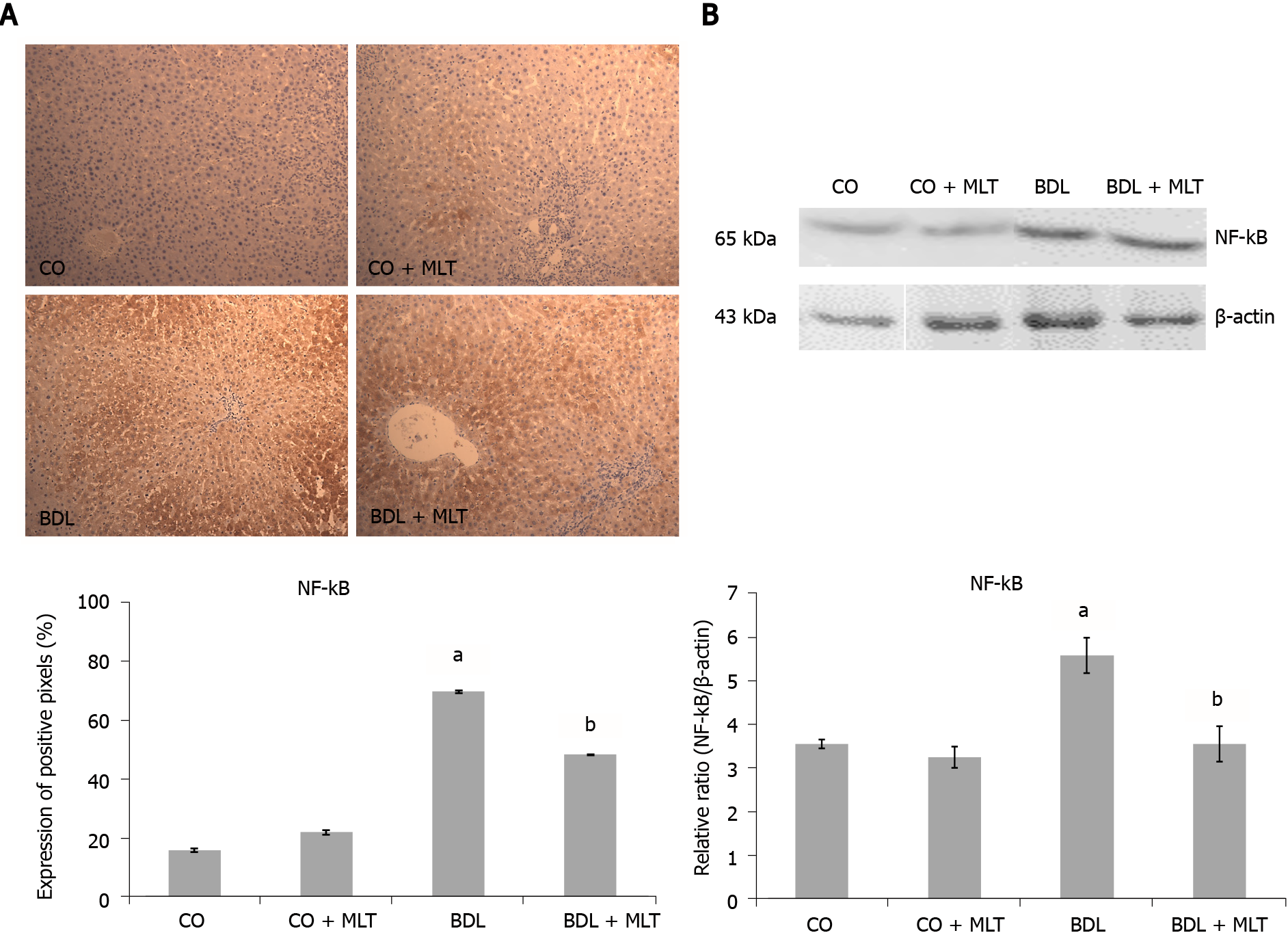
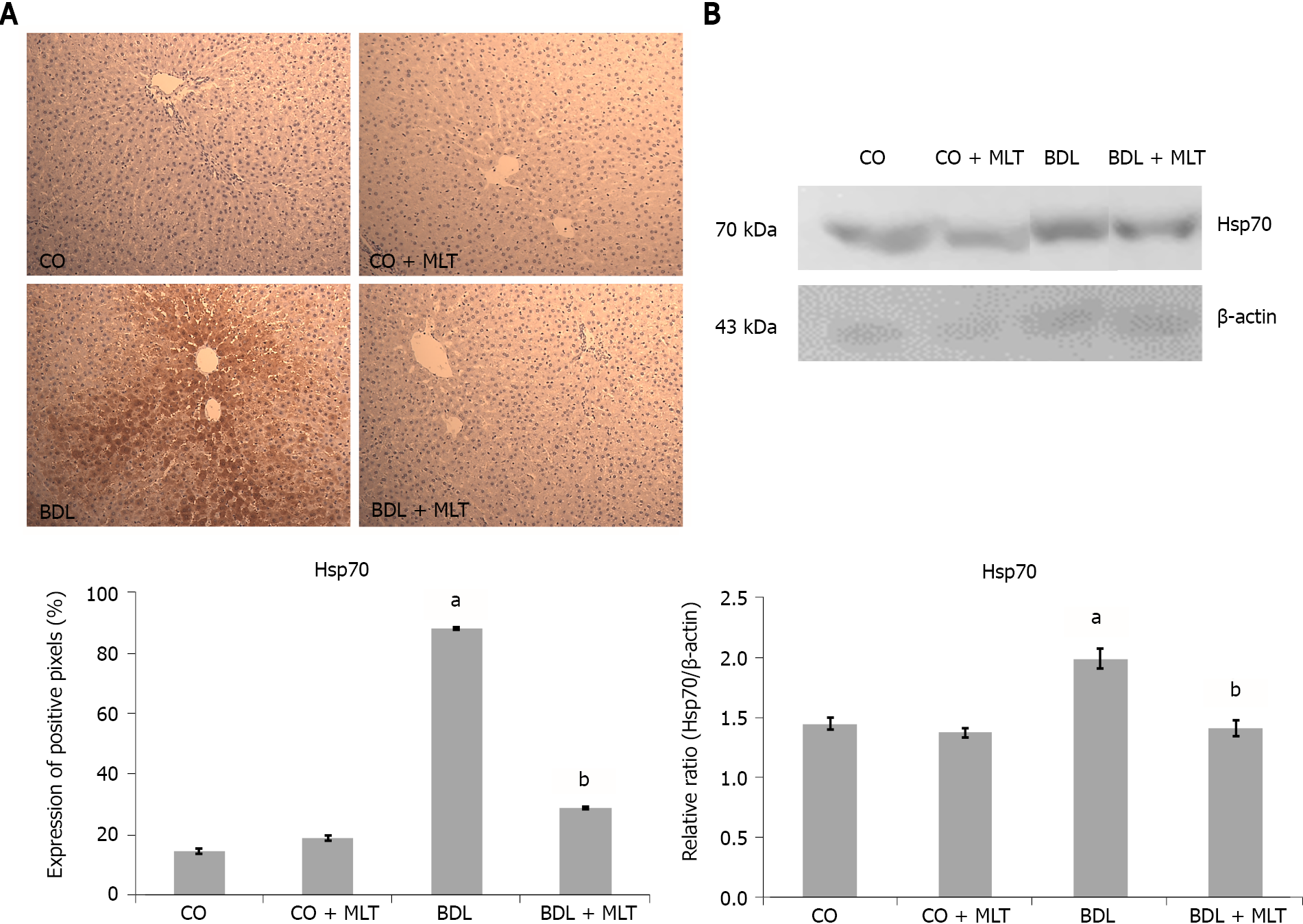
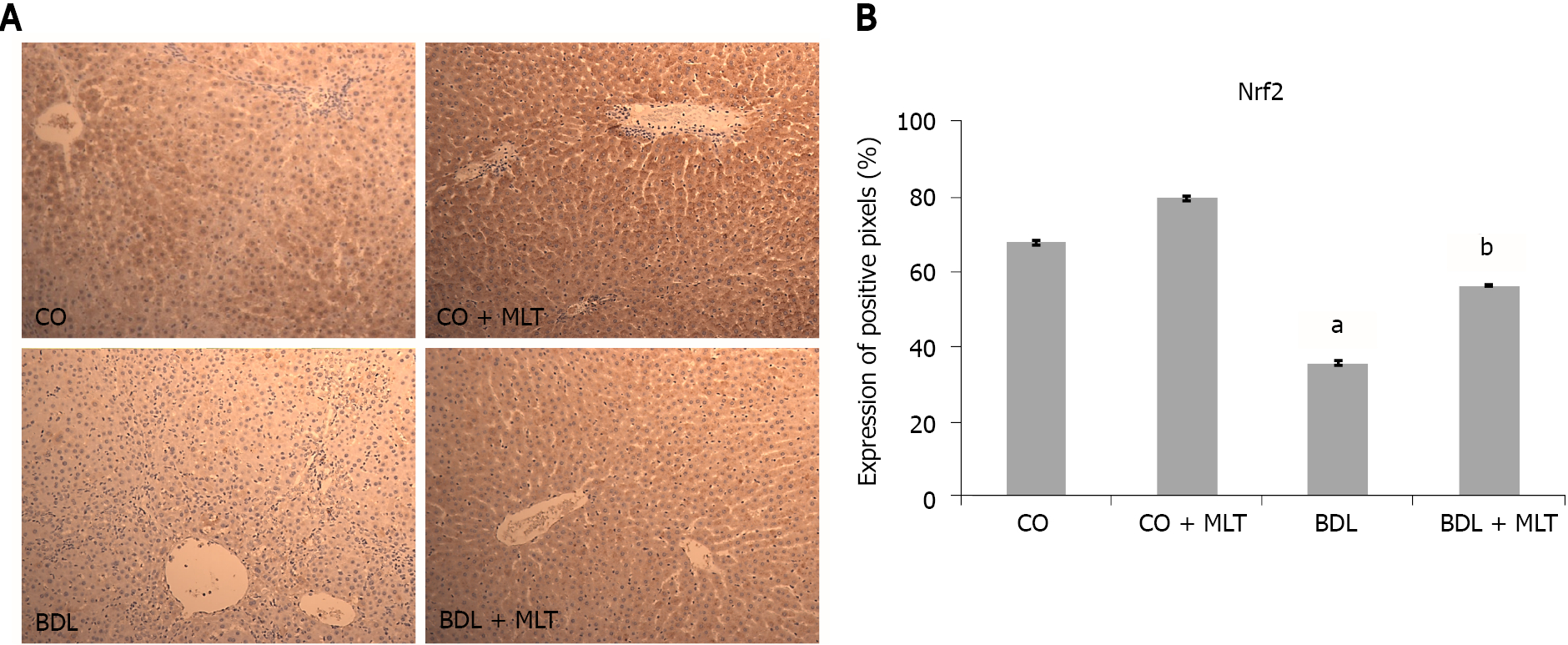
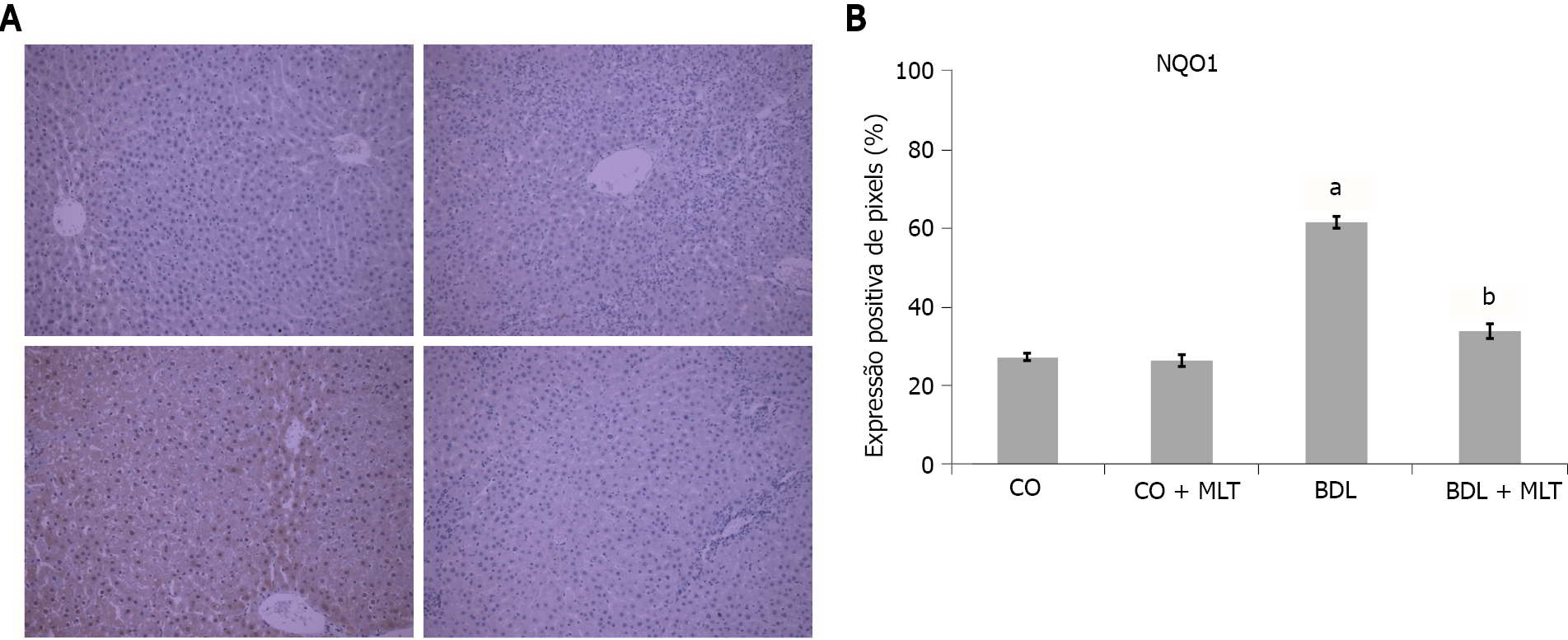
The slides, pre-incubated with 10% rabbit serum at room temperature to block possible unwanted reactions from the secondary antibody, were incubated with monoclonal antibodies (Nrf2, NQO1, NF-ҡB and Hsp70) (Santa Cruz Biotechnology, Santa Cruz, CA, United States) overnight at 4 ºC, followed by incubation with a secondary antibody for one hour at room temperature. After 60 min at room temperature, they were treated with EnVision reagent and washed three times with phosphate-buffered saline. The nuclei were counterstained with hematoxylin. The primary antibody was diluted in phosphate-buffered saline, which contained bovine albumin as a negative control. The results were evaluated by blinded pathologists using a microscope equipped with a digital camera and Image-Plus software (Media Cybernetics).
Cytoplasmic and nuclear extracts were prepared from liver homogenates using a specific lysis buffer and protease inhibitors[35]. The supernatant fraction was collected and stored in aliquots at -80 °C for further analysis. The lysed proteins were separated by dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were then blocked with 5% skim milk in Tris buffer containing 0.05% Tween 20 (TTBS) for 60 min at 37 °C. Thereafter, the primary antibodies were incubated and stirred overnight at 4 ºC. The following proteins were evaluated: NF-kB (65 kDa and Hsp70; 70 kDa) (Santa Cruz Biotechnology, Santa Cruz, CA, United States) diluted from 1:200 to 1:1000 with Tris-buffered saline in skim milk at 5%. HRP-antibody protein biomarker detection was performed with an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). The density of specific bands was quantified through image densitometry software (Scion Image, Frederick, MD, United States)[36,37].
The quantitative data were expressed as mean ± standard error. The groups were compared using unilateral analysis of variance. The Student-Newman-Keuls procedure was used to find differences in means (SPSS, version 17.0). The Tukey test was used for the comet assay. The data were analyzed in GraphPad InsTat 3.1, with P < 0.05 considered significant.
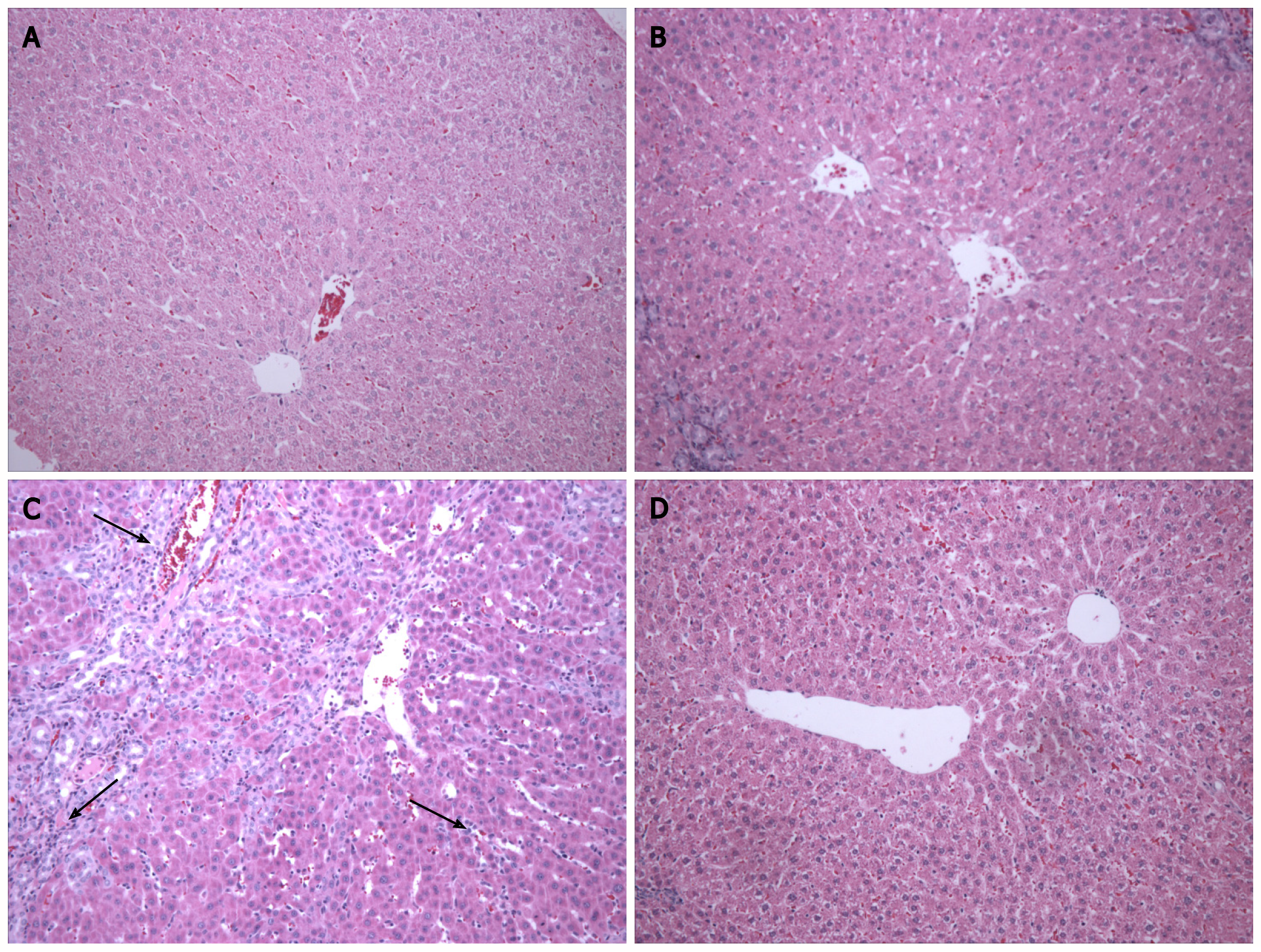
In the histological analysis, hematoxylin-eosin staining in the BDL group revealed changes in the liver parenchyma, a loss of hepatocyte cords, and the presence of inflammatory infiltrate (Figure 1, black arrows). In the BDL + MLT group, we observed restructuring of these changes, including the formation of hepatocyte cords, decreased inflammatory infiltrate, and preserved hepatocytes (Figure 1). In the CO and CO + MLT groups, the liver parenchyma was unchanged.
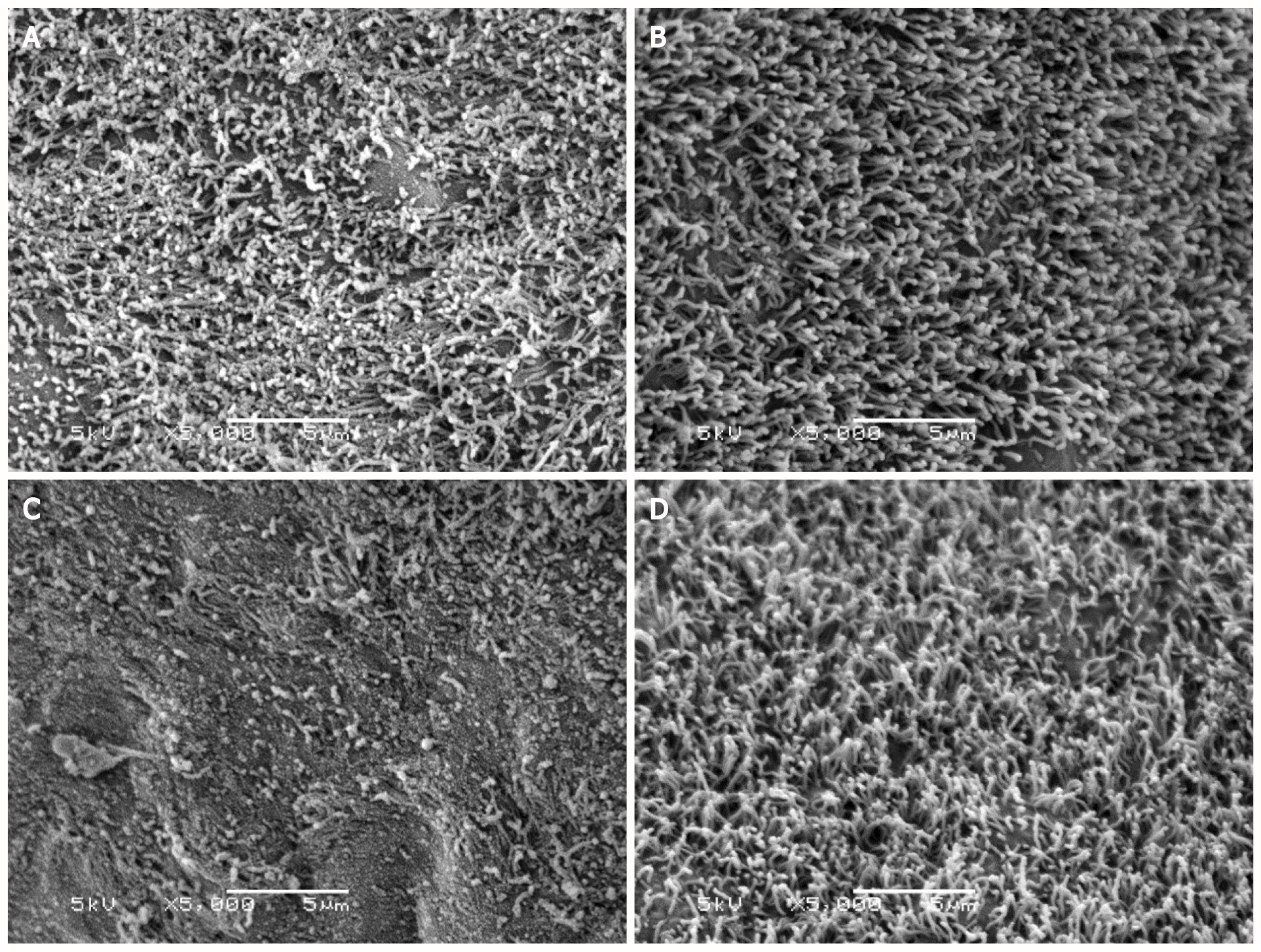
According to scanning electron microscopy of the liver samples, the ciliated membrane, which covers hepatocytes involved in inflammatory process signaling in response to damage, was intact in the CO and the CO + MLT groups. This membrane was damaged in the BDL group, although in the BDL + MLT group the membrane had been restructured (Figure 2).
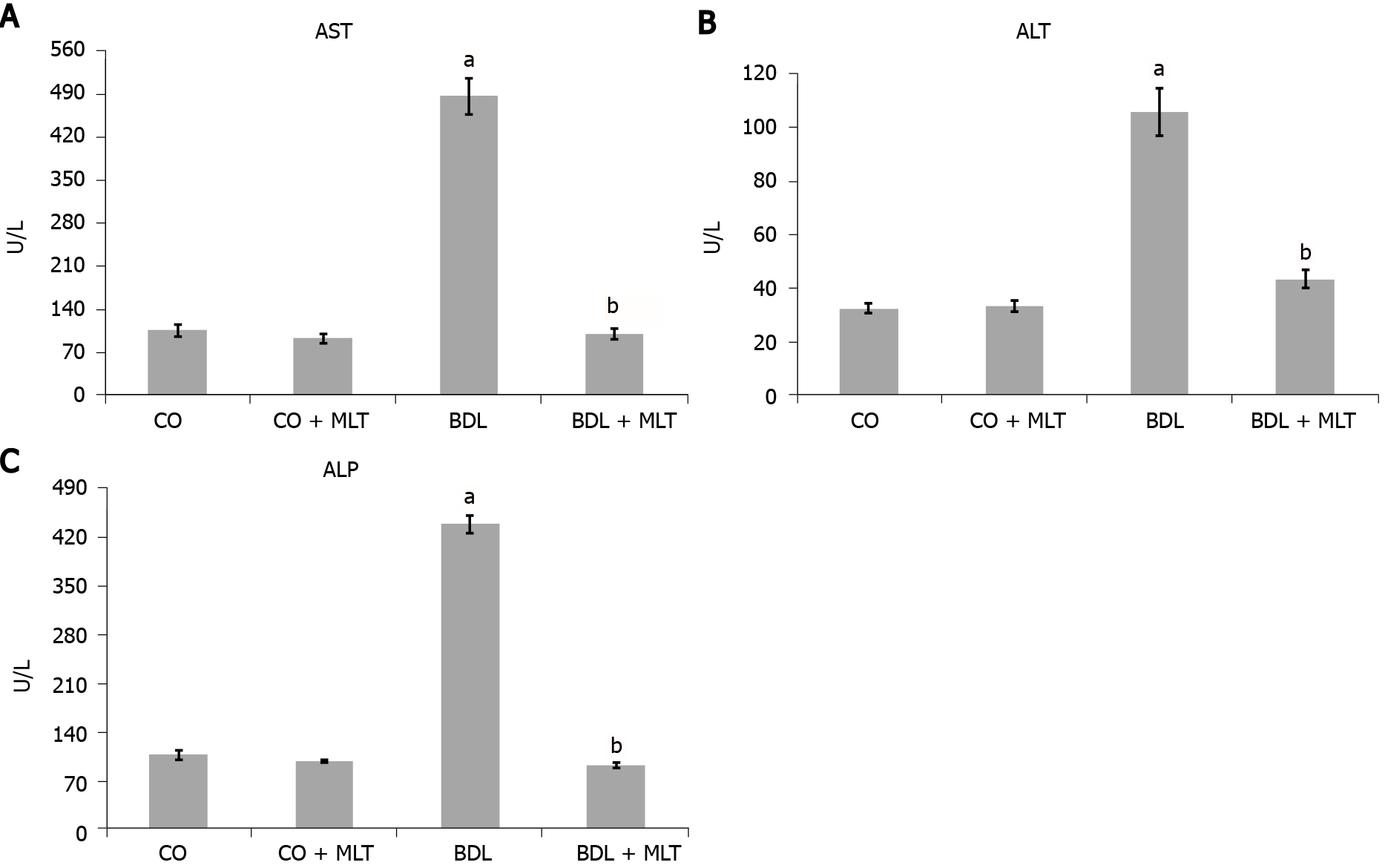
Liver enzyme alterations: All plasma liver enzymes in the BDL group were significantly higher than in the control groups (CO and CO + MLT), and these values were significantly lower in the BDL + MLT group than the BDL group (P < 0.001) (Figure 3).
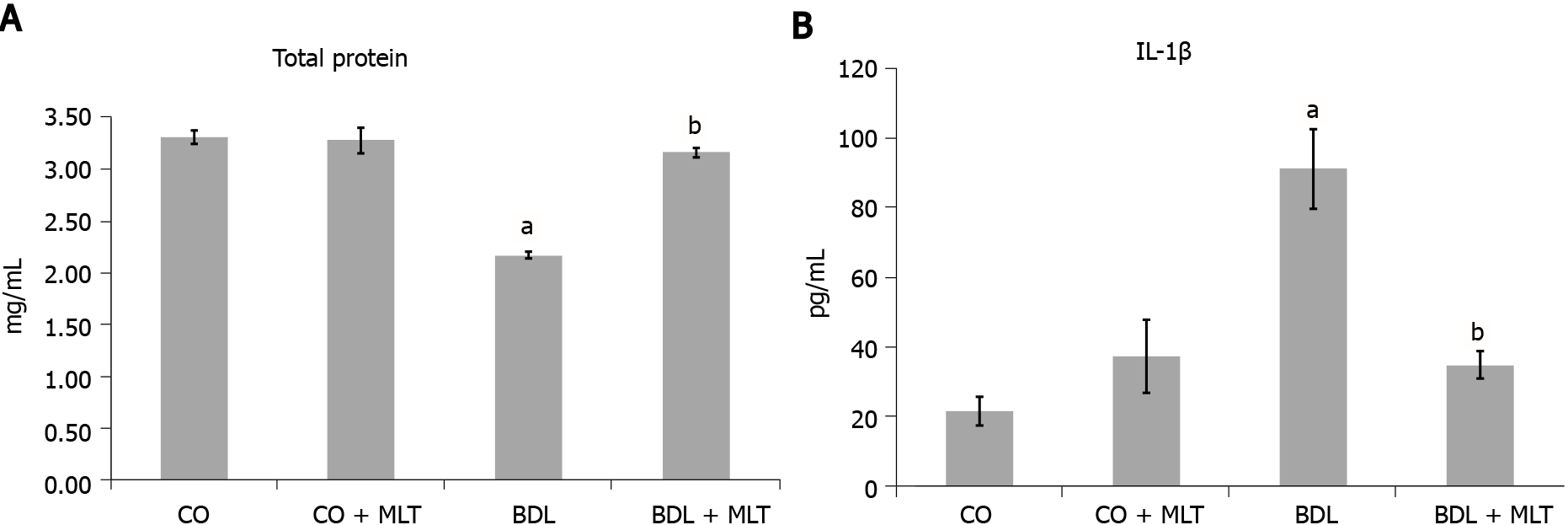
Protein evaluation, LPO, antioxidant enzyme SOD and nitric oxide: The total protein levels (Figure 4A) in liver homogenate were significantly higher in the BDL group than the control groups (P < 0.001) and were significantly higher in the BDL + MLT group than the BDL group (P < 0.001).
The LPO level was significantly higher in the BDL group than the CO and CO + MLT groups and was significantly lower in the BDL + MLT group than the BDL group (P < 0.001) (Table 1).
There was significantly less SOD activity in the BDL group than the control groups (CO and CO + MLT) and significantly more activity in the BDL + MLT group than the BDL group (P < 0.001) (Table 1).
The levels of nitric oxide metabolites (nitrites and nitrates) were significantly higher in the BDL group than the CO and CO + MLT groups, but they were significantly lower in the BDL + MLT group than the BDL group (P < 0.001) (Table 1).
In the comet assay analysis, the BDL group had a significantly higher damage index and damage frequency than the CO and CO + MLT groups. These parameters were significantly lower in the BDL + MLT group than the BDL group (P < 0.001) (Table 2).
The micronucleus frequency was significantly higher in the BDL group than the CO and CO + MLT groups and was significantly lower in the BDL + MLT group than the BDL group (P < 0.001) (Table 3). No significant differences were found between the groups in the polychromatic/normochromatic erythrocyte ratio, which indicated no toxicity in the bone marrow.
The pro-inflammatory cytokine IL-1β levels were significantly higher in the BDL group than the CO and CO + MLT groups (P < 0.001) and were significantly lower in the BDL + MLT group than the BDL group (P < 0.001), as can be seen in Figure 4B.
We observed significantly higher expression of NF-kB (Figure 5A) and Hsp70 (Figure 6A) in the BDL group than the control groups and significantly lower expression in the BDL + MLT group than the BDL group (P < 0.001).
There was significantly lower expression of Nrf2 in the BDL group than in the CO and CO + MLT groups but significantly higher expression in the BDL + MLT group than the BDL group (Figure 7). There was significantly higher expression of NQO1 in the BDL group than the CO and CO + MLT groups but significantly lower expression in the BDL + MLT group than the BDL group (P < 0.001)(Figure 8).
Common bile duct obstruction causes hepatocellular damage and an inflammatory response due to the accumulation of bile salts in the liver, which promotes cytokine production, hepatocellular injury, and the healing process, leading to an accumulation of collagen, fibrosis, and liver cirrhosis[38]. The BDL model has been used to study the numerous molecular signaling pathways involved in secondary biliary cirrhosis[3].
MLT has shown protective effects in different models, inhibiting oxidative stress, inflammatory signaling, autophagy, hepatocyte apoptosis, cell and tissue damage[3,16,17,39-41], and it seems safe and effective in the short term as a sedative in patients with CTP classes A and B cirrhosis and SD. This finding may have clinical applications in the holistic management of patients with cirrhosis[23].
The present study investigated the effects of MLT on oxidative, inflammatory, tissue, and cellular injury in an experimental model of secondary biliary cirrhosis. It was found that a dose of 20 mg/kg of MLT, which has already been investigated in other studies by our group, was effective in reducing or modulating oxidative stress, inflammatory processes, and DNA damage. The animals that underwent BDL surgery had a higher expression of the enzymes AST, alanine aminotransferase and alkaline phosphatase than the other groups, indicating possible damage to hepatocyte membranes. When MLT was administered to these animals, we observed a significant decrease in the expression of these enzymes, possibly due to hepatocyte membrane restructuring and reduced liver damage. This corroborates the results of Wu et al[12] in a model of BDL and CCL4-induced hepatic fibrosis. These authors found a significant increase in AST and alanine aminotransferase expression, as well as an equally significant reduction in expression after treatment with the antioxidant quercetin. In a model of severe acute liver failure, Schemitt et al[42] found an increase in AST, alanine aminotransferase and alkaline phosphatase expression, indicating a loss of liver integrity; high serum levels of these enzymes were related to cell damage and liver cell necrosis, which was reversed with glutamine[42].
Changes in the hepatic parenchyma, including the formation of fibrotic septa and necrosis, are often associated with the process of cirrhosis[12]. Hematoxylin-eosin staining showed disorganized liver tissue in the BDL group, including a loss of hepatocyte cords and the presence of inflammatory infiltrate and fibrosis. However, tissue restructuring had occurred in the BDL + MLT group, and organized tissue was observed in the CO and CO + MLT groups. Scanning electron microscopy revealed that in the control groups (CO and CO + MLT) the ciliated membrane of hepatocytes involved in inflammatory process signaling was intact. In the BDL group, however, this membrane was damaged, and in the BDL + MLT group it had been restructured (Figure 2).
In 2016, Wree and Marra[43] described the surface of hepatocytes and their association with reduced cell permeability, as well as a consequent increase in mediators involved in the fibrotic and inflammatory process, which was associated with changes in the ciliated membrane and inflammasome. According to Gonzalez-Navajas[44], inflammation is involved in the pathogenesis of many liver diseases, including cirrhosis, in which many inflammatory cytokines are produced after activation of a multiprotein complex known as inflammasome. However, the origin and mechanisms of hepatic damage mediated by inflammasome are little known[43]. Most acute or chronic liver diseases are accompanied by inflammation, a complex process in response to liver aggression, which causes serious damage to the liver parenchyma[45-47].
In 2017, Giusto et al[48] showed that mice with cirrhosis induced by BDL and CCL4 had fibrotic nodules and cellular changes. In 2013, Mazo et al[4] observed that in rats submitted in experimental nonalcoholic steatohepatitis, including fibrosis, the administration of N-acetylcysteine restored liver parenchyma.
Studies report that in the pathophysiology of biliary cirrhosis, liver damage is maximized by the action of free radicals. LPO causes the disorganization of cell membranes, resulting in increased membrane permeability and consequent enzyme leakage, leading to cell death. Studies show that plasma malondialdehyde levels may be associated with increased LPO[3,5,42].
In our study, LPO was analyzed with a TBARS assay, and it was significantly higher in the cirrhotic group (BDL) than the other groups, which may be associated with damage to cell membranes. LPO was significantly lower in the BDL + MLT group than the BDL group, which suggests that MLT plays a protective role. These data corroborate a study by Zhu et al[49], who, in rats with hepatopulmonary syndrome-BDL induced, observed higher LPO in the cirrhotic group and that Tea polyphenols significantly decreased LPO. Similar data were observed in other experimental models that administered MLT[3,41,42].
Other authors have observed LPO in the lungs of animals with secondary biliary cirrhosis due to BDL, considering it characteristic of the damage to cell membranes in this experimental model[3,49].
In addition to the lipid damage, which is assessed by increased LPO, reactive oxygen species can damage DNA. Oxidative damage to DNA is common, being the main cause of genomic instability[31]. We observed a significant increase in liver DNA damage in the BDL group, as well as in the frequency of micronuclei in the bone marrow, which suggests increased genomic instability. The significantly better micronucleus and comet assay results in the MLT-treated group suggests that MLT prevented cytogenetic damage, as well as strand breakage and DNA base oxidation in this model. The results of the present study corroborate those of Moreira et al[40], who used an experimental model of diethylnitrosamine-induced hepatocellular carcinoma, finding a lower damage index and frequency in the MLT-treated group, possibly due to MLT’s antioxidant capacity to regulate several key genes involved in DNA repair pathways, in addition to lower oxidative damage, inflammatory processes and tissue damage, as observed in our study. The pathophysiological mechanisms of cirrhosis initiate several signaling pathways, such as the Nrf2 pathway, which has a protective effect against oxidative damage. Under stress conditions, Nrf2 is translocated to the nucleus and activates the expression of genes that encode various antioxidant enzymes, such as SOD and NQO1[17,42,50]. Nuclear expression of Nrf2 was significantly lower in the BDL group in our study. Likewise, we observed that MLT treatment significantly increased Nrf2 expression and reestablished the expression of NQO1 and SOD, which suggests that MLT restored the antioxidant system, reducing oxidative stress by modulating the Nrf2 pathway. These results were similar to those of Schemitt et al 2019[42], who evaluated the effects of glutamine in a severe acute liver failure model and observed increased expression of NQO1 and SOD through regulation of the Nrf2-mediated antioxidant system.
In the present study, increased pro-inflammatory cytokine IL-1β levels were observed in the BDL group, as well as increased expression of NF-kB. MLT reversed the inflammatory process, which was demonstrated by lower IL-1β levels and reduced NF-kB expression. Colares et al[3] found increased expression of TNF-α and inducible nitric oxide synthase in their BDL group. Inducible nitric oxide synthase is expressed in inflammatory conditions in response to pro-inflammatory cytokines and is associated with increased nitric oxide levels, which we evaluated in the present study. Our results suggest that, due to its anti-inflammatory effect, MLT modulated the NF-kB pathway during inflammation, thus reducing the expression of genes involved in the inflammatory process, such as inducible nitric oxide synthase, nitric oxide and pro-inflammatory cytokines (IL-1β and TNF-α).
In a liver fibrosis model in rats, Czechowska et al[51] demonstrated that MLT inhibited the release of NF-kB and, consequently, reduced production of pro-inflammatory cytokines, probably due to its anti-inflammatory antioxidant action. In CCL4-induced liver cirrhosis model in rats, Hardeland[18] observed that MLT reduced the expression of NF-kB, as well as the expression of inducible nitric oxide synthase.
Heat shock proteins are extremely important for the protection of cells. In particular, Hsp70, which has cytoprotective functions, acts on protein folding, transport and degradation and can be induced in response to various stresses, including trauma, inflammatory diseases, oxidative stress and liver cirrhosis[52]. We observed an increase in the expression of Hsp70 in the animals of the BDL group, however, treatment with MLT led to a significant reduction in the expression of Hsp70. This suggests that MLT, possibly due to its important antioxidant effect, effectively regulated Hsp70 in the BDL + MLT group. In 2015, Moreira et al[40] reported an increase in Hsp70 expression in a model of hepatocellular carcinoma in rats treated with MLT. In 2019, Schemitt et al[42] evaluated a model of liver toxicity and observed that the expression of Hsp70 was reduced, possibly due to the increase in oxidative stress, thus contributing to disease worsening.
Biosynthesis of MLT by cholangiocytes is essential for maintaining biliary epithelium function and this cytoprotective mechanism, which appears to be impaired by decreased biliary MLT synthesis in biliary duct obstruction, exacerbates biliary damage and liver fibrosis. Concomitant with enhanced liver fibrosis, we observed increased biliary senescence[20].
MLT has been demonstrated to ameliorate liver damage by decreasing oxidative stress, inflammatory responses, and bile acid-induced apoptosis. The inadequate expression of MLT predisposes liver cells to immune- and oxidative stress-related damage[53].
In cholangiocytes exposed to mitochondrial oxidative stress, MLT decreased the expression of proapoptotic stimuli, which was accompanied by the inhibition of NFκB-p65, a pivotal mediator of inflammatory response, activation of antiapoptotic signaling, and increased biliary senescence and ROS, which activated HSCs by a paracrine mechanism, directly interacting with MLT on HSCs[20]. In human cholangiopathies such as PBC and PSC, an initial balance between cholangiocyte apoptosis and compensatory cholangiocyte proliferation is followed by a failure in cholangiocyte proliferative capacity, and enhanced apoptosis favors evolution toward ductopenia[20].
It can be concluded from our results that MLT treatment reduced tissue and cellular lesions in the liver, inhibited lipoperoxidation and DNA damage and reduced NO levels. In addition, MLT regulated cytoprotective capacity, regulating the Nrf2 pathway and restoring the enzymes NQO1 and SOD in the livers of treated animals.
Our results suggest that MLT has potential for clinical practice, although which patients might benefit, when treatment should begin, the dosage, and treatment duration must still be determined. Thus, larger studies assessing the efficacy and safety of MLT in the long term and in the later stages of cirrhosis are required before its clinical use can be recommended.
Liver cirrhosis, which causes millions of deaths per year, is characterized by the appearance of fibrotic nodules and septa caused by chronic harmful stimuli. Oxidative damage may play a key role in the development and progression of cirrhosis. Thus, promoting the identification of new antioxidant compounds can contribute to enriching the available therapeutic arsenal.
Numerous studies using different experimental models have reported melatonin (MLT)’s protective effects on the liver. The antioxidant effect of MLT is related to its high solubility in lipids, which facilitates its passage through cell membranes. Thus, understanding the mechanisms involved in the protective action of MLT in cirrhosis can lead to the development of new therapeutic strategies that can lead to improved medical care.
The aim of the study was to evaluate the protective action of MLT in cirrhosis induced by bile duct ligation (BDL) in rats.
Wistar rats were divided into a control group, a MLT control group, a BDL group, and a BDL group treated with MLT. Intraperitoneal administration of MLT at a dose of 20 mg/kg of body weight started on the 15th day after the beginning of the experiment and continued daily for 14 d. At the end of the experiment, the animals were euthanized. Blood was collected for liver integrity tests and the liver was collected for histological analysis, DNA damage assessment, and biochemical and Western blot analysis of proteins related to oxidative stress and the inflammatory process.
MLT promoted a significant improvement in the biochemical parameters of oxidative stress markers and the inflammatory process. DNA damage was also lower in animals treated with MLT after undergoing BDL. Tissue damage and protein expression assessed by immunohistochemistry and Western blot analysis were significantly lower in animals treated with MLT.
According to the results obtained in the evaluated parameters, treatment with MLT reduced tissue and cell damage in the liver. Our results suggest that MLT may be of use of in patients with cirrhosis.
Further studies are needed to assess the long-term efficacy and safety of MLT administration in cirrhotic patients before it can be recommended in clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-reviewer’s classification of scientific quality
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goyal AK, Skrypnik D S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Stalker MJ, Hayes MA. Liver and biliary system. Jubb, Kennedy, and Palmer’s pathology of domestic animals 2007; 2: 297-388. |
| 2. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 781] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 3. | Colares JR, Schemitt EG, Hartmann RM, Licks F, Soares MD, Bosco AD, Marroni NP. Antioxidant and anti-inflammatory action of melatonin in an experimental model of secondary biliary cirrhosis induced by bile duct ligation. World J Gastroenterol. 2016;22:8918-8928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 4. | Mazo DF, de Oliveira MG, Pereira IV, Cogliati B, Stefano JT, de Souza GF, Rabelo F, Lima FR, Ferreira Alves VA, Carrilho FJ, de Oliveira CP. S-nitroso-N-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des Devel Ther. 2013;7:553-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Barnett R. Liver cirrhosis. Lancet. 2018;392:275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Padma VV, Baskaran R, Roopesh RS, Poornima P. Quercetin attenuates lindane induced oxidative stress in Wistar rats. Mol Biol Rep. 2012;39:6895-6905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Schemitt EG, Colares JR, Hartmann RM, Morgan-Martins MI, Marroni CA; Tuñón JM, Marroni NP. Effect of glutamine on oxidative stress and inflammation in a rat model of fulminant hepatic failure. Nutr Hosp. 2016;33:210-219. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 1008] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 9. | Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA, Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Tang W, Jiang YF, Ponnusamy M, Diallo M. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20:13079-13087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 11. | Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1168] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 12. | Wu L, Zhang Q, Mo W, Feng J, Li S, Li J, Liu T, Xu S, Wang W, Lu X, Yu Q, Chen K, Xia Y, Lu J, Xu L, Zhou Y, Fan X, Guo C. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 13. | Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K, Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol. 2010;30:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Bozaykut P, Ozer NK, Karademir B. Regulation of protein turnover by heat shock proteins. Free Radic Biol Med. 2014;77:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr Med Chem. 2015;22:2690-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Sato K, Meng F, Francis H, Wu N, Chen L, Kennedy L, Zhou T, Franchitto A, Onori P, Gaudio E, Glaser S, Alpini G. Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J Pineal Res. 2020;68:e12639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Hu C, Zhao L, Tao J, Li L. Protective role of melatonin in early-stage and end-stage liver cirrhosis. J Cell Mol Med. 2019;23:7151-7162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Hardeland R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 19. | Song Z, Humar B, Gupta A, Maurizio E, Borgeaud N, Graf R, Clavien PA, Tian Y. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J Pineal Res. 2018;65:e12486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Ostrycharz E, Wasik U, Kempinska-Podhorodecka A, Banales JM, Milkiewicz P, Milkiewicz M. Melatonin Protects Cholangiocytes from Oxidative Stress-Induced Proapoptotic and Proinflammatory Stimuli via miR-132 and miR-34. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ, Korolczuk A. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol. 2014;65:75-82. [PubMed] |
| 22. | Pakravan H, Ahmadian M, Fani A, Aghaee D, Brumanad S, Pakzad B. The Effects of Melatonin in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. Adv Biomed Res. 2017;6:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | De Silva AP, Niriella MA, Ediriweera DS, De Alwis JP, Liyanage IK, Ettickan U, Liyanapathirana KV, Undugodage C, de Silva HA, de Silva HJ. Low-dose melatonin for sleep disturbances in early-stage cirrhosis: A randomized, placebo-controlled, cross-over trial. JGH Open. 2020;4:749-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Lorente L, Rodriguez ST, Sanz P, Abreu-González P, González-Rivero AF, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Cerro P, Portero J, Barrera MA. Low Serum Melatonin Levels Prior to Liver Transplantation in Patients with Hepatocellular Carcinoma are Associated with Lower Survival after Liver Transplantation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305-311. [PubMed] |
| 26. | Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4'-epiadriamycin in mice. Tumori. 1985;71:241-249. [PubMed] |
| 27. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8015] [Cited by in RCA: 40250] [Article Influence: 821.4] [Reference Citation Analysis (0)] |
| 28. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7899] [Cited by in RCA: 7981] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 29. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 30. | Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206-221. [PubMed] [DOI] [Full Text] |
| 32. | Picada JN, Flores DG, Zettler CG, Marroni NP, Roesler R, Henriques JA. DNA damage in brain cells of mice treated with an oxidized form of apomorphine. Brain Res Mol Brain Res. 2003;114:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Mavournin KH, Blakey DH, Cimino MC, Salamone MF, Heddle JA. The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1990;239:29-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 312] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Picada JN, da Silva KV, Erdtmann B, Henriques AT, Henriques JA. Genotoxic effects of structurally related beta-carboline alkaloids. Mutat Res. 1997;379:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Laemmli UK, Mölbert E, Showe M, Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970;49:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 209] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350-4354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33019] [Cited by in RCA: 36592] [Article Influence: 795.5] [Reference Citation Analysis (0)] |
| 38. | Rocha EG, Pereira MLD. Social representations on alcoholic liver cirrhosis elaborated by its carriers. Esc Anna Nery. 2007;11:670-676. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Salvi JO, Schemitt E, Fonseca SR, Hartmann RM, Colares JR, Marroni CA, Picada JN, Marroni NAP. Melatonin modulates antioxidant response and protects hepatocytes in rats with severe acute liver failure. South Am J Bas Educ Tech Technol. 2020;7:280-312. [DOI] [Full Text] |
| 40. | Moreira AJ, Ordoñez R, Cerski CT, Picada JN, García-Palomo A, Marroni NP, Mauriz JL, González-Gallego J. Melatonin Activates Endoplasmic Reticulum Stress and Apoptosis in Rats with Diethylnitrosamine-Induced Hepatocarcinogenesis. PLoS One. 2015;10:e0144517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Salvi JO, Schemitt EG, Colares JR, Hartmann RM, Marroni CA, Marroni NP. Action of Melatonin on Severe Acute Liver Failure in Rats. J Pharm Bio. 2017;12:62-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Schemitt EG, Hartmann RM, Colares JR, Licks F, Salvi JO, Marroni CA, Marroni NP. Protective action of glutamine in rats with severe acute liver failure. World J Hepatol. 2019;11:273-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Wree A, Marra F. The inflammasome in liver disease. J Hepatol. 2016;65:1055-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | González-Navajas JM. Inflammasome activation in decompensated liver cirrhosis. World J Hepatol. 2016;8:207-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19:4850-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 46. | Lebda MA, Sadek KM, Abouzed TK, Tohamy HG, El-Sayed YS. Melatonin mitigates thioacetamide-induced hepatic fibrosis via antioxidant activity and modulation of proinflammatory cytokines and fibrogenic genes. Life Sci. 2018;192:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Woolbright BL, Jaeschke H. Inflammation and Cell Death During Cholestasis: The Evolving Role of Bile Acids. Gene Expr. 2019;19:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Giusto M, Barberi L, Di Sario F, Rizzuto E, Nicoletti C, Ascenzi F, Renzi A, Caporaso N, D'Argenio G, Gaudio E, Musarò A, Merli M. Skeletal muscle myopenia in mice model of bile duct ligation and carbon tetrachloride-induced liver cirrhosis. Physiol Rep. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Zhu J, Qiu J, Chen K, Wang W, Zheng S. Tea polyphenols and Levofloxacin alleviate the lung injury of hepatopulmonary syndrome in common bile duct ligation rats through Endotoxin -TNF signaling. Biomed Pharmacother. 2021;137:111263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Hong RT, Xu JM, Mei Q. Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2009;15:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (4)] |
| 51. | Czechowska G, Celinski K, Korolczuk A, Wojcicka G, Dudka J, Bojarska A, Reiter RJ. Protective effects of melatonin against thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol. 2015;66:567-579. [PubMed] |
| 52. | Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8:e1000410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Chen L, Zhou T, Wu N, O'Brien A, Venter J, Ceci L, Kyritsi K, Onori P, Gaudio E, Sybenga A, Xie L, Wu C, Fabris L, Invernizzi P, Zawieja D, Liangpunsakul S, Meng F, Francis H, Alpini G, Huang Q, Glaser S. Pinealectomy or light exposure exacerbates biliary damage and liver fibrosis in cholestatic rats through decreased melatonin synthesis. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1525-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |