Published online Jan 21, 2022. doi: 10.3748/wjg.v28.i3.290
Peer-review started: June 3, 2021
First decision: July 27, 2021
Revised: August 12, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: January 21, 2022
Processing time: 223 Days and 20.2 Hours
Viral hepatitis infections are a great burden in children who have received liver transplant. Hepatotropic viruses can cause liver inflammation that can develop into liver graft fibrosis and cirrhosis over the long term. Immunological reactions due to viral hepatitis infections are associated with or can mimic graft rejection, rendering the condition difficult to manage. Prevention strategies using vaccinations are agreeable to patients, safe, cost-effective and practical. Hence, strategies to eliminate viral hepatitis A and B focus mainly on immunization programmes for children who have received a liver transplant. Although a vaccine has been developed to prevent hepatitis C and E viruses, its use is not licensed worldwide. Consequently, eliminating hepatitis C and E viruses mainly involves early detection in children with suspected cases and effective treatment with antiviral therapy. Good hygiene and sanitation are also important to prevent hepatitis A and E infections. Donor blood products and liver grafts should be screened for hepatitis B, C and E in children who are undergoing liver transplantation. Future research on early detection of viral hepatitis infections should include molecular techniques for detecting hepatitis B and E. Moreover, novel antiviral drugs for eradicating viral hepatitis that are highly effective and safe are needed for children who have undergone liver transplantation.
Core Tip: Viral hepatitis infections are a great burden for pediatric liver transplant recipients. Strategies to prevent infection include immunization, good sanitation and screening donor blood products and liver grafts for hepatitis B, C and E. In children infected with viral hepatitis who have received a liver transplant, early detection is crucial to guide proper management, as the infection can mimic or cause graft rejection. Effective antiviral therapy should be initiated when treating children with hepatitis B and C. Patients infected with hepatitis B who have undergone successful viral eradication should be revaccinated to maintain high hepatitis B surface antibodies to guarantee immunoprotection.
- Citation: Sintusek P, Thanapirom K, Komolmit P, Poovorawan Y. Eliminating viral hepatitis in children after liver transplants: How to reach the goal by 2030. World J Gastroenterol 2022; 28(3): 290-309
- URL: https://www.wjgnet.com/1007-9327/full/v28/i3/290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i3.290
Viral hepatitis is an infectious disease leading to high morbidity and mortality, especially in endemic areas such as Asia. Hepatitis viruses are hepatotropic and are classified into types A, B, C, D and E. In immunocompromised patients, including children who have undergone liver transplantation (LT) and typically receive lifelong immunosuppressants, nearly all viral hepatitis infections are chronic, progressing to liver fibrosis and cirrhosis in the long term. Hepatitis A is the only hepatitis virus that presents as an acute self-limiting infection but that is more severe in immunocompromised patients than in healthy individuals. Because viral hepatitis places a heavy burden on patients, strategies for prevention, early detection and prompt, effective management are crucial for graft survival and long-term outcomes in children after LT. In this review, we focus on lessons learned and future opportunities to develop effective strategies to eliminate hepatitis A, B, C and E in children after LT.
Yong Poovorawan (Figure 1), MD is currently the Professor and the head of the Center of Excellence in Clinical Virology at the Faculty of Medicine, Chulalongkorn University, Bangkok. Professor Poovorawan obtained the medical degree in 1974 and his specialization in paediatrics in 1978 from King Chulalongkorn Memorial Hospital, Chulalongkorn University. In 1984, he became a research fellow on the field of paediatric hepatology at King’s College Hospital Medical School, London. Professor Poovorawan has been working in the Department of Pediatrics at Chulalongkorn University, beginning as a lecturer and becoming Professor in 1991. Professor Poovorawan has received many research awards and honours, including the Outstanding Researcher Award in 1997 from the National Research Council of Thailand, Outstanding Scientist Award in 1997 from the Foundation for the Promotion of Science and Technology under the Patronage of His Majesty the King, Mahidol University-B-Braun Award in 2002, Thailand Research Fund Award in 2004 and has been nominated Senior Research Scholar by the Thailand Research Fund since 1997. He also received the Outstanding Best Teachers Award in 2004 from the Thailand National University Teacher Association. He is a leader who has been working on Viral hepatitis in Thailand. Outstanding Prof. Thailand Research Fund (2012-2014), Research Chair Grant, NSTDA (2014), Outstanding Achievement Doctor from the Medical Council of Thailand (2018), Achievement Award in Virology, Genetics Society of Thailand (2018), Achievement Award from the National Vaccine Institute of Thailand (2019). His work on avian influenza in Thailand also received outstanding research awards from the Thailand Research Fund in 2004 and the National Research Council in 2006. He is a member of the expanded programme on immunization vaccine, viral hepatitis and emerging diseases of the Center Disease Control, Ministry of Public Health. Professor Poovorawan has authored and co-authored more than 614 publications in the fields of hepatitis, paediatric hepatology and virology, with H-index 66 on Google Scholar.
Manifestations of hepatitis A virus (HAV) infection mainly derive from the immuno
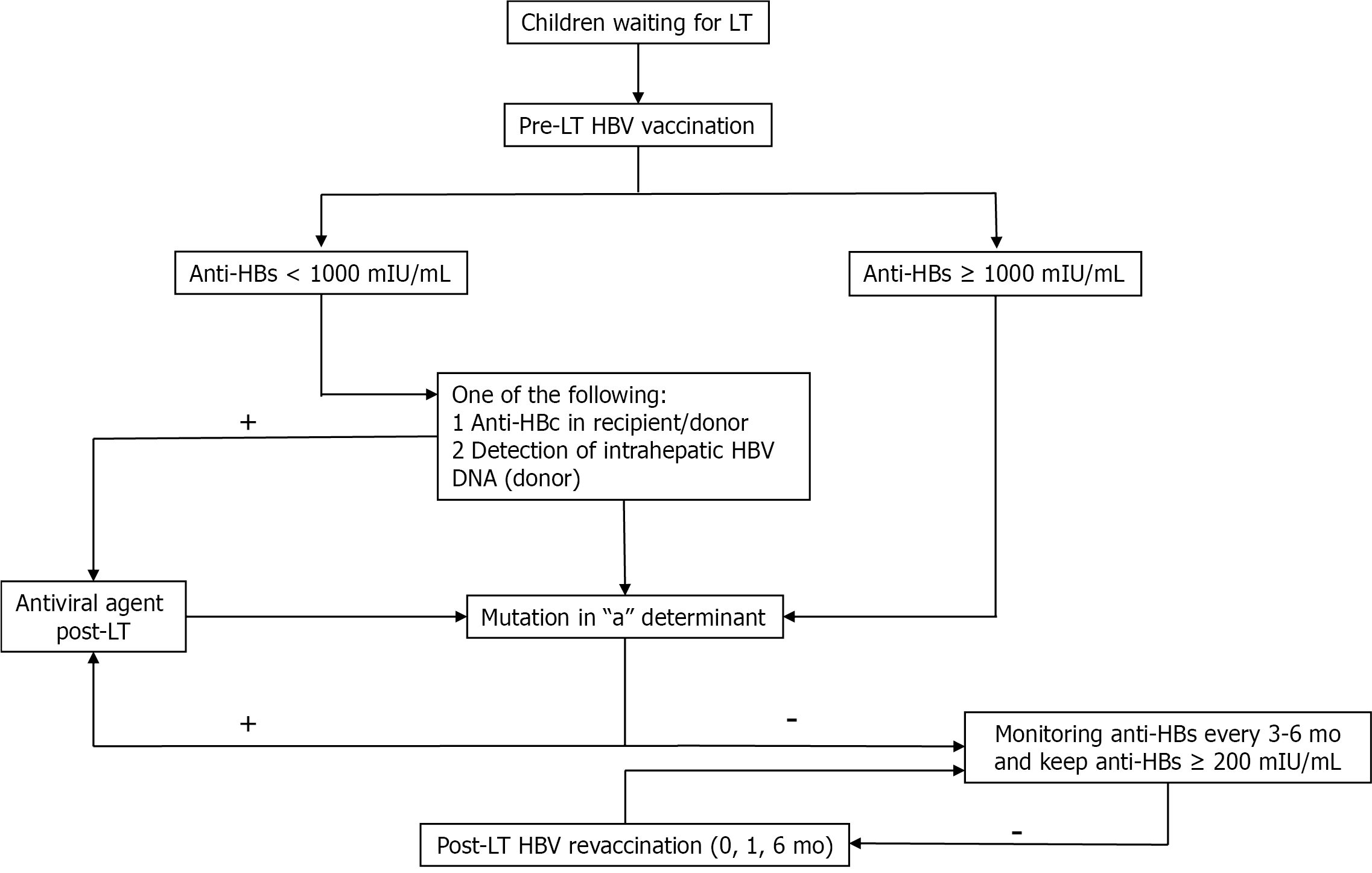
Pre-LT: Since universal hepatitis B virus (HBV) vaccination programmes began in the 1990s[15], HBV infection prevalence has rapidly decreased worldwide. HBV vaccine series that include vaccines at birth, 1-2 and 6-12 mo can reduce mother-to-child transmission, the major mode of HBV transmission in children, from 65%-90% to 3.6%-4.0%[16,17]. Indeed, the seroprotective rate after a complete HBV series is > 95%[18]. In our cohort study, although seroprotective rates decreased to 44% over a 20-year follow-up, 93.1% of the children exhibited seroconversion after a booster dose[19]. The presence of immune memory cells after the booster dose confirmed waning immunity with an anamnestic response, indicating increased levels of hepatitis B surface antibodies (anti-HBs)[19]. However, in immunocompromised patients with chronic liver diseases or cirrhosis, revaccination yields unsatisfactory outcomes, with seroconversion rates of 37.0%-90.9% on conventional schedules[20-28] and 16%-72% on accelerated/super-accelerated[29-37] schedules. Many studies on HBV schedules have been conducted to improve immunologic responses after revaccination in nonresponders, mainly using adult data, with different doses, routes, vaccine types, numbers and injection intervals. Regardless, no differences in the efficacy of these regimens have been shown[38]. Overall, time is a concern for participants awaiting LT, and super-accelerated or accelerated regimens should be considered for short-term prevention of HBV infections during and after LT[39].
During LT: External sources of HBV transmission, such as blood products, medical instruments and transmission by hospital personnel or close contacts, are concerns. Anti-HBs may decline after excessive plasma loss during surgery, and occult HBV infections from positive hepatitis B core antibody (anti-HBc) blood products have been reported[40].
Post-LT: Immunologic loss of HBV is common after LT [41], and de novo hepatitis B infection (DNH) was observed in our paediatric LT centre[42]. DNH is likely related to acquired HBV infections from endemic environments or from HBV reactivation from positive anti-HBc allografts during immunologic loss[43-47]. In our centre, the anti-HBs loss rate increased rapidly after LT, and 46%, 57% and 82% of patients had anti-HBs levels of < 10 mIU/mL at 1 year, 2 years and > 3 years after LT, respectively. One case of DNH was detected at 3 years after LT, though anti-HBs levels were > 1000 mIU/mL before LT[42]. Hence, regular monitoring for anti-HBs and revaccination after LT are crucial. Studies of immunogenicity to HBV revaccination after LT have reported higher humoral immune responses in children than in adults (up to 100% vs 33.3%-63.8%); however, immunity waned, and the patients needed frequent booster doses to maintain high seroprotective levels[43]. In healthy adults not responding to conventional vaccine schedules, a systematic review found no differences in seroconversion rates according to dosage or vaccine administration route[38]. However, to date, no study has been conducted involving children in this population. We conducted studies of immunologic responses to standard vs double-dose HBV vaccine series (at 0, 1 and 6 mo) in children after LT exhibiting anti-HBs loss and found response rates of 91.6% and 85% after a 6-mo follow-up, with no statistically significant difference in anti-HBs level between the two regimens (unpublished data). Hence, short-term assessment revealed that HBV revaccinations in children after LT are highly effective and safe.
Positive anti-HBc allografts are considered a major risk factor of DNH after LT, especially in patients without prior seroprotection or rapid anti-HBs loss after LT. In addition to being revaccinated 3-6 mo after LT, other strategies to prevent DNH include antiviral therapy and/or passive immunity with hepatitis B immunoglobulin (HBIG). Unlike the many studies that have used adult data and investigated several strategies, few studies of prophylactic strategies against DNH have been conducted in children who receive positive anti-HBc allografts[43,44,48-51]. Song et al[48] reported the efficacy of pre- and post-LT HBV vaccinations to prevent DNH and recommended a prophylactic strategy to maintain anti-HBs levels at ≥ 1000 mIU/mL pre-LT and ≥ 200 mIU/mL post-LT without antiviral consideration. The DNH rate when using this strategy was 1.3%[48]. However, anti-HBs levels may rapidly decline after LT owing to the massive immunosuppression involved. In such cases, antiviral therapy should be added in parallel until the appropriate revaccination time after LT (usually 3-6 mo) and until anti-HBs levels increase to ≥ 200 mIU/mL after revaccination.
Children with chronic HBV infections are rarely indicated for LT because they are usually asymptomatic in the stage of hepatitis B e-antigen (HBeAg)-positive chronic infection or HBeAg-positive chronic hepatitis. Thus far, immunoprophylaxis data on recurrent HBV infections after LT are mainly based on adult data.
In summary, strategies to prevent HBV infection before, during and after LT mainly include active immunization. Super-accelerated and accelerated vaccines may be considered for timely protection prior to LT (to keep anti-HBs levels ≥ 1000 mIU/mL if possible). However, in children, anti-HBs levels should be regularly monitored, and revaccinations should be provided to maintain high anti-HBs levels (≥ 200 mIU/mL).
Despite antiviral HBIG and active HBV immunization strategies, DNH has been reported in 0.9%-4.0% of both paediatric and adult LT patients[48,52,53]. Table 1 summarizes the risk factors for DNH. An escape mutation in the “a” determinant region within the hepatitis B surface antigen (HBsAg) that develops before LT, after HBV vaccination, or after HBIG administration post-LT should be considered[54]. In this situation, antiviral agents play a major role in preventing DNH, and long-term assessment for drug resistance should be considered. We recommend including pre-LT evaluations for HBV by serological, molecular and virological methods. Liver donors and allografts should be evaluated for covalently closed circular DNA (cccDNA) and HBV viral loads in cases of suspected occult infection with an escape mutant.
| Risk factors |
| Positive anti-HBc donor[40] |
| Positive-intrahepatic HBV DNA[40] |
| Liver graft HBV DNA > 1000 copies[40] |
| Intraoperative fresh-frozen plasma transfusion > 400 mL[40] |
| Positive-anti-HBc recipients[40] |
| Pre-operative anti-HBs < 1000 mIU/mL[40,43,48] |
| Post-operative anti-HBs < 100-200 mIU/mL[48,53] |
| Hepatitis B surface mutation (within the “a” determinant region[54]) |
Su et al[54] found that after DNH occurred in children post-LT, more than half (5/9) exhibit seroconversion after lamivudine therapy. However, one child carried a tyrosine-methionine-aspartic acid-aspartic acid (YMDD) motif mutation, and the authors switched antiviral agent from lamivudine to adefovir dipivoxil. To date, no consensus treatment for DNH has been reached[43,54-56]. Antiviral therapy for DNH might follow the guidelines for treating HBV infections in children (Table 2). In our unit, one patient with DNH was treated with interferon-α for 1 year without a response, even though this child exhibited HBsAg seroconversion after 6 mo of entecavir therapy. We revaccinated him against HBV after HBsAg clearance following entecavir therapy. This child received an HBV revaccination series (0, 1 and 6 mo) and maintained anti-HB levels of > 1000 mIU/mL without a rebooster at a 44-mo follow-up. Further study on the efficacy of antiviral therapy for DNH and other novel antiviral therapies with less drug resistance and high efficacy in children with DNH should be conducted to determine the best endpoints of HBsAg clearance and anti-HBs appearance. Tenofovir alafenamide (TAF) is a novel tenofovir product with improved properties for avoiding kidney and bone-related adverse events due to tenofovir disoproxil fumarate (TDF). Compared with TDF, TAF has non-inferior efficacy and a good safety profile[57]. Nevertheless, data for post-transplant adults and children receiving TAF are lacking. Only a small single-centre study of adult liver-transplant recipients found that TAF (25 mg/d) displayed high antiviral efficacy in preventing HBV recurrence without affecting immunosuppressive medications or graft functioning and had a good safety profile[58]. TAF is a promising antiviral therapy for adolescents diagnosed with DNH.
| Medication | Licensing | Dose and duration | HBsAg loss (%) | Resistance (%) |
| IFN-α-2b | ≥ 1 yr | 6 million IU/m2 three times weekly for 6 mo | 1-2 | 0 |
| Lamivudine | ≥ 2 yr | 3 mg/kg daily for ≥ 1 yr | 0 | 19-64 |
| Entecavir | ≥ 2 yr | 0.25-0.5 mg daily for ≥ 1 yr | 0.52 | 0.7-1.2 |
| Tenofovir dipovaxil fumarate | ≥ 12 yr | 300 mg daily for ≥ 1 yr | 0.02 | 0 |
| Adefovir | ≥ 12 yr | 10 mg daily for ≥ 1 yr | 0 | 0.9-20 |
As mentioned, current HBV prophylaxis and therapies do not completely eradicate HBV infections in most cases, requiring lifelong medication. Thus, effective and finite HBV treatment remains an unmet medical need, and new therapeutic approaches and drugs are necessary to achieve a functional cure (mainly defined as a loss of when HBsAgs off therapy). Multiple novel drugs targeting different steps in the HBV life cycle are being developed. Antiviral and host-targeting agents are the two main drugs being studied. The major HBV-target-specific categories of antiviral drugs are hepatocyte-entry receptor inhibitors (e.g., bulevirtide, formerly myrcludex B)[59,60], cccDNA inhibitors, nucleocapsid-assembly modulators (core protein allosteric modulators, e.g., JNE-56136379)[61], post-transcriptional control inhibitors (RNA interference drugs, e.g., ARC-520)[62], HBsAg-release inhibitors (nucleic-acid polymers, e.g., REP 2139 and 2165)[63] and HBV DNA polymerase inhibitors. Therapies that target host immune responses include Toll-like receptor (TLR)-7 (e.g., GS-9620, vesatolimod)[64], TLR-8 (GS-9688, selgantolimod)[65], and TLR-9 agonists, checkpoint inhibitors (anti-programmed death 1 and anti-programmed death-ligand 1)[66] and therapeutic vaccines[67]. These drugs are currently in phase I and II clinical trials that mainly include non-transplant adult patients and indicate a promising future for HBV eradication. No data are available on the efficacy of these new drugs against HBV recurrence or de novo infection in children after LT. Further studies are needed to determine the impact of the new drugs on these patient groups.
Based on current knowledge of the human immunodeficiency virus (HIV) and hepatitis C virus (HCV), immunomodulators and combination treatments targeting several steps in HBV replication will likely be required to achieve a functional cure for HBV. Preclinical studies are applying this strategy in animal models[68], and clinical trials are investigating combinations of several antiviral drugs or immune boosters with antiviral agents. This new approach using combination therapies will need to be individualized, but many patients may be eligible.
In summary, strategies to eliminate HBV in paediatric liver transplant recipients include HBV immunization both pre- and post-LT. Early detection of HBV infections, especially of escape mutants, which lead to vaccine failure in recipients, and of cccDNA in the livers of positive anti-HBc donors, should be evaluated via molecular and viral genetic analysis in the liver tissues of both the donors and recipients. Patients with vaccine failure or DNH should promptly undergo antiviral therapy. Figure 2 shows the proposed strategies to eliminate HBV in children post-LT.
HCV infections are a global health problem, with an estimated 71 million people being chronically infected in 2016 and 400000 deaths annually worldwide[69]. Therefore, in 2016, the World Health Organization (WHO) set the goal of eliminating HCV by 2030. There has been significant progress towards this goal in screening policies, improving access to care, and reducing the costs of direct-acting antivirals (DAAs). Compared with adult patients, little attention has been paid to diagnosis, therapy, and prevention for children and adolescents. One reason is that prior to 2017, no DAAs were licensed for use in patients under 18 years old, and evidence was lacking to support paediatric management guidelines and policies. The majority of national HCV policies do not include explicit recommendations for HCV testing and treatment in children and adolescents[70]
In 2018, the global prevalence of HCV viraemia in populations under 18 years old was 0.13%, with an overall burden of 3.3 million cases[71]. The true HCV infection prevalence in paediatric populations is unknown due to a lack of universal screening strategies. Perinatal transmission is a major cause of recognized HCV infections in children, with transmission rates of 5% from HCV-infected mothers and 10% from HCV-HIV-coinfected mothers[72,73]. Moreover, the opioid epidemic is associated with an expanding ongoing risk of HCV transmission from mothers to children[74]. In the United States, nearly 29000 HCV-infected women gave birth annually from 2011-2014[75]. Moreover, the transmission risk increases with higher maternal HCV viral loads, HIV coinfections, longer labour durations, amniocentesis or foetal-scalp monitoring, and prolonged membrane rupture[72,76-78]. Several studies from developed countries have reported increased injection drug use as a risk factor of HCV and HIV infections among adolescents[79,80]. Sexual transmission of HCV is also a major factor in men who have sex with men, including those infected with HIV or those who have received a pre-exposure prophylaxis for HIV[81,82].
After vertical HCV transmission, 25%-40% of patients spontaneously clear the infection within the first 4 years of life[83]. Approximately half of infants born with HCV will develop chronic disease that may lead to cirrhosis and hepatocellular carcinoma in late childhood[84]. The natural history of paediatric HCV differs from that of HCV acquired in adulthood. Host factors (e.g., rs12979860 mutation in the IL28B gene[85], natural killer cell cytolytic functions[86]) and viral factors (e.g., HCV genotype)[87] are associated with spontaneous clearance of HCV infections. Children with chronic HCV infections are mostly asymptomatic, with mild degrees of hepatitis and fibrosis during childhood and higher rates of spontaneous HCV clearance. Therefore, it is uncommon for children and adolescents to develop HCV-associated end-stage liver disease or hepatocellular carcinoma (HCC)[88]. Comorbidities, including haematological disease with iron overload, obesity, alcohol use, and concomitant viral infections (e.g., HBV or HCV), are associated with accelerated liver fibrosis and cirrhosis development[89]. HCV-related extrahepatic manifestations are less common in paediatric patients than in adult patients[90]. In general, HCV infections in children and adolescents are related to poor life quality and reduced cognitive functioning[84].
Several current international guidelines recommend anti-HCV testing (with a confirmatory nucleic acid assay for a positive result) for all pregnant women, especially those in high-risk groups, including those with past or current injection drug use, incarceration history, unregulated tattoos/piercings, receipt of contaminated blood products, or exposure in HCV-endemic areas[91-93]. HCV RNA can be found in breast milk and colostrum, but breastfeeding does not increase HCV transmission rates except in HCV-HIV coinfected mothers[94]. All children born to HCV-infected mothers should be tested for HCV infection before 18 mo of age. Because anti-HCV antibodies passed from mothers can persist until 18 mo of age, HCV infection in children younger than 18 mo can be diagnosed by detecting HCV RNA. High-risk adolescents, including those who with histories of injection drug use and men who have sex with men, should be tested for HCV infection[95].
The asymptomatic nature of HCV infection and the high cost of diagnostic screening are the important barriers to detecting and treating HCV-infected patients[96]. Thus, a simple, cost-effective diagnostic method for routine HCV screening especially for low- to middle- income countries is needed. The core antigen of HCV (HCV Ag) is an alternative for screening and diagnosis. This test can be used as a supplemental marker after anti-HCV testing to reduce the requirement of further confirmatory HCV RNA assays[97]. Point-of-care tests of viraemia are related with improvement in access to testing [98].
Advancement of oral DAA therapies has resulted in a paradigm shift in treating HCV, with cure rates of > 90% and few adverse effects. DAAs with pan genotypic activity are recommended as preferred regimens for all treatment-naïve and treatment-experienced HCV patients, regardless of age, sex, stage of liver fibrosis, or HIV coinfection[93,99,100]. Conversely, pegylated-interferon-based regimens are no longer recommended. DAA treatment with an approved regimen is recommended for all children and adolescents ≥ 3 years old with HCV infection, regardless of disease severity[101,102]. Early antiviral treatment should be administered to reduce morbidity and mortality if extrahepatic manifestations occur (e.g., glomerulonephritis and cryoglobulinemia).
Adolescents aged 12-17 years who are treatment-naïve or -experienced, without cirrhosis or with compensated cirrhosis (Child-Pugh A) should be treated according to the recommendations for adult patients. For pangenotypic HCV, two DAA regimens are recommended: sofosbuvir (400 mg)/velpatasvir (100 mg) once daily for 8-12 wk, achieving a 95% sustained virological response (SVR)-12 rate (97/102; 1 virological failure) with mild-to-moderate adverse events[103]; a fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) once daily for 8 wk, achieving a 100% SVR-12 rate with a good safety profile[104]. Although the clinical trial for glecaprevir /pibrentasvir included only adolescents with HCV genotypes 1-4, this drug was approved by the Food and Drug Administration (FDA) for adults with all genotypes. In 2019, the FDA approved treating genotype-specific HCV with sofosbuvir (400 mg)/ledipasvir (90 mg) for 12 wk in adolescents aged 12-17 years or weighing at least 35 kg with genotypes 1, 4, 5, or 6, without cirrhosis or with compensated cirrhosis[105, 106].
Children aged 3-11 years who are treatment-naïve or treatment-experienced, without cirrhosis or with compensated cirrhosis (Child-Pugh A) with any HCV genotypes should be treated with the FDA-approved regimens of a fixed-dose combination of sofosbuvir (200 mg)/velpatasvir (50 mg) for those aged > 6 years weighing ≥ 17 kg and sofosbuvir (150 mg)/velpatasvir (37.5 mg) for 12 wk[103] in those with weighing < 17 kg. One trial found that for children aged 3-11 years, the fixed-dose combination of glecaprevir (250 mg)/pibrentasvir (100 mg) for those weighing 30-44 kg, glecaprevir (200 mg)/pibrentasvir (80 mg) for those weighing 20-29 kg, and glecaprevir (150 mg)/pibrentasvir (60 mg) for those weighing 12-19 kg for 8-16 wk achieved a 96% SVR-12 rate, without drug-related severe adverse events[107]. However, this formulation is not yet FDA approved.
Overall, DAA-experienced children and adolescent patients with HCV are rare in clinical practice (Table 3). Because data for these populations are limited, DAA-experienced paediatric patients with HCV infections should be treated using the guidelines for adult patients.
| Age | Genotype | No cirrhosis/ cirrhosis | Recommended regimens of DAAs | Duration (wk) |
| 12-17 yr | Pan-genotypes | No cirrhosis | Sofosbuvir 400 mg/ velpatasvir 100 mg | 12 |
| Compensated cirrhosis (Child-Pugh A) | Glecaprevir 300 mg/pibrentasvir 120 mg | 8-12 | ||
| 12-17 yr or BW ≥ 35 kg | 1, 4, 5, 6 | No cirrhosis | Sofosbuvir 400 mg/ledipasvir 90 mg | 12 |
| Compensated cirrhosis (Child-Pugh A) | Sofosbuvir 200 mg/velpatasvir 50 mg (BW ≥ 17 kg) | |||
| 3-11 yr | Pan-genotypes | No cirrhosis | Sofosbuvir 150 mg/velpatasvir 37.5 mg (BW < 17 kg) | 12 |
| Compensated cirrhosis (Child-Pugh A) | Glecaprevir 250 mg/pibrentasvir 100 mg (BW 30-44 kg); Glecaprevir 200 mg/pibrentasvir 80 mg (BW 20-29 kg); Glecaprevir 150 mg/pibrentasvir 60 mg (BW 12-19 kg) | 12; 8-16; 8-16; 8-16; |
Children and adolescents with chronic HCV infections rarely require LT for complications from liver cirrhosis or HCC; recurrent HCV after LT is also clinically rare. In a retrospective study of the United Network of Organ Sharing database, Gupta et al[108] found that 120 paediatric patients received transplants for chronic HCV infections in 1994-2010. One-year and 3-year survival rates were 97% and 89%, respectively, in patients with post-paediatric end-stage liver diseases. Pre-LT recipient factors, good surgical technique, and effective treatment for HCV infections are associated with good prognostic outcomes in paediatric patients after LT[108]. Patients who achieve an SVR have less mortality than do those without SVR after treatment. Treatment has better effects on disease outcomes if it is started before cirrhosis[109]. To prevent long-term liver disease and HCV spread, antiviral therapy should be available in childhood.
LT for HCV-related diseases has decreased in the era of DAA treatment. Treating patients before LT reduces the chance of graft dysfunction after LT and may stabilize or improve liver function. SVR before LT may lead to the delisting of some patients[110]. Patients with decompensated (Child-Pugh B or C) cirrhosis who have model for end-stage liver disease (MELD) scores of < 20 without HCC and are awaiting LT should be treated with DAAs before LT. The recommended regimen is sofosbuvir (400 mg)/velpatasvir (100 mg) plus weight-based ribavirin 1000-1200 mg/day for 12 wk or sofosbuvir (400 mg)/velpatasvir (100 mg) for 24 wk in those with contraindications for ribavirin. Patients with MELD scores > 20 should undergo transplantation first and treated for HCV infection after LT if the waiting time is < 6 mo[111, 112].
Vaccines: Despite the high curative rate of HCV infections by DAAs, high-risk populations remain at risk of reinfection, even after successful treatment. Preventing new HCV infections is vital and may result in the WHO’s 2030 global elimination goal. A prophylactic HCV vaccine might also help to achieve this goal by preventing transmission. Nevertheless, no vaccine for preventing HCV infections has been approved to date.
A recent phase 1-2, randomized, double-blind, placebo-controlled trial by Page et al[113] enrolled adults aged 18-45 years who had injected drugs within 90 d. These adults received either an intramuscular injection of a recombinant chimpanzee adenovirus type-3 vector-priming vaccination (ChAd3-NSmut vaccine) on day 0 and a recombinant modified vaccine (Ankara, MVA-NSmut vaccine) booster on day 56 (vaccine group) or a saline placebo on days 0 and 56. Despite inducing HCV-specific T-cell responses and lowering peak HCV RNA levels, the vaccine failed to prevent chronic HCV infection compared with placebo[113]. The innate variability of HCV enveloped proteins and the limited knowledge of HCV protein structures are barriers to developing an HCV vaccine. Future work should determine the optimal HCV epitopes to target vaccine development.
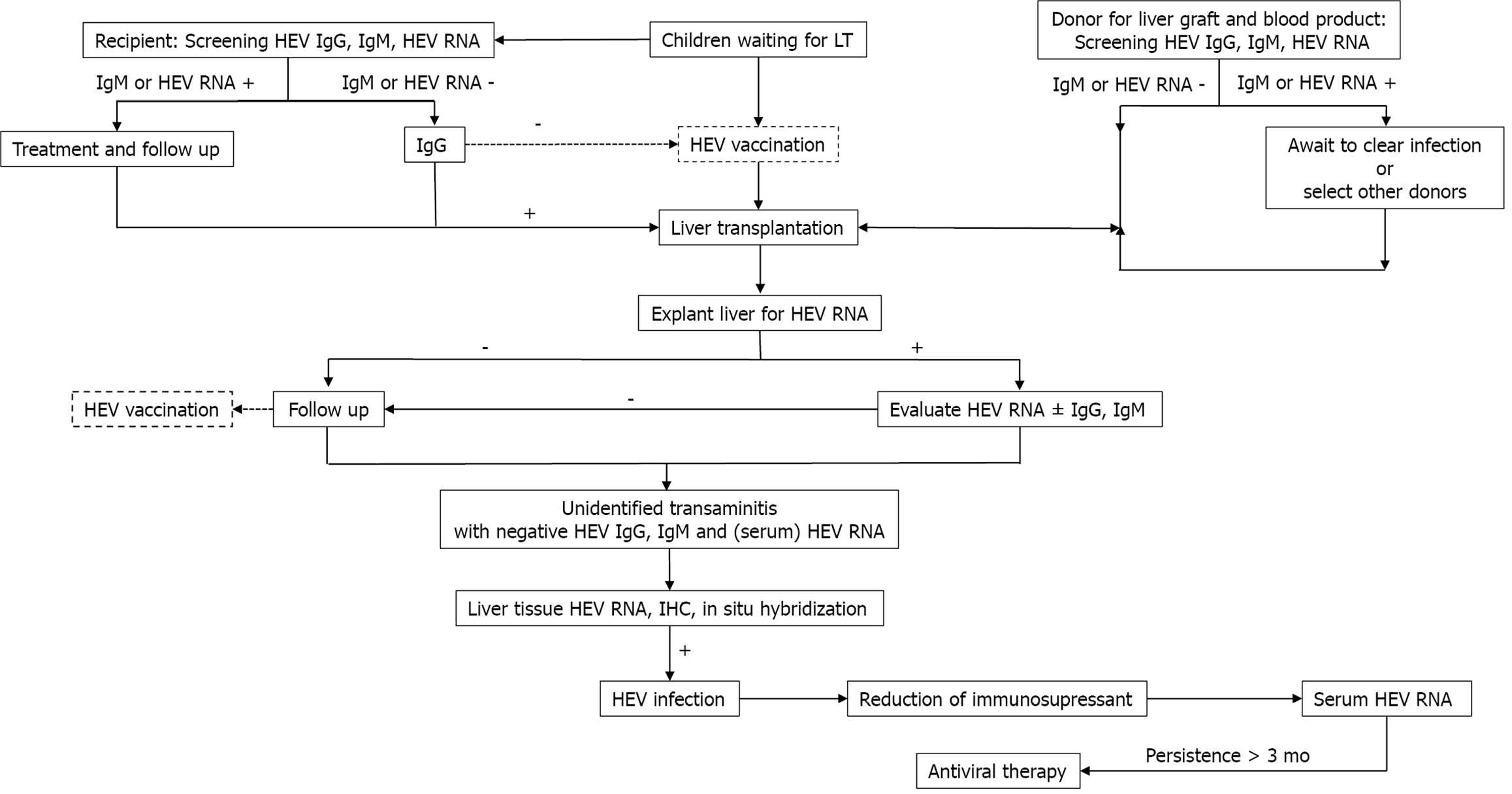
Hepatitis E virus (HEV) was first discovered in the 1980s and normally manifests as an acute self-limited condition[114], though chronic HEV infection courses were recognized in 2008 in organ-transplant recipients[115]. HEV infection seroprevalence varies from 0.3%-75.6% depending on the area and diagnostic method[116-121]. HEV is transmitted mainly via the faecal-oral route, but mother-to-child[122], liver graft-to-recipient and plasma-derived-product transmission[123,124] have been reported. One study reported HEV transmission via liver graft[125], and several cases of HEV infections transmitted by blood transfusion have been reported. These findings have led to universal HEV-RNA testing in blood donors in many countries[123,124,126,127]. To detect HEV infection in immunocompetent children, primary testing with anti-HEV IgG and IgM is reasonable, and HEV RNA in stool and serum samples should be assessed in highly suspected cases that yielded negative results by serological methods[128]. However, serum HEV RNA analysis is preferable in immunocompromised patients, as they cannot mount an antibody response[129]. HEV infection has clinical impacts in immunocompromised hosts, especially in those needing organ transplantation. Moreover, HEV infections can be asymptomatic pre-existing chronic liver diseases or solid organ transplantation[130-133], or liver-associated morbidity due to progressive fibrosis and cirrhosis may be present[134]. Additionally, these conditions increase the risk of graft rejection[132]. In 2014, the European Association for the Study of the Liver proposed a well-organized stepwise plan for managing HEV infections in both adults and children after organ trans
| Ref. | Year | Country | Participants | Seroprevalence of HEV infection | Methods | Comments | |
| HEV IgM/G | HEV RNA | ||||||
| 1[130] | 2012 | Canada | Gr 1; N: 66 with normal LFT, aged 13.7 yr (1.8-25.5); Gr 2; N: 14 with transaminitis, aged 17.4 yr (5.9-19.8) | Gr 1: 10/66 (15%) with IgG +, none had IgM, HEV RNA +; Gr 2: 12/14 (86%) with IgG+; 9/12 (75%) with IgM+; 1/12 (0.8%) with HEV RNA + | Feldan Bio Inc, Saint-Augustin | Serum nested RT-qPCR | All in Gr 2 showed a trend toward chronic hepatitis and fibrosis; An 8-yr-old girl had chronic HEV infection (genotype 3) for > 10 yr and developed cirrhosis |
| 2[131] | 2012 | Germany | N: 41 liver-transplanted children, aged 8.8 ± 4.2 yr | 2/41 (4.9%) IgG +0/41 stool HEV RNA + | Mikrogen | Stool RT-qPCR | No case with chronic HEV infection |
| 3[132] | 2013 | Germany | N: 22 liver-transplanted children, aged 6.7 yr (1.4-17.2) | 1/22 (0.45%) IgG + by Wantai assay and HEV RNA + in serum | Wantai assay | Serum or stool PCR | 10-year-old boy with HEV infection that had persistent transaminitis after 2-mo immunosuppressive reduction. Ribavirin 15 mg/kg/d was started for 6 mo. Normal LFT and undetectable serum and stool HEV RNA at day 42 of treatment. |
| 4[139] | 2014 | Brazil | One liver-transplanted child: case report | HEV IgG/IgM and HEV RNA in serum and liver tissue at 6-10 yr after liver transplantation | Mikrogen | Liver and serum RT-PCR | A 4-yr-old girl with transaminitis from ACR at 6 yr after LT, had transaminitis off and on and HEV IgG/IgM and HEV RNA was detected 9-10 yr after LT. Chronic HEV infection was successful treatment with ribavirin for 10 mo. |
| 5[133] | 2015 | France | 84 liver-transplanted children, aged 12.3 yr | 8/84 (8.3%) HEV IgG+ | Wantai assay | Ceeram Tools® kit for HEV-RNA detection | None had HEV IgM/RNA +; No case of chronic infection |
| 6[140] | 2020 | France | 80 liver-transplanted children, aged 3.5 ± 4 yr | 6/80 (8%) with HEV IgG+ | Wantai assay | Ceeram Tools® kit for HEV-RNA detection | None had HEV IgM/RNA +; No case of chronic infection; 4/6 had undetectable HEV IgG after follow-up (3-42 mo) |
| 7 | 2021 | Thailand | 30 liver-transplanted children with transaminitis, aged 1.2-17.6 yr | 14/30 (45.2%) with HEV IgG+, 4 (13%) with HEV IgM+ and one case with HEV RNA in stool | Euroimmun kit | Stool PCR | All of them had persistence of HEV IgM from 5 to 44 mo and transaminitis from 4 to 30 mo before HEV testing. The previous treatment included graft rejection, de novo autoimmune hepatitis and CMV viremia. |
Strategies to eliminate HEV infection include prevention by implementing hygienic measures and thoroughly cooking food, screening plasma-derived products from immunocompromised patients and, developing an HEV vaccine. Early detection and effective treatment with antiviral agents in infected patients are also crucial.
Diagnostic testing for HEV infection: As chronic HEV infections in children after LT lead to progressive hepatitis and liver fibrosis, suspected cases should be tested. As serologic testing is insufficient to detect HEV infections in immunosuppressed patients, HEV infections should be diagnosed based on HEV-RNA detection in specimens. Protzer et al[141] reported molecular detection of HEV in liver-biopsied tissues from four liver-transplanted patients, whereas serology only detected two (Mikrogen assay). Prost et al[142] compared HEV-RNA detection in clinical liver-biopsy tissues between in situ testing and qPCR from paraffin-embedded liver tissues and found that qPCR was more effective. Additionally, Ankavay et al[143] found that detecting the open reading frame 2 (ORF2) protein of HEV via immunohistochemistry of liver tissues can be used as a rapid histopathological method to diagnose HEV infections. The sensitivity and specificity of this technique were the same as those of tissue PCR for HEV RNA. The ORF2 clone 1E6 antibody yielded the highest diagnostic accuracy and was more sensitive for HEV serotypes 1 and 3 in the livers of both immunocompromised and immunocompetent patients[143]. Detecting HEV in liver tissues may be more reliable and may correlate directly with liver inflammation and damage in the immunocompromised. Regardless, a limitation of ORF2 clone 1E6 staining is that it is less sensitive for HEV genotypes 2 and 4. A cost-effective method of detecting HEV infection with high efficacy is still needed. Table 5 summarizes the HEV detection methods and their diagnostic value[144,145].
| Detection | Technique | Specimen |
| Virus or its components (direct method) | HEV nucleic acid: (1) RT-PCR; (2) Realtime RT-PCR; and (3) Loop-mediated isothermal amplification assay. HEV RNA: (1) In situ hybridization; (2) HEV viral protein (antigen); (3) EIA; and (4) IHC. | Serum, stool, bile, liver tissue |
| Host immune response (indirect method) | Specific anti-HEV antibodies (IgM and IgG) (sensitivity 72%-98% and specificity 78%-96%): (1) Indirect EIA; (2) Immunochromatographic assays; (3) Double-antigen sandwich-based EIAs; (4) μ capture EIAs for IgM anti-HEV; (5) Specific cellular immune response; and (6) ELISpot assays. | Serum, peripheral blood mononuclear cells |
Antiviral therapy for HEV infection: In addition to ribavirin, other medications used in HEV-infected adults include pegylated interferon-α and add-on effects of sofosbuvir with ribavirin[128,146]. Recent data show that interferon λ1-3 inhibits HEV replication in an in vitro culture system and may be effective for treating HEV infections[147]. Another proposed medication is zinc salt. In a human hepatoma cell study, zinc salt dose-dependently inhibited replication of HEV genotypes 1 and 3[148]. In fact, zinc can directly decrease HEV replication by suppressing viral translation and processing of nonstructural proteins encoded by ORF1 and by inhibiting IFN-λ3 from binding to its receptor[149,150]. Moreover, zinc has an indirect effect by modulating host immune responses and is a cofactor in host cellular processes[150]. Hence, zinc is a promising drug for HEV therapy without serious adverse effects. Clinical and basic research are needed regarding the therapeutic benefits of zinc in HEV infections.
Prevention with an HEV vaccine: Since 2001, several vaccines based on virus-like particles have been developed[151], and there have been clinical trials on three vaccine candidates[136,137,152]. One is licensed in China, with 100% efficacy over 12 mo after 3 injections[137]. Moreover, the efficacy remained high at 86.8% after a 4.5-year follow-up[138]. However, these three vaccines mainly protect against genotypes 1 and 4 but cannot protect against genotype 3, which is the main genotype causing chronic HEV infections in patients after LT[153]. In 2019, an HEV vaccine was initiated and is progressing in clinical trials in the United States[153]. In general, an HEV vaccine will be a powerful weapon in public health for protecting against HEV infections (Figure 3).
To eliminate viral hepatitis in paediatric liver-transplant recipients, multiple strategies must be integrated into clinical practice. Similar to the prevention of HAV infections, immunization is the mainstay of prevention against HBV infection in children with liver transplants. Regular monitoring of humoral immunity for HBV and HAV and revaccination programmes in cases with immunity loss are necessary. Antiviral therapy plays a major role in HBV and HCV infections. For HEV infection, molecular techniques for early detection in children with liver transplant with unidentified causes of hepatitis should be developed to guide proper management of HEV infection.
We would like to express our sincere respect and gratitude to Professor Poovorawan Y, our mentor and friend, for devoting nearly all of his life to Thai research development. He is our role model and truly inspires us to conduct more bench and bedside integrated research in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elshimi E S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Koul R, Lal BB, Pamecha V, Sarin S, Alam S. Liver Transplantation Reverses Hepatic Myelopathy in 2 Children With Hepatitis A Infection. Child Neurol Open. 2021;8:2329048X20983763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Pramoolsinsap C. Acute hepatitis A and acquired immunity to hepatitis A virus in hepatitis B virus (HBV) carriers and in HBV- or hepatitis C virus-related chronic liver diseases in Thailand. J Viral Hepat. 2000;7 Suppl 1:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Ruddy SJ, Johnson RF, Mosley JW, Atwater JB, Rossetti MA, Hart JC. An epidemic of clam-associated hepatitis. JAMA. 1969;208:649-655. [PubMed] |
| 4. | Gao S, Nishinari K. Effect of degree of acetylation on gelation of konjac glucomannan. Biomacromolecules. 2004;5:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Sintusek P, Poovorawan Y. Immunization status and hospitalization for vaccine-preventable and non-vaccine-preventable infections in liver-transplanted children. World J Hepatol. 2021;13:120-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Sa-nguanmoo P, Posuwan N, Vichaiwattana P, Vuthitanachot V, Saelao S, Foonoi M, Fakthongyoo A, Makaroon J, Srisingh K, Asawarachun D, Owatanapanich S, Wutthiratkowit N, Tohtubtiang K, Vongpunsawad S, Yoocharoen P, Poovorawan Y. Declining Trend of Hepatitis A Seroepidemiology in Association with Improved Public Health and Economic Status of Thailand. PLoS One. 2016;11:e0151304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Eisenbach C, Longerich T, Fickenscher H, Schalasta G, Stremmel W, Encke J. Recurrence of clinically significant hepatitis A following liver transplantation for fulminant hepatitis A. J Clin Virol. 2006;35:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Günther M, Stark K, Neuhaus R, Reinke P, Schröder K, Bienzle U. Rapid decline of antibodies after hepatitis A immunization in liver and renal transplant recipients. Transplantation. 2001;71:477-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Stark K, Günther M, Neuhaus R, Reinke P, Schröder K, Linnig S, Bienzle U. Immunogenicity and safety of hepatitis A vaccine in liver and renal transplant recipients. J Infect Dis. 1999;180:2014-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Dumot JA, Barnes DS, Younossi Z, Gordon SM, Avery RK, Domen RE, Henderson JM, Carey WD. Immunogenicity of hepatitis A vaccine in decompensated liver disease. Am J Gastroenterol. 1999;94:1601-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Irving GJ, Holden J, Yang R, Pope D. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev. 2019;12:CD009051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Ferreira CT, da Silveira TR, Vieira SM, Taniguchi A, Pereira-Lima J. Immunogenicity and safety of hepatitis A vaccine in children with chronic liver disease. J Pediatr Gastroenterol Nutr. 2003;37:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Arslan M, Wiesner RH, Poterucha JJ, Gross JB Jr, Zein NN. Hepatitis A antibodies in liver transplant recipients: evidence for loss of immunity posttransplantation. Liver Transpl. 2000;6:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Zhu J, Alalkim F, Hussaini T, Erb SR, Marquez V, Krajden M, Webber D, Yoshida EM. In-hospital post-transplant acute hepatitis A viral (HAV) infection in a liver transplant recipient who was HAV seropositive pre-transplant. Saudi J Gastroenterol. 2019;25:67-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Maupas P, Goudeau A, Coursaget P, Drucker J, Bagros P. Hepatitis B vaccine: efficacy in high-risk settings, a two-year study. Intervirology. 1978;10:196-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1-31. [PubMed] |
| 17. | Poovorawan Y, Sanpavat S, Pongpunlert W, Chumdermpadetsuk S, Sentrakul P, Safary A. Protective efficacy of a recombinant DNA hepatitis B vaccine in neonates of HBe antigen-positive mothers. JAMA. 1989;261:3278-3281. [PubMed] |
| 18. | Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013;31:2506-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother. 2013;9:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Lee SD, Chan CY, Yu MI, Lu RH, Chang FY, Lo KJ. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59:463-468. [PubMed] |
| 21. | Liu J, Wu H, Chen H. Immune response to hepatitis B vaccine in patients with chronic hepatitis C infection: A systematic review and meta-analysis. Hepatol Res. 2018;48:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Mattos AA, Gomes EB, Tovo CV, Alexandre CO, Remião JO. Hepatitis B vaccine efficacy in patients with chronic liver disease by hepatitis C virus. Arq Gastroenterol. 2004;41:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ashhab AA, Rodin H, Campos M, Abu-Sulb A, Hall JA, Powell J, Debes JD. Response to hepatitis B virus vaccination in individuals with chronic hepatitis C virus infection. PLoS One. 2020;15:e0237398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Daryani NE, Nassiri-Toosi M, Rashidi A, Khodarahmi I. Immunogenicity of recombinant hepatitis B virus vaccine in patients with and without chronic hepatitis C virus infection: a case-control study. World J Gastroenterol. 2007;13:294-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Bonazzi PR, Bacchella T, Freitas AC, Osaki KT, Lopes MH, Freire MP, Machado MC, Abdala E. Double-dose hepatitis B vaccination in cirrhotic patients on a liver transplant waiting list. Braz J Infect Dis. 2008;12:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Pascasio JM, Aoufi S, Gash A, Sousa JM, Perea R, Sayago M, Ferrer MT, Valencia R, Gómez-Bravo MA, Bernardos A, Márquez JL. Response to a vaccination schedule with 4 doses of 40 microg against hepatitis B virus in cirrhotic patients evaluated for liver transplantation. Transplant Proc. 2008;40:2943-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Khokhar N, Niazi TK, Qureshi MO. Effect of hepatitis B vaccination in patients with chronic hepatitis C. J Coll Physicians Surg Pak. 2014;24:392-395. [PubMed] |
| 28. | Minakari M, Tahmasebi A, Motlagh MH, Ataei B, Yaran M, Kalantari H, Tavakkoli H. Efficacy of double dose recombinant hepatitis B vaccination in chronic hepatitis C patients, compared to standard dose vaccination. Int J Prev Med. 2014;5:145-151. [PubMed] |
| 29. | Van Thiel DH, el-Ashmawy L, Love K, Gavaler JS, Starzl TE. Response to hepatitis B vaccination by liver transplant candidates. Dig Dis Sci. 1992;37:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | I Gutierrez Domingo, J M Pascasio Acevedo, A Alcalde Vargas, A Ramos Cuadra, M T Ferrer Ríos, J M Sousa Martin, M Sayago Mota, A Giráldez Gallego, G Suárez Artacho. Response to vaccination against hepatitis B virus with a schedule of four 40-μg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc. 2012;6:1499-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Kallinowski B, Benz C, Buchholz L, Stremmel W. Accelerated schedule of hepatitis B vaccination in liver transplant candidates. Transplant Proc. 1998;30:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Castells L, Esteban R. Hepatitis B vaccination in liver transplant candidates. Eur J Gastroenterol Hepatol. 2001;13:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Villeneuve E, Vincelette J, Villeneuve JP. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol. 2000;14 Suppl B:59B-62B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Sticchi L, Iavarone IG, Durando P, Di Biagio A, Schiavetti I, Murgia F, Icardi G. The role of hepatitis B vaccine challenge dose in patients with underlying health conditions. Hum Vaccin Immunother. 2021;17:575-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Arbizu EA, Marugán RB, Grijalba JY, Serrano PL, Grande LG, Del Campo Terrón S. Intramuscular versus intradermal administration of anti-hepatitis B vaccine in non-cirrhotic hepatitis C patients. Vaccine. 2003;21:2747-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Idilman R, De MN, Colantoni A, Nadir A, Van Thiel DH. The effect of high dose and short interval HBV vaccination in individuals with chronic hepatitis C. Am J Gastroenterol. 2002;97:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | David MC, Ha SH, Paynter S, Lau C. A systematic review and meta-analysis of management options for adults who respond poorly to hepatitis B vaccination. Vaccine. 2015;33:6564-6569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Rodrigues IC, Silva RCMAD, Felício HCC, Silva RFD. NEW IMMUNIZATION SCHEDULE EFFECTIVENESS AGAINST HEPATITIS B IN LIVER TRANSPLANTATION PATIENTS. Arq Gastroenterol. 2019;56:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Dong C, Song Z, Chen J, Ma N, Meng X, Sun C, Duan K, Bi B, Wang K, Qin H, Han C, Yang Y, Zhang F, Zheng W, Gao W. Risk factors of de novo hepatitis B virus infection in pediatric hepatitis B core antibody positive liver graft recipients under prophylactic therapy. J Gastroenterol Hepatol. 2020;35:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Leung DH, Ton-That M, Economides JM, Healy CM. High prevalence of hepatitis B nonimmunity in vaccinated pediatric liver transplant recipients. Am J Transplant. 2015;15:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Sintusek P, Posuwan N, Wanawongsawad P, Jitraruch S, Poovorawan Y, Chongsrisawat V. High prevalence of hepatitis B-antibody loss and a case report of de novo hepatitis B virus infection in a child after living-donor liver transplantation. World J Gastroenterol. 2018;24:752-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B, Cheng YF, Eng HL. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. 2007;7:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Komatsu H, Inui A, Yoshio S, Fujisawa T. Pharmacotherapy options for managing hepatitis B in children. Expert Opin Pharmacother. 2012;4:449-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Chen YS, Wang CC, de Villa VH, Wang SH, Cheng YF, Huang TL, Jawan B, Chiu KW, Chen CL. Prevention of de novo hepatitis B virus infection in living donor liver transplantation using hepatitis B core antibody positive donors. Clin Transplant. 2002;16:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Takemura N, Sugawara Y, Tamura S, Makuuchi M. Liver transplantation using hepatitis B core antibody-positive grafts: review and university of Tokyo experience. Dig Dis Sci. 2007;52:2472-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Maehara Y, Kuwano H. Prevention of hepatitis B virus infection from hepatitis B core antibody-positive donor graft using hepatitis B immune globulin and lamivudine in living donor liver transplantation. Liver Int. 2005;25:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Song Z, Dong C, Meng X, Sun C, Wang K, Qin H, Han C, Yang Y, Zhang F, Zheng W, Chen J, Duan K, Bi B, Gao W. Prophylactic Strategy Against De Novo Hepatitis B Virus Infection for Pediatric Recipients Who Receive Hepatitis B Core Antibody-Positive Liver Grafts. Liver Transpl. 2021;27:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 49. | Lin CC, Yong CC, Chen CL. Active vaccination to prevent de novo hepatitis B virus infection in liver transplantation. World J Gastroenterol. 2015;21:11112-11117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Xinias I, Vasilaki K, Argiropoulou E, Mavroudi A, Tsiatsiou O, Roilides E. De novo HBV Hepatitis in a Child with Liver Transplantation. Maedica (Bucur). 2021;16:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | S H Engler, P W Sauer, M Golling, E A Klar, C Benz, W Stremmel, B Kallinowski. Immunogenicity of two accelerated hepatitis B vaccination protocols in liver transplant candidates. Eur J Gastroenterol Hepatol. 2001;4:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts - a systematic analysis. Clin Transplant. 2011;25:E243-E249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Park JB, Kwon CH, Lee KW, Choi GS, Kim DJ, Seo JM, Kim SJ, Joh JW, Lee SK. Hepatitis B virus vaccine switch program for prevention of de novo hepatitis B virus infection in pediatric patients. Transpl Int. 2008;21:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Su WJ, Ho MC, Ni YH, Wu JF, Jeng YM, Chen HL, Wu YM, Hu RH, Chang MH, Lee PH. Clinical course of de novo hepatitis B infection after pediatric liver transplantation. Liver Transpl. 2010;16:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Jae Hyun Han, Dong Goo Kim, Gun Hyung Na, Eun Young Kim, Soo Ho Lee, Tae Ho Hong, Young Kyoung You, Jong Young Choi, Seung Kew Yoon. De novo hepatitis B virus infection developing after liver transplantation using a graft positive for hepatitis B core antibody. Ann Surg Treat Res. 2015;3:145-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Toniutto P, Fumo E, Caldato M, Apollonio L, Perin A, Pirisi M. Favourable outcome of adefovir-dipivoxil treatment in acute de novo hepatitis B after liver transplantation. Transplantation. 2004;77:472-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, Acharya SK, Flaherty JF, Massetto B, Cathcart AL, Kim K, Gaggar A, Subramanian GM, McHutchison JG, Pan CQ, Brunetto M, Izumi N, Marcellin P; GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 58. | Saab S, Song D, Challita YP, Xiwen Zhou T, Saab EG, Viramontes MR, Choi G, Durazo FA, Han SB, El Kabany MM, Jackson NJ, Busuttil RW. Long-term outcomes with oral therapy in liver transplant recipients with hepatitis B. Clin Transplant. 2019;33:e13740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 60. | Thanapirom K, Suksawatamnuay S, Sukeepaisarnjaroen W, Treeprasertsuk S, Tanwandee T, Charatcharoenwitthaya P, Thongsawat S, Leerapun A, Piratvisuth T, Boonsirichan R, Bunchorntavakul C, Pattanasirigool C, Pornthisarn B, Tuntipanichteerakul S, Sripariwuth E, Jeamsripong W, Sanpajit T, Poovorawan Y, Komolmit P. Association of the S267F variant on NTCP gene and treatment response to pegylated interferon in patients with chronic hepatitis B: a multicentre study. Antivir Ther. 2018;23:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J, Manuel Pascasio J, Sarrazin C, Vanwolleghem T, Shukla U, Fry J, Yogaratnam JZ. JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients With Chronic Infection. Gastroenterology. 2020;159:521-533.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 62. | Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai CL, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 63. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 64. | Kayesh MEH, Kohara M, Tsukiyama-Kohara K. Toll-Like Receptor Response to Hepatitis B Virus Infection and Potential of TLR Agonists as Immunomodulators for Treating Chronic Hepatitis B: An Overview. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Mackman RL, Mish M, Chin G, Perry JK, Appleby T, Aktoudianakis V, Metobo S, Pyun P, Niu C, Daffis S, Yu H, Zheng J, Villasenor AG, Zablocki J, Chamberlain J, Jin H, Lee G, Suekawa-Pirrone K, Santos R, Delaney WE 4th, Fletcher SP. Discovery of GS-9688 (Selgantolimod) as a Potent and Selective Oral Toll-Like Receptor 8 Agonist for the Treatment of Chronic Hepatitis B. J Med Chem. 2020;63:10188-10203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 66. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 67. | Lim SG, Agcaoili J, De Souza NNA, Chan E. Therapeutic vaccination for chronic hepatitis B: A systematic review and meta-analysis. J Viral Hepat. 2019;26:803-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Dusheiko G. Will we need novel combinations to cure HBV infection? Liver Int. 2020;40 Suppl 1:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Basyte-Bacevice V, Kupcinskas J. Evolution and Revolution of Hepatitis C Management: From Non-A, Non-B Hepatitis Toward Global Elimination. Dig Dis. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 70. | Malik F, Bailey H, Chan P, Collins IJ, Mozalevskis A, Thorne C, Easterbrook P. Where are the children in national hepatitis C policies? JHEP Rep. 2021;3:100227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M, Estes C, Gamkrelidze I, Jerabek K, Ma S, Montoya S, Razavi-Shearer D, Razavi-Shearer K, Robbins-Scott S, Razavi H, El Sayed MH. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol. 2020;5:374-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 72. | Indolfi G, Azzari C, Resti M. Perinatal transmission of hepatitis C virus. J Pediatr. 2013;163:1549-1552.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 74. | Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, Huang X, Leake JA, Ward JW, Vellozzi C. Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep. 2016;65:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 75. | Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C Virus Infection Among Reproductive-Aged Women and Children in the United States, 2006 to 2014. Ann Intern Med. 2017;166:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 76. | Dal Molin G, D'Agaro P, Ansaldi F, Ciana G, Fertz C, Alberico S, Campello C. Mother-to-infant transmission of hepatitis C virus: rate of infection and assessment of viral load and IgM anti-HCV as risk factors. J Med Virol. 2002;67:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, Manolaki N. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis. 2005;37:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, Vierucci A. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 180] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 79. | Prussing C, Bornschlegel K, Balter S. Hepatitis C surveillance among youth and young adults in New York City, 2009-2013. J Urban Health. 2015;92:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Centers for Disease Control and Prevention (CDC). Hepatitis C virus infection among adolescents and young adults:Massachusetts, 2002-2009. MMWR Morb Mortal Wkly Rep. 2011;60:537-541. [PubMed] |
| 81. | Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 82. | Danta M, Dusheiko GM. Acute HCV in HIV-positive individuals - a review. Curr Pharm Des. 2008;14:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Bortolotti F, Verucchi G, Cammà C, Cabibbo G, Zancan L, Indolfi G, Giacchino R, Marcellini M, Marazzi MG, Barbera C, Maggiore G, Vajro P, Bartolacci S, Balli F, Maccabruni A, Guido M; Italian Observatory for HCV Infection and Hepatitis C in Children. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. 2008;134:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 84. | Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, Narkewicz MR, Rosenthal P, Smith LJ, Schwarz KB, Robuck P, Barton B, González-Peralta RP. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48:341-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Compagnone A, Catenazzi P, Riccardi R, Zuppa AA. Mother-to-child transmission of hepatitis C virus. Minerva Pediatr. 2019;71:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Indolfi G, Mangone G, Moriondo M, Serranti D, Bartolini E, Azzari C, Resti M. Altered natural killer cells subsets distribution in children with hepatitis C following vertical transmission. Aliment Pharmacol Ther. 2016;43:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Resti M, Jara P, Hierro L, Azzari C, Giacchino R, Zuin G, Zancan L, Pedditzi S, Bortolotti F. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Indolfi G, Mangone G, Bartolini E, Moriondo M, Azzari C, Resti M. Hepatitis C viraemia after apparent spontaneous clearance in a vertically infected child. Lancet. 2016;387:1967-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Guido M, Bortolotti F, Leandro G, Jara P, Hierro L, Larrauri J, Barbera C, Giacchino R, Zancan L, Balli F, Crivellaro C, Cristina E, Pucci A, Rugge M. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time? Am J Gastroenterol. 2003;98:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | Indolfi G, Stagi S, Bartolini E, Salti R, de Martino M, Azzari C, Resti M. Thyroid function and anti-thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clin Endocrinol (Oxf). 2008;68:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | CanHepC. The Canadian Network on Hepatitis C BlueprintWriting Committee andWorking Groups. Blueprint to inform Hepatitis C Elimination Efforts in Canada. 2019. |
| 92. |
|
| 93. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 94. | Ruiz-Extremera A, Salmerón J, Torres C, De Rueda PM, Giménez F, Robles C, Miranda MT. Follow-up of transmission of hepatitis C to babies of human immunodeficiency virus-negative women: the role of breast-feeding in transmission. Pediatr Infect Dis J. 2000;19:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization; 2017 Feb- . [PubMed] |
| 96. | Cox AL. MEDICINE. Global control of hepatitis C virus. Science. 2015;349:790-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 97. | Wasitthankasem R, Vichaiwattana P, Auphimai C, Siripon N, Klinfueng S, Tangkijvanich P, Vongpunsawad S, Poovorawan Y. HCV core antigen is an alternative marker to HCV RNA for evaluating active HCV infection: implications for improved diagnostic option in an era of affordable DAAs. PeerJ. 2017;5:e4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Peeling RW, Boeras DI, Marinucci F, Easterbrook P. The future of viral hepatitis testing: innovations in testing technologies and approaches. BMC Infect Dis. 2017;17:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection [Internet]. Geneva: World Health Organization; 2018 Jul- . [PubMed] |
| 100. | Hopkins L, Dunlap T, Cline H. Pharmacology Update for the Treatment of Hepatitis C Virus. Nurs Clin North Am. 2020;55:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 102. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 103. | Zignego AL, Monti M, Gragnani L. Sofosbuvir/Velpatasvir for the treatment of Hepatitis C Virus infection. Acta Biomed. 2018;89:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 104. | Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C, Hierro L, Kelly D, Ling SC, Strokova T, Del Valle-Segarra A, Lovell S, Liu W, Ng TI, Porcalla A, Gonzalez YS, Burroughs M, Sokal E. Pharmacokinetics, Safety, and Efficacy of Glecaprevir/Pibrentasvir in Adolescents With Chronic Hepatitis C Virus: Part 1 of the DORA Study. Hepatology. 2020;71:456-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 105. | El-Araby HA, Behairy BE, El-Guindi MA, Adawy NM, Allam AA, Sira AM, Khedr MA, Elhenawy IA, Sobhy GA, Basiouny HEDM, Salem ME, Abdel-Aziz SA, Fouad OA, Ayoub BA. Generic sofosbuvir/ledipasvir for the treatment of genotype 4 chronic hepatitis C in Egyptian children (9-12 years) and adolescents. Hepatol Int. 2019;13:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Pokorska-Śpiewak M, Dobrzeniecka A, Aniszewska M, Marczyńska M. Real-Life Experience with Ledipasvir/Sofosbuvir for the Treatment of Chronic Hepatitis C Virus Infection with Genotypes 1 and 4 in Children Aged 12 to 17 Years-Results of the POLAC Project. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Jonas MM, Rhee S, Kelly DA, Del Valle-Segarra A, Feiterna-Sperling C, Gilmour S, Gonzalez-Peralta RP, Hierro L, Leung DH, Ling SC, Lobzin Y, Lobritto S, Mizuochi T, Narkewicz MR, Sabharwal V, Wen J, Kei Lon H, Marcinak J, Topp A, Tripathi R, Sokal E. Pharmacokinetics, Safety, and Efficacy of Glecaprevir/Pibrentasvir in Children With Chronic HCV: Part 2 of the DORA Study. Hepatology. 2021;74:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 108. | Gupta M, Bahirwani R, Levine MH, Malik S, Goldberg D, Reddy KR, Shaked A. Outcomes in pediatric hepatitis C transplant recipients: analysis of the UNOS database. Pediatr Transplant. 2015;19:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Shahid I, Alzahrani AR, Al-Ghamdi SS, Alanazi IM, Rehman S, Hassan S. Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 110. | Corson M, Moch A, Saab S. Hepatitis C Virus Treatment in Patients With Chronic Kidney Disease and in Kidney Transplant Recipients. Gastroenterol Hepatol (N Y). 2018;14:280-285. [PubMed] |
| 111. | Cortesi PA, Belli LS, Facchetti R, Mazzarelli C, Perricone G, De Nicola S, Cesana G, Duvoux C, Mantovani LG, Strazzabosco M; European Liver and Intestine Transplant Association (ELITA). The optimal timing of hepatitis C therapy in liver transplant-eligible patients: Cost-effectiveness analysis of new opportunities. J Viral Hepat. 2018;25:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, Hur C, Donnell DMS, Chung RT, Chhatwal J. Cost Effectiveness of Pre- vs Post-Liver Transplant Hepatitis C Treatment With Direct-Acting Antivirals. Clin Gastroenterol Hepatol. 2018;16:115-122.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, Osburn WO, Forman M, Thomas E, Thornton K, Wagner K, Vassilev V, Lin L, Lum PJ, Giudice LC, Stein E, Asher A, Chang S, Gorman R, Ghany MG, Liang TJ, Wierzbicki MR, Scarselli E, Nicosia A, Folgori A, Capone S, Cox AL. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N Engl J Med. 2021;384:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 114. | Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 562] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 115. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 116. | Bouamra Y, Gérolami R, Arzouni JP, Grimaud JC, Lafforgue P, Nelli M, Tivoli N, Ferretti A, Motte A, Colson P. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology. 2014;57:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Farshadpour F, Taherkhani R, Makvandi M. Prevalence of Hepatitis E Virus among Adults in South-West of Iran. Hepat Res Treat. 2015;2015:759589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Mathur P, Arora NK, Panda SK, Kapoor SK, Jailkhani BL, Irshad M. Sero-epidemiology of hepatitis E virus (HEV) in urban and rural children of North India. Indian Pediatr. 2001;38:461-475. [PubMed] |
| 119. | Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis. 2009;200:48-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 120. | Verhoef L, Koopmans M, Duizer E, Bakker J, Reimerink J, Van Pelt W. Seroprevalence of hepatitis E antibodies and risk profile of HEV seropositivity in The Netherlands, 2006-2007. Epidemiol Infect. 2012;140:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Izopet J, Labrique AB, Basnyat B, Dalton HR, Kmush B, Heaney CD, Nelson KE, Ahmed ZB, Zaman K, Mansuy JM, Bendall R, Sauné K, Kamar N, Arjyal A, Karkey A, Dongol S, Prajapati KG, Adhikary D. Hepatitis E virus seroprevalence in three hyperendemic areas: Nepal, Bangladesh and southwest France. J Clin Virol. 2015;70:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 122. | Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother-to-child transmission of hepatitis E virus infection. Indian J Pediatr. 2003;70:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 123. | Coilly A, Haïm-Boukobza S, Roche B, Antonini TM, Pause A, Mokhtari C, Becq A, Farahmand H, Hauser L, Duclos-Vallée JC, Samuel D, Adam R, Roque-Afonso AM. Posttransplantation hepatitis E: transfusion-transmitted hepatitis rising from the ashes. Transplantation. 2013;96:e4-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K, Maguchi H, Yoshida J, Maekubo H, Mishiro S, Ikeda H. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 125. | Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 126. | Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 127. | Hitzler WE, Runkel S. Prevalence, persistence and liver enzyme levels of HGV RNA-positive blood donors determined by large-scale screening and transmission by blood components. Clin Lab. 2004;50:25-31. [PubMed] |
| 128. | Fischler B, Baumann U, Dezsofi A, Hadzic N, Hierro L, Jahnel J, McLin V, Nobili V, Smets F, Verkade H, Debray D. Hepatitis E in Children: A Position Paper by the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2016;63:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 412] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 130. | Halac U, Béland K, Lapierre P, Patey N, Ward P, Brassard J, Houde A, Alvarez F. Chronic hepatitis E infection in children with liver transplantation. Gut. 2012;61:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Hoerning A, Hegen B, Wingen AM, Cetiner M, Lainka E, Kathemann S, Fiedler M, Timm J, Wenzel JJ, Hoyer PF, Gerner P. Prevalence of hepatitis E virus infection in pediatric solid organ transplant recipients--a single-center experience. Pediatr Transplant. 2012;16:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Junge N, Pischke S, Baumann U, Goldschmidt I, Manns M, Wedemeyer H, Pfister ED. Results of single-center screening for chronic hepatitis E in children after liver transplantation and report on successful treatment with ribavirin. Pediatr Transplant. 2013;17:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 133. | Laverdure N, Scholtès-Brunel C, Rivet C, Heissat S, Restier L, Bacchetta J, Boillot O, Dumortier J, Lachaux A. Paediatric liver transplanted patients and prevalence of hepatitis E virus. J Clin Virol. 2015;69:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Halac U, Béland K, Lapierre P, Patey N, Ward P, Brassard J, Houde A, Alvarez F. Cirrhosis due to chronic hepatitis E infection in a child post-bone marrow transplant. J Pediatr. 2012;160:871-4.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Rivero-Juarez A, Vallejo N, Lopez-Lopez P, Díaz-Mareque AI, Frias M, Vallejo A, Caballero-Gómez J, Rodríguez-Velasco M, Molina E, Aguilera A. Ribavirin as a First Treatment Approach for Hepatitis E Virus Infection in Transplant Recipient Patients. Microorganisms. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Zaman K, Dudman S, Stene-Johansen K, Qadri F, Yunus M, Sandbu S, Gurley ES, Overbo J, Julin CH, Dembinski JL, Nahar Q, Rahman A, Bhuiyan TR, Rahman M, Haque W, Khan J, Aziz A, Khanam M, Streatfield PK, Clemens JD. HEV study protocol : design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open. 2020;10:e033702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 137. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 555] [Article Influence: 37.0] [Reference Citation Analysis (1)] |
| 138. | Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, Guo M, Liu XH, Li JX, Yang CL, Tang Q, Jiang RJ, Pan HR, Li YM, Shih JW, Ng MH, Zhu FC, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 139. | Passos-Castilho AM, Porta G, Miura IK, Pugliese RP, Danesi VL, Porta A, Guimarães T, Seda J, Antunes E, Granato CF. Chronic hepatitis E virus infection in a pediatric female liver transplant recipient. J Clin Microbiol. 2014;52:4425-4427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Abi Nader E, Girard M, Leruez-Ville M, Sissaoui S, Lacaille F, Roque-Afonso AM, Debray D. Seroprevalence of Hepatitis E virus infection in children after liver transplantation: A single-center experience in France. Clin Res Hepatol Gastroenterol. 2020;44:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 141. | Protzer U, Böhm F, Longerich T, Seebach J, Heidary Navid M, Friemel J, Marques-Maggio E, Bawohl M, Heikenwalder M, Schirmacher P, Dutkowski P, Clavien PA, Schemmer P, Schnitzler P, Gotthardt D, Müllhaupt B, Weber A. Molecular detection of hepatitis E virus (HEV) in liver biopsies after liver transplantation. Mod Pathol. 2015;28:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 142. | Prost S, Crossan CL, Dalton HR, De Man RA, Kamar N, Selves J, Dhaliwal C, Scobie L, Bellamy COC. Detection of viral hepatitis E in clinical liver biopsies. Histopathology. 2017;71:580-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 143. | Ankavay M, Montpellier C, Sayed IM, Saliou JM, Wychowski C, Saas L, Duvet S, Aliouat-Denis CM, Farhat R, de Masson d'Autume V, Meuleman P, Dubuisson J, Cocquerel L. New insights into the ORF2 capsid protein, a key player of the hepatitis E virus lifecycle. Sci Rep. 2019;9:6243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 144. | Drobeniuc J, Meng J, Reuter G, Greene-Montfort T, Khudyakova N, Dimitrova Z, Kamili S, Teo CG. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin Infect Dis. 2010;51:e24-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 145. | Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2013;10:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 146. | Perisetti A, Laoveeravat P, Inamdar S, Tharian B, Thandassery R, Goyal H. Hepatitis E virus infection in liver transplant recipients: a descriptive literature review. Eur J Gastroenterol Hepatol. 2020;32:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 147. | Nishiyama T, Kobayashi T, Jirintai S, Kii I, Nagashima S, Prathiwi Primadharsini P, Nishizawa T, Okamoto H. Screening of novel drugs for inhibiting hepatitis E virus replication. J Virol Methods. 2019;270:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 148. | Kaushik N, Subramani C, Anang S, Muthumohan R, Shalimar, Nayak B, Ranjith-Kumar CT, Surjit M. Zinc Salts Block Hepatitis E Virus Replication by Inhibiting the Activity of Viral RNA-Dependent RNA Polymerase. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 149. | Kanade GD, Pingale KD, Karpe YA. Activities of Thrombin and Factor Xa Are Essential for Replication of Hepatitis E Virus and Are Possibly Implicated in ORF1 Polyprotein Processing. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 150. | Kaushik N, Anang S, Ganti KP, Surjit M. Zinc: A Potential Antiviral Against Hepatitis E Virus Infection? DNA Cell Biol. 2018;37:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 151. | Cao Y, Bing Z, Guan S, Zhang Z, Wang X. Development of new hepatitis E vaccines. Hum Vaccin Immunother. 2018;14:2254-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 152. | Zhang J, Zhao Q, Xia N. Prophylactic Hepatitis E Vaccine. Adv Exp Med Biol. 2016;948:223-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 153. | Li Y, Huang X, Zhang Z, Li S, Zhang J, Xia N, Zhao Q. Prophylactic Hepatitis E Vaccines: Antigenic Analysis and Serological Evaluation. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |