Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.4007
Peer-review started: March 30, 2022
First decision: April 25, 2022
Revised: May 11, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: August 7, 2022
Processing time: 126 Days and 1.4 Hours
Complete polyp resection is the main goal of endoscopic removal of large colonic polyps. Resection techniques have evolved in recent years and endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR) with margin ablation, cold snare polypectomy (CSP), cold EMR, and underwater EMR have been introduced. Yet, efficacy of these techniques with regard to local recurrence rates (LRRs) vs traditional hot snare polypectomy and standard EMR remains unclear.
To analyze LRR of large colonic polyps in a systematic review and meta-analysis.
MEDLINE, EMBASE, EBM Reviews, and CINAHL were searched for prospective studies reporting LRR or incomplete resection rate (IRR) after colonic polypectomy of polyps ≥ 10 mm, published between January 2011 and July 2021. Primary outcome was LRR for polyps ≥ 10 mm.
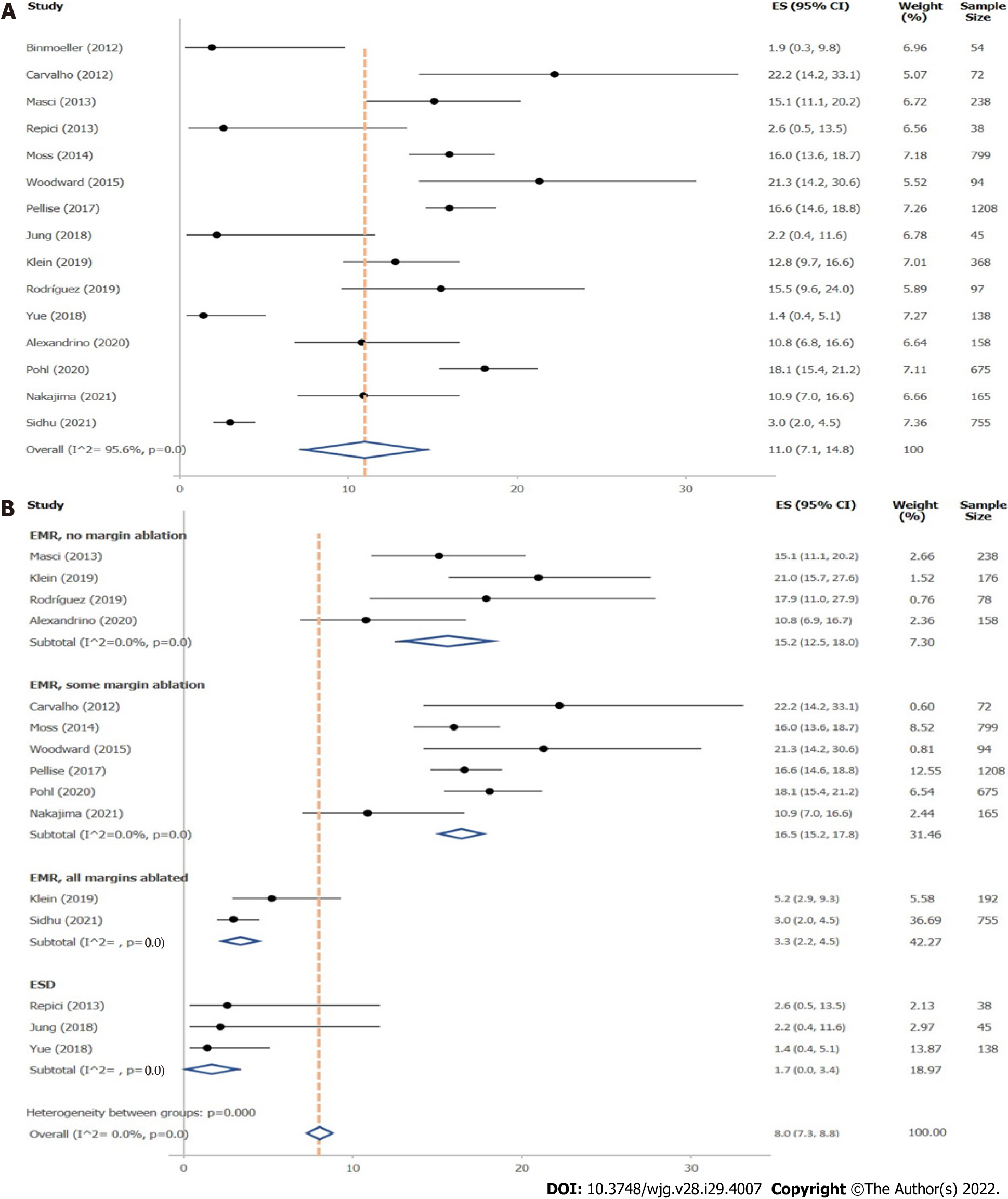
Six thousand nine hundred and twenty-eight publications were identified, of which 34 prospective studies were included. LRR for polyps ≥ 10 mm at up to 12 mo’ follow-up was 11.0% (95%CI, 7.1%-14.8%; 15 studies; 4904 polyps). ESD (1.7%; 95%CI, 0%-3.4%; 3 studies, 221 polyps) and endoscopic mucosal resection with margin ablation (3.3%; 95%CI, 2.2%-4.5%; 2 studies, 947 polyps) significantly reduced LRR vs standard EMR without (15.2%; 95%CI, 12.5%-18.0%; 4 studies, 650 polyps) or with unsystematic margin ablation (16.5%; 95%CI, 15.2%-17.8%; 6 studies, 3031 polyps).
LRR is significantly lower after ESD or EMR with routine margin ablation; thus, these techniques should be considered standard for endoscopic removal of large colorectal polyps. Other techniques, such as CSP, cold EMR, and underwater EMR require further evaluation in prospective studies before their routine implementation in clinical practice can be recommended.
Core Tip: Complete polyp resection is the main goal of endoscopic removal of large colonic polyps. Resection techniques have evolved in recent years and endoscopic submucosal dissection, endoscopic mucosal resection (EMR) with margin ablation, cold snare polypectomy, cold EMR, and underwater EMR have been introduced. Yet, efficacy of these techniques with regard to local recurrence rates (LRRs) vs traditional hot snare polypectomy and standard EMR remains unclear. We aimed to analyze LRR of large colonic polyps in a systematic review and meta- analysis.
- Citation: Rotermund C, Djinbachian R, Taghiakbari M, Enderle MD, Eickhoff A, von Renteln D. Recurrence rates after endoscopic resection of large colorectal polyps: A systematic review and meta-analysis. World J Gastroenterol 2022; 28(29): 4007-4018
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/4007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.4007
Complete endoscopic polyp removal is especially important for large colorectal polyps in order to prevent local polyp recurrence and progression to colorectal cancer[1]. Evidence is growing that polyp removal is frequently incomplete, putting patients at risk of developing post-colonoscopy cancer[2-4]. A meta-analysis published in 2020 found that after snare resection, 15.9% of diminutive and small polyps and 20.8% of polyps 10–19 mm are removed incompletely[5]. For polyps 20 mm or larger, a meta-analysis published in 2014 demonstrated a recurrence rate of 15% after endoscopic mucosal resection (EMR)[6].
In recent years, different techniques for endoscopic resection of large colorectal polyps have evolved or been developed. Cold snare polypectomy (CSP) has been introduced and its use expanded to include the removal of large colorectal polyps[7]. Endoscopic submucosal dissection (ESD) has gained traction in Western countries and EMR has undergone technical modifications by introducing margin ablation or underwater EMR[8,9].
These developments have sparked our interest in providing an up-to-date meta-analysis of local recurrence rates (LRRs) and incomplete resection rates (IRRs) for large (≥ 10 mm) colorectal polyps, and to evaluate the impact of the novel or modified endoscopic resection techniques on LRRs.
The analysis was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement[10].
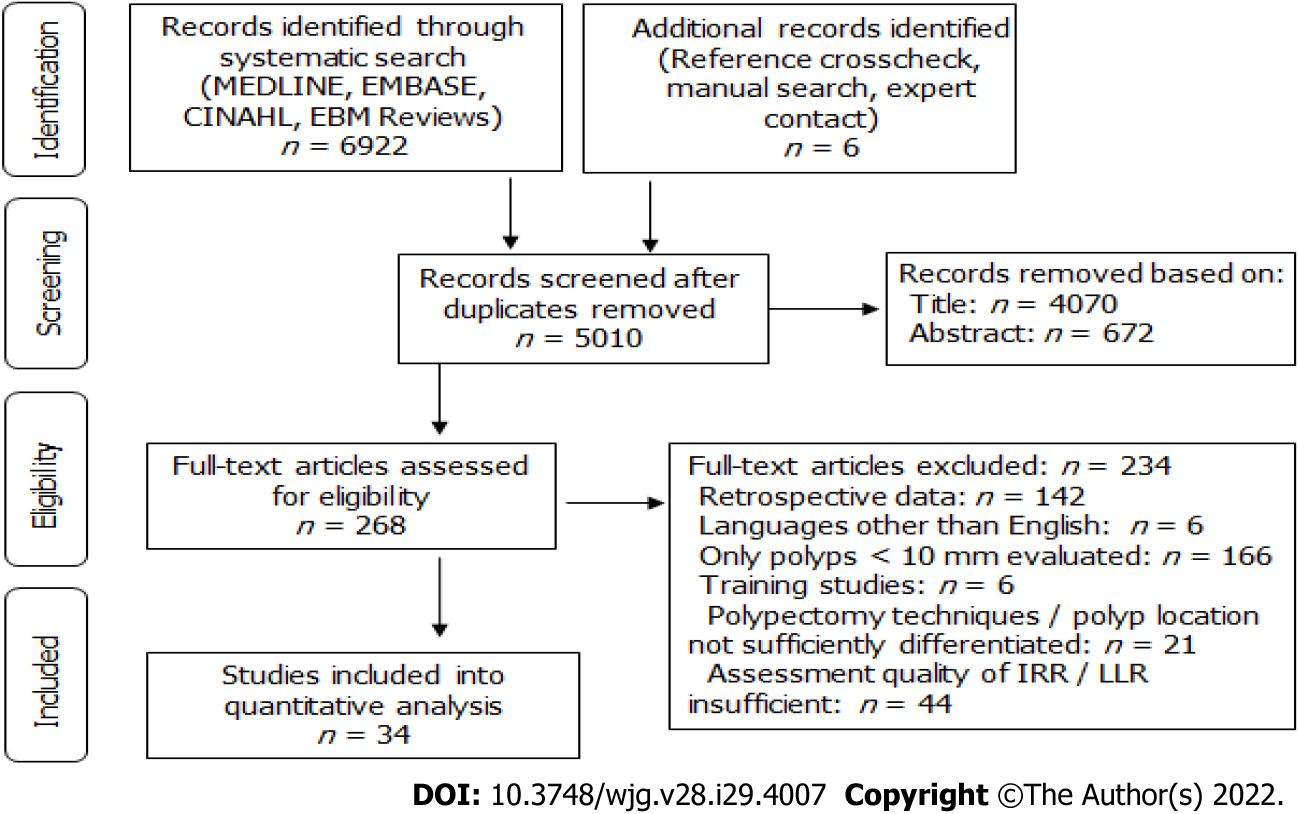
A systematic literature search was performed within MEDLINE, EMBASE, EBM Reviews, and CINAHL databases. All articles published between January 2011 and July 2021 reporting on IRR and/or LRR for colorectal polyps 10 mm or larger removed by endoscopic resection techniques were included in the search. For specific search terms, see Supplementary Table 1. Additionally, a secondary search was performed to identify further records using article reference crosscheck, manual searching, and expert contact.
Articles retrieved by the systematic search were collected and duplicates removed. Two researchers (Rotermund C and Taghiakbari M) assessed all articles independently and decided upon inclusion and exclusion. In cases of disagreement, a third researcher (von Renteln D) was consulted.
Inclusion criteria were full-text articles of prospectively performed clinical studies reporting on either LRR or IRR evaluated by margin assessment or margin biopsy of endoscopically removed polyps ≥ 10 mm. Even though often of larger sample size, publications with retrospective study design were excluded from the analysis, as risk for selection bias and risk for missed data is usually higher.
Exclusion criteria were retrospective study design, polyps < 10 mm, IRR evaluated by visual margin assessment, data from first follow-up that exceeded 12 mo, publications solely evaluating difficult polypectomies, publication languages other than English, articles reporting on training of a certain technique, and articles in which results from different polypectomy techniques were not clearly distinguishable.
Relevant data retrieved from the evaluated study included author, year of publication, country, study type, study quality, polyp size, polyp morphology, polyp histology, polyp resection method and adjunct therapy, LRR, IRR, IRR assessment method, submucosal injection rate and solution, en bloc resection rate, and endoscopist number and experience level. For analyses, polyps were subdivided according to size: 10–19 mm, ≥ 20 mm (not including polyps < 20 mm), and all polyps ≥ 10 mm (including polyps ≥ 20 mm). Data were retrieved by one author (Rotermund C) and correct retrieval confirmed by a second author (Djinbachian R).
Primary outcome was LRR for polyps ≥ 10 mm. Local recurrence was defined as the presence of recurrent polyp at the resection site, detected during follow-up examination. Publications, in which the appointments for follow-up examinations exceeded 12 mo between the different patients, were excluded from the analysis. Secondary outcomes were IRR evaluated by either margin assessment or margin biopsy for polyps ≥ 10 mm, as well as factors influencing LRR and IRR, including polyp resection technique [hot snare polypectomy (HSP), CSP, hot and cold EMR, underwater EMR, ESD], adjunct therapy, margin assessment method, submucosal injection status, polyp size, polyp morphology and histology, endoscopist experience and number of endoscopists involved. IRR assessment method was defined as (1) Biopsy from the resection margin (=“margin biopsy”); (2) Histologic assessment of polyp margin (= “margin assessment”); and (3) En bloc resection and histologic assessment of polyp margin (= “en bloc and margin assessment”). Endoscopist experience was defined as (1) Less experienced for EMR, when a fellow was included in the study or < 2000 colonoscopies had been performed by the endoscopist; (2) Experienced for EMR, when only expert endoscopists (> 2000 colonoscopies) were involved; (3) Less experienced for ESD, when fellows for ESD (< 200 cases) were included in the study; and (4) Experts for ESD (> 200 cases).
Study quality was assessed independently by two researchers (Taghiakbari M and Rotermund C). In cases of disagreement, a third researcher (von Renteln D) was consulted. For evaluation, National Institutes of Health quality assessment forms for case series and randomized controlled trials (RCTs) were used[11]. For RCTs (maximum score: 14), a score of 11–14 was rated as good, a score of 8–10 as fair, and a score below 8 as poor quality. For prospective case series (maximum score: 9), a score of 7–9 was rated as good, a score of 4–6 as fair, and a score below 4 as poor quality. Detailed information on criteria for low and high quality are given in Supplementary Tables 2 and 3.
A sensitivity analysis was performed to determine the effects of excluding poor-quality studies and publication bias was assessed using funnel plots (Supplementary Figure 1). The graph was plotted as proportion vs. sample size instead of log odds vs 1/standard error, as this method has been shown to be more accurate in predicting risk of publication bias for meta-analyses of proportions[12].
Proportions were meta-analyzed using the metaprop command of Stata version 16 (StataCorp, College Station, TX, United States), and tests of heterogeneity were performed using the I2 statistic. Either a random-effects model or a fixed-effect model was used for the analyses. Proportions were reported with their associated 95% confidence intervals (CIs) with an alpha level of < 0.05 used for statistical significance.
Systematic literature search yielded 6922 hits and 6 additional records were identified through reference crosscheck, manual search, and expert contact (Figure 1, Supplementary Table 1). After removal of duplicates, 5010 publications remained. Of these, 4070 were excluded based on title and 672 based on abstract, so that 268 full-text records were evaluated for eligibility. Ultimately, 34 publications were included in the analysis, with 19 reporting on IRR, 13 on LRR, and 2 on both (Figure 1). All studies were prospective and 14 were RCTs.
Included studies showed symmetrical distribution for both assessments of LRR and IRR, with no publication bias detected (Supplementary Figure 1). Quality assessment revealed 23 studies of good quality, 10 studies of fair quality, and 1 study of poor quality (Supplementary Tables 2 and 3). Sensitivity analyses did not show statistically different results or decreased heterogeneity when excluding poor- or fair-quality studies (Supplementary Figures 2 and 3).
A total of 15 studies reported on LRR after removal of large colonic polyps ≥ 10 mm. Of these, 15 studies stated LRR obtained during follow-up examinations up to 12 mo, 7 during follow-up up to 24 mo, and 3 from follow-up after more than 24 mo (Supplementary Table 4). Definitions of LRR given in the original studies are presented in Supplementary Table 5. Mean overall LRR at up to 12 mo’ follow-up was 11.0% (95%CI, 7.1%–14.8%; 4904 polyps) (Figure 2A). Overall LRR for follow-up up to 24 mo was 14.6% (95%CI, 8.4%–20.8%; 7 studies) (Supplementary Figure 4).
Local recurrence rate up to 12 mo’ follow-up: Influence of resection method: Resection method was found to exhibit major influence on LRR of polyps ≥ 10 mm (Figure 2B, Table 1). ESD (1.7%; 95%CI, 0–3.4%; 3 studies) and EMR with margin ablation (3.3%; 95%CI, 2.2%–4.5%; 2 studies) significantly reduced LRR compared with EMR in which margin ablation was not performed (15.2%; 95%CI, 12.5%–18.0%; 4 studies) or only used in some cases (16.5%; 95%CI, 15.2%–17.8%; 6 studies). No prospective studies were found evaluating LRR after HSP, CSP, or cold EMR within the search period. Two studies evaluated LRR after underwater EMR; however, heterogeneity between studies was high, so that a valid analysis could not be performed.
| Subgroups | LRR % (95%CI) | I2 % | Studies, n | Polyps, n |
| Resection method, polyps ≥ 10 mm | ||||
| Hot EMR, no margin ablation | 15.2 (12.5–18.0) | 0 | 4 | 650 |
| Hot EMR, some margin ablation | 16.5 (15.2–17.8) | 0 | 6 | 3013 |
| Hot EMR, with margin ablation | 3.3 (2.2–4.5) | NA | 2 | 947 |
| ESD | 1.7 (0.0–3.4) | NA | 3 | 221 |
| Resection method, polyps ≥ 20 mm | ||||
| Hot EMR, no margin ablation | 14.8 (11.0–18.5) | NA | 2 | 334 |
| Hot EMR, some margin ablation | 16.5 (15.2–17.8) | 0 | 6 | 3013 |
| Hot EMR, with margin ablation | 3.3 (2.2–4.5) | NA | 2 | 947 |
| ESD | 2.4 (0–5.7) | NA | 2 | 83 |
| Polyp size | ||||
| ≥ 10 mm | 11.0 (7.1–14.8) | 95.6 | 15 | 4904 |
| ≥ 20 mm | 11.2 (6.8–15.6) | 95.8 | 12 | 4431 |
| Expert level | ||||
| Only expert endoscopists | 13.3 (11.1–15.6) | NA | 2 | 3712 |
| Including non-expert endoscopists | 11.8 (6.8–16.8) | 95.8 | 9 | 837 |
| Not defined | 9.2 (1.5–16.9) | 93.7 | 4 | 524 |
Similarly, when only results for polyps ≥ 20 mm were evaluated, ESD and EMR with margin ablation yielded lower LRRs compared with EMR without margin ablation (Table 1). No prospective studies were found evaluating HSP, CSP, or cold EMR.
Local recurrence rate up to 12 mo’ follow-up: further influencing factors: Polyp size did not influence LRR (≥ 10 mm: 11.0%; 95%CI, 7.1%–14.8%; 15 studies vs ≥ 20 mm: 11.2%; 95%CI, 6.8%–15.6%; 12 studies) (Table 1). Similarly, expert status of the endoscopist was not found to influence LRR (Table 1); however, as only two expert studies were found, the data set was small. The data set was also insufficient for analysis of the influence of polyp morphology or histology on LRR. Only one study included pedunculated polyps (12.1% of all resected polyps); however, the reported LRR was comparable to the rate observed in other studies[13]. Most studies included sessile serrated adenoma/polyps (SSA/Ps). One study compared LRR after EMR removal of SSA/Ps vs conventional adenomas and reported significantly reduced LRR for SSA/Ps[14].
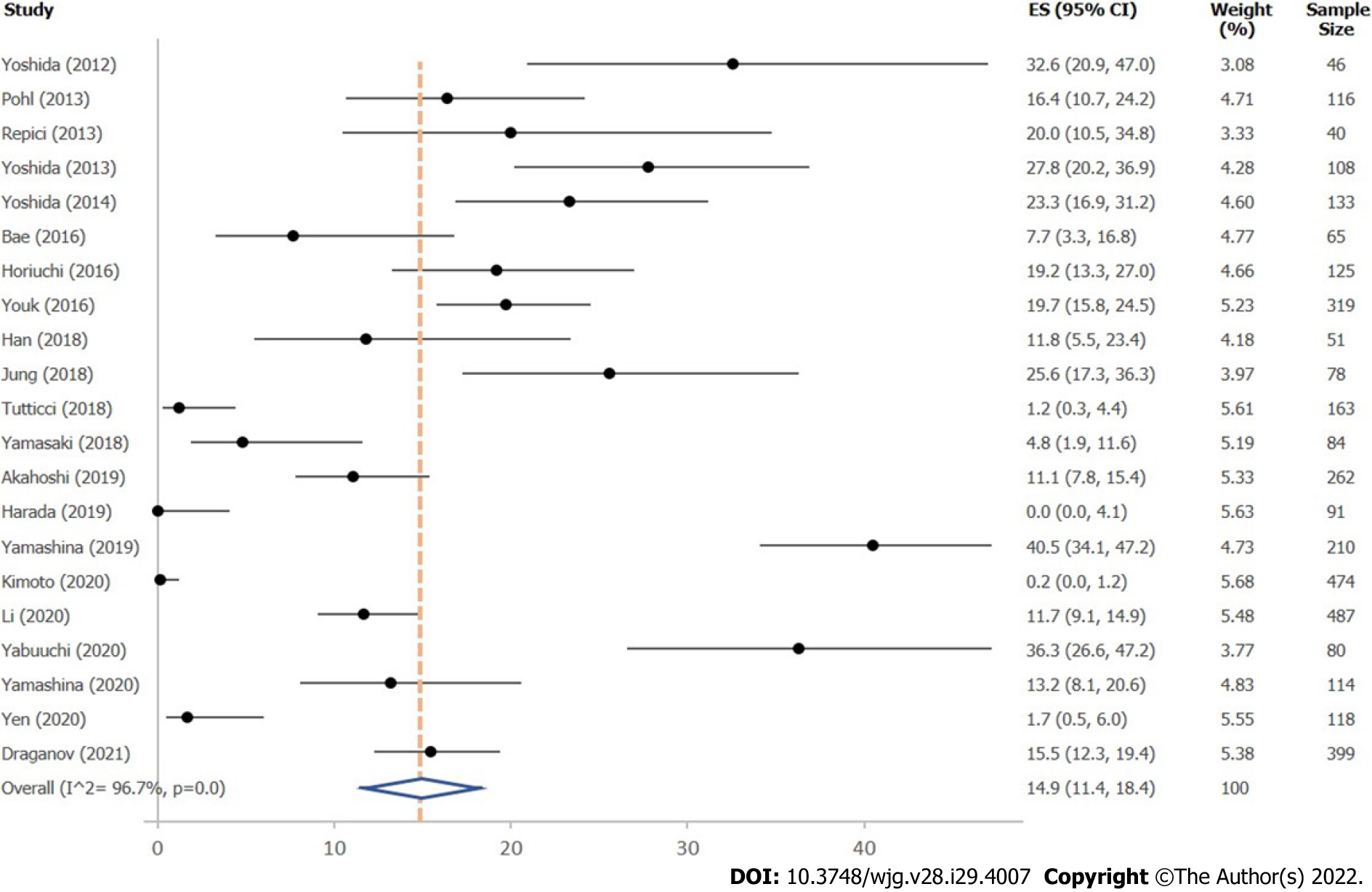
A total of 21 studies reported on IRR after removal of large colonic polyps ≥ 10 mm, using either margin assessment or margin biopsy for evaluation (Supplementary Table 6). Mean overall IRR for all polyps ≥ 10 mm was 14.9% (95%CI, 11.4%–18.4%; 21 studies; 3563 polyps) (Figure 3). Twelve studies indicated IRR for polyps 10–19 mm, resulting in a mean IRR of 16.0% (95%CI, 10.4-21.7%) (Supplemen
Incomplete resection rate: Influence of resection method: Resection method was not found to significantly influence IRR of polyps 10–19 mm, comparing hot EMR (18.5%; 95%CI, 8.9%–28.1%; 8 studies), HSP (16.2%; 95%CI, 10.6%–21.7%; 2 studies), underwater EMR (25.5%; 95%CI, 18.9%–32.2%; 2 studies), and cold EMR (14.0%; 95%CI, 1.8%–26.3%; 3 studies), with studies on cold EMR exhibiting high variability (Table 2). Only two studies evaluated CSP, showing high heterogeneity, so that a valid analysis could not be performed.
| Subgroups | IRR % (95%CI) | I2 % | Studies, n | Polyps, n |
| Resection method, polyps 10–19 mm | ||||
| Hot EMR | 18.5 (8.9–28.1) | 93.2 | 8 | 655 |
| HSP | 16.2 (10.6–21.7) | NA | 2 | 167 |
| U-EMR | 25.5 (18.9–32.2) | NA | 2 | 160 |
| Cold EMR1 | 14.01 (1.8–26.3) | NA | 3 | 334 |
| Resection method, polyps ≥ 20 mm | ||||
| ESD | 12.5 (6.2–18.8) | 95.0 | 9 | 1452 |
| Hot EMR | 29.3 (19.3–39.2) | NA | 3 | 88 |
| Submucosal injection, polyps 10–19 mm | ||||
| No injection | 14.4 (5.4–23.3) | 95.8 | 6 | 836 |
| Injection | 20.0 (11.9–28.0) | 93.9 | 10 | 989 |
| Submucosal injection, polyps ≥ 20 mm | ||||
| No injection | 32.4 (0–76.3) | 96.2 | 3 | 124 |
| Injection | 12.6 (7.7–17.6) | 94.4 | 13 | 1614 |
| Injection solution, polyps ≥ 10 mm | ||||
| Saline solution | 15.8 (7.1–24.6) | 95. 6 | 6 | 774 |
| Hyaluronic acid | 16.3 (8.5–24.1) | 95.1 | 8 | 916 |
| Expert level | ||||
| Only expert endoscopists | 7.0 (3.5, 10.4) | 93. 7 | 8 | 1451 |
| Including non-expert endoscopists | 20.3 (13.5–27.1) | 96.0 | 13 | 2092 |
| Method of margin evaluation, polyps 10–19 mm | ||||
| Margin assessment | 18.6 (10.9, 26.2) | 75.1 | 5 | 380 |
| Margin biopsy | 5.7 (1.1, 10.3) | 95.1 | 5 | 1150 |
| Method of margin evaluation, polyps ≥ 20 mm | ||||
| Margin assessment | 21.8 (9.4–34.2) | 92.1 | 4 | 429 |
| Margin assessment and en bloc resection | 14.1 (5.7–22.6) | 96.0 | 7 | 1106 |
| Margin biopsy | 0.4 (0–2.5) | 55.8 | 3 | 203 |
Comparison of ESD and EMR for polyps ≥ 20 mm showed a lower IRR for ESD (12.5%; 95%CI, 6.2%–18.8%; 9 studies) than for EMR (29.3%; 95%CI, 19.3%–39.2%; 3 studies) (Table 2). Only two studies evaluated IRR for polyps ≥ 20 mm with HSP, yielding high heterogeneity, so that a valid analysis could not be performed. No data were found reporting IRR after CSP or cold EMR for polyps ≥ 20 mm.
Incomplete resection rate: Further influencing factors: For polyps sized 10–19 mm, submucosal injection status did not influence IRR. Mean IRR after resection with submucosal injection was 20.0% (95%CI, 11.9%–28.0%; 10 studies) compared with 14.4% (95%CI, 5.4%–23.3%; 6 studies) after resection without submucosal injection (Table 2). For polyps ≥20 mm, IRR was lower after resection with prior submucosal injection (Table 2). Mean IRR after submucosal injection was 12.6% (95%CI 7.7–17.6; 13 studies), compared to 32.4% (95%CI, 0-76.3%; 3 studies) after resection without injection.
The solution used for submucosal injection was not found to influence IRR, yielding comparable results for saline solution (15.8%, 95%CI, 7.1%–24.8%; 6 studies) and hyaluronic acid (16.3%, 95%CI, 8.5%–24.1%; 8 studies) (Table 2).
There was a strong trend toward lower IRR when considering endoscopist experience (Table 2, Supplementary Figure 7). The mean IRR was 7.0% (95%Cl, 3.5%–10.4%; 8 studies) when only expert endoscopists were involved in the study, and 20.3% (95%Cl, 13.5%–27.1%; 13 studies) when non-experts were included.
Insufficient data were available for analysis of the influence of polyp morphology or histology on IRR. Three studies included around 50% or more pedunculated polyps; two analyzed hot EMR[15,16] , and the third analyzed hot and cold EMR and CSP[17]. Three further studies included smaller numbers of pedunculated polyps, using cold EMR[18], ESD[19], and underwater EMR[20]. Most studies included 10% or less SSA/Ps, while two studies investigating CSP and cold EMR evaluated results from SSA/Ps only[21,22]. The latter two studies reported exceptionally low IRR of less than 1.5%.
This meta-analysis confirms the high risk for recurrence after standard EMR resection of large colonic polyps. When standard EMR without routine margin ablation is used, we found a 12-month recurrence rate of 15.2%. This is comparable to the results found in the two available meta-analyses published in 2014 and 2021, which reported recurrence rates of 15%[6] and 10%[23], respectively. However, since then, many new or modified endoscopic removal techniques have been developed. These novel developments include cold EMR, hot snare with margin ablation, and an increasing body of literature on ESD for colorectal polyps coming from Asian, European and North American centers. We found that two of these modalities resulted in significantly lower LRRs compared with standard EMR. ESD was associated with an LRR of only 1.7%, and the LRR after EMR with routine ablation of the complete margin was 3.3%. However, ESD requires advanced endoscopy skills, adequate training, and the technique is associated with an increased risk for complications[24-26]. Furthermore, significant differences in safety and efficacy of ESD have been shown between Asian and non-Asian countries[27], so that EMR has remained the standard for large polyp resection in Western countries to date.
The other modality that shows significantly reduced recurrence rates is the combination of hot EMR with routine margin ablation[9,28]. Thus, ESD or EMR with routine margin ablation currently seem to be the best approaches for endoscopic removal of large colorectal polyps in order to avoid recurrence. We found that systematic margin ablation after EMR can reduce the LRR to rates similar to those of ESD. These results originate from two recent Australian prospective studies, in which snare tip soft coagulation (STSC) was routinely performed after EMR[9,28]. As these studies included only polyps ≥ 20 mm further studies evaluating the effect of margin ablation on polyps sized 10-19 mm may be of additional value. The positive effect of margin ablation has also been shown in a retrospective US study, evaluating systematic application of argon plasma coagulation (APC) after EMR in 246 patients[29]. The authors found an LRR of 5% at < 12 mo FU, which is comparable to the rates found by Klein (5%) [9] and Sidhu (3%)[28]. However, the results for using APC margin ablation still need to be confirmed by prospective studies. A recently completed prospective, multicenter study evaluating resection of large colonic lesions ≥ 20 mm in 76 patients using EMR and hybrid APC for margin and base ablation found a LRR of 2.2%[30]. This indicates that APC ablation can reduce local recurrence comparable to ESD and EMR with STSC.
Importantly, margin ablation should be performed systematically and completely, as visual margin assessment may underestimate incomplete resection[4]. This is confirmed by our analysis, which showed that studies using unsystematic or incomplete margin ablation were not able to reduce the LRR[9,14,31].
Notably, even though use of cold snare resection techniques for large colonic polyps is increasingly reported, at present no prospective studies have been published reporting LRR for CSP or cold EMR for large colorectal polyps. Furthermore, recent retrospective studies have indicated that these techniques might potentially increase the risk for local recurrence. In the largest retrospective series published to date, Suresh et al[32] reported an LRR of 34.8% after cold EMR. Therefore, caution is warranted for routine use of cold EMR outside of clinical studies until data from ongoing RCTs comparing hot with cold EMR become available.
For polyps 10–19 mm, follow-up examination is often performed years after the index colonoscopy. Therefore, data on LRR for 10–19 mm colorectal polyps are sparse, and we used IRR to estimate the risk of local recurrence for this subgroup. In our analysis, overall IRR for polyps sized 10–19 mm was 16.0%. This rate was similar to the IRR found in a previous meta-analysis (20.8%)[5] and in one of the landmark studies on IRR (CARE study)[4]. The CARE study reported that even though endoscopists rated resection as complete by visual assessment, 10.1% of cases showed residual tissue on margin biopsy. Compared with the previous meta-analysis, our analysis included more data, especially regarding cold snare resection techniques[5]. However, adding the recently published data on cold snare resection did not significantly alter overall IRR of polyps 10–19 mm. Furthermore, IRR of EMR, cold EMR, HSP, and underwater EMR were similar[17,18,21]. Only two prospective studies evaluated IRR for large colonic polyps after CSP resection[17,21]. These studies showed high variability in IRR, and while one study found rates comparable to those obtained with other techniques[17], rates reported in the second were extremely low[21]. This is likely based on the fact that in this study only expert endoscopists were involved and only SSA/Ps were removed by wide-field style cold snare resection. A previous meta-analysis has already demonstrated that expert endoscopists achieve lower IRRs[5], and this was confirmed in our analysis, with an IRR of 7.0% for expert endoscopists and 20.3% for those studies in which less experienced endoscopists were included. Furthermore, the exclusive inclusion of SSA/Ps introduced a further bias into the study, as removal of SSA/Ps generally yields better results[14]. This renders the generalizability of CSP to general clinical practice difficult, and more prospective studies are needed to establish IRR, and especially LRR risk, after CPS and cold EMR removal of large colorectal polyps including all pathology types.
For polyps ≥ 20 mm, the mean IRR was 11.7% in this analysis. Interestingly, this rate is lower than the results obtained for polyps sized 10–19 mm (16.0%). This effect was based on the good results obtained with ESD, and is most likely also associated with the fact that 10–19 mm polyps are usually resected in general endoscopic practice, whereas polyps ≥ 20 mm are often referred for expert resection.
Recent guidelines recommend HSP, EMR [European Society of Gastrointestinal Endoscopy, American Gastroenterological Association (AGA)][33,34] and CSP (AGA)[34] for resection of 10–19 mm polyps. Our data show that EMR with margin ablation is an important new development that warrants further study. For lesions ≥ 20 mm, guidelines recommend ESD in specific cases, and the need for a skilled endoscopist to perform the procedure is highlighted[25,26,35]. The importance of endoscopist skill level is also supported by our data. Furthermore, our data suggest that EMR with systematic and complete margin ablation may be an appropriate alternative to ESD, especially considering the low complication rates reported in the available studies[9,28,29].
However, safety profiles should also be taken into account when evaluating different polypectomy techniques. Known complications occurring during and after polypectomy are immediate and delayed bleeding as well as perforation and post-coagulation syndrome[33,34]. A meta-analysis from 2016 evaluating endoscopic resection of polyps ≥ 20 mm found perforation occurring in 1.5% and bleeding in 6.5% of polyps. Mortality was indicated as 0.08%[36]. Yet, an up-to-date analysis comparing safety profiles of CSP, HSP, EMR with and without margin ablation, underwater EMR, and ESD still has to be performed.
Our analysis has several strengths, including the robustness of the literature search with a large number of publications screened (6928 publications). Of these, 34 prospective studies with a total of 10268 polyp resections were included. As only prospective data were evaluated, we were able to perform a high-quality analysis, as retrospective studies reporting on LRR and IRR are unsystematic in the ascertainment of the main outcomes, thus having a high likelihood of bias. Furthermore, the retrieval of granular data allowed us to perform multiple analyses.
The main limitation of the study is that by exclusion of retrospective studies, the data set was reduced. Furthermore, publications were excluded in which IRR was determined by visual assessment or in which appointments for follow-up examinations for LRR assessment exceeded 12 mo. While this reduced the amount of studies included into the analysis, it ensured a better overview of the high-quality data available in the literature. Additionally, it shows that for some techniques data of sufficient quality is sparse and that there is a need for further studies. Second, expert endoscopist status was difficult to determine, as there is no published consensus definition, and some studies do not clarify the expertise of the involved endoscopists. However, expert and non-expert status were systematically assessed and discussed for this meta-analysis. Third, as assessment for IRR is not standardized, different methods, including margin assessment and margin biopsy, are used for its estimation. However, margin assessment is likely to overestimate IRR, as lesions resected in piecemeal fashion may be resected completely, but will appear with positive resection margins. Margin biopsy, on the other hand, is likely to underestimate IRR, as only sample parts of the margins are examined[37]. Therefore, true IRR will be located somewhere in between the values found with margin biopsy and margin assessment. Another limitation is the elevated heterogeneity in some reported outcomes. For LRR, most of the observed heterogeneity was due to combining different resection techniques into the same analysis. When stratifying for resection technique and use of margin ablation, we found very low heterogeneity in the reported outcomes. For IRR, the differing use of wide field resection before biopsies and the number of margin biopsies taken could explain some of the heterogeneity reported.
In conclusion, we found that local recurrence after resection of large colonic polyps occurs frequently when standard EMR is used. Local polyp recurrence can be reduced by performing ESD or EMR with routine and complete margin ablation. Other techniques, such as CSP, cold EMR, and underwater EMR require further evaluation in prospective studies before their routine implementation in clinical practice can be recommended.
Complete resection is the aim of endoscopic therapy for large colonic polyps. Endoscopic mucosal resection (EMR) is the most common endoscopic treatment for such polyps. In recent years, endoscopic resection techniques have evolved, including cold snare polypectomy (CSP), cold EMR, EMR with margin ablation, underwater EMR, and endoscopic submucosal dissection (ESD).
Efficacy of these newer polypectomy techniques with regard to local recurrence rates (LRRs) vs traditional hot snare polypectomy and standard EMR remains unclear.
These developments have sparked our interest in providing an up-to-date meta-analysis of LRRs and incomplete resection rates (IRRs) for large (≥ 10 mm) colorectal polyps, and to evaluate the impact of the novel or modified endoscopic resection techniques on LRRs.
A systematic literature search was performed within MEDLINE, EMBASE, EBM Reviews, and CINAHL databases. All articles published between January 2011 and July 2021 reporting on IRR and/or LRR for colorectal polyps 10 mm or larger removed by endoscopic resection techniques were included in the search.
LRR were lowest when EMR with systematic margin ablation (3.3%) or ESD (1.7%) were used for endoscopic removal of large (> 10 mm) colorectal polyps. When standard EMR (without margin ablation) or with partial margin ablation were used, LRRs were high (15.2% and 16.5%, respectively).
Local recurrence after resection of large colonic polyps occurs frequently when standard EMR is used, but can be reduced by performing ESD or EMR with routine and complete margin ablation. Other techniques, such as CSP, cold EMR, and underwater EMR require further evaluation in prospective studies before their routine implementation in clinical practice can be recommended.
ESD or EMR with margin ablation should be considered standard of care for endoscopic removal of large colorectal polyps in order to avoid recurrence. At present, cold snare resection techniques or underwater EMR should only be performed within clinical trials, pending the availability of high-quality evidence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Yu T, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Pohl H, Anderson JC, Aguilera-Fish A, Calderwood AH, Mackenzie TA, Robertson DJ. Recurrence of Colorectal Neoplastic Polyps After Incomplete Resection. Ann Intern Med. 2021;174:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 3. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 4. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 5. | Djinbachian R, Iratni R, Durand M, Marques P, von Renteln D. Rates of Incomplete Resection of 1- to 20-mm Colorectal Polyps: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;159:904-914.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 7. | Thoguluva Chandrasekar V, Spadaccini M, Aziz M, Maselli R, Hassan S, Fuccio L, Duvvuri A, Frazzoni L, Desai M, Fugazza A, Jegadeesan R, Colombo M, Dasari CS, Hassan C, Sharma P, Repici A. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc. 2019;89:929-936.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Klein A, Tate DJ, Jayasekeran V, Hourigan L, Singh R, Brown G, Bahin FF, Burgess N, Williams SJ, Lee E, Sidhu M, Byth K, Bourke MJ. Thermal Ablation of Mucosal Defect Margins Reduces Adenoma Recurrence After Colonic Endoscopic Mucosal Resection. Gastroenterology. 2019;156:604-613.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 1308] [Article Influence: 327.0] [Reference Citation Analysis (1)] |
| 11. | National Institutes of Health. NIH Study Quality Assessment Tool 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. |
| 12. | Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 571] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 13. | Woodward T, Crook JE, Raimondo M, Wallace M. Improving complete EMR of colorectal neoplasia: a randomized trial comparing snares and injectate in the resection of large sessile colon polyps. Gastrointest Endosc. 2015;81:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Pellise M, Burgess NG, Tutticci N, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, P'Ng H, Mahajan H, McLeod D, Bourke MJ. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut. 2017;66:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Han SJ, Jung Y, Cho YS, Chung IK, Kim JY, Eun JY, Lee SH, Ko GB, Lee TH, Park SH, Cho HD, Kim SJ. Clinical Effectiveness of Submucosal Injection with Indigo Carmine Mixed Solution for Colon Endoscopic Mucosal Resection. Dig Dis Sci. 2018;63:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yoshida N, Saito Y, Hirose R, Ogiso K, Inada Y, Yagi N, Naito Y, Otake Y, Nakajima T, Matsuda T, Yanagisawa A, Itoh Y. Endoscopic mucosal resection for middle and large colorectal polyps with a double-loop snare. Digestion. 2014;90:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Li D, Wang W, Xie J, Liu G, Wang R, Jiang C, Ye Z, Xu B, He X, Hong D. Efficacy and safety of three different endoscopic methods in treatment of 6-20 mm colorectal polyps. Scand J Gastroenterol. 2020;55:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Yabuuchi Y, Imai K, Hotta K, Ito S, Kishida Y, Yoshida M, Kawata N, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Aizawa D, Oishi T, Imai T, Ono H. Efficacy and safety of cold-snare endoscopic mucosal resection for colorectal adenomas 10 to 14 mm in size: a prospective observational study. Gastrointest Endosc. 2020;92:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology. 2021;160:2317-2327.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Yamashina T, Uedo N, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Takeuchi Y, Fujii S, Kusaka T, Shimokawa T. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology. 2019;157:451-461.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Kimoto Y, Sakai E, Inamoto R, Kurebayashi M, Takayanagi S, Hirata T, Suzuki Y, Ishii R, Konishi T, Kanda K, Negishi R, Takita M, Ono K, Minato Y, Muramoto T, Ohata K. Safety and Efficacy of Cold Snare Polypectomy Without Submucosal Injection for Large Sessile Serrated Lesions: A Prospective Study. Clin Gastroenterol Hepatol. 2022;20:e132-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Tutticci NJ, Hewett DG. Cold EMR of large sessile serrated polyps at colonoscopy (with video). Gastrointest Endosc. 2018;87:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Lim XC, Nistala KRY, Ng CH, Lin SY, Tan DJH, Ho KY, Chong CS, Muthiah M. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal polyps: A meta-analysis and meta-regression with single arm analysis. World J Gastroenterol. 2021;27:3925-3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 26. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 27. | Daoud DC, Suter N, Durand M, Bouin M, Faulques B, von Renteln D. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:2518-2536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 28. | Sidhu M, Shahidi N, Gupta S, Desomer L, Vosko S, Arnout van Hattem W, Hourigan LF, Lee EYT, Moss A, Raftopoulos S, Heitman SJ, Williams SJ, Zanati S, Tate DJ, Burgess N, Bourke MJ. Outcomes of Thermal Ablation of the Mucosal Defect Margin After Endoscopic Mucosal Resection: A Prospective, International, Multicenter Trial of 1000 Large Nonpedunculated Colorectal Polyps. Gastroenterology. 2021;161:163-170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | Raju GS, Lum P, Abu-Sbeih H, Ross WA, Thirumurthi S, Miller E, Lynch P, Lee J, Bhutani MS, Shafi M, Weston B, Rashid A, Wang Y, Chang GJ, Carlson R, Hagan K, Davila M, Stroehlein J. Cap-fitted endoscopic mucosal resection of ≥ 20 mm colon flat lesions followed by argon plasma coagulation results in a low adenoma recurrence rate. Endosc Int Open. 2020;8:E115-E121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Motchum L, Levenick J, Djinbachian R, Moyer M, Bouchard S, Taghiakbari M, Repici A, Deslandres E, Renteln D von. Endoscopic mucosal resection combined with hybrid argon plasma coagulation to prevent recurrence of large nonpedunculated colorectal polyps. Gastrointestinal Endoscopy, under Revision 2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Pohl H, Grimm IS, Moyer MT, Hasan MK, Pleskow D, Elmunzer BJ, Khashab MA, Sanaei O, Al-Kawas FH, Gordon SR, Mathew A, Levenick JM, Aslanian HR, Antaki F, von Renteln D, Crockett SD, Rastogi A, Gill JA, Law RJ, Elias PA, Pellise M, Mackenzie TA, Rex DK. Effects of Blended (Yellow) vs Forced Coagulation (Blue) Currents on Adverse Events, Complete Resection, or Polyp Recurrence After Polypectomy in a Large Randomized Trial. Gastroenterology. 2020;159:119-128.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 32. | Suresh S, Zhang J, Ahmed A, Abu Ghanimeh M, Elbanna A, Kaur R, Isseh M, Watson A, Dang DT, Chathadi KV, Pompa R, Singla S, Piraka C, Zuchelli T. Risk factors associated with adenoma recurrence following cold snare endoscopic mucosal resection of polyps ≥ 20 mm: a retrospective chart review. Endosc Int Open. 2021;9:E867-E873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 34. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1095-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (3)] |
| 35. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 36. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 37. | Djinbachian R, Renteln DV. Endoscopic Polypectomy: How Should We Determine Complete Resection Status? Clin Gastroenterol Hepatol. 2022;20:242-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |