Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3825
Peer-review started: September 18, 2021
First decision: December 4, 2021
Revised: December 15, 2021
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: August 7, 2022
Processing time: 319 Days and 6.4 Hours
Recent studies have demonstrated that dysfunction of the intestinal barrier is a significant contributing factor to the development of severe acute pancreatitis (SAP). A stable intestinal mucosa barrier functions as a major anatomic and functional barrier, owing to the balance between intestinal epithelial cell (IEC) proliferation and apoptosis. There is some evidence that calcium overload may trigger IEC apoptosis and that calcineurin (CaN)/nuclear factor of activated T-cells (NFAT) signaling might play an important role in calcium-mediated apoptosis.
To investigate the potential mechanisms underlying the therapeutic effect of Qingyi decoction (QYD) in SAP.
A rat model of SAP was created via retrograde infusion of sodium deoxycholate. Serum levels of amylase, tumor necrosis factor (TNF-α), interleukin (IL)-6, D-lactic acid, and diamine oxidase (DAO); histological changes; and apoptosis of IECs were examined in rats with or without QYD treatment. The expression of the two subunits of CaN and NFAT in intestinal tissue was measured via quantitative real-time polymerase chain reaction and western blotting. For in vitro studies, Caco-2 cells were treated with lipopolysaccharide (LPS) and QYD serum, and then cell viability and intracellular calcium levels were detected.
Retrograde infusion of sodium deoxycholate increased the severity of pancreatic and intestinal pathology and the levels of serum amylase, TNF-α, and IL-6. Both the indicators of intestinal mucosa damage (D-lactic acid and DAO) and the levels of IEC apoptosis were elevated in the SAP group. QYD treatment reduced the serum levels of amylase, TNF-α, IL-6, D-lactic acid, and DAO and attenuated the histological findings. IEC apoptosis associated with SAP was ameliorated under QYD treatment. In addition, the protein expression levels of the two subunits of CaN were remarkably elevated in the SAP group, and the NFATc3 gene was significantly upregulated at both the transcript and protein levels in the SAP group compared with the control group. QYD significantly restrained CaN and NFATc3 gene expression in the intestine, which was upregulated in the SAP group. Furthermore, QYD serum significantly decreased the LPS-induced elevation in intracellular free Ca2+ levels and inhibited cell death.
QYD can exert protective effects against intestinal mucosa damage caused by SAP and the protective effects are mediated, at least partially, by restraining IEC apoptosis via the CaN/NFATc3 pathway.
Core Tip: This manuscript investigated the role of the calcineurin (CaN)/nuclear factor of activated T-cells (NFATc3) pathway in the apoptosis of intestinal epithelial cells (IECs) in severe acute pancreatitis (SAP) and the potential mechanisms underlying the therapeutic effect of Qingyi decoction (QYD). QYD significantly restrained CaN and NFATc3 gene expression in the intestine, ameliorated IEC apoptosis associated with SAP, and decreased the lipopolysaccharide-induced elevation in intracellular free Ca2+ levels and cell death. These findings suggest that the protective effects of QYD might be mediated, at least partially, by downregulating IEC apoptosis via the CaN/NFATc3 pathway.
- Citation: Wang GY, Shang D, Zhang GX, Song HY, Jiang N, Liu HH, Chen HL. Qingyi decoction attenuates intestinal epithelial cell injury via the calcineurin/nuclear factor of activated T-cells pathway. World J Gastroenterol 2022; 28(29): 3825-3837
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3825
Severe acute pancreatitis (SAP) is a severe acute abdominal disease characterized by high morbidity and mortality that can occur as a consequence of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)[1]. A considerable amount of literature has been published on SAP. These studies have demonstrated that dysfunction of the intestinal barrier is a significant contributing factor to the development of SAP. Disruption of the intestinal barrier can give rise to gut bacteria and endotoxin translocation, thus creating secondary infections, SIRS, and MODS, leading to SAP[2]. A stable intestinal mucosa barrier functions as a major anatomic and functional barrier, owing to the balance between intestinal epithelial cell (IEC) proliferation and apoptosis. Recent research has revealed that IEC apoptosis plays an essential role in the development of SAP, and increased IEC apoptosis has been confirmed to contribute to intestinal injury, mucosal atrophy, bacterial translocation, and barrier dysfunction in SAP[3-5].
As a ubiquitous second messenger, Ca2+ has a significant impact on a variety of cellular processes in nearly all cell types. Calcineurin (CaN), a unique calcium-activated serine/threonine phosphatase, is a dominant factor in calcium-dependent signal transduction pathways. Nuclear factor of activated T-cells (NFAT) is a substrate phosphorylated by CaN, that is completely dependent on Ca2+/CaN signaling; thus, NFAT is remarkably responsive to intracellular Ca2+ oscillations[6]. There is some evidence that calcium overload may trigger IEC apoptosis and that CaN/NFAT signaling might play an important role in calcium-mediated apoptosis[7,8].
Qingyidecoction (QYD), a Chinese herbal medicine consisting of Radix Bupleuri (Chaihu, Bupleurum scorzonerifolium Willd.), Scutellariae Radix (Huangqin, Scutellaria baicalensis Georgi.), Aucklandiae Radix (Muxiang, Aucklandia lappa Decne.), Rhizoma Corydalis (Yanhusuo, Corydalis acropteryx Fedde), Coptidis Rhizoma (Huanglian, Coptis chinensis Franch.), Radix Paeoniae Alba (Baishao, Paeonia lactiflora Pall.), Rhei Radix Et Rhizoma (Dahuang, Rheum palmatum L.) and Natrii Sulfas (Mangxiao, Mirabilite) has been widely utilized for several decades in the treatment of acute pancreatitis in China[9]. QYD, as an organic combination of many effective components, plays a multitarget role in acute pancreatitis treatment through multiple pathways, including protecting the intestinal barrier. The efficacy of QYD has been demonstrated to involve its ability to moderate endotoxin generation, restrict excessive neutrophil activation, minimize the release of inflammatory cytokines, and inhibit IEC apoptosis[10]. The present study aimed to investigate the role of the CaN/NFATc3 pathway in the apoptosis of IECs in SAP and explore the potential mechanisms underlying the therapeutic effect of QYD.
Male Sprague-Dawley (SD) rats, 180-220 g, were obtained from the specific-pathogen-free Animal Center of Dalian Medical University. This study was approved by the Ethics Committee of Dalian Medical University. Thirty male SD rats were randomly divided into an SAP group, a QYD treatment group, and a control group, with ten rats per group. The SAP model was established using a method previously described[9]. The rats in the QYD group were gavaged with three doses of QYD (10 mL/kg body weight/dose, the First Affiliated Hospital of Dalian Medical University, Dalian, China) 0.5 h before and 6 and 12 h after surgery. The rats in the control group received only sham surgery[9]. The rats were anesthetized with 10% chloralhydrate via intraperitoneal injection at 3 mL per kg bodyweight 24 h post operation. Abdominal aorta blood samples and tissue samples were collected immediately.
Serum amylase was detected using a spectrophotometric method with a commercial kit (Jiancheng, Nanjing, China). Briefly, 0.5 mL of starch reagent and 0.10 mL of serum samples were added to a 5 mL graduated tube. After incubating for 7.5 min at 37 °C, 0.5 mL of iodine reagent and 3.0 mL water were added immediately. The absorbance was measured at 660 nm. Enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor (TNF-α) (Lengton, Shanghai, China), D-lactic acid (Goybio, Shanghai, China), diamine oxidase (DAO) (Jiancheng, Nanjing, China), interleukin (IL)-6 (Lengton, Shanghai, China) were used to evaluate the levels in rat serum according to the manufacturer’s instructions.
Pancreatic and intestinal tissues were fixed in 10% formaldehyde. After dehydration in gradient alcohol and transparentizing in xylene, the tissues were embedded in paraffin and cut into 5 μm thick slices. The slices were stained with hematoxylin and eosin for histological examination. The tissues were scored under an optical microscope (Leica, Solms, Germany) by a pathologist blinded to the experimental design. Five histopathological parameters (edema, inflammatory infiltration, hemorrhage, and acinar necrosis) of the pancreatic tissue were evaluated, each on a scale of 0-4 using a previously described method[11]. Mucosal injury, inflammatory infiltration, and hemorrhage were graded, each on a scale of 0-5, using a method described in a previous article to assess intestinal tissue[12].
Fixed rat intestinal sections with 10% formaldehyde were deparaffinized in xylene and rehydrated through a graded ethanol series. A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay kit (Keygen, Nanjing, China) was applied to assess apoptosis in intestinal tissue. Briefly, rat intestinal sections were treated with 50 μL of the TUNEL reaction mixture and then incubated at 37 °C for 60 min. After rinsing with phosphate buffer solution, tissue sections were stained with 4’,6-diamidino-2-phenylindole (DAPI) and observed with fluorescence microscopy.
For immunofluorescence analysis, intestinal sections were incubated in a xylol-ethanol gradient to remove the paraffin. The slides were incubated with primary antibody against NFATc3 (Santa Cruz, CA, United States) and subsequently incubated with Alexa Fluor goat anti-rabbit immunoglobulin G (Life Technologies), and then observed using fluorescence microscopy following staining of the nuclei with DAPI (Sigma-Aldrich, St. Louis, MO). The specificity of the signals was monitored by blank staining without primary antibodies.
Western blotting was performed as previously described[13]. Total protein from intestinal tissue was extracted with a protein extraction kit (KeyGen, Nanjing, China). Protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane. After nonspecific binding was blocked, the membranes were incubated with primary antibodies against calcineurin A (CnA, 1:2000, Abcam, United Kingdom), calcineurin B (CnB, 1:500, Abcam, United Kingdom), and β-actin (1:1000). Immunoreactive proteins were visualized using enhanced chemiluminescence detection (Bioworld Technology, Nanjing, China). ImageJ software was used for statistical analyses of the band intensities normalized to β-actin.
Total RNA extraction from intestinal tissue was performed with NAiso Plus (TaKaRa, Dalian, China), and then a PrimeScriptTMRT MasterMix kit (TaKaRa, Dalian, China) was used to synthesize cDNA. Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out in a 7300 Real-time PCR System using a SYBR® Premix Ex TaqTMIIKit (TaKaRa). The primer sequences were as follows: NFATC3 forward 5’-GGTGGCCATCCTGTTGTGAA-3’ and reverse 5’-CAGTAATGCGATGCACCTGGTAA-3’; β-actin forward 5’- GGAGATTACTGCCCTGGCTCCTA-3’ and reverse 5’-GACTCATCGTACTCCTGCTTGCTG-3’. The 2-ΔΔCt method was used for statistical analyses of the mRNA levels normalized to β-actin.
Drug-containing serum was prepared as described previously[14]. Ten SD rats were divided into two groups: A normal group and a QYD serum group. In the QYD serum group, QYD (the First Affiliated Hospital of Dalian Medical University, Dalian, China) was administered to SD rats via gavage at 10 mL/dose/kg body weight twice per day for 5 d. The rats in the normal group were treated with the same volume of saline. After 5 d of treatment, blood samples were extracted from the abdominal arteries. Under anesthesia, serum was isolated from both the normal group and the QYD group based on a previously described method[15].
Caco-2 cells were randomly divided into the following groups: The rat serum group, in which the cells were treated with 10% normal rat serum for 24 h; the rat serum + lipopolysaccharide (LPS) group, in which the cells were exposed to 100 ng/mL LPS and 10% normal rat serum for 24 h; and the QYD serum + LPS group, in which the cells were treated with 100 ng/mL LPS and 10% QYD serum for 24 h.
Caco-2 cell viability was assessed using MTT assays (Sigma, St Louis, MO, United States). After incubation with MTT dye (5 mg/mL) for 4 h at 37 °C, DMSO (150 μL/well) was added. The optical absorbance was evaluated with a microplate reader at 490 nm to measure cell viability.
Intracellular Ca2+ levels were monitored using the Ca2+-sensitive fluorescent indicator Fluo-3. Flou-3 can enter cells through the medium of lipophilic AM and combine with intracellular free Ca2+. The fluorescence intensity of Flou-3 is positively correlated with the concentration of intracellular free Ca2+. Caco-2 cells were seeded in HBSS buffer with Fluo-3/AM at 5 μmol/L for 30 min. The cells were washed twice in fresh HBSS and then treated with 0.5 mL of HBSS buffer. Finally, a confocal laser scanning microscope (BioRad Radiance 2100) was used to assess the fluorescence intensity.
Data are expressed as the mean ± SD. SPSS v16.0 software was used for data analysis. One-way ANOVA was applied to assess significant differences between groups; P < 0.05 was considered to indicate a statistically significant difference.
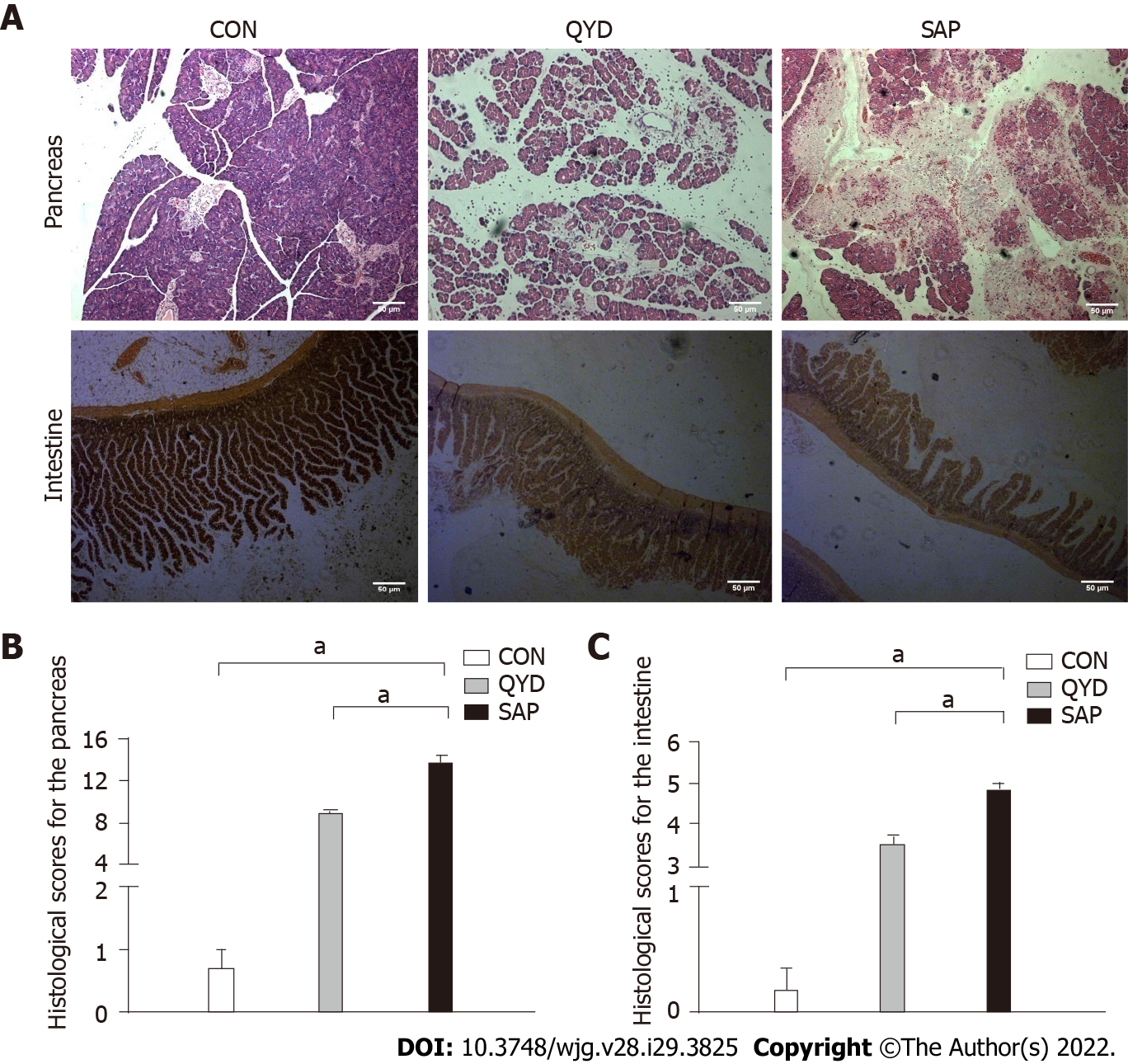
As shown in Figure 1A, edema, inflammatory infiltration, hemorrhage, and acinar necrosis were observed in the pancreas as the characteristic morphological features of SAP. Interstitial edema, irregular villi, mucosal erosion, and inflammatory infiltration were exhibited in the SAP group, with necrosis and shedding of the intestinal epithelium. The pancreatic and intestinal histological scores were significantly higher according to the standards of Anthony and Chiu, respectively. However, QYD treatment significantly ameliorated the pancreatic and intestinal pathology caused by SAP (Figures 1B and 1C).
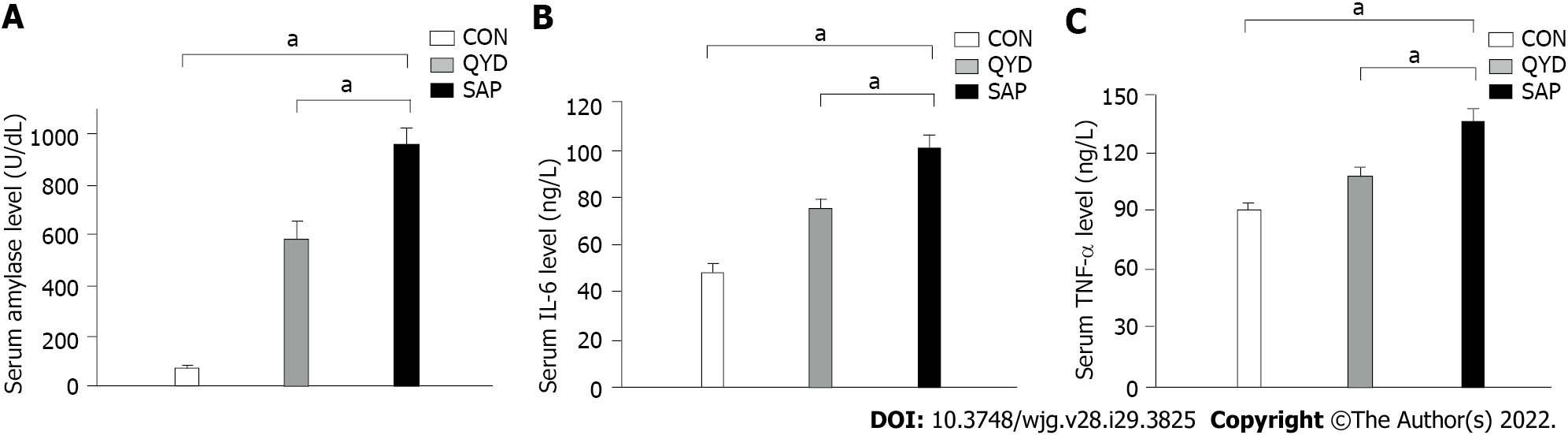
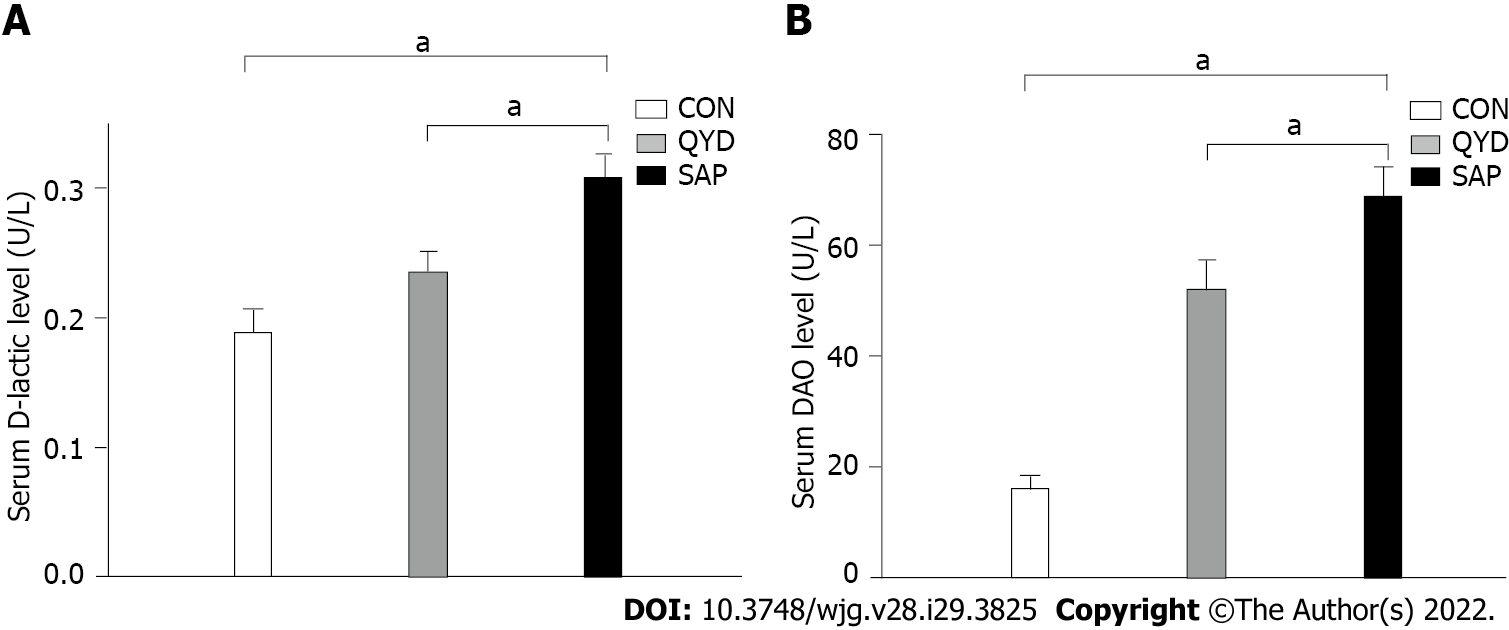
It can be seen from the data in Figure 2 that the serum levels of amylase (Figure 2A), IL-6 (Figure 2B), and TNF-α (Figure 2C) were increased significantly in the SAP group compared with the control group. Consistent with previous research results, QYD treatment ameliorated the increases in amylase, IL-6, and TNF-α serum levels observed in the SAP group. D-lactic acid and DAO, which can serve as markers of intestinal barrier dysfunction and tissue injury, were significantly increased in the SAP group compared with the control group. However, the levels of serum D-lactic acid and DAO in the QYD group were significantly decreased compared with those in the SAP group (Figures 3A and 3B).
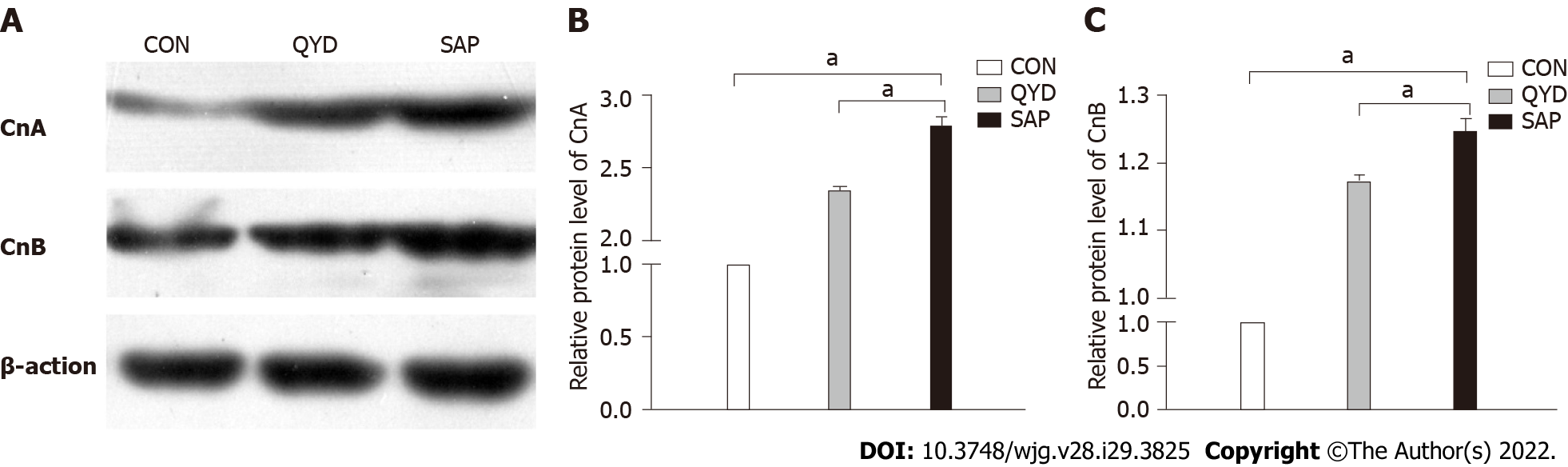
Following QYD treatment, the expression levels of CnA and CnB were detected via western blot analysis. The results, shown in Figure 4A, demonstrated that the expression levels of CnA and CnB were increased in the SAP group. The band intensities of CnA and CnB in the SAP group were 2.78 and 1.24, respectively, normalized to β-actin. QYD treatment significantly reduced the protein expression levels of CnA and CnB (Figures 4B and 4C).
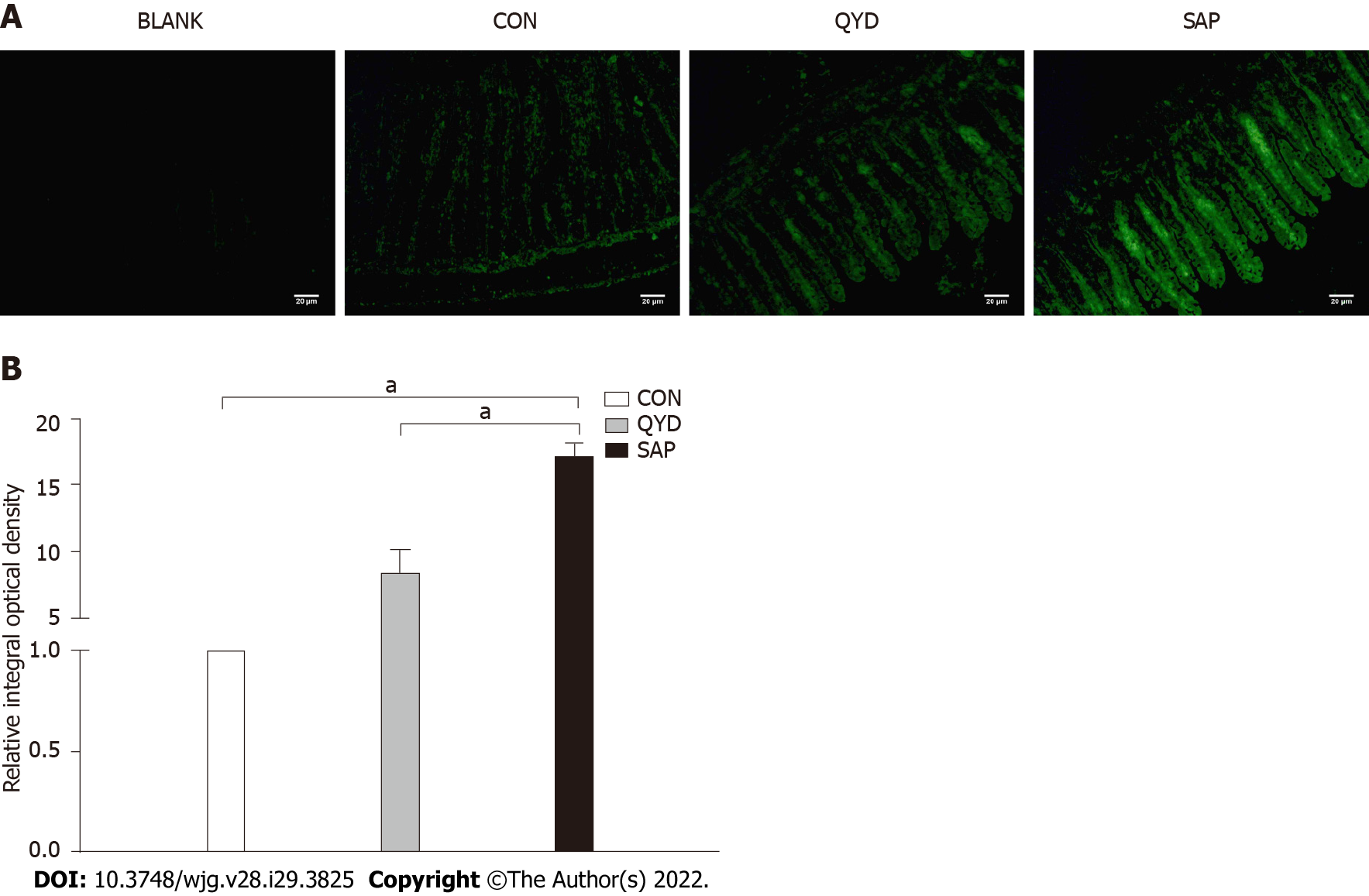
Furthermore, we examined NFATc3 protein expression in intestinal tissues using immunofluorescence. NFATc3 was highly expressed in the SAP group compared with the control group but significantly downregulated under QYD treatment (Figure 5A). Semiquantitative analysis showed that the relative integral optical density (IOD) value was higher in the SAP group than in the control group. However, the relative IOD value was lower in the QYD group than in the SAP group (Figure 5B).
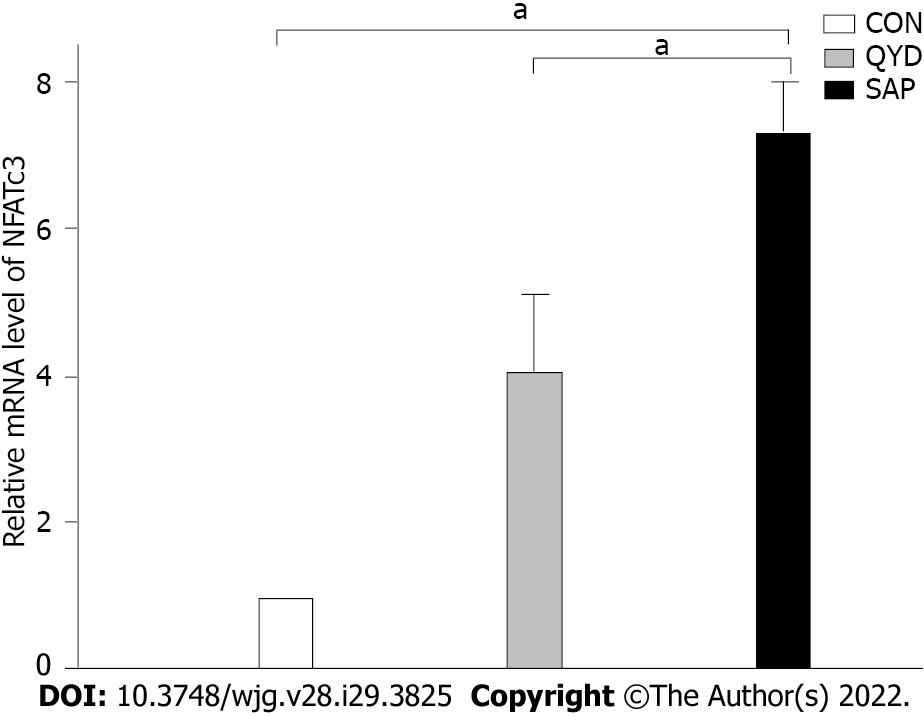
Compared with the control group, qRT-PCR analysis revealed that the level of NFATc3 mRNA transcription was obviously increased in the SAP group. QYD treatment decreased the mRNA level compared with that in the SAP group (Figure 6).
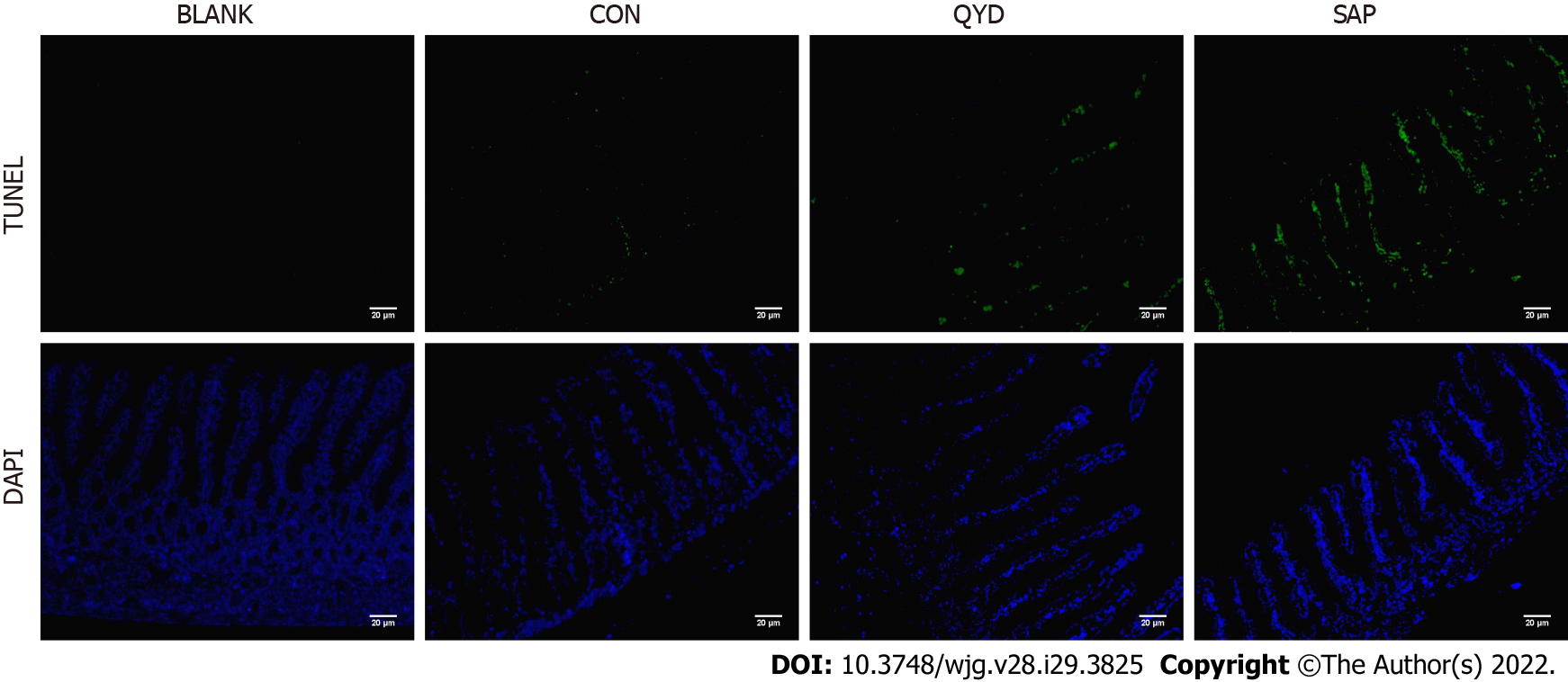
TUNEL staining results of rat intestinal tissue slices revealed that retrograde infusion of sodium deoxycholate increased ICE apoptosis compared with the control rats (Figure 7). However, compared with that in the SAP group, the level of ICE apoptosis in the QYD groups was significantly lower.
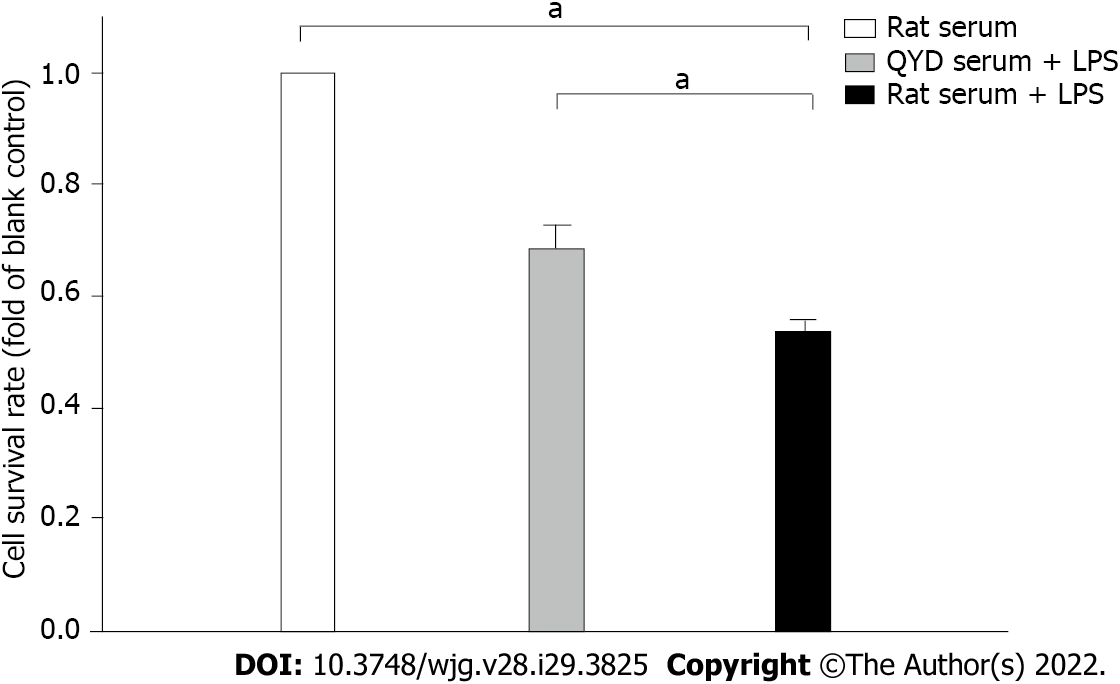
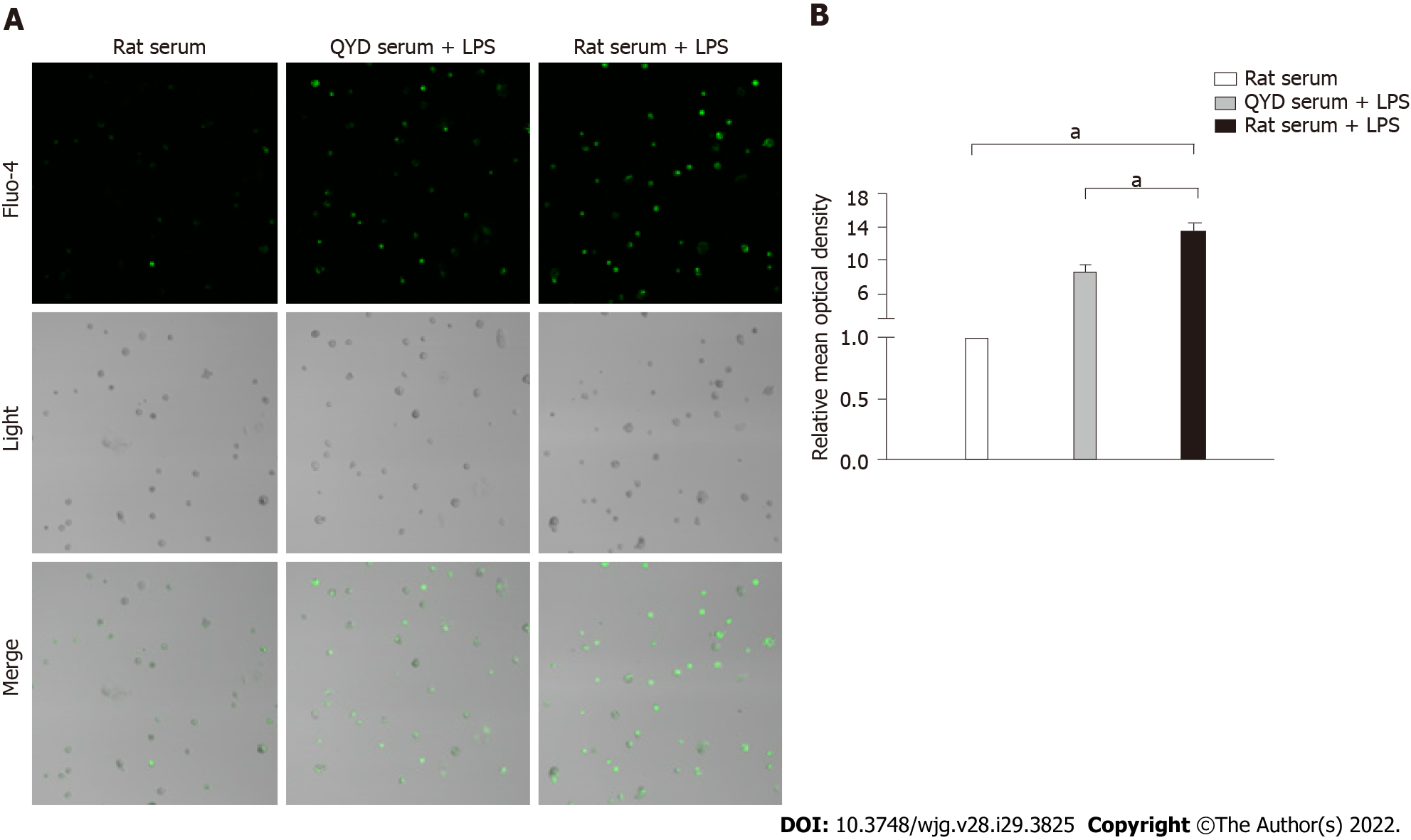
A significant decline in cell viability after LPS treatment was verified by MTT assays (Figure 8), but QYD serum inhibited LPS-induced cell death. LPS induced a significant increase in Ca2+ levels. Treatment with QYD serum significantly attenuated the increase in Ca2+ levels caused by LPS-induced injury (Figure 9A). The relative mean optical density value was higher in the LPS group than in the control group, but QYD serum treatment decreased the relative mean optical density induced by LPS (Figure 9B).
SAP refers to an acute abdominal disease characterized by rapid onset, rapid progression, and a high fatality rate. Prior studies have noted the importance of the intestinal barrier in the progression of SAP, which involves alterations in its mechanical, immune, chemical, and biological barriers[16]. Bacterial and/or endotoxin translocation from the gut lumen caused by intestinal barrier functional disturbance has a major influence on the prognosis of SAP[17]. The intestinal epithelium functions as the first barrier to defend against the invasion of overcrowded intestinal bacteria owing to intestinal motor dysfunction in SAP. Therefore, conservation and renovation of the intestinal epithelial function may have a positive effect on SAP and reduce SAP-mediated septic morbidity and mortality[18]. As a specific form of cell death in terms of its morphology and biochemistry, apoptosis has been thought of as a key factor in the physiological processes of IEC biology[19]. Recently, investigators have examined the effects of IEC apoptosis on intestinal integrity[18]. TNF-α, secreted from pancreatic acinar cells, monocytes and macrophages, not only mediates primary inflammation directly but also contributes to the occurrence of secondary inflammation[20,21]. High concentrations of TNF-α can be detected in serum as well as in pancreatic parenchyma, ascitic fluid, and lymphatic drainage in the early stages of AP. Many studies have confirmed that high serum levels of TNF-α are related to the severity of AP[22,23]. IL-6 is a phosphorylated glycoprotein of 185 amino acids encoded by the human IL-6 gene. The IL-6 gene has been mapped to human chromosome 7p21-24[24]. Current evidence has clarified that IL-6 is a vital cytokine in AP development; moreover, the serum level of IL-6 is associated with AP severity[25]. DAO is an intestinal mucosal enzyme that plays a key role in digestion and absorption and maintenance of the mucosal barrier. DAO activity in the mature upper villus cells of the intestinal mucosa is high, but it is very low in all other tissues under normal physiological conditions. However, DAO activity increases in the blood plasma and intestinal lumen when the intestinal mucosa is damaged[26,27]. D-lactic acid is a metabolite produced by the fermentation of intestinal bacteria. When the permeability of the intestinal mucosa increases as a result of infection with bacteria, D-lactic acid can enter the blood circulation and be detected in the peripheral blood. Consequently, D-lactic acid can be used as an early indicator of intestinal mucosa damage with high specificity[28]. In accordance with previous studies, the present results confirmed that retrograde infusion of sodium deoxycholate successfully created a rat model of SAP. Retrograde infusion of sodium deoxycholate increased the severity of pancreatic and intestinal pathology and the levels of serum amylase, TNF-α, and IL-6. Moreover, both the indicators of intestinal mucosa damage (D-lactic acid and DAO) and the levels of IEC apoptosis were elevated in the SAP group.
CaN, a serine/threonine phosphatase, can be defined as a central calcium-responsive signaling molecule that has a significant impact on immune responses. CaN contains two subunits, CnA (the large catalytic subunit) and CnB (the smaller regulatory subunit). As a result, the formation of a heterodimer determines the activity of CaN[29]. It is now well established based on a variety of studies that CaN is a significant contributing factor to the development of acute pancreatitis as a unique target of aberrant calcium signaling[30-32]. For example, bile acid-induced pancreatic injury, transient high ductal pressure-induced pancreatic inflammation, and pancreatic inflammation caused by radiocontrast agents are all dependent on CaN activation[31,33]. CaN can dephosphorylate several substrates; one of the most important of these substrates is NFATc[34]. CaN/NFAT signaling can accurately adjust gene expression to alter cell differentiation and development, dependent on its remarkable ability to sense Ca2+ oscillations in cells[35]. When phosphorylated, all NFAT members reside in the cytosol of resting cells. However, upon dephosphorylation in response to high intracellular levels of Ca2+, cytosolic CaN binds to NFAT, leading to nuclear translocation and transcription. The calcium-CaN-activated NFAT family contains four members (NFATc1, NFATc2, NFATc3, and NFATc4), among which the NFATc3 gene has been identified to be expressed in mammalian intestinal tissues[36]. The effects of NFATc3 in AP were described in a report by Awla et al[37]. Their results demonstrated that NFATc3 could adjust to trypsinogen activation, inflammation, and pancreatic tissue damage during the process of AP. Consequently, NFATc3 activity might be a therapeutic target[37]. In this study, we found that the protein expression levels of the two subunits of CaN were remarkably elevated in the SAP group. Furthermore, we examined NFATc3 expression using qRT-PCR and immunofluorescence analyses. The results suggested that the NFATc3 gene was significantly upregulated at both the transcript and protein levels in the SAP group compared with the control group.
The therapeutic effects and associated mechanisms of QYD may lie in clearing fever, detoxifying, soothing the liver, regulating the flow of vital energy and removing obstructions, invigorating the circulation of blood, dissipating blood stasis, and dredging urination and defecation, according to traditional Chinese medicine’s holistic thoughts[38]. QYD, as an organic combination of many effective components, plays a multitarget role in acute pancreatitis treatment through multiple pathways, including defending the intestinal barrier. It has been proven that QYD can suppress intestinal bacterial translocation, moderate inflammatory factor release, and prohibit intestinal mucosa destruction by inhibiting the expression of intestinal secreted phospholipase A2[39], suppressing the Toll-like receptor 4/nuclear factor-kappa B signaling pathway and stimulating Zona occludens 1 expression[9]. Serum pharmacology, first brought forward by the famous Japanese medical scientist Masakazu Tashiro, was introduced into the field of TCM medical research in 1997[40]. Serum pharmacology can broadly be defined as a new experimental method in which the target drug is administered orally to experimental animals, and then the serum is separated for in vitro pharmacological testing after a certain period[41]. Currently, rabbits and rats are mainly chosen for the preparation of drug-containing serum as a result of their biological characteristics similar to those of humans[42]. After investigation of many of the pharmacokinetic parameters of TCM, an optimal medication plan (medicating 2 times a day, 3 d to 5 d continuously, and exsanguination 1 h after the last medication) was proven feasible. Owing to better reflecting the metabolism of TCM in the human body, serum pharmacology can provide new directions for pharmacological analysis of TCM[43]. In this study, the severity of SAP in the QYD group was attenuated compared with that in the untreated SAP group. In addition, the levels of IEC apoptosis declined in the QYD group relative to the untreated group. QYD significantly alleviated the upregulation of CaN and NFATc3 gene expression in the intestine in the SAP group. Further study indicated that QYD serum significantly decreased the LPS-induced elevation of intracellular free Ca2+ levels and cell death.
In summary, the results of this research demonstrated that the CaN/NFATc3 pathway might play a key role in IEC injury caused by SAP and that QYD can exert protective effects. Moreover, QYD can regulate CaN and NFATc3 gene expression. The findings suggest that the protective effects of QYD might be mediated, at least partially, by restraining IEC apoptosis via the CaN/NFATc3 pathway.
Severe acute pancreatitis (SAP) is a severe acute abdominal disease characterized by high morbidity and mortality. A considerable amount of literature has demonstrated that intestinal barrier dysfunction is a significant contributory factor to SAP development. Qingyi decoction (QYD) has been used to treat acute pancreatitis in China for many years.
The protective functions of QYD against intestinal mucosa injuries caused by SAP will provide new therapeutic information on SAP.
To research the function and mechanism of QYD in treating intestinal mucosa injuries caused by SAP.
A rat model of SAP was created. Hematoxylin and eosin staining of pancreatic and intestinal tissue was performed. Enzyme-linked immunosorbent assay was used to estimate the concentrations of tumor necrosis factor (TNF-α), interleukin (IL)-6, D-lactic acid, and diamine oxidase (DAO). Terminal deoxynucleotidyl transferase dUTP nick-end labeling was carried out to assess intestinal epithelial cell (IEC) apoptosis. Quantitative real-time polymerase chain reaction, western blotting, and immunofluorescence were used to determine the expression of calcineurin (CaN) and nuclear factor of activated T-cells (NFATc3). MTT and confocal laser scanning microscope were used to detect cell viability and intracellular calcium levels in vitro studies.
In this study, the severity of SAP in the QYD group was attenuated. In addition, the levels of IEC apoptosis declined in the QYD group. QYD significantly restrained CaN and NFATc3 gene expression in the intestine. Further study indicated that QYD serum significantly decreased the lipopolysaccharide-induced elevation in intracellular free Ca2+ levels and cell death.
This research demonstrated that the CaN/NFATc3 pathway might play a key role in IEC injury caused by SAP and that QYD can exert protective effects, at least partially, by restraining IEC apoptosis via the CaN/NFATc3 pathway.
This study provides insight into the function and mechanism of QYD in the treatment of intestinal mucosa injuries caused by SAP in vivo and in vitro experiments, thereby providing theoretical support for the clinical application of QYD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France; Ma W, China; Surbatovic M, Serbia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879-13892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (5)] |
| 2. | Zhang JX, Dang SC, Yin K, Jiang DL. Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2011;10:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res. 2006;135:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Kishi S, Sawa H, Nakajima T, Kuroda Y. Protective effect of caspase inhibitor on intestinal integrity in experimental severe acute pancreatitis. J Surg Res. 2007;138:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109 (Pt 9):2287-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 454] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Sommerer C, Meuer S, Zeier M, Giese T. Calcineurin inhibitors and NFAT-regulated gene expression. Clin Chim Acta. 2012;413:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Jia Z, Chen Q, Qin H. Ischemia-induced apoptosis of intestinal epithelial cells correlates with altered integrin distribution and disassembly of F-actin triggered by calcium overload. J Biomed Biotechnol. 2012;2012:617539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Shati AA. Doxorubicin-induces NFAT/Fas/FasL cardiac apoptosis in rats through activation of calcineurin and P38 MAPK and inhibition of mTOR signalling pathways. Clin Exp PharmacolPhysiol. 2020;47:660-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 2015;21:3537-3546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Yang DY, Duan SB, Aili JT. [Effect of qingyi decoction in treating severe acute pancreatitis and its impacts on blood level of tumor necrosis factor-alpha, interleukin-6 and inteleukin-8]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:1122-1124. [PubMed] |
| 11. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg. 1970;101:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Wang G, Zhang J, Xu C, Han X, Gao Y, Chen H. Inhibition of SOCs Attenuates Acute Lung Injury Induced by Severe Acute Pancreatitis in Rats and PMVECs Injury Induced by Lipopolysaccharide. Inflammation. 2016;39:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Guo SG, Zhang W, Jiang T, Dai M, Zhang LF, Meng YC, Zhao LY, Niu JZ. Influence of serum collected from rat perfused with compound Biejiaruangan drug on hepatic stellate cells. World J Gastroenterol. 2004;10:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhang GX, Zhan C, Wang K, Han J, Shang D, Chen HI. Qingyi Decoction amerliorates acute biliary pancreatitis by targeting Gpbar1/NF-kb pathway. Front Biosci (Landmark Ed). 2019;24:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113 (Pt 10):1771-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 324] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Chen X, Zhao HX, Fu XS, Li CP, Zhong XL. Glucagonlike peptide 2 protects intestinal barrier in severe acute pancreatitis through regulating intestinal epithelial cell proliferation and apoptosis. Pancreas. 2012;41:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Wang X, Wang B, Wu K, Xu M, Gong Z. Growth hormone downregulated the excessive apoptosis of ileal intestinal epithelial cells in rats during the early course of acute necrotizing pancreatitis. Pancreas. 2002;25:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174-G1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. TNF-alpha as a therapeutic target in acute pancreatitis--lessons from experimental models. ScientificWorldJournal. 2007;7:431-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Wendling D, Prati C. Paradoxical effects of anti-TNF-α agents in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Shen Y, Cui N, Miao B, Zhao E. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci. 2009;54:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 25. | Hansen M, Nielsen AR, Vilsbøll T, Lund A, Krarup T, Knop FK, Vestergaard H. Increased levels of YKL-40 and interleukin 6 in patients with chronic pancreatitis and secondary diabetes. Pancreas. 2012;41:1316-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Thompson JS, Vaughan WP, Forst CF, Jacobs DL, Weekly JS, Rikkers LF. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. JPEN J Parenter Enteral Nutr. 1987;11:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Bragg LE, Thompson JS, West WW. Intestinal diamine oxidase levels reflect ischemic injury. J Surg Res. 1991;50:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Smith SM, Eng RH, Buccini F. Use of D-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis. 1986;154:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 29. | Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1056] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 30. | Orabi AI, Wen L, Javed TA, Le T, Guo P, Sanker S, Ricks D, Boggs K, Eisses JF, Castro C, Xiao X, Prasadan K, Esni F, Gittes GK, Husain SZ. Targeted inhibition of pancreatic acinar cell calcineurin is a novel strategy to prevent post-ERCP pancreatitis. Cell Mol Gastroenterol Hepatol. 2017;3:119-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Muili KA, Wang D, Orabi AI, Sarwar S, Luo Y, Javed TA, Eisses JF, Mahmood SM, Jin S, Singh VP, Ananthanaravanan M, Perides G, Williams JA, Molkentin JD, Husain SZ. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2013;288:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Shah AU, Liu J, Mistry PK, Jain D, Husain SZ. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G967-G973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Wen L, Javed TA, Yimlamai D, Mukherjee A, Xiao X, Husain SZ. Transient High Pressure in Pancreatic Ducts Promotes Inflammation and Alters Tight Junctions via Calcineurin Signaling in Mice. Gastroenterology. 2018;155:1250-1263.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1192] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 35. | Müller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 36. | Angulo C, Alamillo E, Ascencio F, Reyes-Becerril M. Characterization of nuclear factor of activated T-cells-c3 (NFATc3) and gene expression of upstream-downstream signaling molecules in response to immunostimulants in Pacific red snapper cells. Dev Comp Immunol. 2018;78:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Awla D, Zetterqvist AV, Abdulla A, Camello C, Berglund LM, Spégel P, Pozo MJ, Camello PJ, Regnér S, Gomez MF, Thorlacius H. NFATc3 regulates trypsinogen activation, neutrophil recruitment, and tissue damage in acute pancreatitis in mice. Gastroenterology. 2012;143:1352-1360.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | HanSL. Efficacy of Qingyi decoction in the treatment of severe acute pancreatitis: systematic review and meta-analysis. J Pract Med. 2008;. |
| 39. | Su S, Liang T, Zhou X, He K, Li B, Xia X. Qingyi decoction attenuates severe acute pancreatitis in rats via inhibition of inflammation and protection of the intestinal barrier. J Int Med Res. 2019;47:2215-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Zhang WX, Feng M, Mao YL, Zhang WZ, Ni Y. Review on the Application of Serum Pharmacochemistry of Traditional Chinese Medicine. Drug Evaluation Res. 2019;42:1448-1453. |
| 41. | Wu XW, Hao YY, Nie CX, Ni Y, Hao XL, Liu C. An overview of methodology and research progress on application of serum pharmacochemistry of traditional Chinese medicine. Chin J Exp Tradit Med Form. 2019;24:173-179. |
| 42. | Tao S. Research on rapid discovery method of Chinese medicine effective substances based on chemometrics and immobilized enzyme. Zhejiang University, 2015. |
| 43. | Chen Q, Huang L, Wang R, Pang C. Research Status of Serum Pharmacology and Serum Pharmacochemistry of Traditional Chinese Medicine. Agric Biotechnol. 2021;10:126-128. |