Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3732
Peer-review started: April 7, 2022
First decision: May 9, 2022
Revised: May 21, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: July 28, 2022
Processing time: 110 Days and 14.4 Hours
Secondary sclerosing cholangitis, characterized by biliary obstruction, can be caused by drugs such as immune checkpoint inhibitors (ICIs). While there a few reports of sclerosing cholangitis after immune checkpoint inhibitor administration, no case has been reported after discontinuation of such drugs.
A 68-year-old man who underwent chemotherapy for lung adenocarcinoma with bone metastasis presented with abdominal pain and fever 4 mo after the final administration of pembrolizumab. Computed tomography revealed thickening of the gallbladder wall and dilatation of the common bile duct. Endoscopic retro-grade cholangiopancreatography revealed an irregularly narrowed intrahepatic bile duct. Biopsy of the bile duct demonstrated that CD8+ T cells were predominant over CD4+ T cells. Liver biopsy showed dominant infiltration of CD8+ T in the portal tract, but onion-skin lesions were not observed. The patient was diagnosed with immune-related sclerosing cholangitis induced by pembrolizumab. Administration of methylprednisolone and endoscopic nasobiliary drainage were performed, but the cholangiography and laboratory test findings did not improve. No further treatment was administered due to disease progression, and the patient was referred for palliative care.
Immune-related sclerosing cholangitis may have a late onset, and such cases occurring after discontinuation of ICIs should be carefully managed.
Core Tip: Immune checkpoint inhibitors have become a new standard in cancer treatment, but have often been reported to induce adverse events, called immune-related adverse events (irAEs). Biliary system complications, such as irAEs, remain rare, and the management strategy remains unclear. We present herein, a rare case of delayed immune-related sclerosing cholangitis (SC) after discontinuation of pembrolizumab. Our case emphasizes that immune-related SC can occur later, but the mechanisms have not yet been elucidated. As such, our case contributes to new knowledge in the hopes of being able to establish proper diagnostic criteria and management strategies for similar patients.
- Citation: Tanaka T, Sakai A, Tsujimae M, Yamada Y, Kobayashi T, Masuda A, Kodama Y. Delayed immune-related sclerosing cholangitis after discontinuation of pembrolizumab: A case report. World J Gastroenterol 2022; 28(28): 3732-3738
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3732
Secondary sclerosing cholangitis (SC) is a chronic disease characterized by biliary obstruction and can be caused by a variety of specific etiologies such as infections, immune-related factors, toxicity, obstruction, ischemic injury, or drugs, including immune checkpoint inhibitors (ICIs)[1]. ICIs have transformed the treatment landscape for patients with many advanced malignancies but have often been reported to induce adverse events, called immune-related adverse events (irAEs)[2]. The irAEs differ from toxicities caused by cytotoxic or molecularly targeted agents, and the time to toxicity may be delayed and not follow a cyclical pattern, as observed with conventional cytotoxic agents[3]. Biliary system complications, such as irAEs, remain rare, and the management strategy remains unclear. There are a few reports of SC induced by ICIs; however, no cases developed immune-related SC after discontinuation of ICIs. Herein, we report a case of delayed immune-related SC caused by pembrolizumab.
A 68-year-old male patient was admitted to our hospital with abdominal pain and fever.
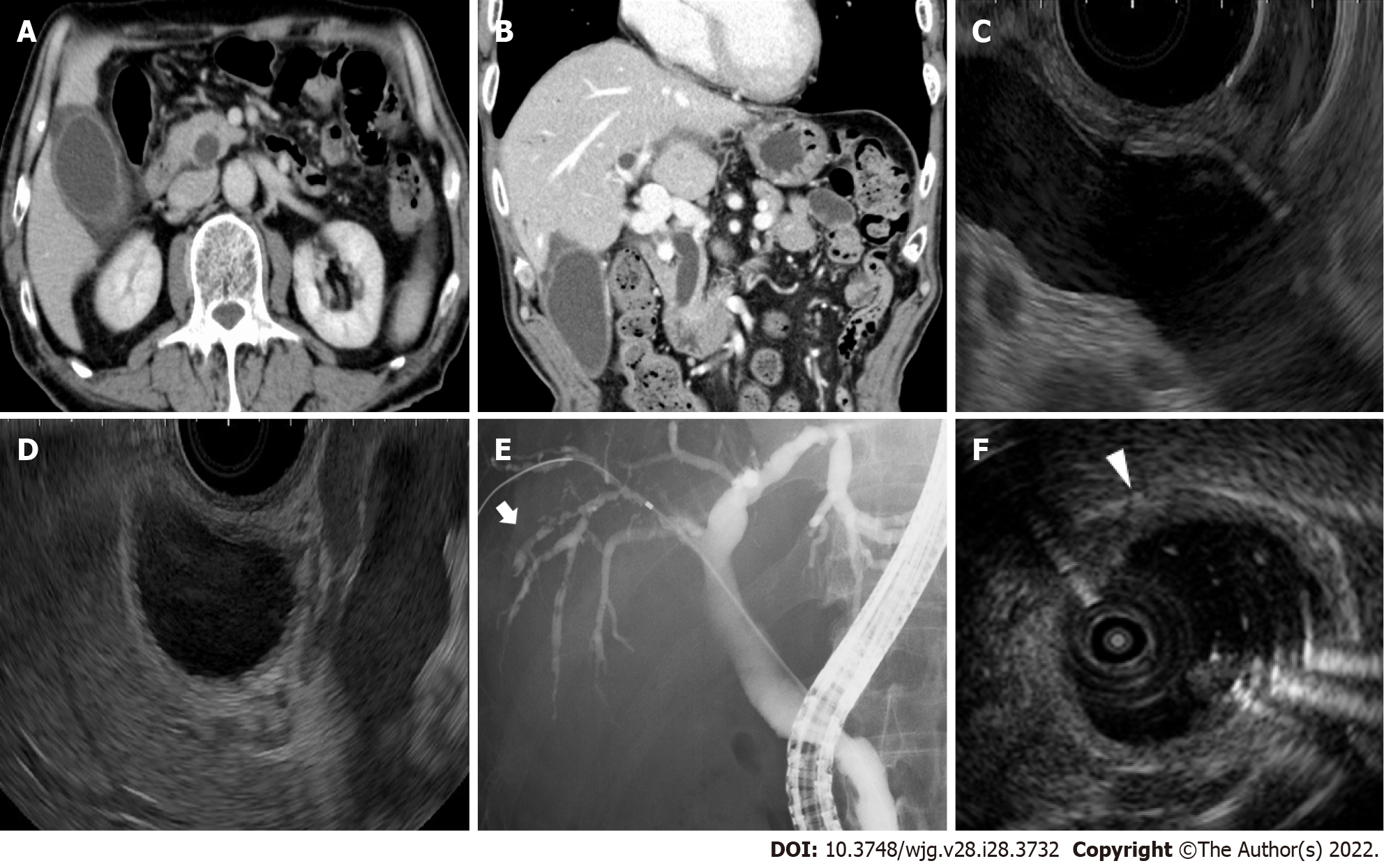
Contrast-enhanced computed tomography and endoscopic ultrasonography revealed swelling and wall thickening of the gallbladder and dilatation of the common bile duct without obstruction (Figure 1A-D). Endoscopic retrograde cholangiopancreatography revealed a dilated common bile duct and irregularly narrowed right intrahepatic bile duct (Figure 1E). Intraductal ultrasonography demonstrated diffuse wall thickening from the right bile duct to the common bile duct (Figure 1F).
Laboratory tests revealed increased levels of white blood cells [10100/μL (3300-8600/μL)], C-reactive protein [4.88 mg/dL (0.00-0.18 mg/dL)], aspartate transaminase [31 U/L (13–30 U/L)], alanine transaminase (ALT) [50 U/L (7–23 U/L)], gamma-glutamyl transpeptidase [205 U/L (13-64 U/L)], and alkaline phosphatase (ALP) [996 U/L (38-113 U/L)]. Total bilirubin was within the normal range [0.7 mg/dL (0.4-1.5 mg/dL)], and blood cultures showed no bacterial infection. The serum immunological markers, including antinuclear antibody, antimitochondrial antibody, and anti-smooth muscle antibody were negative, and the immunoglobulin G4 Level (59 mg/dL) was within the normal range.
The patient's body temperature was 38.9 °C, but the other vital signs were stable. Regarding the pulmonary and cardiac examination, no obvious abnormality was observed. Physical examination revealed epigastric tenderness without rebound tenderness or Murphy’s sign. No jaundice or palpable masses were observed.
No information was available regarding his family history.
The patient has laparoscopic inguinal hernia repair twenty years ago.
The patient was diagnosed with stage IV lung adenocarcinoma (wild-type epidermal growth factor receptor and anaplastic lymphoma kinase, programmed death ligand 1 positive expression) and received first-line treatment with cisplatin and pemetrexed sodium hydrate. Four months after the initiation of first-line chemotherapy, the patient received second-line treatment with pembrolizumab because of disease progression. Three months later (three cycles of pembrolizumab), carboplatin and nab-paclitaxel were administered as third-line treatments. Four months after discontinuation of pembrolizumab (3 mo after initiation of third-line chemotherapy), the patient was admitted to our hospital with abdominal pain and fever.
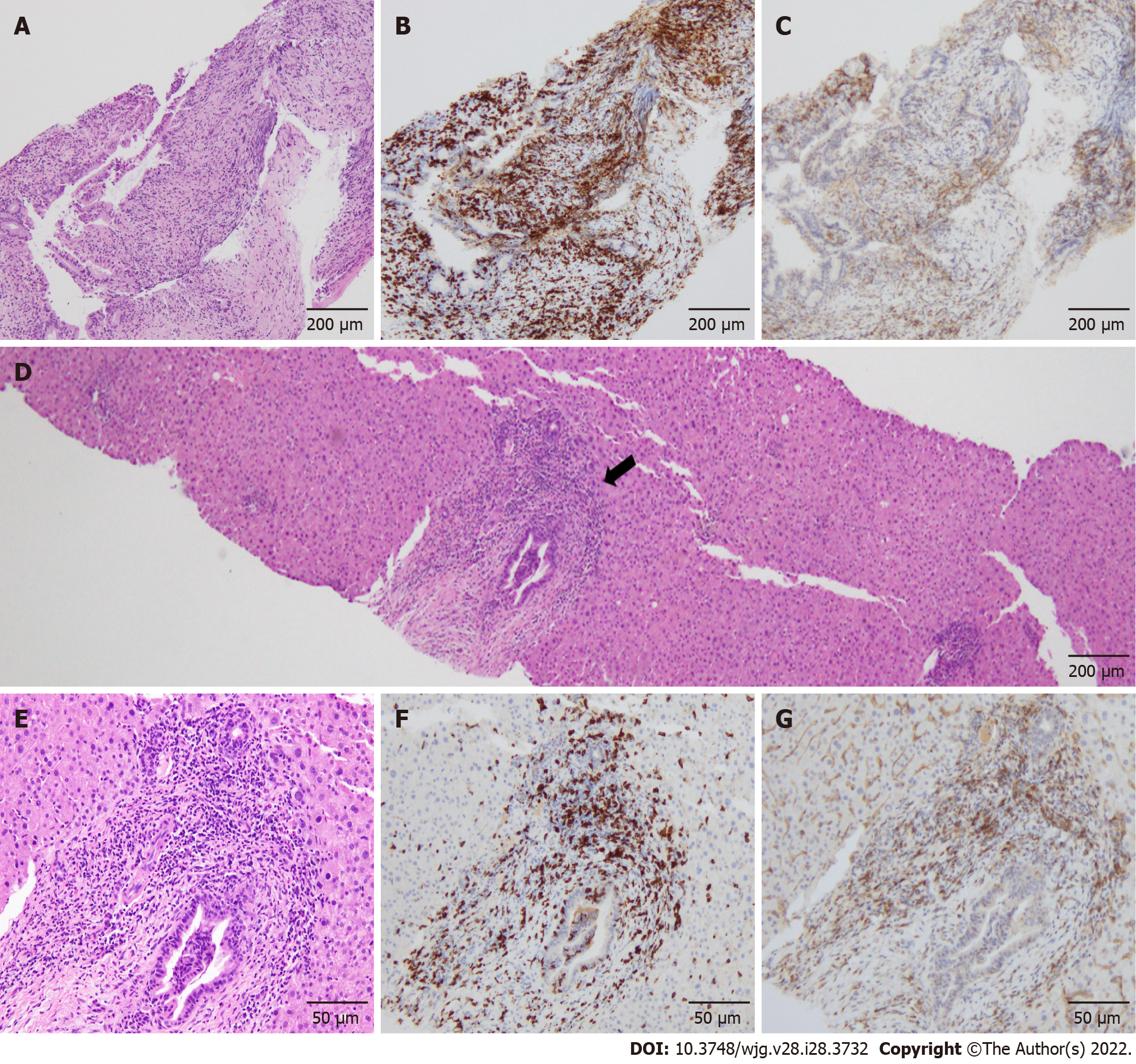
The common bile duct biopsy showed intraepithelial infiltration of lymphocytes, and CD8+ T cells were more predominant in the biliary epithelium than CD4+ T cells (Figure 2A-C). Percutaneous ultrasonography-guided liver biopsy was also performed for differential diagnosis of other liver diseases. The liver biopsy showed infiltration of predominantly CD8+ T cells in the portal area, but the periductal "onion-skin" fibrosis characteristic of primary sclerosing cholangiopathies, and panlobular hepatitis or isolated central zonal necrosis characteristic of acute hepatitis were not detected (Figure 2D-G). According to these imaging and pathological findings, the patient was diagnosed with immune-related SC induced by pembrolizumab.
Endoscopic nasobiliary drainage was performed and administration of 25 mg (0.5 mg/kg) of methylprednisolone was initiated. However, grade 3 elevation of ALP and grade 1 elevation of ALT were still present, and the cholangiography findings did not improve.
Because of disease progression, no further treatment was administered, and the patient was referred for palliative care.
ICIs enhance antitumor immunity by blocking negative regulators of T cell function that exist on both immune and tumor cells. Although these agents can lead to remarkable responses, their use may also be associated with irAEs. A previous study showed that most irAEs occur in the first few months of treatment, but late-onset toxicity even after ICI discontinuation is also possible[4]. To the best of our knowledge, no case of immune-related SC after pembrolizumab discontinuation has been reported.
Kawakami et al[5] revealed that immune-related cholangitis was characterized by: (1) Localized extrahepatic bile duct dilation without obstruction; (2) Diffuse hypertrophy of the extrahepatic bile duct wall; (3) A dominant increase in the biliary tract enzymes ALP and gamma-glutamyl transpeptidase relative to the hepatic enzymes aspartate and alanine aminotransferase; (4) Normal or reduced levels of the serum immunological markers antinuclear antibody, antimitochondrial antibody, smooth muscle antibody, and immunoglobulin G4; (5) Pathological findings of biliary tract cluster of differentiation CD8+ T cell infiltration from liver biopsy; and (6) A moderate to poor response to steroid therapy. The present case met all these characteristics of immune-related cholangitis.
In contrast, SC developed while carboplatin and nab-paclitaxel were administered. Previous studies reported that chemotherapy-induced SC and drug-induced SC appeared to be one or more strictures of the large bile ducts, mainly the common hepatic duct and the right and left hepatic ducts, usually sparing the common bile duct and smaller intrahepatic ducts[6,7]. Histologically, Weber et al[8] showed that a shift in the CD4+/CD8+ cell ratio in favor of CD8+ cytotoxic T lymphocytes is useful in discriminating irAEs from other conditions (e.g., autoimmune diseases and drug-induced injury). In our case, an irregularly narrowed right intrahepatic bile duct and infiltration of predominantly CD8+ T cells were observed. Therefore, we diagnosed the patient with SC induced by pembrolizumab on the basis of the imaging and pathological findings of previous reports.
Recently, new reports of irAEs emerging after discontinuation of immunotherapy, a clinical diagnostic complex termed delayed immune-related events (DIRE), have surfaced[9]. The first case of DIRE after discontinuation of pembrolizumab was reported in 2013[10], and various late-onset irAEs have also been reported in the literature[11]. Anti-PD-1 antibodies continue to bind to lymphocytes several weeks after the last infusion of PD-1 inhibitors, which explains the development of irAEs after discontinuation of ICI therapy[12]. Although there have been some reports of DIRE after discontinuation of pembrolizumab, no cases of delayed immune-related SC induced by pembrolizumab have been reported.
Previous studies showed that steroid therapy was recommended for the treatment of irAEs, but was not useful for the treatment of immune-related SC[4,13]. Ursodeoxycholic acid (UDCA) and other anti-inflammatory agents, including immunomodulators and infliximab, have been considered for the treatment of irAEs, but their efficacy and response have been reported to be insufficient for the treatment of immune-related SC[14,15], and more cases may be needed to evaluate the usefulness of these drugs for immune-related SC. Biliary drainage has been reported to be ineffective for immune-related SCs[5,16,17]. It remains uncertain why there is a poor response to steroid therapy and biliary drainage for immune-related SC, and further studies are necessary to establish the treatment for immune-related SC.
This indicates that immune-related SC can occur later, similar to other hepatic irAEs. The imaging features and pathological findings of immune-related SC have become almost clear, but the mechanism and onset time of immune-related SC have not been elucidated. Further studies and accumulation of cases are necessary to establish appropriate diagnostic criteria and management strategies, and caution should be exercised for late-onset irAEs after discontinuation of ICIs.
Immune-related sclerosing cholangitis may have a late onset, and such cases occurring after discontinuation of immune checkpoint inhibitors should be carefully managed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YH, China; Martin M S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Brooling J, Leal R. Secondary Sclerosing Cholangitis: a Review of Recent Literature. Curr Gastroenterol Rep. 2017;19:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 478] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 3. | Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 645] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 4. | de La Rochefoucauld J, Noël N, Lambotte O. Management of immune-related adverse events associated with immune checkpoint inhibitors in cancer patients: a patient-centred approach. Intern Emerg Med. 2020;15:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, Kamata K, Takenaka M, Kimura M, Chikugo T, Sato T, Kudo M, Ito A, Nakagawa K. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs. 2017;35:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Sandrasegaran K, Alazmi WM, Tann M, Fogel EL, McHenry L, Lehman GA. Chemotherapy-induced sclerosing cholangitis. Clin Radiol. 2006;61:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 523] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 9. | Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD, Urba WJ, Leidner RS. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Hanrahan P, Van Der Westhuizen A, Collins S, Owens D, Hersey P. Delayed autoimmune effects in patients treated with antibodies against the checkpoint inhibitor PD1. J Dtsch Dermatol Ges. 2013;11:16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kanaoka K, Moriizumi K, Okada H, Iwahashi K, Tsuji H, Yasuoka H, Minami S. Pembrolizumab-Induced Delayed-Onset Hepatitis. Case Rep Gastroenterol. 2020;14:586-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, Hirata H, Yano Y, Yokota S, Kinehara Y, Naito Y, Otsuka T, Kanazu M, Kuroyama M, Hamaguchi M, Koba T, Futami Y, Ishijima M, Suga Y, Akazawa Y, Machiyama H, Iwahori K, Takamatsu H, Nagatomo I, Takeda Y, Kida H, Akbay EA, Hammerman PS, Wong KK, Dranoff G, Mori M, Kijima T, Kumanogoh A. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 13. | Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, Kawata S, Matsumoto K, Isomoto H. Programmed cell death-1 inhibitor-related sclerosing cholangitis: A systematic review. World J Gastroenterol. 2020;26:353-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 14. | Zhu GQ, Shi KQ, Huang GQ, Wang LR, Lin YQ, Braddock M, Chen YP, Zhou MT, Zheng MH. A network meta-analysis of the efficacy and side effects of UDCA-based therapies for primary sclerosing cholangitis. Oncotarget. 2015;6:26757-26769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1421] [Article Influence: 177.6] [Reference Citation Analysis (0)] |
| 16. | Kashima J, Okuma Y, Shimizuguchi R, Chiba K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother. 2018;67:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 17. | Koya Y, Shibata M, Shinohara N, Nebuya S, Oe S, Honma Y, Senju M, Sato N, Harada M. Secondary sclerosing cholangitis with hemobilia induced by pembrolizumab: Case report and review of published work. Hepatol Res. 2019;49:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |