Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3637
Peer-review started: April 1, 2022
First decision: May 9, 2022
Revised: June 6, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: July 28, 2022
Processing time: 116 Days and 18.2 Hours
Pancreatic cancer is highly aggressive and lethal. Due to the lack of effective methods for detecting the disease at an early stage, pancreatic cancer is frequently diagnosed late. Gemcitabine has been the standard chemotherapy drug for patients with pancreatic cancer for over 20 years, but its anti-tumor effect is limited. Therefore, FOLFIRINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) as well as combination therapies using gemcitabine and conventional agents, such as cisplatin and capecitabine, has also been administered; however, these have not resulted in complete remission. Therefore, there is a need to develop novel and effective therapies for pancreatic cancer. Recently, some studies have reported that combinations of gemcitabine and targeted drugs have had significant anti-tumor effects on pancreatic cancer cells. As gemcitabine induced DNA damage response, the proteins related to DNA damage response can be suitable additional targets for novel gemcitabine-based combination therapy. Furthermore, KRAS/ RAF/MEK/ERK signaling triggered by oncogenic mutated KRAS and autophagy are frequently activated in pancreatic cancer. Therefore, these characteristics of pancreatic cancer are potential targets for developing effective novel therapies.
In this minireview, combinations of gemcitabine and targeted drugs to these characteristics, combinations of targeted drugs, combinations of natural products and anti-cancer agents, including gemcitabine, and combinations among natural products are discussed.
Core Tip: Gemcitabine has been the standard chemotherapy drug for patients with pancreatic cancer; however, its effectiveness is limited. Therefore, various combination therapies involving gemcitabine and targeted drugs are being explored. A review of combination therapies based mainly on clinical studies has been published recently; therefore, this minireview focuses on the findings of basic studies and discusses combinations of gemcitabine and targeted drugs, combinations of targeted drugs, combinations of natural products and anti-cancer agents, including gemcitabine, and combinations among natural products.
- Citation: Nishimoto A. Effective combinations of anti-cancer and targeted drugs for pancreatic cancer treatment. World J Gastroenterol 2022; 28(28): 3637-3643
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3637.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3637
Pancreatic cancer is fatal and has a 5-year survival rate of approximate 10%[1]. It is estimated that pancreatic cancer will become the second most common cause of cancer-related deaths in the United States by 2030[2]. Pancreatic cancer is frequently diagnosed at a late stage owing to the lack of effective methods for detecting it at earlier stages and non-specific symptoms. Therefore, there is an urgent need to develop both novel effective therapies for pancreatic cancer that has progressed to a late stage and effective methods for detecting pancreatic cancer at an early stage.
Gemcitabine is the standard treatment for patients with pancreatic cancer. However, as the anti-tumor effect of gemcitabine is limited, FOLFIRINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) as well as combination therapies of gemcitabine and conventional agents, such as cisplatin and capecitabine, has also been administered[3-5]. Although these combination therapies improved overall survival compared to gemcitabine alone, they did not achieve complete remission. In addition, the incidence of toxicity associated with these combination therapies has increased[3-6].
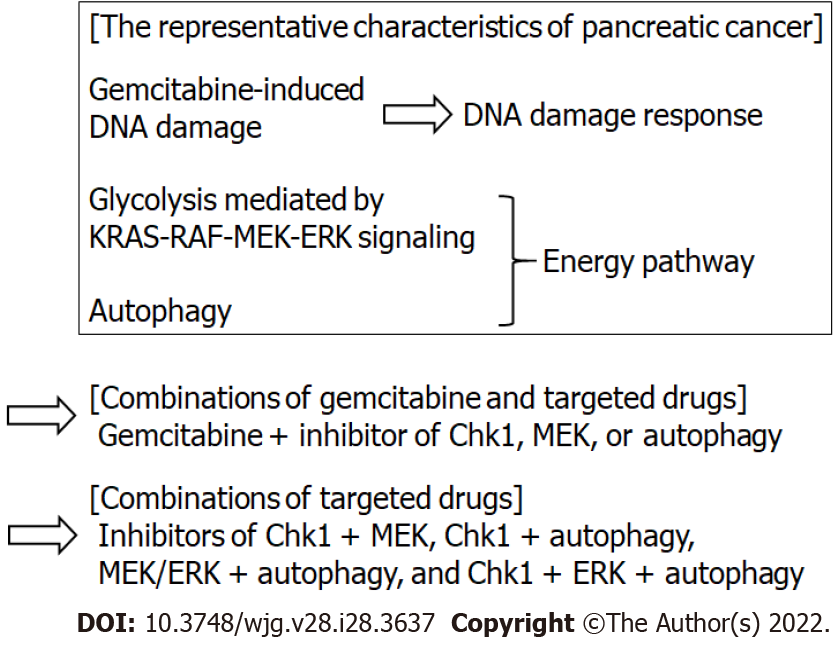
Recent studies have attempted to identify effective combinations of gemcitabine and targeted drugs for pancreatic cancer. As gemcitabine, a well-known DNA-damaging agent, induced DNA damage response, the proteins related to DNA damage response can be suitable additional targets for novel gemcitabine-based combination therapy. Furthermore, KRAS/RAF/MEK/ERK signaling triggered by oncogenic mutated KRAS and autophagy, which are described as the characteristics of pancreatic ductal adenocarcinoma (PDAC), are frequently activated. Therefore, these characteristics are promising targets for effective novel therapeutic strategies[7-12]. An excellent review on effective combination therapies for pancreatic cancer was published recently[13]. This review was based mainly on the findings of preclinical and clinical studies[13]; therefore, this minireview focuses on the findings of basic studies and discusses combinations of gemcitabine and targeted drugs, combinations of targeted drugs, combinations of natural products and anti-cancer agents, including gemcitabine, and combinations among natural products.
Gemcitabine, a well-known DNA-damaging agent, has been the standard first-line drug for patients with pancreatic cancer. However, the efficacy of gemcitabine in pancreatic cancer is limited and a novel gemcitabine-based combination therapy is required. In this section and Figure 1, combinations of gemcitabine and targeted drugs to enhance the anti-tumor effect are summarized.
Quinone-methide triterpenoid pristimerin induces lysosomal degradation of checkpoint kinase 1 (Chk1) and augments the expression of γ-H2AX, which is a biomarker of DNA damage following gemcitabine treatment[14]. Furthermore, the combination of gemcitabine and pristimerin was shown to increase apoptosis of pancreatic cancer cells[14]. The Chk1 inhibitor MK-8776 also enhances the sensitivity of multiple human cancer cell lines, including pancreatic cancer cells, to gemcitabine[15]. The DNA damage response mediated by Chk1 in pancreatic cancer stem cells was greater than that in non-pancreatic cancer stem cells, indicating that Chk1 inhibition selectively sensitizes pancreatic cancer stem cells to gemcitabine[16]. The combination of Chk1 inhibition and gemcitabine reduces the ability of tumor initiation in pancreatic cancer stem cells[16]. The anti-tumor effect of the combination of gemcitabine and doublecortin-like kinase 1 (Dclk1) inhibitor has also been reported[17]. The latter significantly decreased the expression of gemcitabine-induced phosphorylated Chk1 in pancreatic cancer cells. The combination of gemcitabine and Dclk1 inhibitor did not arrest the cell cycle at the S phase and allowed cell cycle progression[17]. In addition, the combination of gemcitabine and Dclk1 inhibitor increased the rate of γ-H2AX-positive cells compared to individual treatments. The combination of gemcitabine and Dclk1 inhibitor induced PARP1 cleavage as well as caspase-3 activation and significantly decreased the survival rate of pancreatic cancer cells compared to gemcitabine treatment alone[17].
Oncogenic KRAS mutations are present in approximately 90% of pancreatic cancer cases. Consequently, KRAS and its downstream proteins, such as RAF, MEK, and ERK, are activated in pancreatic cancer cases and contribute to the progression of the disease. Therefore, inhibitors targeting these proteins may be effective in inhibiting this progression.
Antibodies that bind intracellularly to the activated GTP-bound form of oncogenic KRAS mutants have been developed and significantly sensitize pancreatic cancer cells to gemcitabine[18,19]. These antibodies synergistically increase the anti-tumor effect of gemcitabine by inhibiting the RAF/MEK/ERK signaling pathway downstream of KRAS[18,19]. The antibodies are internalized in the cytoplasm by endocytosis through the tumor-associated receptors of extracellular epithelial cell adhesion molecules[19]. These antibodies synergistically increase the anti-tumor effect of gemcitabine by inhibiting KRAS/RAF/MEK/ERK signaling in pancreatic cancer cells[18,19].
The MEK inhibitor, trametinib, both alone and in combination with gemcitabine, was shown to exhibit significantly enhanced anti-tumor effects compared to gemcitabine alone[20]. The combination of gemcitabine and trametinib also increased the inhibition of tumor growth in pancreatic cancer patient-derived orthotopic xenografts in nude mice compared to trametinib alone[20]. Moreover, the combination of the MEK inhibitors, trametinib and cobimetinib, prevented tumor growth in gemcitabine-resistant pancreatic cancer patient-derived orthotopic xenografts in nude mice[21]. These results suggest that such combinations have therapeutic potential against pancreatic cancer.
Gemcitabine has significantly been shown to increase autophagy induction in human pancreatic cancer cells, and combined treatment with gemcitabine and chloroquine, an autophagy inhibitor, triggered a marked boost in reactive oxygen species (ROS) levels and increased lysosomal membrane permeability[22]. Consequently, proteases, including cathepsins, are released from lysosomes into the cytoplasm, leading to apoptosis. Thus, the combination of gemcitabine and chloroquine has an anti-tumor effect on pancreatic cancer cells through the apoptotic pathway by lysosomal dysfunction via a marked boost of ROS[22]. Cancer stem cells are considered to be responsible for the recurrence and chemoresistance of cancer. The expression of the markers of cancer stem cells, aldehyde dehydrogenase 1, CD44, and CD133, was found to be positively correlated with the expression of LC3 type II, an autophagy marker, in pancreatic cancer tissues[23]; this suggests an association between autophagy and cancer stem cells. Indeed, autophagy inhibition decreased the activity of sphere formation of pancreatic cancer stem cells, and gemcitabine and autophagy inhibition markedly reduced the populations of cancer stem cells[23].
Recent studies have demonstrated that combinations of targeted drugs have potential for developing novel and effective therapy for pancreatic cancer. In this section and Figure 1, combinations of targeted drugs for PDAC therapy are summarized.
KRAS suppression or ERK inhibition was shown to decrease both glycolytic and mitochondrial functions and to increase autophagic flux in PDAC, suggesting that ERK inhibition enhances dependence on autophagy[24]. The combination of ERK and autophagy inhibitors synergistically enhanced anti-tumor activity in KRAS-driven PDAC via the dysfunction of the energy pathways consisting of glycolysis and autophagy[24]. It has been reported that inhibition of the KRAS/ RAF/MEK/ERK signaling pathway elicits autophagy, resulting in protection of PDAC cells from cytotoxic effects[25]. The combination of MEK1/2 and autophagy inhibitors showed synergistic anti-tumor effects against PDAC cells in vitro and promoted the regression of patient-derived xenografts of PDAC in mice[25]. Furthermore, the effect of the combination of trametinib and chloroquine was not limited to PDAC and resulted in similar responses in patient-derived xenografts of BRAF-mutated colorectal cancer and NRAS-mutated melanoma[25].
Screening of druggable genes by genetic loss-of-function using CRISPR-Cas9 and small interfering RNA revealed that components of the ATR-Chk1 DNA damage response pathway were modulators of sensitivity to ERK inhibitor treatment in KRAS-mutant PDAC[26]. Chk1 inhibition suppressed the growth of both PDAC cell lines and organoids and activated ERK signaling and autophagy, suggesting that Chk1 inhibition enhances dependence on ERK signaling and autophagy[26]. These findings provide a mechanistic basis for the effectiveness of the inhibition of Chk1, ERK, and autophagy. Indeed, this triple combination of inhibitors synergistically enhanced the anti-tumor effect in KRAS-mutant PDAC[26].
Pooled shRNA library screening was used in an orthotopic xenograft model to identify multiple glycolysis genes as potential targets that may sensitize PDAC cells to MEK inhibition[27]. Apoptosis in KrasG12D-driven PDAC cells was synergistically induced, in vitro, via the combination of 2-deoxyglucose, a glycolysis inhibitor, and a MEK inhibitor; the same also inhibited tumor growth of PDAC xenografts, leading to prolonged overall survival in a genetically engineered PDAC mouse model[27]. Molecular and metabolic analyses revealed that the combined inhibition of glycolysis and ERK signaling synergistically caused apoptosis by inducing lethal stress in the endoplasmic reticulum. These results indicate that the combination of 2-deoxyglucose and a MEK inhibitor may be an effective approach for targeting KRAS-driven PDAC[27].
PDAC exhibits high basal lysosomal activity and relies on lysosome-dependent recycling pathways, such as autophagy, to generate substrates for metabolism[28]. Kinase inhibitor screening revealed that the replication stress response inhibitor and chloroquine, an autophagy inhibitor that works via the functional inhibition of lysosomes, were synthetically lethal in PDAC cells[28]. Chloroquine induces replication stress due to aspartate depletion, and a replication stress response inhibitor and chloroquine synergistically inhibit tumor growth in PDAC[28].
Major histocompatibility complex class I (MHC-I) molecules are selectively degraded via autophagy in PDAC[29]. Consequently, the expression of MHC-I at the cell surface is reduced, and MHC-I is localized predominantly within autophagosomes and lysosomes. Autophagy inhibition was shown to restore the expression of MHC-I at the cell surface and improve antigen presentation[29]. Furthermore, autophagy inhibition synergizes with dual immune checkpoint blockade therapy (anti-PD1 and anti-CTLA4 antibodies) to enhance the anti-tumor immune response and reduce tumor growth in syngeneic host mice[29].
The use of natural products as adjunctive therapies for pancreatic cancer has a great potential due to the anti-cancer efficacy and low toxicity. Yue et al[30] summarized combinations of natural products and anti-cancer agents, including gemcitabine, and combinations among natural products. They focused on the following: combinations of natural products and gemcitabine (for example, cucurbitacin B and gemcitabine[31], glaucarubinone and gemcitabine[32], escin and gemcitabine[33], and gum mastic and gemcitabine[34]); combinations of natural products and other agents, such as all-trans retinoic acid and sulindac (for example, 12-O-tetradecanoylphorbol-13-acetate and all-trans retinoic acid[35], parthenolide and sulindac[36], and triptolide and hydroxycamptothecin[37]); and combinations among natural products (for example, sulforaphane and quercetin[38], wogonin, apigenin, and chrysin[39], and metformin and aspirin[40]).
While agents from purified chemical compounds generally target single molecules, natural products mostly consist of multiple components that concurrently act on various molecular targets. Therefore, natural products are expected to have various functions, including improvement of anti-cancer efficacy, enhancement of immune system, and reduction of side effects[41].
In general, it is widely accepted that combination therapy is more effective than monotherapy. In this minireview, combinations of gemcitabine and targeted drugs, combinations of targeted drugs, combinations of natural products and anti-cancer agents, including gemcitabine, and combinations among natural products are described. Hereafter, preclinical and clinical studies are needed to examine the possibility for clinical applications. Concurrently, additional basic studies that attempt to identify combinations that synergize anti-cancer effects are needed to find the better treatment options.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen H, China; lee Y, South Korea S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11347] [Article Influence: 3782.3] [Reference Citation Analysis (4)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5115] [Article Influence: 465.0] [Reference Citation Analysis (0)] |
| 3. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1389] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 4. | Ouyang G, Liu Z, Huang S, Li Q, Xiong L, Miao X, Wen Y. Gemcitabine plus cisplatin versus gemcitabine alone in the treatment of pancreatic cancer: a meta-analysis. World J Surg Oncol. 2016;14:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5609] [Article Influence: 400.6] [Reference Citation Analysis (1)] |
| 6. | Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill S, Ruan JY, Cheung WY. Eligibility of Metastatic Pancreatic Cancer Patients for First-Line Palliative Intent nab-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am J Clin Oncol. 2017;40:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 592] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 8. | Bannoura SF, Uddin MH, Nagasaka M, Fazili F, Al-Hallak MN, Philip PA, El-Rayes B, Azmi AS. Targeting KRAS in pancreatic cancer: new drugs on the horizon. Cancer Metastasis Rev. 2021;40:819-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Mann KM, Ying H, Juan J, Jenkins NA, Copeland NG. KRAS-related proteins in pancreatic cancer. Pharmacol Ther. 2016;168:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Raufi AG, Liguori NR, Carlsen L, Parker C, Hernandez Borrero L, Zhang S, Tian X, Louie A, Zhou L, Seyhan AA, El-Deiry WS. Therapeutic Targeting of Autophagy in Pancreatic Ductal Adenocarcinoma. Front Pharmacol. 2021;12:751568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Piffoux M, Eriau E, Cassier PA. Autophagy as a therapeutic target in pancreatic cancer. Br J Cancer. 2021;124:333-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Miller AL, Garcia PL, Yoon KJ. Developing effective combination therapy for pancreatic cancer: An overview. Pharmacol Res. 2020;155:104740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Jiang Z, Zhao Y, Liu Y, Tao L. Pristimerin synergizes with gemcitabine through abrogating Chk1/53BP1-mediated DNA repair in pancreatic cancer cells. Food Chem Toxicol. 2021;147:111919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Engelke CG, Parsels LA, Qian Y, Zhang Q, Karnak D, Robertson JR, Tanska DM, Wei D, Davis MA, Parsels JD, Zhao L, Greenson JK, Lawrence TS, Maybaum J, Morgan MA. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res. 2013;19:4412-4421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Venkatesha VA, Parsels LA, Parsels JD, Zhao L, Zabludoff SD, Simeone DM, Maybaum J, Lawrence TS, Morgan MA. Sensitization of pancreatic cancer stem cells to gemcitabine by Chk1 inhibition. Neoplasia. 2012;14:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Kawamura D, Takemoto Y, Nishimoto A, Ueno K, Hosoyama T, Shirasawa B, Tanaka T, Kugimiya N, Harada E, Hamano K. Enhancement of cytotoxic effects of gemcitabine by Dclk1 inhibition through suppression of Chk1 phosphorylation in human pancreatic cancer cells. Oncol Rep. 2017;38:3238-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kang YW, Lee JE, Jung KH, Son MK, Shin SM, Kim SJ, Fang Z, Yan HH, Park JH, Han B, Cheon MJ, Woo MG, Lim JH, Kim YS, Hong SS. KRAS targeting antibody synergizes anti-cancer activity of gemcitabine against pancreatic cancer. Cancer Lett. 2018;438:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Lee JE, Kang YW, Jung KH, Son MK, Shin SM, Kim JS, Kim SJ, Fang Z, Yan HH, Park JH, Yoon YC, Han B, Cheon MJ, Woo MG, Seo MS, Lim JH, Kim YS, Hong SS. Intracellular KRAS-specific antibody enhances the anti-tumor efficacy of gemcitabine in pancreatic cancer by inducing endosomal escape. Cancer Lett. 2021;507:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kawaguchi K, Igarashi K, Miyake K, Lwin TM, Miyake M, Kiyuna T, Hwang HK, Murakami T, Delong JC, Singh SR, Clary B, Bouvet M, Unno M, Hoffman RM. MEK inhibitor trametinib in combination with gemcitabine regresses a patient-derived orthotopic xenograft (PDOX) pancreatic cancer nude mouse model. Tissue Cell. 2018;52:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Kawaguchi K, Igarashi K, Murakami T, Kiyuna T, Lwin TM, Hwang HK, Delong JC, Clary BM, Bouvet M, Unno M, Hoffman RM. MEK inhibitors cobimetinib and trametinib, regressed a gemcitabine-resistant pancreatic-cancer patient-derived orthotopic xenograft (PDOX). Oncotarget. 2017;8:47490-47496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Fu Z, Cheng X, Kuang J, Feng H, Chen L, Liang J, Shen X, Yuen S, Peng C, Shen B, Jin Z, Qiu W. CQ sensitizes human pancreatic cancer cells to gemcitabine through the lysosomal apoptotic pathway via reactive oxygen species. Mol Oncol. 2018;12:529-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Yang MC, Wang HC, Hou YC, Tung HL, Chiu TJ, Shan YS. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer. 2015;14:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang GF, Witkiewicz AK, Knudsen ES, Petricoin EF 3rd, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD, Der CJ. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 25. | Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT, Burrell LD, Lum DH, Whisenant JR, Gilcrease GW 3rd, Cavalieri CC, Rehbein KM, Cutler SL, Affolter KE, Welm AL, Welm BE, Scaife CL, Snyder EL, McMahon M. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 486] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 26. | Klomp JE, Lee YS, Goodwin CM, Papke B, Klomp JA, Waters AM, Stalnecker CA, DeLiberty JM, Drizyte-Miller K, Yang R, Diehl JN, Yin HH, Pierobon M, Baldelli E, Ryan MB, Li S, Peterson J, Smith AR, Neal JT, McCormick AK, Kuo CJ, Counter CM, Petricoin EF 3rd, Cox AD, Bryant KL, Der CJ. CHK1 protects oncogenic KRAS-expressing cells from DNA damage and is a target for pancreatic cancer treatment. Cell Rep. 2021;37:110060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Yan L, Tu B, Yao J, Gong J, Carugo A, Bristow CA, Wang Q, Zhu C, Dai B, Kang Y, Han L, Feng N, Jin Y, Fleming J, Heffernan TP, Yao W, Ying H. Targeting Glucose Metabolism Sensitizes Pancreatic Cancer to MEK Inhibition. Cancer Res. 2021;81:4054-4065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Elliott IA, Dann AM, Xu S, Kim SS, Abt ER, Kim W, Poddar S, Moore A, Zhou L, Williams JL, Capri JR, Ghukasyan R, Matsumura C, Tucker DA, Armstrong WR, Cabebe AE, Wu N, Li L, Le TM, Radu CG, Donahue TR. Lysosome inhibition sensitizes pancreatic cancer to replication stress by aspartate depletion. Proc Natl Acad Sci U S A. 2019;116:6842-6847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath J, Kim GE, Mancias JD, Fearon DT, Perera RM, Kimmelman AC. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 822] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 30. | Yue Q, Gao G, Zou G, Yu H, Zheng X. Natural Products as Adjunctive Treatment for Pancreatic Cancer: Recent Trends and Advancements. Biomed Res Int. 2017;2017:8412508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, Said JW, Koeffler HP. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009;69:5876-5884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Yeo D, Huynh N, Beutler JA, Christophi C, Shulkes A, Baldwin GS, Nikfarjam M, He H. Glaucarubinone and gemcitabine synergistically reduce pancreatic cancer growth via down-regulation of P21-activated kinases. Cancer Lett. 2014;346:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Huang XY, Wang HC, Yuan Z, Li A, He ML, Ai KX, Zheng Q, Qin HL. Gemcitabine combined with gum mastic causes potent growth inhibition and apoptosis of pancreatic cancer cells. Acta Pharmacol Sin. 2010;31:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Avila GE, Zheng X, Cui XX, Ryan AD, Hansson A, Suh J, Rabson AB, Chang RL, Shih WJ, Lin Y, Crowell P, Lu YP, Lou YR, Conney AH. Inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans retinoic acid on the growth of cultured human pancreas cancer cells and pancreas tumor xenografts in immunodeficient mice. J Pharmacol Exp Ther. 2005;315:170-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther. 2005;4:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Yang SW, Wang W, Xie XY, Zhu WP, Li FQ. In vitro synergistic cytotoxic effect of triptolide combined with hydroxycamptothecin on pancreatic cancer cells. Am J Chin Med. 2011;39:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Ding J, Polier G, Köhler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem. 2012;287:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Yue W, Zheng X, Lin Y, Yang CS, Xu Q, Carpizo D, Huang H, DiPaola RS, Tan XL. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget. 2015;6:21208-21224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Li L, Leung PS. Use of herbal medicines and natural products: an alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol. 2014;53:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |