Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3627
Peer-review started: March 24, 2022
First decision: April 25, 2022
Revised: May 8, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 28, 2022
Processing time: 124 Days and 5.9 Hours
Acute liver failure (ALF) is a severe and life-threatening condition in which rapid deterioration of liver function develops in a patient who has no preexisting liver disease. Mesenchymal stem cells (MSCs) are immunoregulatory stem cells which are able to modulate phenotype and function of all immune cells that play pathogenic role in the development and progression of ALF. MSCs in juxtacrine and paracrine manner attenuate antigen-presenting properties of dendritic cells and macrophages, reduce production of inflammatory cytokines in T lymphocytes, suppress hepatotoxicity of natural killer T (NKT) cells and promote generation and expansion of immunosuppressive T, B and NKT regulatory cells in acutely inflamed liver. Due to their nano-sized dimension and lipid envelope, intravenously injected MSC-derived exosomes (MSC-Exos) may by-pass all biological barriers to deliver MSC-sourced immunoregulatoy factors directly into the liver-infiltrated immune cells and injured hepatocytes. Results obtained by us and others revealed that intravenous administration of MSCs and MSC-Exos efficiently attenuated detrimental immune response and acute inflammation in the liver, suggesting that MSCs and MSC-Exos could be considered as potentially new remedies in the immunotherapy of ALF. In this review, we emphasize the current knowledge about molecular and cellular mechanisms which are responsible for MSC-based modulation of liver-infiltrated immune cells and we discuss different insights regarding the therapeutic potential of MSCs in liver regeneration.
Core Tip: Due to their potent immunoregulatory, angiomodulatory and hepatoprotective properties, mesenchymal stem cells and their exosomes suppress detrimental immune response, prevent apoptosis and promote survival of injured hepatocytes which results in an enhanced repair and regeneration of acutely injured liver.
- Citation: Harrell CR, Pavlovic D, Djonov V, Volarevic V. Therapeutic potential of mesenchymal stem cells in the treatment of acute liver failure. World J Gastroenterol 2022; 28(28): 3627-3636
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3627.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3627
The liver is the largest solid organ in the human body. It consists of large sections (lobes) which are divided in a thousand, hexagonally-shaped small units (lobules)[1]. The lobule is the main structural unit of the liver consisting of a portal triad, hepatocytes and a central vein[1]. The liver is essential organ which performs many functions including bile production, removal of waste products and toxins from the bloodstream, regulation of blood clotting, storage of fat-soluble vitamins, minerals (copper, iron) and glycogen[1]. The liver conjugates bilirubin, handles cholesterol homeostasis, regulates sex hormone metabolism and produces proteins which are important for optimal functioning of endocrine, gastrointestinal, reproductive and immune system. Since liver regulates many vital functions, severe and massive liver injury results in the development of life-threatening multi-organ dysfunction[1].
Acute liver failure (ALF) is a clinical syndrome characterized by rapid deterioration of liver function followed by ascites, coagulopathy, hepatic encephalopathy and multi-organ failure which occur in a patient who has no preexisting liver disease[2]. An annual incidence of ALF is 5.5-6.2 cases per million population per year[3]. ALF is a consequence of severe drug-induced liver injury, hepatic ischemia, invasion of hepatotropic viruses and develops due to the generation of detrimental immune response against foreign or self-antigens, released from injured hepatocytes[4].
Pathogen-associated molecular patterns expressed on invading pathogens or damage-associated molecular patterns and alarmins released from damaged-hepatocytes activate liver resident professional antigen presenting cells [dendritic cells (DCs) and Kupffer cells][2,5]. Activated DCs present lipid antigens to the liver natural killer T (NKT) cells in CD1-dependent manner, enabling their activation and polarization in inflammatory, interferon gamma (IFN-γ)-producing NKT1 and interleukin (IL)-17-producing NKT17 cells[6]. Additionally, activated liver DCs capture microbial proteins and self-proteins released from injured hepatocytes, and present them to the antigen-specific naïve CD4+ and CD8+ T cells[7]. DC-derived IL-12 induce activation and differentiation of naïve CD4+T cells in effector, IFN-γ-producing Th1 cells while DC-sourced IL-1, IL-6 and IL-23 induce generation of IL-17 and IL-22-producing effector Th17 cells[7].
Activated Kupffer cells produce tumor necrosis factor alpha (TNF-α) and IL-1β which induce enhanced expression of E and P selectins on liver endothelial cells, enabling massive influx of circulating neutrophils and monocytes in the injured livers[2,5]. Additionally, activated Kupffer cells release Th1 and Th17 cell-attracting chemokines (CXCL3 and CCL20) and recruit effector CXCR3-expressing CD4+Th1 cells and CCR6-expressing CD4+Th17 cells in the inflamed livers[6]. NKT1/Th1 cell-derived IFN-γ and NKT17/Th17 cell-sourced IL-17 induce enhanced synthesis of hepatotoxic TNF-α, nitric oxide (NO) and reactive oxygen species in Kupffer cells and liver-infiltrated neutrophils, resulting in massive necrosis of hepatocytes[6,7].
Transplantation of bio-artificial livers and hepatocytes could provide support of liver function temporarily and are therefore used as therapeutic approaches for the treatment of ALF[8]. Restored liver function and reduced incidence of extra hepatic complications were observed in ALF patients who underwent transplantation of hepatocytes[2]. However, short-term viability of engrafted hepatocytes and immune rejection against major histocompatibility complex (MHC) miss-matched transplanted hepatic cells significantly limited therapeutic efficacy of liver transplantation. Immunosuppressive therapy managed to temporarily improve survival of ALF patients who underwent liver transplantation[8,9]. Nevertheless, continuous and long-term use of immunosuppressive drugs may induce severe and life-threatening immunosuppression which may be fatal for ALF-treated patients[9]. Accordingly, there is an urgent need for the development and therapeutic use of new immunoregulatory and hepatoprotective agent which could create immunosuppressive microenvironment in injured liver, suppress detrimental immune response, alleviate liver inflammation and, at the same time, promote hepatocyte proliferation without causing severe, systemic immunosuppression.
Mesenchymal stem cells (MSCs) are adult stem cells that reside in almost all postnatal organs, where participate in the elimination of microbial pathogens, suppress detrimental immune response, promote survival of injured parenchymal cells and enhance tissue repair and regeneration[10]. A large number of experimental and clinical studies demonstrated therapeutic efficacy of MSCs and their secretome in the treatment of ALF[11,12]. MSCs were either directly transplanted in the injured livers or were infused via peripheral, portal, splenic veins and hepatic artery. Sang et al[13] and Cao et al[14] demonstrated that MSCs administered via portal vein had better capability to attenuate liver inflammation, reduce necrosis and promote liver regeneration than MSCs which were transplanted via hepatic artery, peripheral vein or MSCs which were directly injected in the damaged livers. Teshima and colleagues observed that MSCs which were transplanted via splenic vein more efficiently suppressed liver inflammation and enhanced liver regeneration than MSCs which were injected via peripheral vein[15]. Opposite to these findings were results reported by Sun et al[16] who showed that there was no significant difference in the regeneration of acutely injured livers of animals that received MSCs via hepatic artery, portal or peripheral vein, suggesting that route of MSCs injection was not crucially important for their therapeutic effects in the treatment of ALF.
mmUpon engraftment, transplanted MSCs in juxtacrine (cell-to-cell contact-dependent signaling) and paracrine manner [through the activity of MSC-sourced soluble factors: Prostaglandin E2 (PGE2), IL-10, transforming growth factor beta (TGF-β), indoleamine 2, 3 dyoxigenase (IDO), NO, hepatic growth factor (HGF), IL-1 receptor antagonist] modulate phenotype and function of all immune cells that play pathogenic role in the development and progression of acute liver injury[11,12]. MSCs also produce various growth and angiomodulatory factors [HGF, IL-6, vascular endothelial growth factor, basic fibroblast growth factor (bFGF)] which support survival and proliferation of injured hepatocytes[10,13]. Herewith, in this review article, we summarized current knowledge about molecular and cellular pathways that were responsible for hepatoprotective, immunoregulatory and angiomodulatory effects of MSCs and their secretome in the therapy of ALF. An extensive literature review was carried out in March 2022 across several databases (MEDLINE, EMBASE, Google Scholar, ClinicalTrials.gov). Keywords used in the selection were: “mesenchymal stem cells”, “secretome”, “exosomes”, “acute liver injury”, “immunomodulation”, “immunosuppression”, “hepatocytes”, “differentiation”, “ischemia”, “angiomodulation”, “therapeutic potential”, “liver repair and regeneration”. All journals were considered, and an initial search retrieved 125 articles. The abstracts of all these articles were subsequently reviewed by two of the authors (CRH and VV) to check their relevance to the subject of this manuscript. Eligible studies had to delineate the effects of MSCs and their secretome on hepatocyte survival, phenotype and function of liver-infiltrated immune cells, and their findings were analyzed in this review.
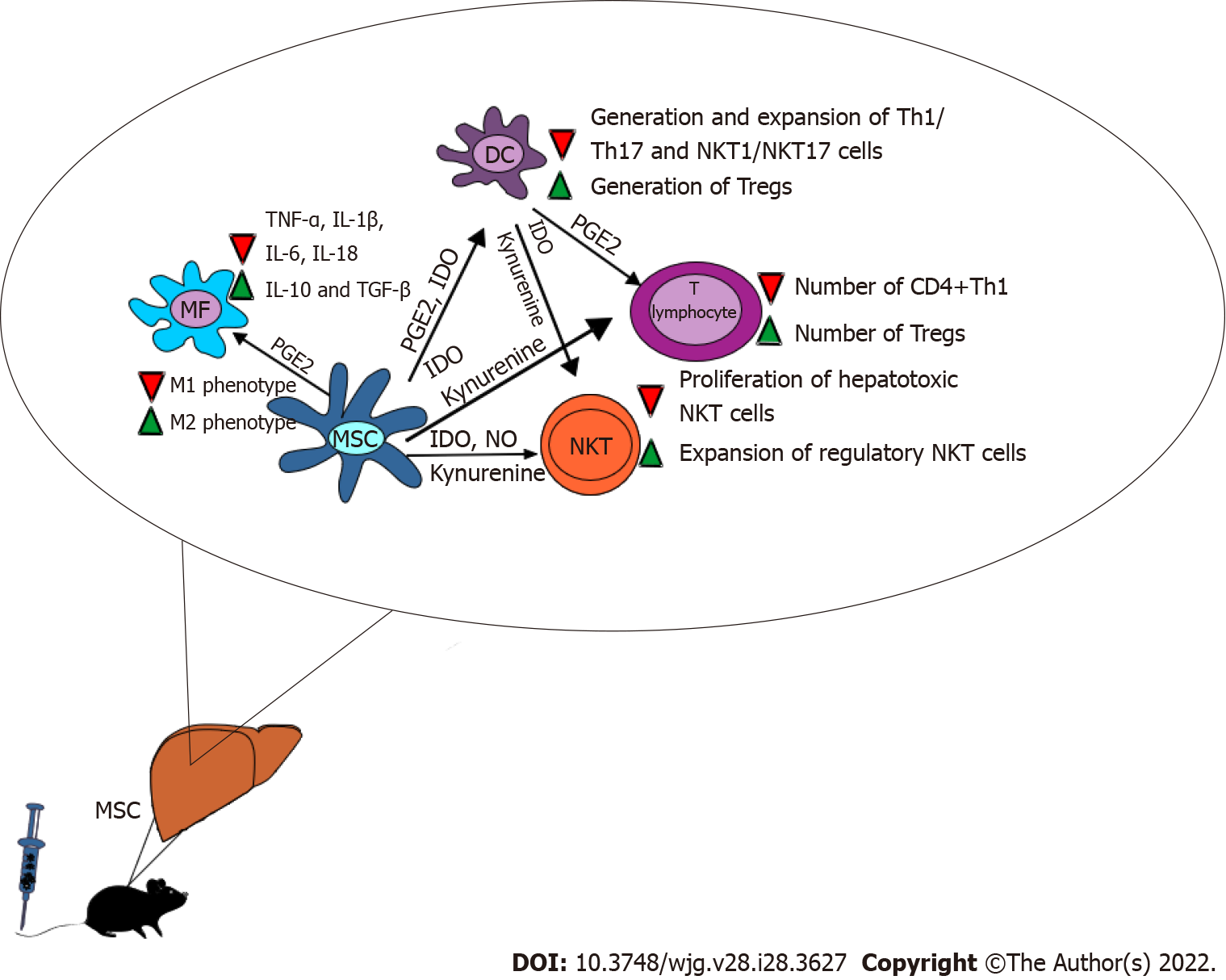
A large number of experimental studies demonstrated that hepatoprotective effects of MSCs were mainly relied on the immunoregulatory properties of MSCs[17-20]. MSCs attenuated acute liver inflammation and promoted liver regeneration by inducing immunosuppressive phenotype of liver macrophages, DCs, T lymphocytes and NKT cells[21-24].
Wang and colleagues showed that beneficial effects of MSCs in the attenuation of ALF were relied on the suppression of inflammatory M1 macrophages which hold central function in initiating acute liver inflammation[17]. Murine BM-MSCs alleviated murine D-galactosamine (D-Gal)-induced ALF by inducing generation of immunosuppressive M2 phenotype in liver macrophages in PGE2-dependent manner (Figure 1). MSC-derived PGE2 bound to the EP4 receptor and inhibited TGF-β-activated kinase 1-driven activation of nucleotide-binding and oligomerization domain-like receptor 3 (NLRP3) inflammasome in Kupffer cells[17]. PGE2-dependent suppression of NLRP3 inflammasome in liver macrophages resulted in down-regulated secretion of inflammatory cytokines (IL-1β, IL-6 and IL-18) which led to the alleviation of acute liver inflammation[17]. Additionally, MSC-sourced PGE2 induced phosphorylation and activation of transcriptional factor STAT6 which modulated mammalian target of rapamycin (mTOR) signaling that resulted in the generation of immunosuppressive M2 phenotype in liver macrophages. By producing anti-inflammatory cytokines (IL-10 and TGF-β), M2 macrophages attenuated on-going inflammation and promoted repair and regeneration of D-Gal-injured livers[17]. Similar findings were reported by Miao and colleagues who provided evidence that PGE2-dependent repression of NLRP3 inflammasome in Kupffer cells was mainly responsible for beneficial effects of BM-MSCs in the attenuation of acute liver injury during lipopolysaccharide (LPS)-induced sepsis in mice[18]. BM-MSC-sourced PGE2 generated immunosuppressive phenotype and increased IL-10 production by modulating activity of extracellular signal-regulated kinase 1 (ERK1) in Kupffer cells[18].
Liu et al[19] who analyzed the frequency of F4/80-expressing macrophages in the livers of CCL4+MSC-treated mice confirmed that MSC-based therapy significantly altered phenotype and function of Kupffer cells in inflamed livers. During the initial phase of ALF (first 48 h after CCL4 injection), significantly higher number of inflammatory M1 macrophages was observed in injured livers while immunosuppressive M2 macrophages were dominant subpopulation of liver macrophages during the recovery phase (7 d after CCL4 injection)[19]. Intravenous injection of murine MSCs (5 × 105 cells/mice) which were obtained from compact bone attenuated liver inflammation and completely regenerate CCL4-injured livers. During the injured phase, the number of inflammatory monocyte-derived macrophages was significantly increased by MSC treatment while during the recovery phase of acute liver injury MSCs favored generation and expansion of immunosuppressive M2 macrophages, crucially contributed to the creation of immunosuppressive microenvironment in regenerated livers[19].
Hua et al[20] demonstrated that amnion-derived MSCs (A-MSCs) suppressed inducible nitric oxide synthase (iNOS) activity and NO production, attenuated synthesis of inflammatory cytokines (TNF-α, IL-6) and induced generation of anti-inflammatory phenotype in liver macrophages by promoting production of immunosuppressive IL-10. A-MSC-dependent modulation of liver macrophages was relied on modulation of autophagy, as evidenced by down-regulated expression of microtubule-associated protein 1 light chain 3 in A-MSC-treated Kupffer cells[20]. Single injection of A-MSCs (1 × 106 cells/mice) alleviated acetaminophen (APAP)-induced ALF in mice by inducing alternative activation of Kupffer cells. Liposome-induced macrophage depletion almost completely diminished beneficial effects of A-MSCs, indicating that macrophages were the main cellular targets of A-MSCs in the treatment of APAP-induced ALF[20].
We and others demonstrated that MSCs and their secretome [MSC-derived conditioned medium (MSC-CM)] attenuated antigen-presenting properties of liver-infiltrated DCs, reducing their capacity for the generation and expansion of inflammatory Th1/Th17 and NKT1/NKT17 cells in injured livers[21-24]. MSC-sourced PGE2 and IDO were mainly responsible for the suppression of DC-dependent activation of liver T and NKT cells (Figure 1)[21-24].
Zhang et al[21] showed that intravenously injected BM-MSCs prevented progression of Propionibacterium acnes (P. acnes)-induced ALF in mice by inducing regulatory phenotype in liver DCs. MSCs-treated livers had normal morphology and structure without any signs of massive hepatocyte injury. MSCs down-regulated production of Th1 cell-attracting chemokines (CXCL9, CXCL10, CCL3, CCL21) which resulted in reduced influx of CXCR3 and CCR5-expressing effector CD4+ Th1 cells and CCR7-expressing memory CD4+ Th1 cells in the livers of P. acnes-treated mice[21]. Accordingly, only small number of liver-infiltrating lymphocytes was observed around the central veins of P. acnes + MSC-treated mice. Flow cytometry analysis of liver-infiltrated CD4+T cells revealed decreased expression of activating markers (CD44, CD69), indicating that MSCs suppressed activation of CD4+ T cells in P. acnes-treated animals[21]. MSCs attenuated ALF in mice by suppressing production of inflammatory and hepatotoxic cytokines (TNF-α and IFN-γ) in liver CD4+Th1 cells which was followed by the attenuation of systemic inflammation and by complete restoration of liver function[21]. Reduced number of CD4+ Th1 cells was accompanied with increased presence of FoxP3-expressing CD4+ CD25+ T regulatory cells (Tregs) in the livers of P. acnes + MSC-treated mice. Suppression of Th1 cell-driven liver inflammation was a consequence of MSC-dependent modulation of hepatic DCs[21]. DCs isolated from the livers of P. acnes + MSC-treated animals had regulatory phenotype, characterized by low expression of co-stimulatory molecules (CD80, CD86), decreased production of Th1 cell-related inflammatory cytokines (TNF-α, IL-1β, IL-12) and increased production of immunosuppressive cytokines (IL-10 and TGF-β) which induced generation and expansion of FoxP3-expressing CD4+ CD25+ Tregs in injured livers[21]. MSCs induced generation of regulatory phenotype in CD11c+ B220- precursor DCs through the activation of PI3K signaling pathway. By activating EP4 receptor, MSC-sourced PGE2 induced phosphorylation of PI3K and ERK1/2 kinases and elicited generation of tolerogenic, immunosuppressive phenotype in liver DCs[21]. Importantly, MSC-generated DCs possessed potent immunosuppressive and hepatoprotective properties. Passive transfer of MSC-generated regulatory DCs induced generation of hepatic Tregs, attenuated liver inflammation and prevented development of ALF in P. acnes-treated mice[21]. MSCs and DC-dependent expansion of Tregs was responsible for beneficial effects of MSCs since Treg depletion completely abrogated MSC-based attenuation of acute liver inflammation[21,22].
By using Concanavalin (Con A) and alpha-galactoceramide (α-GalCer)-induced hepatitis as murine models of ALF, we showed that MSC-dependent modulation of the cross-talk between DCs and NKT play important role in MSC-based attenuation of ALF[22-25]. Intravenously injected BM-MSCs significantly alleviated serum levels of AST and ALT, reduced apoptosis of hepatocytes and improved survival of Con A- and α-GalCer-treated mice[22-24]. Cellular make-up of the livers of MSC-treated animals revealed significantly reduced number of hepatotoxic, FasL, NKG2D and CD107-expressing NKT cells, indicating that MSCs suppressed cytotoxic properties of NKT cells[23]. The same phenomenon was observed in Con A- and α-GalCer-treated mice that received BM-MSC-CM, suggesting that MSCs suppressed NKT cell-driven liver inflammation in paracrine manner[22,23]. Among various MSC-derived immunosuppressive factors, NO and Kynurenine (IDO metabolite) were present in the highest concentration in BM-MSC-CM[23]. Additionally, inhibition of iNOS and IDO activity completely diminished hepatoprotective properties of MSCs, confirming the importance of NO and IDO/Kynurenine for MSC-dependent suppression of ALF[23,24]. MSC-sourced NO inhibited proliferation of hepatotoxic NKT cells while MSC-derived IDO induced generation of regulatory and immunosuppressive phenotype in NKT cells[23,25]. Additionally, MSC-sourced IDO prevented trans-differentiation of NKT regulatory cells (NKTregs) in inflammatory NKT17 cells by activating general control nonderepressible 2 (GCN2) kinase which suppressed protein kinase B/mTOR-dependent synthesis of inflammatory cytokines IL-17 and IL-22 in NKTregs[23,25].
In addition to their direct immunoregulatory effects on hepatotoic NKT cells, BM-MSCs and BM-MSC-CM suppressed effector functions of liver NKT cells indirectly, through the modulation of phenotype and function of hepatic DCs[25]. Liver DCs play important role in MSC-dependent suppression of liver NKT cells since their depletion abrogated hepatoprotective effects of MSCs and aggravated liver injury in MSC-treated mice[23,25]. MSCs attenuated antigen-presenting properties of hepatic DCs by down-regulating expression of MHC class II and co-stimulatory molecules on their membranes and by reducing synthesis of NKT1 and NKT17 cell- related inflammatory cytokines (TNF-α, IL-β, IL-12, IL-23, IFN-γ, IL-17)[22-25]. In similar manner as it was observed by Zhang et al[16], we also noticed that MSCs induced generation of regulatory and tolerogenic phenotype in hepatic DCs[22-25]. Increased IDO activity and enhanced production of anti-inflammatory cytokines (TGF-β and IL-10) were observed in the DCs which were isolated from the livers of Con A + MSCs and α-GalCer + MSCs-treated mice[23]. MSC-generated regulatory DCs induced generation and expansion of IL-10 and TGF-β-producing NKTregs, contributing to the creation of immunosuppressive microenvironment in injured livers[24]. Additionally, BM-MSCs reduced presence of inflammatory, IFN-γ and TNF-α-producing NKT1 and IL-17-producing NKT17 cells in the livers of Con A + MSCs and α-GalCer + MSCs-treated mice which resulted in alleviated liver inflammation[24]. MSC-sourced IDO and its metabolite Kynurenine were mainly responsible for MSC-dependent generation of regulatory DCs[24,25]. Inhibition of IDO activity in MSCs attenuated their capacity for the generation of tolerogenic phenotype in hepatic DCs and diminished their hepatoprotective and immunosuppressive effects[24].
MSCs represent heterogeneous population of immunoregulatory and angiomodulatory stem cells which possess enormous differentiation potential[25]. All subpopulations of MSCs spontaneously differentiate into the cells of mesodermal origin[25]. However, under appropriate conditions, MSCs may generate cells of ectodermal and endodermal origin, including hepatocyte-like cells (HLCs)[26-29].
Local tissue microenvironment, particularly oxygen supply and cytokines/growth factors released by parenchymal and tissue-resident immune cells modulate phenotype of MSCs[25,26]. MSCs are usually derived from bone marrow, adipose tissue, amniotic fluid, placenta, umbilical cord, dental pulp, compact bone and peripheral blood[10]. The development origin significantly affects potential for differentiation and other functional characteristics of tissue-specific MSCs[26]. BM-MSCs possess great genomic stability after long-term propagation[26]. An improved engraftment and increased life span are the main characteristics of adipose tissue-derived MSCs (AT-MSCs)[26]. Dental pulp-derived MSCs, amniotic fluid derived MSCs and placental derived MSCs have increased capacity for differentiation in neural cells, while BM-MSCs, AT-MSCs and umbilical cord derived MSCs (UC-MSCs) have great differentiation potential towards the cells of endodermal origin, including HLCs[26].
Accordingly, a large number of evidence demonstrated that human BM-MSC-, AT-MSC- and UC-MSC-derived HLCs represent valuable and promising cell source for the regeneration of injured livers[25-29].
Two step protocol is the most usually used for the generation of human MSC-derived HLCs[29]. After isolation from BM, AT and UC, MSCs are, under standard conditions (at 37 ℃ in a humidified atmosphere containing 5% CO2), cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (FBS) until they reach 75%-80% of confluence. Then, MSCs are cultured for 48 h in the FBS-free DMEM medium supplemented with endothelial growth factor (20 ng/mL) and basic fibroblast growth factor (bFGF, 10 ng/mL) to be committed towards endodermal lineage[29]. Afterwards, MSCs were seven days exposed to HLC step-1 differentiation medium, consisting of HGF (20 ng/mL), bFGF (10 ng/mL) and nicotin-amide (0.61 g/L). By binding to the c-met receptor on differentiated cells, HGF activates intracellular signaling molecules (Grb2-associated binder-1, son of sevenless), ERK kinase and mitogen-activated protein kinase which are important in the early stages of hepatogenesis[29]. Intracellular signaling elicited by HGF/c-met are enhanced by nicotin-amide which acts as selective kinase inhibitor that improves cell survival and proliferation during the initial stage of endodermal patterning. Finally, MSCs are cultured in HLC step-2 differentiation medium consisting of oncostatin (OSM, 20 ng/mL), dexamethasone (1 mmol/L) and insulin, transferrin and selenium (ITS, 50 mg/mL). OSM promotes maturation of hepatocytes, dexamethasone induces enhanced expression of liver-enriched transcription factors [CCAAT/enhancer-binding protein alpha (C/EBPα), hepatocyte nuclear factor (HNF)-4α], while ITS promotes the proliferation and survival of differentiated HLCs[29]. At the end of the differentiation process, HLCs generated from BM-, AT- and UC-MSCs, fully obtain hepatocyte-like polygonal morphology with cytoplasmic granulation and prominent centrally positioned nucleus[29]. Exposition to HLC differentiation mediums do not alter telomerase activity and do not induce genetic instability in human BM-, AT- and UC-MSC-derived HLCs[29].
MSC-derived HLCs had gene expression profile similar to fetal or adult hepatocytes[25-29]. During the early stage of differentiation, MSC-derived HLCs express several early hepatocyte differentiation markers [alfa-fetoprotein, cytokeratin (CK)7, GATA4, HNF1α, HNF3β, HNF4α, C/EBPα] while during the late stage of differentiation, MSC-derived HLCs express cell markers of mature hepatocytes (forkhead transcription factor which has a crucial role in hepatocyte differentiation), hepatocyte-specific gap junction protein connexin 32 and hepatocyte paraffin 1, localized in hepatocytes mitochondria). Finally, at the end of differentiation, MSC-derived HLCs are able to produce hepatocyte-specific proteins (albumin, fibrinogen, transferrin) and enzymes (cytochrome P450 subtypes 3A4 and 1A1, phosphoenolpyruvate carboxykinase 1 and carbamoylphosphate synthetase[26-29].
The analysis of functional characteristics of BM-, AT- and UC-MSCs-derived HLCs revealed that AT-MSC- and BM-MSC-derived HLCs had the higher capacity for glycogen storage than UC-MSCs-derived HLCs and their gene expressions were more consistent with the normal hepatocyte-differentiation profile[30]. Albumin gene expression increased progressively during the differentiation process of AT-MSC- and BM-MSC-derived HLCs. In contrast, only transitory expression of albumin was observed in UC-MSC-derived HLCs (between day 7 and day 25). Additionally, HLCs which were generated from AT-MSCs and BM-MSCs displayed clusters arrangements and had increased proliferation rate compared to UC-MSC-derived HLCs which were not expanded as expected, hampering their potential for in vivo use[30]. Accordingly, only therapeutic potential of AT-MSC-derived HLCs and BM-MSC-derived HLCs was examined in vivo, in murine model of CCL4-induced acute liver injury[30]. Intravenously injected human AT-MSC-and BM-MSC-derived HLCs (1 × 106 cells), managed to completely regenerate CCL4-injured livers of immunodeficient mice. Hepatocytes isolated from the livers of CCL4-treated mice displayed normal morphology two weeks after the transplantation of human AT-MSC- and BM-MSC-derived HLCs. Importantly, hepatocytes in the regenerated livers of experimental mice express human albumin and CK19 confirming that they were generated from transplanted human AT-MSC- and BM-MSC-derived HLCs[30]. Accordingly, both AT-MSCs and BM-MSCs represent valuable cell source for the generation of functional HLCs which may repopulate and regenerate injured livers of ALF patients[30]. Nevertheless, it should be noted that progression of ALF is mainly a consequence of immune cell-dependent massive damage of hepatocytes[2,6]. Accordingly, detrimental immune response could induce injury of engrafted HLCs as well. Therefore, co-transplantation of MSC-derived HLCs and immunosuppressive MSCs should be explored as new therapeutic approach which could successfully regenerate injured livers of ALF patients.
Although results obtained in animal studies strongly suggest that MSCs could be considered as new therapeutic agents in regenerative hepatology, there are several safety issues which limit clinical use of MSCs[31]. Firstly, MSCs are not constitutively immunosuppressive cells[32]. MSCs may adopt phenotype and function under the influence of biological factors to which they are exposed. When MSCs engraft in the tissue with low level of TNF-α and IFN-γ, they obtain pro-inflammatory MSC1 phenotype and secrete large number of bioactive factors which aggravate on-going inflammation. On the contrary, when MSCs are exposed to the high levels of TNF-α and IFN-γ, they acquire immunosuppressive MSC2 phenotype and produce immunoregulatory factors that suppress inflammatory immune cells[32]. Since local tissue concentration of TNF-α and IFN-γ differ in initial and recovery phases of acute liver inflammation, there is a concern that MSCs which will be engrafted in the injured livers with low level of TNF-α and IFN-γ may obtain pro-inflammatory MSC1 phenotype and may aggravate on-going inflammation[31,32]. Additionally, TGF-β and bone morphogenetic protein, released by macrophages in inflamed and regenerated tissues, may induce spontaneous and unwanted chondrogenic and osteogenic differentiation of MSCs[33]. Possible unwanted differentiation of transplanted MSCs represents an important safety concern which limits clinical use of MSCs in regenerative hepatology[33].
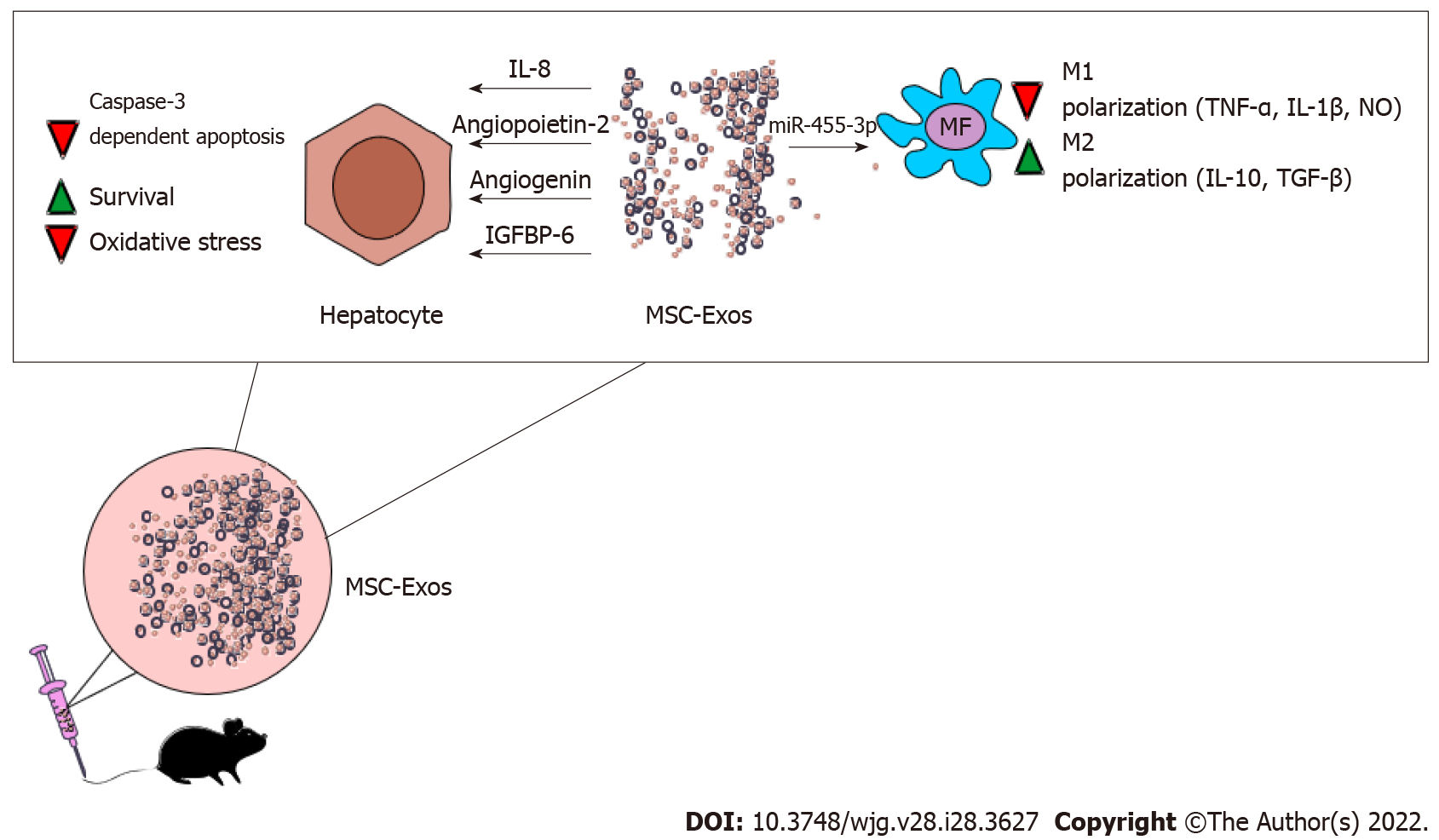
MSC-Exos are nano-sized extracellular vesicles which contain all of MSC-sourced immunoregulatory and angiomodulatory factors[34]. Due to their nano-sized dimension and lipid envelope, intravenously injected MSC-Exos by-pass all biological barriers and deliver their cargo (miRNAs) anti-inflammatory cytokines, growth factors) directly into the target liver-infiltrated immune cells and injured hepatocytes. Since the majority of MSC-dependent beneficial effects in attenuation of liver inflammation were relied on the activity of MSCs-sourced bioactive factors[25], administration of MSC-Exos is considered as an alternative therapeutic approach to MSC-based therapy since it addresses all safety concerns related to the transplantation of MSCs[34]. A detailed proteomic analysis revealed that MSC-Exos contained proteins that regulate hepatocyte survival, oxidative stress, tissue remodeling, activation and migration of immune cells[34]. Accordingly, several experimental studies demonstrated therapeutic potential of MSC-Exos in the treatment of ALF[35-37].
After intravenous injection, MSC-Exos obtained from MB-MSC-Exos migrated in the liver, attenuated D-Gal/LPS-induced acute liver inflammation and improved survival of in D-Gal/LPS-treated mice suffering from ALF[35]. By delivering immunosuppressive molecules and trophic factors in inflamed livers [IL-8, angiopoietin-2, angiogenin, insulin-like growth factor binding protein-6 (IGFBP-6)] MB-MSC-Exos attenuated caspase-3-dependent apoptosis of injured hepatocytes[35]. MB-MSCs-sourced IL-8 inhibited TNF-α-induced activation of caspase-3 and prevented apoptosis of hepatocytes while angiopoietin-2, angiogenin and IGFBP-6 promoted survival of hepatocytes by preventing the progression of ischemia-induced injury in inflamed livers[35].
The importance of anti-apoptotic effects of MSC-Exos in the treatment of ALF was confirmed in murine model of CCL4-induced acute liver injury[36]. Jiang and colleagues demonstrated that hepatoprotective effects of Exos isolated from the secretome of human UC-MSCs (UC-MSC-Exos) were relied on their capacity to inhibit caspase 3 and Bcl-2-associated X protein-driven apoptosis of hepatocytes[36]. Additionally, significant decrease of oxidase metabolite 8-hydroxy-2-deoxyguanosine was observed in CCL4-injured livers 24 h after administration of UC-MSC-Exos, indicating that UC-MSC-Exos-dependent inhibition of oxidative stress in injured hepatocytes was, at least partially, responsible for the alleviation of ALF in experimental mice[36].
As evidenced by Shao and colleagues, MSC-Exo-sourced miR-455-3p was crucially responsible for MSC-dependent immunosuppression in ALF[37]. MSC-derived miR-455-3p modulated activation of PI3K and attenuated synthesis of inflammatory cytokines IL-6, G-CSF, IL-17, IP-10, and MCP-1 in liver macrophages[37]. Significantly reduced necrosis of hepatocytes, down-regulated serum levels of aspartate aminotransferase, alanine transaminase and bilirubin were observed in CCL4 + miR-455-3p-treated mice. Additionally, miR-455-3p significantly reduced presence of CD68-expressing macrophages in the liver and decreased total number of circulating inflammatory CD14+ CD16+ and CCR2+ CD16+ monocytes in peripheral blood of CCL4-treated animals, indicating that beneficial effects of miR-455-3p was mainly relied on the suppression of inflammatory monocytes/macrophages[37] (Figure 2).
MSCs possess potent immunoregulatory, angiomodulatory and hepatoprotective properties[25,38]. Results obtained in experimental studies showed that MSCs and MSC-Exos were able to efficiently attenuate acute liver inflammation and to promote enhanced repair and regeneration of injured liver tissue, suggesting their potential clinical use in the therapy of ALF[25,38].
However, it should be noted that there are several issues that need to be addressed before MSCs and their exosomes could be offered as new remedy in regenerative hepatology. The optimal tissue source, dose, and frequency of MSCs/MSC-Exos should be defined. Up-coming studies should also precisely determine all MSC-sourced immunoregulatory and hepatoprotective factors which are responsible for their beneficial effects. Afterwards, bioengineering of MSCs which will massively produce these bioactive molecules should be performed. It is highly expected that these newly developed MSCs and their exosomes will have significantly enhanced efficacy in in the treatment of ALF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salim A, Pakistan; Lu CX, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 900] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 2. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 3. | Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Lemmer P, Pospiech JC, Canbay A. Liver failure-future challenges and remaining questions. Ann Transl Med. 2021;9:734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Dong V, Nanchal R, Karvellas CJ. Pathophysiology of Acute Liver Failure. Nutr Clin Pract. 2020;35:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Zhang H, Jiang Z, Zhang L. Dual effect of T helper cell 17 (Th17) and regulatory T cell (Treg) in liver pathological process: From occurrence to end stage of disease. Int Immunopharmacol. 2019;69:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Przybyl H, Grindler J, Lauer D. Unfreezing What's Hot in Liver Transplantation: A Review of Current Trends. AACN Adv Crit Care. 2022;33:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Biolato M, Bianco A, Lucchini M, Gasbarrini A, Mirabella M, Grieco A. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs. 2021;35:861-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Hu C, Li L. Improvement of mesenchymal stromal cells and their derivatives for treating acute liver failure. J Mol Med (Berl). 2019;97:1065-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Hu C, Wu Z, Li L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci. 2020;16:893-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Zhou JH, Lu X, Yan CL, Sheng XY, Cao HC. Mesenchymal stromal cell-dependent immunoregulation in chemically-induced acute liver failure. World J Stem Cells. 2021;13:208-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wu R, Fan X, Wang Y, Shen M, Zheng Y, Zhao S, Yang L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front Immunol. 2022;13:833878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 13. | Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, Ding YT. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int. 2016;15:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, Li Y, Pan X, Wang Y, Li L. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Teshima T, Matsumoto H, Michishita M, Matsuoka A, Shiba M, Nagashima T, Koyama H. Allogenic Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorate Acute Hepatic Injury in Dogs. Stem Cells Int. 2017;2017:3892514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Sun L, Fan X, Zhang L, Shi G, Aili M, Lu X, Jiang T, Zhang Y. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med. 2014;34:987-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Wang J, Liu Y, Ding H, Shi X, Ren H. Mesenchymal stem cell-secreted prostaglandin E2 ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 2021;12:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Miao CM, Jiang XW, He K, Li PZ, Liu ZJ, Cao D, Ou ZB, Gong JP, Liu CA, Cheng Y. Bone marrow stromal cells attenuate LPS-induced mouse acute liver injury via the prostaglandin E 2-dependent repression of the NLRP3 inflammasome in Kupffer cells. Immunol Lett. 2016;179:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Liu J, Feng B, Xu Y, Zhu J, Feng X, Chen W, Sheng X, Shi X, Pan Q, Yu J, Zeng X, Cao H, Li L. Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis. Stem Cell Res Ther. 2019;10:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Hua D, Ju Z, Gan X, Wang Q, Luo C, Gu J, Yu Y. Human amniotic mesenchymal stromal cells alleviate acute liver injury by inhibiting the pro-inflammatory response of liver resident macrophage through autophagy. Ann Transl Med. 2019;7:392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, Zhao F, Liu Q, Wei X, Jin M, Wu C, Xie Q, Zhang Y, Wan B. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59:671-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Gazdic M, Markovic BS, Arsenijevic A, Jovicic N, Acovic A, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N, Lukic ML, Volarevic V. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl. 2018;24:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Gazdic M, Simovic Markovic B, Vucicevic L, Nikolic T, Djonov V, Arsenijevic N, Trajkovic V, Lukic ML, Volarevic V. Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase- and indoleamine 2,3-dioxygenase-dependent manner. J Tissue Eng Regen Med. 2018;12:e1173-e1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, Djonov V, Lukic ML, Volarevic V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017;23:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Gazdic M, Arsenijevic A, Markovic BS, Volarevic A, Dimova I, Djonov V, Arsenijevic N, Stojkovic M, Volarevic V. Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int J Biol Sci. 2017;13:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med. 2006;141:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Taléns-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gómez-Lechón MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12:5834-5845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 183] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Sawitza I, Kordes C, Götze S, Herebian D, Häussinger D. Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep. 2015;5:13320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Chivu M, Dima SO, Stancu CI, Dobrea C, Uscatescu V, Necula LG, Bleotu C, Tanase C, Albulescu R, Ardeleanu C, Popescu I. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl Res. 2009;154:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Manzini BM, da Silva Santos Duarte A, Sankaramanivel S, Ramos AL, Latuf-Filho P, Escanhoela C, Kharmandayan P, Olalla Saad ST, Boin I, Malheiros Luzo ÂC. Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis. Cytotherapy. 2015;17:1052-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 527] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 32. | Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 33. | Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 34. | Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 533] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 35. | Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 36. | Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018;2018:6079642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 37. | Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, Chen M, Zhang B, Zhou Y, Yao R, Shi Y, Bu H. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 38. | Harrell CR, Djonov V, Volarevic V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |