Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3620
Peer-review started: February 21, 2022
First decision: May 9, 2022
Revised: May 23, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 28, 2022
Processing time: 155 Days and 11.3 Hours
Multidisciplinary pediatric aerodigestive centers have been proposed to address the needs of children with complex multi-system problems affecting the respiratory and upper gastrointestinal tracts. The setup of a multidisciplinary service allows for the complex coordination needed between different subspecialties. This allows for rapid communication and family-centered decision making and agreement on further diagnostic and/or therapeutic next steps such as offering triple endoscopy when indicated. Triple endoscopy entails performing rigid upper airway assessment, flexible bronchoscopy and upper gastrointestinal endoscopy and has been linked to reduced time to diagnosis/treatment, reduced costs and anesthesia exposure. This review summarizes the available literature on the structure and benefits of multidisciplinary pediatric aerodigestive services.
Core Tip: Multidisciplinary pediatric aerodigestive programs serve patients with multisystem problems affecting the respiratory and upper gastrointestinal tracts and require the participation and expertise of essential subspecialists including gastroenterologists. This setup allows for coordinated diagnostic and therapeutic interventions such as triple endoscopy. Benefits include reducing time to diagnosis and/or treatment, reducing healthcare costs and limiting exposure to radiation and anesthesia, providing a strong example of value-based healthcare delivery.
- Citation: Kanotra SP, Weiner R, Rahhal R. Making the case for multidisciplinary pediatric aerodigestive programs. World J Gastroenterol 2022; 28(28): 3620-3626
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3620.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3620
Multidisciplinary programs for complex medical conditions such as cystic fibrosis and asthma have been shown to optimize the value of healthcare delivery by improving outcomes and reducing cost[1,2]. There is growing literature supporting this concept for aerodigestive programs[3,4]. Multidisciplinary pediatric aerodigestive programs have been proposed to address the specific needs of children with complex multi-system problems affecting the respiratory and upper gastrointestinal (GI) tracts. Conditions managed by aerodigestive programs are diverse and may include structural or physiological airway disease, chronic parenchymal lung disease, lung injury from aspiration or infection, dysphagia, and esophageal mucosal, motility and anatomic abnormalities[1,5]. One main aim of this coordinated interdisciplinary approach is to allow for expedited patient evaluation in a single clinical setting, including diagnostic and therapeutic interventions. Several benefits have been attributed to aerodigestive programs, as opposed to independent evaluations by different subspecialists at different times. Benefits include shorter time to diagnosis, reduced radiation and anesthesia exposure and lower healthcare related costs. The purpose of the review is to shed light on the structure, services and impacts of aerodigestive programs and to highlight the need for gastroenterology expertise and involvement within these programs.
Advances in the care of critically ill infants and children have created a growing population of patients with complex chronic multi-system diseases requiring a collaborative multispecialty approach to their management[6]. This includes multidisciplinary aerodigestive programs that focus on children with a combination of multiple and interrelated congenital and/or acquired conditions affecting airway, breathing, feeding, and concerns about growth/nutrition (Table 1). Key service components of an aerodigestive program include a pediatric otolaryngologist, pediatric pulmonologist and pediatric gastroenterologist along with a speech language pathologist, social worker and nutritionist. The first consensus statement on aerodigestive programs, published in 2018, provided a definition of an aerodigestive patient, specified needed participation of vital pediatric disciplines and their levels of expertise, and highlighted essential care components, assessments and therapies including endoscopic procedures[5].
| Pulmonary | Otolaryngology | Gastroenterology |
| Chronic cough | Tracheostomy dependence | Dysphagia |
| Stridor | Stridor | Feeding intolerance |
| Wheezing | Noisy breathing | Gastroesophageal reflux disease |
| Recurrent pneumonia | Recurrent croup | Eosinophilic esophagitis |
| Tracheomalacia | Laryngeal cleft | Esophageal stricture |
| Bronchomalacia | Laryngeal stenosis | Congenital esophageal anomalies |
| Chronic lung disease | Laryngomalacia | Malnutrition |
| Need for ventilatory support | Vocal cord paralysis | Feeding tube dependence |
| Subglottic stenosis | ||
| Tracheal stenosis |
Results of a survey published in 2019 noted the number of pediatric aerodigestive programs to be rapidly proliferating with diffuse geographic presence throughout the United States, with the largest subset operating in academic centers[7]. This was a recent phenomenon as a considerable percent of these programs had been operating for just a few years.
Aerodigestive programs not only offer expertise from different specialties in a single setting, but also enable the coordination of evaluation and intervention. Depending on patient presentation and the differential diagnoses, management includes education, discussion of potential treatment options and risks and benefits of treatment modalities and further testing. Evaluation can include a list of airway and GI interventions offered individually or in combination.
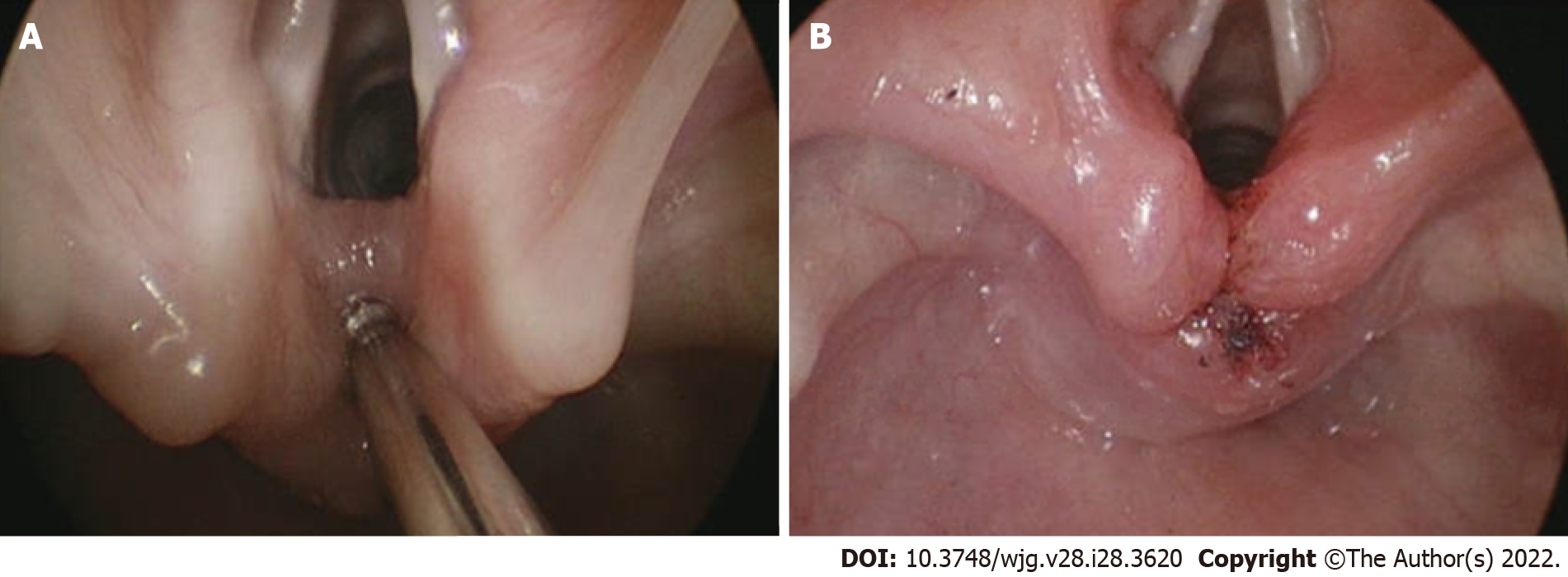
From the airway perspective, evaluation can include awake in-office flexible laryngoscopy and swallow assessment. Further workup may include drug induced sleep endoscopy, direct laryngoscopy and rigid bronchoscopy, while therapeutic interventions targeting specific pathology such as endoscopic repair of laryngeal cleft (Figure 1), laser excision of cysts, balloon dilation of subglottic stenosis and more advanced open airway interventions. These procedures have benefits and risks which should be taken in consideration and clearly discussed with caregivers. Some evaluation can take place without sedation while others require various levels of sedation, often performed in the operating room with the assistance of anesthesia services.
Awake in-office flexible laryngoscopy allows for a non-sedated evaluation of the upper airway. This allows for the assessment of upper airway dynamics and abnormalities such as vocal cord lesions and vocal cord movement, tongue base obstruction, hypopharyngeal collapse, adenoid or tonsillar hypertrophy and laryngomalacia. Assessment of dysphagia and aspiration can also be undertaken during in-office flexible laryngoscopy, via a procedure referred to as Fiberoptic Endoscopic Evaluation of Swallowing. Even though an invaluable tool for assessment of airway and swallowing abnormalities in the children, in-office flexible laryngoscopy has some disadvantages including limited assessment of structures below the vocal cords and dependence on patient compliance. In addition, therapeutic airway interventions are not possible in the office setting.
Drug induced sleep endoscopy (DISE) allows for upper airway evaluation using a flexible endoscope while a patient is in a pharmacologically induced sleep-like state. During DISE, an endoscope is passed through the nares to assess the nasopharynx, oropharynx, larynx, and in some cases the trachea. The procedure is often carried out either with propofol or dexmedetomidine sedation, which simulates natural sleep and can demonstrate lesions causing obstruction that may not be evident on an awake in-office flexible laryngoscopy[8].
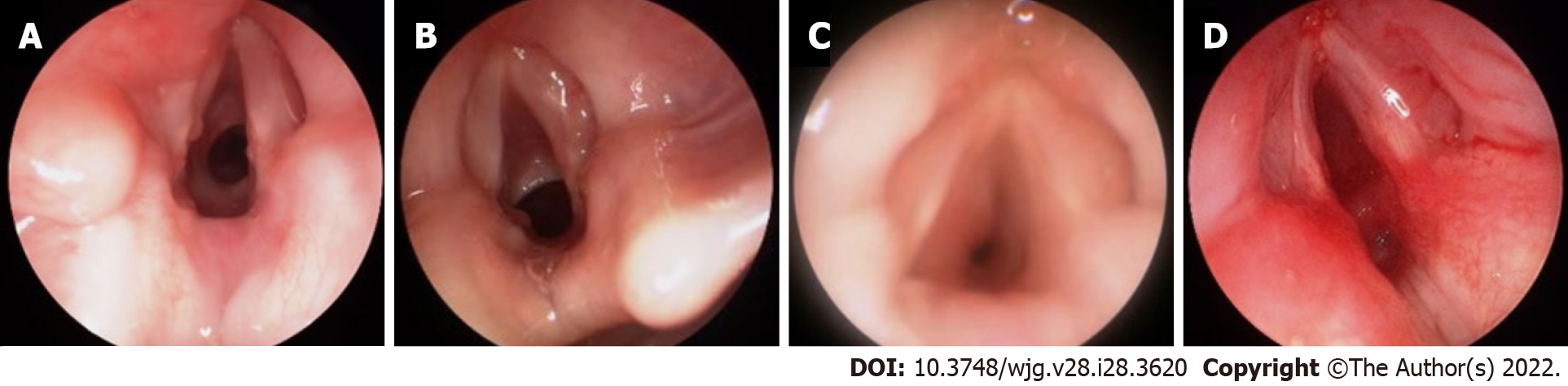
Rigid endoscopy is considered the gold standard for diagnostic airway assessment of structures below the vocal cords, including trachea and bronchi. It offers better optics, ability to size areas of narrowing and provides an opportunity to perform a variety of interventions (Figure 2). This procedure requires patient sedation and thus is not hampered by patient tolerance or compliance. Spontaneous breathing can be maintained, which allows for examination of the dynamic status of the airway. The sedated status allows for surgical interventions such as biopsy, laryngeal cleft injection or suturing, epiglottopexy, balloon dilation, vocal cord injection, and laryngotracheal reconstruction[9,10].
Upper GI evaluation may include video fluoroscopic evaluation of swallowing, contrast imaging for esophageal strictures, impedance pH testing for gastroesophageal reflux disease (GERD), esophageal manometry for motility disorders and mucosal endoscopic assessment for esophagitis including eosinophilic esophagitis (EoE). Aerodigestive patients often have feeding problems and are at high risk for malnutrition, necessitating feeding tube placement and use[11]. Some patients can be managed with nasogastric and gastrostomy tubes while others require jejunal feeds due to persistent GERD and/or intolerance to intragastric feeds. The choice, placement and maintenance of feeding tubes can be managed by a pediatric gastroenterologist within an aerodigestive program[12].
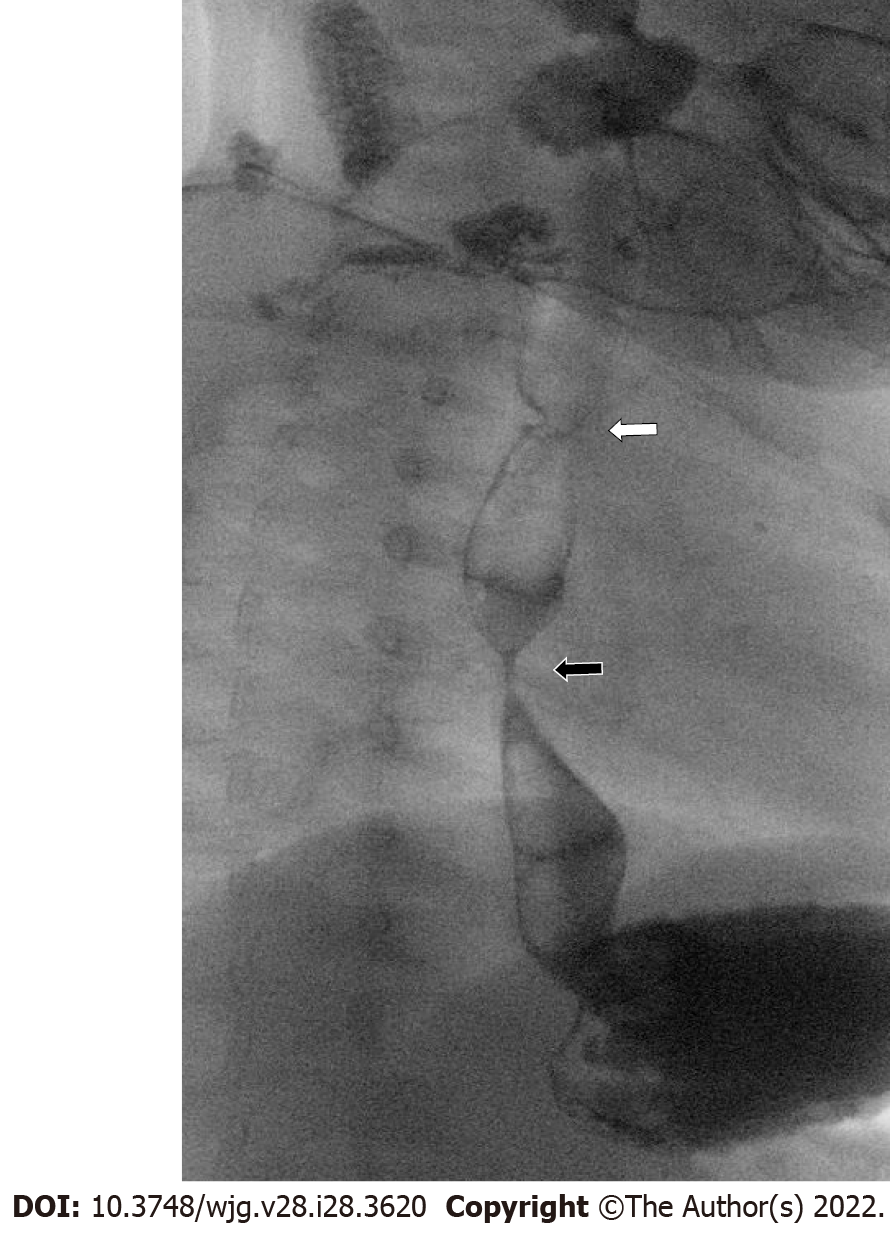
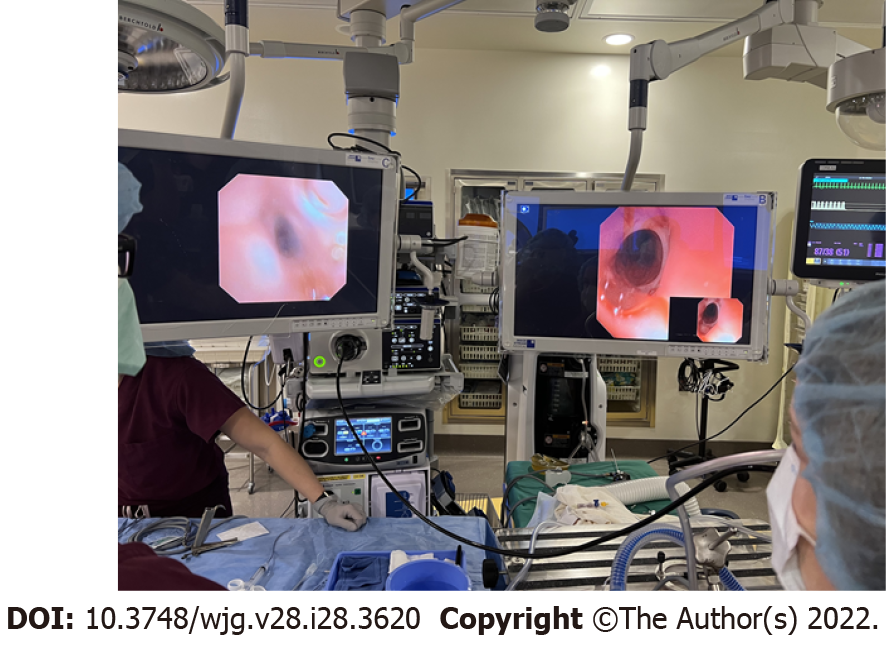
Specific patient categories presenting to an aerodigestive clinic may have a particularly higher risk for upper GI abnormalities contributing the symptoms. These include patients previously treated for esophageal atresia and tracheoesophageal fistula (Figure 3). Common complications among these patients include esophagitis[13], recurrent respiratory tract infection, tracheomalacia and poor growth with increased healthcare utilization[14-16]. Pediatric gastroenterology input can be crucial to evaluate for anatomic and mucosal esophageal abnormalities and for persistent tracheoesophageal fistula, which can be difficult to diagnosis. Simultaneous tracheal and esophageal endoscopic assessment can help in challenging cases when imaging modalities are not definitive (Figure 4).
In some patients, flexible bronchoscopy, flexible upper GI endoscopy, direct laryngoscopy, and rigid bronchoscopy are indicated and can be offered in combination aptly named as “triple endoscopy”. A triple endoscopy provides efficient, organized and thorough evaluation of the patient’s disorders without requiring multiple, separate procedures under anesthesia. This allows for complementary modalities for evaluation of upper GI and airway structures and dynamics and facilitates real-time discovery, discussion, and planning amongst all the specialists involved, allowing for excellent, patient-centric care. A consensus on the core data elements to be gathered during triple endoscopy has been published[17]. From the gastroenterology standpoint, the importance of assessing for EoE is highlighted due to potential contribution to aerodigestive symptoms. An upper endoscopy should therefore be considered as part of a triple endoscopy in patients presenting with symptoms potentailly arising from esophageal mucosal disease (especially EoE) as well as anatomic GI disorders (such as esophageal strictures). Another common indication for triple endoscopy is in the preoperative evaluation of children undergoing open airway reconstructive surgery (like patients with tracheostomies moving towards decannulation). Triple endoscopy has also shown high yield in the evaluation of chronic cough, aspiration, apparent life-threatening episodes, recurrent croup and recurrent pneumonias. Triple endoscopy can assess factors that can predict outcomes of open airway reconstruction such as laryngotracheal reconstruction, cricotracheal resections and tracheal resections, allowing for risk modification to optimize patient outcomes. Triple endoscopy serves to provide a comprehensive assessment including evaluating grade of subglottic stenosis, ruling out secondary airway lesions (such as tracheobronchomalacia). Active esophageal disorders, like EoE and GERD, have been linked to unfavorable effect outcomes of open airway reconstruction so it is important for identify these conditions and optimize their management[18,19]. In this patient population, flexible bronchoscopy with bronchoalveolar lavage provides evidence of bacterial colonization which can direct prophylactic antibiotics. Patients with tracheostomies, frequently evaluated by aerodigestive programs, commonly have airway colonization with Pseudomonas aeruginosa. Pseudomonal wound infection during open airway reconstruction surgeries leads to increased morbidity[20,21]. Implementation of culture directed antibiotics prior to or during open airway reconstruction surgery can therefore improve outcomes. Studies have demonstrated that specific subsets of aerodigestive patients are more likely to have abnormal mucosal histology on upper GI endoscopy and/or abnormal impedance pH testing[22,23]. This includes those with previously diagnosed asthma, feeding difficulty, and esophageal atresia with or without tracheoesophageal fistula, with 40%-50% yield on upper GI testing[22]. Findings noted included reflux esophagitis and EoE. It is important to note that in many studies a large percent of patients (> 50%) were on acid suppressive therapy which may have treated underlying proton pump inhibitor responsive eosinophilia (now considered a subset of EoE) which requires prolonged treatment and monitoring[24].
As aerodigestive programs expand, there has been more emphasis on measuring program impact on patients, their families and the healthcare system. Clinical outcomes typically followed can include documented improvement in swallowing (control of aspiration), frequency of respiratory infections, rate of decannulation in patients with tracheostomies and overall patient mortality. Other important outcomes studied include time to reach a diagnosis and/or initiate treatment, impact of health-related quality of life, healthcare costs and utilization.
Studies have demonstrated numerous positive impacts of aerodigestive programs. These include lower technical direct cost (by as much as 70%) and fewer inpatient days after enrolling patients in an aerodigestive program[3]. Frequency of unnecessary evaluation (swallow studies and impedance pH testing) dropped when such programs were established[24,25]. Aerodigestive programs have been linked to improved patient quality of life and reduced caregiver burden[11]. Studies have also noted shortened time to diagnosis (6 d vs 150 d, P < 0.001), fewer specialist consultations, lower hospital charges, less radiation exposure and reduced anesthesia exposure when aerodigestive programs were established[4,26]. The benefit of reducing anesthesia exposure is of particular interest in infants and young children in terms of diminishing neurocognitive risks associated with repeated exposure to anesthetics[27].
Further advantages of triple endoscopy include a significant reduction in mean anesthesia time (54 min vs 89 min, P < 0.0001) compared to having the 3 procedures done separately[28]. Charges and direct costs for triple endoscopy were also significantly lower as compared to performing these as separate procedures[28]. It is important to note that not all patients who present to an aerodigestive require a triple endoscopy. Patients with isolated surgical pathology such as cystic lesions (for example vallecular cyst) are likely to only require otolaryngologist intervention only.
Aerodigestive programs have specific requirements for essential subspecialist participation and expertise which includes gastroenterology services. These programs offer numerous advantages in the management of complex multi-system problems affecting the respiratory and upper GI tracts especially with the diverse and overlapping contributions of conditions leading to a range of patient signs and symptoms. The setup of an aerodigestive program allows for diagnostic and therapeutic interventions, offered individually or in combination. Benefits of aerodigestive programs have been demonstrated and include reducing time to diagnosis and/or initiating of treatment, reducing healthcare costs and limiting patient radiation and anesthesia exposure, providing a strong example of value-based healthcare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Pandey A, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Condren M, Boger JA. Impact of a pediatric clinic-based multidisciplinary asthma education and management program. J Pediatr Pharmacol Ther. 2005;10:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Sawicki GS, Goss CH. Tackling the increasing complexity of CF care. Pediatr Pulmonol. 2015;50 Suppl 40:S74-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Appachi S, Banas A, Feinberg L, Henry D, Kenny D, Kraynack N, Rosneck A, Carl J, Krakovitz P. Association of Enrollment in an Aerodigestive Clinic With Reduced Hospital Stay for Children With Special Health Care Needs. JAMA Otolaryngol Head Neck Surg. 2017;143:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Collaco JM, Aherrera AD, Au Yeung KJ, Lefton-Greif MA, Hoch J, Skinner ML. Interdisciplinary pediatric aerodigestive care and reduction in health care costs and burden. JAMA Otolaryngol Head Neck Surg. 2015;141:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Boesch RP, Balakrishnan K, Acra S, Benscoter DT, Cofer SA, Collaco JM, Dahl JP, Daines CL, DeAlarcon A, DeBoer EM, Deterding RR, Friedlander JA, Gold BD, Grothe RM, Hart CK, Kazachkov M, Lefton-Greif MA, Miller CK, Moore PE, Pentiuk S, Peterson-Carmichael S, Piccione J, Prager JD, Putnam PE, Rosen R, Rutter MJ, Ryan MJ, Skinner ML, Torres-Silva C, Wootten CT, Zur KB, Cotton RT, Wood RE. Structure and Functions of Pediatric Aerodigestive Programs: A Consensus Statement. Pediatrics. 2018;141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Gumer L, Rosen R, Gold BD, Chiou EH, Greifer M, Cohen S, Friedlander JA. Size and Prevalence of Pediatric Aerodigestive Programs in 2017. J Pediatr Gastroenterol Nutr. 2019;68:e72-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Arganbright JM, Lee JC, Weatherly RA. Pediatric drug-induced sleep endoscopy: An updated review of the literature. World J Otorhinolaryngol Head Neck Surg. 2021;7:221-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Wineski RE, Panico E, Karas A, Rosen P, Van Diver B, Norwood TG, Grayson JW, Beltran-Ale G, Dimmitt R, Kassel R, Rogers A, Leonard M, Chapman A, Boehm L, Wiatrak B, Harris WT, Smith N. Optimal timing and technique for endoscopic management of dysphagia in pediatric aerodigestive patients. Int J Pediatr Otorhinolaryngol. 2021;150:110874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Day KE, Smith NJ, Kulbersh BD. Early surgical intervention in type I laryngeal cleft. Int J Pediatr Otorhinolaryngol. 2016;90:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kim S, Park M, Kim E, Kim GE, Jung JH, Kim SY, Kim MJ, Kim DH, Park S, Koh H, Ho IG, Kim SK, Hwang S, Shin KH, Lee H, Lee B, Sohn MH, Rha DW, Kim KW. Development of a Multidisciplinary Aerodigestive Program: An Institutional Experience. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Al-Zubeidi D, Demir H, Bishop WP, Rahhal RM. Gastrojejunal feeding tube use by gastroenterologists in a pediatric academic center. J Pediatr Gastroenterol Nutr. 2013;56:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 13. | Tambucci R, Rea F, Angelino G, Malamisura M, Mennini M, Riccardi C, Farello G, Valfré L, Dall'Oglio L, Markowitz JE, Fiocchi AG, De Angelis P. Eosinophilic esophagitis in esophageal atresia: Tertiary care experience of a "selective" approach for biopsy sampling. World Allergy Organ J. 2020;13:100116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Tuğba RG, Tuğba ŞE, Ayşe TA, Zeynep RO, Pelin A, Cem K, Ramazan K. Review of Complications of Operated Esophageal Atresia and Tracheoesophageal Fistula Patients. Turk Arch Pediatr. 2021;56:380-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Leibovitch L, Zohar I, Maayan-Mazger A, Mazkereth R, Strauss T, Bilik R. Infants Born with Esophageal Atresia with or without Tracheo-Esophageal Fistula: Short- and Long-Term Outcomes. Isr Med Assoc J. 2018;20:161-166. [PubMed] |
| 16. | Cartabuke RH, Lopez R, Thota PN. Long-term esophageal and respiratory outcomes in children with esophageal atresia and tracheoesophageal fistula. Gastroenterol Rep (Oxf). 2016;4:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Boesch RP, de Alarcon A, Piccione J, Prager J, Rosen R, Sidell DR, Wootten C, Balakrishnan K; Aerodigestive Research Collaborative. Consensus on Triple Endoscopy Data Elements Preparatory to Development of an Aerodigestive Registry. Laryngoscope. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ludemann JP, Hughes CA, Noah Z, Holinger LD. Complications of pediatric laryngotracheal reconstruction: prevention strategies. Ann Otol Rhinol Laryngol. 1999;108:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Halstead LA. Gastroesophageal reflux: A critical factor in pediatric subglottic stenosis. Otolaryngol Head Neck Surg. 1999;120:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Sasaki CT, Horiuchi M, Koss N. Tracheostomy-related subglottic stenosis: bacteriologic pathogenesis. Laryngoscope. 1979;89:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Gray S, Miller R, Myer CM 3rd, Cotton RT. Adjunctive measures for successful laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 1987;96:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | DeBoer EM, Kinder S, Duggar A, Prager JD, Soden J, Deterding RR, Ruiz AG, Jensen EL, Weinman J, Wine T, Fortunato JE, Friedlander JA. Evaluating the yield of gastrointestinal testing in pediatric patients in aerodigestive clinic. Pediatr Pulmonol. 2018;53:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Rosen R, Amirault J, Johnston N, Haver K, Khatwa U, Rubinstein E, Nurko S. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2014;49:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Hart CK, de Alarcon A, Tabangin ME, Hamilton S, Rutter MJ, Pentiuk SP, Garza JM. Impedance probe testing prior to pediatric airway reconstruction. Ann Otol Rhinol Laryngol. 2014;123:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Wentland C, Hersh C, Sally S, Fracchia MS, Hardy S, Liu B, Garcia JA, Hartnick CJ. Modified Best-Practice Algorithm to Reduce the Number of Postoperative Videofluoroscopic Swallow Studies in Patients With Type 1 Laryngeal Cleft Repair. JAMA Otolaryngol Head Neck Surg. 2016;142:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Boesch RP, Balakrishnan K, Grothe RM, Driscoll SW, Knoebel EE, Visscher SL, Cofer SA. Interdisciplinary aerodigestive care model improves risk, cost, and efficiency. Int J Pediatr Otorhinolaryngol. 2018;113:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Gascon E, Klauser P, Kiss JZ, Vutskits L. Potentially toxic effects of anaesthetics on the developing central nervous system. Eur J Anaesthesiol. 2007;24:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Ruiz AG, Bhatt JM, DeBoer EM, Friedlander J, Janosy N, Peterson MB, Wine T, Deterding R, Prager JD. Demonstrating the benefits of a multidisciplinary aerodigestive program. Laryngoscope. 2020;130:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |