Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3608
Peer-review started: February 16, 2022
First decision: April 12, 2022
Revised: April 24, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 28, 2022
Processing time: 160 Days and 23.1 Hours
Proton pump inhibitors (PPIs), the most commonly used antisecretory medi-cations in the management of reflux illness, virtually eliminate elective surgery for ulcer disease, and relegate anti-reflux surgery to patients with gastroesophageal reflux disease (GERD) who are inadequately managed by medical therapy. However, PPI medications still leave some therapeutic demands of GERD unmet. Furthermore, up to 40%-55% of daily PPI users have chronic symptoms, due to PPI refractoriness. Potassium-competitive acid blockers (P-CABs) transcend many of the problems and limits of PPIs, delivering quick, powerful, and extended acid suppression and allowing for treatment of numerous unmet needs. Recently, it has become clear that compromised mucosal integrity plays a role in the etiology of GERD. As a result, esophageal mucosal protection has emerged as a novel and potential treatment approach. An increasing body of research demonstrates that when P-CABs are used as primary drugs or add-on drugs (to regular treatment), they provide a considerable extra benefit, particularly in alleviating symptoms that do not respond to PPI therapy.
Core Tip: The potassium-competitive acid blocker (P-CAB) has been discovered as a possible acid suppression therapeutic option. At the enzyme level, P-CABs compete with K+ to suppress acid formation; the binding location of these compounds is separate from the probable pocket that K+ occupies. When the P-CAB binds to the enzyme, it stops K+ from attaching and activating it. According to clinical trials, P-CABs are extremely selective for gastric H+, K+-ATPase, restricting stomach acid production while acting quickly. Such medicines, which can have a rapid onset of action and a longer duration, may offer considerable advantages to individuals suffering from gastroesophageal reflux disease and other acid-related disorders.
- Citation: Leowattana W, Leowattana T. Potassium-competitive acid blockers and gastroesophageal reflux disease. World J Gastroenterol 2022; 28(28): 3608-3619
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3608
Gastroesophageal reflux, the reflux of stomach contents into the esophagus, usually occurs after large and fatty meals. Nonetheless, under normal settings, efficient esophageal cleaning mechanisms return the bulk of the refluxed material to the stomach, and symptoms do not occur. However, when stomach reflux is severe or physically aggressive, it causes symptoms and difficulties, as well as a decrease in quality of life, thus creating gastroesophageal reflux disease (GERD)[1,2]. Since the 1990s, the prevalence of GERD has grown consistently, particularly in East Asia and North America (2.5%-7.8%, and 10%-20%, respectively)[3-5]. Heartburn and regurgitation are common esophageal symptoms, accompanied by consequent chest discomfort and dysphagia. Chronic cough, hoarseness of voice, and asthma are other extra-esophageal symptoms that have been linked to GERD in population-based research. The three GERD phenotypes are erosive esophagitis (EE), non-erosive reflux disease (NERD), and Barrett's esophagitis. Their responses to therapy vary greatly. Of note, the majority (70%) of GERD patients have an endoscopically normal mucosa and are regarded as having NERD.
Pharmacologic, endoscopic, and surgical options for GERD treatment are now available. The most often recommended drugs for GERD treatment in clinical practice are proton pump inhibitors (PPIs) and histamine-2 receptor antagonists. Other, less potent agents typically used to treat mild or intermittent symptoms or as adjunct therapies include antacids, sucralfate, baclofen, alginate, and prokinetics[6,7]. The goals of GERD management include symptom relief, esophageal inflammation healing, esophageal inflammation maintenance and prevention, and quality of life enhancement. PPIs have long been thought to be the basis of GERD treatment. Because of their significant and consistent antisecretory action, PPIs have proven particularly useful in treating EE, managing symptoms, and preventing GERD-related complications such as esophageal ulcers, esophageal bleeding, and peptic stricture. Overall, PPIs have been regarded as a relatively safe class of drugs, with many patients taking them for an extended time. Despite the success of PPIs in addressing many aspects of GERD, there are still many unmet needs[8-10]. Advanced EE, NERD, overnight heartburn, maintenance therapy, and refractory GERD are among them.
Furthermore, PPIs are ineffective for postprandial heartburn and are currently not recommended for atypical or extra-esophageal GERD presentations as well as GERD complications[11,12]. Importantly, prolonged PPI medications have been linked to side effects, generating worries among physicians and patients alike about their safety profile. This review looks at the development and quality of present and potential acid suppression medications, focusing on the potassium-competitive acid blocker (P-CAB) class and exploring their clinical studies.
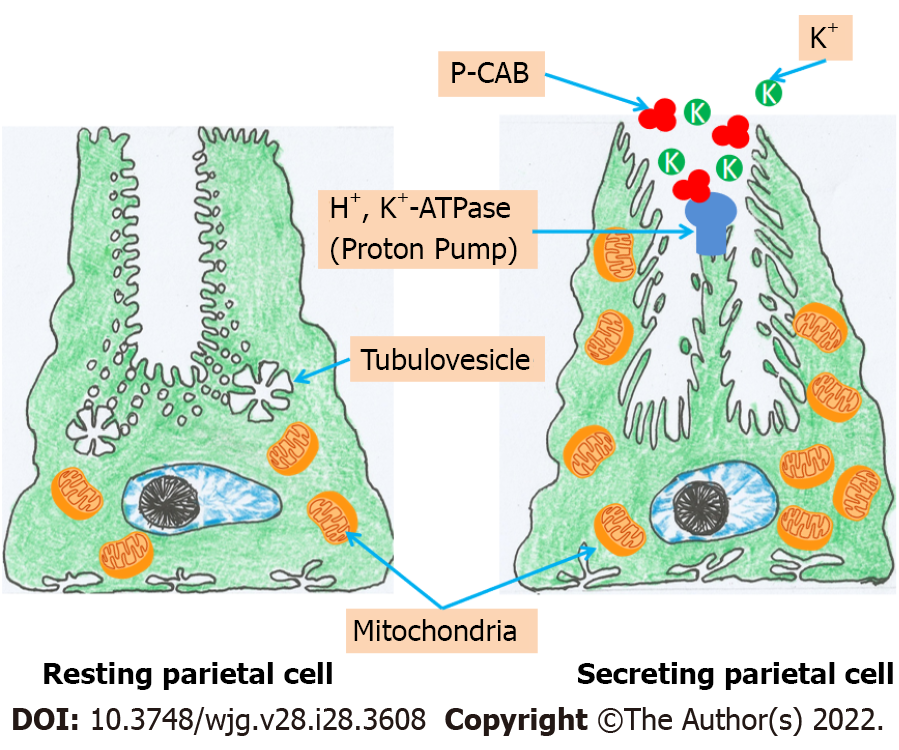
Gastric acid is essential for food and water purification and digestion. Gastric juice has an extremely low pH (pH = 1) because parietal cells in the oxyntic mucosa of the stomach secrete H+ and Cl- ions to make hydrochloric acid. These cells secrete 1-2 L of hydrochloric acid every day. The action of gastric H+/K+-ATPase in the apical membrane of parietal cells promotes a very high concentration of H+ in the lumen compared to plasma. The potassium ion plays an essential role in activating gastric H+/K+-ATPase and is required for the enzyme to function. At rest, H+/K+-ATPase is confined to tubulovesicular regions of a parietal cell with low K+ concentrations and membranes that are impermeable to K+. As a result, the enzyme is incapable of activating and transporting H+ ions. When the parietal cell is stimulated, the tubulovesicular components merge with the cell’s apical membrane. After being exposed to K+-containing luminal fluid, the H+/K+-ATPase enzyme can begin to exchange H+ for K+[13-15]. Due to its important involvement in the production of gastric juice, K+ is a possible therapeutic target for acid blockers. One technique is to block the K+ channels that allow ions to flow through the apical membrane of parietal cells, while another is to compete with K+ at the level of the parietal cells' H+/K+-ATPase.
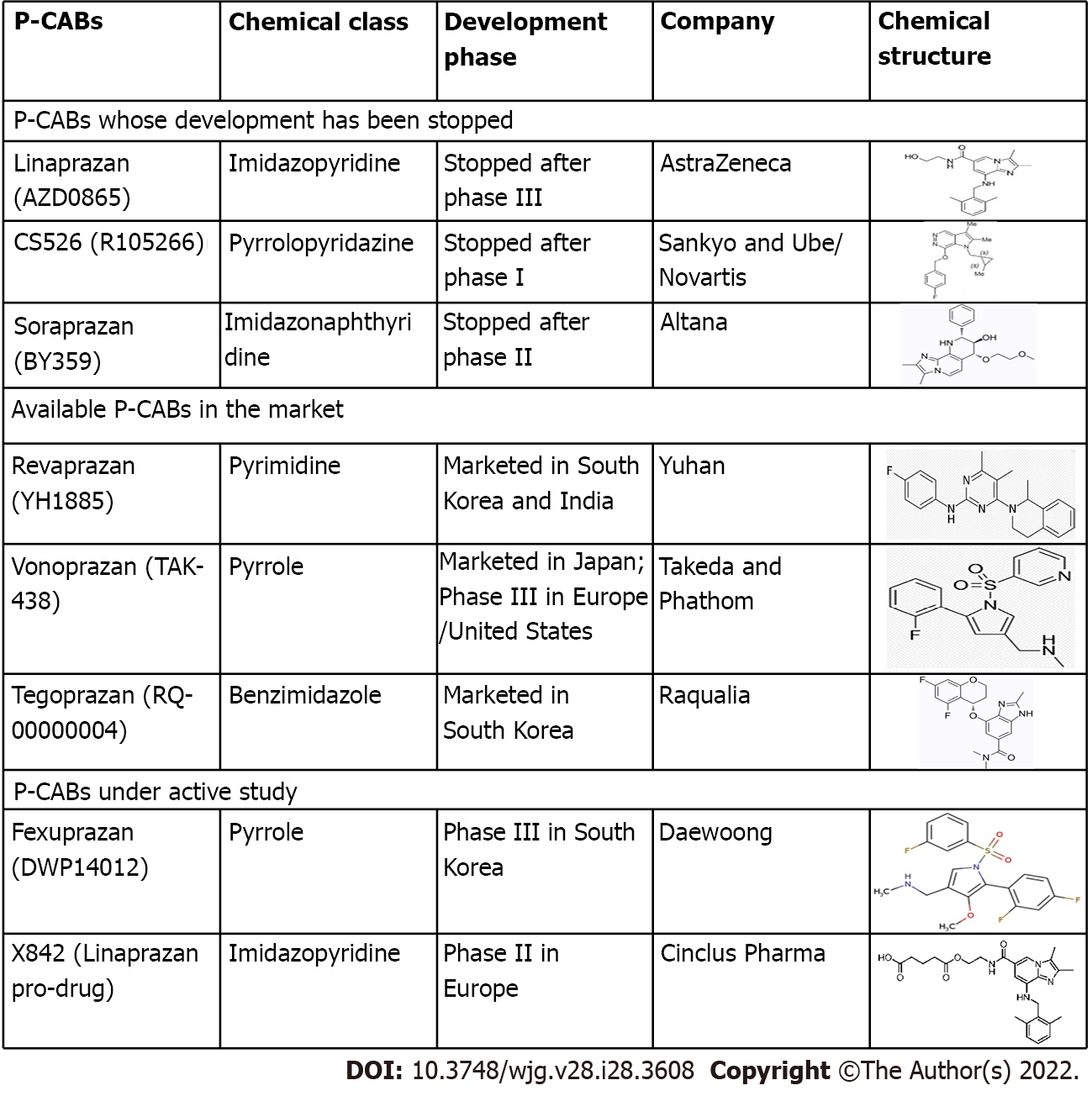
The surface of the stomach H+/K+-ATPase (the proton pump) is exposed to the extremely acidic parietal cell canaliculus, which has a high affinity for K+ during acid secretion. Given the significance of the cation for enzyme activity, drugs that compete for K+ binding have the potential to inhibit acid secretion. The mechanism of action of P-CAB is based on this premise. P-CABs were initially developed in the 1980s and have been studied by many pharmaceutical companies worldwide due to their ability to block the proton pump quickly, efficiently, and reversibly. Schering-Plough was one of those companies that developed a prototype P-CAB, SCH28080. Although the mechanism of action was not fully known at that time, this drug was discovered to lower stomach acid output in humans[16,17]. SCH28080 was later demonstrated to inhibit gastric H+/K+-ATPase by competing with K+[18,19]. However, clinical development of SCH28080 was halted due to hepatotoxicity produced by repeated treatment. This condition sparked research into several kinds of SCH28080 derivatives, such as imidazopyridine derivatives (BY841), imidazo-naphthyridine derivatives (soraprazan), imidazo-thienopyridines (SPI-447), quinolone derivatives (SK&F96067)[20-23], pyrrolo-pyridazine derivatives (CS-526), pyrimidine derivatives (revaprazan), and pyrrole derivatives [vonoprazanm (VPZ)][24-26].
These novel antisecretory medicines vary from PPIs in that they compete with K+ and cause a dose-dependent selective and reversible inhibition of the proton pump. Because they aren't prodrugs that need to be activated in parietal cells like PPIs, they start working right away, and control of acid secretion begins within the first day of treatment after starting the first dose. In addition, their dissociation from the proton pump is sluggish, and they can stay in the stomach mucosa for up to 24 h. As a result, unlike PPIs, which are less effective at night, their acid inhibitory action persists throughout the day and night[27,28]. Table 1 compares the key differences in the mechanisms of action of P-CABs and PPIs.
| Proton pump inhibitors | Potassium-competitive acid blockers |
| Prodrug requires transformation to the active form, sulphenamide | Direct action on the H+/K+ ATPase after protonation |
| Sulphenamide binds covalently to H+/K+-ATPase | P-CABs bind competitively to the K+ binding site of H+/K+-ATPase |
| Irreversible binding to the proton pump | Reversible binding to the proton pump |
| The full effect after repeated doses | The full effect after the first dose |
| PK affected by genetic polymorphism | PK not affected by genetic polymorphism |
| PD effect more significant during the daytime | PD effect lasting for both the daytime and nocturnal hours |
| Meal-dependent antisecretory activity | Meal-independent antisecretory activity |
| Concentrate in parietal cell acid space (1000-fold higher than in plasma) | Super concentrates in parietal cell acid space (100000-fold higher than in plasma) |
| Duration of effect related to the half-life of the sulphenamide-enzyme complex | Duration of effect related to the half-life of the drug in plasma |
P-CABs are classified into many chemical classes (Figure 1). They are a varied class of medications while having a similar mechanism of action. P-CABs are lipophilic, weak bases with limited pH stability and high pKa values. They may concentrate in acidic environments because of the combination of these properties. In the parietal cell canaliculi (pH = 1), a P-CAB with pKa of 6.0 would be 100000-fold larger than in the plasma (pH = 7.4). In vitro and in vivo investigations using AZD0865 and revaprazan showed a concentrated P-CAB in the gastric mucosa[29-31]. By binding ionically to the enzyme, P-CAB inhibits gastric H+/K+-ATPase and prevents further activation by K+. P-CABs are expected to bind at or near the K+ binding site, preventing the K+ from accessing the site (Figure 2). Although these novel drugs exhibit quick and potent antisecretory action, not all of their attractive pharmacodynamic features have translated into therapeutic advantages due to their hepatotoxicity and insufficient efficacy. In addition, linaprazan (AZD0865) failed to outperform standard-dose PPIs in the treatment of peptic ulcers and reflux esophagitis (RE)[32,33]. Soraprazan (BY359) and CS526 (R105366) have fulfilled their proof of effectiveness and safety goals in phase II tests, but data on these medicines have not been published[34].
P-CABs reach peak plasma concentrations quickly after oral administration, in part because they are acid-stable and may be administered as immediate-release formulations. In healthy men, a single dose of revaprazan (100 to 200 mg) resulted in peak plasma concentrations at 1.7 to 1.8 h, which declined to a mean elimination time (T1/2) of 2.2 h to 2.4 h with repeated administration. The pharmacokinetics and concentration-time profiles of revaprazan were comparable following repeated administration (on day 7) to those reported after the initial dosage on day 1[35]. In 2004, Yu et al[36] conducted a single-blind, dose-rising, parallel-group, randomized, placebo-controlled trial in 46 healthy subjects. In the single-dose research, plasma concentrations of revaprazan attained peak values 1.3 h to 2.5 h after administration and thereafter decreased mono-exponentially with a terminal T1/2 of 2.2 h to 2.4 h in dosage groups up to 200 mg. Revaprazan has linear pharmacokinetic properties, with negligible accumulation after numerous doses. The onset of pharmacological effects was quick, with the greatest benefits shown on the first day of treatment with repeated doses. They concluded that revaprazan was safe, well-tolerated, and efficiently suppressed acid secretion by increasing intragastric pH in a dose-dependent manner.
In 2015, Sakurai et al[37] conducted 2 phase I, single rising-dose, randomized, double-blind, placebo-controlled trials in 84 volunteers from Japan using 1-120 mg VPZ and 63 healthy males from the United Kingdom using 1-40 mg VPZ to evaluate the pharmacokinetics, pharmacodynamics, safety, and tolerability. They discovered that VPZ plasma concentration-time profiles exhibited fast absorption at all doses, with a median Tmax of up to 2 h. T1/2 was predicted to be up to 9 h. The acid suppression effect was dose-dependent, with a 24-h intragastric pH 4 holding time for 40 mg VPZ being 92% in Japanese males and 87% in British males. They determined that single oral doses of 20-120 mg VPZ suppressed gastric acid production in healthy male participants in a quick, deep, and 24-h manner. Furthermore, VPZ was well tolerated at all dosages tested, suggesting that it might be used as an alternate therapy for acid-related diseases.
Recently, Han et al[38] performed a phase I, randomized, placebo-controlled study in 56 healthy volunteers without Helicobacter pylori infection to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of tegoprazan (TPZ). They found that TPZ was well tolerated. The majority of the adverse events were mild and resolved without any long-term consequences. On day 7, despite multiple dosages of TPZ, there was no accumulation in the plasma. The pharmacodynamics study found that TPZ suppressed gastric acid rapidly in a dose-dependent manner. They concluded that TPZ was well tolerated and demonstrated rapid and potent gastric acid suppression.
Sunwoo et al[39] conducted a randomized, double-blind, double-dummy, placebo-controlled study to evaluate a single ascending dose and multiple ascending doses of fexuprazan (FPZ) in 120 healthy male subjects without Helicobacter pylori (H. pylori) infection. They discovered that FPZ was well tolerated and suppressed gastric acid output for 24 h after delivery. In the multiple ascending doses trial, FPZ plasma concentrations grew in a dose-proportional way, but it did not appreciably accumulate in the plasma in the single ascending dosage study. They concluded that FPZ showed an immediate, long-lasting gastric acid suppression effect and was safe.
In 2020, Hwang et al[40] conducted a single- and multiple-dose, randomized, double-blind, placebo-controlled trial to elucidate the pharmacodynamics, pharmacokinetics, and safety of FPZ among healthy volunteers of Korean, Caucasian, and Japanese descent. FPZ (40, 60, or 80 mg for Koreans; 40 or 80 mg for Caucasians; 20, 40, or 80 mg for Japanese) or a placebo were given to ten participants in each group at random. Gastric acid suppression was shown to be consistent across ethnic groups. After successive doses of 40 mg, the mean percentages of time when the intragastric pH was over 4 in Korean, Caucasian, and Japanese participants were 64.3 percent, 62.8 percent, and 70.3 percent, respectively. Furthermore, the 80 mg dose that could effectively suppress gastric secretion was 94.8%, 90.6%, and 90.6% for the Korean, Caucasian, and Japanese subjects, respectively. They determined that FPZ suppressed stomach acid in the same way in Korean, Caucasian, and Japanese patients, and that the pharmacokinetic and pharmacodynamic correlations, as well as the safety, were the same in all three ethnic groups. The FPZ could be utilized regardless of ethnicity.
X842 is a linaprazan prodrug that is currently under development. X842 has a T1/2 of 10 h and enables improved intragastric pH regulation over 24 h. Linaprazan was established in a phase I study that used a single dose or multiple escalating dosage design to assess the drug’s safety and tolerability in healthy volunteers as the primary endpoint[41]. X842 was shown to be safe and well tolerated by the participants in the study. During the trial, no severe or significant adverse events were recorded. When both pharmacodynamics and pharmacokinetics were evaluated, precise dose linearity was identified. The mean median intragastric pH at each X842 dosage was never less than 4.
In 2015, Ashida et al[42] undertook a parallel-group, dose-ranging, multicenter, randomized, double-blind trial in 732 patients with EE to assess the effectiveness and safety of VPZ vs lansoprazole (LPZ). The eligible EE individuals were randomized 1:1:1:1:1 to undergo an 8-wk therapy with LPZ 30 mg, VPZ 5 mg, VPZ 10 mg, VPZ 20 mg, and VPZ 40 mg. They discovered that with VPZ 5, 10, 20, and 40 mg, and LPZ 30 mg, the proportion of healed EE individuals at week 4 was 92.3%, 92.5%, 94.4%, 97.0% and 93.2%, respectively. When corrected for baseline Los Angeles (LA) grades A/B and C/D, all VPZ regimens were non-inferior to LPZ 30 mg therapy. With VPZ 5, 10, 20, 40 mg, and LPZ 30 mg, the proportions of healed EE individuals were 87.3%, 86.4%, 100%, 96.0%, and 87.0%, respectively, for LA grades C/D. The incidence of adverse events was the same in all groups. They indicated that VPZ was efficacious and comparable to LPZ in the treatment of EE. For severe EE (LA grades C/D), they advised VPZ doses of 20 mg or more.
Furthermore, Ashida et al[43] performed another parallel-group comparison, multicenter, randomized, double-blind trial to evaluate the non-inferiority, long-term efficacy, and safety as maintenance therapy of VPZ 20 mg compared with LPZ 30 mg in 409 endoscopically confirmed EE patients (LA grades A-D). They discovered that the proportion of patients with healed EE up to week 8 for VPZ (203/205) and 95.5% for LPZ (190/199) was 99.0% for VPZ (203/205) and 95.5% for LPZ (190/199), demonstrating the non-inferiority of VPZ 20 mg therapy. There were a few EE recurrences (< 10%) for long-term maintenance evaluation in patients treated with VPZ 10 mg or 20 mg. They stated that the comparative trial demonstrated the non-inferiority of VPZ 20 mg to LPZ 30 mg in EE, and that VPZ was well tolerated and efficacious among long-term maintenance EE patients (Table 2).
| Ref. | Country/region | Study design | Patients, n | Diagnosis | Treatment groups | Duration of treatment, wk | Clinical outcomes |
| Ashida et al[42] | Japan | Multicenter, randomized, double-blind | 732 | EE | LPZ 30 mg; VPZ 5 mg; VPZ 10 mg; VPZ 20 mg; VPZ 40 mg | 8 | All VPZ regimens were non-inferior to LPZ 30 mg treatment. The proportions of healed EE subjects were 87.3%, 86.4%, 100%, 96.0%, and 87.0% with VPZ 5, 10, 20, 40 mg, and LPZ 30 mg, respectively |
| Ashida et al[43] | Japan | Multicenter, randomized, double-blind | 607 | EE | LPZ 15 mg; VPZ 10 mg; VPZ 20 mg | 24 | The EE recurrence rates were 16.8%, 5.1%, and 2.0% with LPZ 15 mg, VPZ 10 mg, and VPZ 20 mg, respectively, over the 24-wk maintenance period |
| Shinozaki et al[47] | Japan | Retrospective cohort | 55 | NERD = 30; EE = 25 | VPZ 10 mg | 4 | The VPZ 10 mg for 1-mo alleviated GERD symptoms in 89% and were sustained in 82% after 1 yr without further therapy |
| Oshima et al[48] | Japan | Randomized placebo control | 32 | EE | LPZ 30 mg; VPZ 20 mg | 2 | The heartburn was relieved earlier with VPZ than with LPZ, and it was completely relieved in 31.3% and 12.5% of patients on day 1 with VPZ and LPZ, respectively |
| Akiyama et al[49] | Japan | Retrospective cohort with prospective study | 13 | PPI-refractory GERD | PPIs switch to VPZ 20 mg | 8 | The median gastric acid exposure times of the VPZ 20 mg were lower than the median gastric acid exposure times of the PPI treatment in both daytime and nocturnal observations. The VPZ 20 mg outperforms PPIs in stomach acid suppression, EAE control, symptom alleviation, and esophagitis healing in patients with PPI-refractory GERD |
| Mizuno et al[50] | Japan | Open-label, single-center, prospective study | 50 | PPI-refractory RE | PPIs switch to VPZ 20 mg for 4 wk and VPZ 10 mg | 48 | VPZ 10 mg prevented the recurrence of esophageal mucosal breaks in 43 of 50 (86.0%) patients. VPZ 10 mg is clinically efficacious for the long-term maintenance of healed RE |
| Xiao et al[51] | China, South Korea, Taiwan, Malaysia | Phase III, double-blind, multicenter study | 481 | EE | LPZ 30 mg; VPZ 20 mg | 8 | The 8-wk EE healing rates in the VPZ and LPZ groups were 92.4% and 91.3%, respectively. VPZ 20 mg was not inferior to LPZ 30 mg in EE healing at 8 wk |
| Okanobu et al[52] | Japan | Randomized control study | 73 | EE | VPZ 10 mg; VPZ 20 mg | Each dose for 4 wk and VPZ 10 mg for 8 wk | VPZ 10 mg had the same result as VPZ 20 mg in mucosal healing and symptom reduction at 4 wk and throughout the trial |
| Matsuda et al[53] | Japan | Multicenter, randomized, cross-over study | 122 | Erosive GERD | VPZ 10 mg; LPZ 15 mg | Every 2nd day for 4 wk and cross-over for more 4 wk | GERD symptoms were significantly reduced with VPZ 10 mg every other day, as measured by the FSSG and the gastrointestinal symptom rating scale |
| Lee et al[54] | South Korea | Randomized, double-blind, parallel-group comparison study | 302 | EE | TPZ 50 mg; TPZ 100 mg; EPZ 40 mg | 4 and 8 | At week 8, the cumulative healing rates for TPZ 50 mg, TPZ 100 mg, and EPZ 40 mg were 98.9%, 98.9%, and 98.9%, respectively |
| Kim et al[55] | South Korea | Phase III, double-blind, placebo-controlled, multicenter study | 324 | NERD | TPZ 50 mg; TPZ 100 mg; placebo | 4 | The proportions of heartburn-free days and full-resolution rates of heartburn were significantly higher in both TPZ groups than in the placebo group |
| Lee et al[56] | South Korea | Phase III, multicenter, randomized, double-blind trial | 260 | EE | FPZ 40 mg; EPZ 40 mg | 8 | FPZ 40 mg was non-inferior to EPZ 40 mg. FPZ 40 mg provided better symptom relief in patients with moderate to severe heartburn, with the effect lasting throughout the night |
In 2017, Iwakiri et al[44] conducted a randomized, double-blind, multicenter trial of VPZ 20 mg or 40 mg in 19 patients with PPI-resistant (LPZ 30 mg) EE to measure stomach and esophageal pH over 24 h. Patients with endoscopically proven PPI-resistant EE received VPZ 20 mg (9 cases) or VPZ 40 mg (10 cases) for 8 wk after a 7- to 14-d run-in period with LPZ 30 mg therapy. The percentage of stomach pH 4 [pH 4 holding time ratio (HTR)] increased significantly in both groups from baseline: In the 20 mg group, it increased from 73.21% to 96.46%, and in the 40 mg group, it increased from 69.97% to 100.00%. There were no significant increases in esophageal pH 4 HTRs from baseline. After 8 wk of treatment, the healing rate in patients with baseline EE grades A-D was 60.0% in the 20 mg group and 71.4% in the 40 mg group. They concluded that VPZ 20 mg and 40 mg substantially suppressed gastric acid secretion over 24 h with considerably higher gastric pH at 4 HTR, resulting in an EE repair rate greater than 60%.
In the same year, Yamashita et al[45] conducted a study to evaluate the effects of VPZ and PPIs in 8 RE patients using multichannel intraluminal impedance-pH. They found that the mucosal lesions in 7/8 patients (87.5%) with persistent gastric mucosal injury after completing an 8-wk standard PPI therapy had entirely healed after VPZ therapy. From 26.5% to 78.0%, there was a considerable rise in stomach pH > 4 HTR. In addition, a decrease in esophageal pH of 4 HTR was seen, although it was not statistically significant. The overall number of reflux events, comprising acid and proximal reflux episodes, as well as the time it took for acid to clear, were both dramatically reduced. They concluded that VPZ should be used in patients with PPI-refractory RE.
In 2018, Ashida et al[46] conducted a study to compare VPZ 10 mg (n = 202) and 20 mg (n = 204) with LPZ 15 mg (n = 201) as maintenance treatment in 607 patients with healed EE. EE recurrence rates with LPZ 15 mg, VPZ 10 mg, and VPZ 20 mg were 16.8%, 5.1%, and 2.0%, respectively, throughout a 24-wk maintenance period. VPZ was shown to be non-inferior to 15 mg LPZ. The 10 mg and 20 mg VPZ were significantly lower than the LPZ at 15 mg. The EE recurrence rate, on the other hand, did not change substantially between the two VPZ dosages. They determined that VPZ 10 mg and 20 mg were non-inferior to LPZ 15 mg as maintenance treatment for individuals with healed EE.
A retrospective cohort study that recruited 55 patients with symptomatic GERD (NERD = 30, EE = 25) treated with VPZ 10 mg who had been followed for more than 1 year was conducted by Shinozaki et al[47]. They discovered that taking VPZ 10 mg for one month relieved GERD symptoms in 89% of patients and was maintained in 82% after one year without further treatment. In 47% of cases, 1-year maintenance treatment resulted in long-term relief of GERD symptoms. Nine of the forty-nine responders experienced a return of GERD symptoms, while VPZ dosage escalation relieved symptoms in 67% (6/9) of patients. Postprandial discomfort, postprandial distress, constipation, and diarrhea significantly decreased after 1 m and remained stable after a year. After 1 year of therapy, the endoscopic healing rate of EE was 95%. They concluded that 1 year of VPZ therapy reduces GERD symptoms significantly, and endoscopic healing of EE is good. VPZ is an efficient and beneficial long-term treatment for GERD.
In 2019, Oshima et al[48] conducted a randomized, placebo-controlled experiment in 32 patients with endoscopically proven EE who had heartburn at least once a week to see how quickly VPZ and LPZ relieve heartburn. For 14 d, the patients were given either VPZ 20 mg or LPZ 30 mg before breakfast. They found that heartburn was relieved earlier with VPZ than with LPZ. Furthermore, on day 1, VPZ and LPZ totally cured heartburn in 31.3% and 12.5% of patients, respectively. With VPZ medication, significantly more patients experienced full nighttime heartburn resolution compared with LPZ treatment. They concluded that during the first week of therapy, VPZ provided more prolonged heartburn relief than LPZ.
Recently, Akiyama et al[49] studied the efficacy of VPZ 20 mg in 13 patients with PPI-refractory GERD who exhibited continued pathological esophageal acid exposure (EAE). Among 13 patients who were observed by multichannel intraluminal impedance-pH at baseline (PPI treatment) and after VPZ 20 mg therapy, the median gastric acid exposure times of the VPZ group were lower than those of the PPI group, both during daytime and nocturnal observations. Furthermore, during the 24-h monitoring period, VPZ 20 mg treatment resulted in lower median EAE values (4.5%) than PPI therapy (10.6%). EAE normalization occurred in 46% of VPZ-treated people, and it was connected to complete stomach acid reduction (P = 0.005). Reflux symptoms (P = 0.01) and EE (P = 0.01) improved after switching to VPZ 20 mg. They concluded that VPZ 20 mg outperforms PPIs in stomach acid suppression, EAE control, symptom alleviation, and esophagitis healing in patients with PPI-refractory GERD.
Mizuno et al[50] carried out a study to assess the effectiveness of VPZ 10 mg as a maintenance treatment for healed RE in 50 patients who completed 48-wk maintenance therapy. Maintenance therapy with VPZ 10 mg at 48 wk avoided the recurrence of esophageal mucosal breaches in 43 of 50 patients (86.0%). Throughout the 48-wk maintenance therapy, the symptomatic non-relapse rate for acid reflux-related symptom score on the frequency scale for GERD symptoms and acid reflux score on the gastrointestinal symptom rating scale was 70.0% and 72.0%, respectively. They determined that VPZ 10 mg is clinically efficacious for the long-term maintenance of healed RE.
In 2020, Xiao et al[51] conducted a phase III, double-blind, multicenter study in 468 endoscopically confirmed EE patients and randomly assigned them to take either VPZ 20 mg (238) or LPZ 30 mg (230) once daily for 8 wk. They found that the 8-wk EE healing rates in the VPZ and LPZ groups were 92.4% and 91.3%, respectively. Moreover, in patients with a baseline LA classification of C/D, VPZ had higher 2-wk, 4-wk, and 8-wk EE healing rates than LPZ. Overall, the rates of EE healing appeared to be greater with VPZ than with LPZ. They concluded that VPZ is not inferior to LPZ in EE healing at 8 wk, and the two treatment groups had identical safety results.
Last year, Okanobu et al[52] performed a randomized control study to evaluate the efficacy of VPZ 10 mg (n = 36) compared with VPZ 20 mg (n = 37) in 73 patients with EE. The patients were given each dose for four weeks as an initial treatment, followed by eight weeks of maintenance therapy with VPZ 10 mg. The endoscopic healing rates of the 20 mg and 10 mg groups were 94.6% and 94.4%, respectively, after four wk. In both treatment groups, the frequency scale for GERD symptoms decreased significantly, from 13 to 4 and 14 to 3, respectively, in the 20 mg and 10 mg groups. The scores have plummeted to 2 after 12 wk. They determined that after 4 wk and throughout the experiment, VPZ 10 mg medication had a similar therapeutic response to VPZ 20 mg treatment in terms of mucosal repair and symptom reduction. These results were also the same in LA classification grade A/B patients but not in grade C/D patients.
A prospective, multicenter, open-label, randomized cross-over trial with two periods was conducted by Matsuda et al[53] to clarify the efficacy and superiority of VPZ 10 mg every other day over LPZ 15 mg in the maintenance management of 122 erosive GERD patients. They observed that 93.6% of the VPZ group and 82.1% of the LPZ group had well-controlled symptoms, with a significant difference (P = 0.003) using McNemar's test. The VPZ-LPZ and LPZ-VPZ groups, respectively, had 96.7% and 80.0% of patients well managed throughout the first four weeks (P = 0.007). For the second 4 wk, 94.4% of patients in the VPZ-LPZ and 76.7% of patients in the LPZ-VPZ groups were well controlled following 6 consecutive days a week (P = 0.009). They found that taking VPZ 10 mg every other day decreased GERD symptoms considerably, as indicated by the frequency scale for the gastrointestinal symptom rating scale and GERD symptoms.
Lee et al[54] conducted a parallel-group, multicenter, randomized, double-blind comparative trial in 302 Korean patients with endoscopically confirmed EE in 2019. The patients were randomly assigned to receive TPZ 50 mg, TPZ 100 mg, or EPZ 40 mg for 4 wk or 8 wk. They confirmed that at week 8, the cumulative healing rates for TPZ 50 mg, TPZ 100 mg, and EPZ 40 mg were 98.9% (91/92), 98.9% (90/91), and 98.9% (87/88), respectively. Both TPZ dosages were non-inferior to EPZ 40 mg, and TPZ was well tolerated.
Kim et al[55] undertook a multicenter, double-blind, placebo-controlled, phase III trial in 324 Korean patients with NERD in 2021 to investigate the safety and effectiveness characteristics of TPZ relative to placebo. They were randomized into three groups with TPZ 50 mg, TPZ 100 mg, and placebo once daily for 4 wk. After 4 wk of therapy with TPZ 50 mg, TPZ 100 mg, or placebo, 42.5% (45/106), 48.5% (48/99), and 24.2% (24/99) of patients achieved full relief of significant symptoms. TPZ 50 mg and 100 mg performed better than the placebo by a substantial statistical difference. Both the TPZ and placebo groups had significantly greater percentages of heartburn-free days and full-resolution rates of heartburn. Furthermore, no apparent change in the occurrence of treatment-emergent adverse events was observed.
In a phase III, multicenter, randomized, double-blind trial, FPZ, the antisecretory action of a pyrrole derivative with a quick and full onset was tested. A total of 260 adult patients with endoscopically proven EE (LA grades A to D) were given either FPZ 40 mg or EPZ 40 mg per day. The cumulative proportion of patients with healed mucosal breaks, as evaluated by endoscopy, was the main outcome measure at week 8. The healing rate, symptoms, and quality of life were assessed at week 4, and it was discovered that FPZ was non-inferior to EPZ, with the groups having identical cumulative healing rates at 8 wk and equivalent rates at 4 wk. In individuals with moderate to severe heartburn, FPZ provided superior symptom alleviation that lasted throughout the night. The medication was well tolerated, with similar rates of side effects across treatment groups[56].
Despite the fact that PPIs are one of the safest pharmacological classes available and have been used for almost 30 years, the number of papers on delayed-release PPI safety has skyrocketed, with many extensively discussed subjects appearing in high-profile journals[57,58]. Clinical studies and subsequent meta-analyses have revealed that VPZ has outstanding short and medium-term safety when compared to the performance of PPIs[42-53,59,60]. Because serum gastrin and pepsinogen I levels in healthy volunteers and GERD patients mirrored the antisecretory effect of VPZ, hypergastrinemia associated with long-term therapy may be a concern[61,62]. Clinical studies and subsequent meta-analyses have revealed that VPZ has outstanding short and medium-term safety when compared to the performance of PPIs[63]. Long-term PPI therapy has been associated with dysbiosis and changes in the gut microbiome, and similar changes have now been reported with VPZ[64-66]. Lipopolysaccharide biosynthesis proteins and lipopolysaccharide biosynthesis were the most dramatically elevated pathways in response to VPZ. They are most likely caused by an increase in intraluminal pH and are analogous to H. pylori responses to external pH changes. Because lipopolysaccharide is a potent immune response stimulator generated by Gram-negative bacteria, these data imply that VPZ may increase bacterial growth[67-69].
Many of the disadvantages and limitations of delayed-release PPIs are overcome by P-CABs. Mucosal healing in acid-related disorders is linked to the duration and degree of acid suppression, and also the length of treatment. Given the challenges in achieving good symptom management, particularly at night, with presently available delayed-release PPIs once daily, this novel family of medicines, P-CABs, delivers immediate, powerful, and extended acid suppression. They have the potential to address many of the unmet therapeutic needs in GERD, such as obtaining immediate heartburn relief and fast and specific healing of severe RE. The benefits of long-term acid suppression extend to H. pylori eradication, where intragastric pH control, especially at night, is crucial. Moreover, VPZ may be an optimum dual therapy as a straightforward, dependable, and successful first-line treatment for GERD. More thorough evaluations of VPZ, TPZ, and FPZ are needed, especially in Europe and North America. After extensive worldwide use of P-CABs, clinicians will justify whether it is effective, safe, and superior to currently available treatment with PPIs. In our opinion, VPZ or other P-CABs should be reserved for difficult-to-treat acid-related illnesses and unmet requirements, where the benefit-to-risk ratio is predicted to be the best.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Togo
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lan C, China; Sintusek P, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Chatila AT, Nguyen MTT, Krill T, Roark R, Bilal M, Reep G. Natural history, pathophysiology and evaluation of gastroesophageal reflux disease. Dis Mon. 2020;66:100848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1265] [Article Influence: 115.0] [Reference Citation Analysis (2)] |
| 4. | Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 5. | Fock KM, Talley N, Goh KL, Sugano K, Katelaris P, Holtmann G, Pandolfino JE, Sharma P, Ang TL, Hongo M, Wu J, Chen M, Choi MG, Law NM, Sheu BS, Zhang J, Ho KY, Sollano J, Rani AA, Kositchaiwat C, Bhatia S. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett's oesophagus. Gut. 2016;65:1402-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Hershcovici T, Fass R. Gastro-oesophageal reflux disease: beyond proton pump inhibitor therapy. Drugs. 2011;71:2381-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Shibli F, Kitayama Y, Fass R. Novel Therapies for Gastroesophageal Reflux Disease: Beyond Proton Pump Inhibitors. Curr Gastroenterol Rep. 2020;22:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Spechler SJ. Evaluation and Treatment of Patients with Persistent Reflux Symptoms Despite Proton Pump Inhibitor Treatment. Gastroenterol Clin North Am. 2020;49:437-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Wijarnpreecha K, Thongprayoon C, Chesdachai S, Panjawatanana P, Ungprasert P, Cheungpasitporn W. Associations of Proton-Pump Inhibitors and H2 Receptor Antagonists with Chronic Kidney Disease: A Meta-Analysis. Dig Dis Sci. 2017;62:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Yadlapati R, DeLay K. Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Med Clin North Am. 2019;103:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Moore JM, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease: real or imagined? Curr Opin Gastroenterol. 2010;26:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Rettura F, Bronzini F, Campigotto M, Lambiase C, Pancetti A, Berti G, Marchi S, de Bortoli N, Zerbib F, Savarino E, Bellini M. Refractory Gastroesophageal Reflux Disease: A Management Update. Front Med (Lausanne). 2021;8:765061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 13. | Engevik AC, Kaji I, Goldenring JR. The Physiology of the Gastric Parietal Cell. Physiol Rev. 2020;100:573-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 14. | DuBose TD Jr, Codina J. H,K-ATPase. Curr Opin Nephrol Hypertens. 1996;5:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Sakai H, Fujii T, Takeguchi N. Proton-Potassium (H(+)/K(+)) ATPases: Properties and Roles in Health and Diseases. Met Ions Life Sci. 2016;16:459-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ene MD, Khan-Daneshmend T, Roberts CJ. A study of the inhibitory effects of SCH 28080 on gastric secretion in man. Br J Pharmacol. 1982;76:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Long JF, Chiu PJ, Derelanko MJ, Steinberg M. Gastric antisecretory and cytoprotective activities of SCH 28080. J Pharmacol Exp Ther. 1983;226:114-120. [PubMed] |
| 18. | Beil W, Hackbarth I, Sewing KF. Mechanism of gastric antisecretory effect of SCH 28080. Br J Pharmacol. 1986;88:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Kaunitz JD, Sachs G. Identification of a vanadate-sensitive potassium-dependent proton pump from rabbit colon. J Biol Chem. 1986;261:14005-14010. [PubMed] |
| 20. | Keeling DJ, Malcolm RC, Laing SM, Ife RJ, Leach CA. SK&F 96067 is a reversible, lumenally acting inhibitor of the gastric (H+ + K+)-ATPase. Biochem Pharmacol. 1991;42:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tsukimi Y, Ushiro T, Yamazaki T, Ishikawa H, Hirase J, Narita M, Nishigaito T, Banno K, Ichihara T, Tanaka H. Studies on the mechanism of action of the gastric H+,K(+)-ATPase inhibitor SPI-447. Jpn J Pharmacol. 2000;82:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wurst W, Hartmann M. Current status of acid pump antagonists (reversible PPIs). Yale J Biol Med. 1996;69:233-243. [PubMed] |
| 23. | Simon WA, Herrmann M, Klein T, Shin JM, Huber R, Senn-Bilfinger J, Postius S. Soraprazan: setting new standards in inhibition of gastric acid secretion. J Pharmacol Exp Ther. 2007;321:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Ito K, Kinoshita K, Tomizawa A, Morikawa-Inomata Y, Inaba F, Fujita Y, Tabata K, Shibakawa N. The effect of subchronic administration of 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-{[(1S,2S)-2-methylcyclopropyl]methyl}-1H-pyrrolo[2,3-d]pyridazine (CS-526), a novel acid pump antagonist, on gastric acid secretion and gastrin levels in rats. J Pharmacol Exp Ther. 2008;326:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Mori H, Tonai-Kachi H, Ochi Y, Taniguchi Y, Ohshiro H, Takahashi N, Aihara T, Hirao A, Kato T, Sakakibara M, Kurebayashi Y. N-(2-hydroxyethyl)-N,2-dimethyl-8-{[(4R)-5-methyl-3,4-dihydro-2H-chromen-4-yl]amino}imidazo[1,2-a]pyridine-6-carboxamide (PF-03716556), a novel, potent, and selective acid pump antagonist for the treatment of gastroesophageal reflux disease. J Pharmacol Exp Ther. 2009;328:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Arikawa Y, Nishida H, Kurasawa O, Hasuoka A, Hirase K, Inatomi N, Hori Y, Matsukawa J, Imanishi A, Kondo M, Tarui N, Hamada T, Takagi T, Takeuchi T, Kajino M. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem. 2012;55:4446-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Scarpignato C, Pelosini I, Di Mario F. Acid suppression therapy: where do we go from here? Dig Dis. 2006;24:11-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 28. | Savarino V, Marabotto E, Zentilin P, Demarzo MG, de Bortoli N, Savarino E. Pharmacological Management of Gastro-Esophageal Reflux Disease: An Update of the State-of-the-Art. Drug Des Devel Ther. 2021;15:1609-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Gedda K, Briving C, Svensson K, Maxvall I, Andersson K. Mechanism of action of AZD0865, a K+-competitive inhibitor of gastric H+,K+-ATPase. Biochem Pharmacol. 2007;73:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Kirchhoff P, Andersson K, Socrates T, Sidani S, Kosiek O, Geibel JP. Characteristics of the K+-competitive H+,K+-ATPase inhibitor AZD0865 in isolated rat gastric glands. Am J Physiol Gastrointest Liver Physiol. 2006;291:G838-G843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Sunwoo J, Ji SC, Oh J, Ban MS, Nam JY, Kim B, Song GS, Yu KS, Jang IJ, Lee S. Pharmacodynamics of tegoprazan and revaprazan after single and multiple oral doses in healthy subjects. Aliment Pharmacol Ther. 2020;52:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 32. | Kahrilas PJ, Dent J, Lauritsen K, Malfertheiner P, Denison H, Franzén S, Hasselgren G. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Dent J, Kahrilas PJ, Hatlebakk J, Vakil N, Denison H, Franzén S, Lundborg P. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Metz DC, Vakil N, Keeffe EB, Lichtenstein GR. Advances in gastrointestinal pharmacotherapy. Clin Gastroenterol Hepatol. 2005;3:1167-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, Chae HS, Kim JK, Chung IS. Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects. J Gastroenterol Hepatol. 2010;25:1618-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, Lim HS, Jang IJ, Shin SG, Song KS, Moon BS. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol. 2004;44:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Sakurai Y, Nishimura A, Kennedy G, Hibberd M, Jenkins R, Okamoto H, Yoneyama T, Jenkins H, Ashida K, Irie S, Täubel J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Rising TAK-438 (Vonoprazan) Doses in Healthy Male Japanese/non-Japanese Subjects. Clin Transl Gastroenterol. 2015;6:e94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 38. | Han S, Choi HY, Kim YH, Nam JY, Kim B, Song GS, Lim HS, Bae KS. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Sunwoo J, Oh J, Moon SJ, Ji SC, Lee SH, Yu KS, Kim HS, Lee A, Jang IJ. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Hwang JG, Jeon I, Park SA, Lee A, Yu KS, Jang IJ, Lee S. Pharmacodynamics and pharmacokinetics of DWP14012 (fexuprazan) in healthy subjects with different ethnicities. Aliment Pharmacol Ther. 2020;52:1648-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 41. | Unge P, Andersson K. A first-in-human, open-label, healthy volunteer study of the new P-CAB X842 demonstrating 24h acid control for treatment of acid related diseases. Gastroenterology. 2017;154:S238. [DOI] [Full Text] |
| 42. | Ashida K, Sakurai Y, Nishimura A, Kudou K, Hiramatsu N, Umegaki E, Iwakiri K, Chiba T. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42:685-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 43. | Ashida K, Sakurai Y, Hori T, Kudou K, Nishimura A, Hiramatsu N, Umegaki E, Iwakiri K. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43:240-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 44. | Iwakiri K, Sakurai Y, Shiino M, Okamoto H, Kudou K, Nishimura A, Hiramatsu N, Umegaki E, Ashida K. A randomized, double-blind study to evaluate the acid-inhibitory effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol. 2017;10:439-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Yamashita H, Kanamori A, Kano C, Hashimura H, Matsumoto K, Tsujimae M, Yoshizaki T, Momose K, Obata D, Eguchi T, Fujita M, Okada A. The Effects of Switching to Vonoprazan, a Novel Potassium-Competitive Acid Blocker, on Gastric Acidity and Reflux Patterns in Patients with Erosive Esophagitis Refractory to Proton Pump Inhibitors. Digestion. 2017;96:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Ashida K, Iwakiri K, Hiramatsu N, Sakurai Y, Hori T, Kudou K, Nishimura A, Umegaki E. Maintenance for healed erosive esophagitis: Phase III comparison of vonoprazan with lansoprazole. World J Gastroenterol. 2018;24:1550-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 47. | Shinozaki S, Osawa H, Kobayashi Y, Sakamoto H, Hayashi Y, Miura Y, Kawarai Lefor A, Yamamoto H. Long-term outcomes of patients with symptomatic gastroesophageal reflux disease treated with vonoprazan. Scand J Gastroenterol. 2018;53:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Oshima T, Arai E, Taki M, Kondo T, Tomita T, Fukui H, Watari J, Miwa H. Randomised clinical trial: vonoprazan vs lansoprazole for the initial relief of heartburn in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Akiyama J, Hosaka H, Kuribayashi S, Moriyasu S, Hisada Y, Okubo H, Watanabe K, Imbe K, Nagata N, Kojima Y, Yokoi C, Uemura N, Shimoyama Y, Kawamura O, Yamada M, Kusano M. Efficacy of Vonoprazan, a Novel Potassium-Competitive Acid Blocker, in Patients with Proton Pump Inhibitor-Refractory Acid Reflux. Digestion. 2020;101:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Mizuno H, Nishino M, Yamada K, Kamiyamamoto S, Hinoue Y. Efficacy of Vonoprazan for 48-Week Maintenance Therapy of Patients with Healed Reflux Esophagitis. Digestion. 2020;101:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Xiao Y, Zhang S, Dai N, Fei G, Goh KL, Chun HJ, Sheu BS, Chong CF, Funao N, Zhou W, Chen M. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut. 2020;69:224-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 52. | Okanobu H, Kohno T, Mouri R, Hatsushika Y, Yamashita Y, Miyaki E, Fukuhara T, Okazaki A, Sakano A, Urabe A, Takaki S, Mori N, Tsuji K, Ochi H, Furukawa Y. Efficacy of vonoprazan 10 mg compared with 20 mg for the initial treatment in patients with erosive esophagitis: a randomized pilot study. Esophagus. 2021;18:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Matsuda S, Kato M, Sakakibara Y, Hamada H, Sasaki Y, Mori H, Hirai Y, Inoue S, Toyokawa T, Kagaya T, Kuwai T, Esaka N, Yamashita H, Watanabe N, Matsumoto M, Fujii H, Demura M, Kubo K, Mabe K, Harada N. A study for every second day administration of vonoprazan for maintenance treatment of erosive GERD (ESD von GERD): a multicenter randomized cross-over study. J Gastroenterol. 2022;57:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Lee KJ, Son BK, Kim GH, Jung HK, Jung HY, Chung IK, Sung IK, Kim JI, Kim JH, Lee JS, Kwon JG, Park JH, Huh KC, Park KS, Park MI, Kim N, Lee OY, Jee SR, Lee SK, Youn SJ, Kim SK, Lee ST, Hong SJ, Choi SC, Kim TN, Youn YH, Park HJ, Kang MJ, Park CH, Kim BT, Youn S, Song GS, Rhee PL. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:864-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Kim SH, Cho KB, Chun HJ, Lee SW, Kwon JG, Lee DH, Kim SG, Jung HY, Kim JW, Lee JS, Park H, Choi SC, Jee SR, Kim HS, Ko KH, Park SJ, Lee YC, Park SH, Kim AR, Kim EJ, Park HW, Kim BT, Song GS. Randomised clinical trial: comparison of tegoprazan and placebo in non-erosive reflux disease. Aliment Pharmacol Ther. 2021;54:402-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Lee OY, Chun HJ, Kim JJ, Kim JI, Kim SK, Lee SW, Park KS, Lee KL, Choi SC, Jang JY. A phase 3, non-inferiority randomized controlled trial with fexuprazan, a novel potassium competitive acid blocker vs. esomeprazole in patients with erosive esophagitis. Gastroenterology. 2020;158:S1072. |
| 57. | Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 58. | Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 59. | Cheng Y, Liu J, Tan X, Dai Y, Xie C, Li X, Lu Q, Kou F, Jiang H, Li J. Direct Comparison of the Efficacy and Safety of Vonoprazan Versus Proton-Pump Inhibitors for Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;66:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 60. | Miwa H, Igarashi A, Teng L, Uda A, Deguchi H, Tango T. Systematic review with network meta-analysis: indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease. J Gastroenterol. 2019;54:718-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Kojima Y, Takeuchi T, Sanomura M, Higashino K, Kojima K, Fukumoto K, Takata K, Sakamoto H, Sakaguchi M, Tominaga K, Higuchi K. Does the Novel Potassium-Competitive Acid Blocker Vonoprazan Cause More Hypergastrinemia than Conventional Proton Pump Inhibitors? Digestion. 2018;97:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Uemura N, Kinoshita Y, Haruma K, Yao T, Kushima R, Kanoo T. Rationale and design of the VISION study: a randomized, open-label study to evaluate the long-term safety of vonoprazan as maintenance treatment in patients with erosive esophagitis. Clin Exp Gastroenterol. 2018;11:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Hafiz RA, Wong C, Paynter S, David M, Peeters G. The Risk of Community-Acquired Enteric Infection in Proton Pump Inhibitor Therapy: Systematic Review and Meta-analysis. Ann Pharmacother. 2018;52:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Naito Y, Kashiwagi K, Takagi T, Andoh A, Inoue R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion. 2018;97:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 65. | Singh A, Cresci GA, Kirby DF. Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr Clin Pract. 2018;33:614-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Kakiuchi T, Yamamoto K, Imamura I, Hashiguchi K, Kawakubo H, Yamaguchi D, Fujioka Y, Okuda M. Gut microbiota changes related to Helicobacter pylori eradication with vonoprazan containing triple therapy among adolescents: a prospective multicenter study. Sci Rep. 2021;11:755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Otsuka T, Sugimoto M, Inoue R, Ohno M, Ban H, Nishida A, Inatomi O, Takahashi S, Naito Y, Andoh A. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut. 2017;66:1723-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012;36:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 69. | Cochet F, Peri F. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |