Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3555
Peer-review started: April 4, 2022
First decision: May 9, 2022
Revised: June 6, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 28, 2022
Processing time: 113 Days and 16.8 Hours
Hepatitis B virus (HBV) has posed a threat to public health, mainly resulting in liver damage. With long-term accumulation of extracellular matrix, patients with chronic hepatitis B are at high risk of developing into liver fibrosis and cirrhosis and even life-threatening hepatic carcinoma. The occurrence of complications such as spontaneous bacterial peritonitis and hepatic encephalopathy greatly increases disability and mortality. With deeper understanding of the bidirectional interaction between the liver and the gut (gut-liver axis), there is a growing consensus that the human health closely relates to the gut microbiota. Supported by animal and human studies, the gut microbiota alters as the HBV-related liver fibrosis initials and progresses, characterized as the decrease of the ratio between “good” and “potentially pathogenic” microbes. When the primary disease is controlled via antiviral treatment, the gut microbiota dysfunction tends to be improved. Conversely, the recovery of gut microbiota can promote the regression of liver fibrosis. Therapeutic strategies targeted on gut microbiota (rifaximin, probiotics, engineered probiotics and fecal microbiota transplantation) have been applied to animal models and patients, obtaining satisfactory results.
Core Tip: Intimate connection between the gut microbiota alteration and hepatitis B virus (HBV)-related fibrosis and complications has been supported by animal and human studies. Researchers and clinicians are making effort to control and reverse fibrosis by rebuilding a healthy gut microbiota. We herein discuss the gut microbiota alteration in HBV-related fibrosis and therapies targeted on reconstruction of gut microbiota homeostasis.
- Citation: Li YG, Yu ZJ, Li A, Ren ZG. Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications: Molecular mechanisms and therapeutic inventions. World J Gastroenterol 2022; 28(28): 3555-3572
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3555
Hepatitis B virus (HBV) has brought about substantial global health problems, giving rise to approximately 1.5 million new infections in 2019[1]. Balancing the pathogenic ability and immunity defense, some patients may experience chronic HBV infection, and even chronic hepatitis B (CHB). The different phrases are designed by the presence of hepatitis B e antigen (HBeAg), HBV DNA levels, alanine aminotransferase (ALT) values and liver inflammation, and CHB is mainly characterized by elevated ALT levels and moderate/severe liver diseases[2]. Chronic HBV infection tends to be asymptomatic initially, however, tissue repair against chronic inflammation may result in an immoderate accumulation of extracellular matrix (ECM), so CHB patients are at high risk of developing progressive fibrosis and life-threatening cirrhosis. Complications, such as portal hypertension, spontaneous bacterial peritonitis (SBP)[3] and hepatic encephalopathy (HE)[4], are difficult to prevent and address. With hepatocellular carcinoma (HCC) coming along stealthily[5], approximately 820000 deaths were caused by HBV infection–related causes in 2019[1].
The human intestine, as an organ directly connected with the outside world, is colonized by microbes progressively after birth[6]. The human gut microbiota is now considered to be composed of approximately 1014 bacteria[7], 200-300 fungal species[8] and abundant bacteriophages[9], and is increasingly seen as a significant superorganism[10]. Predominant strains in the adult intestine belong to
The liver is closely connected with the gut via the gut-liver axis, defined as the bidirectional interaction between the liver and the gut via transport of bile acids, immunoreactive substances, nutrients, etc.[17]. When impairment of intestinal barriers and disturbances of the gut microbiota occur, gut-derived microbe/antigen translocation may lead to invasion of the liver. The association between gut microbiota alterations and chronic liver diseases (CLDs) has received great attention.
This review will concentrate on gut microbiota alterations in HBV-related liver fibrosis and summarize the cutting edge of new therapeutic strategies. We will summarize and discuss: (1) Gut microbiota alteration in HBV-related liver fibrosis; (2) Gut microbiota-related mechanisms of liver fibrosis; (3) Gut microbiota dysfunction in liver fibrosis complications; and (4) Gut microbiota-related treatment toward HBV-related fibrosis and complications.
HBV-infected populations tend to obtain a gut microbiota that differs from that of healthy people (Table 1). Depending on host and viral factors, patients with HBV infection may experience different phrases[2]. In this part, gut microbiota alteration in the HBV persistence and different stages of HBV infection will be discussed.
| Ref. | Population (n) | Detection method | Gut microbiota alteration | Additional findings |
| Lu et al[30] | Healthy volunteers (n = 32); HBV carriers (n = 30); CHB (n = 31); Decompensated HBV-LC (n = 31) | qPCR | Phylum | Copies of operons that code for virulence factors markedly increased. Fecal sIgA and TNF-α in decompensated HBV-LC patients were higher than other groups |
| Bacteroidetes ↓ | ||||
| Firmicutes ↓ | ||||
| Family | ||||
| Bifidobacteria/Enterobacteriaceae ↓ | ||||
| Xu et al[142] | Healthy volunteers (n = 15); CHB (n = 16); HBV-LC (n = 16) | qPCR | Species | B. dentium, which was considered to be an opportunistic pathogen, increased in HBV-LC patients. Species composition of Bifidobacterium shifted from beneficial to pathogenic |
| (Bifidobacterium specific) | ||||
| B. catenulatum ↓ | ||||
| B. longum ↓ | ||||
| B. dentinum ↑ | ||||
| Wu et al[143] | Healthy volunteers (n = 38); Decompensated HBV-LC (n = 61); HBV-LT (after LC) (n =74) | qPCR | Species (Lactobacilli specific) | Less complex fecal lactobacilli composition was found especially in decompensated HBV-LC patients |
| L. rhamnosus ↓ | ||||
| L. fermentus ↓ | ||||
| Wei et al[38] | Healthy volunteers (n = 120); HBV-LC (n = 120): CTP-A (n = 40); CTP-B (n = 40); CTP-C (n = 40) | Solexa sequencing | Phylum | A negative correlation was observed between the Child-Turcotte-Pugh scores and Bacteroidetes (P < 0.01) |
| Bacteroidetes ↓ | ||||
| Proteobacteria ↑ | ||||
| Family | ||||
| Enterobacteriaceae ↑ | ||||
| Genera | ||||
| Veillonella ↑ | ||||
| Wang et al[23] | Healthy volunteers (n = 22); CHB (n = 85): CP-A (n = 76); CP-B (n = 9) | 16S rRNA sequencing | Family | Streptococcus, Veillonella, Streptococcus and Haemophilus had strong correlations with liver function indices and serum metabolites. They were significantly higher in patients with higher Child-Pugh scores. The gut microbiota may be partially involved in the abnormal accumulation of serum metabolites |
| Lachnospiraceae ↓ | ||||
| Rikenellaceae, ↓ | ||||
| Porphyromonadaceae ↓ | ||||
| Ruminococcaceae ↓ | ||||
| Veillonellaceae ↑ | ||||
| Deng et al[29] | Healthy volunteers (n = 20); HBV-LC (n = 80): CP-A (n = 30); CP-B (n = 31); CP-C (n = 19) | 16S rRNA sequencing | Phylum | Gut microbiota alteration mentioned on the left were all independent risk or protective factors for HBV-LC. Serum endotoxin increased in patients with higher CP classes (P = 0.000) |
| Firmicutes/Bacteroidetes ↑ | ||||
| Genera | ||||
| Megamonas ↓ | ||||
| Veillonella ↓ | ||||
| Zeng et al[140] | Healthy volunteers (n = 15); CHB (n = 21); HBV-LC (n = 25); HBV-HCC (n = 21) | 16S rRNA sequencing | Phylum | Higher Bacteroidetes/firmicutes ratio represented for higher LPS exposure |
| Proteobacteria ↑ | ||||
| Bacteroidetes ↑ | ||||
| Firmicutes ↓ | ||||
| Family | ||||
| Bifidobacteria/Enterobacteriaceae ↓ | ||||
| Wang et al[59] | Healthy volunteers (n = 21); CHB (n = 69); F0-1 (n = 25); F2-4 (n = 44) | 16S rRNA sequencing | Genera | Genera responsible for bile acid metabolism decreased in CHB fibrosis patients |
| Prevotella ↑ | ||||
| Bacteroides ↓ | ||||
| Ruminococcus ↓ | ||||
| Chen et al[28] | Healthy volunteers (n = 21); HBV carriers (n = 23); CHB (n = 28); HBV-LC (n = 25) | 16S rRNA sequencing | Phylum | HBV-LC patients had higher bacterial network complexity with lower abundance of potential beneficial bacterial taxa |
| Actinobacteria ↑ | ||||
| Bacteroidetes ↓ | ||||
| Firmicutes ↓ | ||||
| Proteobacteria ↑ | ||||
| Yang et al[27] | Healthy volunteers (n = 31); HBV carriers (n = 24); CHB (n = 56); HBV-LC (n = 54); HBV-ACLF (n = 52) | 16S rRNA sequencing | There are fluctuations in the changes | HBV carriers might be the most suitable donors for FMT for higher α diversity and abundance of potential beneficial bacteria |
| Wang et al[37] | Healthy volunteers (n = 877); CHB (n = 252); HBV-LC (n = 162); HBV-ACLF (n = 212) | 16S rRNA sequencing; metagenomic sequencing | Species | High abundance of Enterococcus is associated with progression while that of Faecalibacterium is associated with regression of HBV-ACLF |
| Enterococcus faecium ↑ |
After the infection, HBV may be spontaneously cleared or cause chronic infection in different individuals: 95% of adult-acquired infections result in spontaneous clearance, while over 90% of newborn infections lead to chronic infections[18]. The same phenomenon has been observed in animal experiments, in which hepatitis B surface antigen (HBsAg) of immature mice remained positive[19]. The age-related difference in immune clearance of HBV is consistent with the stabilization time of the gut microbiota, and maturation appears to facilitate HBV clearance by diminishing the tolerance phenotype and stimulating the immunoreactive pathway[19,20]. Similarly, if the gut microbiota was greatly imbalanced by antibiotics, the depletion can impair intestinal barrier function and weaken the ability of humoral and cellular immunity to clear HBV: adult mice with a mature gut microbiota managed to clear HBV after 6 wk of infection, while they failed to do so after antibiotic use[19,21].
Due to the difficulty of studying acute HBV infection in humans, animal studies have been used: the ratio of Firmicutes/Bacteroides increased in the early stages of infection (Day 14) and decreased significantly over time (Day 49) in two mouse groups that were constructed with different plasmids[22].
Compositional changes have already occurred in the gut microbiota in early-stage CHB patients: in the Child-Pugh A and B groups, the abundance of 5 operational taxonomic units (OTUs) belonging to Actinomyces, Clostridium sensu stricto, unclassified Lachnospiraceae and Megamonas increased, while 27 OTUs decreased, which belong to Alistipes, Asaccharobacter, Bacteroides, Butyricimonas, Clostridium IV, etc.[23].
To further understand the gut microbiota dynamics in chronic HBV infection and CHB, there are also studies concentrating on the association with clinical indicators reflecting liver function and infection state. The gut microbiota of subjects from the chronic HBV infection group with normal ALT (NALT) levels was rather similar to those from the healthy volunteers, while significantly different from those from the high ALT level group[24]; however, in a recent study, the authors presented a slightly different perspective that the microbial diversity and abundance of Lactobacillus, Clostridium, and Bifidobacterium were lower in CHB-NALT patients than in healthy volunteers[25]. Streptococcus, Veillonella, Streptococcus and Haemophilus showed high correlations with some serum metabolites, including aromatic amino acids (phenylalanine and tyrosine), which are assumed to play pathogenic roles the progression of CHB[23]. The gut microbiota also varies according to viral load: HBV-infected individuals with a low viral load showed high diversity and carry a predominance of taxa associated with fatty acid and lipid metabolism[26].
As the disease progresses, the gut microbiota changes dynamically: the α diversity of asymptomatic HBV carriers slightly increased compared with that of healthy donators, while that of patients in the other three groups (CHB, liver cirrhosis, and acute-on-chronic liver failure (ACLF)) decreased with the severity of the disease[27]. The gut microbiota of patients with liver cirrhosis showed lower diversity and higher network complexity[28]. Veillonellaceae and Lachnospiraceae families were depleted in patients with liver cirrhosis compared with those in healthy volunteers, while Megamonas and Veillonella genera were depleted and enriched in patients, respectively[29]. Additionally, copy numbers of Enterobacteriaceae increased and lactic acid bacteria were depleted, with marked variation in the intestinal community of CHB patients[30]. The Bifidobacteria/Enterobacteriaceae ratio can be used for tracing the progression of liver disease[30]. With the magnitude of severity of liver disease (estimated as increasing liver Child-Pugh score), partial functional genes were correlated, such as those encoding aspartate-ammonia ligase, transaldolase, adenylosuccinate synthetase and IMP dehydrogenase[31]. According to the combined results of multiple studies, there is a well-acknowledged decrease in Firmicutes abundance and increase in Proteobacteria during the progression of HBV-related fibrosis.
Liver cirrhosis is a dangerous premalignant condition with an increasing incidence of genetic aberrations and an elevated risk of HCC[32,33]. HCC patients tend to present a distempered gut microbiota and abnormal metabolites[34]. The butyrate-producing genera were depleted, while lipopolysaccharide (LPS)-producing genera were enriched in liver cirrhosis and HCC patients, and Clostridioides abundance was generally observed to be positively related to the tumor size of HCC[35]. In another study, Bacteroides, Lachnospiracea incertae sedis, and Clostridium XIVa were enriched in HCC patients, and there was a consistency of positive correlation with the tumor burden[36]. By integrating the clinical characteristics and database analysis, serum bile acids may be the communication mediators between these three genera and the host transcriptome[36]. HCC can be secondary to a number of causes, including HBV, Hepatitis C virus (HCV) and so on. Compared with non-HBV non-HCV HCC, the abundance of Prevotella was much greater in HBV-related HCC group[34]. HBV-related HCC group had higher abundance of pathways related to DNA formation and function (including chaperones and folding catalysts, DNA replication proteins and chromosome), which supported that HBV can impair the normal function of DNA[34].
Additionally, dynamic alteration of gut microbiota is a valuable indicator to predict the prognosis of end-stage liver disease. The richness of Enterococcus was significantly higher in the HBV-related ACLF progression group, while a high abundance of Faecalibacterium was associated with regression (groups were divided according to the model for end-stage liver disease at discharge)[37]; a higher abundance of E. coli is consistent with an increasing level of LPS ligand in the circulation of patients with end-stage liver disease[38-40].
Liver fibrosis is fibrous scar caused by excess accumulation of ECM[41]. It is driven by the chronic and persistent occurrence of parenchymal injury and the activation of the inflammatory response, followed by a continuous repair reaction and liver fibrogenesis[42]. For HBV infection, liver infringement comes from not only HBV but also gut-derived microbe/antigen translocation and abnormal metabolites.
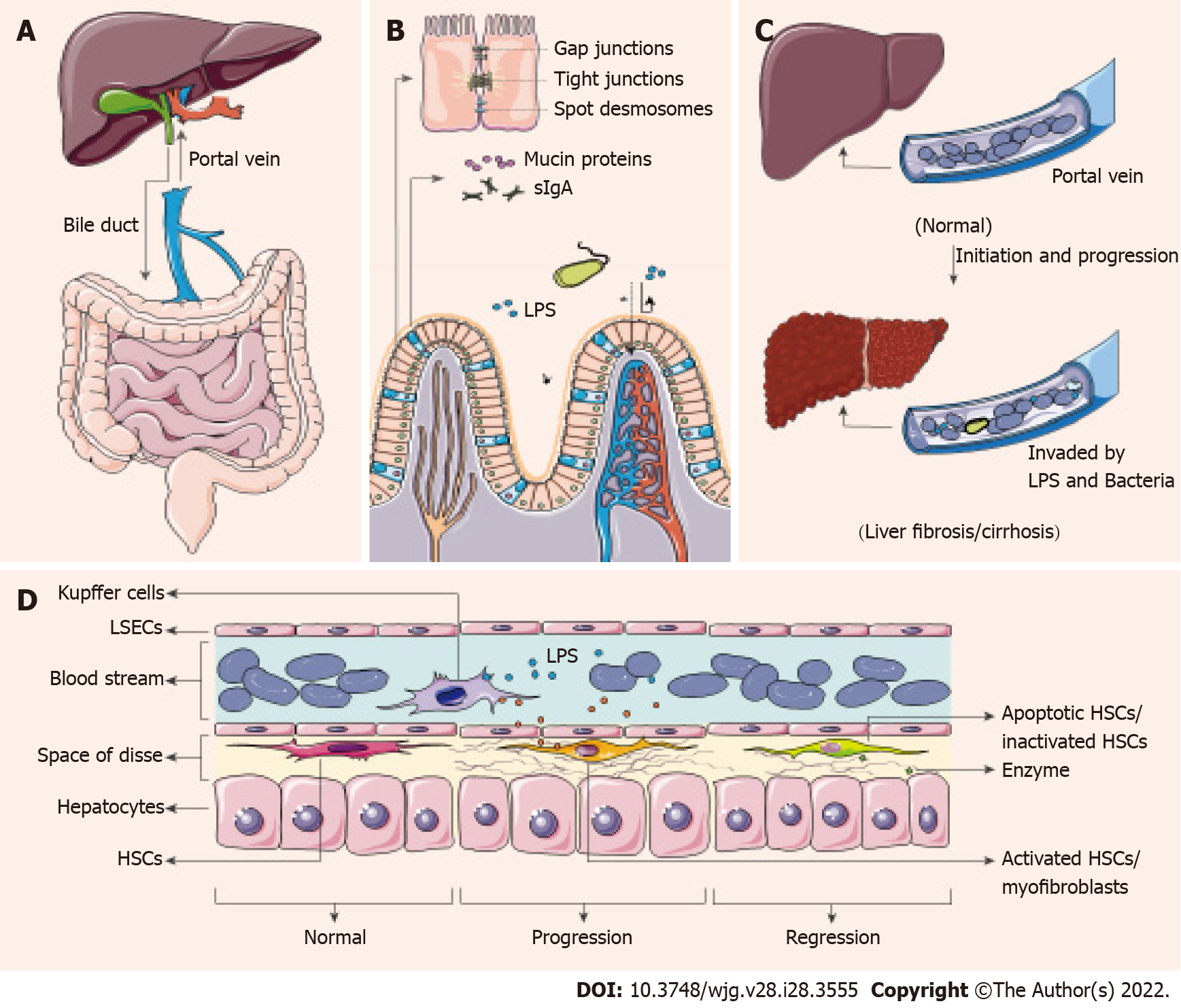
There is a close connection between the gut and liver through known organic pipelines (bile duct and portal vein)[43], and whether there are detours needs further study. The liver produces and sends primary bile acids (BAs) and immunologic active materials (some antimicrobial peptides) through the biliary tract to assist in intestinal digestion and immunity. Conversely, the portal vein carries secondary BAs, nutrients, gastrointestinal metabolites from the gut to the liver, to provide nutrients and get detoxification and biotransformation[17,44] (Figure 1A).
In a non-disease state, intestinal physical and chemical barriers effectively block pathogens or toxic substances and decrease bacterial colonization. The barriers mainly include mucin proteins secreted by goblet cells, secretory IgA (sIgA) secreted by plasma cells in lymphoid follicles of the lamina propria and tight junctions between intestinal epithelial cells (IECs)[45] (Figure 1B). Disorders of these barriers can lead to increased intestinal permeability and translocation of microbial components or metabolites (LPS, microbial DNA) in CLD patients, allowing microbes and antigens to translocate into the portal vein[45], and subsequently induce chronic or acute inflammatory responses of different tissues and organs[46] (Figure 1C).
The gastrointestinal mucus layer is the first line of defense against microbes, and the mobility enables the layer to carry pathogens distally and reduce microbial colonization[47]. The experimental mouse models with liver cirrhosis [induced by bile-duct ligation (BDL) or tetrachloromethane (CCl4)] show a reduced thickness of the mucus layer, with loss of goblet cells[48]. These cirrhotic mice show pathological bacterial translocation, which has not been found in healthy or pre-hepatic portal-hypertensive mice[48]. sIgA is the predominant contributor to mucosal immunity, recognizing and eliminating bacterial protein antigens, and it also participates in barrier layer limitation of microbe/antigen translocation[49]. Patients with HBV-induced decompensated cirrhosis have increased sIgA content in blood and stool[30], consistent with the increased bacterial migration. Simultaneously, intestinal tight junctions are weakened in patients with liver cirrhosis, and the expression of tight junction proteins is decreased[50,51]. Zonulin, an effective physiological regulator of tight junctions, is one of the markers of intestinal permeability[52]. Serum zonulin content is significantly increased in HBV-related liver cirrhosis and HCC patients and the levels are correlated with the stages[53].
The impairment of the intestinal barrier greatly reduces the efficiency of blocking microbe/antigen translocation. Gut-derived microbes or fragments and metabolites enter the venous system, travel through the portal vein to invade the liver. Diversity of circulating bacteria in cirrhosis patients is consistent with the presence of dysbiosis[54]. Recent studies have also supported that the occurrence of intestinal bacterial overgrowth and bacterial translocation in cirrhosis using methods such as bacterial DNA sequencing[55] and fluorescence microscopy[21] and suggested that the mechanism is associated with antimicrobial host defense[56]. Simultaneously, LPS is one of the component of the outer membrane of Gram-negative bacteria, mainly from Enterobacteriaceae[57]. The dysbiosis of the gut microbiota in mice leads to endotoxemia, which may bring about Kupffer Cell (KC) IL-10 production and KC-mediated T cell suppression[57]. And endotoxemia is highly related to the severity in liver diseases and complications[58].
Additionally, abnormal composition of the gut microbiota results in metabolic disorders, among which the metabolism of BAs has aroused great concern[25]. The level of fecal total BAs decreased and the ratio of conjugated and primary BAs increased in CHB patients without liver cirrhosis, which may be the prelude of following changes[25]. And there is a trend that abundance of the bacteria genera responsible for BA metabolism is decreased in CHB patients with moderate/advanced fibrosis[59,60]. There is also a link between gut bacteria-controlled BA metabolism and liver antitumor immunosurveillance via natural killer T (NKT) cells[61].
Pattern recognition receptors (PRRs) are highly conserved host sensors that are able to recognize exogenous and endogenous antigens, including pathogen-associated molecular patterns (PAMPs) and host-derived damage-associated molecular patterns (DAMPs)[62]. PRRs are expressed by a plethora of immune cells, especially macrophages[63]. Macrophages could be at the center of innate immune regulation, linking microbe/antigen translocation and liver inflammation or fibrosis[64]. Recognition of PRRs sends the initial signal to active downstream adaptor proteins to undergo maturation and assemble transcription factors, such as nuclear factor (NF)-κB[65,66]. The produced cytokines then recruit inflammatory cells, drive antimicrobial activities and promote myofibroblast formation[67].
Myofibroblasts, the collagen-producing cells, are not present in healthy livers[68]. In response to toxic liver injury, myofibroblasts are mainly transformed from activated hepatic stellate cells (HSCs)[69]. There are four different stages of HSCs, namely, quiescent, activated (equivalent to collagen type I-producing myofibroblasts), inactivated and senescent[41]. Under physiological conditions, quiescent HSCs stay in the space of Disse and function as the major vitamin A storage site[70]. Simulated by several cytokines (especially transforming growth factor (TGF)-β)[71], quiescent HSCs modulate phenotypes and transform into activated HSCs, and the activated HSCs migrate and secrete ECM to produce a fibrous scar[41]. After removing the initial driver, there is a decrease in the levels of pro-inflammatory cytokines (interleukin-6, interleukin-1β and tumor necrosis factor) and TGF-β, and a rapid decline of the counts of activated HSCs[41]. Activated HSCs can be transformed into inactivated or senescent cells, and stop producing type-I collagen fibers[72]. Later, when fiber degradation by matrix metalloproteinases overwhelms fiber formation, liver fibrosis can be controlled, regressed and even reversed[73].
In conclusion, increased microbe and endotoxin loads in the portal vein cause PRR activation on immune cells, especially on macrophages, which leads to the activation of quiescent HSCs into activated HSCs[44,66]. Later, activated HSCs proliferate in response to various cytokines, secrete type-I collagen fiber and make liver fibrotic[41]. Upon cessation of underlying injury, myofibroblasts undergo inactivation or apoptosis, and fibrosis can be discontinued or reversed[41] (Figure 1D). This is the mechanism of effective treatment to control and regress liver fibrosis.
As mentioned above, gut microbiota alterations may drive immune-related inflammation and fibrosis in the liver. Due to the accumulation of collagen fiber, liver stiffness is increased, bloodstream transport is blocked, healthy liver parenchyma is replaced and liver biotransformation and detoxification abilities are weakened[74]. As the disease progresses into the decompensation stage, patients may experience deadly complications, such as portal hypertension, spontaneous bacterial peritonitis (SBP) and HE. The relationship among gut microbiota alteration, liver fibrosis and portal hypertension is similar to the question of the chicken and the egg, as they drive and affect each other[75]. Compared with compensated cirrhosis, gut microbiota composition is characterized by an increase in the abundance of potentially pathogenic bacteria in the decompensation stage, especially Alcaligenaceae, Porphyromonadaceae, Veillonellaceae and Enterobacteriaceae[76].
SBP refers to the infection of ascites without an apparent intra-abdominal focus[77]. It is a severe infection and is often fatal in patients with cirrhosis and ascites[78]. The pathogen of SBP in liver cirrhosis patients is mainly from the intestinal tract.
More than two decades ago, DNA fragments of 30 bacterial isolated from ascites, mesenteric lymph nodes, portal blood, and ileal flora were compared[79]. The same bacterial strain was simultaneously isolated in ascites and in mesenteric lymph nodes and/or the ileum in 7/8 (87%) instances[79]. Intraperitoneal LPS increased TLR4 (Toll-like receptor 4, the canonical PRR for LPS) expression and amplified portal hypertension in rat liver fibrosis[80].
HE is a fatal central nervous system complication caused by acute and chronic hepatitis or decompensated cirrhosis[81], which is considered consciousness disturbance after ammonia-related cerebral edema[82]. HE patients tend to have a poor prognosis and high mortality and recurrence rates, with greatly increasing economic and nursing burdens[83].
Currently, there is an increasing consensus that the gut microbiota and gastrointestinal metabolites play an important role in the initiation and progress of HE. On the basis of the gut-liver axis mentioned above, researchers proposed the concept of the gut-brain-liver axis to describe the role of the gut microbiota[84]. Cognitive dysfunction in cirrhosis is related to a decrease in the abundance of autochthonous families and an increase in Alcaligenaceae and Porphyromonadaceae[85,86].
On the one hand, gut microbiota alteration in the decompensation stage is consistent with the accumulation of microbe-derived products, including ammonia, mercaptans, benzodiazepine-like substances, and indoles[76]. These products can pass the blood-brain barrier and alter astrocyte function, resulting in osmotic or oxidative stress, mitochondrial dysfunction, neurotransmission disorder, etc.[81]. On the other hand, neurotransmitters produced by the microbiota, including serotonin, dopamine, and aminobutyric acid, can act on specific receptors of exogenous primary afferent neuron cells, or cross the blood-brain barrier to act as active neurotransmitters[87]. The complex network among the enteric nervous system, the autonomic nervous system and the neuroendocrine and neuroimmunity systems of the central nervous system has a mutual impact on the gut microbiota, and the up-down or down-up regulation mechanisms need further exploration[84].
Based on the fibrosis regression theory mentioned above, removing the cause is the key to controlling and reversing liver fibrosis (Tables 2 and 3). For more than a decade, antiviral therapy has been recognized as an effective method to prevent, control and even reverse fibrosis and cirrhosis[88]. Rifaximin reduces the virulence of the overgrown gut microbiota[89]. With further understanding of the connection between the gut microbiota and HBV-related fibrosis, scientists have suggested that host health depends on the balance of the composition of the entire microbial community rather than one or a few dominant organisms[90]. New therapeutic strategies for HBV-related fibrosis, cirrhosis and complications have been broadened to regulate the gut microbiota through probiotic supplementation and microbiota transplantation from healthy donors.
| Ref. | Study populations (n) | Treatment and grouping (n) | Conclusions |
| Antiviral therapy | |||
| Li et al[96] | AAV-mediated persistent HBV infection (AAV-HBV) mice (n = 10) | 35 d after HBV infection, 4 wk of daily ETV treatment. ETV (n = 5) | Gut microbiota dysbiosis of the AAV-HBV mice was reversed by ETV treatment with restored α diversity and changed proportion of Akkermansia, Lacnospiracea and Marvinbryantia |
| Rifaximin | |||
| Kang et al[105] | Germ-free mice (n = 16) | 15 d of rifaximin 100 mg/(kg·d), or humanized with stools from a HCV-induced cirrhotic patient with MHE. Rifaximin (n = 4); Humanized (n = 4); Rifaximin + humanized (n = 4) | Rifaximin beneficially altered intestinal ammonia generation by regulating intestinal glutaminase expression independent of gut microbiota. MHE-associated fecal colonization resulted in intestinal and systemic inflammation. It was ameliorated with rifaximin |
| Engineered probiotics | |||
| Nicaise et al[120] | Ornithine transcarbamoylase-deficient Sparse-fur mice; Carbon tetrachloride rats; Thioacetamide-induced acute liver failure mice | NCIMB8826 (wild-type strain Lactobacillus plantarum), or EV101 (engineered Lactobacillus plantarum, LDH-/AlaD+) oral and intrarectal administration | EV101 administration was effective in controlling hyperammonemia in constitutive animal models with a significant effect on survival, which might be involved with direct ammonia consumption in the gut |
| Kurtz et al[121] | Ornithine transcarbamylase-deficient spfash mice; Thioacetamide-induced acute liver failure mice; Healthy volunteers (n = 52) | Non-modified Escherichia coli Nissle 1917 (EcN), SYNB1020 (engineered EcN, ΔargR, ΔthyA, malEK:PfnrS-argAfbr) administration | SYNB1020 converted NH3 to l-arginine in vitro, and reduced systemic hyperammonemia, improved survival in mouse models. SYNB1020 was well tolerated in healthy volunteers |
| Ochoa-Sanchez et al[122] | Bile-duct ligated rats | Non-modified EcN, S-ARG, or S-ARG + BUT administration | S-ARG converted ammonia to arginine, it was further modified to additionally synthesize butyrate, which had the potential to prevent HE |
| FMT | |||
| Liu et al[134] | Germ-free mice | Sterile supernatant or entire stool from pre-FMT and post-FMT cirrhotic patients with HE was transplanted to Germ-free mice | Transferred microbiota mediated neuroinflammation. Cirrhosis-associated dysregulation of gut microbiota was related with frontal cortical inflammation |
| Ref. | Study populations (n) | Treatment and grouping (n) | Conclusions |
| Antiviral therapy | |||
| Lu et al[97] | Healthy volunteers (n = 30); CHB (n = 30) | 8 wk of daily ETV treatment. ETV (n = 30) | After ETV treatment, gut microbiota abundance increased markedly, blood biochemical, immunological and virological responses improved significantly |
| Lu et al[98] | Healthy volunteers (n = 30); CHB patients (n = 60) | 8 wk of daily ETV treatment, or with additional CB. ETV (n = 30); ETV + CB (n = 30) | Additional CB fail to improve blood biochemical, immunological and virological responses, but affects the gut microbiota in the CHB patients treated with ETV |
| Rifaximin | |||
| Bajaj et al[104] | Decompensated LC patients with MHE (n = 20):CHB (NM) | 8 wk of rifaximin 550-mg BD. Rifaximin (n = 20) | Rifaximin affected little on gut microbiota, there was just a modest decrease in Veillonellaceae and increase in Eubacteriaceae. Rifaximin significantly improved cognition and endotoxemia, it increased increase in serum saturated and unsaturated fatty acids post-rifaximin |
| Lutz et al[144] | Decompensated LC patients with ascites (n = 152): Viral hepatitis (n = 35) | Prophylactic antibiotic treatment before the time of paracentesis. Rifaximin (n = 27); Other systemic antibiotics (n = 17) | Prophylactic rifaximin did not reduce SBP occurrence. Prophylactic rifaximin was associated with the dominant bacteria in ascites: Escherichia coli and enterococci were dominant of patients without prophylaxis, klebsiella species were mostly recovered from the rifaximin group |
| Kimer et al[102] | Decompensated LC patients (n = 54): CHB (NM) | 4 wk of rifaximin 550-mg BD or placebo BD. Rifaximin (n = 36); Placebo (n = 18) | Rifaximin had minor effects on bacteria translocation and gut microbiota. Rifaximin showed little impact on the inflammatory state (reflected as the level of TNF-α, IL-6, IL-10, IL-18, SDF-1α, TGF-1β, CRP) |
| Kaji et al[103] | Decompensated LC patients (n = 30): CHB (n = 4) | 4 wk of rifaximin 1200 mg/d. Rifaximin (n = 30) | Rifaximin alleviated HE and endotoxemia with improved intestinal hyperpermeability, and it is involved in a gut microbial change. Rifaximin didn’t affect serum proinflammatory cytokine levels (TNF-α, IL-6, IFN-γ, IL-10) |
| Probiotics | |||
| Agrawal et al[117] | LC patients with recovered HE (n = 235): CHB (n = 49) | 3 mo of lactulose 30–60 mL/d, or 3 capsules of probiotics per day, which contained 4 strains of Lactobacillus. Lactulose (n = 80); Probiotics (n = 77) | Lactulose and probiotics were effective for secondary prophylaxis of HE in cirrhotic patients |
| Ziada et al[115] | Decompensated LC patients with MHE (n = 90): CHB (NM) | 4 wk of lactulose 30–60 mL/d, or 3 capsules of probiotics per day, which contained Lactobacillus acidophilus. Lactulose (n = 30); Probiotics (n = 30) | Probiotic was better tolerated than lactulose. Both of them can improve blood ammonia and psychometric tests and reduce the risk of developing overt HE. Magnetic resonance spectroscopy showed more improvement in the levels of brain neurometabolites in the probiotic group |
| Xia et al[116] | Decompensated HBV-LC patients with MHE (n = 67) | 3 mo of probiotics 1500-mg TD, which contained Clostridium butyricum combined with Bifidobacterium infantis. Probiotics (n = 30) | After probiotics treatment, the therapeutic bacteria were significantly enriched, while Enterococcus and Enterobacteriaceae were decreased. Probiotics contributed to the improved cognition and the decreased ammonia levels |
| FMT | |||
| Ren et al[132] | CHB with positive HBeAg, received over 3 yr of antiviral treatment (n = 18) | FMT was performed by gastroscope every 4 wk until HBeAg clearance. FMT (n = 5) | FMT was effective on HBeAg-positive CHB, especially in patients who could not cease the oral antiviral treatment even after long-term treatment |
| Bajaj et al[135] | Decompensated LC patients with recurrent HE (n = 20). CHB (NM) | After 5 d of antibiotics, FMT was performed by enema, or standard of care (SOC, rifaximin/lactulose) was applied. FMT (n = 10); SOC (n = 10) | FMT increased diversity and beneficial taxa of gut microbiota, improved cognition and showed good tolerance, other than SOC |
| Bajaj et al[136] | Decompensated LC patients with recurrent HE (n = 20). CHB (NM) | FMT was performed by enema, or standard of care (SOC, rifaximin/lactulose) was applied. FMT (n = 10); SOC (n = 10) | Oral FMT capsules are safe and well tolerated. Post-FMT, duodenal mucosal diversity increased with higher Ruminococcaceae and Bifidobacteriaceae and lower Streptococcaceae and Veillonellaceae. Reduction in Veillonellaceae were noted post-FMT in sigmoid and stool |
| Chauhan et al[133] | CHB with positive HBeAg, received over 1 years of antiviral treatment (n = 18) | 6 FMTs were performed by gastroscope every 4 wk FMT (n = 12) | FMT appeared to be safe and effective on HBeAg-positive CHB in viral suppression and HBeAg clearance |
At present, the main endpoint of all current treatment strategies is to maintain long-term suppression of HBV replication[2]. Two main options are nucleoside analogs (NAs) and interferon alpha[91]. NAs with a high barrier to HBV resistance, including entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF), are believed to be favorably safe and long-acting[92]. Antiviral treatment (AVT) exerts a positive influence on survival rate and quality of life by preventing disease progression, reversing and degrading fibrosis and cirrhosis[93,94], and even reducing HCC incidence and mortality in CHB patients[95].
ETV therapy reverses gut microbiota dysbiosis induced by HBV infection in a mouse model[96]. And in a controlled cross-sectional and longitudinal real-world study, the species abundance of the gut microbiota increased markedly after ETV treatment[97]. After 8 wk of ETV treatment, the abundance of Clostridium sensu stricto 1, Erysipelotrichaceae UCG-007 and Intestinibacter increased significantly, and that of Streptococcus, Atopobium and Murdochiella was markedly reduced[97]. Although the addition of Clostridium butyricum (CB) to ETV failed to improve the serum biochemical, immunologic and virologic variables, addition of CB affected the gut microbiota in CHB patients treated with ETV[98]. While there is a lack of dynamic and synergetic studies on liver fibrosis outcomes and gut microbiota alterations during AVT, collaborative microbes contributing the most to antiviral-intervened HBV-related fibrosis cannot be pinpointed definitively.
Rifaximin is a rifamycin-based nonsystemic antibiotic with low gastrointestinal absorption and good antibacterial activity[89,99]. The gastrointestinal tract is the main therapeutic target of rifaximin, and it has been widely used in controlling HE with infrequent side effects and a favorable long-term safety profile[100,101].
Current ideas suggest that rifaximin may have positive implications for liver cirrhosis and complications by acting on the gut microbiota. However, according to a randomized trial, there seems to be a minor impact on the composition of the gut microbiota[102]. Enrolled patients with cirrhosis and ascites were divided into two groups to receive rifaximin or placebo for 4 wk. Rifaximin decreased gut bacterial abundance, while no effect on particular species was observed; blood bacterial richness was decreased and the difference in Pseudomonadales abundance was relatively obvious[102]. And there was no difference in circulating markers of inflammation between the two groups[102]. Two additional studies also supported that rifaximin has little influence on gut microbiota abundance[103], but the metabolite levels altered: after rifaximin application, endotoxemia was relieved, and serum saturated and unsaturated fatty acid levels were increased significantly[104]. The former conclusion agreed with a study on experimental mice[105]. Therefore, rather than having a bactericidal effect, rifaximin seems to have direct effects on bacterial function and virulence[89].
Probiotics are living nonpathogenic microorganisms, and treatment doses (at least 106 viable CFU/g) may help temper the gut microbiota[106]. Lactobacillus and Bifidobacterium genera are widely reported as clinically available probiotics[107]. In recent studies, probiotics have been broadly used to regulate the gut microbiota for further positive influences on primary diseases, such as gastrointestinal dysfunctions[108,109], metabolic diseases[110,111] and psychoneurotic disorders[112,113].
The role of probiotics in complications of HBV-related fibrosis and cirrhosis has been validated, especially for HE. Probiotics can drive the gut microbiota, triggering emotional brain signatures[114]. For minimal HE, probiotic therapy (Lactobacillus acidophilus) can improve blood ammonia and psychometric tests and reduce the risk of overt encephalopathy deterioration[115]. Further studies confirmed that patients’ cognition, venous ammonia level and intestinal mucosal barrier function were significantly improved after 3 mo of probiotic use (Clostridium butyricum combined with Bifidobacterium infantis), and the predominant bacteria (Clostridium cluster I and Bifidobacterium) were obviously enriched in the probiotic-treated group, while Enterococcus and Enterobacteriaceae were depleted[116]. The combination of probiotics and lactulose is effective for the secondary prophylaxis of HE patients with cirrhosis[117]. Simultaneously, probiotics may work by promoting the growth of beneficial microbes and preventing PAMP-mediated liver inflammation and the anti-proliferative, anti-angiogenic, and anti-metastatic effects of the antioxidant can block the progress of HCC[118].
Additionally, rapid progress in synthetic biology has brought more options, which makes engineered live biotherapeutics an available and promising strategy[119]. More than one decade ago, the genetically engineered ammonia-hyperconsuming strain NCIMB8826 was verified to exhibit a beneficial effect at a lower dose than its wild-type counterpart[120]. In recent years, more engineered bacteria have been constructed to accelerate ammonia metabolism, reduce blood ammonia concentration and reduce HE incidence[121,122]. One team from Synlogic Inc. engineered oral probiotic Escherichia coli Nissle 1917 (Ecn) to create strain SYNB1020[121]. SYNB1020 is able to convert NH3 to L-arginine in vivo and reduce hyperammonemia in two mouse models (ornithine transcarbamylase-deficient spfash mice and thioacetamide-induced liver injury mice). Satisfyingly, it showed metabolic activity and good tolerance in a phase 1 clinical study of 52 healthy adult volunteers. Later, another group modified Ecn to consume and convert ammonia to arginine, which was further modified to additionally synthesize butyrate[122]. Both of these studies showed that engineered probiotics have positive therapeutic significance for hyperammonemia and underlying potential for HE prevention. However, these strains have not progressed to clinical studies in hyperammonemia patients, and the clinical effects need further study.
Fecal microbiota transplantation (FMT) is an emerging treatment method that transfers the gut microbiota from a healthy donor to a patient[123]. Due to its ability to directly reshape or rebuild the recipient’s gut microbial communities, FMT is one of the most promising therapies balancing and stabilizing the gut microbiota[76], and it has been applied to research-based treatment in animal models of a variety of diseases[124,125] and to study the mechanisms[126,127]. In recent years, FMT has been expanded to clinical treatment for human disease as a noninvasive strategy for conditions including recurrent Clostridium difficile infection[128], inflammatory bowel disease[129], severe obesity and metabolic syndrome[130]. Regarding the mechanism, the gut microbiota structure can be improved by FMT, and a clinical trial employing autologous FMT supported this point[131].
Clinical trials have also aimed to determine whether CHB patients can benefit from FMT therapy. In a pilot study carried out in China, FMT showed the potential to induce HBeAg clearance in HBeAg-positive CHB patients after long-term AVT: There was a significant HBeAg level decline in the FMT group (FMT combined with AVT), while no decline in the control group (AVT only) was found[132]. The results were consistent with a nonrandomized controlled clinical trial carried out in India: after 1 year of FMT therapy for 6 terms, the FMT group (FMT + AVT) seemed to show potential effectiveness and safety compared with those of the AVT group (AVT only)[133]. Some researchers have also hypothesized that FMT of some potential beneficial bacteria can change the occurrence of disease, and HBV carriers might be the most suitable donors for slightly higher microbiota abundance[27]. However, due to the limitations of a small number of participants and a lack of randomized clinical trials, further well-designed clinical trials are needed to confirm the initial assumptions and promote clinical practicability.
Studies on FMT for HE animal models or patients show satisfactory results. In animal experiments, neuroinflammation alleviation was found in cirrhosis model mice receiving FMT[134]. In a randomized clinical trial, FMT from rationally selected donors helped reduce and improve hospitalizations and improve cognition and dysbiosis for cirrhosis with recurrent HE[135]. Later, the same team verified the safety of FMT capsules through a phase 1, randomized and placebo-controlled clinical trial[136]. In addition to integral inoculation, selective inoculation of specific strains also plays an ameliorating role. Transplanting low-urease altered Schaedler flora to mice prepared with a depleted microbiota leaded to durable reduction in fecal urease activity and ammonia production[137]. The symbiotic pair of Lactobacillus reuteri JBD400 and Streptococcus rubneri JBD420 cooperatively improved transplantation efficiency 2.3 × 103 times more than that of sole transplantation and significantly lowered blood ammonia levels[138].
Consequently, gut microbiota alteration has been observed to be related to HBV-related fibrosis initiation and progression, and it is a promising therapeutic target. According to current studies, HBV persistence and clearance show consistency with the maturity and health of the gut microbiota[19,21]. With an increase of Child-Pugh scores and the model for end-stage liver disease, the gut microbiota is characterized by a decrease in the ratio of “good” to “potentially pathogenic” bacteria, and species diversity tends to decrease[139,140]. However, it is difficult to clarify which is the initiating factor between gut microbiota alteration and HBV-related fibrosis progression. Existing studies tend to be descriptive and lack HBV-specific exploration. Gut microbiota-related mechanisms are based on the gut-liver axis and immune-mediated response, briefly including intestinal barrier impairment, PRR activation, cytokine production, HSC activation and transformation, and fiber secretion and formation[41]. When the driver is removed, activated HSCs are inhibited or become apoptotic, and fiber scars are degraded, resulting in fibrosis regression[41].
Beyond theory, quite a few studies have begun examining therapeutic inventions. AVT can effectively control or even reverse HBV-related liver fibrosis, during which the gut microbiota gradually returns to homeostasis[96,97]. Rifaximin may decrease the virulence of the overgrown gut microbiota[89]. Probiotics and FMT are the most popular gut microbiota targeted therapies, and they are moving from the laboratory to the clinic. In addition, synthetic probiotics and selective microbiota transplantation may make these therapies more precise, and bring fewer side effects.
However, current studies do have limitations. There is a lack of in-depth research on the specific molecular mechanisms of the gut microbiota. Further clinical studies are needed to determine its effectiveness in patients with HBV-induced liver cirrhosis in the real world[141]. We must also admit that age, host location, dietary habits have a great impact on the gut microbiota, which leads to the lack of consistency and comparability of the alterations in gut microbiota in different studies. Therefore, diagnosis potential of microbial markers should be considered the factors mentioned above. We are looking forward to more powerful studies to strengthen the theoretical foundation and promote clinical application.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gerlich W, Germany; Han G S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. 2021. Available from: https://www.who.int/publications/i/item/9789240027077. |
| 2. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 3. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 5. | Yang R, Xu Y, Dai Z, Lin X, Wang H. The Immunologic Role of Gut Microbiota in Patients with Chronic HBV Infection. J Immunol Res. 2018;2018:2361963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 6. | Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1146] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 7. | Doré J, Simrén M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013;1:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017;32:300-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 9. | Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol. 2012;10:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015;174:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1563] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 12. | Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 974] [Article Influence: 108.2] [Reference Citation Analysis (2)] |
| 13. | Chen L, Zhang YH, Huang T, Cai YD. Gene expression profiling gut microbiota in different races of humans. Sci Rep. 2016;6:23075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 15. | Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 725] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 16. | Jones RM, Neish AS. Gut Microbiota in Intestinal and Liver Disease. Annu Rev Pathol. 2021;16:251-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 970] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 18. | Wu LL, Huang TS, Shyu YC, Wang CL, Wang HY, Chen PJ. Gut microbiota in the innate immunity against hepatitis B virus - implication in age-dependent HBV clearance. Curr Opin Virol. 2021;49:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, Ni YH, Tseng HT, Wu D, Lu X, Wang HY, Chen PJ, Chen DS. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A. 2015;112:2175-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Xu D, Huang Y, Wang J. Gut microbiota modulate the immune effect against hepatitis B virus infection. Eur J Clin Microbiol Infect Dis. 2015;34:2139-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Guo W, Zhou X, Li X, Zhu Q, Peng J, Zhu B, Zheng X, Lu Y, Yang D, Wang B, Wang J. Depletion of Gut Microbiota Impairs Gut Barrier Function and Antiviral Immune Defense in the Liver. Front Immunol. 2021;12:636803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Zhu Q, Xia P, Zhou X, Li X, Guo W, Zhu B, Zheng X, Wang B, Yang D, Wang J. Hepatitis B Virus Infection Alters Gut Microbiota Composition in Mice. Front Cell Infect Microbiol. 2019;9:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Wang J, Wang Y, Zhang X, Liu J, Zhang Q, Zhao Y, Peng J, Feng Q, Dai J, Sun S, Zhao L, Zhang Y, Hu Y, Zhang M. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front Microbiol. 2017;8:2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Yun Y, Chang Y, Kim HN, Ryu S, Kwon MJ, Cho YK, Kim HL, Cheong HS, Joo EJ. Alterations of the Gut Microbiome in Chronic Hepatitis B Virus Infection Associated with Alanine Aminotransferase Level. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Sun Z, Huang C, Shi Y, Wang R, Fan J, Yu Y, Zhang Z, Zhu K, Li M, Ni Q, Chen Z, Zheng M, Yang Z. Distinct Bile Acid Profiles in Patients With Chronic Hepatitis B Virus Infection Reveal Metabolic Interplay Between Host, Virus and Gut Microbiome. Front Med (Lausanne). 2021;8:708495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Joo EJ, Cheong HS, Kwon MJ, Sohn W, Kim HN, Cho YK. Relationship between gut microbiome diversity and hepatitis B viral load in patients with chronic hepatitis B. Gut Pathog. 2021;13:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Yang XA, Lv F, Wang R, Chang Y, Zhao Y, Cui X, Li H, Yang S, Li S, Zhao X, Mo Z, Yang F. Potential role of intestinal microflora in disease progression among patients with different stages of Hepatitis B. Gut Pathog. 2020;12:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Deng YD, Peng XB, Zhao RR, Ma CQ, Li JN, Yao LQ. The intestinal microbial community dissimilarity in hepatitis B virus-related liver cirrhosis patients with and without at alcohol consumption. Gut Pathog. 2019;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Qin N, Guo J, Qian G, Fang D, Shi D, Xu M, Yang F, He Z, Van Nostrand JD, Yuan T, Deng Y, Zhou J, Li L. Functional gene arrays-based analysis of fecal microbiomes in patients with liver cirrhosis. BMC Genomics. 2014;15:753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Ganesan M, Eikenberry A, Poluektova LY, Kharbanda KK, Osna NA. Role of alcohol in pathogenesis of hepatitis B virus infection. World J Gastroenterol. 2020;26:883-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Tang Y, Zhou H, Xiang Y, Cui F. The diagnostic potential of gut microbiome for early hepatitis B virus-related hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2021;33:e167-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 35. | Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, Zuo X, Pan H, Zheng J, Wang F. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232-4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, Lu H, Yin S, Ji J, Zhou L, Zheng S. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 37. | Wang K, Zhang Z, Mo ZS, Yang XH, Lin BL, Peng L, Xu Y, Lei CY, Zhuang XD, Lu L, Yang RF, Chen T, Gao ZL. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure. Gut Microbes. 2021;13:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, Wang S, Li X, Han J, Huang L, Yuan J. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 2013;13:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014;5:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Mohamadkhani A. On the potential role of intestinal microbial community in hepatocarcinogenesis in chronic hepatitis B. Cancer Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1166] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 42. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 781] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 43. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 44. | Hrncir T, Hrncirova L, Kverka M, Hromadka R, Machova V, Trckova E, Kostovcikova K, Kralickova P, Krejsek J, Tlaskalova-Hogenova H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 45. | Chen D, Le TH, Shahidipour H, Read SA, Ahlenstiel G. The Role of Gut-Derived Microbial Antigens on Liver Fibrosis Initiation and Progression. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Nagpal R, Yadav H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann Nutr Metab. 2017;71 Suppl 1:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 47. | Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 986] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 48. | Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, Moghadamrad S, Hassan M, Albillos A, Francés R, Juanola O, Spadoni I, Rescigno M, Wiest R. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71:1126-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 49. | Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1106] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 50. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 51. | Pijls KE, Koek GH, Elamin EE, de Vries H, Masclee AA, Jonkers DM. Large intestine permeability is increased in patients with compensated liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G147-G153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518-1519. [PubMed] |
| 53. | Wang X, Li MM, Niu Y, Zhang X, Yin JB, Zhao CJ, Wang RT. Serum Zonulin in HBV-Associated Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma. Dis Markers. 2019;2019:5945721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Kajihara M, Koido S, Kanai T, Ito Z, Matsumoto Y, Takakura K, Saruta M, Kato K, Odamaki T, Xiao JZ, Sato N, Ohkusa T. Characterisation of blood microbiota in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 57. | Zhou W, Luo J, Xie X, Yang S, Zhu D, Huang H, Yang D, Liu J. Gut Microbiota Dysbiosis Strengthens Kupffer Cell-mediated Hepatitis B Virus Persistence through Inducing Endotoxemia in Mice. J Clin Transl Hepatol. 2022;10:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 268] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Wang X, Chen L, Wang H, Cai W, Xie Q. Modulation of bile acid profile by gut microbiota in chronic hepatitis B. J Cell Mol Med. 2020;24:2573-2581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 61. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1037] [Article Influence: 148.1] [Reference Citation Analysis (0)] |
| 62. | Paludan SR, Pradeu T, Masters SL, Mogensen TH. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat Rev Immunol. 2021;21:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 63. | Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 733] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 64. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 813] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 65. | Żeromski J, Kierepa A, Brzezicha B, Kowala-Piaskowska A, Mozer-Lisewska I. Pattern Recognition Receptors: Significance of Expression in the Liver. Arch Immunol Ther Exp (Warsz). 2020;68:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Kassa Y, Million Y, Gedefie A, Moges F. Alteration of Gut Microbiota and Its Impact on Immune Response in Patients with Chronic HBV Infection: A Review. Infect Drug Resist. 2021;14:2571-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020;180:1044-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 1408] [Article Influence: 281.6] [Reference Citation Analysis (0)] |
| 68. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 69. | Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:E3297-E3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 70. | Senoo H, Kojima N, Sato M. Vitamin A-storing cells (stellate cells). Vitam Horm. 2007;75:131-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 72. | Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 74. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 856] [Article Influence: 214.0] [Reference Citation Analysis (1)] |
| 75. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 76. | Paratore M, Santopaolo F, Cammarota G, Pompili M, Gasbarrini A, Ponziani FR. Fecal Microbiota Transplantation in Patients with HBV Infection or Other Chronic Liver Diseases: Update on Current Knowledge and Future Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 78. | Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 79. | Llovet JM, Bartolí R, March F, Planas R, Viñado B, Cabré E, Arnal J, Coll P, Ausina V, Gassull MA. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J Hepatol. 1998;28:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Steib CJ, Hartmann AC, v Hesler C, Benesic A, Hennenberg M, Bilzer M, Gerbes AL. Intraperitoneal LPS amplifies portal hypertension in rat liver fibrosis. Lab Invest. 2010;90:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 82. | Gu X, Lu Q, Zhang C, Tang Z, Chu L. Clinical Application and Progress of Fecal Microbiota Transplantation in Liver Diseases: A Review. Semin Liver Dis. 2021;41:495-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Retraction notice to: Clinical significance of the best response during repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma [J. Hepatol. 2014; 60: 1212–1218]. J Hepatol. 2015;62:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Ding JH, Jin Z, Yang XX, Lou J, Shan WX, Hu YX, Du Q, Liao QS, Xie R, Xu JY. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26:6141-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (6)] |
| 85. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 86. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Johnson KV, Foster KR. Why does the microbiome affect behaviour? Nat Rev Microbiol. 2018;16:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 88. | Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 89. | Caraceni P, Vargas V, Solà E, Alessandria C, de Wit K, Trebicka J, Angeli P, Mookerjee RP, Durand F, Pose E, Krag A, Bajaj JS, Beuers U, Ginès P; Liverhope Consortium. The Use of Rifaximin in Patients With Cirrhosis. Hepatology. 2021;74:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 90. | Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 91. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 92. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 93. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 94. | Liaw YF. Reversal of cirrhosis: an achievable goal of hepatitis B antiviral therapy. J Hepatol. 2013;59:880-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Chen MY, Peng CY, Chien RN, Huang YW, Wang HY, Lin CL, Yang SS, Chen TM, Mo LR, Hsu SJ, Tseng KC, Hsieh TY, Suk FM, Hu CT, Bair MJ, Liang CC, Lei YC, Tseng TC, Chen CL, Kao JH; C-TEAM study group and the Taiwan Liver Diseases Consortium. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 96. | Li X, Wu S, Du Y, Yang L, Li Y, Hong B. Entecavir therapy reverses gut microbiota dysbiosis induced by hepatitis B virus infection in a mouse model. Int J Antimicrob Agents. 2020;56:106000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 97. | Lu YX, He CZ, Wang YX, Ai ZS, Liang P, Yang CQ. Effect of Entecavir on the Intestinal Microflora in Patients with Chronic Hepatitis B: A Controlled Cross-Sectional and Longitudinal Real-World Study. Infect Dis Ther. 2021;10:241-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Lu YX, Chang YZ, Liang P, Yang CQ. Effect of Additional Clostridium butyricum on the Intestinal Flora of Chronic Hepatitis B Patients Treated with Entecavir. Infect Dis Ther. 2021;10:1519-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Alimirah M, Sadiq O, Gordon SC. Novel Therapies in Hepatic Encephalopathy. Clin Liver Dis. 2020;24:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [PubMed] |
| 101. | Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Fuchs M, Luketic V, Sanyal AJ. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478-487.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 102. | Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, Al-Soud WA, Mortensen MS, Sørensen SJ, Møller S, Bendtsen F; members of the CoRif study group. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J Gastroenterol Hepatol. 2018;33:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 103. | Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 105. | Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, Carroll I, Yang J, Gillevet PM, Jiao C, Takei H, Pandak WM, Iida T, Heuman DM, Fan S, Fiehn O, Kurosawa T, Sikaroodi M, Sartor RB, Bajaj JS. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol. 2016;7:e187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe. 2011;17:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Stavropoulou E, Bezirtzoglou E. Probiotics in Medicine: A Long Debate. Front Immunol. 2020;11:2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 108. | Lai HH, Chiu CH, Kong MS, Chang CJ, Chen CC. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 109. | Korpela K, Salonen A, Vepsäläinen O, Suomalainen M, Kolmeder C, Varjosalo M, Miettinen S, Kukkonen K, Savilahti E, Kuitunen M, de Vos WM. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 110. | Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, Yang J, Chen L, Chen G, Qu S, Liang J, Qin L, Huang Q, Peng Y, Li Q, Wang X, Kong P, Hou G, Gao M, Shi Z, Li X, Qiu Y, Zou Y, Yang H, Wang J, Xu G, Lai S, Li J, Ning G, Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 111. | Sergeev IN, Aljutaily T, Walton G, Huarte E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 112. | Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 799] [Cited by in RCA: 874] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 113. | Chahwan B, Kwan S, Isik A, van Hemert S, Burke C, Roberts L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J Affect Disord. 2019;253:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 114. | Bagga D, Reichert JL, Koschutnig K, Aigner CS, Holzer P, Koskinen K, Moissl-Eichinger C, Schöpf V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 115. | Ziada DH, Soliman HH, El Yamany SA, Hamisa MF, Hasan AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? Arab J Gastroenterol. 2013;14:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 117. | Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 118. | Thilakarathna WPDW, Rupasinghe HPV, Ridgway ND. Mechanisms by Which Probiotic Bacteria Attenuate the Risk of Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Ozdemir T, Fedorec AJH, Danino T, Barnes CP. Synthetic Biology and Engineered Live Biotherapeutics: Toward Increasing System Complexity. Cell Syst. 2018;7:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 120. | Nicaise C, Prozzi D, Viaene E, Moreno C, Gustot T, Quertinmont E, Demetter P, Suain V, Goffin P, Devière J, Hols P. Control of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents. Hepatology. 2008;48:1184-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, Kotula JW, Antipov E, Dagon Y, Denney WS, Wagner DA, West KA, Degar AJ, Brennan AM, Miller PF. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 122. | Ochoa-Sanchez R, Oliveira MM, Tremblay M, Petrazzo G, Pant A, Bosoi CR, Perreault M, Querbes W, Kurtz CB, Rose CF. Genetically engineered E. coli Nissle attenuates hyperammonemia and prevents memory impairment in bile-duct ligated rats. Liver Int. 2021;41:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 123. | Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 390] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 124. | Marotz CA, Zarrinpar A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J Biol Med. 2016;89:383-388. [PubMed] |
| 125. | Niina A, Kibe R, Suzuki R, Yuchi Y, Teshima T, Matsumoto H, Kataoka Y, Koyama H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci Microbiota Food Health. 2021;40:98-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Geng S, Cheng S, Li Y, Wen Z, Ma X, Jiang X, Wang Y, Han X. Faecal Microbiota Transplantation Reduces Susceptibility to Epithelial Injury and Modulates Tryptophan Metabolism of the Microbial Community in a Piglet Model. J Crohns Colitis. 2018;12:1359-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 498] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 128. | Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, Reinert SE, Machan JT, Brandt LJ. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med. 2016;165:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 129. | Khanna S, Vazquez-Baeza Y, González A, Weiss S, Schmidt B, Muñiz-Pedrogo DA, Rainey JF 3rd, Kammer P, Nelson H, Sadowsky M, Khoruts A, Farrugia SL, Knight R, Pardi DS, Kashyap PC. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017;5:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 130. | Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, Birch DW, Samarasinghe KK, Walter J, Madsen KL. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 131. | Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174:1406-1423.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 739] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 132. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 133. | Chauhan A, Kumar R, Sharma S, Mahanta M, Vayuuru SK, Nayak B, Kumar S, Shalimar. Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig Dis Sci. 2021;66:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 134. | Liu R, Kang JD, Sartor RB, Sikaroodi M, Fagan A, Gavis EA, Zhou H, Hylemon PB, Herzog JW, Li X, Lippman RH, Gonzalez-Maeso J, Wade JB, Ghosh S, Gurley E, Gillevet PM, Bajaj JS. Neuroinflammation in Murine Cirrhosis Is Dependent on the Gut Microbiome and Is Attenuated by Fecal Transplant. Hepatology. 2020;71:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 135. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 136. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 137. | Shen TC, Albenberg L, Bittinger K, Chehoud C, Chen YY, Judge CA, Chau L, Ni J, Sheng M, Lin A, Wilkins BJ, Buza EL, Lewis JD, Daikhin Y, Nissim I, Yudkoff M, Bushman FD, Wu GD. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015;125:2841-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 138. | Liu J, Zhai C, Rho JR, Lee S, Heo HJ, Kim S, Kim HJ, Hong ST. Treatment of Hyperammonemia by Transplanting a Symbiotic Pair of Intestinal Microbes. Front Cell Infect Microbiol. 2021;11:696044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Ponziani FR, Gerardi V, Pecere S, D'Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322-12333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 141. | Kang Y, Cai Y. Gut microbiota and hepatitis-B-virus-induced chronic liver disease: implications for faecal microbiota transplantation therapy. J Hosp Infect. 2017;96:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 142. | Xu M, Wang B, Fu Y, Chen Y, Yang F, Lu H, Xu J, Li L. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb Ecol. 2012;63:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 143. | Wu ZW, Lu HF, Wu J, Zuo J, Chen P, Sheng JF, Zheng SS, Li LJ. Assessment of the fecal lactobacilli population in patients with hepatitis B virus-related decompensated cirrhosis and hepatitis B cirrhosis treated with liver transplant. Microb Ecol. 2012;63:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Lutz P, Parcina M, Bekeredjian-Ding I, Nischalke HD, Nattermann J, Sauerbruch T, Hoerauf A, Strassburg CP, Spengler U. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One. 2014;9:e93909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |