Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3535
Peer-review started: January 17, 2022
First decision: April 11, 2022
Revised: April 25, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 28, 2022
Processing time: 190 Days and 14.2 Hours
Hepatocellular carcinoma (HCC) is the most common type of liver cancer worldwide. Viral hepatitis is a significant risk factor for HCC, although metabolic syndrome and diabetes are more frequently associated with the HCC. With increasing prevalence, there is expected to be > 1 million cases annually by 2025. Therefore, there is an urgent need to establish potential therapeutic targets to cure this disease. Peroxisome-proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor that plays a crucial role in the patho-physiology of HCC. Many synthetic agonists of PPARγ suppress HCC in experimental studies and clinical trials. These synthetic agonists have shown promising results by inducing cell cycle arrest and apoptosis in HCC cells and preventing the invasion and metastasis of HCC. However, some synthetic agonists also pose severe side effects in addition to their therapeutic efficacy. Thus natural PPARγ agonists can be an alternative to exploit this potential target for HCC treatment. In this review, the regulatory role of PPARγ in the pathogenesis of HCC is elucidated. Furthermore, the experimental and clinical scenario of both synthetic and natural PPARγ agonists against HCC is discussed. Most of the available literature advocates PPARγ as a potential therapeutic target for the treatment of HCC.
Core Tip: Hepatocellular carcinoma (HCC) is the most common type of liver cancer worldwide. Viral infections and metabolic syndrome are the major risk factors for HCC, and its incidence is expected to increase to > 1 million cases annually by 2025. The crucial role of peroxisome-proliferator-activated receptor gamma (PPARγ) in HCC pathophysiology makes it a potential therapeutic target. Along with synthetic agonists, natural PPARγ agonists provide alternative and safer options for HCC treatment; however, they need to be validated clinically. This review discusses the regulatory role of PPARγ in HCC pathogenesis and experimental and clinical scenarios of PPARγ agonists in HCC treatment.
- Citation: Katoch S, Sharma V, Patial V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: Experimental and clinical scenarios. World J Gastroenterol 2022; 28(28): 3535-3554
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3535.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3535
Liver cancer is the sixth most common cause of cancer-related death worldwide, with a higher prevalence in men than women. Hepatocellular carcinoma (HCC) incidence was expected to increase to > 1 million individuals annually by 2025[1]. HCC, a primary subtype of liver cancer, primarily occurs in Asia and Africa due to the high prevalence of hepatitis B virus (HBV), hepatitis C virus, and diabetes[2]. These conditions are linked to the inflammatory response in the liver, leading to the development of HCC. Furthermore, other conditions such as obesity, dietary mycotoxin exposure, and excessive alcohol consumption are also among the risk factors for the development of HCC. These factors lead to the development of cirrhosis in 70%-80% of HCC patients. Liver transplantation is currently the best option for curing HCC, but there is a limitation to the availability of donors[3]. During the last two decades, the understanding and management of HCC have changed dramatically due to the extensive basic and clinical research, which may further help to reveal potential targets for the treatment of HCC. Sorafenib is the first-line defense therapy approved by the United States food and Drug Administration (FDA) for the advanced stages of HCC. It is a type of multikinase inhibitor that shows tumor-suppressing activity via targeting vascular endothelial growth factor receptor, adenosine monophosphate-activated protein kinase (AMPK), and platelet-derived growth factor receptor[4]. Apart from their therapeutic potential, sorafenib shows acquired resistance in HCC cells. The low response rate indicates that patients sensitive to sorafenib during the treatment will develop resistance within 6 mo. These negative impacts of approved drugs prompted many researchers to find novel drugs or targets to cure HCC[5].
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated nuclear receptor activated by synthetic and natural agonists[6]. It is highly expressed in adipose tissue, where it plays a central role in regulating adipose tissue function. Many studies have established the role of PPARγ in the pathophysiology of HCC. In vitro and in vivo data have shown the inhibitory role of PPARγ activation in tumor cell growth, migration, and invasion suggesting its therapeutic role in the growth regulation of HCC[7,8]. The antitumor effects of PPARγ are fulfilled by various mechanisms, including the induction of cell cycle arrest and activation of genes/proteins involved in immune and inflammatory responses[9]. Previous reports have revealed the mechanism underlying the development of HCC and suggested the presence of PPARγ in human HCC tissues, which shows a dose-dependent decrease in the growth of HCC cell lines[10]. Thus, molecules modulating PPARγ signaling pathways will provide a novel solution for the effective treatment of HCC. This review focuses on the role of PPARγ in the HCC pathophysiology and the experimental and clinical status of PPARγ agonists in the treatment of HCC.
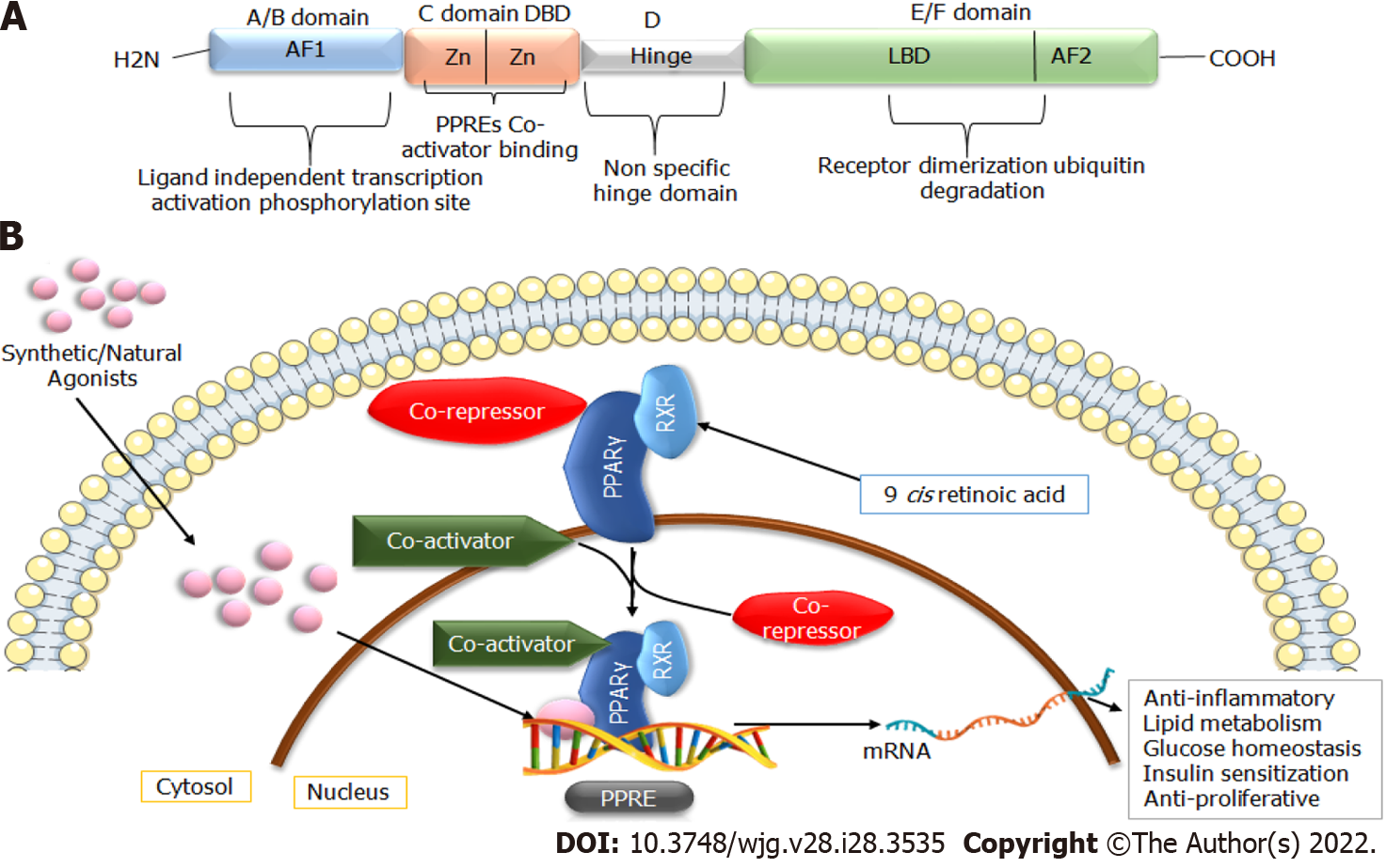
The PPARs protein belong to the superfamily of nuclear hormone factors containing 48 members. PPARs were mainly recognized for their proficiency in promoting peroxisome proliferation in the liver, and their expression is mainly regulated in response to ligand binding[11]. Three isoforms of PPARs, namely PPARα, PPARγ, and PPARδ, have been studied to a large extent. Of these isoforms, PPARγ is highly expressed in adipose tissue, where it plays a vital role in regulating lipid homeostasis, energy balance, adipogenesis, and inflammation. Due to the presence of different promotor regions and 5’ exons, PPARγ has three distinct mRNAs (PPARγ1, PPARγ2, and PPARγ3). The translation products of PPARγ1 and PPARγ3 yield identical proteins; however, PPARγ2 results in a product with an additional N-terminal region[12]. PPARγ1 and PPARγ3 are biologically expressed in different tissues (hepatocytes, muscles, and endothelial cells), whereas PPARγ2 is only widely expressed in adipose tissue[9,13]. PPARγ plays a significant role in maintaining metabolic alterations, inflammation, glucose homeostasis, cell cycle regulation, differentiation, and migration, making it a potential therapeutic target for treating metabolic disorders and cancers[14]. The structural arrangement of PPARs is similar to steroid and thyroid hormone receptors. Its ligand-binding cavity is 3- to 4 -times higher than that of the other nuclear receptors. They can be activated by various natural and synthetic agonists, such as essential fatty acids[15,16]. The three-dimensional structure of PPARγ consists of a canonical domain shared with other nuclear receptors, named A-E from N to C terminus (Figure 1). These domains include the amino-terminal AF-1 domain, a DNA-binding domain with two zinc finger motifs, and a ligand-binding domain (LBD or E/F domain) at the C-terminus responsible for specific ligand binding at the peroxisome proliferator response element (PPRE)[16,17]. After interaction with specific ligands, the LBD facilitates the heterodimerization of PPARs with retinoid X receptor (RXR), which subsequently binds to the PPRE of the target gene. RXR is activated by the natural ligand 9-cis-retinoic acid receptor and synthetic retinoids receptors. However, in the absence of specific ligands, heterodimers bind with co-repressors, ultimately inhibiting the gene[12]. This complex subsequently recruits coactivation or co-repressors to regulate the expression of targets genes related to lipid glucose metabolisms and inflammation (Figure 1)[6].
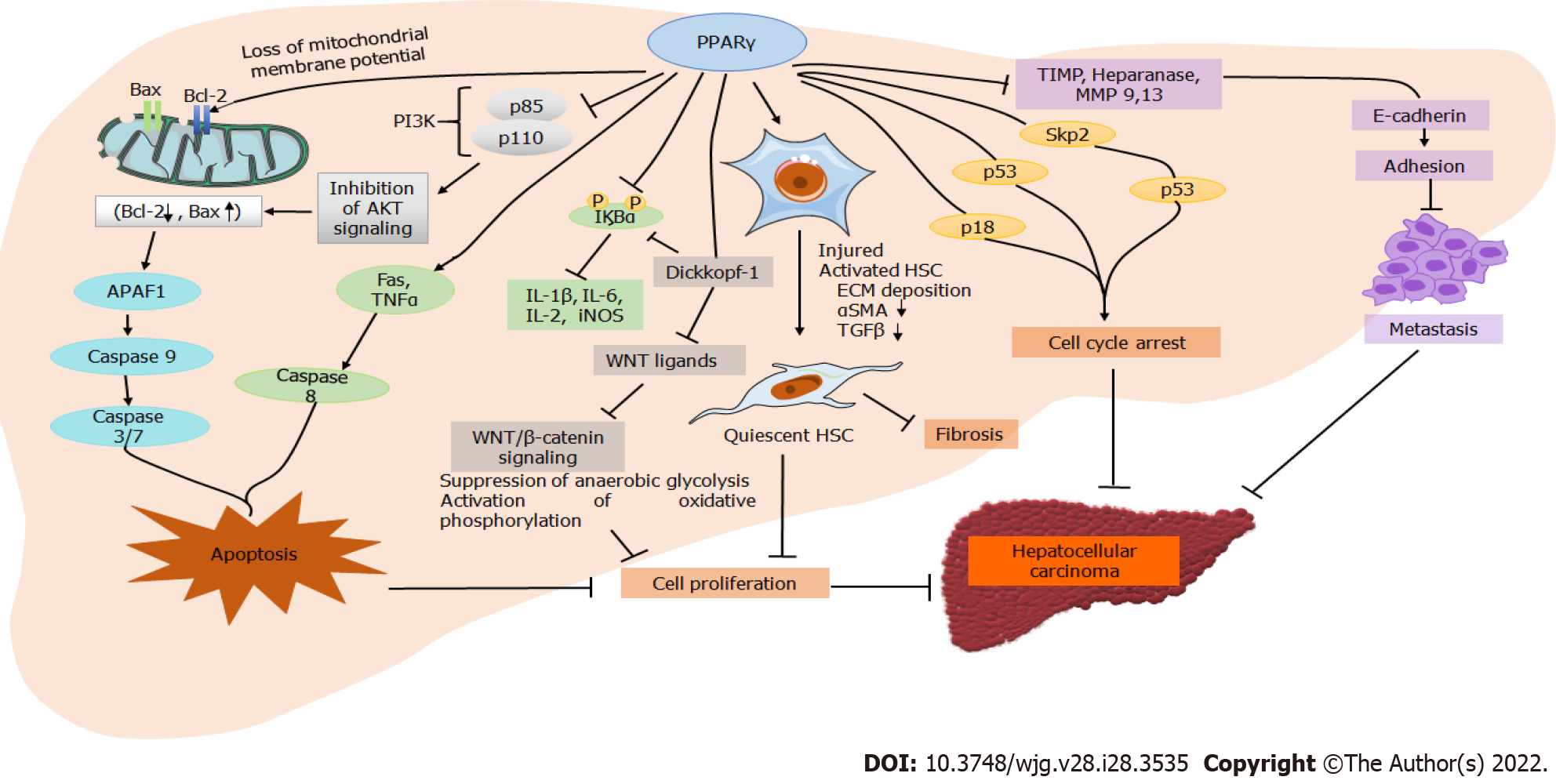
PPARγ plays a multifunctional role in many tissues and cell types such as adipocytes, pancreas, macrophages, liver, kidney, and skeletal muscle. It plays a regulatory role in adipocyte differentiation, lipid metabolism, and insulin sensitivity via downregulating leptin concentration[7]. Despite the low expression in the healthy liver, PPARγ plays a significant role in several hepatic conditions such as fatty liver, fibrosis, and HCC. Many in vitro and in vivo studies have reported that natural and synthetic PPARγ agonists inhibit tumor growth and cell migration in HCC[18]. The activation of PPARγ inhibits cell growth by inducing G0/G1 cell cycle arrest in HCC cells, which is suggested to be associated with p21, p27, and p18 upregulation (Figure 2). Furthermore, p27 upregulation downregulates S-phase kinase-associated protein-2 (Skp2) in HCC, an F-box protein component of the Skp, Cullin, F-box ubiquitin-ligase complex. p27 plays a vital role in G0/G1 arrest instead of p21[10,19]. The direct overexpression of PPARγ in hepatic cancerous cells also inhibits cell growth; however, the cells are arrested in the G2/M phase instead of the G0/G1 phase after PPARγ agonist treatment. G2/M phase arrest in PPARγ overexpression is attributed to activating cell division cycle 25C phosphatase by Ser216 phosphorylation and preventing premature mitosis[20]. Compared to wild-type mice, another study on PPARγ-deficient mice showed increased hepatocarcinogenesis after treatment of diethylnitrosamine (DENA). Growth differentiation factor 15 (GDF 15) is a target gene of PPARγ and is induced by its activation. GDF 15 overexpression in many cancers is associated with an antitumorigenic response, as it was suggested to reduce cancer cell viability and induce cell apoptosis. PPARγ activation by agonist or direct overexpression induces apoptosis by intrinsic and extrinsic pathways[21]. Activation of the extrinsic apoptosis pathway by PPARγ overexpression is attributed to the induction of tumor necrosis factor α (TNFα) and Fas, leading to the activation of downstream caspases (Figure 2). In the intrinsic pathway, PPARγ overexpression stimulates B-cell lymphoma 2 (Bcl-2)-associated X protein transcription and release into the cytosol, activating apoptotic protease activating factor 1 and caspase-9 complex, which further triggers caspase 3 and 7 to induce apoptosis[6,21]. The antitumorigenic effect of PPARγ in HCC is also suggested via modulation of the phosphoinositide 3-kinase (PI3K)/Akt pathway[22]. PPARγ activation attenuates p85 activation, which is essential for Akt induction, thus inhibiting PI3K/Akt signaling and inducing apoptosis[23].
Hepatic inflammation is crucial in the progression of HCC, and PPARγ plays a central role in regulating inflammation. PPARγ inhibits inflammation by interfering with nuclear factor-kappa B (NF-κB) and suppressing the production of proinflammatory cytokines (TNFα and interleukin 1 beta [IL-1β]). Activation of PPARγ by specific ligands in T-cell differentiation promotes an inflammatory response, thereby playing a significant role in the adaptive immune response. Thus, PPARγ act as an important therapeutic target for regulating inflammatory markers (TNFα, IL-2, IL-1β, and IL-6) against the progression of several diseases[7,16]. Hepatic stellate cell (HSC) activation and fibrogenic factor significantly contribute to the development of HCC (Figure 2). PPARγ is highly expressed in quiescent HSCs and has a role in their transdifferentiation. HSC activation and PPARγ are inversely related as increased expression of PPARγ inhibits HSC proliferation and induces apoptosis in activated HSCs[24]. It also reduces the expression of alpha-smooth muscle actin (αSMA) and hydroxyproline to inhibit hepatic fibrosis. Hepatic injury induces microvascular complications in the liver, stimulating various sinusoidal cells such as HSCs, liver sinusoidal endothelial cells, and Kupffer cells. PPARγ regulates the role of these cells in liver inflammation and fibrosis. The deactivation of HSCs by PPARγ agonists further reduces extracellular matrix deposition and expression patterns of matrix metalloproteinase (MMP)/tissue inhibitors of MMPs (TIMP). The expression of MMP9 and MMP13, TIMP, heparinase, and E-cadherin is associated with cancer cell migration and metastasis[25]. The expression patterns of these markers are directly linked to PPARγ activation. Reports also link PPARγ activation with autophagy in HCC. Autophagy is thought to be inhibited after autophagosome formation in the absence of PPARγ, resulting in increased light chain 3 protein expression and accumulation of p62 in the autophagosome[26,27]. Therefore, induction of autophagy in HCC is linked to the activation of PPARγ in HCC. A recent study elucidated the role of PPARγ coactivator-1α (PGC1α) in suppressing HCC metastasis. The levels of PGC1α were downregulated in human HCC and associated with a poor prognosis, large tumor size, and vascular invasion[28]. However, PGC1α overexpression in the HCC cells inhibited tumor cell migration and invasion. The suppression of metastasis by PGC1α overexpression was suggested due to PPARγ-dependent downregulation of pyruvate dehydrogenase kinase isozyme 1 and inhibition of aerobic glycolysis through Wnt/β-catenin/pyruvate dehydrogenase kinase-1 (PDK1) axis regulation[29].
Zinc finger protein 746 (ZNF746) is a Parkin-interacting substrate (PARIS), acting as a transcriptional regulator of PPARγ co-activator 1 alpha (PGC1α) which further regulates the activity of PPARγ and is involved in the onset of HCC. The elevated levels of insoluble parkin with PARIS accretion in the hepatic cells of diethylnitrosamine (DEN)-injected mice were observed with the downregulation of PGC1α and NRF1. Moreover, Chang liver cells treated with hydrogen peroxide showed PARIS accretion and alleviation of PGC1α. As the co-activator, PGC1α is directly linked to PPARγ regulation, further monitoring the oncogenic stress promoting cancer development. Thus, the modulation of PPARγ and its co-activators can be a promising therapeutic target for HCC[30]. In a clinical study, it was subsequently observed that the expression of PGC1α is negatively associated with tumor size and vascular influx. The increased expression of PGC1α could elevate the degree of oxidative phosphorylation, further slowing down the rate of metastasis and the Warburg effect of HCC cells[31]. Rapid proliferation is the prime feature of cancerous cells for which cells need to meet the high energy demand through the aerobic glycolysis pathway rather than the pyruvate oxidation pathway. The canonical Wnt/β-catenin signaling was also targeted to observe the expression of PDK1 in the PGC1α knockdown model by employing two popular inhibitors of this signaling pathway (XAV-939 and ICG-001). Gene Set Enrichment Analysis indicated that these inhibitors alleviate the overexpression of extracellular lactate, suggesting the possible role of PGC1α in the inhibition of aerobic glycolysis via Wnt/β-catenin signaling. Dual-luciferase reporter assays showed that the transcriptional actions of PPARγ are significantly increased in HCCLM3 and MHCC97H cells with PGC1α augmentation. These results show that the tumor-suppressive activity of PGC1α depends on PPARγ, which makes PPARγ a key regulator of HCC[29,32]. An earlier report revealed the role of PPARγ in HCC by analyzing the mRNA and protein expression in 20 patients with cirrhosis and chronic hepatitis. The results indicated a statistically pronounced drop in levels of PPARγ in HCC compared to the non-tumorous liver tissue[33]. A report confirmed that miR-130b aids cell aggressiveness by suppressing PPARγ in human HCC[34]. Similarly, evidence on the oncogenic role of miR-1468 in HCC via activating the PPARγ/Akt pathway was also recently confirmed. The increased levels of miR-1468 elevated the malignant prognostic features and improved survival. Carboxy-terminal domain 2 and UPF1 RNA Helicase And ATPase were identified as the downstream targets for miR-1468, which regulate PPARγ/Akt pathway activation. Restoration of the expression of these targets partially abolished the effects of miR-1468, explaining the regulation via PPARγ/Akt signaling[35].
Many studies have explored the therapeutic effects of synthetic and natural PPARγ agonists against HCC in preclinical and clinical trials. The activation of PPARγ significantly suppresses HCC progression and invasion. Several findings have identified PPARγ as a target for tumor suppression, a mediator of apoptosis, and a suppressor of carcinogenesis and metastasis by triggering intrinsic pathways and mainly inhibiting the PI3K/Akt survival pathway[8,21,36]. The various synthetic and natural PPARγ agonists used for HCC are listed in Table 1.
| Agonist name | Drug bank/ PubChem ID | Model | Concentration/dose of agonist | Effects | Ref. |
| Synthetic agonists | |||||
| Pioglitazone | DB01132 | In vivo (Rats, and Mice) | 3 mg/kg; 10 mg/kg | Reduced HCC progression and decreased tumor size and volume | [44] |
| Rosiglitazone | DB00412 | In vivo (Orthotopic Mice)In vitro (MHCC97L, and BEL-7404) | 50 µmol/L | Decreased HCC migration, and invasiveness | [25] |
| In vitro (HepG2 and PC3) | 0.1, 1, 10, 100 µmol/L | Reduced cancer growth, Increased apoptosis | [49] | ||
| In vitro (HepG2 and Hep3B) | 80 µmol/L | Restricted the oncogenic activity of SEPT2 | [50] | ||
| Telmisartan | DB00966 | In vitro (HLF, HLE, HuH-7, PLC/PRF/5, and HepG2) | 10, 50 or 100 µmol/L | Inhibit proliferation, induce cell cycle arrest | [53] |
| In vivo (Mice) | 15 mg/kg | Reversed malignant anomalies, antioxidant, anti-inflammatory | [54] | ||
| Troglitazone | DB00197 | In vitro (Hep G2, HuH-7, KYN-1, and KYN-2) | 5, 10, 25 µmol/L | Reduced cell proliferation and increased apoptosis | [56] |
| In vitro (HepG2) | 5, 10, 20, 40, 80, and 100 µmol/L | Apoptosis and growth inhibition | [57] | ||
| In vitro (Hep G2, HuH-7, KYN-1, and KYN-2) | 5, 10, and 25 µmol/L | Inhibited DNA synthesis, cell cycle growth, and α-fetoprotein levels | [58] | ||
| In vitro (PLC/PRF/5, and HuH-7) | 5, 10, 20, 40, 60, 80, and 100 µmol/L | Reduced cell proliferation and increased apoptosis | [59] | ||
| In vitro (HLF, HAK-1A, HAK-1B, and HAK-5) | 10, 20, 30, 40, and 50 µmol/L | Reduced cell proliferation and increased apoptosis | [19] | ||
| Saroglitazar | DB13115 | In vivo (Mice) | 4 mg/kg | Reduced inflammation in hepatic lobules, hepatocellular ballooning, and steatosis | [61] |
| In vivo (Rats) | 4 mg/kg | Improved lipid profile, and histopathological changes | [62] | ||
| Natural agonists | |||||
| Cannabinol, Cannabinoids | DB14737 | In vitro (HepG2 and HUH-7); In vivo (Mice) | 8 µmol/L; 15 mg/kg | Increased apoptosis, autophagy, anti-proliferative | [66] |
| In vitro (HEK-293T and Neuro-2a); In vivo (Mice) | 1, 5, 10, 25 µmol/L; 20 mg/kg | Antitumor, antioxidant, anti-inflammatory | [68] | ||
| Capsaicin | DB06774 | In vivo (Rats) | 0.5 and 1 mg/kg | Inhibit hepatic injury, and collagen deposition, anti-inflammatory | [71] |
| Curcumin | DB11672 | In vivo (Rats) | 20 mg/kg | Attenuated histopathological, serological, proliferative, and apoptotic parameters | [77] |
| In vitro (H22); In vivo (Mice) | 5, 10, 20, 40, and 80 µmol/L; 50, 100 mg/kg | Antiproliferative, decrease tumor growth, induce apoptosis | [78] | ||
| In vivo (Mice) | 150 mg/kg | Reduced inflammation, and tumor size | [79] | ||
| In vivo (Rats) | 0.5, 1, 2, 5, 10, 15, and 20 ng/mL | Interrupted TGFβ signaling, activated hepatic stellate cells | [80] | ||
| In vitro (SMMC7721 and Huh-7) | 10, 20, 40, 80, and 160 µmol/L | Suppressed cellular proliferation | [82] | ||
| Hesperidin | DB04703 | In vivo (Rats) | 50 and 100 mg/kg | Suppressed TGFβ signaling and hepatocarcinogenesis | [85] |
| In vivo (Rats) | 200 mg/kg | Inhibited PI3K/Akt pathway, Antioxidant | [86] | ||
| In vitro (HepG2); In vivo (Rats) | 100 µmol/L; 150 mg/kg | Inhibited Wnt3a/5a signaling pathway, anti-inflammatory | [87] | ||
| Hispidulin | DB14008 | In vitro (SMMC7721 and Bel7402); In vivo (mouse tumor xenograft) | 10 and 20 µmol/L; 20 and 40 mg /kg | Anticancerous, inhibited cell migration | [89] |
| In vitro (NCI-H460 and A549) | 4, 8, 15, 30, and 60 µmol/L | Induced ROS-mediated apoptosis, anti-cancerous | [90] | ||
| Isoflavone | DB12007 | In vivo (Bel-7402 and SK-Hep-1)In vivo (Mice) | 75 and 12 µmol/L resp.; 25 and 7.5 mg/kg resp. | Anti-inflammatory, anti-tumorigenic, reduced the size and volume of tumor | [94] |
| In vitro (Hepa 1-6 cells) | 1, 5, 10, 15, 20, 25, 50, 75, and 100 μmol/L | Antitumorigenic and antiproliferative | [95] | ||
| In vitro (HCC-LM3, SMMC-7721, Hep3B, Bel-7402, and Huh-7)In vivo (Mice) | 40, 60, and 80 μmol/L; 20, 40, and 80 mg/kg | Suppressed aerobic glycolysis and increased apoptotic rate | [96] | ||
| Oroxyloside | 14655551 | In vitro (HepG2) and SMMC-7721); In vivo (Mice) | 100, 200, and 300 μmol/L; 90 mg/kg | Cell cycle arrest and growth repression | [100] |
| Resveratrol | DB02709 | In vivo (Rats) | 100 mg/kg | Antioxidant, anti-inflammatory, anticancer | [101] |
| In vitro (HepG2); In vivo (Rats) | 7.81, 15.63, 31.25, 62.5, 125, and 250 µg/mL; 20 mg/kg | Attenuated histopathological, serological, proliferative, and apoptotic parameters | [102] | ||
| Miscellaneous | |||||
| Avicularin | 5490064 | In vitro (HuH-7) | 25, 50, and 100 µg/mL | Decreased the cell migration and invasiveness | [107] |
| Honokiol | 72303 | In vitro (HEK-293 and 3T3-L1); In vivo (Mice) | 1, 3, and 10 μmol/L; 100 mg/kg | Activated PPARγ/RXR heterodimers; Reduced hyperglycemia | [108] |
| Chrysin | DB15581 | In vitro (MDA-MB-231 and HepG2)In vivo (Mice) | 10 µmol/L; 10 mg/kg | Increased apoptosis | [112] |
| Quercetin | DB04216 | In vitro (HepG2 and SMCC-7721); In vivo (Mice) | 0.05, 0.1, and 0.15 mmol/L; 40 mg/kg | Promoted the autophagy | [114] |
| In vitro (PATU-8988 and PANC-1) | 20, 40, 80, and 160 µmol/L | Suppressed HCC via STAT3 pathway | [117] | ||
| In vitro (LM3); In vivo (Mice) | 40, 80, and 120 µmol/L; 100 mg/kg | Reduced invasiveness, Cell cycle regulation | [118] | ||
| Clinical trials | |||||
| Population type | No. of patients | ||||
| Thiazolidinediones | NA | Hongkong | 1153 | Reduce the synergistic effect of diabetes with liver disorders; Reduced risk of HCC | [38],[39],[40],[41] |
| Taiwanese | 77396 | ||||
| 32891 | |||||
| 76349 | |||||
| Pioglitazone | DB01132 | Chinese | 75 | Blocked RAGE signaling; Reduced HCC | [45] |
| Japanese | 85 | Reduced growth and invasion of HCC cells | [46] | ||
| Thai | 10000 | Reduced risk of HCC | [47] | ||
| Rosiglitazone | DB00412 | French | 44 | Reduced NASH activity and ballooning score, Ameliorated histopathological aberrations | [51] |
| Saroglitazar | DB13115 | Indian | 30 | Improved glycemic index and liver stiffness | [63] |
| 90 | Improved fibrosis score | [64] | |||
| Isoflavone | DB12007 | Japanese | 302 | Antioxidant, reduced risk of HCC | [97] |
| 191 | Antioxidant, reduced risk of HCC | [98] | |||
PPARγ itself and its agonists have anticancer activities, such as growth inhibition, induction of apoptosis, and cell differentiation. Thiazolidinediones (TZDs) are a class of synthetic PPARγ agonists, and many compounds of this class have been studied for their efficacy in experimental models and clinical trials. These compounds were used as a bioregulatory remedial approach to target the communicative framework of HCC in patients with non-curative HCC[37]. TZDs are also effective for glycemic control and the likelihood of HCC and hepatic manifestation in diabetic patients with chronic hepatitis B (CHB). Of the 28999 patients with CHB, 3963 patients developed HCC at a median follow-up of 7.1 years, whereas 1153 patients were administered TZD during the follow-ups. The findings showed the co-relation of TZD use with lowering the risk of poor hepatic manifestations in diabetic patients with CHB[38]. A population-based case-control study performed in 23580 diabetic patients demonstrated the negative relationship between the risk of HCC and use of TZDs. There is a time-dependent effect of TZD use on the risk of HCC. The longer the duration of TZD use, the lower the risk of HCC[39-41]. Many other reports have also suggested that the administration of PPARγ agonists ameliorates several types of cancers, i.e. colorectal, bladder, lung, and liver cancers. The effects are more substantial at higher cumulative dosages with longer durations[42].
Pioglitazone (PGZ), a PPARγ ligand, works by improving the insulin sensitivity of tissues and exhibits anticancer activity. It selectively stimulates PPARγ via modulating the transcriptional alterations of genes involved in glucose metabolism and insulin resistance and further decreasing the gluconeogenesis and levels of glycated hemoglobin in the bloodstream[43]. PGZ treatment inhibits fibrosis progression and HCC development and reduces tumor size in DENA-induced rats at 3 mg/kg and mice at 10 mg/kg. PGZ is suggested to exhibit protective effects by reducing mitogen-activated protein kinase (MAPK) and upregulating adiponectin levels, resulting in activation of the hepatoprotective AMPK pathway[44].
The anticancer activity of PGZ is attributed to the pathological receptors for advanced glycation end products (RAGE). HCC tissues from 75 patients showed high expression of RAGE in HCC tissues, which was closely linked to pathological staging and lymph-vascular space influx. However, PGZ treatment suppressed cellular proliferation, ameliorated apoptosis, and cell cycle arrest, which further elevated PPARγ expression and decreased the expression of RAGE, NF-κB, high mobility group box 1, p38MAPK Ki-67, MMP2, and cyclin D1. The results demonstrated that PGZ as a PPARγ agonist possibly slows down the growth and invasion of HCC cells by blocking RAGE signaling[45]. Another prospective study confirmed the effect of PGZ on HCC by investigating 85 patients with HCC and hepatitis C virus infection to investigate recurrence-free survival. The spline-model analysis showed that the lessened risk of HCC recurrence is associated with increased body weight and body mass index ≥ 23. PGZ was also observed to alleviate insulin resistance and serum adiponectin levels[46]. A lifetime Markov model was employed among the population of Thailand to study the life expectancy, quality-adjusted life years, lifetime costs, and the incremental cost-effectiveness ratios in HCC patients. The weight reduction program with the administration of PGZ demonstrated that PGZ can reduce the number of HCC cases[47]. These therapeutic potentials also have limitations. PGZ has adverse effects such as body weight gain, peripheral edema, bone loss, and heart failure. Additionally, the risk of bladder cancer significantly limits the use of this agonist in the medical field[48].
Rosiglitazone is a member of the TZD class of insulin-sensitizing PPARγ agonists. An inhibitory effect of PPARγ was reported on the invasive and metastatic potential of HCC in vitro (MHCC97L and BEL-7404 cell lines) and in vivo (orthotopic HCC mouse model). A pronounced expression of PPARγ was demonstrated in HCC cell lines treated with adenovirus-expressing mouse PPARγ1 (Ad-PPARγ), rosiglitazone (50 µmol/L), or Ad-PPARγ plus rosiglitazone. The induction of PPARγ markedly repressed HCC cell migration, invasiveness, levels of pro-metastatic genes (MMP9, MMP13, heparanase [HPSE]), and hepatocyte growth factor. However, the levels of cell adhesion genes (E-cadherin and SYP), extracellular matrix regulator TIMP3, and tumor suppressor gene retinoblastoma 1 were elevated. Additionally, direct transcriptional regulation of the genes TIMP3, MMP9, MMP13, and HPSE regulating PPARγ levels was also validated by chromatin immunoprecipitation-PCR[25]. Bcl-2 is a well-known family of anti-apoptotic proteins regulating endogenous apoptotic pathways and are highly expressed in carcinomas. (-)-gossypol ((-)-G) is the (-) enantiomer of gossypol that acts as a small molecule to induce apoptosis in several types of cancers by inhibiting Bcl-2 proteins. In a study, rosiglitazone was employed to sensitize (-)-G to induce apoptosis at different concentrations (0.1, 1, 10, 100 µmol/L). The (-)-G induced Mcl-1 (myeloid cell leukemia-1) stability was the prime concern for its apoptotic activity. However, rosiglitazone attenuated this stability via Janus kinase phosphorylation, further repressing cancer growth. These results suggest that rosiglitazone can reduce cancer growth and sensitize the other apoptotic factors for performing a similar activity. The study also provides insights into the novel cancer therapeutic activity of BH3 mimetics in the case of carcinomas based on the combination of PPARγ agonists and BH3 mimetics[49]. Rosiglitazone (80 µmol/L) also inhibits HCC cell growth by restricting the oncogenic activity of septin 2[50].
A long-term clinical trial was conducted in which 53 patients underwent liver biopsies and were further treated with rosiglitazone (8 mg/d) for the next 2 years. Forty-four patients fulfilled the criteria of the extension period and underwent another biopsy. During the extension phase, serum insulin and alanine aminotransferase (ALT) levels were decreased by 26% and 24%, respectively. Non-alcoholic steatohepatitis activity, ballooning, and fibrotic stage were decreased but not on a significant scale. The treatment was continued for another 2 years, but no significant results were obtained, showing that rosiglitazone does attenuate insulin sensitivity and transaminase levels but might not significantly improve other histopathological parameters. However, additional targets were suggested to be explored[51]. However, there is increasing evidence of bone fractures in females medicated with rosiglitazone after menopause, limiting its use. In September 2010, the FDA restricted the use of rosiglitazone based on meta-analyses of mostly short-term randomized controlled trials, which showed evidence of myocardial infection risk. However, these restrictions were removed in 2013 based on other large clinical trials by Duke Clinical Research Institute, which showed no complications regarding heart failure[52].
Telmisartan (TEL) is an angiotensin II receptor blocker with a high affinity for the angiotensin II receptor type 1, whose impromptu link with HCC has been discovered; however, the underlying mechanism is not clear. TEL shows basal resemblance with a well-known PPARγ agonist, PGZ. TEL (at concentrations of 10, 50, or 100 µmol/L) inhibits the proliferation and G0 to G1 cell cycle transition leading to G0/G1 cell cycle arrest in hepatic cancer cells (HLF, HLE, HuH-7, PLC/PRF/5, and HepG2) in a dose-dependent manner. The cell cycle arrest was accompanied by reduced cell cycle-related proteins, including cyclin D1 and cyclin E. Further TEL was suggested to increase the activity of AMPK and inhibit the mammalian target of rapamycin (mTOR) pathway[53]. Another study used a DENA-induced HCC mouse model to evaluate the effects of TEL (15 mg/kg), sorafenib (SRF) (30 mg/kg), and a combination of these two agonists. The treatment downregulated the mRNA expression of NF-кBp65, AFP, TNFα, and transforming growth factor beta 1 (TGFβ1) resulting in the reversion of malignant anomalies and suppression of extracellular signalregulated protein kinase 1/2 (ERK1/2) activation. SRF and TEL showed antiproliferative, antimetastatic, and anti-angiogenic effects by improving the expression of hepatic cyclin D1, MMP2, and vascular endothelial growth factor (VEGF). However, only TEL has exhibited agonistic activity for PPARγ receptors, as indicated by the elevated PPARγ DNA-binding activity, mRNA expression of cluster of differentiation 36, heme oxygenase 1, and enhanced hepatic antioxidant capacity. Moreover, TEL and SRF both ameliorate phosphorylation-induced activation of TGFβ-activated kinase 1 (TAK1), suggesting that TAK1 might act as the core mediator for the interaction between ERK1/2 and NF-кB. TEL exerts its anticancer effects by modulating the ERK1/2, TAK1, and NF-кB signaling axis from the perspective of its PPARγ agonistic activity. Thus, TEL may be a useful PPARγ agonist for further clinical studies in the context of HCC treatment[54]. Despite its potential, it has adverse effects including headaches, dizziness, fatigue, upper respiratory tract or stomach-related infections, sinusitis, nonspecific pain, and diarrhea[55].
Troglitazone (TGZ) is a member of the TZD class of drugs and acts as a PPARγ agonist. The antiproliferative and antitumorigenic effects of TGZ were studied in the BEL-7402 HCC cell line at 5, 10, and 25 µmol/L concentrations. TGZ induced cell death in a concentration-dependent manner resulting in the increased presence of fragmented DNA and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells. TGZ enhanced cell cycle arrest in the G0/G1 phase and increased caspase activities (caspase 3, 6, 7, and 9), indicating increased cell apoptosis[56]. In another study, the HepG2 cell line treated with TGZ showed significant growth inhibition in a dose-dependent manner. The TUNEL assay and immunohistochemistry showed apoptosis induction and elevated expression of apoptotic proteins such as caspase 3 and survivin[57]. PPARγ was functionally expressed in hepatic cancer cell lines (HepG2, HuH-7, KYN-1, and KYN-2) with TGZ treatment. This was followed by the profound inhibition of cellular proliferation, DNA synthesis, cell cycle growth, and α-fetoprotein levels[58]. Similar results have also been shown by other groups that used other HCC cell lines such as PLC/PRF/5, HuH-7[59], HLF, HAK-1A, HAK-1B, and HAK-5 with TGZ[10,19]. The reduction in cell proliferation and increased apoptosis in most of these studies demonstrated the usefulness of TGZ for chemoprevention in HCC. Some recent studies showed the hepatotoxic effect of TGZ on diabetic patients. There is a significant elevation in liver enzymes level (ALT and aspartate aminotransferase [AST]) in 1.9% of patients with diabetes treated with TGZ for 24 to 48 wk. Furthermore, the cost of TGZ is much higher than that of other oral antihyperglycemic agents or insulin, which also limits the use of TGZ[60].
Saroglitazar is a first-class drug that acts as a dual PPARα/γ agonist. It is indicated for enhanced diabetic dyslipidemia, inflammation, steatosis, ballooning, and fibrosis progression. The agonistic effects of this drug have a favorable impact on insulin resistance and lipid profile. Saroglitazar treatment is thought to ameliorate high-fat diet-induced aberrations. The improvements were observed in hepatic lobular inflammation, hepatocellular ballooning, steatosis, and fibrosis. The effects of saroglitazar were more pronounced compared to PGZ. Transcriptomic analyses revealed the elevated expression of PPARγ in hepatic tissue with the anti-inflammatory effects of saroglitazar treatment[61]. Similarly, saroglitazar improved liver function parameters, degenerative changes, glucose and insulin levels, and lipid profile in high-fat emulsion plus lipopolysaccharide (LPS)-treated rats. The positive effects on serum leptin, TNFα, and adiponectin levels were also observed. The multiple protective roles of PPARα/γ agonists in liver disorders suggest the usefulness of saroglitazar in managing liver cancer[62].
In a prospective observational study, 30 diabetic patients with liver fibrosis were enrolled and treated with 4 mg saroglitazar daily for 6 mo. A profound improvement in glycemic index, liver stiffness, and serum triglyceride levels of the patients was observed with no significant adverse side effects[63]. Another study conducted in 90 NAFLD patients who underwent liver biopsies, fibrosis scores, and other non-invasive parameters showed that saroglitazar treatment significantly improved the serum biomarker levels and fibrosis score. The study concluded the reversal effect of saroglitazar on fibrosis and advocated its use in treating HCC[64]. The most common adverse events associated with saroglitazar included asthenia, gastritis, chest discomfort, peripheral edema, dizziness, and tremors[65].
Natural PPARγ agonists have many beneficial properties including antioxidant, anti-inflammatory, antifibrotic, and antitumor effects. In addition to therapeutic effects, synthetic drugs have many adverse effects due to full PPARγ activation. Therefore, researchers are exploring potential natural PPARγ modulators with high specificity in terms of their binding at the active site and improving drug safety. The PPARγ-activating effect of natural products is recognized as having great potential in developing anticancer therapy. There are many reports on the natural PPARγ agonist against HCC in various experimental models.
The hemp plant Cannabis sativa L. produces approximately 60 unique compounds known as cannabinoids, of which Δ9-tetrahydrocannabinol (THC) is the most important due to its high potency and abundance in cannabis. Various studies have reported the fair safety profile of cannabinoids, in accordance with its probable antiproliferative activity on cancerous cells, may set the basis for future trials to evaluate the potential antitumor activity of cannabinoids. Vara et al[66] reported that cannabinoids THC and JWH-015 increased the intracellular mRNA and protein levels of PPARγ in HCC cells, and inhibition of PPARγ decreased cannabinoid-induced cell death and apoptosis. Further, increased PPARγ levels were correlated with endoplasmic reticulum stress and autophagy in HCC cells, suggesting the antiproliferative effects of cannabinoids through PPARγ-dependent pathways. The antitumor activity of THC was evaluated in patients who had failed standard therapy norms. In vitro studies have shown the suppression of tumor cell proliferation, and Ki67 immunostaining exhibits a reduced number of tumor cells[67]. THC is suggested to induce transcriptional modulation of the PPARγ pathway, and the activation is much more potent by cannabinoid acids than its decarboxylated products, indicating that cannabinoids act as a PPARγ agonist[68]. Cannabis contains some psychoactive agents that increase sociability and exert euphoric effects. Repeated use of cannabis has been linked to short- and long-term side effects, including respiratory and cardiovascular disorders, cognitive alterations, psychosis, schizophrenia, and mood disorders[69]. A recent study highlighted the side effects of a common preparation from C. sativa named marijuana. This study gave the putative association of the use of cannabis with a higher risk of gingival and periodontal diseases, oral infection, and cancer of the oral cavity[70]. Given the growing popularity of cannabinoid-based drugs for recreational and medical purposes and their potentially harmful effects, there is a need for further investigation in this field.
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a vital constituent of chili peppers belonging to the family of Capsicum. These phytoconstituents possess anti-inflammatory and chemopreventive properties. They counter various compounds’ mutagenic properties and exert anticancer effects on breast, colon, prostate, and hepatic cancers. The DENA-induced models of HCC in rats and hepatic stellate cell lines were used to study the effects of capsaicin. Capsaicin was observed to inhibit hepatic injury, NF-κB activation, and collagen deposition. It has also ameliorated the levels of α-SMA, collagen type I, MMP2, TGFβ1, and TNFα. Furthermore, TGFβ1 expression and the phosphorylation of Smad2/3 were also inhibited through induction of PPARγ expression. The findings showed that capsaicin attenuates hepatic fibrosis by upregulating PPARγ expression[71]. The limitations of this natural PPARγ agonist should be mentioned. Capsaicin is a well-known irritant responsible for producing a painful, burning sensation when applied to the skin. Exposure to the eyes is painful and causes tearing, conjunctivitis, and blepharospasm[72]. Capsaicin is also a tussive agent, and inhaled capsaicin can be used to induce cough under experimental conditions. In humans, inhaled capsaicin induces a cough response immediately upon administration[73,74]. Interestingly, there is evidence that topical capsaicin can exacerbate angiotensin-converting enzyme (ACE) inhibitor-induced cough. A patient taking an ACE inhibitor for several years with no complaint of coughing reported coughing associated with applying a 0.075% capsaicin cream[75]. Additionally, oral administration of the ACE inhibitor captopril was found to cause a shift in the dose-response curve of inhaled capsaicin-induced cough in a trial with healthy adults[76].
Curcumin is a polyphenol compound present in Curcuma longa and is well known for its multiple therapeutic effects. Our previous study reported the effect of curcumin and piperine on DENA-induced HCC in rats. Curcumin prevented HCC progression by improving hepatic pathology, apoptosis induction, and inhibiting cell proliferation. However, the synergistic effect on HCC suppression was observed with the combination of curcumin and piperine[77]. Similarly, another study also reported the inhibition of cell proliferation, tumor growth, and apoptosis induction by curcumin treatment in HCC. The effect was suggested to decrease VEGF expression and PI3K/Akt signaling[78]. A study in a transgenic mouse model (expressing double HBV oncoproteins, HBx and pre-S2 in the liver) of HBV-related HCC reported the protective effects of phytosomal curcumin via targeting PPARγ as a key regulator. Curcumin decreased HCC formation and reduced the tumor size. Moreover, considerably more potent effects were observed on activation of PPARγ and inhibition of NF-κB. The report suggested that curcumin is an agonist for PPARγ, upregulating the genes involved in lipid metabolism, antiproliferation, and anti-inflammation. Furthermore, PPARγ activation regulates the suppression of NF-κB and subsequent pro-inflammatory cytokines. In addition, curcumin also is suggested to repress mTOR[79]. Recently, the antitumor effect of curcumin on HCC was suggested due to the involvement of miR-21 targeting TIMP3 and inhibition of the TGFβ1/Smad3 signaling pathway. The inhibition of TGFβ1/Smad3 signaling by curcumin is reportedly linked to activation of the PPARγ gene[80,81]. It was further suggested to suppress cell proliferation through long non-coding RNA downregulation and inhibition of Wnt/β-catenin signaling[82]. The major disadvantage of this medication is the usage of high doses, which ultimately leads to liver injury in humans and experimental animals. A study showed that curcumin supplementation with paracetamol at doses of 50 and 100 mg/kg per day in experimental rabbits showed elevation of liver injury markers (ALT, AST, ALP, total protein, and albumin level) in plasma. Furthermore, levels of red blood cells and platelets were raised[83]. Also, the poor bioavailability of curcumin leads to its combined usage with other drugs such as piperine, which reportedly causes adverse drug reactions[84].
Hesperidin is a flavanone glycoside found in the rind of citrus fruits including oranges and lemon. It possesses several pharmacological activities including antioxidant, anti-inflammatory, and anticancer effects. The chemopreventive efficacy of hesperidin was evaluated in DENA-induced HCC in rats. The hesperidin significantly reduced hepatic serological and tumor biomarkers along with TNFα. Furthermore, it also reduced the hepatic degenerative changes, oxidative stress, collagen deposition, TGFβ1, and NF-κB expression. However, the upregulated expression of nuclear factor erythroid 2–related factor 2, HO-1, and PPARγ suggested the effect of hesperidin via suppressing TGFβ signaling and subsequently activating PPARγ[85]. Another study investigated the efficacy of hesperidin via the PI3K/Akt pathway as a probable mechanism for curing HCC. Treatment with hesperidin elevated the protein levels of PI3K, Akt, and cyclin-dependent kinase 2 and ameliorated HCC progression[86]. In addition, hesperidin reportedly alters Wnt3a/β-catenin signaling in preventing HCC[87]. There are few reports on the bioavailability and solubility of hesperidin. Ameer et al[88] reported that hesperidin is absorbed across the gastrointestinal tract on oral administration, but cumulative recovery indicates low bioavailability. The factors limiting the bioavailability of hesperidin are poor water solubility and its precipitation in an acidic environment.
Hispidulin, a phenolic flavonoid, exhibits anticancer activity against several types of cancers. The effect of hispidulin on HCC was studied in tumor cell lines (SMMC7721 and Bel7402) and mouse tumor xenograft models. Hispidulin activates caspase 3, triggers apoptosis, and inhibits cell migration via PPARγ activation, which is further linked to escalated phosphorylation of AMPK, ERK, and JNK
Isoflavones are a group of phytochemicals, a type of naturally occurring isoflavonoids. Studies have shown the anticancer effects of different isoflavones in the case of HCC[93]. A combination of two well-known isoflavones, Biochanin A and SB590885, was evaluated for their anticancer activities in HCC. The combination showed synergistic inhibition of cell growth and induced cell cycle arrest and apoptosis in vitro. The inhibition of cellular proliferation and tumor suppression were attributed to the aberration of ERK MAPK and PI3K/Akt pathways. In vivo, a profound reduction in the size and volume of HCC tumors was noted, indicating the combination therapy of isoflavones as a potential lead for the management and treatment of advanced HCC[94]. The antitumorigenic and antiproliferative role of genistein was also studied in HCC in vitro. The isoflavone suppressed the proliferation of Hepa 1-6 cells and caused apoptosis in time- and dose-dependent manners[95]. In another study, genistein treatment suppressed aerobic glycolysis and increased the apoptotic rate in HCC cell lines. Additionally, genistein exhibited inhibitory effects on tumor progression and aerobic glycolysis. This may be identified as an effective treatment for advanced HCC[96]. Studies have reported the PPARγ-modulating effect of isoflavones and inhibition of HCC through inhibition of the PI3K/Akt pathway, and aerobic glycolysis further validates the involvement of PPARγ signaling. Clinical studies have also suggested that the more the dietary intake of flavonoids, the lesser the risk of developing HCC. In the Japanese population, a correlation between the isoflavone-rich diet and risk of HCC was observed[97,98]. Despite the therapeutic potential, some contentious health issues are associated with their intake. Soy proteins rich in isoflavones showed unfavorable effects at a higher dose, including gastrointestinal upset, constipation, nausea, allergic reactions, and loss of appetite. In animals, the intake of isoflavone (genistein) reportedly impacts the fertility and morphogenesis of ovaries. In addition, long-term use of soy extract may result in abnormal tissue growth in the uterus[99].
Oroxyloside (OAG), a flavonoid, was explored as a new dual agonist of PPARγ/α, which acts as a potent cell proliferation inhibitor in HCC-based metabolic transition. It regulates the glycolipid metabolic enzymes (PPAR-dependent or PPAR-independent), inhibits the breakdown of glucose, and promotes fatty acid oxidation, which generates acetyl-CoA for the tricarboxylic acid cycle and oxidative phosphorylation. The metabolic transition produced by OAG exhibits a profound generation of reactive oxygen species, leading to G1 cell cycle arrest and growth repression of HCC cells. OAG requires pyruvate dehydrogenase kinase 4 and β-oxidation to inhibit cell proliferation, explaining its PPARγ agonistic behavior. OAG is a new PPARγ/α agonist drug candidate and an effective therapeutic approach for HCC based on metabolic reprogramming[100]. Although many bioactive flavones' sources are very well known, information on their bioavailability and their active forms in vivo is limited. In particular, most flavonoid agents' absorption, metabolism, and blood delivery are poorly understood. Due to limited literature, it is difficult to elucidate the whole molecular mechanism. Hence, further studies are required to uncover their therapeutic potential against liver diseases.
Resveratrol (RS) is a popular natural polyphenolic PPARγ agonist, well known for its anticancer properties, and has been recognized as the alternate mode in cancer treatment. A study revealed the effect of RS against alcohol-aflatoxin B1-induced HCC. During the progression of HCC, a decline in the antioxidant markers was effectively restored by resveratrol treatment. RS modulated the activity of the sirtuin 1 (SIRT1) enzyme in HCC by negatively regulating the levels of NF-κB, and cross-talk between this PPARγ agonist and SIRT1 signaling was observed[101]. A nano-formulation of RS using liposomes was developed to establish a specific drug delivery system for managing HCC. In vitro studies have revealed the increased internalization and enhanced anticancer activity of liposomal formulation (RL5) compared to naïve RS. A profound reduction in liver injury markers, hepatocyte nodules, and degenerative changes in the liver was observed in an in vivo HCC model. The results indicated the promising action of nano-formulation of RS and its substantial activity in controlling the severity of HCC[102]. Earlier, similar approaches were briefly reviewed by Santos et al[103] to study the pharmacokinetics of RS-loaded nanoparticles (RS-NPs) and study their effects on cancer tissue. A comprehensive analysis was carried out in various in vivo models, which revealed the markedly enhanced anticancer activity of RS-NPs. However, the poor bioavailability and rapid metabolism restricted the successful translation of resveratrol to clinical form. The in vivo efficacy of RS is affected due to its low solubility and low bioavailability. Oral intake of 25 mg of RS showed extremely low bioavailability; only a trace amount of unmetabolized RS was detected in plasma. The gastrointestinal tract absorbs approximately 70% of RS, but it is further metabolized by three distinct metabolic pathways leading to low bioavailability[104].
Avicularin (quercetin-3-α L arabinofuranoside), a glycoside related to quercetin, reportedly reduces obesity, inflammation, and drug resistance[105,106]. It also induces cytotoxicity in cancer cells by promoting intrinsic apoptosis pathways. One study investigated the activity of avicularin in HCC by employing HuH-7 cell lines. Avicularin inhibited cell proliferation in a dose-dependent manner and markedly decreased the cell migration and invasiveness of the cancer cells. Gene and protein expression studies revealed reduced levels of NF-κB, cyclooxygenase 2, and PPARγ. Avicularin may have the potential to modulate PPARγ to induce antineoplastic activity in HCC[107].
Honokiol (C18H18O2) is a bioactive, biphenolic phytoconstituent derived from the bark and leaves of Magnolia Officinalis. Honokiol exhibits various protective activities such as anticarcinogenic, anti-inflammatory, anti-angiogenic, antioxidative, and repressive potency towards the malignant conversion of papillomas to carcinomas without any noticeable toxicity effects. A group of researchers employed a great blend of in silico, in vitro, and in vivo techniques to pinpoint and validate honokiol as a potent lead for being a PPARγ agonist. The binding of honokiol into the ligand-binding pocket of PPARγ was anticipated via various in silico techniques. The luciferase reporter assay confirmed this binding and advocated that honokiol could act as a partial PPARγ agonist. Further, using 3T3-L1 and mouse embryonic cell lines, it was observed that honokiol stimulated basal glucose uptake but did not induce adipogenesis. However, the oral administration of honokiol resulted in reduced hyperglycemia and weight gain[108]. Various studies have suggested that honokiol acts as an RXR agonist forming RXR dimers and activating PPARγ/RXR heterodimers. Additionally, it also potentiates the activation of PPARγ/RXR heterodimers induced by rosiglitazone[109-111]. Also, no peer-reviewed papers proving the abuse, misuse, or dependence on or addiction to avicularin and honokiol have been retrieved yet.
Chrysin is a dihydroxyflavone belonging to the family of flavonoids. A study revealed that chrysin reduced cell viability and promoted apoptosis in all cell lines via inhibiting the Skp2 and low-density lipoprotein receptor-related protein 6 expression. However, reduced MMP2, MMP9, and fibronectin levels were observed[112]. Despite these interesting bioactivities, the clinical applications of chrysin have been constrained by its hydrophobicity, poor bioavailability, and degradation at alkaline pH[113]. Similarly, quercetin (QE) is a classic flavonoid and a yellow crystalline pigment present in plants, used as a food supplement to reduce allergic responses or boost immunity. It has been known to inhibit the development of various types of cancer hepatic conditions[114,115]. QE was suggested to effectively suppress HCC due to its close interaction with the signal transducer and activator of transcription 3 (STAT3) pathway[116,117]. It inhibits cell proliferation, cell cycle regulation, and invasiveness of the cancer cells by promoting the autophagy of HCC[118]. However, the bioavailability of QE is very low due to its poor aqueous solubility and instability, challenging its therapeutic application in the pharma sector[119].
Cancer tissues display metabolic and thermodynamic aberrations with dysregulated cellular growth. Although the role of PPARγ and its agonists in HCC and other cancers have been extensively studied, as discussed above, several conflicting reports exist concerning the PPARγ expression in cancers. It is unclear whether PPARγ induction promotes or suppresses tumor growth and viability. In the case of several cancers, PPARγ mainly exhibits the down-regulated expressions while activating several other pathways like the canonical Wnt/beta-catenin pathway, PI3K/Akt pathway, STAT3 pathway, etc[82,87,118]. The activation of Wnt/β-catenin signaling leads to the upregulated PDK1, which leads to aerobic glycolysis and mitochondrial stress[29]. A recent report by Galbraith et al[120] revealed that the activation of PPARγ, in turn, induced Akt serine/threonine kinase 3 (AKT3), which eventually led to the more aggressive form of cancer. AKT3 enhances PGC1α localization to the nuclear space by repressing chromosome maintenance region 1, while the latter served as the downstream target for PGC1α. All these led to mitochondrial biogenesis, which fueled the progression of the tumor. Previous studies have also reported such inconsistent findings for PPARγ in HCC. Koga et al[10] tested five patients with cirrhotic livers and found no significant change in the PPARγ expressions compared to the surrounding non-cancerous tissue. Another study reported the consistently overexpressed PPARγ in HCC tissue having null expression in the surrounding tissues, even though all the patients were infected with viral hepatitis (B or C)[121]. Although the well-known inhibitory effects of PPARγ agonists are reported, they are also suggested to have PPARγ-independent effects on cancers. Troglitazone, as discussed above, has a prominent antitumorigenic role in HCC. However, there are reports of it exhibiting PPARγ-independent activity. Palakurthi et al[122] studied troglitazone and ciglitazone on both PPARγ-/- and PPARγ+/+ mouse embryonic stem cells considering various concentrations. Both the agonists could inhibit cellular proliferation in a dose-dependent manner by suppressing the G1-S transition. This evidence demonstrated that the antiproliferative effect was induced by suppressing the translation initiation. More similar reports back up the PPARγ-independent antitumorigenic property of PPARγ agonists[123,124]. One of the studies focused on the HCC progression in HBV-transgenic mice demonstrated that the anticancerous, antiproliferative, and apoptotic effects of TZD were more significant in PPARγ-deficient mice in comparison with the control mice, exhibiting normal PPARγ levels[125]. It is well-understood that PPARγ could potentially affect various pathways, so it is vital to understand the underlying mechanisms critically. This understanding is an absolute requirement as PPARγ may be inconsistent. However, it highlights its crucial role in tumor development, suggesting that targeted biomedical research against PPARγ could provide a highly efficacious avenue for treating and managing of HCC and various other cancers.
The majority of current studies support the fact that PPARγ may be a potential target against the progression of HCC. They have extensively explored the various signaling cascades through which PPARγ exerted inhibitory against HCC using synthetic and natural agonists in preclinical and clinical trials. PPARγ was suggested as a potential target as it suppresses cell proliferation, migration, and invasion in HCC cells through different signaling pathways. TZD, a class of synthetic PPARγ agonists, were extensively studied for their efficacy against HCC. TZD showed significant results against the progression of HCC; however, due to their adverse effect on different organs, these drugs are not approved for any cancer treatment. Therefore, increased focus was employed to identify natural and endogenous PPARγ agonists having high bioavailability and specificity in terms of their binding at the active site. Several studies reported the safety profiles and therapeutic role of natural agonists against HCC in various experiment modals. Natural agonists are also effectively reported to mediate apoptosis and inhibit cell proliferation, tumor growth, and metastasis in HCC. Few reports also highlighted the contradictory role of PPARγ in HCC. These contradictions might be due to some unidentified link between PPARγ and cancer. With the well-established role of PPARγ in the progression of HCC, better efficacy of its agonists may be achieved by a complete understanding of underlying mechanisms through which PPARγ showed therapeutic effects. Future studies should be focused on developing novel PPARγ targeting therapy for the treatment of HCC.
The authors are thankful to the Director, CSIR-IHBT, Palampur, India, for his continuous support. The CSIR-IHBT communication number is 5016.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cao X, China; Cao ZF, China; Jeong KY, South Korea S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 2. | Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci. 2019;64:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 3. | Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol. 2019;25:2591-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (3)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 5. | Fan G, Wei X, Xu X. Is the era of sorafenib over? Ther Adv Med Oncol. 2020;12:1758835920927602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Hsu HT, Chi CW. Emerging role of the peroxisome proliferator-activated receptor-gamma in hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:127-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 8. | Wu CW, Farrell GC, Yu J. Functional role of peroxisome-proliferator-activated receptor γ in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1665-1669. [PubMed] [DOI] [Full Text] |
| 9. | Tan Y, Wang M, Yang K, Chi T, Liao Z, Wei P. PPAR-α Modulators as Current and Potential Cancer Treatments. Front Oncol. 2021;11:599995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, Kawaguchi T, Sasatomi K, Kimura R, Hashimoto O, Ueno T, Yano H, Kojiro M, Sata M. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Lee WS, Kim J. Peroxisome Proliferator-Activated Receptors and the Heart: Lessons from the Past and Future Directions. PPAR Res. 2015;2015:271983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem. 2019;166:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 13. | Aouali N, Broukou A, Bosseler M, Keunen O, Schlesser V, Janji B, Palissot V, Stordeur P, Berchem G. Epigenetic Activity of Peroxisome Proliferator-Activated Receptor Gamma Agonists Increases the Anticancer Effect of Histone Deacetylase Inhibitors on Multiple Myeloma Cells. PLoS One. 2015;10:e0130339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 15. | Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 713] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 811] [Cited by in RCA: 880] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 17. | Guan Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J Am Soc Nephrol. 2004;15:2801-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Wu L, Guo C, Wu J. Therapeutic potential of PPARγ natural agonists in liver diseases. J Cell Mol Med. 2020;24:2736-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Koga H, Harada M, Ohtsubo M, Shishido S, Kumemura H, Hanada S, Taniguchi E, Yamashita K, Kumashiro R, Ueno T, Sata M. Troglitazone induces p27Kip1-associated cell-cycle arrest through down-regulating Skp2 in human hepatoma cells. Hepatology. 2003;37:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Cheung KF, Zhao J, Hao Y, Li X, Lowe AW, Cheng AS, Sung JJ, Yu J. CITED2 is a novel direct effector of peroxisome proliferator-activated receptor γ in suppressing hepatocellular carcinoma cell growth. Cancer. 2013;119:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Yu J, Shen B, Chu ES, Teoh N, Cheung KF, Wu CW, Wang S, Lam CN, Feng H, Zhao J, Cheng AS, To KF, Chan HL, Sung JJ. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology. 2010;51:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Yousefnia S, Momenzadeh S, Seyed Forootan F, Ghaedi K, Nasr Esfahani MH. The influence of peroxisome proliferator-activated receptor γ (PPARγ) ligands on cancer cell tumorigenicity. Gene. 2018;649:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Bo QF, Sun XM, Liu J, Sui XM, Li GX. Antitumor action of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone in hepatocellular carcinoma. Oncol Lett. 2015;10:1979-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Xiang S, Liu Q, Gu T, Yao Y, Lu X. PPARγ Antagonizes Hypoxia-Induced Activation of Hepatic Stellate Cell through Cross Mediating PI3K/AKT and cGMP/PKG Signaling. PPAR Res. 2018;2018:6970407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Shen B, Chu ES, Zhao G, Man K, Wu CW, Cheng JT, Li G, Nie Y, Lo CM, Teoh N, Farrell GC, Sung JJ, Yu J. PPAR gamma inhibits hepatocellular carcinoma metastases in vitro and in mice. Br J Cancer. 2012;106:1486-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Mahmood DFD, Jguirim-Souissi I, Khadija EH, Blondeau N, Diderot V, Amrani S, Slimane MN, Syrovets T, Simmet T, Rouis M. Peroxisome proliferator-activated receptor gamma induces apoptosis and inhibits autophagy of human monocyte-derived macrophages via induction of cathepsin L: potential role in atherosclerosis. J Biol Chem. 2011;286:28858-28866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Sun M, Tan L, Hu M. The role of autophagy in hepatic fibrosis. Am J Transl Res. 2021;13:5747-5757. [PubMed] |
| 28. | Mastropasqua F, Girolimetti G, Shoshan M. PGC1α: Friend or Foe in Cancer? Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Cheng Z, Tao X, Yu C, Li B, Yang C, Wang S, Lv Y, Zhao F, Yao M, Cong W, Wang C, Qin W. PPARγ Coactivator-1α Suppresses Metastasis of Hepatocellular Carcinoma by Inhibiting Warburg Effect by PPARγ-Dependent WNT/β-Catenin/Pyruvate Dehydrogenase Kinase Isozyme 1 Axis. Hepatology. 2021;73:644-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 30. | Kim H, Lee JY, Park SJ, Kwag E, Koo O, Shin JH. ZNF746/PARIS promotes the occurrence of hepatocellular carcinoma. Biochem Biophys Res Commun. 2021;563:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Bost F, Kaminski L. The metabolic modulator PGC-1α in cancer. Am J Cancer Res. 2019;9:198-211. [PubMed] |
| 32. | Gerhold DL, Liu F, Jiang G, Li Z, Xu J, Lu M, Sachs JR, Bagchi A, Fridman A, Holder DJ, Doebber TW, Berger J, Elbrecht A, Moller DE, Zhang BB. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrinology. 2002;143:2106-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, Röcken C, Malfertheiner P, Farrell GC. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Tu K, Zheng X, Dou C, Li C, Yang W, Yao Y, Liu Q. MicroRNA-130b promotes cell aggressiveness by inhibiting peroxisome proliferator-activated receptor gamma in human hepatocellular carcinoma. Int J Mol Sci. 2014;15:20486-20499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L, Xu Q, Yang W, Liu Q, Tu K. MicroRNA-1468 promotes tumor progression by activating PPAR-γ-mediated AKT signaling in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Hyun S, Kim MS, Song YS, Bak Y, Ham SY, Lee DH, Hong J, Yoon DY. Peroxisome proliferator-activated receptor-gamma agonist 4-O-methylhonokiol induces apoptosis by triggering the intrinsic apoptosis pathway and inhibiting the PI3K/Akt survival pathway in SiHa human cervical cancer cells. J Microbiol Biotechnol. 2015;25:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Walter I, Schulz U, Vogelhuber M, Wiedmann K, Endlicher E, Klebl F, Andreesen R, Herr W, Ghibelli L, Hackl C, Wiest R, Reichle A. Communicative reprogramming non-curative hepatocellular carcinoma with low-dose metronomic chemotherapy, COX-2 inhibitor and PPAR-gamma agonist: a phase II trial. Med Oncol. 2017;34:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Yip TC, Wong VW, Chan HL, Tse YK, Hui VW, Liang LY, Lee HW, Lui GC, Kong AP, Wong GL. Thiazolidinediones reduce the risk of hepatocellular carcinoma and hepatic events in diabetic patients with chronic hepatitis B. J Viral Hepat. 2020;27:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Lin HC, Hsu YT, Kachingwe BH, Hsu CY, Uang YS, Wang LH. Dose effect of thiazolidinedione on cancer risk in type 2 diabetes mellitus patients: a six-year population-based cohort study. J Clin Pharm Ther. 2014;39:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Huang MY, Chung CH, Chang WK, Lin CS, Chen KW, Hsieh TY, Chien WC, Lin HH. The role of thiazolidinediones in hepatocellular carcinoma risk reduction: a population-based cohort study in Taiwan. Am J Cancer Res. 2017;7:1606-1616. [PubMed] |
| 42. | Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Chan KA. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Yan H, Wu W, Chang X, Xia M, Ma S, Wang L, Gao J. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biol Sex Differ. 2021;12:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ, Lanuti M, Hoshida Y, Baumert TF, Tanabe KK, Fuchs BC. Pioglitazone Reduces Hepatocellular Carcinoma Development in Two Rodent Models of Cirrhosis. J Gastrointest Surg. 2019;23:101-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Yang Y, Zhao LH, Huang B, Wang RY, Yuan SX, Tao QF, Xu Y, Sun HY, Lin C, Zhou WP. Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol Carcinog. 2015;54:1584-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Sumie S, Kawaguchi T, Kawaguchi A, Kuromatsu R, Nakano M, Satani M, Yamada S, Okamura S, Yonezawa Y, Kakuma T, Torimura T, Sata M. Effect of pioglitazone on outcome following curative treatment for hepatocellular carcinoma in patients with hepatitis C virus infection: A prospective study. Mol Clin Oncol. 2015;3:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Chongmelaxme B, Phisalprapa P, Sawangjit R, Dilokthornsakul P, Chaiyakunapruk N. Weight Reduction and Pioglitazone are Cost-Effective for the Treatment of Non-Alcoholic Fatty Liver Disease in Thailand. Pharmacoeconomics. 2019;37:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf. 2010;9:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 49. | Li X, He J, Li B, Gao M, Zeng Y, Lian J, Shi C, Huang Y, He F. The PPARγ agonist rosiglitazone sensitizes the BH3 mimetic (-)-gossypol to induce apoptosis in cancer cells with high level of Bcl-2. Mol Carcinog. 2018;57:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Cao LQ, Shao ZL, Liang HH, Zhang DW, Yang XW, Jiang XF, Xue P. Activation of peroxisome proliferator-activated receptor-γ (PPARγ) inhibits hepatoma cell growth via downregulation of SEPT2 expression. Cancer Lett. 2015;359:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T; LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 52. | Mitka M. Panel recommends easing restrictions on rosiglitazone despite concerns about cardiovascular safety. JAMA. 2013;310:246-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Oura K, Tadokoro T, Fujihara S, Morishita A, Chiyo T, Samukawa E, Yamana Y, Fujita K, Sakamoto T, Nomura T, Yoneyama H, Kobara H, Mori H, Iwama H, Okano K, Suzuki Y, Masaki T. Telmisartan inhibits hepatocellular carcinoma cell proliferation in vitro by inducing cell cycle arrest. Oncol Rep. 2017;38:2825-2835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Saber S, Khodir AE, Soliman WE, Salama MM, Abdo WS, Elsaeed B, Nader K, Abdelnasser A, Megahed N, Basuony M, Shawky A, Mahmoud M, Medhat R, Eldin AS. Telmisartan attenuates N-nitrosodiethylamine-induced hepatocellular carcinoma in mice by modulating the NF-κB-TAK1-ERK1/2 axis in the context of PPARγ agonistic activity. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1591-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Unger T, Schupp M. Telmisartan: from lowering blood pressure to end-organ protection. Future Cardiol. 2005;1:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. Peroxisome proliferator-activated receptor gamma ligands inhibit cell growth and induce apoptosis in human liver cancer BEL-7402 cells. World J Gastroenterol. 2003;9:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Zhou YM, Wen YH, Kang XY, Qian HH, Yang JM, Yin ZF. Troglitazone, a peroxisome proliferator-activated receptor gamma ligand, induces growth inhibition and apoptosis of HepG2 human liver cancer cells. World J Gastroenterol. 2008;14:2168-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Rumi MA, Sato H, Ishihara S, Kawashima K, Hamamoto S, Kazumori H, Okuyama T, Fukuda R, Nagasue N, Kinoshita Y. Peroxisome proliferator-activated receptor gamma ligand-induced growth inhibition of human hepatocellular carcinoma. Br J Cancer. 2001;84:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Toyoda M, Takagi H, Horiguchi N, Kakizaki S, Sato K, Takayama H, Mori M. A ligand for peroxisome proliferator activated receptor gamma inhibits cell growth and induces apoptosis in human liver cancer cells. Gut. 2002;50:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Kores K, Konc J, Bren U. Mechanistic Insights into Side Effects of Troglitazone and Rosiglitazone Using a Novel Inverse Molecular Docking Protocol. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Kumar DP, Caffrey R, Marioneaux J, Santhekadur PK, Bhat M, Alonso C, Koduru SV, Philip B, Jain MR, Giri SR, Bedossa P, Sanyal AJ. The PPAR α/γ Agonist Saroglitazar Improves Insulin Resistance and Steatohepatitis in a Diet Induced Animal Model of Nonalcoholic Fatty Liver Disease. Sci Rep. 2020;10:9330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 62. | Hassan NF, Nada SA, Hassan A, El-Ansary MR, Al-Shorbagy MY, Abdelsalam RM. Saroglitazar Deactivates the Hepatic LPS/TLR4 Signaling Pathway and Ameliorates Adipocyte Dysfunction in Rats with High-Fat Emulsion/LPS Model-Induced Non-alcoholic Steatohepatitis. Inflammation. 2019;42:1056-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Mitra A. An Observational Study of Reduction in Glycemic Parameters and Liver Stiffness by Saroglitazar 4 mg in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Cureus. 2020;12:e9065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Shafi SM, Yattoo GN. Spectrum, Clinico-pathological Profile of Non-Alcoholic Fatty Liver Disease and its Treatment Response with 48 wk Therapy of Saroglitazar. JMS SKIMS. 2021;24. |
| 65. | Sosale A, Saboo B, Sosale B. Saroglitazar for the treatment of hypertrig-lyceridemia in patients with type 2 diabetes: current evidence. Diabetes Metab Syndr Obes. 2015;8:189-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Vara D, Morell C, Rodríguez-Henche N, Diaz-Laviada I. Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 2013;4:e618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 67. | Guzmán M, Duarte MJ, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sánchez C, Velasco G, González-Feria L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 68. | Nadal X, Del Río C, Casano S, Palomares B, Ferreiro-Vera C, Navarrete C, Sánchez-Carnerero C, Cantarero I, Bellido ML, Meyer S, Morello G, Appendino G, Muñoz E. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br J Pharmacol. 2017;174:4263-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Cohen K, Weizman A, Weinstein A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin Pharmacol Ther. 2019;105:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 70. | Bellocchio L, Inchingolo AD, Inchingolo AM, Lorusso F, Malcangi G, Santacroce L, Scarano A, Bordea IR, Hazballa D, D'Oria MT, Isacco CG, Nucci L, Serpico R, Tartaglia GM, Giovanniello D, Contaldo M, Farronato M, Dipalma G, Inchingolo F. Cannabinoids Drugs and Oral Health-From Recreational Side-Effects to Medicinal Purposes: A Systematic Review. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Choi JH, Jin SW, Choi CY, Kim HG, Lee GH, Kim YA, Chung YC, Jeong HG. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J Agric Food Chem. 2017;65:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Clark R, Lee SH. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res. 2016;36:837-843. [PubMed] |
| 73. | Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol. 1984;81:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Midgren B, Hansson L, Karlsson JA, Simonsson BG, Persson CG. Capsaicin-induced cough in humans. Am Rev Respir Dis. 1992;146:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Hakas JF Jr. Topical capsaicin induces cough in patient receiving ACE inhibitor. Ann Allergy. 1990;65:322-323. [PubMed] |
| 76. | Morice AH, Brown MJ, Higenbottam T. Cough associated with angiotensin converting enzyme inhibition. J Cardiovasc Pharmacol. 1989;13 Suppl 3:S59-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Patial V, S M, Sharma S, Pratap K, Singh D, Padwad YS. Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats. Environ Toxicol Pharmacol. 2015;40:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett. 2018;15:4821-4826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Teng CF, Yu CH, Chang HY, Hsieh WC, Wu TH, Lin JH, Wu HC, Jeng LB, Su IJ. Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model. Sci Rep. 2019;9:10338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G113-G123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Li J, Wei H, Liu Y, Li Q, Guo H, Guo Y, Chang Z. Curcumin Inhibits Hepatocellular Carcinoma via Regulating miR-21/TIMP3 Axis. Evid Based Complement Alternat Med. 2020;2020:2892917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Shao J, Shi CJ, Li Y, Zhang FW, Pan FF, Fu WM, Zhang JF. LincROR Mediates the Suppressive Effects of Curcumin on Hepatocellular Carcinoma Through Inactivating Wnt/β-Catenin Signaling. Front Pharmacol. 2020;11:847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Sayed MM, El-Kordy EA. The protective effect of curcumin on paracetamol-induced liver damage in adult male rabbits: biochemical and histological studies. Egyptian J Histol. 2014;37:629-39. |
| 84. | Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3258] [Cited by in RCA: 3640] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 85. | Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact. 2017;277:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 86. | Mo'men YS, Hussein RM, Kandeil MA. Involvement of PI3K/Akt pathway in the protective effect of hesperidin against a chemically induced liver cancer in rats. J Biochem Mol Toxicol. 2019;33:e22305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Zaghloul RA, Elsherbiny NM, Kenawy HI, El-Karef A, Eissa LA, El-Shishtawy MM. Hepatoprotective effect of hesperidin in hepatocellular carcinoma: Involvement of Wnt signaling pathways. Life Sci. 2017;185:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Ther. 1996;60:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Han M, Gao H, Ju P, Gao MQ, Yuan YP, Chen XH, Liu KL, Han YT, Han ZW. Hispidulin inhibits hepatocellular carcinoma growth and metastasis through AMPK and ERK signaling mediated activation of PPARγ. Biomed Pharmacother. 2018;103:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 90. | Lv L, Zhang W, Li T, Jiang L, Lu X, Lin J. Hispidulin exhibits potent anticancer activity in vitro and in vivo through activating ER stress in nonsmallcell lung cancer cells. Oncol Rep. 2020;43:1995-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, Sigel E, Rausch WD, Riederer P, Schreier P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Chen LC, Hsu KC, Chiou LC, Tseng HJ, Huang WJ. Total Synthesis and Metabolic Stability of Hispidulin and Its d-Labelled Derivative. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Moselhy J, Srinivasan S, Ankem MK, Damodaran C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015;35:5773-5788. [PubMed] |
| 94. | Xiao Y, Gong Q, Wang W, Liu F, Kong Q, Pan F, Zhang X, Yu C, Hu S, Fan F, Li S, Liu Y. The combination of Biochanin A and SB590885 potentiates the inhibition of tumour progression in hepatocellular carcinoma. Cancer Cell Int. 2020;20:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Sanaei M, Kavoosi F, Valiani A, Ghobadifar MA. Effect of Genistein on Apoptosis and Proliferation of Hepatocellular Carcinoma Hepa1-6 Cell Line. Int J Prev Med. 2018;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, Lu X, Chen K, Xia Y, Lu J, Zhou Y, Fan X, Mo W, Xu L, Guo C. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 2017;117:1518-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 97. | Sharp GB, Lagarde F, Mizuno T, Sauvaget C, Fukuhara T, Allen N, Suzuki G, Tokuoka S. Relationship of hepatocellular carcinoma to soya food consumption: a cohort-based, case-control study in Japan. Int J Cancer. 2005;115:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Zamora-Ros R, Fedirko V, Trichopoulou A, González CA, Bamia C, Trepo E, Nöthlings U, Duarte-Salles T, Serafini M, Bredsdorff L, Overvad K, Tjønneland A, Halkjaer J, Fagherazzi G, Perquier F, Boutron-Ruault MC, Katzke V, Lukanova A, Floegel A, Boeing H, Lagiou P, Trichopoulos D, Saieva C, Agnoli C, Mattiello A, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Engeset D, Skeie G, Argüelles MV, Molina-Montes E, Dorronsoro M, Tormo MJ, Ardanaz E, Ericson U, Sonestedt E, Sund M, Landberg R, Khaw KT, Wareham NJ, Crowe FL, Riboli E, Jenab M. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2013;133:2429-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Yu J, Bi X, Yu B, Chen D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 100. | Zhou Y, Guo Y, Zhu Y, Sun Y, Li W, Li Z, Wei L. Dual PPARγ/ɑ agonist oroxyloside suppresses cell cycle progression by glycolipid metabolism switch-mediated increase of reactive oxygen species levels. Free Radic Biol Med. 2021;167:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Rawat D, Chhonker SK, Naik RA, Koiri RK. Modulation of antioxidant enzymes, SIRT1 and NF-κB by resveratrol and nicotinamide in alcohol-aflatoxin B1-induced hepatocellular carcinoma. J Biochem Mol Toxicol. 2021;35:e22625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Jagwani S, Jalalpure S, Dhamecha D, Jadhav K, Bohara R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater Sci Eng. 2020;6:4969-4984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 103. | Santos AC, Pereira I, Magalhães M, Pereira-Silva M, Caldas M, Ferreira L, Figueiras A, Ribeiro AJ, Veiga F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019;21:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH, Nasrallah GK, Pintus G. Potential Adverse Effects of Resveratrol: A Literature Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 427] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 105. | Fujimori K, Shibano M. Avicularin, a plant flavonoid, suppresses lipid accumulation through repression of C/EBPα-activated GLUT4-mediated glucose uptake in 3T3-L1 cells. J Agric Food Chem. 2013;61:5139-5147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Guo XF, Liu JP, Ma SQ, Zhang P, Sun WD. Avicularin reversed multidrug-resistance in human gastric cancer through enhancing Bax and BOK expressions. Biomed Pharmacother. 2018;103:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Wang Z, Li F, Quan Y, Shen J. Avicularin ameliorates human hepatocellular carcinoma via the regulation of NFκB/COX2/PPARγ activities. Mol Med Rep. 2019;19:5417-5423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM, Heiss EH. Honokiol: a non-adipogenic PPARγ agonist from nature. Biochim Biophys Acta. 2013;1830:4813-4819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Kotani H, Tanabe H, Mizukami H, Amagaya S, Inoue M. A naturally occurring rexinoid, honokiol, can serve as a regulator of various retinoid x receptor heterodimers. Biol Pharm Bull. 2012;35:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Pérez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta. 2012;1821:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 111. | Kotani H, Tanabe H, Mizukami H, Makishima M, Inoue M. Identification of a naturally occurring rexinoid, honokiol, that activates the retinoid X receptor. J Nat Prod. 2010;73:1332-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Huang C, Wei YX, Shen MC, Tu YH, Wang CC, Huang HC. Chrysin, Abundant in Morinda citrifolia Fruit Water-EtOAc Extracts, Combined with Apigenin Synergistically Induced Apoptosis and Inhibited Migration in Human Breast and Liver Cancer Cells. J Agric Food Chem. 2016;64:4235-4245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 113. | Zhang Y, Zhao J, Afzal O, Kazmi I, Al-Abbasi FA, Altamimi ASA, Yang Z. Neuroprotective role of chrysin-loaded poly(lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J Biochem Mol Toxicol. 2021;35:e22634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Dai W, Gao Q, Qiu J, Yuan J, Wu G, Shen G. Quercetin induces apoptosis and enhances 5-FU therapeutic efficacy in hepatocellular carcinoma. Tumour Biol. 2016;37:6307-6313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 115. | Srisa-Nga K, Mankhetkorn S, Okonogi S, Khonkarn R. Delivery of Superparamagnetic Polymeric Micelles Loaded With Quercetin to Hepatocellular Carcinoma Cells. J Pharm Sci. 2019;108:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 116. | Casella ML, Parody JP, Ceballos MP, Quiroga AD, Ronco MT, Francés DE, Monti JA, Pisani GB, Carnovale CE, Carrillo MC, de Luján Alvarez M. Quercetin prevents liver carcinogenesis by inducing cell cycle arrest, decreasing cell proliferation and enhancing apoptosis. Mol Nutr Food Res. 2014;58:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Yu D, Ye T, Xiang Y, Shi Z, Zhang J, Lou B, Zhang F, Chen B, Zhou M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. Onco Targets Ther. 2017;10:4719-4729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 118. | Wu L, Li J, Liu T, Li S, Feng J, Yu Q, Zhang J, Chen J, Zhou Y, Ji J, Chen K, Mao Y, Wang F, Dai W, Fan X, Wu J, Guo C. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019;8:4806-4820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 119. | Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 120. | Galbraith LCA, Mui E, Nixon C, Hedley A, Strachan D, MacKay G, Sumpton D, Sansom OJ, Leung HY, Ahmad I. PPAR-gamma induced AKT3 expression increases levels of mitochondrial biogenesis driving prostate cancer. Oncogene. 2021;40:2355-2366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 121. | Schaefer KL, Wada K, Takahashi H, Matsuhashi N, Ohnishi S, Wolfe MM, Turner JR, Nakajima A, Borkan SC, Saubermann LJ. Peroxisome proliferator-activated receptor gamma inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res. 2005;65:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 122. | Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor gamma and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213-6218. [PubMed] |
| 123. | Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845-5853. [PubMed] |
| 124. | Gardner OS, Shiau CW, Chen CS, Graves LM. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J Biol Chem. 2005;280:10109-10118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Galli A, Ceni E, Mello T, Polvani S, Tarocchi M, Buccoliero F, Lisi F, Cioni L, Ottanelli B, Foresta V, Mastrobuoni G, Moneti G, Pieraccini G, Surrenti C, Milani S. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor gamma-independent regulation of nucleophosmin. Hepatology. 2010;52:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |