Published online Jul 14, 2022. doi: 10.3748/wjg.v28.i26.3232
Peer-review started: January 22, 2022
First decision: March 8, 2022
Revised: March 17, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 14, 2022
Processing time: 172 Days and 2.9 Hours
Recently, hepatic arterial infusion chemotherapy (HAIC) plus lenvatinib has been frequently used to treat unresectable hepatocellular carcinoma (uHCC) in China. In the clinic, the hepatic arteries of some patients shrink significantly during this treatment, leading to improved short-term efficacy.
To investigate the relationship between the shrinkage of hepatic arteries and the short-term effect of HAIC plus lenvatinib treatment.
Sixty-seven participants with uHCC were enrolled in this retrospective study. The patients received HAIC every 3 wk, followed by oral lenvatinib after the first HAIC course. Hepatic artery diameters were measured on CT before treatment and after 1 and 2 mo of treatment. Meanwhile, the changes in tumor capillaries were also examined on pathological specimens before and after 1 mo of treatment. The antitumor response after 1, 3, and 6 mo of treatment was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). The rela
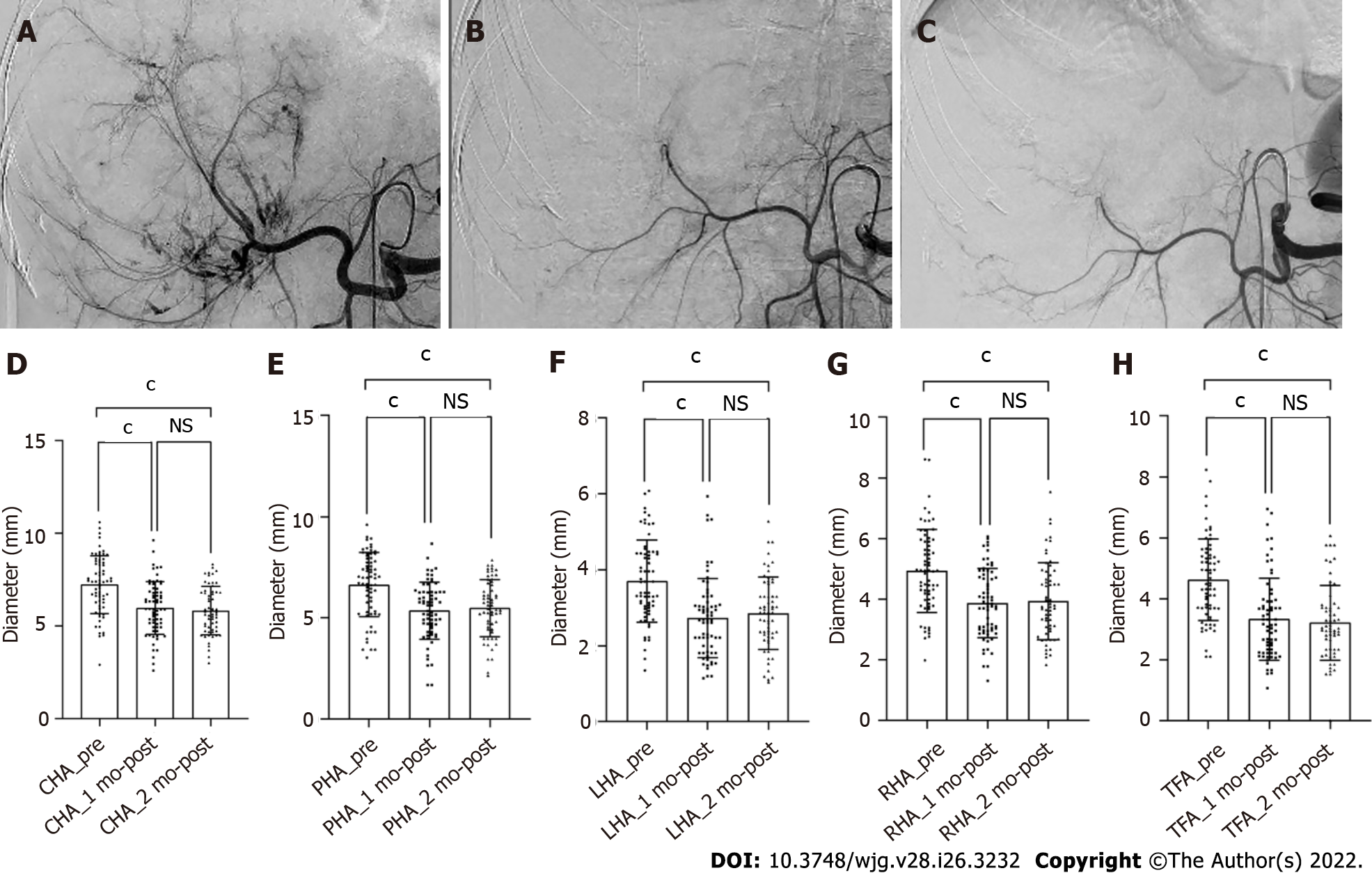
The hepatic artery diameters were all significantly decreased after 1 and 2 mo of treatment (P < 0.001), but there was no difference in the vessel diameters between 1 and 2 mo (P > 0.05). The microvessel density in the tumor lesions decreased significantly after 1 mo of combination treatment (P < 0.001). According to mRECIST, 46, 41, and 24 patients had complete or partial responses after 1, 3, and 6 mo of treatment, respectively, whereas 21, 21, and 32 patients had a stable or progressive disease at these times, respectively. Shrinkage of the tumor-feeding artery was significantly associated with the tumor response after 1, 3, and 6 mo of treatment (P < 0.001, P = 0.004, and P = 0.023, respectively); however, changes in other hepatic arteries were not significantly associated with the tumor response. Furthermore, shrinkage of the tumor-feeding artery was an independent factor for treatment efficacy (P = 0.001, P = 0.001, and P = 0.002 and 1, 3, and 6 mo, respectively).
The hepatic arteries shrank rapidly after treatment with HAIC plus lenvatinib, and shrinkage of the tumor-feeding artery diameter was closely related to improved short-term efficacy.
Core Tip: In this study, it was observed for the first time that the hepatic arteries shrank rapidly after hepatic arterial infusion chemotherapy plus lenvatinib therapy, and the close relationship between shrinkage of the tumor-feeding artery and improved short-term effect in patients with unresectable hepatocellular carcinoma was also confirmed. These findings would be of great significance to physicians for evaluating the effectiveness of this combination therapy earlier in the course of treatment and altering the treatment plan as needed.
- Citation: Wu DD, He XF, Tian C, Peng P, Chen CL, Liu XH, Pang HJ. Tumor-feeding artery diameter reduction is associated with improved short-term effect of hepatic arterial infusion chemotherapy plus lenvatinib treatment. World J Gastroenterol 2022; 28(26): 3232-3242
- URL: https://www.wjgnet.com/1007-9327/full/v28/i26/3232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i26.3232
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, with 850000 new cases reported per year worldwide[1,2]. In total, 70%-80% of patients with HCC in China are ineligible for surgical resection at the time of onset[3]. Recently, hepatic arterial infusion chemotherapy (HAIC) has been increasingly used to treat patients with unresectable HCC (uHCC) in Asian countries[4,5]. HAIC is recommended as a major treatment option in patients with intermediate and advanced HCC by current Japanese and Chinese guidelines[6].
Lenvatinib is an oral multi-target tyrosine kinase inhibitor (TKI) with antiangiogenic effects. Previous studies demonstrated the survival benefit of lenvatinib plus HAIC vs lenvatinib alone in patients with advanced HCC[7]. This combination therapy often requires 6-8 cycles, and early assessment of the antitumor response appears essential for both patients and physicians[8].
Recently, a clinical study demonstrated that the diameters of hepatic arteries are significantly reduced after TKI therapy[9]. Since the early imaging response is often associated with survival benefits, it should be clarified whether the changes in hepatic vessel diameters are related to the efficacy of HAIC combined with lenvatinib. As a result, we conducted a retrospective study of patients with uHCC treated with HAIC plus lenvatinib. The aim of this study was to: (1) Explore the changes in hepatic arteries after combination treatment; and (2) Determine the relationship between the hepatic artery changes and early therapeutic effects.
This was a single-center, retrospective analysis of data from 67 patients with uHCC treated at our institution from January 2019 to October 2020. Approval for the study was obtained from the local ethics committee on human research. The HCC diagnosis was based on the findings of pathological, imaging, and clinical assessments using the European Association for the Study of the Liver criteria[10]. Eligible patients were those aged 18 years or older with uHCC (including patients with large/multifocal HCC and/or with vascular invasion and extrahepatic spread) who received HAIC plus lenvatinib as an initial treatment. Patients who underwent transcatheter arterial chemoembolization or received other TKIs before combination therapy were excluded. Enrolled patients who discontinued treatment during the follow-up period were also excluded.
HAIC was repeated at an interval of 3 wk in enrolled patients in our hospital. A catheter was initially advanced into the celiac or superior mesenteric artery for digital subtraction angiography from the femoral artery; then, a microcatheter was left in the tumor-feeding artery (TFA) for subsequent treatment.
The patients received the following regimen for HAIC through the indwelling microcatheter connected to an artery infusion pump in the ward: oxaliplatin 85 mg/m2 administered intraarterially for 2 h, leucovorin 400 mg/m2 administered intraarterially for 1 h, and 5FU 400 mg/m2 intra-arterial bolus injection on day 1, followed by 5FU 2400 mg/m2 continuous arterial infusion for 46 h. Three days after the first HAIC course, patients began to take lenvatinib (Eisai, Tokyo, Japan) orally at the recommended doses of 12 mg/day for patients weighing ≥ 60 kg and 8 mg/day for patients weighing < 60 kg[11]. Dose adjustment or termination of lenvatinib therapy was allowed when drug-related adverse events (AEs) occurred. Discontinuation of the combination treatment was also allowed for potentially fatal AEs, clinical tumor progression, or the need for conversion therapy.
Therapeutic responses after 1, 3, and 6 mo of combination treatment were assessed according to the modified Response Evaluation Criteria in Solid Tumors using CT (Philips Brilliance iCT; Philips Medical Systems, Best, the Netherlands) images. Treatment responders were defined as patients with a complete response (CR) or partial response (PR). Non-responders were defined as patients with stable disease (SD) or progressive disease (PD). AEs were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0[12].
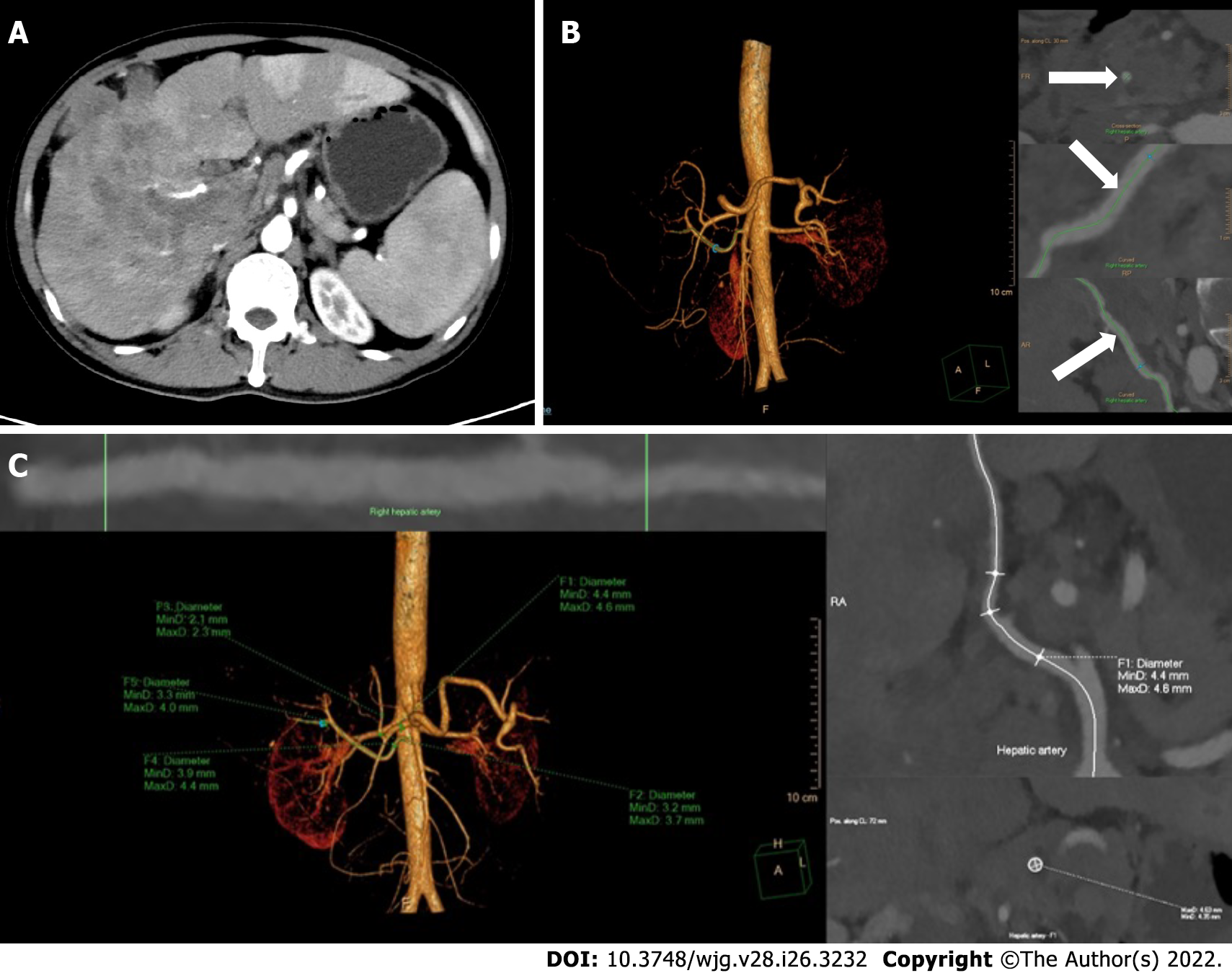
Epigastric enhanced CT Digital Imaging and Communication of Medicine (DICOM) data were collected before treatment and after 1 and 2 mo of treatment and introduced into an image post-processing workstation (Philips IntelliSpace Portal; Koninklijke Philips N.V., Eindhoven, the Netherlands) for three-dimensional reconstruction. On the image post-processing workstation, multiplanar reconstruction images centered on the contrast-enhanced hepatic arteries and the centerline (CLL) of the blood vessels were generated by the software to straighten the curved arteries. The trunk or the thickest branch of the TFA was selected as the object of measurement. The CLL was adjusted manually to measure the largest diameter of the arteries. The diameters of the opening of the common hepatic artery (CHA), proper hepatic artery (PHA), left hepatic artery (LHA), right hepatic artery (RHA), and TFA were further measured by manually drawing the region of interest (Figure 1)[13]. The change in vessel diameters was defined and calculated using the following formula: Δartery = (Dpost - Δpre)/Δpre (Δartery: The rate of change in the diameter of CHA, PHA, LHA, RHA, or TFA; Dpre: The vessel diameter before combination treatment; Dpost: The vessel diameter after combination treatment).
Both the measurement of artery diameters and the evaluation of therapeutic efficacy were individually conducted by two physicians with experience in gastroenterological diagnostics, and differences were resolved by a more experienced physician.
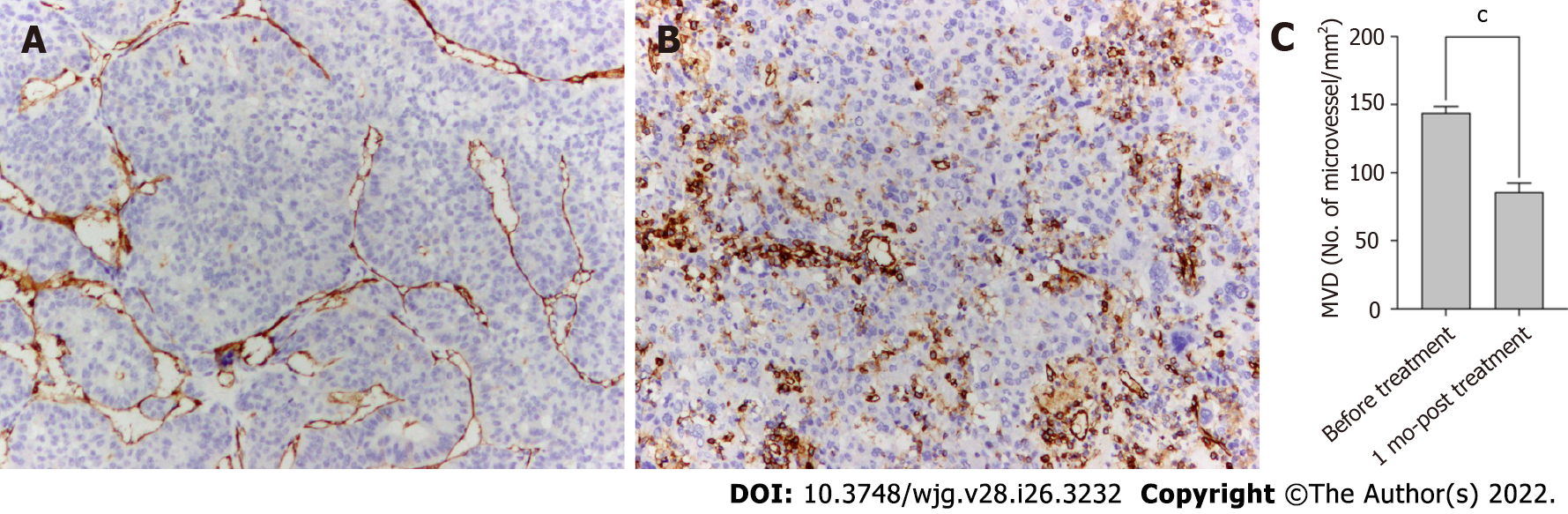
Tumor angiogenesis was evaluated by measuring microvessel density (MVD) after staining using an anti-CD34 antibody. Punch biopsy samples were obtained from tumor lesions before and 1 mo after the start of HAIC plus lenvatinib therapy. The tumor samples were fixed in 10% formalin and embedded in paraffin. After routine laboratory procedures, immunohistochemistry was performed to detect the expression of CD34 (endothelial cell marker) through the avidin–biotin–peroxidase complex[14,15]. MVD was measured at × 100 magnification in five regions of interest using a light microscope. All endothelial cells, including individual cells and grouped cells, were counted as a single vessel if they were separated from the surrounding tumor and other connective tissues[16].
Statistical analyses were performed using IBM SPSS Statistics 26.0. The measurement data were presented as the mean ± SD, and P < 0.05 indicated statistical significance. Considering the loss to follow-up, the changes in the artery diameters were compared using the mixed linear model for repeated measured data, and follow-up pairwise comparison was conducted among the three periods. The independent-samples t-test was used to analyze the changes in MVD. Receiver-operating characteristic (ROC) analysis was used to evaluate the changes in the diameter of all hepatic arteries to differentiate between responders and non-responders and identify the cutoff values. The cutoff was calculated for any factors that were statistically significant in the ROC analysis, which were candidates for univariate and multivariable logistic regression analyses with baseline data.
During the study period, patients meeting the inclusion criteria were followed up for 6 mo after the start of the combination treatment. The study population was predominantly male (59/67, 88.1%), and most of the enrolled patients were infected with hepatitis B virus (53/67, 79.1%). In total, 45 (67.2%) patients were estimated to have Child–Pugh stage A liver function, and 22 (32.8%) patients were categorized as Child–Pugh stage B. Portal vein tumor thrombosis was diagnosed in 44 (65.7%) patients, hepatic vein tumor thrombus in 17 (25.4%) patients, and extrahepatic spread occurred in 13 (19.4%) patients. The characteristics of the patients are summarized in Table 1.
| Characteristics | n (%) |
| Age | |
| ≤ 60 | 52 (77.6) |
| > 60 | 15 (22.4) |
| Gender | |
| Male | 59 (88.1) |
| Female | 8 (11.9) |
| ECOG PS | |
| 0 | 52 (77.6) |
| 1 | 15 (22.4) |
| HBsAg | |
| Positive | 53 (79.1) |
| Negative | 14 (20.9) |
| Child-Pugh Class | |
| Class A | 45 (67.2) |
| Class B | 22 (32.8) |
| Tumor size, cm | |
| ≤ 10 | 43 (64.2) |
| > 10 | 24 (35.8) |
| No. of intrahepatic tumors | |
| ≤ 3 | 32 (47.8) |
| > 3 | 35 (52.2) |
| Baseline AFP level, ng/mL | |
| ≤ 400 | 29 (43.3) |
| > 400 | 38 (56.7) |
| PVTT | |
| Absent | 23 (34.3) |
| Present | 44 (65.7) |
| HVTT | |
| Absent | 50 (74.6) |
| Present | 17 (25.4) |
| Metastasis | |
| Absent | 54 (80.6) |
| Present | 13 (19.4) |
There were no treatment-related deaths in our study. According to CTCAE, grade 1-2 events were observed in 37 patients (55.2%) and grade 3 events in 2 (3.0%) patients, whereas no grade ≥ 4 AEs occurred. The most common grade 1-2 events were abdominal pain (17/67, 25.4%), fever (8/67, 11.9%), and transient nausea and vomiting (8/67, 11.9%). The grade 3 events were liver dysfunction and decreased leukocyte counts, both of which normalized after treatment.
Five patients missed the 3-mo imaging evaluation, and 11 patients missed the 6-mo evaluation because of loss to follow-up. In total, 46, 41, and 24 patients were classified as responders (CR + PR) after 1, 3, and 6 mo of treatment, respectively, whereas the numbers of non-responders (SD + PD) at these time points were 21, 21, and 32, respectively. The overall response rates (ORR) in this study at 1, 3, and 6 mo were 68.7%, 66.1%, and 42.9%, respectively. Eight patients received downstaging therapy within 1 year after treatment, of which six patients underwent hepatectomy and the other two patients underwent radiofrequency ablation.
Vessel diameters were measured in all patients before treatment and after 1 mo of treatment, whereas six patients missed the 2-month measurement. After 1 and 2 mo of treatment, all vessel diameters had gradually decreased compared with the baseline (P < 0.001), but the difference in vessel diameters between 1 and 2 mo was not significant (P > 0.05, Figure 2). According to the ROC analysis, the change in the TFA diameter was significantly different between the responders and non-responders at 1, 3, and 6 mo (P < 0.001, P = 0.004, and P = 0.023, respectively), and the corresponding cutoff values were -0.169, -0.169, and -0.264, respectively. Meanwhile, there were no significant differences in the diameters of other hepatic arteries between the groups (Table 2). Considering the unclear clinical meaning of continuous changes in vessel diameters, the change in the TFA diameter was classified as reduced or unchanged based on the cutoff values.
| Δartery | 1 mo | 3 mo | 6 mo | ||||||
| Responder | Non- responder | P value | Responder | Non- responder | P value | Responder | Non- responder | P value | |
| CHA | - (0.17 ± 0.17) | - (0.14 ± 0.18) | 0.25 | - (0.15 ± 0.16) | - (0.18 ± 0.19) | 1.00 | - (0.18 ± 0.16) | - (0.15 ± 0.18) | 0.51 |
| PHA | - (0.21 ± 0.14) | - (0.13 ± 0.25) | 0.15 | - (0.20 ± 0.15) | - (0.16 ± 0.20) | 0.35 | - (0.16 ± 0.16) | - (0.22 ± 0.17) | 0.30 |
| LHA | - (0.27 ± 0.17) | - (0.21 ± 0.28) | 0.59 | (0.28 ± 0.18) | (0.20 ± 0.27) | 0.43 | - (0.22 ± 0.24) | - (0.30 ± 0.17) | 0.18 |
| RHA | - (0.22 ± 0.17) | - (0.16 ± 0.23) | 0.26 | - (0.20 ± 0.19) | - (0.22 ± 0.20) | 0.58 | - (0.16 ± 0.23) | - (0.24 ± 0.17) | 0.44 |
| TFA | - (0.33 ± 0.20) | - (0.16 ± 0.19) | < 0.001 | - (0.32 ± 0.19) | - (0.18 ± 0.22) | 0.004 | - (0.36 ± 0.16) | - (0.25± 0.23) | 0.023 |
The results of the univariate and multivariable logistic regression analyses of tumor response are listed in Table 3. According to the multivariable analysis, the change in the TFA diameter was an independent factor for the efficacy of HAIC plus lenvatinib after 1 [odds ratio (OR) = 0.12; 95% confidence interval (CI): 0.03-0.43; P = 0.001], 3 (OR = 0.06; 95%CI: 0.01-0.29; P = 0.001), and 6 mo (OR = 0.14; 95%CI: 0.04-0.47; P = 0.002) of treatment. Other independent factors included the alpha-fetoprotein level (OR = 0.17; 95%CI: 0.04-0.73; P = 0.018) at 1 mo and tumor size (OR = 7.65; 95%CI: 1.36-42.88; P = 0.021) at 3 mo.
| 1 mo | 3 mo | 6 mo | |||||||
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||
| P1 value | P2 value | OR (95%CI) | P1 value | P2 value | OR (95%CI) | P1 value | P2 value | OR (95%CI) | |
| TFA (reduced/basically unchanged) | < 0.001 | 0.001 | 0.12 (0.03-0.43) | < 0.001 | 0.001 | 0.06 (0.01-0.29) | 0.002 | 0.002 | 0.14 (0.04-0.47) |
| Sex (male/female) | 0.680 | - | - | 0.349 | - | - | 0.892 | - | - |
| Age (≤ 60/> 60) | 0.850 | - | - | 0.868 | - | - | 0.573 | - | - |
| ECOG (0/1) | 0.412 | - | - | 0.147 | - | - | 0.365 | - | - |
| HBsAg (positive/negative) | 0.122 | - | - | 0.113 | - | - | 0.784 | - | - |
| AFP, ng/mL (≤ 400/> 400) | 0.003 | 0.018 | 0.17 (0.04-0.73) | 0.089 | 0.546 | - | 0.040 | 0.122 | - |
| Child-Pugh (A/B) | 0.384 | - | - | 0.897 | - | - | 0.869 | - | - |
| Tumor size, cm (≤ 10/> 10) | 0.166 | - | - | 0.053 | 0.021 | 7.65 (1.36-42.88) | 0.625 | - | - |
| Tumor number (≤ 3/> 3) | 0.609 | - | - | 0.533 | - | - | 0.280 | - | - |
| PVTT (absent/present) | 0.220 | - | - | 0.169 | - | - | 0.577 | - | - |
| HVTT (absent/present) | 0.842 | - | - | 0.455 | - | - | 1.000 | - | - |
| Metastasis (absent/present) | 0.960 | - | - | 0.694 | - | - | 0.615 | - | - |
The MVD of tumor lesions after the administration of lenvatinib was robustly smaller than that before treatment, suggesting that angiogenesis was strongly suppressed by lenvatinib (P < 0.001, Figure 3).
In the present study, significant shrinkage of hepatic arteries was observed in patients with uHCC after 1 mo of HAIC plus lenvatinib therapy for the first time. We also confirmed that the morphological change in the TFA was closely related to the tumor response after 1, 3, and 6 mo of combination treatment, and this change was an independent factor for improved short-term therapeutic efficacy. These findings would be of great significance to physicians for evaluating the effectiveness of this combination therapy earlier in the course of treatment and altering the treatment plan as needed.
Hepatic arteries were shown to be markedly thin following TKI treatment, and Chen et al[17] attributed these morphological changes to the anti-angiogenic effect of targeted drugs. Notably, lenvatinib more effectively suppresses angiogenesis and inhibits tumor growth than sorafenib by targeting both vascular endothelial growth factor (VEGF) and fibroblast growth factor[18,19]. In our study, shrinkage of hepatic vessels was observed early after the start of HAIC plus lenvatinib therapy, and the powerful antiangiogenic effect of lenvatinib was proven by the decrease in the MVD of the tumor lesions. Meanwhile, anti-VEGF therapies can reverse vessel abnormalities and improve the tumor microenvironment[20]. The decline in local tumor perfusion secondary to the decrease in tumor capillaries and the “normalization hypothesis” may be the main reasons for the conspicuous reduction of hepatic arteries with the combination regimen.
The efficiency of HAIC combined with lenvatinib for intermediate and advanced HCC has been confirmed in previous studies[21,22]. In this study, the ORR reached 68.7%, 66.1%, and 42.9% at 1, 3, and 6 mo, and conversion therapy was achieved in eight patients, which further verified the effectiveness of the combination therapy. Thus, it was necessary to explore the mechanism of action of this combination therapy. A reduction in hepatic artery diameters was observed in most patients in the present study. Kuorda et al[23] also reported that local tumor perfusion declined significantly after oral lenvatinib administration. These findings illustrated that lenvatinib reduces the tumor blood supply after combination therapy. Meanwhile, HAIC has the advantage of achieving high local concentrations in the tumor during arterial infusion[24]. According to the pharmacokinetic characteristics of the intra-arterial infusion[25], a decrease in tumor blood flow can increase the dose of the drug in the target organ, thus enhancing the antitumor effect of HAIC. Furthermore, the normalization and improved functionality of tumor vessels including reduced hypoxia, reduced vascular leakage, and improved vascular permeability can improve the delivery of local chemotherapeutic drugs in tumors[26,27]. The increased drug accumulation in the target organ often results in improved therapeutic efficacy. Since the combined therapy could improve the therapeutic effect, it may also expand the scope of HAIC, which is limited to patients with HCC and multinodule lesions or vascular invasion.
In clinical practice, HAIC often requires 6-8 courses. Moreover, repetitive and inefficient treatment is harmful to patients with uHCC. Therefore, early evaluation of the treatment response is of great significance. In this study, we found that the reduction in the TFA diameter after combination therapy was an independent factor for improved therapeutic efficacy within 6 mo. The morphological change in the TFA is easy to observe during HAIC, and it can help in estimating the early therapeutic effect. Since an improved short-term effect often results in a good prognosis, the observation on thinning of the TFA is significant and more practical for patients with uHCC who are receiving HAIC plus lenvatinib.
The present study had some limitations. This was a single-center retrospective study, and the sample size was small.
In conclusion, lenvatinib can reduce the diameters of hepatic arteries and thus enhance the effect of HAIC. Shrinkage of the TFA after HAIC plus lenvatinib therapy was linked to improved short-term outcomes in patients with uHCC.
Combination of hepatic arterial infusion chemotherapy (HAIC) and lenvatinib has been frequently used to treat unresectable hepatocellular carcinoma (uHCC) in China.
Shrinkage of hepatic arteries after the combination therapy is a common phenomenon and may early reflect the antitumor response, the relationship between which needs further exploration.
To investigate the relationship between hepatic artery diameters reduction and the short-term efficacy of HAIC plus lenvatinib.
Sixty-seven patients with uHCC receiving HAIC plus lenvatinib were analyzed retrospectively. The modified Response Evaluation Criteria in Solid Tumors was used to assess the antitumor response after 1, 3, and 6 mo of treatment. The measurement of hepatic artery diameters before treatment and after 1 and 2 mo of treatment were conducted in a computed tomography image post-processing workstation. Meanwhile, the changes in tumor capillaries were also examined on pathological specimens before and after 1 mo of treatment.
All the hepatic artery diameters and the microvessel density in the tumor lesions were significantly decreased after the combination treatment (all P < 0.001). Shrinkage of the tumor-feeding artery (TFA) was significantly associated with the antitumor response after 1, 3, and 6 mo of treatment (P < 0.001, P = 0.004, and P = 0.023, respectively) and an independent factor for treatment efficacy (P = 0.001, P = 0.001, and P = 0.002 and 1, 3, and 6 mo, respectively).
The retrospective study demonstrated that the shrinkage of the TFA diameter was closely related to improved short-term efficacy of treatment with HAIC plus lenvatinib for the first time.
We believe the findings in this paper will be of interest to the researchers in uHCC. Further, prospective randomized multicenter trials are needed to confirm the relationship between the morphological change in TFA and the early therapeutic effect of uHCC treatment.
All authors are grateful to Dr. Fan XY for assisting with the reconstruction of the CT images.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Tsoulfas G, Greece S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21364] [Article Influence: 2136.4] [Reference Citation Analysis (3)] |
| 2. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1871] [Article Influence: 207.9] [Reference Citation Analysis (4)] |
| 3. | European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236]. J Hepatol. 2019;70:817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Kudo M, Trevisani F, Abou-Alfa GK, Rimassa L. Hepatocellular Carcinoma: Therapeutic Guidelines and Medical Treatment. Liver Cancer. 2016;6:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol. 2018;48:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 7. | He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, Chen MS, Guo RP, Li QJ, Shi M. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Obi S, Sato S, Kawai T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer. 2015;4:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Matsuda N, Imai N, Kuzuya T, Yamamoto K, Ito T, Ishizu Y, Honda T, Ishigami M, Fujishiro M. Progression After Molecular Targeted Agents: Hepatic Arterial Changes and Transarterial Chemoembolization in Hepatocellular Carcinoma. In Vivo. 2021;35:1185-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Jiang H, Liu X, Chen J, Wei Y, Lee JM, Cao L, Wu Y, Duan T, Li X, Ma L, Song B. Man or machine? Cancer Imaging. 2019;19:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Okusaka T, Ikeda K, Kudo M, Finn R, Qin S, Han KH, Cheng AL, Piscaglia F, Kobayashi M, Sung M, Chen M, Wyrwicz L, Yoon JH, Ren Z, Mody K, Dutcus C, Tamai T, Ren M, Hayato S, Kumada H. Safety and efficacy of lenvatinib by starting dose based on body weight in patients with unresectable hepatocellular carcinoma in REFLECT. J Gastroenterol. 2021;56:570-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 13. | Pang H, Chen Y, He X, Tan X, Wang J, Yao Q, Liu X. Twelve-Month Computed Tomography Follow-Up after Thoracic Endovascular Repair for Acute Complicated Aortic Dissection. Ann Vasc Surg. 2021;71:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Matsuki M, Adachi Y, Ozawa Y, Kimura T, Hoshi T, Okamoto K, Tohyama O, Mitsuhashi K, Yamaguchi A, Matsui J, Funahashi Y. Targeting of tumor growth and angiogenesis underlies the enhanced antitumor activity of lenvatinib in combination with everolimus. Cancer Sci. 2017;108:763-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 16. | Mohamed A, Chenna A, Abdelfatah M, Sanjay J, Mohammad MK, Saber I, Kauh J, Elhammali B, Kaseb A. Microvessel density analysis in patients with viral hepatitis-related hepatocellular carcinoma. J Gastrointest Cancer. 2015;46:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Chen L, Zheng Y, Zhang H, Pan H, Liu Q, Zhou X, Wei W, Liu Y, Zhen M, Wang J, Zhou J, Zhao Y. Comparative analysis of tumor-associated vascular changes following TACE alone or in combination with sorafenib treatment in HCC: A retrospective study. Oncol Lett. 2018;16:3690-3698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 19. | Perera S, Kelly D, O'Kane GM. Non-immunotherapy options for the first-line management of hepatocellular carcinoma: exploring the evolving role of sorafenib and lenvatinib in advanced disease. Curr Oncol. 2020;27:S165-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Liu K, Zhang X, Xu W, Chen J, Yu J, Gamble JR, McCaughan GW. Targeting the vasculature in hepatocellular carcinoma treatment: Starving vs normalizing blood supply. Clin Transl Gastroenterol. 2017;8:e98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Shimose S, Iwamoto H, Tanaka M, Niizeki T, Shirono T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, Yamaguchi T, Kawaguchi T, Kuromatsu R, Noguchi K, Koga H, Torimura T. Alternating Lenvatinib and Trans-Arterial Therapy Prolongs Overall Survival in Patients with Inter-Mediate Stage HepatoCellular Carcinoma: A Propensity Score Matching Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Mai QC, Mo ZQ, Shi F, Chen XM. Lenvatinib plus hepatic arterial infusion of modified FOLFOX regime in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2020;38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, Endo K, Oikawa T, Sawara K, Takikawa Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;25:2365-2372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Kondo Y, Fukuda R. [Cutting Edge of Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma]. Gan To Kagaku Ryoho. 2020;47:1-5. [PubMed] |
| 25. | Chen HS, Gross JF. Intra-arterial infusion of anticancer drugs: theoretic aspects of drug delivery and review of responses. Cancer Treat Rep. 1980;64:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1569] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 27. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1442] [Article Influence: 68.7] [Reference Citation Analysis (0)] |