Published online Jul 14, 2022. doi: 10.3748/wjg.v28.i26.3201
Peer-review started: January 13, 2022
First decision: March 8, 2022
Revised: March 22, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: July 14, 2022
Processing time: 180 Days and 23.7 Hours
Endoplasmic reticulum (ER) stress contributes to the pathogenesis of chronic liver diseases, but how hepatocytes respond to ER stress has not been clarified. Alpha-fetoprotein (AFP) is secreted by hepatoma cells and elevated levels of serum AFP are associated with development of liver malignancies.
To investigate whether and how AFP could regulate ER stress and hepatocyte injury.
The distribution of AFP and the degrees of ER stress in liver tissues and liver injury were characterized by histology, immunohistochemistry, and Western blot in biopsied human liver specimens, two mouse models of liver injury and a cellular model. The levels of AFP in sera and the supernatants of cultured cells were quantified by chemiluminescence.
High levels of intracellular AFP were detected in liver tissues, particularly in the necrotic areas, from patients with chronic liver diseases and mice after carbon tetrachloride (CCl4) administration or induction of ER stress, but not from the controls. The induced intracellular AFP was accompanied by elevated activating transcription factor-6 (ATF6) expression and protein kinase R-like ER kinase (PERK) phosphorylation in mouse livers. ER stress induced AFP expression in LO2 cells and decreased their viability. ATF6, but not PERK, silencing mitigated the ER-stress-induced AFP expression in LO2 cells. Conversely, AFP silencing deteriorated the ER stress-mediated LO2 cell injury and CCl4 administration-induced liver damages by increasing levels of cleaved caspase-3, the C/enhancer binding protein homologous protein expression, mixed lineage kinase domain-like pseudokinase and PERK phosphorylation, but decreasing ATF6 expression.
ER stress upregulated intra-hepatocyte AFP expression by activating ATF6 during the process of liver injury and intracellular AFP attenuated hepatocyte apoptosis and necroptosis by alleviating ER stress.
Core Tip: During the process of liver injury, alpha-fetoprotein (AFP) expression was up-regulated in hepatocytes, especially in the necrotic areas, but it did not increase the serum AFP level. Endoplasmic reticulum (ER) stress induced intracellular AFP expression through activating activating transcription factor-6 and the up-regulated intracellular AFP expression attenuated hepatocyte apoptosis and necroptosis by feedback-down-regulating ER stress.
- Citation: Chen YF, Liu SY, Cheng QJ, Wang YJ, Chen S, Zhou YY, Liu X, Jiang ZG, Zhong WW, He YH. Intracellular alpha-fetoprotein mitigates hepatocyte apoptosis and necroptosis by inhibiting endoplasmic reticulum stress. World J Gastroenterol 2022; 28(26): 3201-3217
- URL: https://www.wjgnet.com/1007-9327/full/v28/i26/3201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i26.3201
Chronic liver diseases, such as chronic hepatitis B (CHB), affect many patients, especially in East Asia, such as China. However, the pathogenesis of chronic liver diseases remains unclear. Although human liver has powerful capacity to compensate the disease-related liver injury, how hepatocytes respond and defense against these diseases has not been clarified. Hence, it is of great significance to clarify it for the management of patients with chronic liver diseases.
Alpha-fetoprotein (AFP) is a protein with similar biological function to albumin and secreted mainly by embryonic tissue cells physiologically and malignant hepatocytes pathologically after birth. Elevated levels of serum AFP have been considered as a reliable biomarker for prediction of birth defect and diagnosis of liver cancer, teratoma, renal cell carcinoma, and pancreatic cancer[1] although moderate serum AFP levels can be temporarily detected in individuals with aberrant liver regeneration, hepatitis, and chronic liver disease. Furthermore, low levels of serum AFP have been continually observed in patients with liver cirrhosis and positively correlated with the degrees of liver inflammation and fibrosis[2,3]. The low and moderate levels of serum AFP are likely from the activation and proliferation of liver precursor cells, including oval cells, hepatic progenitor cells, in response to severe liver damages, particularly in liver failure[4-6]. While hepatocyte proliferation usually compensates for mild and moderate liver injury, liver precursor cells can differentiate into hepatocytes and bile duct cells, promoting liver regeneration. The activated liver precursor cells can secrete AFP and high levels of serum AFP have been suggested to be a biomarker of better prognosis of liver failure[7,8]. However, it is unclear whether chronic liver injury can induce AFP expression and secretion in differentiated mature hepatocytes, and how the induced AFP modulates the pathogenic process of chronic liver diseases.
It is notable that endoplasmic reticulum (ER) stress contributes to the pathogenesis of chronic liver diseases[9,10] and is regulated by inositol requiring enzyme-1 and activating transcription factor-6 (ATF6) and protein kinase R-like ER kinase (PERK)/eukaryotic translational initiation factor 2 alpha (eIF2α) signaling[11,12]. Aberrant ER stress can activate cell injury reactions, such as apoptosis and necroptosis by activating the C/enhancer binding protein (EBP)-homologous protein (CHOP) pathway, caspase-3 cleavage and mixed lineage kinase domain-like pseudokinase (MLKL) phosphorylation[13,14]. Our previous study has shown that ER stress inhibits the AFP secretion in hepatoma cells and the increase in the levels of intracellular AFP feedback attenuates the ER stress-related apoptosis and necroptosis in hepatoma cells[15]. However, it is unknown how ER stress can regulate AFP expression and secretion, and how intracellular AFP can modulate the ER stress-induced liver injury during the pathogenic process of chronic liver diseases.
In this research, we focus on whether intracellular AFP exists in hepatocytes during the liver injury, its regulatory relationship with ER stress, and its role in hepatocyte injury through clinical research, in vivo and in vitro experiments.
A total of 34 biopsied liver specimens were collected from CHB patients, eight surgical liver specimens were obtained from patients with hepatic trauma, and another eight surgical liver specimens were obtained from patients with hepatocellular carcinoma (HCC) in the Department of Infectious Diseases, or Hepatobiliary Surgery, the Affiliated Hospital of Zunyi Medical University since 2012. The patients with CHB were diagnosed, according to the Guidelines for Prevention and Treatment of Chronic Hepatitis B revised in 2019[16]. Individual patients were excluded if she/he had current infectious disease, autoimmune liver disease, liver malignant tumor, alcoholic liver disease, drug-induced liver disease, multiple organ dysfunction syndrome, obvious bleeding tendency, deep jaundice, obvious ascites, or another situation not suitable for liver biopsy. Their demographic and clinical data are shown in Table 1. The experimental protocol was approved by the Ethics Committee of Affiliated Hospital of Zunyi Medical University (ZYFYLS[2018] 28).
| Group | CHB | Control | HCC |
| n | 34 | 8 | 8 |
| Age (years; 25%, 75%) | 41.00 (35.00, 48.75) | 32.50 (28.25, 41.85) | 47.00 (40.25, 53.42) |
| ALT (U/L; 25%, 75%) | 137.50 (59.00, 221.50) | 31.5000 (14.5000, 63.1725) | 48.50 (39.00, 65.25) |
| TBil (μmol/L; 25%, 75%) | 16.300 (11.500, 24.225) | 10.80 (9.42, 20.58) | 20.6 (14.5, 32.5) |
| AFP (ng/mL; 25%, 75%) | 4.5400 (2.9850, 13.1725) | 2.02 (1.05, 4.75) | 179.80 (14.65, 207.53) |
Male BALB/c mice (25.0 g ± 3.0 g) were purchased from the Animal Center of Zunyi Medical University (Guizhou Province, China; SYXK[Qin] 2021-0004). The mice were maintained in a specific pathogen-free facility with a controlled temperature (20 °C-24 °C), a 12-h light/dark cycle and allowed free access to food and water ad libitum. The experimental protocol was established, according to the Animal Care and Research guidelines[17] and approved by the Animal Experiment Ethics Committee of Zunyi Medical University (LS[2020] 2-231).
To induce liver injury by carbon tetrachloride (CCl4) administration, the mice were randomized using a random number table into the healthy control group (NC; untreated), solvent control group (olive oil, 5 mL/kg, intraperitoneally, i.p.) and CCl4 group (1 mL/kg mixed with 4 mL of olive oil, i.p.) (n = 12 per group). The mice in the solvent and CCl4 group were administrated with solvent or CCl4 once or twice per week for 8 wk. Their peripheral blood samples were collected 24 h after the last dose, euthanized and their liver tissues were dissected.
To induce ER stress-related liver injury, the mice were randomized into the healthy NC (untreated), solvent control (phosphate buffer saline, PBS, 10 mL/kg) and tunicamycin (TM; an inhibitor of protein glycosylation) groups (2 mg/kg in the same volume of PBS, i.p.; Sigma) once (n = 12 per time point group). One or two days later, their peripheral blood samples were collected, euthanized and their liver tissues were dissected.
To test the role of AFP in the CCl4-induced liver injury, the mice were randomized and treated intravenously with 1×1010 recombinant serotype 8 adeno-associated virus (rAAV8) for expression of control short hairpin RNA (shRNA) or Afp-specific shRNA (Table 2, Genechem, Beijing, China). Six weeks after infection, the levels of AFP expression in the livers of mice were analyzed by Western blot to confirm Afp silencing. The mice with control shRNA or Afp-specific shRNA were administrated with olive oil or CCl4 as the control shRNA + olive oil, CCl4 (control shRNA + CCl4), Afp shRNA + olive oil, or Afp shRNA + CCl4 (Afp shRNA + CCl4). Their peripheral blood samples were collected, euthanized and their livers were dissected at 36 h (n = 12 per group) post CCl4 administration, based on our preliminary studies.
| Insert content | 5'-3' | ||
| Mouse | Afp shRNA | Target sequence | GCATCCATTGCAAAGGAATTA |
| shRNA sequence | GCATCCATTGCAAAGGAATTACGAATAATTCCTTTGCAATGGATGC | ||
| Control shRNA | shRNA sequence | AAACGTGACACGTTCGGAGAACGAATTCTCCGAACGTGTCACGTTT | |
| Human | PERK shRNA | Target sequence | GCACTTTAGATGGGAGAATTG |
| shRNA sequence | GCACTTTAGATGGGAGAATTGCGAACAATTCTCCCATCTAAAGTGC | ||
| Control shRNA | shRNA sequence | AAACGTGACACGTTCGGAGAACGAATTCTCCGAACGTGTCACGTTT | |
| ATF6 shRNA | Target sequence | GCAGGTCCTCCTGTTATTAGA | |
| shRNA sequence | GCAGGTCCTCCTGTTATTAGACGAATCTAATAACAGGAGGACCTGC | ||
| Control shRNA | shRNA sequence | AAACGTGACACGTTCGGAGAA CGAATTCTCCGAACGTGTCACGTTT | |
| AFP shRNA | Target sequence | GCTTCCATATTGGATTCTTAC | |
| shRNA sequence | GCTTCCATATTGGATTCTTAC CGAAGTAAGAATCCAATATGGAAGC | ||
| Control shRNA | shRNA sequence | AAACGTGACACGTTCGGAGAA CGAATTCTCCGAACGTGTCACGTTT | |
Human hepatocyte LO2 and hepatoma HepG2 cell lines were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and identified by STR. The cells were cultured in RPMI-1640 containing 10% fetal bovine serum, 100 Units/mL of penicillin and 100 μg/mL of streptomycin at 37 °C in a 5% CO2 incubator. To induce ER stress, LO2 cells were treated with, or without (NC group), solvent control group (dimethyl sulfoxide, DMSO) and 0.5 μmol/L thapsigargin (TG; an inhibitor of intracellular calcium balance) in DMSO (Sigma, TG group) for 12 h, 24 h and 48 h, respectively.
In addition, LO2 cells (1.2 × 106 cell/well) were cultured in 6-well plates overnight and transfected with plasmids for control shRNA, PERK-specific shRNA and ATF6-specific shRNA (Beijing Genechem) using lipofectamine 3000 (Fisher). Two days later, the cells were treated with vehicle DMSO or TG for 24 h. Additionally, LO2 cells were transfected with plasmid for the expression of control shRNA or AFP-specific shRNA (Beijing Genechem) for 48 h and treated with DMSO or TG for 36 h, respectively.
LO2, HepG2 cells, or individual liver samples were homogenized in immunoprecipitation assay lysis buffer (R0010, Solarbio, Beijing, China). After centrifugation, individual liver lysates (40 μg) were separated in sodium dodecyl sulfate polyacrylamide gel on 10% gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 5% skim dry milk in TBST (Tris-HCl buffer salt solution + Tween 20) and probed with mouse monoclonal antibodies (mAb) against AFP (sc-130302; Santa Cruz Biotechnology, Santa Cruz, CA, United States), ATF6 (sc-166659, 1:1000, Santa Cruz Biotechnology), GAPDH (sc-166545, 1:10000, Santa Cruz Biotechnology), CHOP (GADD153, sc-71136, 1:10000, Santa Cruz Biotechnology), eIF2α (sc-133132, 1:10000, Santa Cruz Biotechnology), phosphorylated PERK (p-PERK, MA5-15033, 1:1000, Thermofisher Scientific, United States), and PERK (sc-377400, 1:10000, Santa Cruz Biotechnology), or rabbit monoclonal antibodies against cleaved caspase-3 (9664, 1:10000, Cell Signaling Technology), and phosphorylated eIF2α (p-eIF2α, 3398, 1:10000, Cell Signaling Technology), or rabbit polyclonal antibodies against phosphorylated MLKL (p-MLKL, PA5-105677, 1:2000, Thermofisher Scientific), and MLKL (PA5-34733, 1:3000, Thermofisher Scientific). After reaction with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit IgG, the immunocomplexes were visualized with enhanced chemiluminescent reagents. Quantity One software (Bio-Rad, Hercules, CA, United States) was used to determine the relative levels of each targeted protein to the control (standardized as 1)[18].
Fresh liver tissues (5 mm × 5 mm in size from each mouse or 1 mm × 30 mm in size from one patient) were fixed in 4% paraformaldehyde for ≥ 24 h, paraffin-embedded and cut. The liver tissue sections (5 μm) were dewaxed, rehydrated and routine-stained with hematoxylin and eosin (H&E). The sections were examined under a light microscope (OLYMPUS CX31) using CaseViewer 2.4 software (3DHISTECH, Hungary). The necrotic areas in the liver were analyzed by Image-Pro Plus 6.0 (Media Cybernetics, United States)[19]. The Histology Activity Index-Knodell scores were determined by two pathologists blindly[20].
The liver tissue sections were dewaxed, rehydrated and blocked with 3% of bovine serum albumin for 20 min. The sections were incubated with mAb against AFP (sc-130302, 1:250) at 4 °C overnight and the bound antibodies were detected with HRP-conjugated anti-mouse IgG, followed by visualizing with diaminobenzidine. The intensity of anti-AFP staining was evaluated by Image Pro Plus 6.0[19].
The effect of Afp silencing on the frequency of apoptotic hepatocytes in liver sections was determined by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyribonucleotide derivative digoxigenin (dUTP) nick end labeling (TUNEL) using a specific kit (Roche, 11684817910), according to the manufacturer’s instruction. Briefly, the paraffin-embedded liver sections were dehydrated, permeabilized, and incubated with a mixture of TdT and dUTP at 1:14 in a humidified chamber for 2 h at 37 °C. The labeled cells were detected with detection solution and after being washed, the sections were counterstained with 4′, 6-diamidino-2-phenylindole. The TUNEL signals were examined under a fluorescent microscope. The apoptotic cells were defined by nuclear green staining while non-apoptotic cells with blue nuclear staining. Five visual fields in each section were randomly selected and the percentages of apoptotic cells in each section were calculated using the formula of positive cells/total cells × 100%.
AFP levels in the supernatant of cultured cells and mouse serum samples were measured by chemiluminescence immunoassay on the Beckman Coulter Auto Analyzer (Model DX1800; 04481798190, Roche Diagnostics GmbH) as previously described[21]. Samples were centrifuged to remove the remaining cells and possible cell debris before testing the AFP concentration.
The levels of serum alanine aminotransferase (ALT), total bilirubin (TBil) were two commonly used measures of liver injury, and analyzed by an auto-analyzer (AU5800, Beckman Coulter, United States)[22].
The impact of specific gene silencing or ER stress on LO2 cell viability was determined using the cell counting kit-8 (CCK-8; Cat. No. 40203ES60; Yeasen Biotechnology, Shanghai, China), per the product instruction. Briefly, LO2 cells (5000 cells/100 μL medium/well in 96-well plates) were treated with, or without, TG for 48 h (5 replicates per sample). During the last one-hour culture, the cells were exposed to CCK-8 and the absorbance of individual wells was detected at a wavelength of 450 nm (Bio-Rad, CA, United States). The relative cell viability (%) = (OD value of the treatment group-OD value of the blank group)/(OD value of the control group-OD value of the blank group) × 100.
Data are representative images or expressed as the mean ± SD of each group from 3 separate experiments. The difference of normally distributed values among groups was analyzed by one-way ANOVA and post hoc Tukey’s method. The difference between groups was analyzed by Student’s t-test. Survival rates were estimated using the Kaplan-Meier method and analyzed by the log-rank test. A P-value less than 0.05 was considered statistically significant.
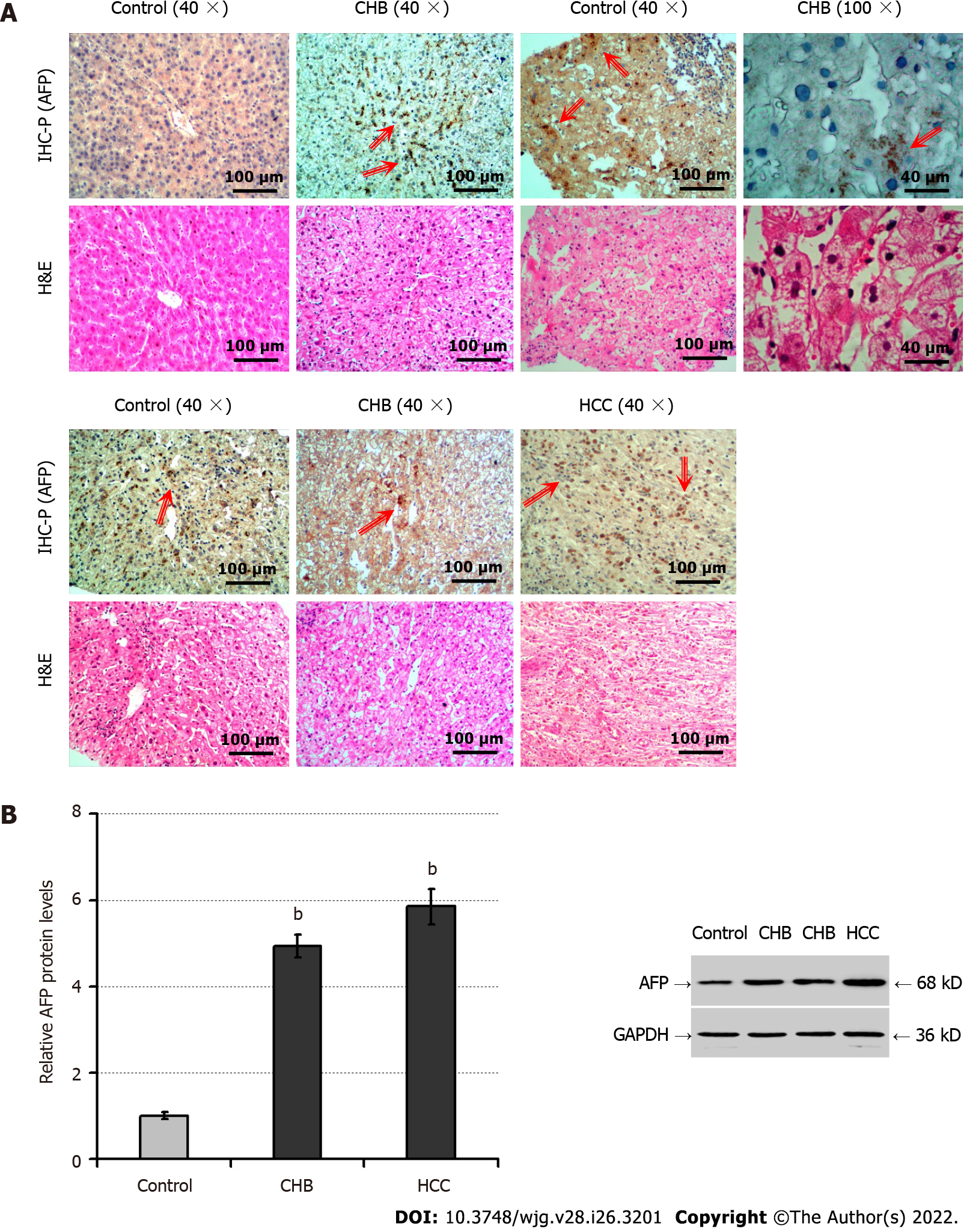
To explore the potential role of AFP in the progression of chronic hepatocyte injury, we analyzed AFP expression in liver tissues of patients with CHB (n = 34), controls (liver trauma; n = 8), and those with HCC (positive controls, n = 8). While there were healthy hepatocytes without degeneration and necrosis in the control liver tissues there were many hepatocytes undergoing degeneration and necrosis (Figure 1A). Immunohistochemistry indicated positive anti-AFP staining in the liver tissues from patients with HCC or CHB, but little in the controls. Interestingly, the positively stained anti-AFP was particularly in the regions with hepatocyte degeneration and necrosis of the liver tissues from CHB patients. Further Western blot displayed significantly increased levels of AFP expression in the livers from HCC or CHB patients, relative to that in the controls (P < 0.01, Figure 1B). Such data indicated that AFP expression was induced in hepatocytes, associated with hepatocyte degeneration and necrosis in humans during the process of hepatocyte injury.
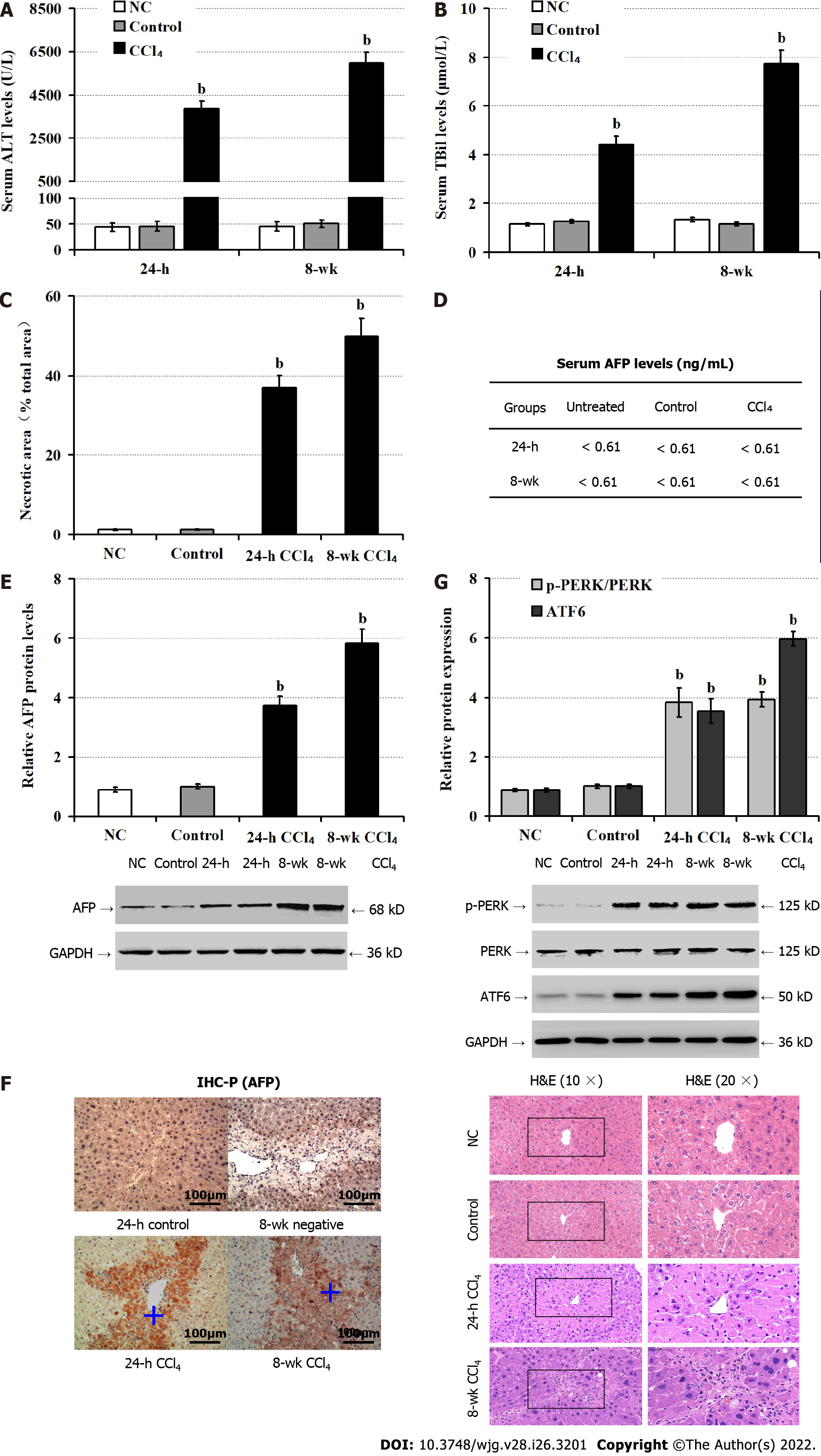
Next, we tested whether AFP expression could be induced in the liver of mouse model of CCl4-induced hepatocyte injury. As expected, CCl4 administration significantly elevated serum ALT (P < 0.05; Figure 2A), TBil (P < 0.05; Figure 2B) levels, accompanied by increased areas of liver tissue necrosis in mice, relative to the NC and the control groups (olive oil) of mice (P < 0.05; Figure 2C), particularly in the livers of mice following administration with CCl4 for 8 wk. There were similarly low levels of serum AFP detected in the different groups of mice (about 0.61 ng/mL, Figure 2D). In contrast, Western blot revealed that CCl4 administration obviously up-regulated AFP expression in the livers of mice in a trend of time-dependence (P < 0.05; Figure 2E). Further immunohistochemistry exhibited that the up-regulated AFP expression was mainly accumulated in the injured liver areas (Figure 2F). Finally, CCl4 administration significantly enhanced the relative levels of PERK phosphorylation and ATF6 expression (P < 0.05; Figure 2G), the hallmarks of enhancing ER stress in the livers of mice. Collectively, CCl4 administration induced hepatocyte injury and ER stress, and up-regulated AFP expression, but not its secretion in mice.
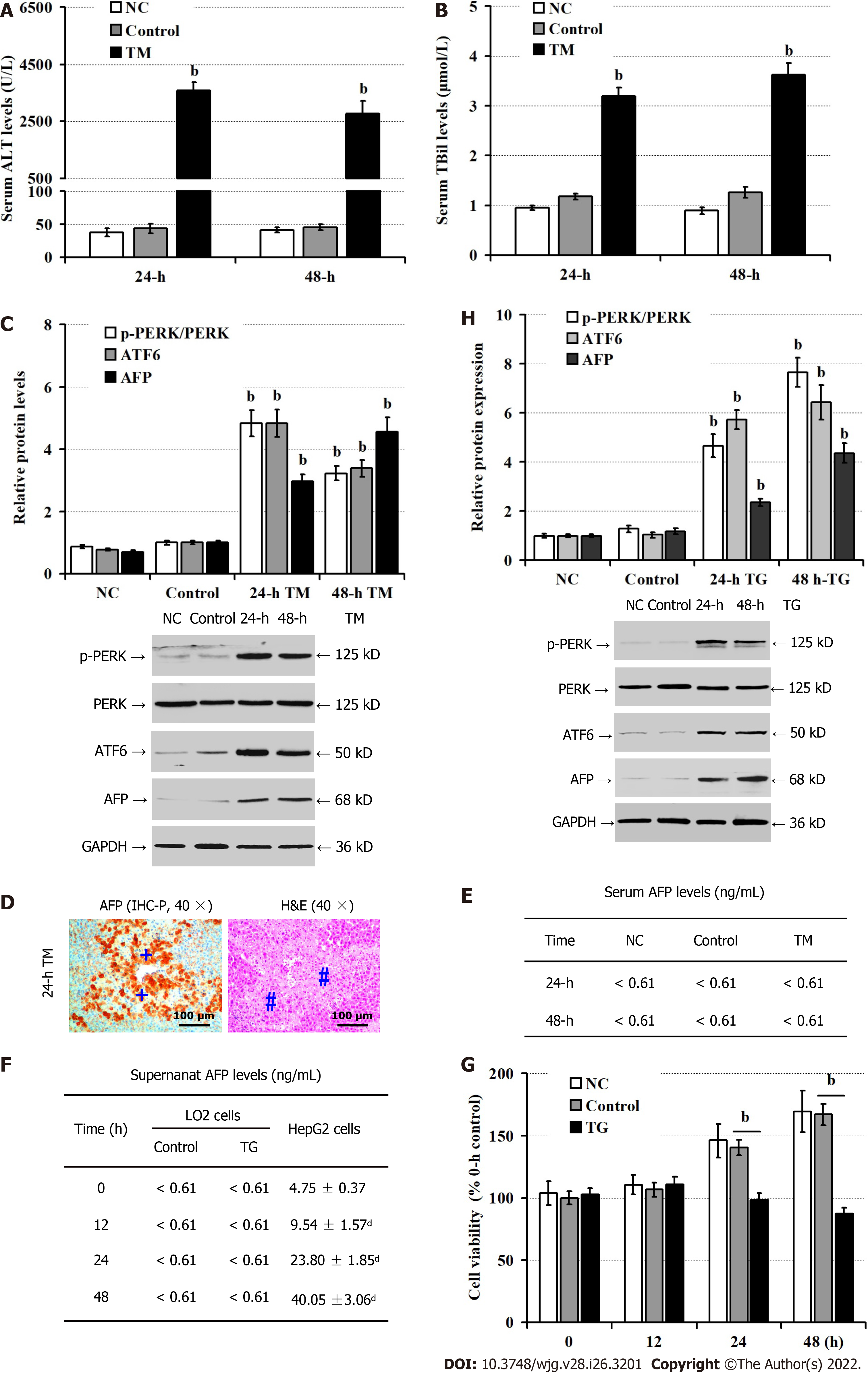
Given that up-regulated AFP expression was associated with enhanced ER stress, we tested whether induction of ER stress could up-regulate AFP expression in TM-injected mice. Compared with the NC and vehicle controls, significantly elevated levels of serum ALT (P < 0.05; Figure 3A) and TBil (P < 0.05; Figure 3B) were detected in mice at 24 h and 48 h post TM injection, implicating that induction of ER stress induced liver damages in mice. Consistently, Western blot revealed that TM injection obviously up-regulated ATF6, and AFP expression and PERK phosphorylation in the livers of mice, relative to that of the controls (P < 0.05; Figure 3C). Interestingly, there were damaged liver areas with strong anti-AFP staining in the mice with TM injection (Figure 3D). However, there were similar levels of serum AFP in the different groups of mice (< 0.61 ng/mL, Figure 3E). Furthermore, while there was no detectable AFP in the supernatants of cultured human non-tumor hepatocyte LO2 cells, even after treatment with TG, the levels of AFP in the supernatants of cultured HepG2 cells increased in a time-dependent manner (Figure 3F). Moreover, TG treatment significantly decreased the viability of LO2 cells at 24 h and 48 h post treatment (P < 0.05, Figure 3G), but TG treatment significantly enhanced the relative levels of AFP and ATF6 expression and PERK phosphorylation in LO2 cells (P < 0.05, Figure 3H). Thus, induction of ER stress induced hepatocyte injury and AFP expression.
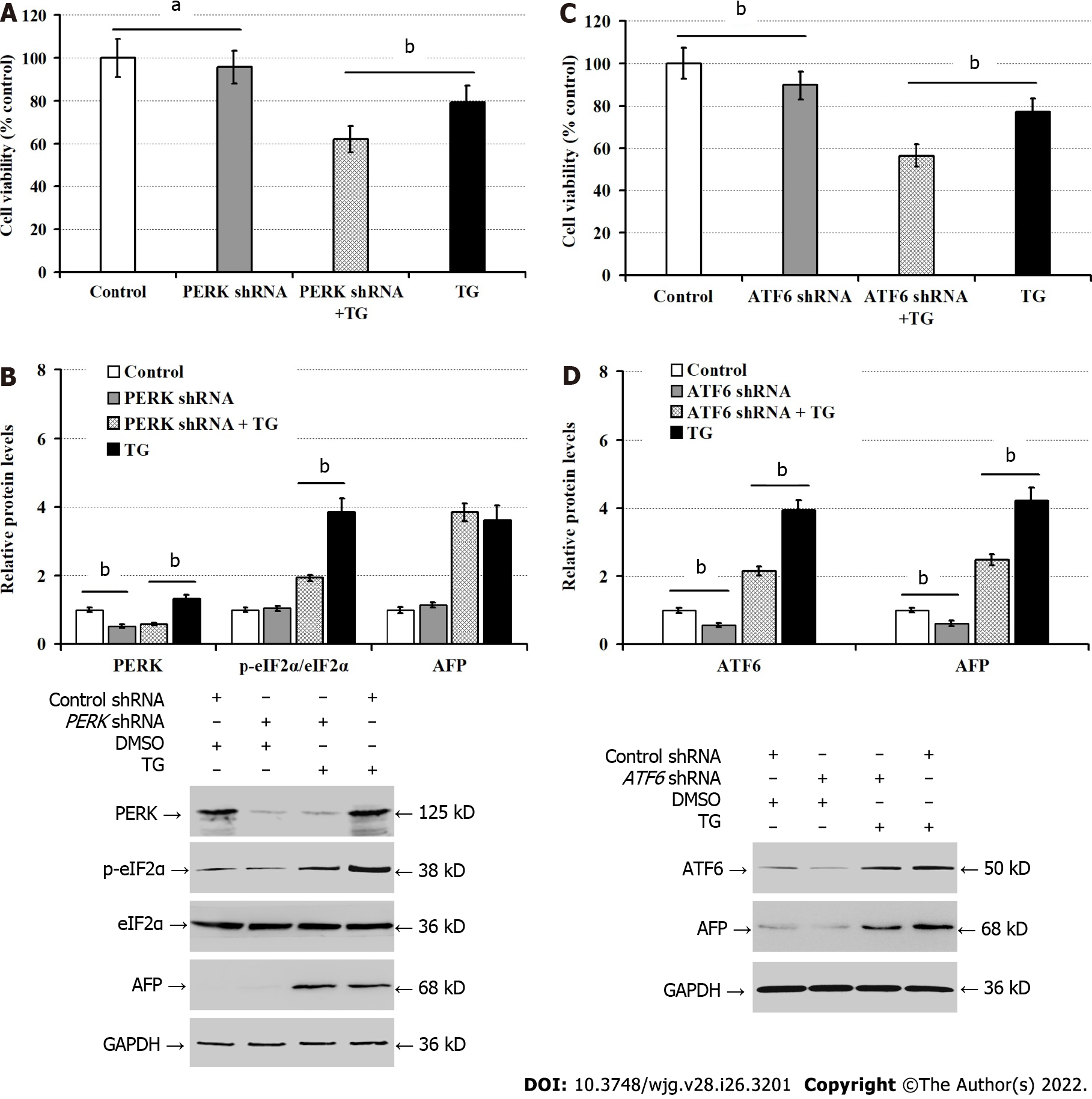
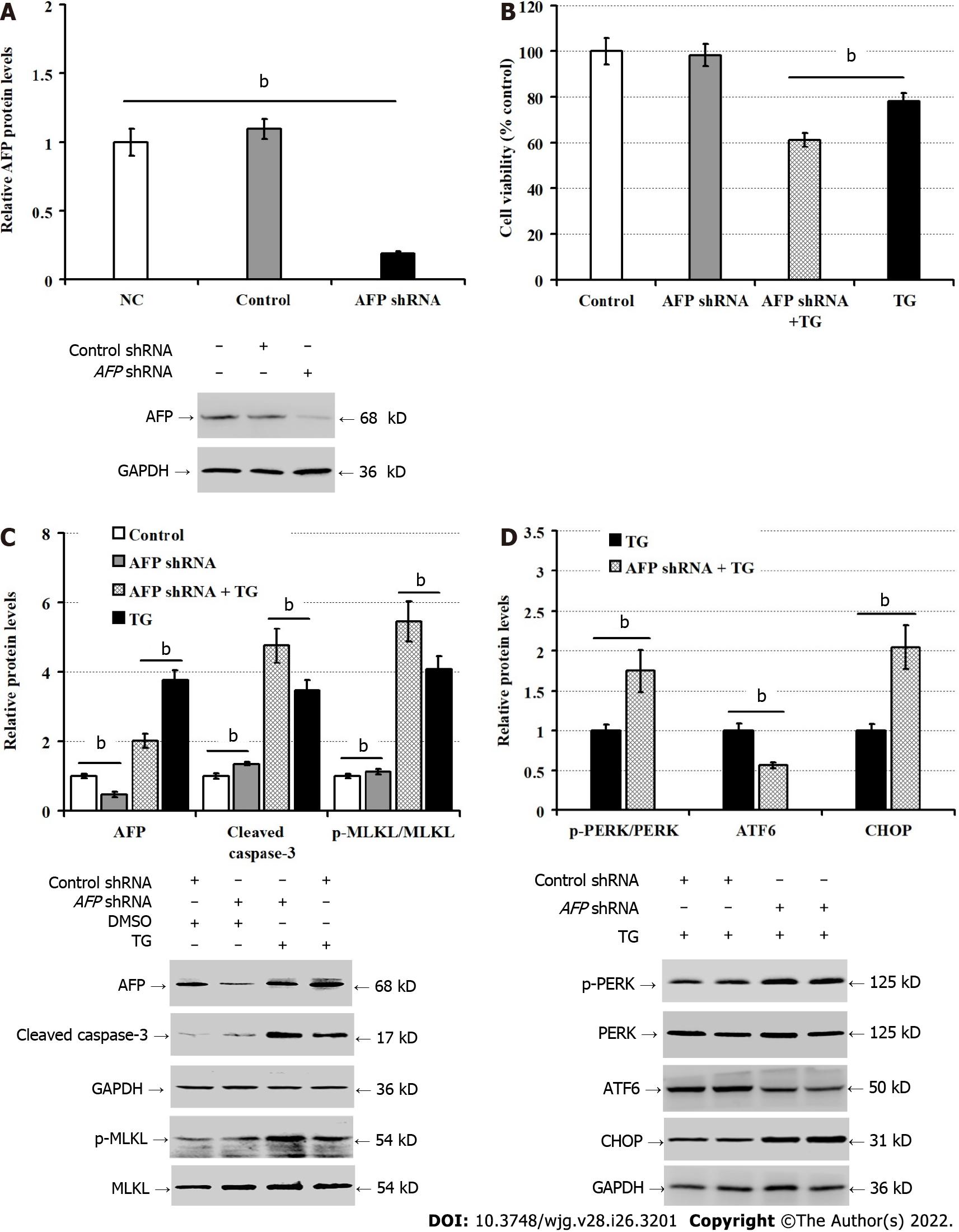
To understand how ER stress promoted AFP expression, we transfected LO2 cells with PERK or ATF6-specific shRNA. We found that PERK silencing significantly reduced the viability of LO2 cells (P < 0.05), and deteriorated the TG-induced damages in LO2 cells (P < 0.01; Figure 4A). While PERK silencing significantly reduced the levels of PERK protein expression in both regular cultures and TG-treated LO2 cells, and mitigated the relative levels of TG-enhanced eIF2α phosphorylation in LO2 cells, PERK silencing failed to significantly alter AFP protein expression in LO2 cells regardless of TG treatment (Figure 4B). In contrast, ATF6 silencing significantly reduced the viability of LO2 cells, but enhanced the TG-induced damages in LO2 cells (P < 0.01; Figure 4C). Moreover, transfection with ATF6 shRNA not only significantly inhibited ATF6 expression, but also attenuated the relative levels of AFP expression in both regular cultures and TG-treated LO2 cells (P < 0.01; Figure 4D). Hence, ATF6 silencing mitigated the TG-induced AFP expression in LO2 cells.
To understand the importance of AFP in ER stress-induced hepatocyte injury, we further explored the impact of AFP silencing on the ER stress-induced hepatocyte injury in vitro. Compared with the control LO2 cells, transfection with AFP-specific shRNA, but not the control shRNA, dramatically reduced AFP expression in LO2 cells (P < 0.01; Figure 5A), and deteriorated the TG-induced damages (P < 0.01), but it did not significantly alter the viability of LO2 cells (P > 0.05, Figure 5B). Furthermore, the AFP silencing significantly increased the levels of cleaved caspase-3 and MLKL phosphorylation regardless of TG treatment (P < 0.05, Figure 5C). Consistently, AFP silencing also significantly increased CHOP expression and PERK phosphorylation, but decreased ATF6 expression in LO2 cells (P < 0.01, Figure 5D). These results clearly indicated that AFP silencing enhanced spontaneous and TG-induced ER stress, apoptosis and necroptosis in LO2 cells.
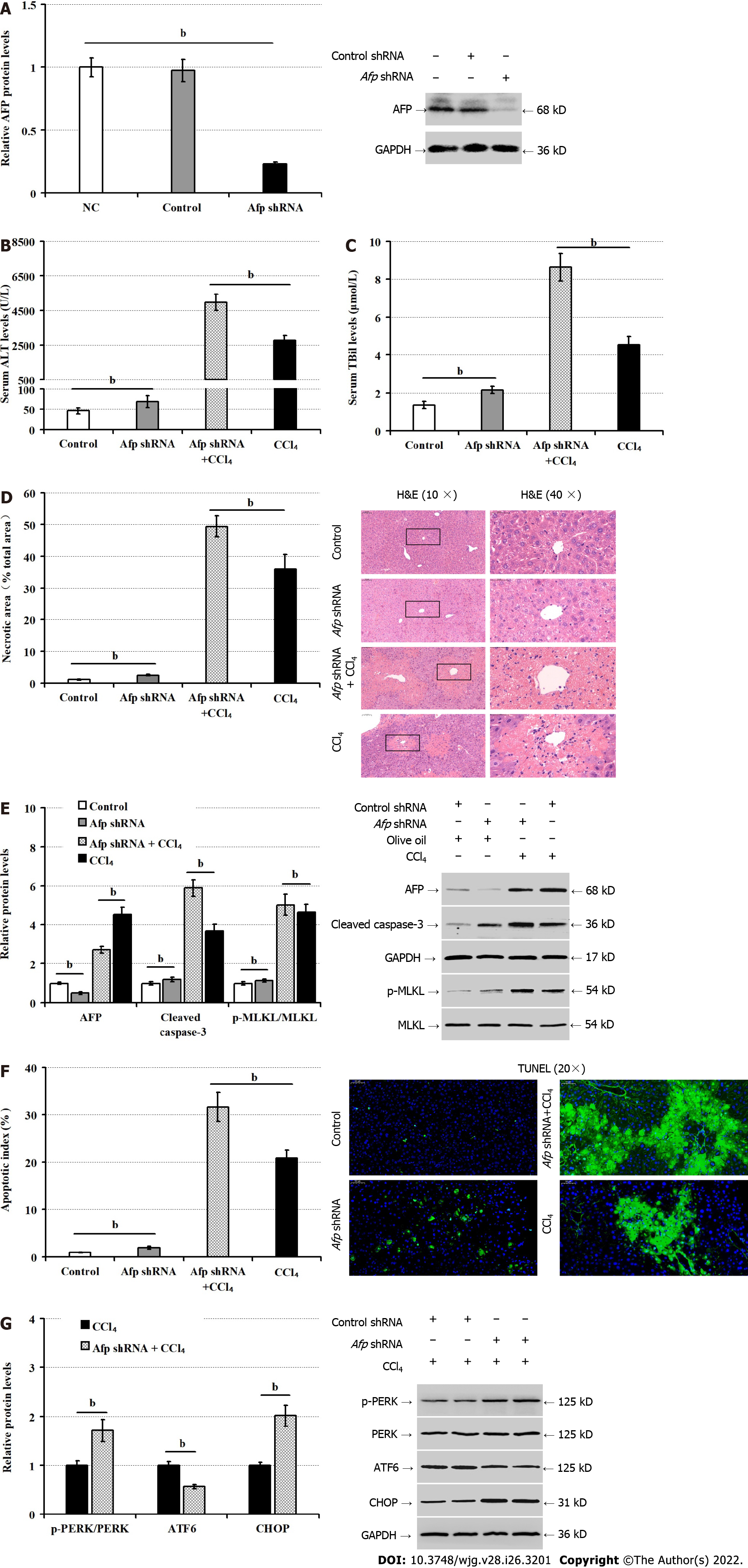
Finally, we tested whether induction of Afp silencing could modulate the CCl4-induced liver injury in mice. After intravenous administration with rAAV8 virus for the expression of control or Afp-specific shRNA for 6 wk, the levels of AFP expression in the liver tissues were reduced dramatically, confirming the Afp silencing (P < 0.01, Figure 6A). Both groups of mice were administrated with vehicle olive oil or CCl4 and 36 h later, we found that Afp silencing increased serum ALT (P < 0.01, Figure 6B), and TBil levels (P < 0.01, Figure 6C), regardless of CCl4 administration. Compared with the control mice, Afp silencing increased the percentages of necrotic areas in the livers of mice (P < 0.01, Figure 6D). Furthermore, Afp silencing decreased AFP expression, but remarkably increased the relative levels of cleaved caspase-3 expression, MLKL phosphorylation (P < 0.01, Figure 6E) and the percentages of apoptotic hepatocytes in the livers of both vehicle and CCl4-treated mice (P < 0.01, Figure 6F). Finally, Afp silencing significantly increased CHOP expression and PERK phosphorylation, but decreased ATF6 expression in the livers of CCl4-treated mice (P < 0.01; Figure 6G). Therefore, Afp silencing enhanced ER stress and liver injury induced by CCl4 in mice.
In this study, we investigated AFP expression, its regulatory mechanism, and its effect on hepatocyte injury during the process of liver injury. We detected high levels of AFP expression in the livers, particularly in the areas of hepatocyte necrosis, of patients with CHB, but not in those with hepatic trauma. Similarly, high levels of AFP expression were observed in the livers of mice following CCl4 administration and ER stress induction. The induced AFP expression was accompanied by liver injury in those patients and mice. Interestingly, there was no significant difference in the levels of serum AFP in those experimental patients and mice, compared to the controls. Moreover, induction of ER stress in human non-tumor hepatocyte LO2 cells also induced AFP expression, hepatocyte apoptosis and necroptosis, but failed to detect AFP in the supernatants of cultured cells. These indicated that during chronic liver diseases, ER stress and other inducers triggered hepatocyte apoptosis and necroptosis and stimulated AFP expression, but limited its secretion, leading to increased levels of intracellular AFP in hepatocytes. These novel data extended our previous study on hepatoma cells[15], and support the notion that AFP can be induced during the process of chronic liver diseases[7,23]. Our findings may shed light on the liver responses to ER stress in the pathogenic process of chronic liver diseases.
Low levels of serum AFP are detected in patients with chronic liver disease, and are positively correlated with the degrees of liver damages[8,24]. Although high levels of AFP expression were detected in mouse livers, we did not detect abnormally high levels of serum AFP in liver-injured mice, consistent with our observation in hepatoma cells[15]. These indicated that ER stress induced intracellular AFP expression by limiting its secretion. Given that healthy hepatocytes do not express AFP in adults it is possible that ER stress-induced hepatocyte injury may also induce compensative hepatocyte proliferation to repair liver damages in these models. However, the induced intracellular AFP is unlikely from the proliferation and differentiation of liver precursor cells. We are interested in further investigating how ER stress limits the secretion of AFP in hepatocytes during the process of chronic liver diseases.
AFP expression is regulated in a manner of tissue-specific and time-restriction[25]. Previous studies have shown that AFP expression is regulated by transcription factors, such as hepatocyte nuclear factor-1 (HNF1), ACCAAT-enhancer binding protein (C/EBP) and NF-1 and their enhancers[26-28]. Furthermore, the mutation in the AFP promoter region can increase the binding affinity of HNF1, leading to sustained increase in the levels of AFP expression[29]. Moreover, the AFP promoter activity is also regulated by the competitive modulation of these transcription factors, activators and inhibitors[30]. In this study, we explored how ER stress induced AFP expression in hepatocytes. ER stress mainly enhances eIF2α activation and regulates the expression of target molecules through ATF4, ATF6, and XBP1 to enhance cell ability to eliminate and degrade misfolded proteins[11]. We found that ATF6, but not PERK, silencing significantly mitigated the ER stress-induced AFP expression in LO2 cells. These data suggest that ATF6 may promote AFP expression in hepatocytes under an ER stress condition.
Functionally, AFP can act as a carrier to maintain plasma colloidal osmotic pressure and transport bilirubin, estrogen, fatty acids, retinoids, steroids and progesterone to regulate hormone homeostasis[31]. Second, AFP can induce immune cell apoptosis and down-regulates the gene expression of a variety of inflammatory factors, inhibiting autoimmunity and aberrant inflammation by protecting the fetus from maternal immune attack and attenuating the immune clearance of tumors[32,33]. Furthermore, AFP can enhance the malignant behavior of hepatoma cells by inhibiting their apoptosis and autophagy[34,35]. Accordingly, AFP can promote the survival and growth of a variety of tumor and non-tumor cells[36,37]. AFP can reduce the tumor necrosis factor alpha (TNF-α)-mediated damages of liver cancer cells or hepatocytes, and promotes their proliferation[38,39]. Studies have found that AFP can enhance the expression of p53, c-fos, c-jun, N-ras and hepatocyte growth factor receptor by binding to its receptors to activate the cyclic adenosine 3’, 5’-monophosphate (cAMP)-protein kinase A (PKA) pathway and induce Ca2+ influx. This process increases intracellular cAMP and PKA, and promotes the proliferation, differentiation and regeneration of hepatocytes. In this study, Afp silencing deteriorated the ER stress-mediated apoptosis and necroptosis of LO2 cells in vitro. Preferable liver Afp silencing also aggravated the CCl4-induced liver damages, hepatocyte apoptosis, and necroptosis in mice. The results suggest that the induced AFP expression in the liver by ER stress may enhance the resistance of hepatocytes to apoptosis and necroptosis stimulators, alleviating liver injury. Interestingly, we found that AFP silencing also exacerbated the ER stress, but lowed ATF6 expression in LO2 cells and in hepatocytes of liver tissues in mice, consistent with our previous observation[15]. These findings suggest that intracellular AFP induced by the ATF6 signaling may feedback-attenuate the ER stress-induced hepatocyte apoptosis and necroptosis, partially by up-regulating ATF6 expression. Given that AFP is not a transcription factor for the ATF6 expression the ER stress-induced AFP expression may through signal-crosstalk induce the ATF6-specific transcription factor and activator expression, indirectly inducing ATF6 expression in hepatocytes in a condition of ER stress.
We have reported that ATF6 is important for hepatocyte apoptosis and programmed necrosis[40]. It is possible that intracellular AFP may interact with intracellular proteins, transcription factors, kinases, co-activators and cell cycle regulators, such as PTEN to activate the PI3K/AKT/mTOR signaling to support the hepatocyte survival[41]. In addition, intracellular AFP may also interact with the apoptosis-related signaling to enhance apoptosis resistance by up-regulating Bcl-2 expression[35]. However, how intracellular AFP mitigates the ER stress-induced hepatocyte injury remains to be further examined.
ER stress induced intracellular AFP expression through activating ATF6 and the induced intracellular AFP feedback-attenuated the ER stress-induced hepatocyte injury. Thus, our findings may shed lights on the molecular regulation by which hepatocytes respond to ER stress, promoting compensative liver repair following ER stress-induced liver injury.
Endoplasmic reticulum (ER) stress plays an important role in the pathogenesis of chronic liver diseases, but how hepatocytes respond to ER stress has not been clarified. Alpha-fetoprotein (AFP) is secreted by hepatoma cells and elevated levels of serum AFP are associated with development of liver malignancies.
Anti-injury response is an important force for hepatocytes to resist liver injury mediated by various reasons, which has a close relationship to the progress and prognosis of liver injury. Studying the anti-injury mechanism of hepatocytes is important for the diagnosis and treatment of liver injury in the clinic.
To investigate whether and how AFP could regulate ER stress and hepatocyte injury.
The distribution of AFP and the degrees of ER stress in liver tissues were characterized by histology, immunohistochemistry, and Western blot in biopsied human liver specimens, two mouse models of liver injury and a cellular model. The levels of AFP in sera and the supernatants of cultured cells were quantified by chemiluminescence.
ER stress induces liver injury and increases intracellular AFP expression in hepatocytes. ER stress up-regulates intracellular AFP expression by up-regulating activating transcription factor-6 (ATF6). Upregulated AFP feedback attenuates ER stress, forming a regulatory loop. Upregulated AFP mitigates the ER stress-induced hepatocyte apoptosis and necroptosis.
ER stress upregulated intracellular AFP expression in hepatocytes by up-regulating ATF6 during the process of liver injury and intracellular AFP feedback-attenuated hepatocyte apoptosis and necroptosis by alleviating ER stress.
Intracellular AFP induced by ER stress alleviates hepatocyte apoptosis and necroptosis by activating ATF6.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K, Japan; Salgado LP, United States S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 397] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 2. | Fouad R, Elsharkawy A, Abdel Alem S, El Kassas M, Alboraie M, Sweedy A, Afify S, Abdellatif Z, Khairy M, Esmat G. Clinical impact of serum α-fetoprotein and its relation on changes in liver fibrosis in hepatitis C virus patients receiving direct-acting antivirals. Eur J Gastroenterol Hepatol. 2019;31:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Tai WC, Hu TH, Wang JH, Hung CH, Lu SN, Changchien CS, Lee CM. Clinical implications of alpha-fetoprotein in chronic hepatitis C. J Formos Med Assoc. 2009;108:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 491] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 6. | Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol. 2006;87:343-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Wang X, Shen C, Yang J, Yang X, Qin S, Zeng H, Wu X, Tang S, Zeng W. Alpha-Fetoprotein as a Predictive Marker for Patients with Hepatitis B-Related Acute-on-Chronic Liver Failure. Can J Gastroenterol Hepatol. 2018;2018:1232785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Huang GQ, Xie YY, Zhu PW, Wang XD, Lin Z, Wang Y, Ye JP, Wang YM, Chen YX, Jin XZ, Van Poucke S, Chen YP, Zheng MH. Stratified alpha-fetoprotein pattern accurately predicts mortality in patients with acute-on-chronic hepatitis B liver failure. Expert Rev Gastroenterol Hepatol. 2018;12:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 10. | Zhong W, Wang X, Rao Z, Pan X, Sun Y, Jiang T, Wang P, Zhou H. Aging aggravated liver ischemia and reperfusion injury by promoting hepatocyte necroptosis in an endoplasmic reticulum stress-dependent manner. Ann Transl Med. 2020;8:869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3048] [Article Influence: 234.5] [Reference Citation Analysis (0)] |
| 12. | Jäger R, Bertrand MJ, Gorman AM, Vandenabeele P, Samali A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell. 2012;104:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Zhao XC, Livingston MJ, Liang XL, Dong Z. Cell Apoptosis and Autophagy in Renal Fibrosis. Adv Exp Med Biol. 2019;1165:557-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 15. | Chen H, Chen GM, Liu YJ, Rao JX, Zhou SZ, Chen S, Chen PT, Yang FW, Cheng QJ, He YH. Alpha-fetoprotein/endoplasmic reticulum stress signaling mitigates injury in hepatoma cells. Neoplasma. 2021;68:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 17. | Simmonds RC. Bioethics and Animal Use in Programs of Research, Teaching, and Testing. In: Weichbrod RH, Thompson GAH, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. Boca Raton (FL), 2018: 35-62. |
| 18. | Chen G, Yang X, He Y, Tang Y, Tian R, Huang W, Chen H, Yang F, Li Y, Lin S. Inhibiting alpha subunit of eukaryotic initiation factor 2 dephosphorylation protects injured hepatocytes and reduces hepatocyte proliferation in acute liver injury. Croat Med J. 2019;60:532-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Tian RD, Chen YQ, He YH, Tang YJ, Chen GM, Yang FW, Li Y, Huang WG, Chen H, Liu X, Lin SD. Phosphorylation of eIF2α mitigates endoplasmic reticulum stress and hepatocyte necroptosis in acute liver injury. Ann Hepatol. 2020;19:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2507] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 21. | Huang H, Zheng XL, Zheng JS, Pan J, Pu XY. Rapid analysis of alpha-fetoprotein by chemiluminescence microfluidic immunoassay system based on super-paramagnetic microbeads. Biomed Microdevices. 2009;11:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lippi G, Dipalo M, Musa R, Avanzini P, Ferrarini C, Pattini A, Aloe R. Evaluation of the analytical performances of the novel Beckman Coulter AU5800. Clin Biochem. 2012;45:502-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kakisaka K, Kataoka K, Onodera M, Suzuki A, Endo K, Tatemichi Y, Kuroda H, Ishida K, Takikawa Y. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol Res. 2015;45:E12-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Yang N, Li Z, Yan M, Xiao W, Zhang W, Long Y, Cheng Y, Ming K, Xu B. Evaluation of Serum Alpha-Fetoprotein Level in Chronic Hepatitis C Patients. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Cao D, Zhou L, Zhang Y, Guo X, Li H, Chen Y, Spear BT, Wu JW, Xie Z, Zhang WJ. ZBTB20 is a sequence-specific transcriptional repressor of alpha-fetoprotein gene. Sci Rep. 2015;5:11979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Lee K. [Regulation of human alpha-fetoprotein gene by C/EBPalpha]. Hokkaido Igaku Zasshi. 2004;79:377-387. [PubMed] |
| 27. | Jose-Estanyol M, Danan JL. A liver-specific factor and nuclear factor I bind to the rat alpha-fetoprotein promoter. J Biol Chem. 1988;263:10865-10871. [PubMed] |
| 28. | Sakata N, Kaneko S, Ikeno S, Miura Y, Nakabayashi H, Dong XY, Dong JT, Tamaoki T, Nakano N, Itoh S. TGF- β Signaling Cooperates with AT Motif-Binding Factor-1 for Repression of the α -Fetoprotein Promoter. J Signal Transduct. 2014;2014:970346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Jeon Y, Choi YS, Jang ES, Kim JW, Jeong SH. Persistent α-Fetoprotein Elevation in Healthy Adults and Mutational Analysis of α-Fetoprotein Promoter, Enhancer, and Silencer Regions. Gut Liver. 2017;11:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Bois-Joyeux B, Danan JL. Members of the CAAT/enhancer-binding protein, hepatocyte nuclear factor-1 and nuclear factor-1 families can differentially modulate the activities of the rat alpha-fetoprotein promoter and enhancer. Biochem J. 1994;301 (Pt 1):49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Terentiev AA, Moldogazieva NT. Structural and functional mapping of alpha-fetoprotein. Biochemistry (Mosc). 2006;71:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Wang X, Wang Q. Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can J Gastroenterol Hepatol. 2018;2018:9049252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Meng W, Bai B, Bai Z, Li Y, Yue P, Li X, Qiao L. The immunosuppression role of alpha-fetoprotein in human hepatocellular carcinoma. Discov Med. 2016;21:489-494. [PubMed] |
| 34. | Wang S, Zhu M, Wang Q, Hou Y, Li L, Weng H, Zhao Y, Chen D, Ding H, Guo J, Li M. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9:1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Li M, Li H, Li C, Zhou S, Guo L, Liu H, Jiang W, Liu X, Li P, McNutt MA, Li G. Alpha fetoprotein is a novel protein-binding partner for caspase-3 and blocks the apoptotic signaling pathway in human hepatoma cells. Int J Cancer. 2009;124:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34:2075-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Yang X, Zhang Y, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008;271:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Semenkova LN, Dudich EI, Dudich IV, Shingarova LN, Korobko VG. Alpha-fetoprotein as a TNF resistance factor for the human hepatocarcinoma cell line HepG2. Tumour Biol. 1997;18:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Cavin LG, Venkatraman M, Factor VM, Kaur S, Schroeder I, Mercurio F, Beg AA, Thorgeirsson SS, Arsura M. Regulation of alpha-fetoprotein by nuclear factor-kappaB protects hepatocytes from tumor necrosis factor-alpha cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030-7038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Huang MY, Wan DW, Deng J, Guo WJ, Huang Y, Chen H, Xu DL, Jiang ZG, Xue Y, He YH. Downregulation of RIP3 Improves the Protective Effect of ATF an Acute Liver Injury Model. Biomed Res Int. 2021;2021:8717565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood). 2001;226:377-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |