Published online Jul 14, 2022. doi: 10.3748/wjg.v28.i26.3150
Peer-review started: October 26, 2021
First decision: April 16, 2022
Revised: April 25, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 14, 2022
Processing time: 260 Days and 4.9 Hours
As the main component of oral contraceptives (OCs), ethinylestradiol (EE) has been widely applied as a model drug to induce murine intrahepatic cholestasis. The clinical counterpart of EE-induced cholestasis includes women who are taking OCs, sex hormone replacement therapy, and susceptible pregnant women. Taking intrahepatic cholestasis of pregnancy (ICP) as an example, ICP consumes the medical system due to its high-risk fetal burden and the impotency of ursodeoxycholic acid in reducing adverse perinatal outcomes.
To explore the mechanisms and therapeutic strategies of EE-induced cholestasis based on the liver immune microenvironment.
Male C57BL/6J mice or invariant natural killer T (iNKT) cell deficiency (Jα18-/- mice) were administered with EE (10 mg/kg, subcutaneous) for 14 d.
Both Th1 and Th2 cytokines produced by NKT cells increased in the liver skewing toward a Th1 bias. The expression of the chemokine/chemokine receptor Cxcr6/Cxcl16, toll-like receptors, Ras/Rad, and PI3K/Bad signaling was upregulated after EE administration. EE also influenced bile acid synthase Cyp7a1, Cyp8b1, and tight junctions ZO-1 and Occludin, which might be associated with EE-induced cholestasis. iNKT cell deficiency (Jα18-/- mice) robustly alleviated cholestatic liver damage and lowered the expression of the abovementioned signaling pathways.
Hepatic NKT cells play a pathogenic role in EE-induced intrahepatic cholestasis. Our research improves the understanding of intrahepatic cholestasis by revealing the hepatic immune microenvironment and also provides a potential clinical treatment by regulating iNKT cells.
Core Tip: In this study, we observed the production of both Th1 and Th2 cytokines by natural killer T (NKT) cells in the liver after 14 d of exposure to ethinylestradiol-induced cholestasis. The liver immune microenvironment was also skewed toward a Th1 bias mainly contributed by NKT cells. Invariant NKT cell deficiency robustly alleviated cholestatic liver damage and downregulated the associated signaling pathways, highlighting the pathogenic role and therapeutic potential of hepatic NKT cells in cholestatic liver diseases.
- Citation: Zou MZ, Kong WC, Cai H, Xing MT, Yu ZX, Chen X, Zhang LY, Wang XZ. Activation of natural killer T cells contributes to Th1 bias in the murine liver after 14 d of ethinylestradiol exposure. World J Gastroenterol 2022; 28(26): 3150-3163
- URL: https://www.wjgnet.com/1007-9327/full/v28/i26/3150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i26.3150
Cholestasis is a mild and common phenomenon during liver diseases but also a crucial triggering element of severe hepatopathy, such as fibrosis, cirrhosis, and hepatic venous thrombosis[1]. Both estrogen and oral contraceptives (OCs) can elicit intrahepatic cholestasis (IHC), whose effects manifest as retention of toxic bile acids in the liver and circulation[2]. 17α-ethinylestradiol (EE) is used as a model drug to induce murine IHC. As the predominant component of OCs and hormone replacement therapy, the clinical counterpart of EE-induced IHC includes women who are taking OCs, postmenopausal replacement therapy and susceptible pregnant women. Sex steroids may lead to alterations in bile components and the elevation of total bile acids (TBAs), which may induce apoptosis and oxidative stress and thus have harmful effects on hepatocytes and other organs[3]. In clinical studies, administration of EE to both males and females leads to increased serum TBA levels and decreased clearance of sulfobromophthalein[4]. In murine studies, the mechanisms involve the activation of AMP-activated protein kinase[5] and estrogen receptor α[6], the inhibition of farnesoid X receptor (FXR), bile acid transporters, bile acid synthase and metabolism enzymes, and inflammatory reactions[7-9]. The first-line therapy ursodeoxycholic acid (UDCA) for cholestatic diseases is a naturally hydrophilic bile acid, although its potency is limited, as approximately 40% of patients are not responsive to UDCA treatment[10].
Among these predisposing factors, the significance of the local immune microenvironment in the liver has been emphasized because sex hormones are immunomodulators that are metabolized in the liver. In a murine model of EE-induced cholestasis, hepatic expression of TNF-α and IL-6 was greatly upregulated[8]. Proinflammatory mediators can affect nuclear receptors and transporters and then cause bile acid equilibrium disorder, increased cytokine secretion, and further exacerbated cholestasis, forming a positive regulation loop[8]. In patients with intrahepatic cholestasis of pregnancy (ICP), an increase in Th1-type cytokines and a decrease in Th2-type cytokines suggest the involvement of proinflammatory and cytotoxic Th1 biases in ICP[11]. However, the impotency of corticosteroids in the treatment of cholestasis indicates that promising therapies or active agents are in heavy demand[12].
Natural killer T (NKT) cells are one of the most abundant lymphocytes in the liver, and their role in cholestasis is noteworthy[13]. NKT cells behave similarly to conventional T cells and function as both effector and regulatory immune cells. Jα18-/- mice are not prone to developing cholestatic liver injury after alpha-naphthylisothiocyanate (ANIT) administration due to the invariant NKT-cell (iNKT cell) knockout-related reduction in cytokines and the restored expression of transporters and bile acid metabolism enzymes[14]. Recent work also demonstrated that IL-4 secreted by type 1 NKT cells (iNKT cells) may inhibit type 2 NKT cells and upregulate immunoregulators, affecting the expression of bile acid nuclear receptors, transporters, and CYP450 enzymes, thus exacerbating triptolide-induced cholestatic liver damage[15]. However, Jα18-/- mice showed aggravation of liver damage after bile duct ligation (BDL) surgery compared with wild-type mice due to the increase in neutrophils, chemokines and cytokines[16]. Moreover, knockout of the bile acid sensor FXR gene increases liver NKT cells and aggravates liver damage, indicating that FXR can regulate the activation of liver NKT cells[17]. Certain antigens that can activate NKT cells exist in the bile of patients with chronic liver diseases[18]. Thus, the aim of the present study was to investigate the effects and mechanisms of NKT cells in a murine model of EE-induced cholestatic hepatotoxicity.
17α-EE (CAS:57-63-6, batch number: E0037, contents > 98.0% (T) high performance liquid chromatography) was purchased from tcichemicals (Shanghai, China). EE was dissolved in an 80% 1,2-propylene glycol solution and diluted with physiological saline to a dosing concentration of 10 mg/kg before the experiment. Anti-CD16/32 antibody (clone: 2.4G2), which was used for blocking before staining, anti-CD3e-FITC antibody (clone: 145-2C11), anti-CD49b-APC antibody (clone: DX5), leukocyte activation cocktail with BD GolgiPlugTM, anti-IFN-γ-PE antibody (clone: XMG1.2), and anti-IL-4-PE antibody (clone: 11B11) were purchased from BD Pharmingen (San Diego, CA, United States).
Male C57BL/6J mice (6-8 wk of age and 18-20 g of weight) were obtained from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). iNKT cell-deficient Jα18-/- mice on a C57BL/6 background (6 to 8 wk old) were kindly provided by Dr. Li Bai (University of Science and Technology of China). All mice were administered physiological saline or EE (10 mg/kg) subcutaneously (s.c.) for 14 continuous d. Each group contained 6 mice. All mice were maintained and bred under controlled conditions (pathogen-free, 22 ± 2 °C, 12:12-h light-dark regular photoperiod) with ad libitum mouse chow and water access. The animals were housed in the laboratory for 1 wk prior to experiments to acclimate. All procedures involved in this study were performed under the Ethical Committee of China Pharmaceutical University and the Laboratory Animal Management Committee of Jiangsu Province guidelines (Approval No.: 2021-10-003).
After perfusing saline solution into the heart to eliminate blood, the mouse liver was minced through a 200-gauge nylon mesh and washed with cold PBS. After centrifugation at 50 × g for 2 min in the crude solution, the separated supernatant was centrifuged for another 10 min at 800 × g. The cell pellets were resuspended in 40% Percoll for centrifugation at 1250 × g for 15 min. After nonparenchymal cell (NPC) isolation from the cell pellets by red blood cell lysis solution (0.15 M NH4Cl and 0.1 mmol/L Na2EDTA) treatment and 2 washes, NPCs were stimulated with leukocyte activation cocktail for 4-5 h (BD Pharmingen).
After stimulation, NPCs were blocked with anti-CD16/32 and then surface-labeled with FITC-conjugated anti-mouse CD3e and APC-CD49b antibodies. NPCs were permeabilized with Cytofix/ Cytoperm (Becton Dickinson) following the manufacturer’s instructions, and intracellular staining was performed by incubation with IFN-γ or IL-4 antibodies. After each step, unbound antibodies were excluded from the system by centrifugation. Then, the cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Palo Alto, CA, United States), with 50000 events recorded per tube. The results were processed using FlowJo version 10 software (FlowJo, Ashland, OR, United States). The gating strategy was as follows: first, NPCs were gated by FSC and SSC, followed by the gating of CD3e+ or CD3e- cells. CD49b+ IFN-γ+, CD49b+ IL-4+ or CD49b- IFN-γ+, and CD49b- IL-4+ cells were then gated and analyzed.
Anticoagulant-free serum was collected and then analyzed for the levels of alkaline phosphatase (ALP), total bile acid (TBA), alanine transaminase (ALT), and aspartate transaminase (AST) using the ALP, ALT, and AST quantification kit (Whitman Biotech, Nanjing, China) and TBA quantification kit (Jiancheng Bioengineering Institute, Nanjing, China).
The liver lobules were fixed, paraffin embedded, sectioned, and stained with hematoxylin and eosin for histopathological examination. Slides were coded, randomized, and assessed by pathologists who, during the evaluation of the slides, were blinded to the treatment groups. Scoring for liver injury was conducted according to the following criteria: no hepatocellular necrosis, proliferation of pseudocholangiolar duct, or inflammatory cell infiltration, 0; mild, 1; moderate, 2; severe, 3. The other sections were subjected to IHC for semiquantification of the expression of toll-like receptor (TLR) 9 (Novus, 26C593.2, dilution percentage: 1:200).
RNA was extracted from liver sections by TRIzol reagent (Vazyme Biotech, Nanjing, China). Isolated RNA was processed by the HiScriptTM Q RT SuperMix for quantitative polymerase chain reaction (qPCR) (+ gDNA wiper) kit (Vazyme Biotech) for cDNA synthesis. A 20 μL real-time PCR system was prepared according to the manufacturer’s instructions. The mRNA levels were normalized to the housekeeping gene Gapdh. The primer sequences used are shown in Table 1.
| Name | Forward (5’ to 3’) | Reserve (5’ to 3’) |
| Gapdh | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| Tlr2 | TGCTCCTGCGAACTCCTATC | CAGACTCCAGACACCAGTGC |
| Tlr4 | AGCTTCTCCAATTTTTCAGAACTTC | TGAGAGGTGGTGTAAGCCATGC |
| Tlr6 | AGCCAAGACAGAAAACCCATC | GGGGTCATGCTTCCGACTAT |
| Tlr7 | CACCACCAATCTTACCCTTACC | CAGATGGTTCAGCCTACGGAA |
| Tlr9 | GCTGTCAATGGCTCTCAGTTCC | CCTGCAACTGTGGTAGCTCACT |
| Cxcr6 | GGTTCTTCCTGCCATTGCTCAC | GCAGGAACACAGCCACTACAAG |
| Cxcl16 | GCAGGGTACTTTGGATCACATCC | AGTTCACGGACCCACTGGTCTT |
| Ras | TCGCACTGTTGAGTCTCGGCAG | TATGCTGCCGAATCTCACGGAC |
| Raf | CGCCAAGTCAATCATCCACAGAG | CACCGAGATTTCACTGTGGCTAG |
| Pi3k | CACCTGAACAGACAAGTAGAGGC | GCAAAGCATCCATGAAGTCTGGC |
| Bad | GGGAGCAACATTCATCAGCAGG | CGTCCTCGAAAAGGGCTAAGCT |
Values are expressed as the means ± SE. The groups were evaluated using Student’s t test. A P value < 0.05 was considered statistically significant.
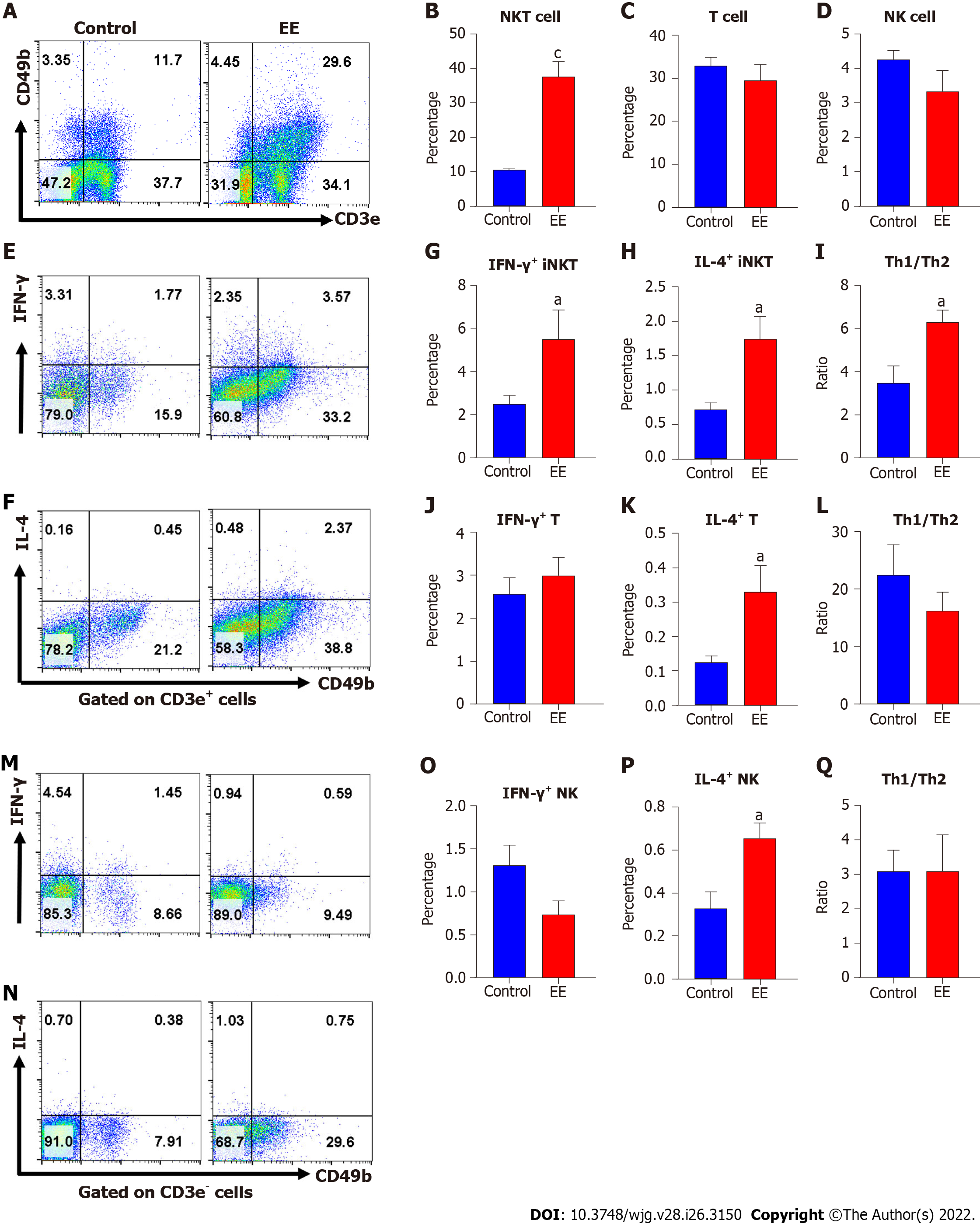
Increased activation of NKT cells, T cells, or NK cells has been reported to contribute to the pathophysiology of cholestasis[15,19,20]. Here, we measured and analyzed their percentages and Th1/Th2 cytokine production to evaluate their activation. Hepatic NKT cells, CD3+ T cells (excluding NKT cells) and NK cells were defined as CD3e+CD49b+ (Figure 1A upper right), CD3e+CD49b- (Figure 1A lower right), and CD3e-CD49b+ (Figure 1A upper left), respectively. After EE administration, the percentage of hepatic NKT cells expanded more than 3-fold compared with the control group, while the frequencies of CD3+ T cells and NK cells showed no significant changes (Figure 1A-D). Both IFN-γ and IL-4 secreted from NKT cells increased, which further indicated NKT-cell activation. Moreover, the upregulated Th1/Th2 ratios indicated that a skewed hepatic Th1 immune response was due to NKT cells (Figure 1E–I). Compared with the control group, IL-4 secreted by CD3+ T cells and NK cells increased, and their IFN-γ secretion and Th1/Th2 ratios remained unaltered (Figure 1J–Q).
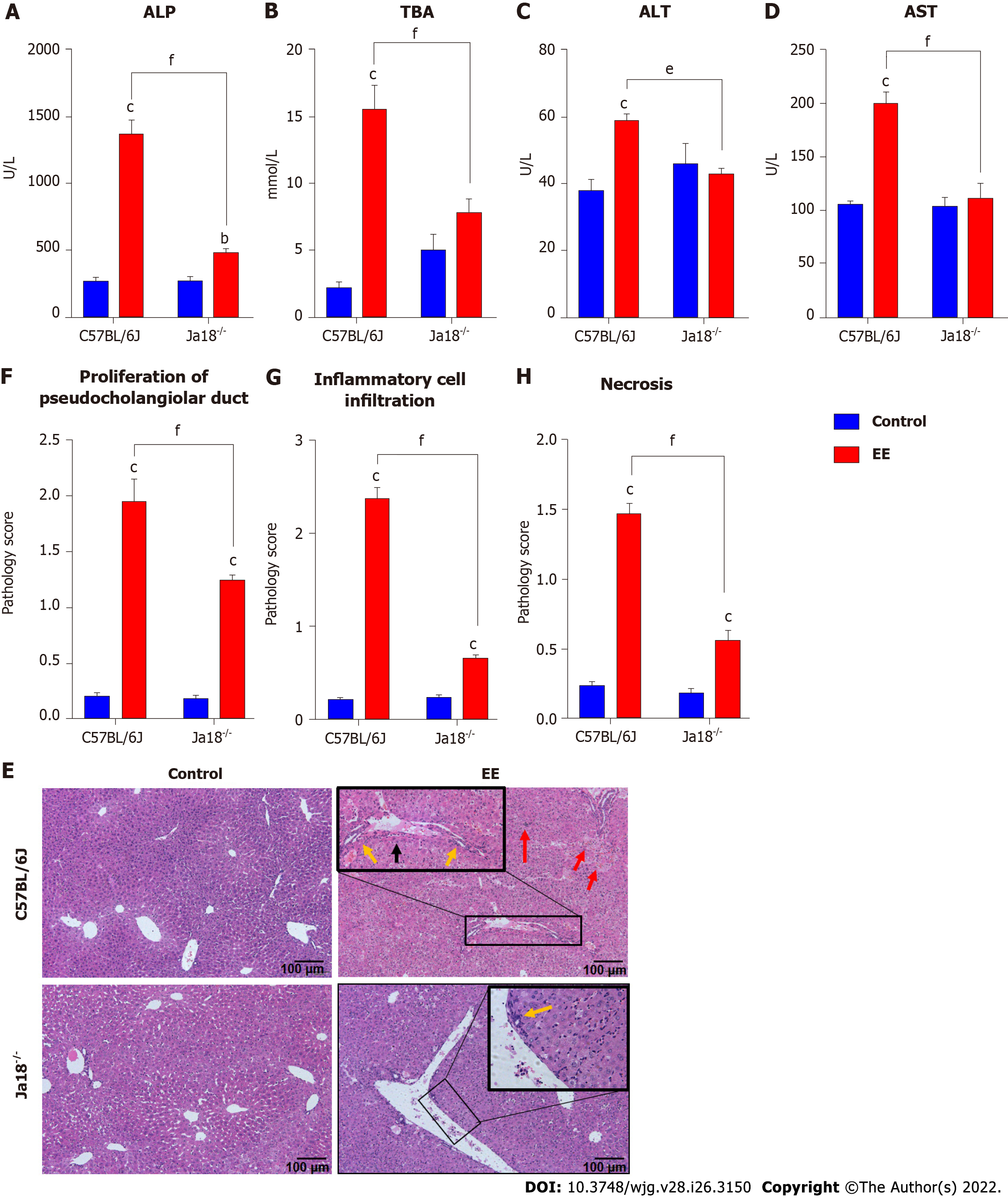
For further investigation of the NKT-cell effect, cholestatic liver injury was compared between iNKT cell knockout mice (Jα18-/- mice) and C57BL/6J mice after EE administration. Compared with the control group, EE induced increased levels of ALP, TBA, ALT, and AST in C57BL/6J mice. Compared with C57BL/6J mice, Jα18-/- mice demonstrated significantly lower levels of ALP, TBA, ALT, and AST (Figure 2A-D). Based on the scoring criteria[21], the histopathological and hepatic injury score results showed proliferation of pseudocholangiolar duct (yellow arrows), inflammatory cell infiltration (red arrows) and hepatocyte necrosis (black arrows) after EE treatment, whereas Jα18-/- mice exhibited reduced proliferation of the pseudocholangiolar duct, inflammatory cell infiltration, and necrosis (Figure 2E-G). These results showed that iNKT cell-deficient mice can inhibit the development of cholestatic hepatotoxicity induced by EE, indicating a pathogenic effect exerted by iNKT cells on EE-induced cholestatic liver damage.
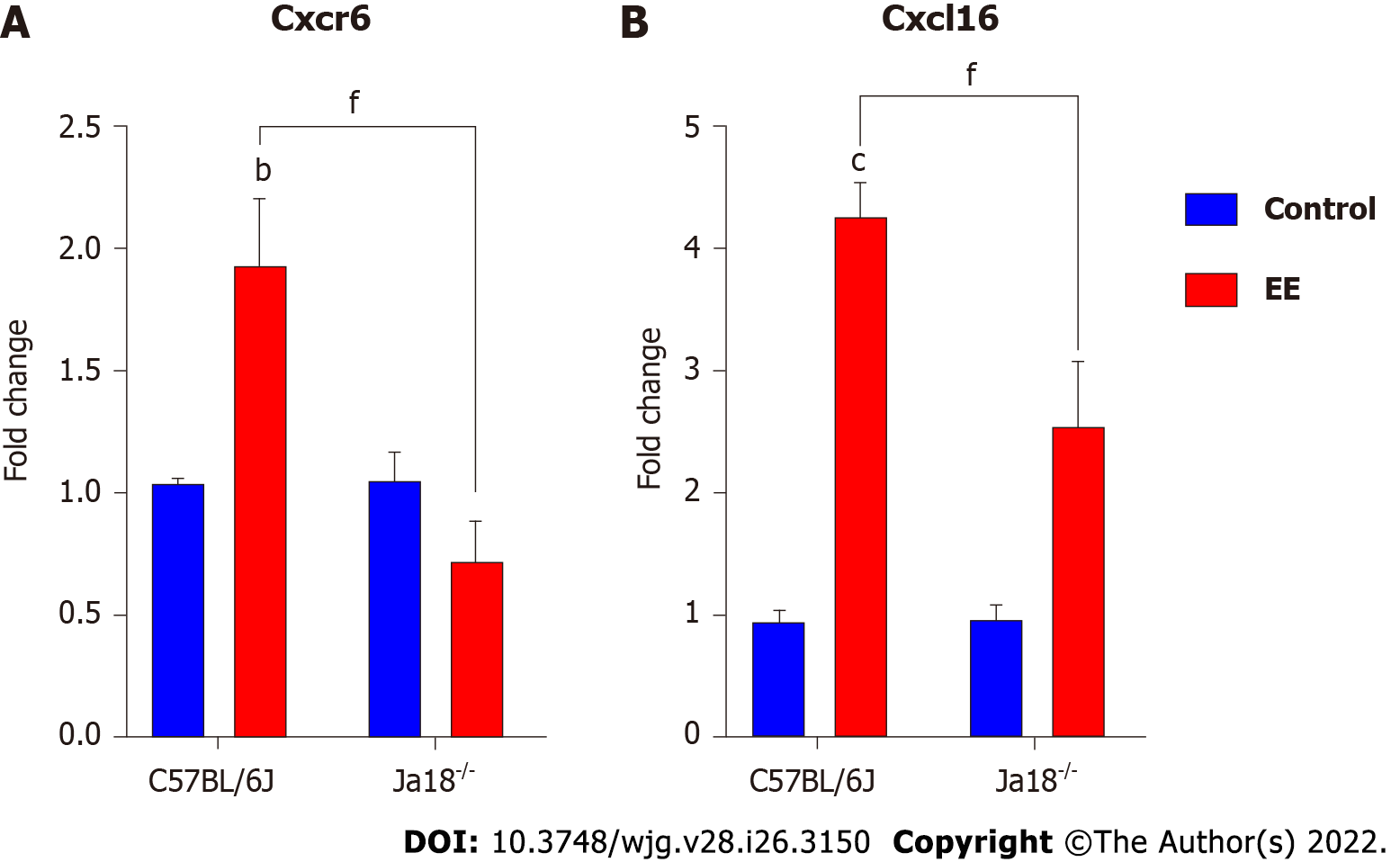
CXCR6, the receptor for chemokine CXCL16, is expressed on the surface of several hepatic lymphocytes, including NKT cells. The CXCR6-dependent infiltration of NKT cells into the liver induces enhanced inflammation in a murine model of chronic hepatic damage[22]. The qPCR results showed that EE promoted the hepatic mRNA expression of Cxcr6/Cxcl16 in C57BL/6J mice. Compared with C57BL/6J mice, iNKT cell deficiency significantly suppressed the mRNA expression of Cxcr6/Cxcl16, which may be related to the absence of the need for hepatic recruitment of NKT cells (Figure 3).
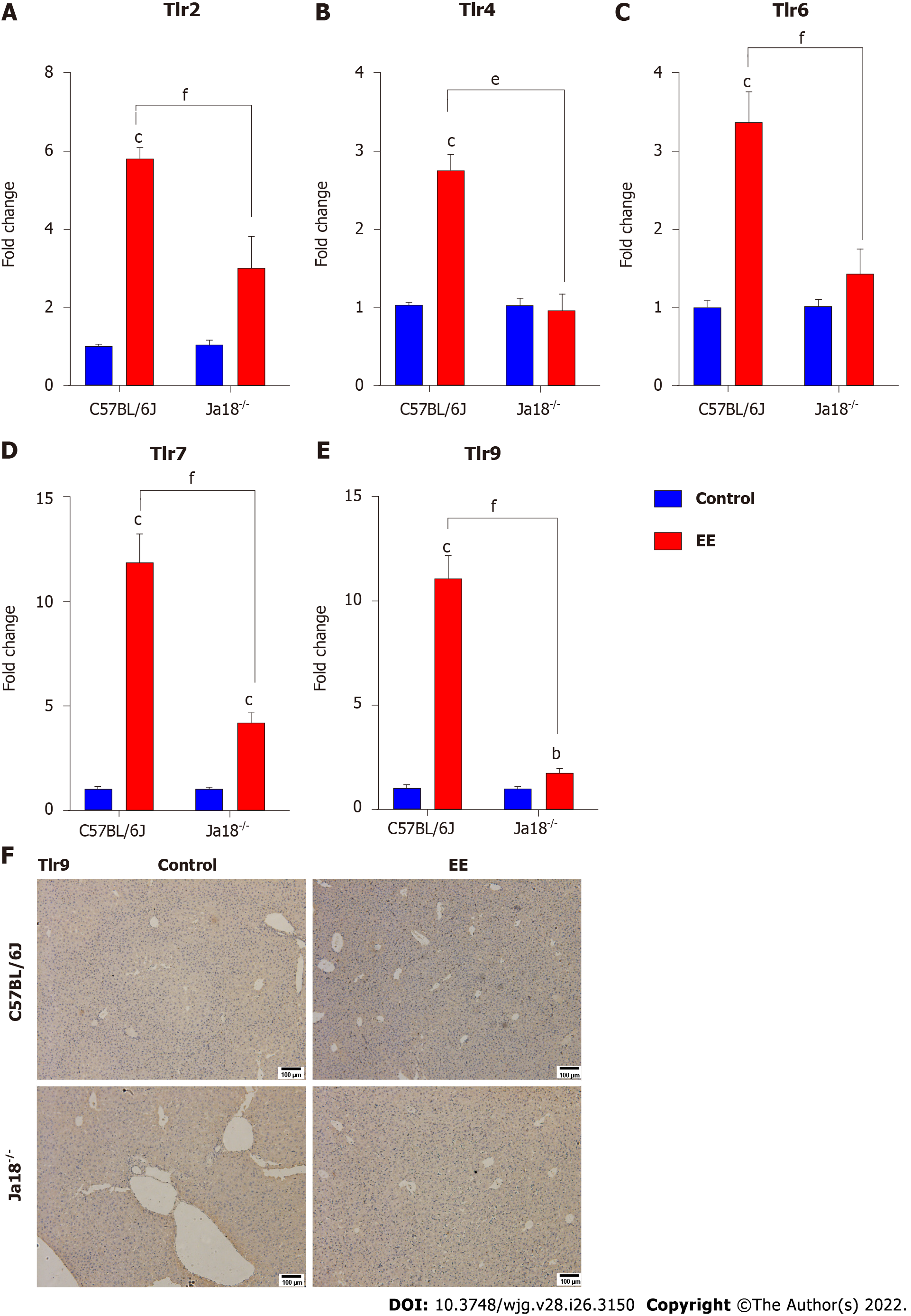
Accumulating evidence suggests that TLRs contribute to the development of hepatobiliary damage[23]. TLRs have also been reported to contribute to the activation and subsequent cytokine production of NKT cells[24]. Intriguingly, iNKT cells have been reported to express certain TLRs, some of which are functional[25]. In the present study, TLRs associated with NKT-cell activation or liver disease were measured and analyzed. The results showed that the mRNA expression levels of Tlr2, Tlr4, Tlr6, Tlr7, and Tlr9 were significantly upregulated after EE administration. Deficiency of iNKT cells significantly downregulated the expression of the above TLRs compared with that in C57BL/6J mice (Figure 4), which suggested that the absence of iNKT cells may influence the expression of TLRs. The hepatic immunohistochemistry results of TLR9 showed a trend similar to that of mRNA (Figure 4F).
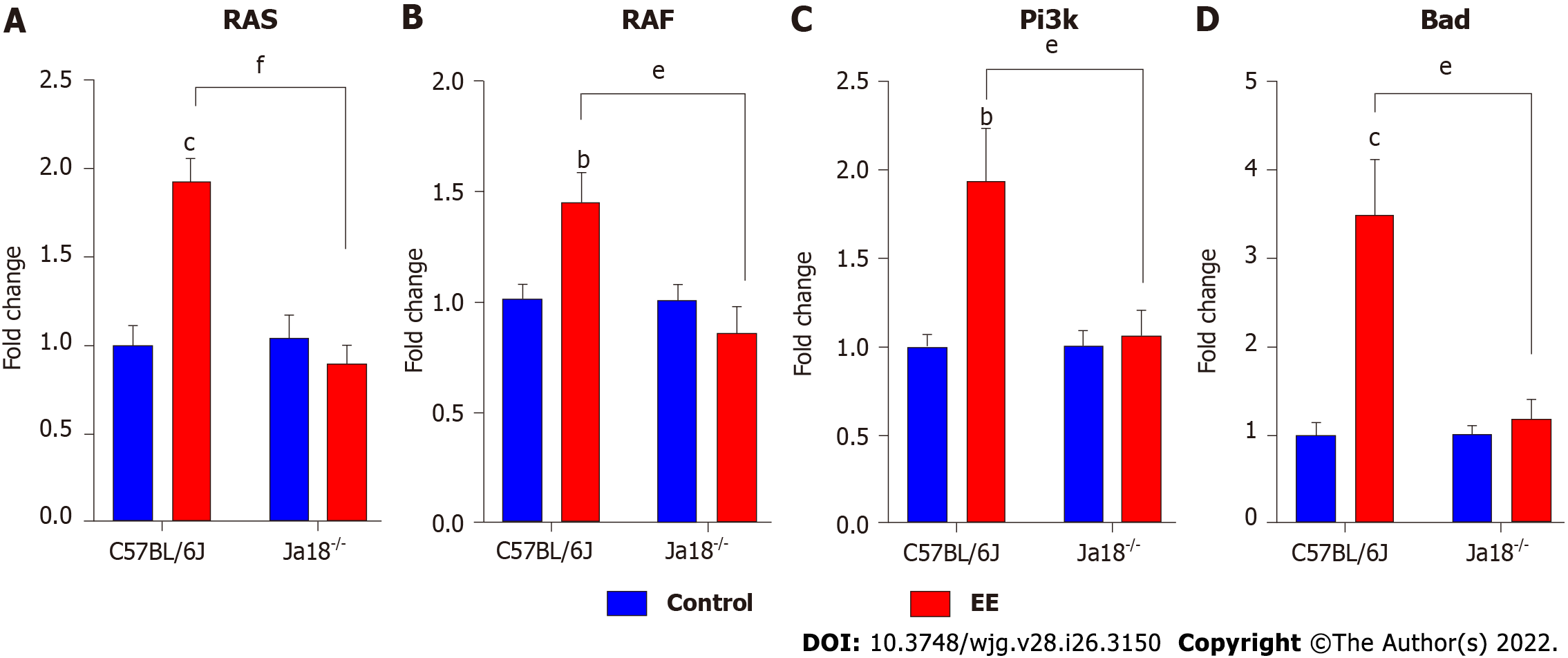
TLRs or T cell receptors (TCRs) can stimulate T cells, leading to MAPK signaling pathway activation, which is required for the effects of T cells[26]. iNKT cell activation by TCR stimulation has also been reported to be mediated by ERK and p38 MAPK[27]. In the present study, the expression of both MAPK and PI3K signaling was investigated after EE administration in C57BL/6J and Jα18-/- mice. The hepatic mRNA expression levels of MAPK upstream Ras and Raf as well as Pi3k and its downstream Bad, which might be associated with TLR activation, were increased in C57BL/6J mice after EE administration. The above mRNA levels were remarkably inhibited in Jα18-/- mice compared with C57BL/6J mice, which suggested that the absence of iNKT cells changes the expression of MAPK and PI3K signaling pathways (Figure 5).
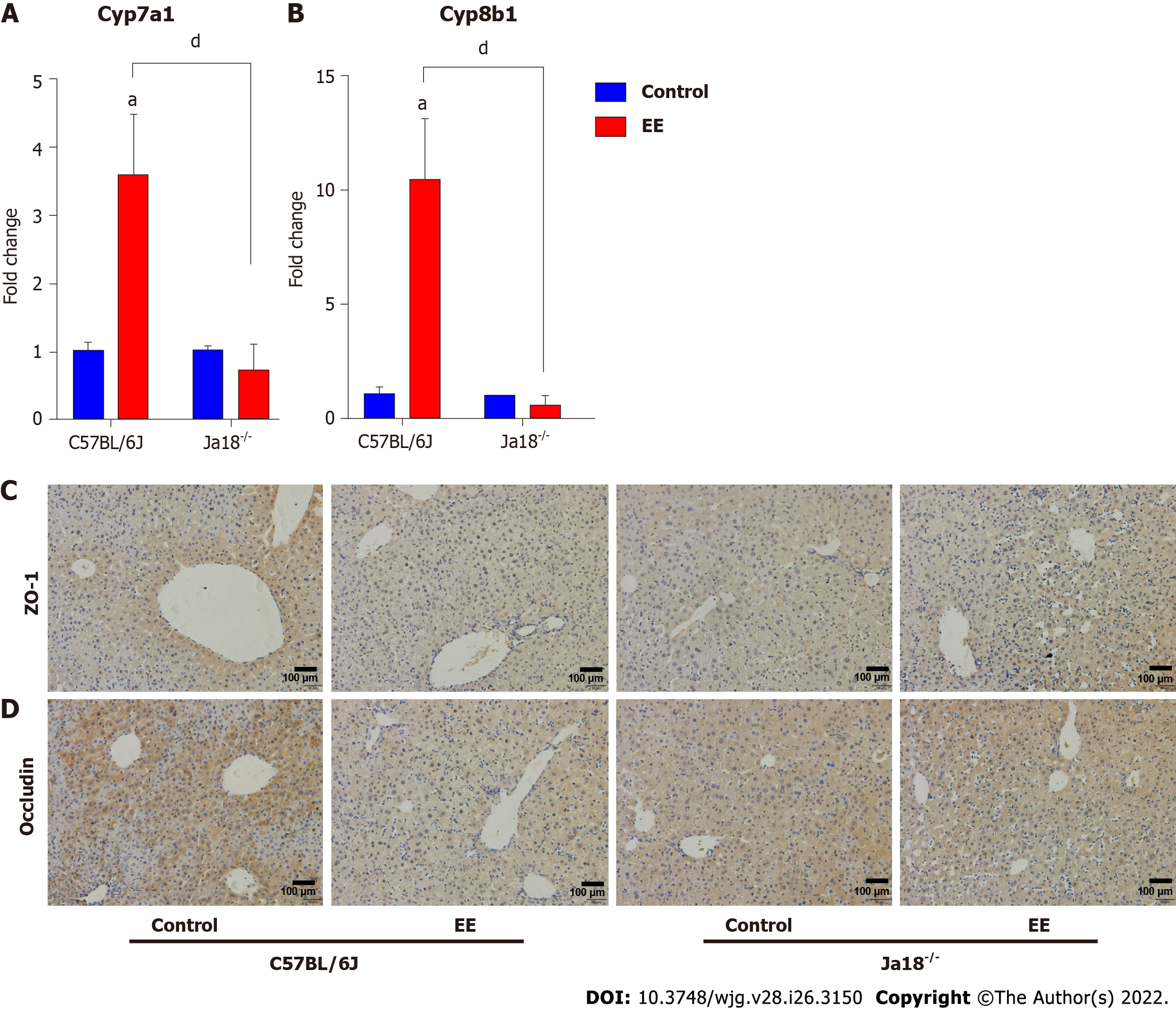
Dysregulated bile acid homeostasis may be attributed to the disorder of bile acid synthesis/metabolism and/or the dysfunction of tight junctions (TJs)[28]. To investigate the possible mechanism of the alleviation of cholestatic liver injury in NKT-cell knockout mice, the levels of CYP450 synthase and TJs were detected. The mRNA levels of Cyp7a1 and Cyp8b1 were upregulated after EE administration in C57BL/6J mice, whereas a nonsignificant difference was shown in Jα18-/- mice. In comparison to C57BL/6J mice, the mRNA expression of Cyp7a1 and Cyp8b1 was inhibited in Jα18-/- mice after EE administration, indicating the relatively reduced synthesis of bile acid (Figure 6A and B). The IHC results showed that, in comparison to the control group, fewer cells positively stained for ZO-1 or Occludin after EE treatment in C57BL/6J mice, and positive cells remained unaltered between the control and EE groups in Jα18-/- mice (Figure 6C and D). These data suggested that knockout of iNKT cells can inhibit bile acid synthase and weaken the inhibition of hepatocyte polarity, thereby reducing cholestatic liver injury.
As the most common hepatic disorder, the principal pathogenic factors of EE-induced IHC include estrogen and toxic bile acid, both of which are correlated with immune imbalance. Increased activation status of NKT cells, T cells, or NK cells has been reported to contribute to the pathophysiology of cholestatic diseases[15,19,20]. Therefore, the percentages of hepatic NKT cells, CD3+ T cells, and NK cells along with their Th1/Th2 cytokine secretion and bias were compared in the present study. Surprisingly, in EE-induced cholestasis, only the NKT-cell percentage and its Th1/Th2 cytokine secretion and ratio significantly increased, indicating their activation and Th1-biased immune response. The percentage of CD3+ T cells and NK cells, along with their secretion of Th1 cytokines, remained unaltered. Th2 cytokines from CD3+ T cells and NK cells showed a rising trend, which did not seem to affect the Th1/Th2 balance (Figure 1). Our results revealed that both Th1 and Th2 cytokines existed in the liver, consistent with previous studies[14,29], and the Th1 bias was mainly contributed by NKT cells. To further confirm the effect of NKT cells in EE-induced cholestasis, cholestatic liver damage was compared between iNKT cell-deficient mice and C57BL/6J mice after EE administration. Jα18-/- mice showed significantly reduced blood biochemistry parameters, including levels of ALP, TBA, ALT, and AST, and alleviated liver histopathological changes, suggesting that NKT cells have a harmful effect on EE-induced cholestasis. NKT cells have been accepted as a key connection between innate and adaptive immunity. Upon activation, NKT cells can activate and influence most innate and adaptive immune cells by rapid and abundant cytokine production[30,31]. NKT cells, whose role is noteworthy in cholestasis, are mostly abundant in the liver rather than other organs. In the PBC mouse model, NKT cells are highly activated and secrete enormous amounts of IFN-γ. In comparison to mice with PBC, cholangitis and liver inflammation are significantly alleviated in mice with NKT-cell deficiency[32]. Our previous investigations also found that iNKT cells and their cytokines aggravate ANIT- and triptolide-induced cholestasis by disrupting bile acid homeostasis[14,15]. Although NKT cells can be protective by suppressing neutrophils in an extrahepatic cholestasis model[16], our research data suggest the harmful effects of NKT cells in EE-induced cholestasis. The above studies indicate that the role of NKT cells may vary in different diseases and models.
Under physiological conditions, CXCR6/CXCL16 regulate the migration of NKT cells in hepatic sinusoids[33]. Under the early pathologic states of chronic liver damage, they specifically mediate the hepatic accumulation of NKT cells, exacerbating hepatic inflammation and promoting fibrogenesis[22]. CXCL16 can be expressed in hepatobiliary tissues of patients with hepatopathy[34] but also in mouse liver sinusoidal endothelial cells triggered by intestinal microorganism-induced bile acids, indicating that its expression is related to hepatobiliary damage[33,35]. Our results showed that the hepatic gene levels of Cxcr6 and Cxcl16 were upregulated (Figure 3). Inflammatory cytokines, such as IFN-γ, which was increased in the present model, are capable of further inducing CXCL16 expression, forming a positive regulation loop[36]. In the present study, iNKT cell deficiency inhibited the mRNA levels of Cxcr6 and Cxcl16, which may be linked to the absence of hepatic infiltration of NKT cells (Figure 3).
Activation of iNKT cells can be caused by direct interaction with TCR or indirect effects of TLR agonists and/or IL-12[37]. TLRs, which are widely expressed in the liver, can stimulate hepatic inflammation and actively participate in the initiation and development of liver damage under pathological conditions[38,39]. Lipopolysaccharide (LPS), a specific TLR4 Ligand, activates hepatic iNKT cells and leads to their secretion of IL-4 within 2 h in vivo or in vitro, demonstrating that TLR4 is expressed and functional in iNKT cells[40]. In alcoholic liver disease, TLR4 inhibition results in drastically reduced levels of hepatic proinflammatory mediators[41]. TLR4 deletion nearly eliminates inflammatory cell infiltration in the liver and hepatocyte injury in a murine model of ischemia reperfusion[42]. In EE-induced cholestatic liver injury, hepatic mRNA levels of Tlr2, 4, 6, 7, and 9 were markedly increased (Figure 4). CD1d knockout mice (lacking NKT cells) exhibit enhanced (> 4-fold) proinflammatory cytokine secretion and higher mRNA levels of TLR4 in the kidney of a nonalcoholic fatty liver disease model[43]. In the present study, iNKT cell deficiency significantly downregulated the expression of the above TLRs compared with C57BL/6J mice (Figure 4), suggesting the effect of iNKT cells on the expression of TLRs. NKT cells have been found to express certain functional TLRs[24]. The IHC results of TLR9 showed a similar trend as the mRNA levels (Figure 4F). In a murine autoimmune hepatitis model induced by concanavalin A, NKT cells infiltrate and are activated, promoted by TLR9 stimulation, thereby leading to aggravated hepatotoxicity[44]. The PI3K/Akt and Ras/Raf/MEK/ERK signaling pathways are considered to be activated and function following inflammation[45], which further upregulates proinflammatory mediators in cholestasis[46]. Our data demonstrated that the mRNA levels of the downstream pathways Ras/Raf and Pi3k/Bad were also upregulated, while iNKT cell deficiency suppressed their upregulation (Figure 5). These results indicated that iNKT cell deletion affected TLRs and their downstream Ras/Raf and PI3K/Bad signaling.
Growing numbers of studies have reported that hepatocyte TJs, constituting hepatocyte polarization, play a pivotal role in the maintenance of the epithelial barrier and permeability; therefore, their structural disruption can lead to the leakage of bile components into blood circulation, bile acid homeostasis disorder, and cholestatic liver injury[47]. TJ damage can be found in obstructive jaundice patients[48] and murine cholestatic models of ANIT[28], carbon tetrachloride[49], BDL[50], and EE[51]. Inflammation has been associated with structural and functional alterations of hepatic TJs[52]. In the present study, the results showed that iNKT cell-deficient mice ameliorated disorders of bile acid synthesis and TJs (Figure 6) and restored previously deteriorated bile acid homeostasis and hepatocyte barrier function, contributing to the mitigation of EE-induced cholestatic hepatotoxicity.
In EE-induced cholestatic hepatotoxicity, EE promoted hepatic NKT-cell proliferation and activation, which contributed to Th1 cytokine bias and influenced the liver immune microenvironment. EE also upregulated the expression of Cxcr6/Cxcl16, TLRs, and downstream Ras/Rad and PI3K/Bad signaling. Moreover, EE influenced the levels of the bile acid synthase Cyp7a1 and Cyp8b1 and the TJs ZO-1 and Occludin. iNKT cell deficiency significantly alleviated cholestatic liver damage and downregulated the abovementioned signaling pathways, indicating the pathogenic effects of hepatic iNKT cells in EE-induced cholestatic liver damage. It is noteworthy that mouse and human NKT cells share similar functions, including the killing of tumor/infected cells by cytotoxicity and their crucial role in autoimmunity[53]. Therefore, regulation of NKT-cell activation may serve as a potential therapeutic strategy with clinical implications for cholestatic diseases.
Cholestasis is mild and common during liver diseases but is also a crucial triggering element of severe hepatopathy. As the predominant component of oral contraceptives (OCs) and hormone replacement therapy, 17α-ethinylestradiol (EE) is used as a model drug to induce murine intrahepatic cholestasis (IHC). The clinical counterpart of EE-induced IHC includes women who are taking OCs, postmenopausal replacement therapy, and susceptible pregnant women.
The significance of the local immune microenvironment in the liver has been emphasized because estrogens are immunomodulators that are metabolized in the liver.
The aim of the present study was to investigate the effects and mechanisms of natural killer T (NKT) cells in a murine model of EE-induced cholestatic hepatotoxicity.
Male C57BL/6J mice or invariant NKT (iNKT) cell deficiency (Jα18-/- mice) were administered with EE (10 mg/kg, subcutaneous) for 14 d.
Both Th1 and Th2 cytokines produced by NKT cells increased in the liver skewing toward a Th1 bias. The expression of the chemokine/chemokine receptor Cxcr6/Cxcl16, toll-like receptors, Ras/Rad, and PI3K/Bad signaling was upregulated after EE administration. EE also influenced bile acid synthase Cyp7a1, Cyp8b1, and tight junctions ZO-1 and Occludin, which might be associated with EE-induced cholestasis. iNKT cell deficiency (Jα18-/- mice) robustly alleviated cholestatic liver damage and lowered the expression of the abovementioned signaling pathways.
The present study demonstrated that hepatic NKT cells play a pathogenic role in EE-induced intrahepatic cholestasis, contributing to the development of the IHC mechanisms and the potential treatment targeting NKT cells.
Hepatic NKT cells and their Th1 cytokine production play a pathogenic role in a 14-d murine model of EE-induced intrahepatic cholestasis.
The authors would like to acknowledge Li Bai (University of Science and Technology of China) for kindly providing iNKT cell-deficient (Jα18-/- mice).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Toxicology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan A-Editor: Lin FY, China S-Editor: Zhang H L-Editor: A P-Editor: Guo X
| 1. | Xiang D, Xu Y, He W, Yang J, Zhang C, Liu D. Bioinformatics-based identification of key pathways and candidate genes for estrogeninduced intrahepatic cholestasis using DNA microarray analysis. Mol Med Rep. 2019;20:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Estrogens and Oral Contraceptives. . [PubMed] |
| 3. | McIlvride S, Dixon PH, Williamson C. Bile acids and gestation. Mol Aspects Med. 2017;56:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Reyes H, Ribalta J, González MC, Segovia N, Oberhauser E. Sulfobromophthalein clearance tests before and after ethinyl estradiol administration, in women and men with familial history of intrahepatic cholestasis of pregnancy. Gastroenterology. 1981;81:226-231. [PubMed] |
| 5. | Li X, Liu R, Luo L, Yu L, Chen X, Sun L, Wang T, Hylemon PB, Zhou H, Jiang Z, Zhang L. Role of AMP-activated protein kinase α1 in 17α-ethinylestradiol-induced cholestasis in rats. Arch Toxicol. 2017;91:481-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006;281:16625-16631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Wang J, Fu T, Dong R, Wang C, Liu K, Sun H, Huo X, Ma X, Yang X, Meng Q. Hepatoprotection of auraptene from the peels of citrus fruits against 17α-ethinylestradiol-induced cholestasis in mice by activating farnesoid X receptor. Food Funct. 2019;10:3839-3850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Yu L, Liu X, Li X, Yuan Z, Yang H, Zhang L, Jiang Z. Protective effects of SRT1720 via the HNF1α/FXR signalling pathway and anti-inflammatory mechanisms in mice with estrogen-induced cholestatic liver injury. Toxicol Lett. 2016;264:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Milona A, Owen BM, Cobbold JF, Willemsen EC, Cox IJ, Boudjelal M, Cairns W, Schoonjans K, Taylor-Robinson SD, Klomp LW, Parker MG, White R, van Mil SW, Williamson C. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology. 2010;52:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Jansen PL, Ghallab A, Vartak N, Reif R, Schaap FG, Hampe J, Hengstler JG. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Peng B, Liu S. [Study of relationship between T helper cell type-1 and type-2 cytokines and intrahepatic cholestasis of pregnancy]. Zhonghua Fu Chan Ke Za Zhi. 2002;37:516-518. [PubMed] |
| 12. | Jin F, Cheng D, Tao JY, Zhang SL, Pang R, Guo YJ, Ye P, Dong JH, Zhao L. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol. 2013;13:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Zou M, Wang A, Wei J, Cai H, Yu Z, Zhang L, Wang X. An insight into the mechanism and molecular basis of dysfunctional immune response involved in cholestasis. Int Immunopharmacol. 2021;92:107328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Nong C, Zou M, Xue R, Bai L, Liu L, Jiang Z, Sun L, Huang X, Zhang L, Wang X. The role of invariant natural killer T cells in experimental xenobiotic-induced cholestatic hepatotoxicity. Biomed Pharmacother. 2020;122:109579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Zou M, Nong C, Yu Z, Cai H, Jiang Z, Xue R, Huang X, Sun L, Zhang L, Wang X. The role of invariant natural killer T cells and associated immunoregulatory factors in triptolide-induced cholestatic liver injury. Food Chem Toxicol. 2020;146:111777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Duwaerts CC, Sun EP, Cheng CW, van Rooijen N, Gregory SH. Cross-activating invariant NKT cells and kupffer cells suppress cholestatic liver injury in a mouse model of biliary obstruction. PLoS One. 2013;8:e79702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Mencarelli A, Renga B, Migliorati M, Cipriani S, Distrutti E, Santucci L, Fiorucci S. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol. 2009;183:6657-6666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Valestrand L, Berntsen NL, Zheng F, Schrumpf E, Hansen SH, Karlsen TH, Blumberg RS, Hov JR, Jiang X, Melum E. Lipid antigens in bile from patients with chronic liver diseases activate natural killer T cells. Clin Exp Immunol. 2021;203:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Sun K, Ma S, Tian S, Zhang M, Liu Y, Li B, Zhou X, Zheng X, Wang L, Han Y. An enhanced level of LAMP-2A participates in CD4+T cell hyperactivity in patients with primary biliary cholangitis. Ann Transl Med. 2021;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hydes TJ, Blunt MD, Naftel J, Vallejo AF, Seumois G, Wang A, Vijayanand P, Polak ME, Khakoo SI. Constitutive Activation of Natural Killer Cells in Primary Biliary Cholangitis. Front Immunol. 2019;10:2633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Zhang YY, Zhang CX, Li Y, Jiang X, Wang YF, Sun Y, Wang J, Ji WY, Liu Y. Development of a novel rat model of heterogeneous hepatic injury by injection with colchicine via the splenic vein. World J Gastroenterol. 2018;24:5005-5012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, Zimmermann HW, Pack O, Gassler N, Hittatiya K, Ludwig A, Luedde T, Trautwein C, Tacke F. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol. 2013;190:5226-5236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Liu W, Jing ZT, Xue CR, Wu SX, Chen WN, Lin XJ, Lin X. PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019;381:114729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Kulkarni RR, Villanueva AI, Read LR, Brisbin JT, Bhaumik SK, LaMarre J, Murali-Krishna K, Sharif S. CpG oligonucleotide-mediated co-stimulation of mouse invariant natural killer T cells negatively regulates their activation status. Cell Tissue Res. 2017;369:541-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Kulkarni R, Behboudi S, Sharif S. Insights into the role of Toll-like receptors in modulation of T cell responses. Cell Tissue Res. 2011;343:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1330] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 27. | Stuart JK, Bisch SP, Leon-Ponte M, Hayatsu J, Mazzuca DM, Maleki Vareki S, Haeryfar SM. Negative modulation of invariant natural killer T cell responses to glycolipid antigens by p38 MAP kinase. Int Immunopharmacol. 2010;10:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Yang T, Wang X, Zhou Y, Yu Q, Heng C, Yang H, Yuan Z, Miao Y, Chai Y, Wu Z, Sun L, Huang X, Liu B, Jiang Z, Zhang L. SEW2871 attenuates ANIT-induced hepatotoxicity by protecting liver barrier function via sphingosine 1-phosphate receptor-1-mediated AMPK signaling pathway. Cell Biol Toxicol. 2021;37:595-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Caielli S, Conforti-Andreoni C, Di Pietro C, Usuelli V, Badami E, Malosio ML, Falcone M. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J Immunol. 2010;185:7317-7329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Zeissig S, Blumberg RS. Analyzing Antigen Recognition by Natural Killer T Cells. Methods Mol Biol. 2019;1988:439-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, Kronenberg M, Flavell RA, Gao B, Gershwin ME. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 532] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 34. | Heydtmann M, Lalor PF, Eksteen JA, Hübscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1037] [Article Influence: 148.1] [Reference Citation Analysis (0)] |
| 36. | Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362-6372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 37. | Anderson CK, Reilly SP, Brossay L. The Invariant NKT Cell Response Has Differential Signaling Requirements during Antigen-Dependent and Antigen-Independent Activation. J Immunol. 2021;206:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J Hepatol. 2016;8:1354-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 39. | Chu AJ. Antagonism by bioactive polyphenols against inflammation: a systematic view. Inflamm Allergy Drug Targets. 2014;13:34-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Askenase PW, Itakura A, Leite-de-Moraes MC, Lisbonne M, Roongapinun S, Goldstein DR, Szczepanik M. TLR-dependent IL-4 production by invariant Valpha14+Jalpha18+ NKT cells to initiate contact sensitivity in vivo. J Immunol. 2005;175:6390-6401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Ishida K, Kaji K, Sato S, Ogawa H, Takagi H, Takaya H, Kawaratani H, Moriya K, Namisaki T, Akahane T, Yoshiji H. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem. 2021;89:108573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 42. | Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, Najafian N, Kupiec-Weglinski JW. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Alhasson F, Dattaroy D, Das S, Chandrashekaran V, Seth RK, Schnellmann RG, Chatterjee S. NKT cell modulates NAFLD potentiation of metabolic oxidative stress-induced mesangial cell activation and proximal tubular toxicity. Am J Physiol Renal Physiol. 2016;310:F85-F101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Jiang W, Sun R, Zhou R, Wei H, Tian Z. TLR-9 activation aggravates concanavalin A-induced hepatitis via promoting accumulation and activation of liver CD4+ NKT cells. J Immunol. 2009;182:3768-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Xiang D, Yang J, Xu Y, Lan L, Li G, Zhang C, Liu D. Estrogen cholestasis induces gut and liver injury in rats involving in activating PI3K/Akt and MAPK signaling pathways. Life Sci. 2021;276:119367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 48. | Assimakopoulos SF, Tsamandas AC, Louvros E, Vagianos CE, Nikolopoulou VN, Thomopoulos KC, Charonis A, Scopa CD. Intestinal epithelial cell proliferation, apoptosis and expression of tight junction proteins in patients with obstructive jaundice. Eur J Clin Invest. 2011;41:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Horikoshi Y, Kitatani K, Toriumi K, Fukunishi N, Itoh Y, Nakamura N, Ohno S, Matsura T, Takekoshi S. Aberrant activation of atypical protein kinase C in carbon tetrachloride-induced oxidative stress provokes a disturbance of cell polarity and sealing of bile canalicular lumen. Am J Pathol. 2015;185:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Fallon MB, Mennone A, Anderson JM. Altered expression and localization of the tight junction protein ZO-1 after common bile duct ligation. Am J Physiol. 1993;264:C1439-C1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Landmann L. Cholestasis-induced alterations of the trans- and paracellular pathways in rat hepatocytes. Histochem Cell Biol. 1995;103:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126-G136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, Juji T, Hirai H. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2002;168:3140-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |