Published online Jun 28, 2022. doi: 10.3748/wjg.v28.i24.2748
Peer-review started: January 16, 2022
First decision: March 8, 2022
Revised: March 14, 2022
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: June 28, 2022
Processing time: 159 Days and 10.5 Hours
Accurate diagnosis of colorectal premalignant polyps, including adenomas, is vital in clinical practice.
To investigate the diagnostic yields of novel findings of brown slits for adenomas.
Patients who underwent colonoscopy at the Toyoshima Endoscopy Clinic were enrolled. Polyps sized ≥ 5 mm suspected of adenomas or clinically significant serrated polyps were included in the study. We defined the surface structures of colorectal polyps, which were brown curves inside and along the tubular glands identified using a combination of a new X1 system (Olympus Corporation) and a conventional magnifying colonoscope with non-staining narrow band imaging (NBI), as brown slits. The brown slits corresponded to slit-like lumens on endocytoscopy and histological crypt openings of an adenoma. We evaluated the diagnostic performance of brown slits for adenoma.
A total of 108 Lesions from 62 patients were eligible. The average age was 60.4 years and 41.9% were male. The mean polyp size was 7.45 ± 2.83 mm. Fifty-seven lesions were positive for brown slits. Histopathological diagnosis comprised 59 low-grade tubular adenomas, 16 sessile serrated lesions, and 33 hyperplastic polyps. Among 59 adenomas, 56 (94.9%) were positive for brown slits. Among 16 sessile serrated lesions, 0 (0%) was positive for brown slits. Among 33 hyperplastic polyps, 1 (3.0%) was positive for brown slits. The sensitivity, specificity, and accuracy of brown slits for adenoma were 94.9%, 98.0%, and 96.3%, respectively. The positive predictive value and negative predictive value of brown slits for adenoma were also excellent for 98.2%, and 94.1%, respectively.
Brown slits on conventional magnifying endoscopy with non-staining NBI using the X1 system were useful for diagnosing colorectal adenoma. The new endoscopy system could be examined using new standards.
Core Tip: We defined the polyp’s surface structure observed on conventional magnifying endoscopy with non-staining narrow band imaging using the X1 system as brown slits. The brown slits corresponded to slit-like lumens on endocytoscopy and histological crypt openings of an adenoma. Brown slits were useful for diagnosing colorectal adenoma.
- Citation: Toyoshima O, Nishizawa T, Yoshida S, Watanabe H, Odawara N, Sakitani K, Arano T, Takiyama H, Kobayashi H, Kogure H, Fujishiro M. Brown slits for colorectal adenoma crypts on conventional magnifying endoscopy with narrow band imaging using the X1 system. World J Gastroenterol 2022; 28(24): 2748-2757
- URL: https://www.wjgnet.com/1007-9327/full/v28/i24/2748.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i24.2748
Colorectal adenoma is a premalignant neoplasm composed of a dysplastic epithelium. Removal of adenomas reduces the risk of colorectal adenocarcinoma[1,2]. Colorectal adenomas are found in ≥ 40% of proper colonoscopies, given that an accurate diagnosis of adenoma is crucial in routine endoscopic practice[3-6]. Colorectal serrated polyps are precursors with different molecular pathways and biological behaviors from adenomas[2,7,8]. Clinically significant serrated polyps (CSSPs), comprising all sessile serrated lesions (SSLs), all traditional serrated adenomas (TSAs), hyperplastic polyps ≥ 10 mm in size anywhere in the colorectum, and hyperplastic polyps ≥ 5 mm in size between the cecum and descending colon are recommended to be endoscopically removed[9-12]. The number, size, grade of dysplasia, and histological subtype of premalignant polyps, including both adenomas and serrated polyps, are the best determinants of the long-term risk of advanced neoplasia and inform surveillance decision-making[7,13-16].
Magnification, dye sprays, and modified light wavelengths, such as narrow band imaging (NBI) and blue laser imaging (BLI), are used to diagnose premalignant polyps better. The criteria for conventional magnifying endoscopy include pit pattern diagnosis using chromoendoscopy[17] and the Japan NBI expert team (JNET) classification using NBI and BLI[18-20]. The standards for ultra-high magnifying endocytoscopy (EC) are as follows: EC classification, which evaluates the glandular structure and cellular atypia after double staining with methylene blue and crystal violet[21-24], and EC vascular classification, which evaluates the microvessels using NBI[25,26]. Slit-like lumens and microvessels with clear margins showing a network are good indicators of adenoma in EC, whereas narrow serrated lumens and obscure microvessels predict hyperplastic polyps. Sasajima et al[21] and Mori et al[23] have elucidated that long tubular pits (slit-like lumens) on EC are histologically equivalent to the crypt opening using the horizontal and vertical sections of specimens, respectively.
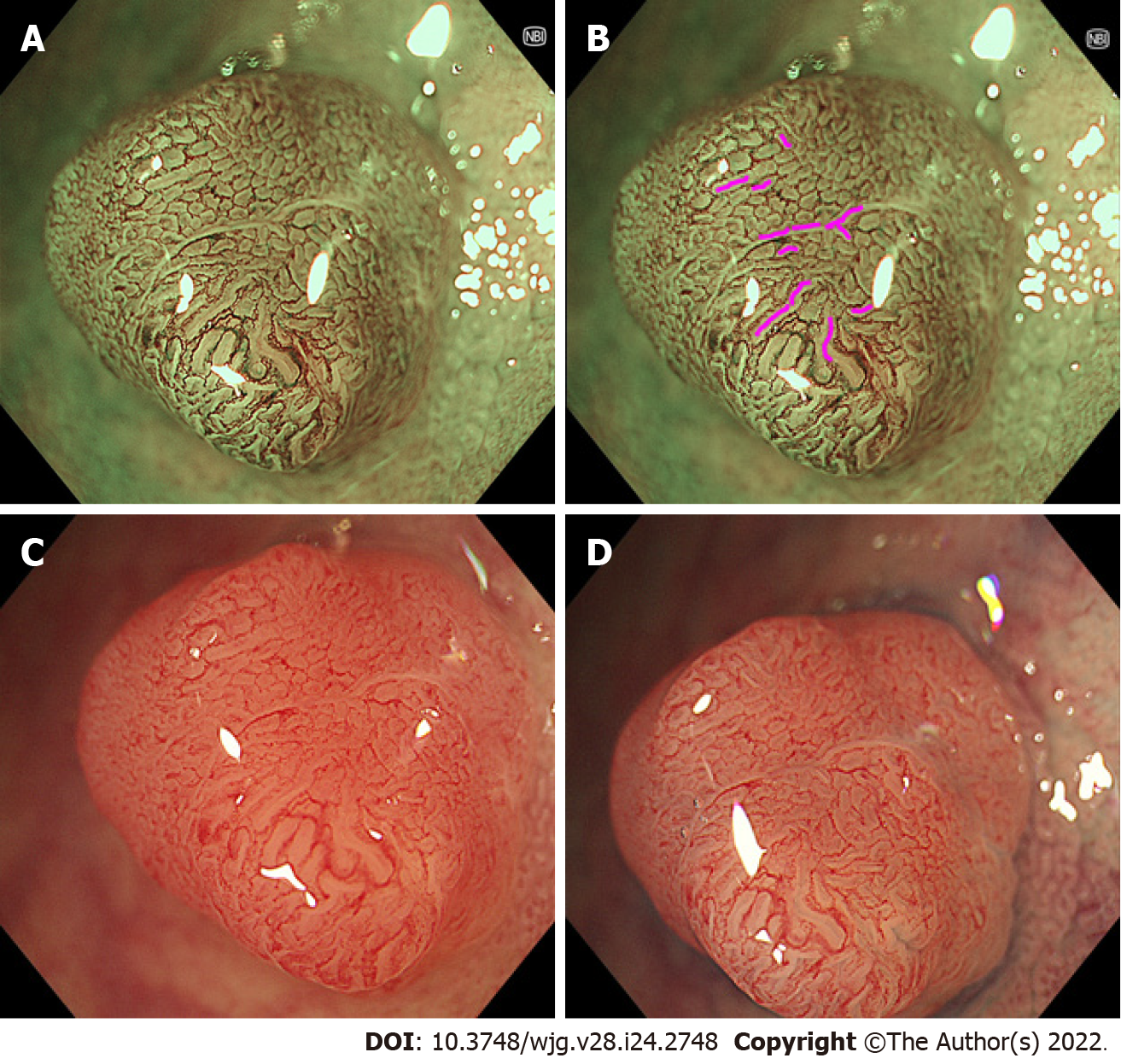
In 2020, a novel endoscopic system, which is a set of an EVIS X1 Video System Center (CV-1500) and a 4K ultra-high definition 32-inch liquid crystal display (LCD) monitor (OEV321UH), was released by Olympus Corporation, Tokyo, Japan. When using the same scope, this system has higher image quality and higher magnification than the previous generation’s Olympus system, which is a set of EVIS Elite Video System Center (CV-290) and a high-definition 26-inch LCD monitor (OEV262H). For example, the magnifications of conventional magnifying endoscopes of Olympus’ CF-HQ290Z and PCF-H290Z were 80 × and 110 ×, respectively, using the Elite system, while the magnifications increased to 95 × and 170 ×, respectively, using the X1 system. Given this, we hypothesized that the new X1 system allows conventional magnifying endoscopy to visualize the structures that could only be observed with EC, and to improve polyp diagnosis. Using a combination of the X1 system and the conventional magnifying endoscope with non-staining NBI, we identified the findings corresponding to the slit-like lumens, which present the crypts of adenoma. Then, we defined the findings as “brown slits” (Figure 1A and B).
To the best of our knowledge, there are no studies in which conventional magnifying endoscopy has verified the evidence accumulated by EC. Therefore, we focused on brown slits detected on conventional magnifying endoscopy using the X1 system and investigated their performance for adenoma diagnosis.
This retrospective study was conducted at the Toyoshima Endoscopy Clinic, a representative outpatient endoscopy-specialized clinic in Japan. This study was conducted in accordance with the ethical guidelines for medical studies in Japan. Written informed consent to use clinical information was obtained from the patients before colonoscopy. The study design was described in a protocol prepared by the Toyoshima Endoscopy Clinic and the Sakitani Endoscopy Clinic, and was approved by the Certified Institutional Review Board, Yoyogi Mental Clinic on July 16, 2021 (approval No. RKK227). We published this study’s protocol on our institute’s website (www.ichou.com) so that patients could opt out of the study if desired. All clinical investigations were performed in accordance with the ethical guidelines of the Declaration of Helsinki.
We enrolled patients who underwent colonoscopy at the Toyoshima Endoscopy Clinic in May and June 2021. Polyps sized ≥ 5 mm suspected of adenomas or CSSPs were included in this study[23,27,28]. The exclusion criteria were ulcerative colitis, poor bowel preparation, lesions for which endoscopic imaging could not be obtained, and lesions for which the specimen could not be collected. We also excluded histologically diagnosed adenocarcinomas, TSAs, and normal mucosa.
Figure 1 shows typical images of a tubular adenoma observed using a combination of the conventional magnifying endoscope (CF-HQ290Z) and the X1 system, at full zoom magnification with NBI and WLI with or without indigo carmine spraying. Brown slits inside and along the tubular glands surrounded by microvessels were identified (pink curves in Figure 1B). We defined this brown pattern as a brown slit. The brown slit was additionally defined as having a length longer than the width of the tubular gland (approximately 100 µm). The brown slits were visible as faint and pale red slits on WLI (Figure 1C). Indigo carmine accumulated in the crypt openings that corresponded to brown slits (Figure 1A, B and D).
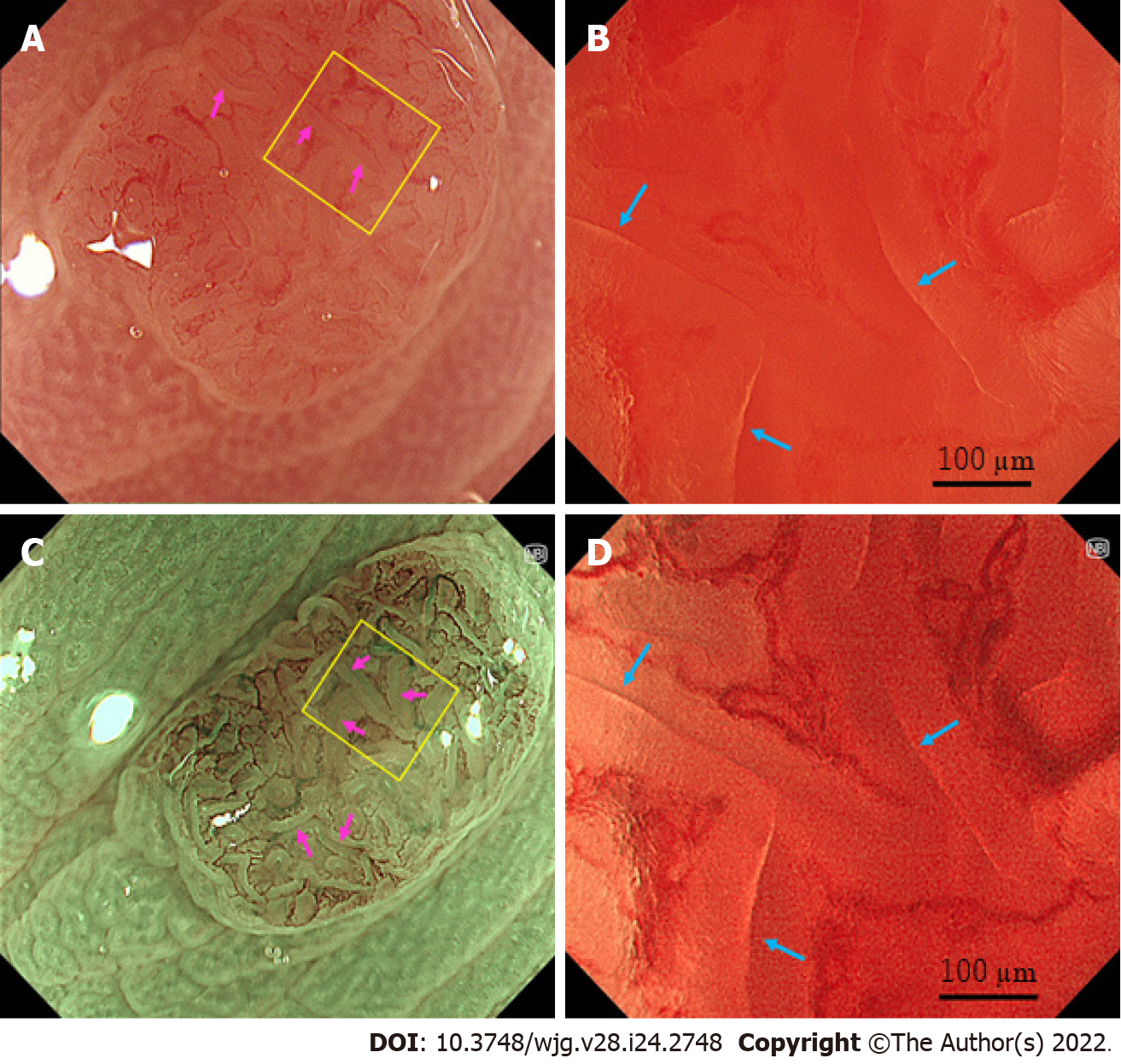
Figure 2 shows representative images of a tubular adenoma using a combination of the endocytoscope (CF-H290EC, Olympus Corporation) and the X1 system. To obtain a fully zoomed image with 790× magnification with a focusing depth of 35 µm, the endoscopist softly contacted the lesion with the endoscope lens[26]. On ultra-high magnifying observation with NBI, slits with a mixture of brown and white colors inside and along the tubular glands were visualized (Figure 2D, blue arrows). This tubular structure corresponded to a slit-like lumen for staining EC[22-25]. The microvessels were clearly identified surrounding the tubular gland and showed a vessel network[25]. On ultra-high magnifying observation with white light imaging (WLI), although microvessels were obscure, slits with a mixture of red and white colors were seen similar to the slits on NBI observation (Figure 2B, blue arrows). We could recognize tubular glands surrounded by the microvessels and brown and pale red slits inside and along the tubular glands with NBI and WLI, respectively, when we observed with approximately 100 × magnification, which was similar to the full zoom of conventional magnifying endoscopy (Figure 2A and C, pink arrows).
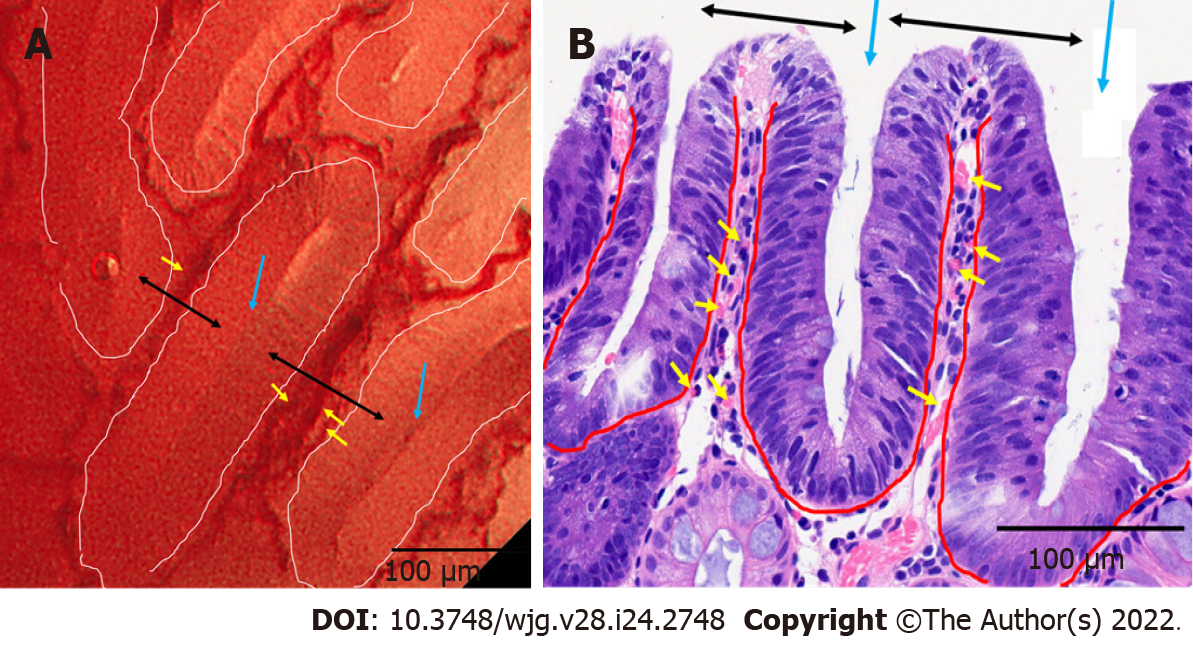
The correspondence between EC and histology is presented in Figure 3. Variably shaped tubular glands (surrounded by white and red curves in Figures 3A and B, respectively) with homogeneous widths of approximately 100 µm were observed. The crypts (blue arrows in Figure 3A and B) were detected as brown and white slits inside and along the tubular glands on EC. The microvessels (yellow arrows in Figure 3) were observed in the intervening part (black arrows in Figure 3). The size and position of the crypt, intervening part, and microvessels were consistent between EC and histology.
From the above, the brown slits on conventional magnifying endoscopy with NBI corresponded to slit-like lumens on EC and crypt openings on histology.
Brown slits were diagnosed in real-time during colonoscopy. Before the colonoscopy, the endoscopists discussed and obtained consensus for the brown slits while viewing the images on non-magnifying, conventional magnifying, and ultra-high magnifying observation. Two expert endoscopists conducted all colonoscopies. An endoscopic set of the X1 system and CF-HQ290Z or PCF-H290Z was used. Colonoscopes without a magnifying function and those with an autofocus function were excluded. The optical enhancement of the NBI was set in mode A8. When a lesion was detected, the surface was washed to remove mucus and dye, and a non-magnifying observation was conducted. Subsequently, the endoscopists focused on and diagnosed brown slits at almost full zoom. The polyp was observed without indigo carmine staining. Location, size, and morphology according to the Paris classification, the presence or absence of brown slits, and endoscopic polyp diagnosis were recorded. The colonoscopists classified each polyp as an adenoma, SSL, or hyperplastic polyp. The lesion size was calibrated by comparison with closed cups of biopsy forceps (approximately 2.5 mm).
Before examination, patients underwent bowel preparation with 2 L of polyethylene glycol solution or 1.8 L of isotonic magnesium citrate. If necessary, additional magnesium citrate or polyethylene glycol was provided. Patients were sedated using midazolam and pethidine hydrochloride. Scopolamine butylbromide or glucagon was intravenously administered to suppress bowel peristalsis. The polyps were resected using snear polypectomy with or without submucosal saline injection.
Fixed specimens were subjected to histological examination. The reference standard was histopathology using standard hematoxylin-eosin staining. All lesions were diagnosed according to World Health Organization criteria[2] by a gastrointestinal pathologist. The pathologist classified each polyp as a tubular adenoma, villous adenoma, adenocarcinoma, SSL, TSA, microvesicular hyperplastic polyp (MVHP), goblet cell-rich hyperplastic polyp (GCHP), or normal mucosa.
The diagnostic performance of brown slits for colorectal adenoma was calculated using BellCurve for Excel (version 3.21; Social Survey Research Information Co., Ltd., Tokyo, Japan).
Of the 115 Lesions in 64 patients who met the criteria, 1 adenocarcinoma in situ, 1 TSA, 4 normal mucosae, and 1 lost specimen were excluded. A total of 108 Lesions in 62 patients were enrolled. Table 1 shows the characteristics of the study participants (mean age, 60.4 years; males, 41.9%). Of the lesions, the mean polyp size was 7.45 mm (range, 5–15 mm), and 57 and 51 were positive and negative for “brown slit” signs, respectively. Histopathological diagnosis of the polyps comprised 59 low-grade tubular adenomas, 16 SSLs, 27 MVHPs, and 6 GCHPs.
| Patient (n) | 62 |
| Age [mean ± SD (range), yr] | 60.4 ± 12.8, (40-89) |
| Sex (male) | 41.9% |
| Polyp (n) | 108 |
| Location (cecum/ascending/transverse/descending/sigmoid/rectum, n) | 13/20/38/7/25/5 |
| Morphology (0-Is/0-IIa, n) | 13/95 |
| Size [mean ± SD (range), mm] | 7.45 (2.83, 5-15) |
| Brown slits (positive/negative, n) | 57/51 |
| Endoscopic diagnosis (adenoma/SSL/hyperplastic polyp, n) | 59/30/19 |
| Histopathological diagnosis (adenoma/SSL/MVHP/GCHP, n) | 591/16/27/6 |
| Endoscopist (1/2, n) | 75/33 |
| Endoscope (CF-HQ290Z/PCF-H290Z, n) | 60/48 |
Table 2 presents the number of lesions in the brown slits. The diagnostic performance of brown slits for colorectal adenomas is shown in Table 3. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were excellent for 94.9%, 98.0%, 96.3%, 98.2%, and 94.1%, respectively.
| Adenoma1 | SSL | MVHP | GCHP | |
| Positive | 56 | 0 | 1 | 0 |
| Negative | 3 | 16 | 26 | 6 |
| Sensitivity | 94.9 (85.9-98.9) |
| Specificity | 98.0 (89.1-99.9) |
| Accuracy | 96.3 (90.8-99.0) |
| Positive predictive value | 98.2 (90.6-100) |
| Negative predictive value | 94.1 (83.8-98.8) |
We defined the polyp’s surface structure observed on conventional magnifying endoscopy with non-staining NBI using the X1 system as brown slits. The brown slits corresponded to the slit-like lumens detected on EC and to the histological crypt opening of the tubular gland of the adenoma. This study elucidated that the brown slits were highly accurate for differentiation between colorectal adenomas and serrated polyps with a sensitivity, specificity, and accuracy of 94.6%, 98.3%, and 96.5%, respectively. Kudo et al[22] reported that the sensitivity, specificity, and accuracy of slit-like lumens were 82.5%, 100%, and 83.9%, respectively, when comparing colorectal adenoma and hyperplastic polyps. The strength of the brown slits is not only the high diagnostic performance, but also the convenience of identification using a conventional magnifying endoscope with non-staining NBI. Kudo et al[25] reported that obtaining a clear ultra-high magnification view using EC was difficult and long. Thus, brown slits are simple and meaningful in daily practice.
Compared with EC, conventional magnifying endoscopy has advantages, such as not requiring contact, thereby reducing bleeding or tissue damage; easy focusing; and high familiarity. Additionally, CF-HQ290Z (4700000 yen: $ 40941) and PCF-H290Z (4500000 yen: $ 39199) are cheaper than CF-H290EC (7900000 yen: $ 68817). In the future, it would be beneficial if the accumulating evidence with the EC could be applied with conventional magnifying endoscopy.
JNET types 1 and 2A correspond to histological classifications of hyperplastic polyp/SSL and low-grade intramucosal neoplasia, respectively. JNET classification was comprehensively judged with two scales: surface pattern and vessel pattern[18,19]. Kobayashi et al[19] reported that the sensitivity, specificity, and accuracy of JNET 2A for low-grade intramucosal neoplasia were 91.4%, 75.1%, and 89.1%, respectively. Because the brown slit sign is based only on the surface pattern, it may be simpler and easier than the JNET classification.
The reasons why the set of X1 system allowed the visualization of the brown slits, which accurately predicted adenomas, were considered as follows: compared to the Elite system, the X1 system has a higher magnification and image quality due to 4K resolution, additional amber color LED, and a new noise reduction system. We previously reported that the new 290 series endoscopic system had advantages in polyp detection and procedure over the previous 260 series model[29]. Improvements of the endoscopic system may increase the visibility of the polyp findings.
The present study had some limitations. The slit-like lumens and a normal pit-like structure on staining EC distinguish low-grade adenoma from advanced neoplasia and allow DISCARD strategy[13,24,30]. Fused gland formations on EC staining can predict the histological grade of differentiation and risk factors for lymph node metastasis in T1 colorectal adenocarcinoma[31]. EC with NBI can examine the diameter and caliber variation of tumor microvessels that are associated with the depth of cancer invasion[32]. EC with NBI also possibly differentiates diminutive hyperplastic polyps from adenomas based on the microvessel pattern[26]. However, the present study excluded carcinomas, TSAs, and diminutive polyps. Furthermore, there were no villous adenomas. Validation of these EC-based evidence by applying conventional magnifying endoscopy in the future is desirable. Second, this was a retrospective study that was limited to single-center expert endoscopists. Prospective analysis at multiple centers, including non-experts, is needed. Third, the NBI mode and 290 series endoscopes were used in this study. Verification using other image-enhancing modalities, such as BLI[20], linked color imaging (LCI, Fujifilm Corporation)[33], texture and color enhancement imaging (TXI, Olympus Corporation), and using new colonoscopes, such as CF-EZ1500D and CF-XZ1200 (Olympus Corporation), are warranted. Fourth, the diagnostic efficacy of brown slit sign has not been proven to be equivalent to that of the JNET classification, although the brown slit sign may be simpler and easier than the JNET classification. The comparison of the diagnostic efficacy between brown slit sign and the JNET classification is future issue.
Brown slits on conventional magnifying NBI endoscopy using the X1 system were useful for diagnosing colorectal adenoma. The new endoscopy system could be examined using new standards.
Accurate diagnosis of colorectal premalignant polyps, including adenomas, is vital in clinical practice.
Using a combination of the X1 system and the conventional magnifying endoscope with non-staining narrow band imaging (NBI), we identified the findings corresponding to the slit-like lumens, which present the crypts of adenoma.
The authors investigated the diagnostic yields of novel findings of brown slits for adenomas.
Patients who underwent colonoscopy at the Toyoshima Endoscopy Clinic were enrolled. Polyps sized ≥ 5 mm suspected of adenomas or clinically significant serrated polyps were included in the study. We defined the surface structures of colorectal polyps, which were brown curves inside and along the tubular glands identified using a combination of a new X1 system (Olympus Corporation) and a conventional magnifying colonoscope with non-staining NBI, as brown slits. The brown slits corresponded to slit-like lumens on endocytoscopy and histological crypt openings of an adenoma. We evaluated the diagnostic performance of brown slits for adenoma.
A total of 108 Lesions from 62 patients were eligible. The average age was 60.4 years and 41.9% were male. The mean polyp size was 7.45 mm. Fifty-seven lesions were positive for brown slits. Histopathological diagnosis comprised 59 low-grade tubular adenomas, 16 sessile serrated lesions, and 33 hyperplastic polyps. The sensitivity, specificity, and accuracy of brown slits for adenoma were 94.9%, 98.0%, and 96.3%, respectively.
Brown slits on conventional magnifying endoscopy with non-staining NBI using the X1 system were useful for diagnosing colorectal adenoma.
The new endoscopy system could be examined using new standards.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan Z, China; Shafaee Z, United States; Sharaf MM, Syria A-Editor: Sahin TT S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1158] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal I, Arends MJ, Odeze RD, Lam AK. Tumours of the colon and rectum. Lyon (France), 2019. |
| 3. | Hilsden RJ, Rose SM, Dube C, Rostom A, Bridges R, McGregor SE, Brenner DR, Heitman SJ. Defining and Applying Locally Relevant Benchmarks for the Adenoma Detection Rate. Am J Gastroenterol. 2019;114:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B, Chaber-Ciopinska A, Pachlewski J, Polkowski M, Regula J. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 370] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 5. | Toyoshima O, Nishizawa T, Yoshida S, Sekiba K, Kataoka Y, Hata K, Watanabe H, Tsuji Y, Koike K. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open. 2020;8:E775-E782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 6. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Arano T, Isomura Y, Kanazawa T, Ando H, Tsuji Y, Koike K. Simple feedback of colonoscopy performance improved the number of adenomas per colonoscopy and serrated polyp detection rate. Endosc Int Open. 2021;9:E1032-E1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 9. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 10. | Anderson JC, Butterly LF, Weiss JE, Robinson CM. Providing data for serrated polyp detection rate benchmarks: an analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2017;85:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Li D, Woolfrey J, Jiang SF, Jensen CD, Zhao WK, Kakar S, Santamaria M, Rumore G, Armstrong MA, Postlethwaite D, Corley DA, Levin TR. Diagnosis and predictors of sessile serrated adenoma after educational training in a large, community-based, integrated healthcare setting. Gastrointest Endosc. 2018;87:755-765.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Klair JS, Ashat M, Johnson D, Arora S, Onteddu N, Machain Palacio JG, Samuel R, Bilal M, Buddam A, Gupta A, Gunderson A, Guturu P, Soota K, Chandra S, Murali AR. Serrated polyp detection rate and advanced adenoma detection rate from a US multicenter cohort. Endoscopy. 2020;52:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, East JE, Maier R, Moorghen M, Muhammad U, Hancock H, Jayaprakash A, MacDonald C, Ramadas A, Dhar A, Mason JM. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut. 2017;66:887-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 14. | Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, Kaye PV, Monahan KJ, Novelli MR, Plumb A, Saunders BP, Thomas-Gibson S, Tolan DJM, Whyte S, Bonnington S, Scope A, Wong R, Hibbert B, Marsh J, Moores B, Cross A, Sharp L. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 15. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 16. | Li D, Liu L, Fevrier HB, Alexeeff SE, Doherty AR, Raju M, Amsden LB, Lee JK, Levin TR, Corley DA, Herrinton LJ. Increased Risk of Colorectal Cancer in Individuals With a History of Serrated Polyps. Gastroenterology. 2020;159:502-511.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 19. | Kobayashi S, Yamada M, Takamaru H, Sakamoto T, Matsuda T, Sekine S, Igarashi Y, Saito Y. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J. 2019;7:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Ito R, Ikematsu H, Murano T, Shinmura K, Kojima M, Kumahara K, Furue Y, Sunakawa H, Minamide T, Sato D, Yamamoto Y, Takashima K, Yoda Y, Hori K, Yano T. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open. 2021;9:E271-E277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, Yamamura F, Ohtsuka K, Inoue H, Hamatani S. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Kudo T, Suzuki K, Mori Y, Misawa M, Ichimasa K, Takeda K, Nakamura H, Maeda Y, Ogawa Y, Hayashi T, Wakamura K, Ishida F, Inoue H, Kudo SE. Endocytoscopy for the differential diagnosis of colorectal low-grade adenoma: a novel possibility for the "resect and discard" strategy. Gastrointest Endosc. 2020;91:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Kudo SE, Misawa M, Wada Y, Nakamura H, Kataoka S, Maeda Y, Toyoshima N, Hayashi S, Kutsukawa M, Oikawa H, Mori Y, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Miyachi H, Ishida F, Inoue H. Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions (with video). Gastrointest Endosc. 2015;82:912-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Kataoka S, Kudo SE, Misawa M, Nakamura H, Takeda K, Toyoshima N, Mori Y, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Baba T, Ishida F. Endocytoscopy with NBI has the potential to correctly diagnose diminutive colorectal polyps that are difficult to diagnose using conventional NBI. Endosc Int Open. 2020;8:E360-E367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Ponugoti P, Rastogi A, Kaltenbach T, MacPhail ME, Sullivan AW, Thygesen JC, Broadley HM, Rex DK. Disagreement between high confidence endoscopic adenoma prediction and histopathological diagnosis in colonic lesions ≤ 3 mm in size. Endoscopy. 2019;51:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Nishizawa T, Yoshida S, Toyoshima A, Yamada T, Sakaguchi Y, Irako T, Ebinuma H, Kanai T, Koike K, Toyoshima O. Endoscopic diagnosis for colorectal sessile serrated lesions. World J Gastroenterol. 2021;27:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Sakitani K, Hata K, Takahashi Y, Fujishiro M, Watanabe H, Koike K. CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score-matching study. Endosc Int Open. 2019;7:E987-E993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Sako T, Kudo SE, Miyachi H, Wakamura K, Igarashi K, Misawa M, Mori Y, Kudo T, Hayashi T, Katagiri A, Ishida F, Azuma T, Inoue H, Hamatani S. A novel ability of endocytoscopy to diagnose histological grade of differentiation in T1 colorectal carcinomas. Endoscopy. 2018;50:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Takeda K, Kudo SE, Misawa M, Mori Y, Kudo T, Kodama K, Wakamura K, Miyachi H, Hidaka E, Ishida F, Inoue H. Comparison of the endocytoscopic and clinicopathologic features of colorectal neoplasms. Endosc Int Open. 2016;4:E397-E402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Paggi S, Radaelli F, Senore C, Maselli R, Amato A, Andrisani G, Di Matteo F, Cecinato P, Grillo S, Sereni G, Sassatelli R, Manfredi G, Alicante S, Buscarini E, Canova D, Milan L, Pallini P, Iwatate M, Rondonotti E, Repici A, Hassan C. Linked-color imaging versus white-light colonoscopy in an organized colorectal cancer screening program. Gastrointest Endosc. 2020;92:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |