Published online Jun 28, 2022. doi: 10.3748/wjg.v28.i24.2705
Peer-review started: December 4, 2021
First decision: January 8, 2022
Revised: January 14, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: June 28, 2022
Processing time: 202 Days and 4.6 Hours
Stool DNA (sDNA) methylation analysis is a promising, noninvasive approach for colorectal cancer screening; however, reliable biomarkers for detecting early-stage colon cancer (ECC) are lacking, particularly in the Chinese population.
To identify a novel stool-based assay that can improve the effectiveness of ECC screening.
A blinded case-control study was performed using archived stool samples from 125 ECC patients, and 125 control subjects with normal colonoscopy. The cohort was randomly divided into training and test sets at a 1.5:1 ratio. Targeted bisulfite sequencing (TBSeq) was conducted on five pairs of preoperative and postop-erative sDNA samples from ECC patients to identify DNA methylation biomarkers, which were validated using pyrosequencing. By logistic regression analysis, a multiplex stool-based assay was developed in the training set, and the detection performance was further assessed in the test set and combined set. The
Following TBSeq, three hypermethylated cytosine-guanine sites were selected as biomarkers, including paired box 8, Ras-association domain family 1 and secreted frizzled-related protein 2, which differed between the groups and were involved in important cancer pathways. An sDNA panel containing the three biomarkers was constructed with a logistic model. Receiver operating characteristic (ROC) analysis revealed that this panel was superior to the fecal immunochemical test (FIT) or serum carcinoembryonic antigen for the detection of ECC. We further found that the combination of the sDNA panel with FIT could improve the screening effectiveness. In the combined set, the sensitivity, specificity and area under the ROC curve for this multiplex assay were 80.0%, 93.6% and 0.918, respectively, and the performance remained excellent in the subgroup analysis by tumor stage. In addition, the detection sensitivity did not differ with tumor site, tumor stage, histological differentiation, age or sex, but was significantly higher in T4 than in T1-3 stage tumors (P = 0.041).
We identified a novel multiplex stool-based assay combining sDNA methylation biomarkers and FIT, which could detect ECC with high sensitivity and specificity throughout the colon, showing a promising application perspective.
Core Tip: Stool DNA (sDNA) methylation analysis has a promising application in the early diagnosis of colorectal cancer. However, reliable biomarkers for detecting early-stage colon cancer (ECC) are lacking. In this study, by targeted bisulfite sequencing, we identified a novel multiplex stool-based assay combining three sDNA methylation biomarkers and fecal immunochemical test. Further validation in larger samples by pyrosequencing showed that it enabled the diagnosis of ECC with high sensitivity and specificity throughout the colon. To our knowledge, this is the first study to focus on ECC screening in China.
- Citation: Jiang HH, Xing SW, Tang X, Chen Y, Lin K, He LW, Lin MB, Tang EJ. Novel multiplex stool-based assay for the detection of early-stage colon cancer in a Chinese population. World J Gastroenterol 2022; 28(24): 2705-2732
- URL: https://www.wjgnet.com/1007-9327/full/v28/i24/2705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i24.2705
Colorectal cancer (CRC) has emerged as a major public health issue in China, with 521400 new cases and 248000 deaths occurring in 2018[1]. The incidence of colon cancer has increased significantly in the past decades, with a trend in younger patients, and most cases are in advanced stages at initial diagnosis[2]. Early screening is the key to improving survival and reducing morbidity[3]. Compared with the high positive rate of rectal palpation in rectal cancer, colonoscopy is currently the main tool and gold standard for detecting colon cancer, but is invasive, costly and poorly tolerated[4]. The fecal immunochemical test (FIT), computed tomography (CT) colonography and blood tumor markers, such as serum carcinoembryonic antigen (CEA), provide relatively noninvasive and painless methods, but their sensitivity for detecting early-stage colon cancer (ECC) is limited[5,6]. Thus, the identification of novel biomarkers that are highly specific, sensitive, and noninvasive is urgently needed for the screening of ECC.
CRC development is characterized by the progressive accumulation of genetic and epigenetic alterations that transform colonic epithelial cells into adenocarcinoma cells[7,8]. These cells are continuously shed into the colonic lumen and mixed with stool[9]. Moreover, the molecular changes caused by CRC tumorigenesis are reportedly present in the stool earlier than in the blood[10]. Hence, detecting aberrant DNA methylation in stool DNA (sDNA) has been proposed as a promising noninvasive alternative for CRC screening. To date, a number of sDNA methylation biomarkers have been reported for the detection of different stages of CRC, including secreted frizzled-related protein 2 (SFRP2), N-myc downstream-regulated gene 4 (NDRG4), ventralis intermedius, COL4A2 and GATA4[11]. Further studies revealed that the combination of multiple biomarkers contributed to a higher diagnostic accuracy than a single biomarker[12]. For instance, a multitarget sDNA test Cologuard, combining NDRG4 and BMP3 methylation, Kirsten rat sarcoma mutations, β-actin and a hemoglobin assay, has been approved for average-risk CRC screening by the US Food and Drug Administration and is now available clinically[13]. However, the reported sensitivity and specificity of the same sDNA methylation biomarker varied greatly among studies, due to the different study populations (mainly the ethnic, geographic and dietary differences), inclusion criteria and levels of examination[14,15]. Moreover, few studies have focused on the sDNA screening test for ECC, especially in the Chinese population.
In this study, using targeted bisulfite sequencing (TBSeq), we identified a novel panel of sDNA methylation biomarkers for ECC detection, which was validated in a training and test design with pyrosequencing (PSQ). We further investigated the detection performance of the sDNA panel combining conventional screening methods, and assessed the effects of clinical covariates on test performance. To our knowledge, this is the first work to focus on ECC screening in China.
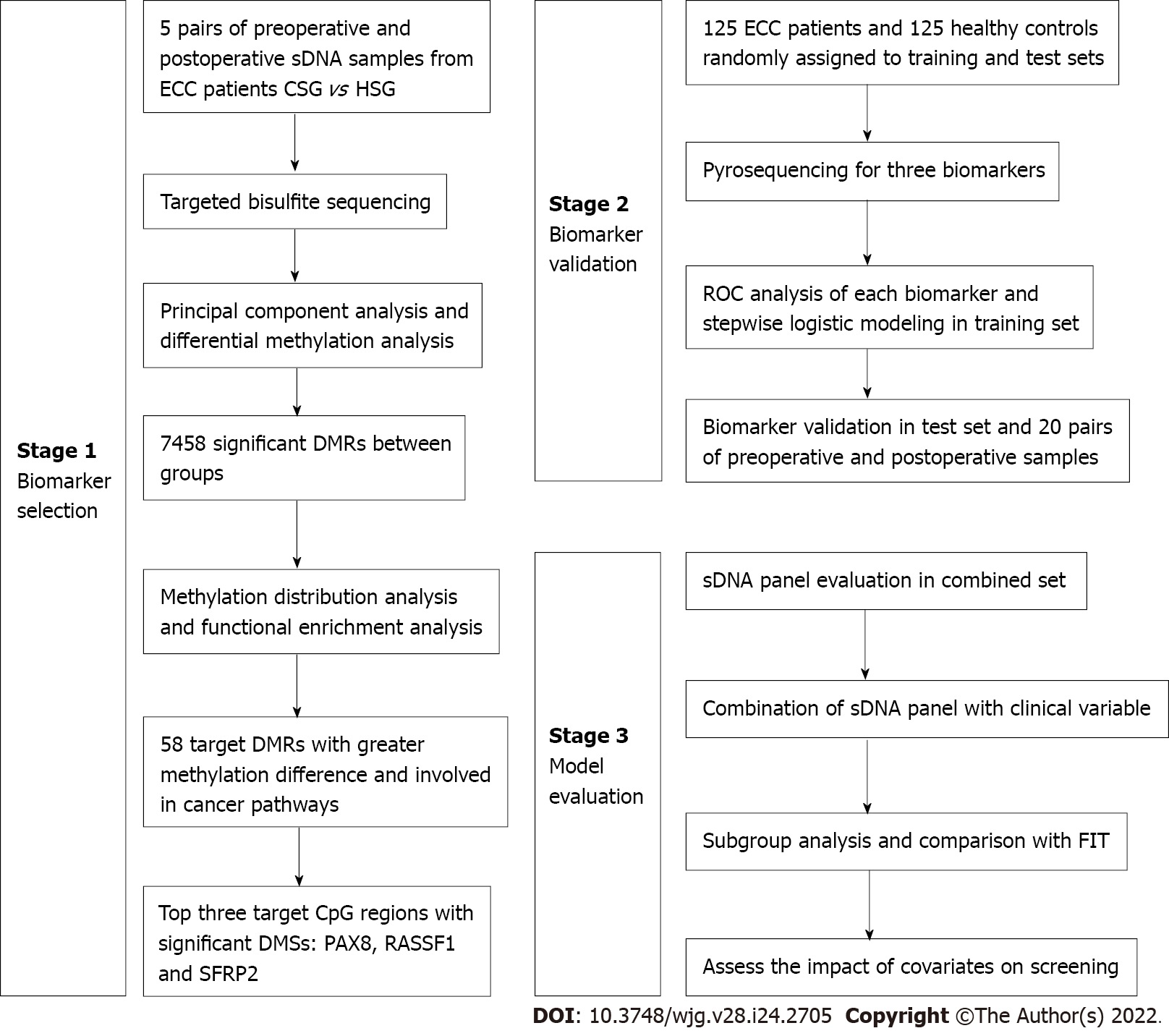
Between November 2018 and June 2020, a single-center, case-control study was performed at Yangpu Hospital Affiliated to Tongji University, using archived stool samples from 125 patients with sporadic ECC and 125 individuals with normal colonoscopy results. The diagnosis of stage I and II colon cancer was histologically confirmed after surgery. All subjects were of Han race living in Shanghai, and the inclusion of patients and controls was carried out to achieve a good match in terms of age and sex. Those who had a history of digestive cancer, inflammatory bowel disease or familial adenomatous polyposis, or an unconfirmed diagnosis were excluded. In addition, clinical information including tumor site, histological differentiation, tumor stage and preoperative serum CEA level was abstracted from medical records. These participants were randomly divided into training and test sets with a sample size ratio of 1.5:1. All subjects provided written informed consent, and this study was approved by the Ethical Committee and Institutional Review Board of our hospital (LL-2018-SCI-003). This study was registered at the Chinese Clinical Trial Registry (ChiCTR1800019552). The study design consisted of three stages: Biomarker selection, biomarker validation and model evaluation, as shown in Figure 1.
All participants were required to undergo screening colonoscopy and provide a fresh stool sample before bowel purgation. In addition, 20 randomly selected ECC patients (6 stage I and 14 stage II cases) were asked to provide stool samples at six months after radical surgery with no tumor recurrence. One part of the stool sample was used for FIT (FASURE; NewScen Coast, China), and the result was evaluated at the manufacturer’s recommended positivity cut-off of 200 ng hemoglobin/mL buffer. The other part (minimum 50 g) was immediately extracted or stored at -80 °C for further use, sDNA was extracted with a QIAamp DNA Fast Stool Mini Kit (Qiagen, Germany), and the quality and concentration were determined by spectrophotometry (NanoDrop 2000; Thermo-Fisher Scientific, United States). Among the 20 pairs of preoperative and postoperative sDNA samples, five pairs (2 stage I and 3 stage II cases) were randomly selected for biomarker identification using TBSeq and named cancer sample group (CSG) and healthy sample group (HSG), respectively.
We used CATCH-Seq target enrichment technology (Novogene, China) to perform bisulfite sequencing, to evaluate 23441 [cytosine-guanine (CpG) islands] (CGIs) (about 83% of the 28226 CGIs in the human genome) and the promoter regions of 19369 RefSeq genes (within 2 kb before the transcription start site)[16,17]. Briefly, according to the manufacturer’s protocol, genomic DNA (1 μg) was fragmented into 200-300 bp fragments using a Covaris S220 (Covarias, United States). The DNA fragments were then sheared, end-repaired and dephosphorylated. The blunt fragments were subsequently A-tailed and ligated to sequencing adaptors that were synthesized with 5’-methylcytosine instead of 5’-cytosine and index sequences. Following the liquid hybridization capture procedure, the target enriched library was bisulfite-converted (EZ DNA Methylation Kit; Zymo Research, United States) and then amplified by polymerase chain reaction (PCR). After quantification and quality control, sequencing was performed on an Illumina Hiseq 2500 platform (Illumina, United States).
Preprocessing included quality control using FastQC, adapter trimming using cutadapt, and read alignment and methylation calling using Bismark with Bowtie2 (hg19/GRCh37). The coverage depth of each base and CpG site was calculated, and the results were filtered by criteria of at least 5X coverage. The CpG sites that were located on the Y chromosome and overlapped with single nucleotide polymorphisms (SNPs) registered in the SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) were excluded from the analysis. For the methylated sites, the methylation level was calculated using the formula: ML = mC/(mC + umC), where ML represents the methylation level, and mC and umC represent the number of methylated and unmethylated C-sites, respectively. Methylation levels of the specified functional regions including CGI, CGI shore (0-2 kb from CGI), CGI shelf (2-4 kb from CGI), promoter, 5’ untranslated regions (UTR), 3’UTR and exon, were summarized to show the distribution[18]. Methylation density was defined as the percentage of methylated CpG sites among all CpG sites within the given region.
In-house scripts were used to identify a differentially methylated site (DMS) by Fisher’s exact test with false discovery rate correction. Statistical significance for DMS between the two groups was determined if the adjusted P value < 0.05 and the difference in methylation level > 0.25. A differentially methylated region (DMR) was identified by swDMR software (http://122.228.158.106/swDMR/) using the sliding-window approach, in which the window was set to 1000 bp and the step length was 100 bp. The DMR should contain at least two CpG sites (all sites are hypermethylated or hypomethylated), and the distance between the adjacent CpG sites was < 100 bp. In addition, the DMRs with an adjusted P value < 0.05 and a difference in methylation level > 0.1 were considered candidate DMRs.
By comparing the methylation status between the CSG and HSG, 7458 DMRs were obtained based on the above standards. To strengthen the robustness of the candidate biomarker, the DMRs located far from CGIs and promoter regions were filtered out. Moreover, to reduce the noise due to sample heterogeneity, it was essential to select biomarkers that had greater methylation difference between groups (P > 0.35) and that were significantly enriched in well-established cancer pathways (adjusted P < 0.05). In total, 58 target DMRs were selected, and each DMR contained at least two significant DMSs. According to the guanine-cytosine percent, primer lengths, amplicon lengths, predicted melting temperatures and the number of SNPs in the primers, the score representing the difficulty levels for all the candidate regions was obtained. Finally, we selected the top three target CpG regions with the best chance of being amplified and conducted in the PSQ assay, and removed the other candidate regions for further validation.
To measure the methylation levels of the three target CpG regions in the 250 stool samples, PSQ was conducted (Oebiotech, China) without knowledge of either the clinical diagnosis or FIT result, sDNA extraction and bisulfite conversion were performed as previously described[19]. The PSQ primers were designed to amplify two to five CpG sites in target sequences using PyroMark Assay Design software (Qiagen, Germany). Primer sequences were listed in Table 1. For methylation-specific PCR (MSP), 50 ng of bisulfite modified DNA was amplified in a 25 μL reaction. The cycling conditions were recommended by the manufacturer and were as follows: A denaturing step of 15 min at 95 °C, then 45 cycles at 94 °C for 30 s, 56 °C for 30 s and 72 °C for 30 s, and a final elongation step of 10 min at 72 °C. PSQ was performed on a PSQ HS96A instrument according to the manufacturer’s guidelines using PyroMark Gold Q96 Reagents (Qiagen, Germany). The methylation index of each gene in each sample was calculated as the mean percentage of mC for all examined CpGs in target regions. All experiments included a negative control without a template[19].
| Target gene | Primer | Sequence | Size (bp) | Target DMS |
| PAX8 | Forward | 5’-GGGGGTTAGGGGATTTTGATTATA-3’ | 166 | chr2:114035984; 114035988; 114035995; 114035998; 114036006 |
| Reverse | Biotin-5’-ATCTCATACCCTTCTCCTAAATTTATAC-3’ | |||
| Sequencing | 5’-ATGGAGTTGTGAGGT-3’ | |||
| RASSF1 | Forward | 5’-TTTATTTATTGGGTGGGGTAGGA-3’ | 141 | chr3:50378714; 50378718 |
| Reverse | Biotin-5’-CCTCAAAATCACCATCCAACCTCTAC-3’ | |||
| Sequencing | 5’-GGGAGATAGGTTAGTAGTTTTA-3’ | |||
| SFRP2 | Forward | 5’-GATTAGGGATAATTAGGTAAAAGGAGTT-3’ | 166 | chr4:154711281; 154711305 |
| Reverse | Biotin-5’-ATTCATCCCCTACCTACCAAAAAACACC-3’ | |||
| Sequencing | 5’-AGTTAGAGATATTAGATTTTAGG-3’ |
Based on the PSQ results, a logistic regression model was developed to define a linear combination of variables that optimized the discrimination between ECC patients and healthy controls. The modeling strategy consisted of age, sex, FIT, preoperative CEA level and sDNA methylation biomarkers in a base model and adding quadratic and pairwise interactions of these variables using backward selection with P < 0.05 for retention. The formula was as follows: Y = β0 + β1X1 + β2X2 + … + βnXn where X represented the exploratory variable[20]. The linear discriminant score with the corresponding cut-off value was then applied to the study population. Receiver operating characteristic (ROC) analysis was applied to compare the accuracy of nested logistic models and to investigate the added value of each variable.
All statistical analyses were conducted using Prism 8 for Windows (GraphPad, United States) and R software v3.5.2 (R Foundation for Statistical Computing, Austria). c2test or Fisher’s exact test was used to compare categorical variables, and Student’s t-test or Mann-Whitney U test was used to compare continuous variables. Principal component analysis (PCA) was performed to visualize the degree of similarity between samples according to their DNA methylation state[21]. A volcano plot and a Circos plot were used to present the differences in DMR methylation levels between groups[22]. To determine the functions and enriched pathways of these DMR-related genes, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed[23]. The sensitivity, specificity, and area under the ROC curve (AUC) with corresponding 95% confidence intervals (CIs) were calculated and compared. The χ2 test was also used to assess the association of detection sensitivity with clinicopathological covariates. For all analyses, P < 0.05 was considered statistically significant.
Overall, this study enrolled 125 ECC patients and 125 healthy controls, with a median age of 69 (range, 48-94) and 68 (range, 42-92) years, respectively; 47.2% of patients and 49.6% of controls were females. Age and sex were well-balanced between patients and controls. With the conventional 5 ng/mL cut-off, serum CEA positivity was observed in 29.6% of patients and 0% of controls, and the difference was statistically significant (P < 0.001). FIT also showed a significantly higher positivity rate in the patients than in the controls (62.4% vs 3.2%, P < 0.001). Of these ECCs, 64 (51.2%) tumors were located proximal to the splenic flexure and 61 (48.8%) were distal. According to the postoperative pathological examination, there were 47 (37.6%) stage I tumors and 78 (62.4%) stage II tumors; 98 (78.4%) cases were well or moderately differentiated, and 27 (21.6%) were poorly differentiated. In addition, study participants were randomly divided into training and test sets with a 1.5:1 split. The differences in all these features between the training and test sets were not significant (all P > 0.05), indicating similar composition and the comparability (Table 2).
| Variable | Total (n = 125) | Training set (n = 75) | Test set (n = 50) |
| Age (yr) | 69 (range, 48-94) | 68 (range, 51-94) | 70 (range, 48-88) |
| Sex | |||
| Male | 66 (52.8) | 39 (52.0) | 27 (54.0) |
| Female | 59 (47.2) | 36 (48.0) | 23 (46.0) |
| Serum CEA (≥ 5 ng/mL) | |||
| Positive | 37 (29.6) | 21 (28.0) | 16 (32.0) |
| Negative | 88 (70.4) | 54 (72.0) | 34 (68.0) |
| FIT (≥ 200 ng/mL) | |||
| Positive | 78 (62.4) | 48 (64.0) | 30 (60.0) |
| Negative | 47 (37.6) | 27 (36.0) | 20 (40.0) |
| Tumor site | |||
| Proximal | 64 (51.2) | 36 (48.0) | 28 (56.0) |
| Distal | 61 (48.8) | 39 (52.0) | 22 (44.0) |
| TNM stage | |||
| I | 47 (37.6) | 30 (40.0) | 17 (34.0) |
| II | 78 (62.4) | 45 (60.0) | 33 (66.0) |
| T stage | |||
| T1-3 | 71 (56.8) | 44 (58.7) | 27 (54.0) |
| T4 | 54 (43.2) | 31 (41.3) | 23 (46.0) |
| Histological differentiation | |||
| Well/moderately | 98 (78.4) | 60 (80.0) | 38 (76.0) |
| Poorly | 27 (21.6) | 15 (20.0) | 12 (24.0) |
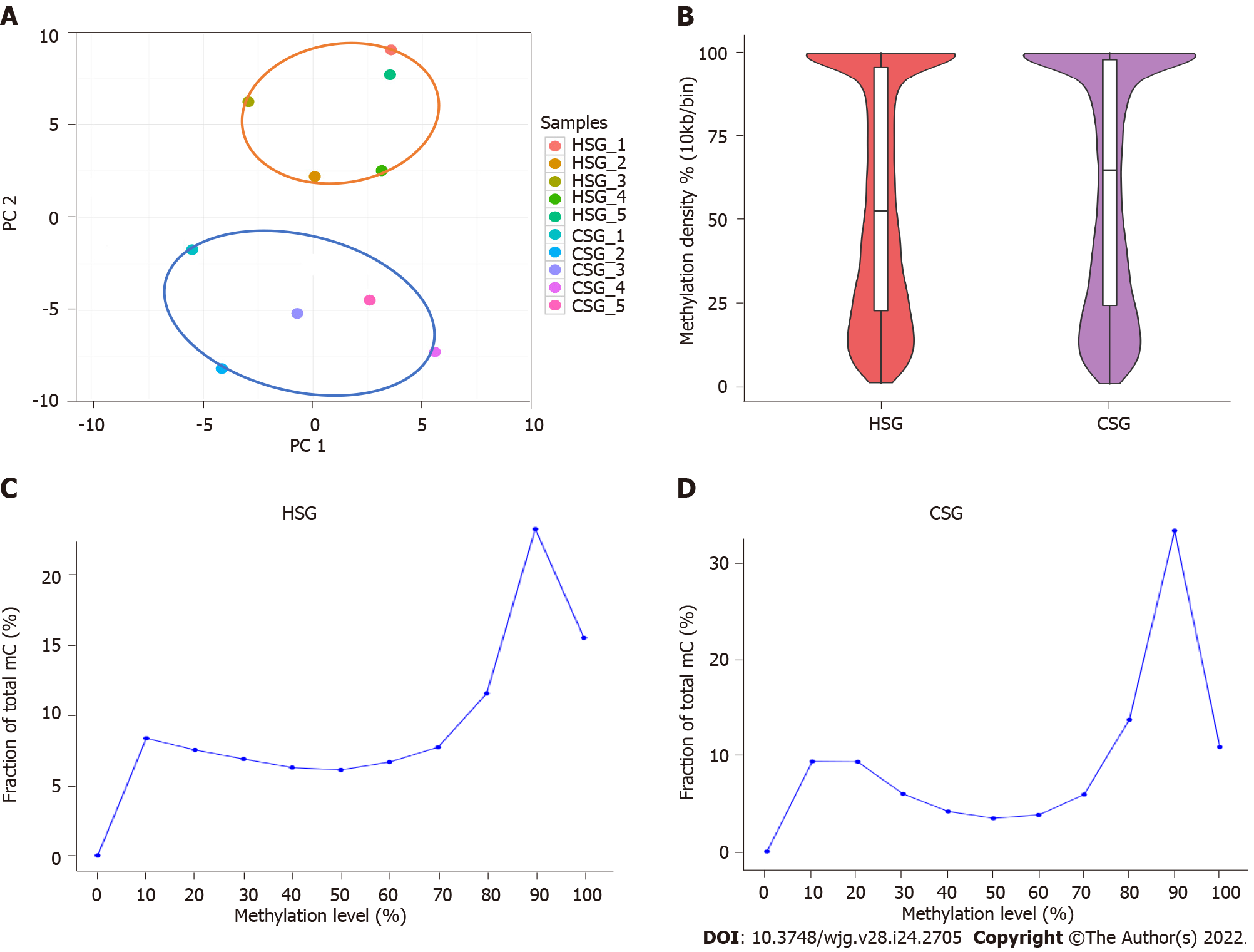
A total of 11.69 G and 11.08 G raw bases were generated on average for the HSG and CSG, respectively. All samples showed a bisulfite conversion rate greater than 99%. After data filtering, approximately 2.8 million CpGs on the target sequencing region were obtained for each sample, and the depth of coverage ranged between 23.66 and 32.24 (Supplementary Table 1). A PCA was performed on the methylation profiles, and demonstrated that the groups had intragroup similarity, but also intergroup dissimilarity (Figure 2A). In addition, the mean methylation densities of the HSG and CSG were 52.8% and 65.4%, respectively (Figure 2B); compared with 50.4% of CpG sites in the HSG, 58.0% of CpG sites in the CSG had a methylation level above 80% (Figure 2C).
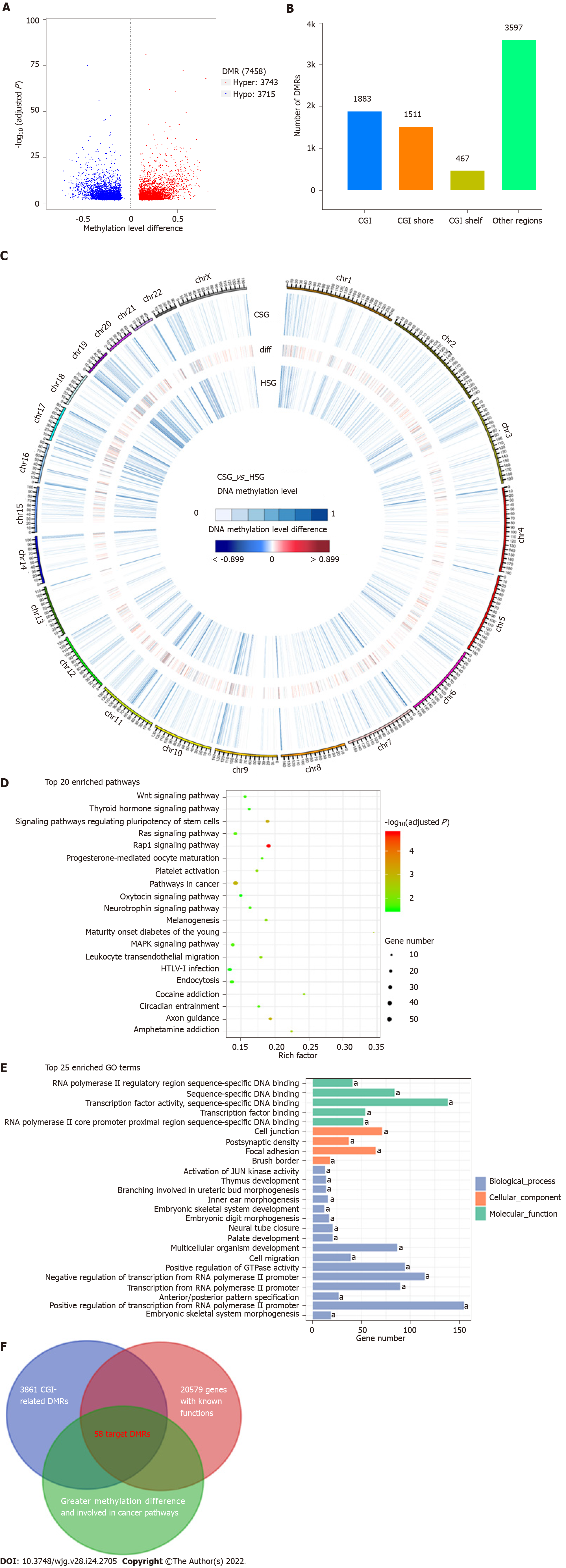
As shown in Figure 3A, in total, 7458 DMRs were identified in the CSG compared with the HSG (adjusted P < 0.05 and methylation level difference > 0.1), including 3743 hypermethylated and 3715 hypomethylated DMRs. Among them, 1883 DMRs (25.3%) were located in the CGIs, and 1978 (26.5%) were located in the regions flanking CGIs (CGI shore and shelf) (Figure 3B). Moreover, of the 3861 CGI-related DMRs, 2531 overlapped with the promoter regions of the 20579 genes with known functions (Figure 3C). To determine changes in the methylation status of gene functions, GO and KEGG pathway analyses were conducted and revealed that these 2062 DMR-related genes were significantly enriched in cell migration, focal adhesion, Wnt, Ras, Rap1 and MAPK signaling pathways, etc (Figures 3D and 3E, adjusted P < 0.05). To mine the data for potential early screening biomarkers, 58 DMR-related genes that differed most in methylation level between the groups and that were involved in the important cancer pathways were selected (Figure 3F). With the PSQ criteria, the top three target CpG regions within the promoters of paired box 8 (PAX8), Ras-association domain family 1 (RASSF1) and SFRP2 were finally identified, and contained five, two and two significant DMSs, respectively (Table 1).
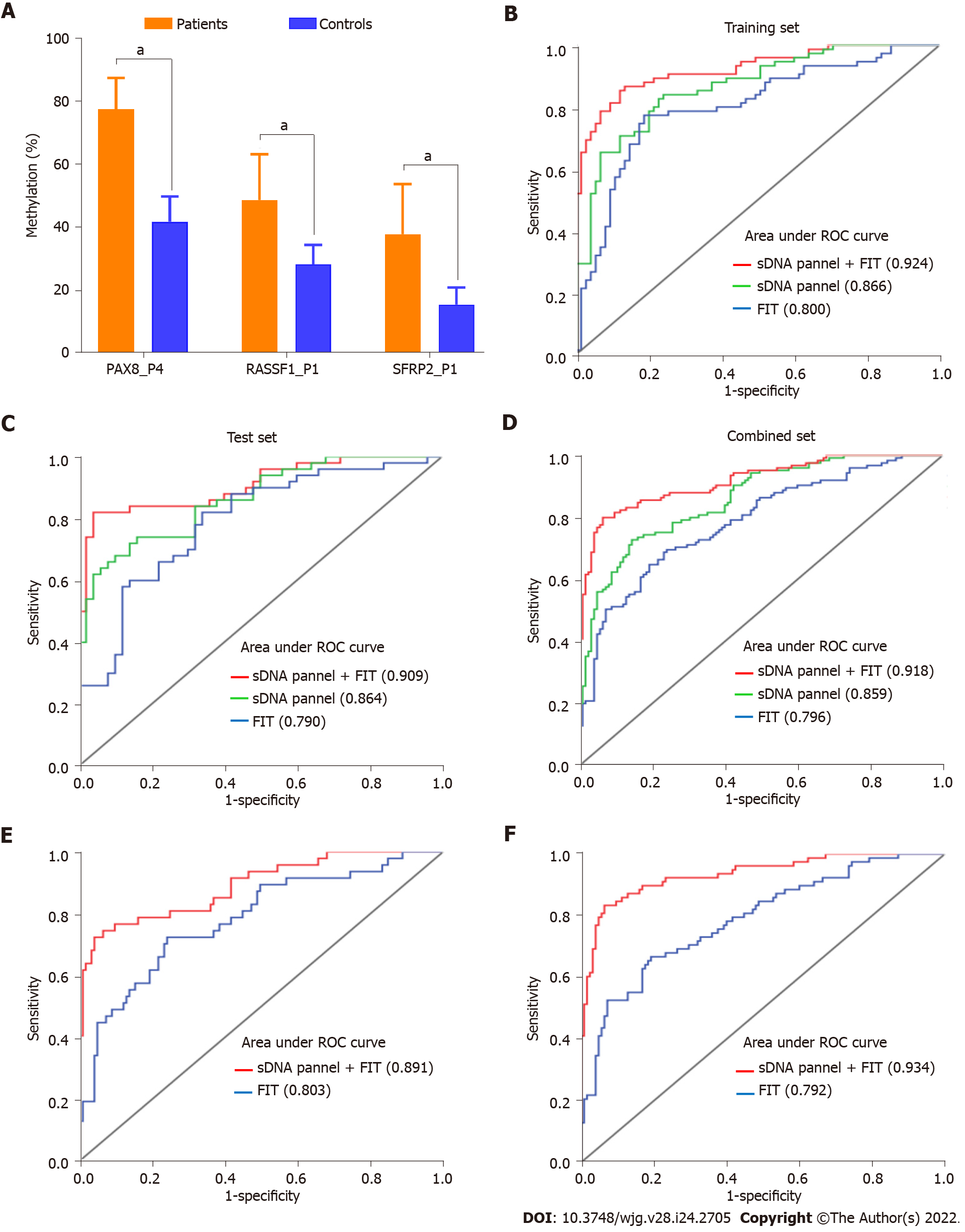
To assess the screening effectiveness of these three biomarkers, PSQ was successfully performed in all sDNA samples. Using the 150 subjects in the training set, ROC analyses showed that the AUCs for the five DMSs of PAX8 ranged from 0.784 to 0.826, the AUCs for the two DMSs of RASSF1 were 0.782 and 0.763, and the AUCs for the two DMSs of SFRP2 were 0.697 and 0.641, respectively (Supple-mentary Figures 1A, 1B and 1C). In addition, serum CEA positivity was detected in 14.0% of the 150 subjects, with a sensitivity of 28.0% and an AUC of 0.640; FIT positivity was detected in 34.0% of the 150 subjects, with a sensitivity of 64.0% and an AUC of 0.800 (Table 3). By stepwise logistic modeling, an sDNA methylation panel including three DMSs was constructed as follows: Y = -10.937 + 0.097 × PAX8_P4 + 0.054 × RASSF1_P1 + 0.058 × SFRP2_P1. These three DMSs showed significantly increased methylation in patients compared with controls (all P < 0.05; Figure 4A) as well as in the comparison between the 20 pairs of preoperative and postoperative patient samples (all P < 0.05; Supplementary Figures 1D, 1E and 1F). The sensitivity and AUC for this sDNA panel were 82.7% and 0.866 (95%CI: 0.810-0.923), respectively, which were superior to any single index above (Figure 4B, Table 3). Subsequently, this panel was validated in an independent test set, and the AUC reached 0.864 (95%CI: 0.795-0.933), also offering an advantage over FIT (Figure 4C).
| Study population | Index | Sensitivity (%) | Specificity (%) | AUC (95%CI) |
| Training set | Serum CEA | 28.0 | 100.0 | 0.640 (0.551-0.729) |
| (n = 150 subjects) | FIT | 64.0 | 96.0 | 0.800 (0.726-0.874) |
| PAX8_P4 | 76.0 | 77.3 | 0.810 (0.741-0.880) | |
| RASSF1_P1 | 50.7 | 93.3 | 0.782 (0.709-0.854) | |
| SFRP2_P1 | 50.7 | 86.7 | 0.697 (0.612-0.782) | |
| sDNA panel | 82.7 | 77.3 | 0.866 (0.810-0.923) | |
| sDNA panel + FIT | 86.7 | 86.7 | 0.924 (0.881-0.966) | |
| Test set | Serum CEA | 32.0 | 100.0 | 0.660 (0.552-0.768) |
| (n = 100 subjects) | FIT | 60.0 | 98.0 | 0.790 (0.697-0.883) |
| sDNA panel | 76.0 | 84.0 | 0.864 (0.795-0.933) | |
| sDNA panel + FIT | 82.0 | 96.0 | 0.909 (0.850-0.967) | |
| Combined set | Serum CEA | 29.6 | 100.0 | 0.648 (0.580-0.716) |
| (n = 250 subjects) | FIT | 62.4 | 96.8 | 0.796 (0.738-0.854) |
| sDNA panel | 75.2 | 84.0 | 0.859 (0.815-0.904) | |
| sDNA panel + FIT | 80.0 | 93.6 | 0.918 (0.884-0.952) | |
| Subgroup for stage I | FIT | 63.8 | 96.8 | 0.803 (0.716-0.891) |
| (n = 172 subjects) | sDNA panel + FIT | 74.5 | 93.6 | 0.891 (0.832-0.950) |
| Subgroup for stage II | FIT | 61.5 | 96.8 | 0.792 (0.721-0.863) |
| (n = 203 subjects) | sDNA panel + FIT | 83.3 | 93.6 | 0.934 (0.899-0.970) |
The diagnostic performance of the sDNA panel was also confirmed in the combined set, achieving an AUC of 0.859 (0.815-0.904) (Figure 4D). In addition, among the 168 subjects with negative FIT and the 213 subjects with negative serum CEA, the AUCs for this panel reached 0.807 (95%CI: 0.734-0.880) and 0.864 (95%CI: 0.816-0.913), respectively (Supplementary Figure 2). We further investigated whether the combination of the sDNA panel with clinical variables could improve the effectiveness of ECC screening. The results showed that the inclusion of FIT was accompanied by a relative increase in sensitivity and AUC, as compared with the sDNA panel, serum CEA or FIT alone (Table 3, Figure 5). In contrast, the contribution of age, sex or preoperative CEA level to aggregate panel discrimination was minimal (data not shown). For example, in the combined set, the sensitivity, specificity and AUC for the sDNA panel combining FIT were up to 80.0%, 93.6% and 0.918 (95%CI: 0.884-0.952), respectively (Figure 4D, Table 3). In the subgroup analysis by tumor stage, this multiplex assay showed excellent performance for both stage I and II ECCs, with an AUC of 0.891 (95%CI: 0.832-0.950) and 0.934 (95%CI: 0.899-0.970), respectively (Figures 4E and 4F).
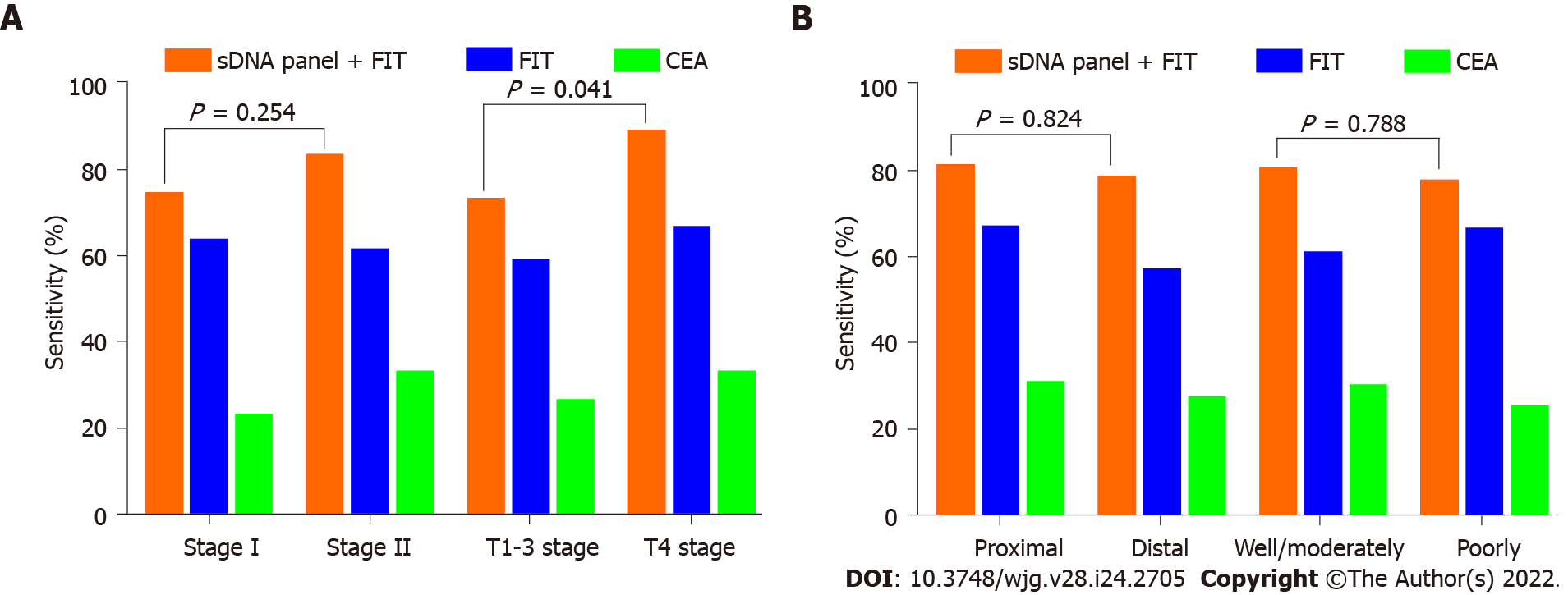
We also analyzed the impact of clinicopathologic covariates on assay screening. First, we found that the detection sensitivities of the sDNA panel combining FIT for stage I and II ECCs were 74.5% and 83.3%, respectively, with no statistical difference (P = 0.254; Figure 5A). However, the detection sensitivity of 88.9% for T4 stage tumors was significantly higher than the aggregate sensitivity of 73.2% for T1-3 stage tumors (P = 0.041; Figure 5A). Second, this multiplex assay detected 81.3% of proximal ECCs vs 78.7% of distal ECCs (P = 0.824; Figure 5B). Third, the detection rate for poorly differentiated tumors was comparable to that for well or moderately differentiated tumors (77.8% vs 80.6%, P = 0.788; Figure 5B). In addition, the sensitivity also did not vary significantly according to age or sex of the patients (data not shown).
CRC is the third most prevalent cancer in China, with an increasing proportion of colon cancer diagnoses over the decades[2]. Effective screening measures are highly needed to lessen this burden, especially for ECC due to its occult onset. Compared with conventional methods, the sDNA test provides a biologically rational approach based on tumor exfoliation and is noninvasive, requires no unpleasant cathartic preparation, no diet or medication restriction[24]. However, reliable biomarkers particularly for ECC detection are lacking. In the present study, we provided a novel multiplex stool-based assay that enabled the diagnosis of ECC with high sensitivity and specificity.
Aberrant DNA methylation is an early and frequent event in carcinogenesis and therefore has great potential for ECC screening[25]. To identify the candidate methylation biomarkers, we performed high throughput TBSeq with five pairs of preoperative and postoperative sDNA samples from ECC patients, where the paired design can help reduce the influence of background noise and improve the feature discriminability. Three of our candidate biomarkers were finally selected: RASSF1, SFRP2 and PAX8. The first two have been identified in various cancers including CRC[26,27], to be the commonly silenced tumor-suppressor genes by promoter hypermethylation[28]. PAX8 is a member of the paired box family of transcription factors[29], and its epigenetic silencing has also been observed in some tumor types, including colon cancer[30]. A recent study found that PAX8 hypermethylation accelerated gastro-intestinal stromal tumor progression by downregulating Wnt4 expression[31]. Anglim et al[32] reported a panel of eight hypermethylated genes containing PAX8, which showed high sensitivity and specificity for the early detection of squamous cell lung cancer.
To date, there have been various methods for detecting abnormal DNA methylation[33,34]. MSP is a simple, rapid and inexpensive method, but is not capable of pattern recognition and identification of CpG sites outside the methylation-specific primers. Bisulfite sequencing was once considered the gold standard for DNA methylation determination. The disadvantage lies in its tedious and time-consuming procedure, and a minimum number of 10-20 clones are required to detect interindividual variabilities in DNA methylation. Methylation-sensitive high-resolution melting is a relatively new technique for methylation assessment based on post-PCR melting curve analysis. It gives a range of methylation estimates rather than a single value, being mainly suitable for large sample screening[35]. MethyLight is a sensitive, high-throughput methylation assay that was developed based on MSP, while the major disadvantage is the high cost of a TaqMan probe[36,37]. In our study, biomarker validation was performed using PSQ, which overcomes the defects of the above methods, combining the measurement of multiple methylation biomarkers with high throughput in a single test[38]. Therefore, PSQ has been suggested as the new gold standard test for methylation detection. The results of PSQ confirmed that the three biomarkers by TBSeq were reliable and feasible.
By logistic regression analysis, a three-DMS panel was developed in this study and was proven more effective than a single biomarker for ECC screening. We further found that the combined detection of the sDNA panel and FIT was characterized by a relative increase in diagnostic performance, which was successfully validated in an independent test set, as well as in the combined dataset. Actually, many attempts at CRC early screening in the Chinese population have been made, but none have gained wide acceptance[12]. Liu et al[39] developed an sDNA methylation panel, including COL4A2 and TLX2, which showed a high sensitivity of 91% for advanced-stage CRC detection with 97% specificity, but the performance for early-stage CRC was significantly weakened, with a sensitivity of 52% and specificity of 86%. In the study by Zhang et al[40], an sDNA panel that examined SFRP2 and WIF-1 promoter methylation was identified, and the sensitivities for detecting stage I and II CRC were 55% and 80%, respectively. Recently, another novel multidimensional assay combining FIT, sDNA mutation, methylation and intestinal bacteria analysis was reported by Mo et al[41]. It displayed the highest sensitivity of 91.89% in stage III CRC, whereas the sensitivity for stage I-II CRC was relatively low (76.27%). Comparatively, the sensitivity, specificity and AUC of our multiplex stool-based assay for detecting ECC reached 80.0%, 93.6% and 0.918, respectively, showing a promising prospect for practical application.
The effect of covariates on test performance was also investigated. It has been suggested that the conventional screening modalities, including colonoscopy and FIT, seem less sensitive for proximal than distal colon neoplasms[42,43]. In this study, our stool multiplex assay showed comparable efficacy for the detection of both proximal and distal ECC, which was consistent with the previous finding by Imperiale et al[13]. Given this performance characteristic, the sDNA test has the potential to improve the screening efficiency for neoplasms throughout the colon, and would be a valuable complement to colonoscopy as an interval test. Furthermore, we found that the sensitivity of this stool multiplex assay for ECC did not vary significantly according to age, sex, histological differentiation or tumor stage, but was significantly higher at T4 stage. Further studies are needed to determine their association and causality. In addition, some studies revealed that the sDNA test sensitivity increased as the tumor size increased[13,41]. Unfortunately, this variable was not included in our study.
The major strength of our study was the training and test set design involving blinded assays in the laboratory, which provided objective data to assess the test performance. The limitations of this study should also be mentioned. First, these analyses were based on data obtained from a single institution in China, and the sample size was relatively small. Second, other factors not included in this study could not be examined for confounding effects. As a result, a large-scale, prospective multicenter study is warranted to further confirm the value of our assay.
In conclusion, we developed and validated a novel multiplex stool-based assay combining sDNA methylation biomarkers and FIT, which could detect ECC with high accuracy throughout the colon.
Colorectal cancer (CRC) is one of the most prevalent malignancies in China with an increasing ratio of colon to rectal cancer over the decades. Early screening is the key to reducing this burden. The traditional screening methods, including colonoscopy, fecal immunochemical test (FIT) and serum carcinoembryonic antigen (CEA), have reached a bottleneck, especially for early-stage colon cancer (ECC) due to its occult onset. Thus, precision and effective non-invasive biomarkers are highly desirable.
Detection of aberrant methylated DNA in stool has been proven to be a promising noninvasive method for the early diagnosis of CRC. However, the reported screening efficacy of the same biomarker varied across different studies. Moreover, effective biomarkers for detecting ECC are scarce, especially in the Chinese population.
The objective of our study was to identify a novel assay based on stool DNA (sDNA) methylation biomarkers which could improve the effectiveness of ECC screening. We also investigated the influence of clinicopathologic covariates on test performance.
We performed a blinded, single-center, case-control study using archived stool samples from 125 ECC patients and 125 individuals with normal colonoscopy (controls); the subjects were randomly assigned to a training or test set at a 1.5:1 ratio. Targeted bisulfite sequencing (TBSeq) was performed on five pairs of preoperative and postoperative sDNA samples from ECC patients to identify DNA methylation biomarkers. Pyrosequencing (PSQ) was used for validation of the candidate biomarkers in large samples. A stepwise logistic regression analysis was applied to the data of the training set to develop a multiplex stool-based assay. The detection performance was further evaluated in the test set and combined set. In addition, the association of detection sensitivity with clinicopathologic covariates were analyzed by χ2 test.
Through TBSeq, the three top hypermethylated genes, paired box 8, Ras-association domain family 1 and secreted frizzled-related protein 2, that were involved in the important cancer pathways were selected as biomarkers. Based on the PSQ results, an sDNA panel containing the three biomarkers was developed by logistic regression modelling. Receiver operating characteristic (ROC) analysis showed that this panel offered an advantage over any single biomarker, FIT or serum CEA in the detection of ECC. Further analysis revealed that the inclusion of FIT could effectively improve the detection accuracy. In the combined set, the sensitivity, specificity and area under the ROC curve for the sDNA panel combining FIT reached 80.0%, 93.6% and 0.918, respectively. Moreover, this multiplex assay maintained excellent performance in the subgroup by tumor stage. Additionally, we found that the detection sensitivity of the multiplex assay was significantly higher in T4 than in T1-3 stage tumors (P = 0.041), but was not affected by tumor site, tumor stage, histological differentiation, age or sex.
The present study identified a novel multiplex stool-based assay, including three sDNA methylation biomarkers and FIT, capable of detecting ECC with high sensitivity and specificity. Importantly, the detection rate by this assay was related to T stage but not tumor site, tumor stage, histological differentiation, etc.
Our study provided a new and promising approach for improvement of ECC screening in the Chinese population. Further demonstrations on a large-scale, prospective multicenter study are needed to conclusively evaluate its value.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiménez Pérez M, Spain; Sharara AI, Lebanon S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1792] [Article Influence: 448.0] [Reference Citation Analysis (1)] |
| 2. | Zhang L, Cao F, Zhang G, Shi L, Chen S, Zhang Z, Zhi W, Ma T. Trends in and Predictions of Colorectal Cancer Incidence and Mortality in China From 1990 to 2025. Front Oncol. 2019;9:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N, Ghai NR, Lee AT, Quesenberry CP, Fireman BH, Doubeni CA. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology. 2018;155:1383-1391.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 4. | Harrison NM, Hjelkrem MC. Bowel cleansing before colonoscopy: Balancing efficacy, safety, cost and patient tolerance. World J Gastrointest Endosc. 2016;8:4-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 5. | Lou S, Shaukat A. Noninvasive strategies for colorectal cancer screening: opportunities and limitations. Curr Opin Gastroenterol. 2021;37:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Bailey JR, Aggarwal A, Imperiale TF. Colorectal Cancer Screening: Stool DNA and Other Noninvasive Modalities. Gut Liver. 2016;10:204-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Hong SN. Genetic and epigenetic alterations of colorectal cancer. Intest Res. 2018;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Ewing I, Hurley JJ, Josephides E, Millar A. The molecular genetics of colorectal cancer. Frontline Gastroenterol. 2014;5:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Boynton KA, Summerhayes IC, Ahlquist DA, Shuber AP. DNA integrity as a potential marker for stool-based detection of colorectal cancer. Clin Chem. 2003;49:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Ahlquist DA. Molecular detection of colorectal neoplasia. Gastroenterology. 2010;138:2127-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 11. | Tse JWT, Jenkins LJ, Chionh F, Mariadason JM. Aberrant DNA Methylation in Colorectal Cancer: What Should We Target? Trends Cancer. 2017;3:698-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Raut JR, Guan Z, Schrotz-King P, Brenner H. Fecal DNA methylation markers for detecting stages of colorectal cancer and its precursors: a systematic review. Clin Epigenetics. 2020;12:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1239] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 14. | Jin H, Wang J, Zhang C. The Value of Multi-targeted Fecal DNA Methylation Detection for Colorectal Cancer Screening in a Chinese Population. J Cancer. 2021;12:1644-1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kim MS, Lee J, Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010;29:181-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Lee EJ, Luo J, Wilson JM, Shi H. Analyzing the cancer methylome through targeted bisulfite sequencing. Cancer Lett. 2013;340:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Morselli M, Farrell C, Rubbi L, Fehling HL, Henkhaus R, Pellegrini M. Targeted bisulfite sequencing for biomarker discovery. Methods. 2021;187:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3811] [Cited by in RCA: 4209] [Article Influence: 323.8] [Reference Citation Analysis (0)] |
| 19. | Delaney C, Garg SK, Yung R. Analysis of DNA Methylation by Pyrosequencing. Methods Mol Biol. 2015;1343:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | LaValley MP. Logistic regression. Circulation. 2008;117:2395-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci. 2016;374:20150202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2837] [Cited by in RCA: 2198] [Article Influence: 244.2] [Reference Citation Analysis (0)] |
| 22. | Zhang H, Meltzer P, Davis S. RCircos: an R package for Circos 2D track plots. BMC Bioinformatics. 2013;14:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 610] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 23. | Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11591] [Cited by in RCA: 22157] [Article Influence: 1704.4] [Reference Citation Analysis (0)] |
| 24. | Ahlquist DA. Multi-target stool DNA test: a new high bar for noninvasive screening. Dig Dis Sci. 2015;60:623-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Verma M, Kumar V. Epigenetic Biomarkers in Colorectal Cancer. Mol Diagn Ther. 2017;21:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Toyota M, Yamamoto E. DNA methylation changes in cancer. Prog Mol Biol Transl Sci. 2011;101:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Strzelczyk JK, Krakowczyk Ł, Owczarek AJ. Methylation status of SFRP1, SFRP2, RASSF1A, RARβ and DAPK1 genes in patients with oral squamous cell carcinoma. Arch Oral Biol. 2019;98:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Rasmussen SL, Krarup HB, Sunesen KG, Pedersen IS, Madsen PH, Thorlacius-Ussing O. Hypermethylated DNA as a biomarker for colorectal cancer: a systematic review. Colorectal Dis. 2016;18:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 29. | Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, Drapkin R, Hirsch MS. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Ehrich M, Turner J, Gibbs P, Lipton L, Giovanneti M, Cantor C, van den Boom D. Cytosine methylation profiling of cancer cell lines. Proc Natl Acad Sci U S A. 2008;105:4844-4849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Miao Z, Wu F, Wei H, Luo Z, Wu K, Zhang J. Enhancer of zeste homolog 2-mediated paired box 8 methylation promotes gastrointestinal stromal tumor progression through Wnt4 downregulation. Cancer Gene Ther. 2021;28:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Anglim PP, Galler JS, Koss MN, Hagen JA, Turla S, Campan M, Weisenberger DJ, Laird PW, Siegmund KD, Laird-Offringa IA. Identification of a panel of sensitive and specific DNA methylation markers for squamous cell lung cancer. Mol Cancer. 2008;7:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Hernández HG, Tse MY, Pang SC, Arboleda H, Forero DA. Optimizing methodologies for PCR-based DNA methylation analysis. Biotechniques. 2013;55:181-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Wani K, Aldape KD. PCR Techniques in Characterizing DNA Methylation. Methods Mol Biol. 2016;1392:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Akika R, Awada Z, Mogharbil N, Zgheib NK. Region of interest methylation analysis: a comparison of MSP with MS-HRM and direct BSP. Mol Biol Rep. 2017;44:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Šestáková Š, Šálek C, Remešová H. DNA Methylation Validation Methods: a Coherent Review with Practical Comparison. Biol Proced Online. 2019;21:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Umer M, Herceg Z. Deciphering the epigenetic code: an overview of DNA methylation analysis methods. Antioxid Redox Signal. 2013;18:1972-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Poulin M, Zhou JY, Yan L, Shioda T. Pyrosequencing Methylation Analysis. Methods Mol Biol. 2018;1856:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Liu X, Wen J, Li C, Wang H, Wang J, Zou H. High-Yield Methylation Markers for Stool-Based Detection of Colorectal Cancer. Dig Dis Sci. 2020;65:1710-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Zhang H, Zhu YQ, Wu YQ, Zhang P, Qi J. Detection of promoter hypermethylation of Wnt antagonist genes in fecal samples for diagnosis of early colorectal cancer. World J Gastroenterol. 2014;20:6329-6335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Mo S, Wang H, Han L, Xiang W, Dai W, Zhao P, Pei F, Su Z, Ma C, Li Q, Wang Z, Cai S, Liu R, Cai G. Fecal Multidimensional Assay for Non-Invasive Detection of Colorectal Cancer: Fecal Immunochemical Test, Stool DNA Mutation, Methylation, and Intestinal Bacteria Analysis. Front Oncol. 2021;11:643136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 362] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 43. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |