Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2546
Peer-review started: January 4, 2022
First decision: March 9, 2022
Revised: March 14, 2022
Accepted: May 7, 2022
Article in press: May 7, 2022
Published online: June 21, 2022
Processing time: 162 Days and 23.9 Hours
With the development of microbiology and metabolomics, the relationship between the intestinal microbiome and intestinal diseases has been revealed. Fecal microbiota transplantation (FMT), as a new treatment method, can affect the course of many chronic diseases such as metabolic syndrome, malignant tumor, autoimmune disease and nervous system disease. Although the mechanism of action of FMT is now well understood, there is some controversy in metabolic diseases, so its clinical application may be limited. Microflora transplantation is recommended by clinical medical guidelines and consensus for the treatment of recurrent or refractory Clostridium difficile infection, and has been gradually promoted for the treatment of other intestinal and extraintestinal diseases. However, the initial results are varied, suggesting that the heterogeneity of the donor stools may affect the efficacy of FMT. The success of FMT depends on the microbial diversity and composition of donor feces. Therefore, clinical trials may fail due to the selection of ineffective donors, and not to faulty indication selection for FMT. A new understanding is that FMT not only improves insulin sensitivity, but may also alter the natural course of type 1 diabetes by modulating autoimmunity. In this review, we focus on the main mechanisms and deficiencies of FMT, and explore the optimal design of FMT research, especially in the field of cardiometabolic diseases.
Core Tip: The success of fecal microbiota transplantation (FMT) depends on the microbial diversity and composition of donor feces. It is newly found that FMT may not only improve insulin sensitivity, but also alter the natural course of type I diabetes by modulating autoimmunity. In this review, we focus on the main mechanisms and deficiencies of FMT, and explore the optimal design of FMT research, especially in the field of cardiometabolic diseases.
- Citation: Zheng L, Ji YY, Wen XL, Duan SL. Fecal microbiota transplantation in the metabolic diseases: Current status and perspectives. World J Gastroenterol 2022; 28(23): 2546-2560
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2546
Most of the research on microorganisms is confined to infectious diseases and the role of microorganisms in human health is largely ignored. The average weight of these microorganisms is about 1.5 kg, equivalent to the weight of the liver. There are 1012–1014 microorganisms, which is 10 times the number of the human body’s own cells, and they are mainly parasitic in the intestinal tract[1]. These symbiotic microorganisms include bacteria, viruses, archaea, fungi and, in some cases, protists, collectively known as the microbiome. The most important advantage of fecal microbiota transplan
During the long process of human evolution, the intestinal flora has coevolved with its host, along with social development, changes in diet, lifestyle and environment. Intestinal symbiotic bacteria can regulate a variety of metabolic activities that cannot be carried out by the human body itself[3]. They can obtain energy by decomposing polysaccharides, proteins and fats in food that cannot be fully digested by the host, and produce a series of metabolites that affect the health of the host. In this process, the intestinal microecosystem is closely related to the host metabolic capacity[4].
As early as 3000 years ago, cow dung was used in India to treat gastrointestinal diseases. As early as the Eastern Jin Dynasty (317–420 AD), a treatment similar to fecal bacterial transplantation, called “Huanglong Soup”, was described in Ge Hong’s “Urgent Prescription for Elbow Reserve”, which was used to treat food poisoning and diarrhea. In traditional Chinese medicine, it is recorded that huanglian and rhubarb, among others, have the curative effect of “quenching thirst” (ancient term for diabetes). Berberine, a monomer component from huanglian, has been recognized internationally for its effect on improving glucose and lipid metabolism earlier. During World War II, German soldiers in North Africa treated diarrhea with camel excrement[5]. At present, FMT is mainly used for the treatment of recurrent Clostridium difficile infection (CDI) in clinical practice, and many clinical trials have confirmed that FMT is a feasible treatment[6].
At present, with the development of fast and accurate high-throughput sequencing technology and the improvement of bioinformatics technology methods, intestinal flora is closely related to metabolic syndrome (MS), type 1 diabetes (T1D) and type 2 diabetes (T2D), various cancers, and autoimmune diseases. Currently, it is believed that the FMT donor should be carefully selected and examined for infectious diseases[7]. However, due to the large difference in metabolism and diet of FMT donors, the effect of transplantation can be different. In this review, the mechanisms and deficiencies of FMT are discussed, and the optimal design of FMT is explored to maximize scientific research and clinical application methods.
The main components of FMT are the gut flora of humans and other species. Humans have evolved to come into contact with a variety of bacteria, including those produced by food fermentation. The oral cavity is an important location of intestinal microbiota, which has an important effect on human health. Studies have shown that children who grow up on farms have a lower risk of asthma; a phenomenon that may be linked to changes in their gut microbiota[8]. In addition, babies born by cesarean section are at increased risk of developing autoimmune diseases, mainly because the initial microbes passed from the vagina to the baby at birth are replaced by skin microbes from the mother and surgical team members, which alter the baby’s gut microbes[9]. An infant’s gut microbiome can be reshaped in breast milk by adding small amounts of bacteria from the mother’s feces, creating a pattern that more closely resembles that of babies born vaginally[10].
FMT has been processed into an odorless and tasteless preparation. In clinical practice, there are three methods of bacterial flora transplantation for patients willing to accept FMT: Upper, middle and lower digestive tract. The methods of transplanting upper digestive tract microflora mainly include oral microflora liquid and oral microflora capsule. The middle digestive tract approach includes a nasointestinal tube, endoscopic biopsy hole, percutaneous endoscopic gastrostomy and jejunal catheterization, endoscopic catheterization such as Transendoscopic enteral tubing (TET)[11]. The lower gastrointestinal pathway includes colonoscopy, colostomy, enema, and colonic pathway TET. Colonic pathway TET is not only used for microflora transplantation, but also for whole colon administration such as mesalazine, hormones and traditional Chinese medicine. As a new endoscopic technique, TET is an important supplement for interventional treatment of inflammatory bowel disease[12].
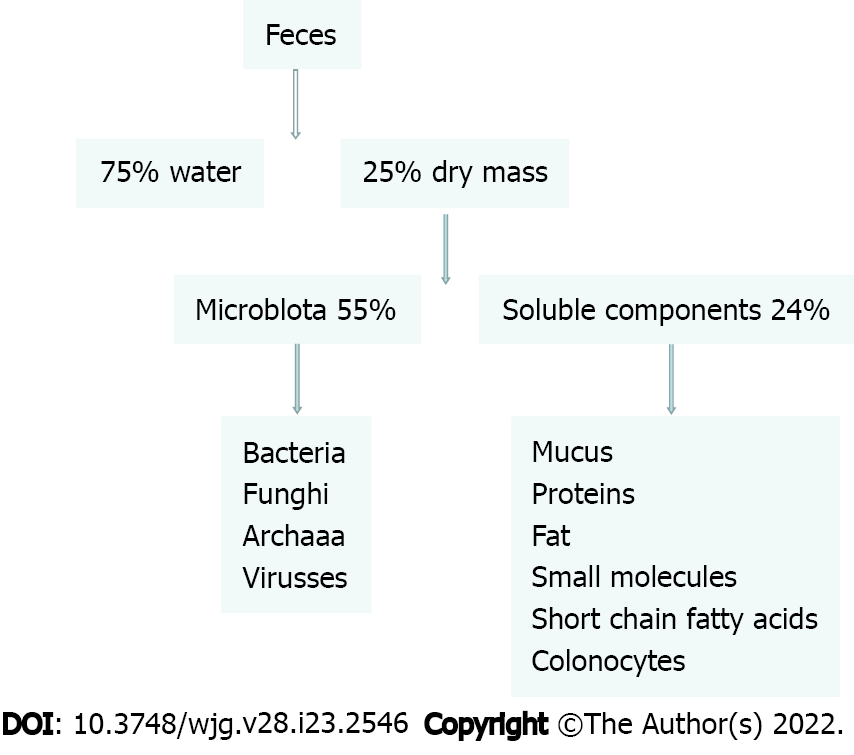
FMT focuses on flora transplantation, but other components, such as phages, should not be ignored, which may be the reason for FMT’s effectiveness in the treatment of recurrent CDI. Therefore, phage research is important, and animal studies have shown that fecal virus transplantation also plays an important role. Analysis of the feces of adults on a classic British diet found that 25% of the 100 g/d excreted was made up of bacteria and 75% of fiber, protein, fat, bile acids and short-chain fatty acids (SCFAs). In most FMT, however, feces are simply mixed with salt water and filtered to remove insoluble substances[13]. Thus, the potential effects of FMT may be partly due to the combined effects of these compounds (Figure 1).
Most studies have focused on fecal transplants from healthy donors (known as FMT allografts). However, autologous fecal transplants have significant advantages[14], such as reducing the risk of infection and increasing the efficiency of transplantation, especially in the treatment of recurrent CDI by freezing their own feces. Autologous fecal transplants are effective in many diseases, but not in disorders caused by intestinal flora disorders, such as inflammatory bowel disease[15]. Studies have shown that disorder of intestinal flora can aggravate the disease, and intestinal inflammation can also affect the composition of intestinal flora[16]. Therefore, it is speculated that fecal biobanks may contain probiotics, which have changed the composition of intestinal flora before the relapse of the disease. This requires further confirmation of the value of probiotics in intestinal flora, but there is insufficient evidence to confirm the value of probiotics collected in clinical remission. Therefore, autologous FMT can improve clinical symptoms by regulating intestinal flora to promote metabolism[17].
In conclusion, regulating the balance of intestinal flora is the primary goal of therapy. Autologous FMT through a duodenal tube or oral capsules can reshape the composition of small intestinal flora, which play an important role in the regulation of autoimmune diseases[18], mainly because the immune system response to antigenic stimuli occurs in the small intestine. It has also been suggested that autologous FMT via the duodenal tube may be valuable in a new method of preserving β-cell function for T1D diagnosis that is more effective than healthy donor FMT[19].
To date, the only reported study of FMT in the treatment of human MS was conducted by Witkowski et al[20]. This study examined the effects of FMT on glucose and lipid metabolism in men with MS in a double-blind randomized controlled trial in nine patients receiving fecal bacteria transplants from lean healthy donors (allograft group) and nine other patients[21]. They received their own fecal bacteria as a control (autologous transplantation group)[22]. After 6 wk of FMT treatment, insulin sensitivity and fecal microbial diversity were significantly increased in the allograft group, while no significant changes were observed in the autograft group. It should be noted that there were individual differences in the efficacy of FMT[23], and Gagliardi et al[24] suggested that the differences might have been more due to different donors than recipients, since the two subjects receiving fecal bacteria from the same donor showed similar benefits[24]. In a randomized, double-blind, controlled trial of fecal bacteria transplan
There is a close relationship between intestinal flora and diabetes mellitus. Some studies have found that the decrease of butyrate- and lactate-producing bacteria is related to the autoimmunity of β cells[28]. In addition, it has been found that the intestinal flora of children with β-cell-related autoimmune diseases lacks Bifidobacterium, and the bacteria producing butyrate and lactate are reduced while Bacteroidetes are increased[24]. Another study in Spain found similar changes in the gut flora of children with T1D, suggesting that structural changes in the gut flora may be associated with T1D[29]. A meta-genomic analysis of the intestinal flora in an included study found that, compared with the control group, patients with T1D had fewer butyrate-producing bacteria and mucin-degrading Prevotella and Akkermansia, and had more lactate-producing bacteria, and bacteria-producing SCFA other than butyrate, such as Bacteroides and Riyanella[30].All of these suggest that gut bacteria may participate in the disorder of immune function in patients with T1D[31].
Dietary fiber can be metabolized and fermented by intestinal bacteria into SCFAs, including acetic acid, propionic acid and butyric acid, which may also be involved in the pathogenesis of metabolic diseases[32]. The types and quantities of SCFAs are thought to vary with the composition of intestinal microbes. In addition to serving as an energy source for intestinal epithelial cells and liver (SCFAs are absorbed by the intestine and transported mainly through the portal vein), SCFAs are thought to have immunomodulatory effects by reducing intestinal permeability[33]. Lipopolysaccharides from intestinal translocation to the portal vein are thought to be involved in obesity-related mild inflammatory responses and insulin resistance in mice[34].
SCFAs produce a small number of microorganisms in T1D, and the incidence of T1D is significantly reduced in nonobese diabetic mice treated with Akkermansia or with a prebiotic diet supplemented with SCFAs[35]. It has been suggested that the restoration of intestinal flora balance through healthy donor FMT may further weaken autoimmune function and β-cell dysfunction[36]. A recent study showed that both healthy autologous and allogeneic FMT attenuates the decline of β-cell function, while donor FMT decreases at a slower rate[37]. Surprisingly, the decay rate of autologous FMT b cells was only 12 mo after three consecutive FMT treatments. Due to the significant changes in microbes from the mouth to the anus during autologous FMT[38], the immune system of the small intestine can be reshaped. Due to the lack of effective immunomodulators to treat T1D, a large number of clinical studies are needed to confirm this[39].
The association between intestinal flora and T2D was first reported by Chinese researchers led by The Shenzhen Huada Institute for Life Sciences and published in Nature in 2012[40]. This study found that the relative abundance of clostridium butyricum and its butyric acid-producing function in Chinese patients with T2D were significantly lower than those in the normal population, and lipopolysaccharide produced by conditional pathogenic Enterobacteriaceae species, hydrogen sulfide proinflammatory function and branched chain amino acid transport function levels were significantly higher than the general population[41]. These changes may be associated with impaired intestinal mucosal barrier function and increased levels of intestinal inflammation in T2D patients[42].
A randomized controlled study showed that autologous FMT can maintain normal metabolism after diet-induced weight loss[43]. It has been observed that obese donor FMT can cause rapid weight gain, so there is a link between the intestinal microbiome, obesity and insulin resistance[44]. On the contrary, non-obese donor FMT can improve insulin resistance in obese patients with MS. Another study found that donor FMT had no effect on glucose metabolism and their diets were metabolically tested. Recent studies have shown that the use of single-dose capsule FMT improves lipid metabolism and insulin resistance, mainly through continuous supplementation of low-fermenter fiber[45]. Therefore, dietary composition may affect insulin resistance of FMT, or metabolites of donor FMT may affect the enteric–brain axis[46].
The relationship between intestinal microflora and nonalcoholic faatty liver disease (NAFLD) is increasingly close, research suggests. Intestinal flora can affect the occurrence and development of NAFLD by changing the composition of intestinal flora, increasing serum endotoxin level and intestinal permeability, producing endogenous alcohol and changing choline metabolism[47]. Animal studies have shown that FMT can improve steatohepatitis in mice induced by high-fat diet, reduce the production of lipids and proinflammatory factors in the liver, regulate the balance of intestinal flora in mice, and increase the abundance of beneficial flora[48]. After FMT treatment, the cecal butyrate concentration and intestinal tight junction protein ZO-1 increased, and the toxin release decreased, thus reducing the inflammatory response[49].
Increased intestinal permeability and metabolic endotoxin caused by changes in intestinal flora composition are involved in the progression of NAFLD in mice, and the severity of NAFLD in mice is increased when special flora are transferred to methionine- and choline-deficient diet, indicating that intestinal flora is involved in the progression of NAFLD[50]. The results of human studies also support the idea that changes in gut flora can contribute to fatty liver disease. Compared with normal subjects, NAFLD patients showed increased intestinal permeability, endotoxemia, increased numbers of g-Proteobacteria, and decreased numbers of Bacteroidetes[51].
Fatty liver often occurs in obese patients. Long-term vegans have a lower risk of NAFLD, which may be related to changes in gut flora. It is suggested that FMT treatment of long-term vegan feces can improve liver inflammation score[52]. Despite the small sample size, this study still found that the inflammatory necrotic tissue score and inflammatory gene expression were reduced after transplantation of vegan fecal flora, which may be an important indicator for predicting the progression of NAFLD to cirrhosis[53]. At the same time, an FMT study in NAFLD patients showed that healthy donor FMT reduced intestinal permeability, which is an important feature that distinguishes NAFLD from other diseases[48]. This study found that magnetic resonance imaging could not make a definitive diagnosis of hepatic adipose degeneration, which must be assessed using gold standard liver histological examination[54].
Since innate and adaptive immune cell reactions occur in the small intestine, immune diseases are usually treated by oral capsules or fresh feces administered through the duodenum under strict anaerobic conditions, in order to ensure that active aerobic and anaerobic bacteria can be transplanted to the maximum extent and thus reshape the intestinal microecological balance[55]. Remodeling of the small intestinal flora is not appropriate for nonimmune diseases or diseases with distal intestinal malformations, but colonic delivery (enema or colonoscopy) may be an option[56].
Whether FMT plays an important role in other diseases besides recurrent CDI needs confirmation. Donor FMT freeze-drying capsules or frozen-solution capsules have been widely used and have gained more support due to their noninvasive administration[57], and it is also convenient for the donor and recipient to make multiple trips to the hospital for transplantation on the same day[58]. Current treatments for CDI include enemas, frozen capsules or freeze-drying formulations. However, it may not be suitable for mild intestinal microecological disorders that do not comply with GMP regulations as compared to fresh feces[59].
Due to the lack of sufficient sample size and establishment of control groups in most clinical studies[55], the conclusions are not reliable, and there is controversy about the preparation of FMT fecal bacteria solution. (1) Selection of stool dilution materials[60]. It is reported that ordinary water (98.5%) has a higher disease remission rate than normal saline (86%) as a stool dilution material, but the recurrence rate of CDI with the former increased > 2 times. Other thinners, such as milk or salt water from plantain, achieved a 94% remission rate; (2) The amount of fecal bacteria liquid transplanted. When the volume of fecal bacteria liquid transplanted is > 500 mL, the remission rate of CDI is 97%, but < 200 mL, the remission rate is only 80%. However, it is difficult to compare the above conclusions because the dilution ratio of feces may vary. Currently, according to the Amsterdam protocol, 200–300 g of donor stool is dissolved in 500 mL normal saline for use (donor stool is preferably fresh within 6 h); and (3) Feasibility of frozen feces. A case report of standardized frozen stool samples used for fecal bacteria transplantation for the treatment of CDI showed that there was no statistical difference in the efficacy of standardized frozen stool compared with fresh stool. Therefore, establishment of stool donation banks and use of standardized frozen stool made fecal bacteria transplantation more feasible in clinical practice[61]. A recent study on the treatment of CDI by oral frozen fecal bacteria capsules showed that no serious adverse reactions occurred in recurrent CDI treated by fecal bacteria transplantation via oral frozen fecal bacteria capsules[62]. The diarrhea relief rate of single administration was 70% (14/20), while four of six patients who did not respond to treatment achieved remission after second administration, resulting in a total remission rate of 90%. This study initially demonstrated the feasibility and safety of fecal bacteria transplantation via frozen fecal bacteria capsules[63].
Protective measures are usually taken to avoid anaerobic bacteria being killed by coming in contact with oxygen[64], but it cannot be completely avoided. Under strict anaerobic conditions, the composition of diluted or filtered microorganisms does not differ significantly before and after the entire procedure, but the activity of the preparation may have been affected[65]. Similarly, prolonged freezing at -80 °C preserved fecal components to a large extent, but whether it had an effect on fecal activity was unclear[66]. However, recent studies have shown that autologous FMT stored in glycerin at -80 °C can completely restore the intestinal lumen and mucosal microbial balance. Whether FMT regulates mild microbial disorders in these studies remains to be confirmed[67].
The diversity of intestinal flora increases with growth and development, and finally forms a complex and relatively stable microbial community at the age of 2–3 years, mainly including bacteria, fungi, viruses and protozoa[68]. There are > 1000 species of bacteria, most of which are obligate anaerobes, including Firmicutes, Bacteroides, Proteobacteria and Actinomycetes, among which Firmicutes and Bacteroides are dominant, accounting for > 90% of all intestinal bacteria[69].
Gut microbiome composition is temporal and spatially specific. Neonatal bacteria from the birth canal colonize the intestine within a few hours after birth. The intestinal microbial composition of early vaginally delivered babies is similar to that of the mother’s vagina, while that of cesarean delivery babies is different[70]. A baby’s gut microbiota can reach the level of a healthy adult at about age 1 year. Most of the microorganisms in the human intestine colonize the colon[71], and the number is 1012 cfu/mL. The microbial content of the jejunum, ileum and duodenum decreases successively, and there are 107, 104 and 103 cfu/mL, respectively. There are also differences in the types of microorganisms rich in each part. In addition, the composition of intestinal mucosa and fecal-associated microorganisms varies[72].
Individuals in the same area may have different gut microbiota. The composition and diversity of intestinal microbiota may influence the therapeutic effect of donor FMT[73], or even insulin resistance. In the past few decades, with the westernization of China’s diet, intestinal microbial diversity has decreased. Preselection of donor FMT may be a feasible way to improve clinical outcomes based on the presence of a specific biological chain. Therefore, an important method to study FMT is to carefully study the baseline data of patients and the microbial composition after FMT[74]. By comparing the baseline data of the donor and the recipient and the microbial composition during a certain period of time, the number of microbes transplanted from the donor to the recipient can be calculated[75]. The most common method is fecal metagenomic sequencing, which identifies microbial species based on specific mononucleotide degeneration[76]. Sequencing techniques combined with bioinformatics analysis reveal the duration of similarity between donor and recipient strains, and how many of the transplanted microbes are likely to restore the original microbial composition[77].
FMT is thought to restore disturbed gut flora to a healthy state either by implanting a donor strain or by other donor-dependent traits, such as the amount of nonbacterial components[78]. However, not all donor gut microbiota are uniform, and comparison of gut microbiota from different donors suggests that microbial diversity and metabolites may be predictors of the success of FMT[79]. In some studies, donor microbiome and metabolomic characteristics may be associated with FMT treatment response. Therefore, the selection of appropriate donor feces is a key factor in the success of FMT[80]. However, few studies of FMT have considered the influence of the variation characteristics of the intestinal microbiome and metabolome of the donor on clinical efficacy[81].
Current studies have confirmed that after FMT treatment, the intestinal flora diversity of the recipient is significantly increased and tends to be the flora characteristics of the donor[82]. Cases that respond to FMT treatment typically show higher microbial diversity. It has been confirmed that intestinal bacterial abundance in donors that respond to FMT is significantly higher than that in donors that do not respond[83].
To date, donor selection methods in FMT studies have included the use of a single donor or the random selection of multiple donors from a group of screened eligible donors[84]. In 2019, Zheng et al[85] first proposed the concept of super donors and believed that the success of FMT depends on the donor[85]. However, the definition of super donor has not been established, and the clinical efficacy of donors before FMT treatment cannot be predictedv[86]. However, the failure of randomly selected single donors may be due to the selection of ineffective donors rather than the incorrect indication selection of FMT[87]. Therefore, an alternative approach is to expose each patient to multiple donors (multidonor transplants) to reduce the risk of receiving FMT from an invalid donor[88]. However, FMT is still in the clinical research stage. Single donors can provide clearer evidence for clinical studies, while multiple donors lead to false negatives or false positives in clinical studies, thus hindering the development of the FMT field and the development of new microbiome therapies[89].
Studies have shown a surprising match between donor and recipient transplants and FMT[90]. The ability to secrete blood group antigens is associated with a reduction in gut microbial diversity, which in turn determines the likelihood of successful transplantation from nonsecreting blood group donors to secreting blood group receptors, which may also apply to human leukocyte antigens (HLAs)[91]. HLA haploidy is an important risk factor for autoimmune diseases such as T1D, and infants with HLA haploidy associated with an increased risk of T1D do form a unique microbiome[92]. What remains to be proven, however, is whether FMT can correct the high-risk microbiome associated with specific HLA haplotypes later in life[93]. In addition, it needs to be considered that intestinal immunoglobulin-secretion-binding bacteria and their components may contribute to the therapeutic effect of donor FMT[94].
To facilitate colonization (also known as transplantation), the recipient’s gut is usually cleaned, most commonly by enemas, laxatives, or broad-spectrum antibiotics[95]. In patients with ulcerative colitis, antibiotic administration after FMT increases the risk of transplant failure, although there is evidence that antibiotic pretreatment improves the efficacy of FMT[96]. Previous studies have shown that antibiotics, metformin, berberine and other drugs can change the intestinal flora, thus affecting the state of the body[97]. A study of Finnish children aged 2–7 years found that macrolide use was associated with subsequent long-term changes in intestinal microbiota composition and metabolism: A decrease in Actinobacteria and an increase in Bacteroidetes and Proteobacteria, decreased biliary saline hydrolyase and increased resistance to macrolides[98]. It is associated with an increased risk of asthma and weight gain. The effect of penicillin on intestinal flora was weaker than that of macrolides[99]. In addition to antibiotics, many Chinese herbal extracts can alter the intestinal flora[100]. A study in Taiwan Chang Gyeong Hospital found that ganoderma lucidum extract can reduce the body weight of high-fat-diet mice, reduce inflammatory response and insulin resistance, reduce intestinal flora Firmi
When a donor transplants microbes to a recipient, the difference in microbial composition between the two may be partly due to lifestyle differences between the donor and recipient[105]. If the recipient’s lifestyle does not change to that of the donor after FMT, then the effect on the recipient’s microbial makeup may disappear over time. People living in different continents and regions have their own unique dietary habits[106]. The diet of Europeans is rich in cheese, butter and other high-fat and high-calorie foods, while the diet of Africans is low in calories and high in dietary fiber[107]. High-throughput sequencing comparing the intestinal microbiota of European children with that of rural children from Burkina Faso, a landlocked country in Western Africa, revealed significant differences between the two[108]. Prevotella and Xylos bacteria, which are associated with cellulose and xylan hydrolysis, were completely absent in the intestinal flora of European children on a high-calorie and high-fat diet, while the intestinal flora of African children on a low-calorie and high-fiber diet was rich in Bacteroidetes, especially Prevotella and Xylos bacteria, while Firmicutes were relatively rare[109]. In addition, African children were found to have significantly more SCFAs in their intestines than European children had, and the abundance of Enterobacteriaceae (mainly Shigella and E. coli) was found to be lower than that of European children[110]. These results suggest that the intestinal flora of African children has adapted to a diet rich in polysaccharides to ensure adequate energy intake from a fiber-rich diet and to reduce the incidence of intestinal inflammatory and infectious diseases[111]. The lack of dietary fiber in the diet of European children may be responsible for the loss of prevosiella and Xylos bacteria associated with cellulose and xylan hydrolysis[112]. Another study found that increased dietary fiber intake increased the diversity of the gut flora, as well as Prevotella abundance[113].
Numerous studies have shown that changes in diet determine microbial composition[114]. When autologous FMT is administered during a particular diet, the beneficial effects of the diet persist even if the diet is no longer continued[115]. Conversely, changes in an individual’s microbial composition also affect diet. A large number of studies have shown that dietary response to FMT may be related to changes in microbial composition[116]. Thus, FMT transplantation using a standardized diet during clinical interventions may be more effective because an important source of microbiome variation has been eliminated, but it has been overlooked in many studies[117].
Although the causal relationship between intestinal microbiota and disease is still unclear, it is sufficient to inspire researchers to implement strategies for disease management by regulating intestinal microbiota[118]. Dietary management, antibiotics, probiotics and other interventions can directly or indirectly enable the reconstruction of intestinal flora[119]. FMT is based on microbiota treatment, in which the isolated functional bacteria are transplanted into the patient to reconstruct intestinal flora and achieve a steady state of the gut microbes so as to attain the purpose of disease treatment[120]. The specific mechanism of FMT has not been clarified yet, and its complex mechanism cannot be replaced and explained by a single strain or single signal[121]. In 2007, the “Human Microbiome Project”, also known as the “Human second Genome Project”, became the cornerstone of human exploration and understanding of intestinal microbes[122]. With the in-depth study of intestinal flora, the causal relationship between intestinal microbes and diseases will become more clear, and the specific action mechanism of FMT will become more clear.
FMT has made great progress in the treatment of recurrent CDI. However, due to infection and repeated use of antibiotics, the diversity of intestinal flora is low and the interactions between microorganisms are affected. Studies have shown that FMT with a healthy diversity of microbiome may increase microbial diversity levels to normal levels and enhance microbial interactions[123]. Recent studies have shown that microorganisms produced by biliary saltase may help improve the efficacy of FMT in the treatment of recurrent CDI, as the enzyme degrades taurocholic acid and effectively inhibits C. difficile[124]. Other studies have shown that FMT treatment can cause some subsequent problems, such as the use of antibiotics to cause bacterial dysregulation, leading to non-C. difficile-dependent colitis recurrence, and thus requiring new FMT corrective treatment[125].
Studies have shown that the most important source of fecal genes is prokaryotic viruses (phages)[126]. Phages are probably also the most overlooked in terms of FMT. Because diarrhea is partially relieved in patients with a small amount of microbial FMT during recurrent CDI, this suggests that phages may play an important role in maintaining host health by regulating gut microbiome composition and its phenotype[127]. Phages play an important role in gene expression of host bacteria and even determine their survival. Thus, FMT may function through donor phage regulation of the recipient flora. Currently, phage transplantation is done through aseptic filtration, which has the advantage of reducing bacterial infection. In addition, a large number of clinical studies are needed to show that phage transplantation has greater application value and potential in some diseases.
The mechanism of action of FMT therapy may be realized through multiple pathways, which may vary according to the FMT condition. However, one of the important mechanisms may be altered microbial metabolite production. This may occur during transplantation or subsequently by newly colonized microorganisms. The effect of the production of large quantities of small molecules by microorganisms on the host needs to be further clarified[128]. The most significant is SCFA butyrate, produced mainly by fibrinolytic enzyme strains, which reduces intestinal permeability and provides nutrients to intestinal cells, producing epigenetic effects[129]. In addition, fibrinolytic enzyme strains has anti-inflammatory properties and can reduce the incidence of T1D in NOD mice[130]. Therefore, FMT may modulate immune activity through autoantigens. In addition, studies have shown reduced production of butyrate strains in T1D[131]. However, when T1D patients were given high concentrations of butyrate, no significant changes were detected in immune cells, and when T1D patients were given FMT, butyrate as an active regulator of protective b cells and immune cells was not detected by metabolomics[132]. As a result, the research impact of using noninvasive biomarkers for microbial metabolism has been largely underestimated[133]. This phenomenon may partly explain some of the differences between rodent and human studies. In addition, it should be made clear that interventional studies cannot completely exclude the potential mechanism of action of butyrate in T1D[134]. Therefore, it is a long and tortuous road to find meaningful microorganisms from clinical observational studies to improve clinical outcomes.
FMT is a new theory and technology that has prospects in the treatment of intestinal microbiome disorders. However, the mechanism of action, ethical issues and effects of FMT are still controversial. The methodology of donor screening, the preparation and state of fecal bacteria solutions, and the approaches to transplantation are not uniform, and there are different reports on the safety and efficacy of FMT treatment[135]. In the future, more and higher-quality randomized controlled clinical trials should be carried out to address the above problems, so as to provide more adequate evidence-based medical evidence[136]. It is certain that with the deepening of scientific research, the mechanism of FMT will be gradually clarified; the intestinal microbial spectrum, microbial metabolites and their association with diseases will be more clear; and the FMT methodology will be more standardized[137]. Despite its limitations, FMT is currently one of the most important tools for studying the role of microorganisms in the pathogenesis of a range of chronic diseases. To improve the effectiveness of studies, further standardization of FMT should be carried out, such as dosage, transplantation method, and whether to use alternate pretreatment of fresh or frozen preparations[138]. In addition, accurate assessments and calculations are required to avoid type I errors in order to accurately assess efficacy. Of course, many meetings and forums are needed to reach consensus.
Donor FMT can restore intestinal microbial function and improve clinical outcomes. Therefore, the question in the future is whether the addition of specific strains of FMT to microbial-targeted therapies can help improve diet and drug therapy to improve human health. Therefore, in order to improve the clinical treatment of recurrent CDI, there is a need for more standardized FMT techniques. Rapid advances in untargeted molecules and bioinformatics have made it possible to analyze in detail the potential mechanisms of action of FMT. These results can identify important microorganisms and their metabolites, which may be used as probiotics, probiotics and epigenetic bacteria to enhance the therapeutic effect of FMT, or even replace FMT, for treatment of metabolic diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dhaliwal A, United States; Romano M, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050-4057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 450] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 2. | Marotz CA, Zarrinpar A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J Biol Med. 2016;89:383-388. [PubMed] |
| 3. | de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. 2017;8:253-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 4. | Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, Paramsothy R, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Lin E, Borody TJ, Wilkins MR, Colombel JF, Mitchell HM, Kaakoush NO. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology. 2019;156:1440-1454.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 5. | Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT, Salazar N, Nogacka AM, Garatachea N, Bossut N, Aprahamian F, Lucia A, Kroemer G, Freije JMP, Quirós PM, López-Otín C. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25:1234-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 6. | Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152:327-339.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 611] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 7. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1564] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 8. | Kang Y, Cai Y. Gut microbiota and obesity: implications for fecal microbiota transplantation therapy. Hormones (Athens). 2017;16:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Sebastián Domingo JJ, Sánchez Sánchez C. From the intestinal flora to the microbiome. Rev Esp Enferm Dig. 2018;110:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Zhang PP, Li LL, Han X, Li QW, Zhang XH, Liu JJ, Wang Y. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta Pharmacol Sin. 2020;41:678-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Song M, Chan AT, Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology. 2020;158:322-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (2)] |
| 12. | Pushpanathan P, Mathew GS, Selvarajan S, Seshadri KG, Srikanth P. Gut microbiota and its mysteries. Indian J Med Microbiol. 2019;37:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Chen X, Devaraj S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr Diab Rep. 2018;18:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Muñoz-Garach A, Diaz-Perdigones C, Tinahones FJ. Gut microbiota and type 2 diabetes mellitus. Endocrinol Nutr. 2016;63:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL, Nieuwdorp M. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 16. | Sidhu M, van der Poorten D. The gut microbiome. Aust Fam Physician. 2017;46:206-211. [PubMed] |
| 17. | Ballini A, Scacco S, Boccellino M, Santacroce L, Arrigoni R. Microbiota and Obesity: Where Are We Now? Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z, Wang T, Luo L, Wang C, Zhao B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019;117:109138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 19. | Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, Aubert G, Alonzo F 3rd, Calcutt NA, Mansuy-Aubert V. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci U S A. 2020;117:26482-26493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127:553-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 614] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 21. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 22. | Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 683] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 23. | Proença IM, Allegretti JR, Bernardo WM, de Moura DTH, Ponte Neto AM, Matsubayashi CO, Flor MM, Kotinda APST, de Moura EGH. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr Res. 2020;83:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, Bonfiglio G, Trancassini M, Passariello C, Pantanella F, Schippa S. Rebuilding the Gut Microbiota Ecosystem. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Illiano P, Brambilla R, Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287:833-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 26. | Woting A, Blaut M. The Intestinal Microbiota in Metabolic Disease. Nutrients. 2016;8:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 27. | Cheng S, Ma X, Geng S, Jiang X, Li Y, Hu L, Li J, Wang Y, Han X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Zhang F, Cui B, He X, Nie Y, Wu K, Fan D; FMT-standardization Study Group. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 29. | Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1375] [Article Influence: 137.5] [Reference Citation Analysis (0)] |
| 30. | Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, Colao A; on behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut microbiota: a new path to treat obesity. Int J Obes Suppl. 2019;9:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 31. | Roman P, Cardona D, Sempere L, Carvajal F. Microbiota and organophosphates. Neurotoxicology. 2019;75:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Haber SL, Raney CRK, Larson TL, Lau JP. Fecal microbiota transplantation for recurrent Clostridioides difficile infection. Am J Health Syst Pharm. 2019;76:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Vangoitsenhoven R, Cresci GAM. Role of Microbiome and Antibiotics in Autoimmune Diseases. Nutr Clin Pract. 2020;35:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Bokoliya SC, Dorsett Y, Panier H, Zhou Y. Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies. Front Cell Infect Microbiol. 2021;11:711055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 35. | Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 36. | Lee P, Yacyshyn BR, Yacyshyn MB. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes Metab. 2019;21:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 37. | Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, Walter J, Madsen KL. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 38. | Wortelboer K, Nieuwdorp M, Herrema H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine. 2019;44:716-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 39. | Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, Ford CB, Bryant JA, Henn MR, Hohmann EL. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17:e1003051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 40. | Ianiro G, Segal JP, Mullish BH, Quraishi MN, Porcari S, Fabiani G, Gasbarrini A, Cammarota G. Fecal microbiota transplantation in gastrointestinal and extraintestinal disorders. Future Microbiol. 2020;15:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60:943-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 42. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 43. | Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 942] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 44. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1137] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 45. | De Musis C, Granata L, Dallio M, Miranda A, Gravina AG, Romano M. Inflammatory Bowel Diseases: The Role of Gut Microbiota. Curr Pharm Des. 2020;26:2951-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Zhang X, Tian H, Chen Q, Qin H, Li N. Fecal microbiota transplantation: standardization or diversification? Sci China Life Sci. 2019;62:1714-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 48. | Burberry A, Wells MF, Limone F, Couto A, Smith KS, Keaney J, Gillet G, van Gastel N, Wang JY, Pietilainen O, Qian M, Eggan P, Cantrell C, Mok J, Kadiu I, Scadden DT, Eggan K. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature. 2020;582:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 49. | Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, Bäckhed F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018;23:27-40.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 512] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 50. | Zhou L, Foster JA. Psychobiotics and the gut-brain axis: in the pursuit of happiness. Neuropsychiatr Dis Treat. 2015;11:715-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Bicknell B, Liebert A, Johnstone D, Kiat H. Photobiomodulation of the microbiome: implications for metabolic and inflammatory diseases. Lasers Med Sci. 2019;34:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Leshem A, Horesh N, Elinav E. Fecal Microbial Transplantation and Its Potential Application in Cardiometabolic Syndrome. Front Immunol. 2019;10:1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Cohen NA, Maharshak N. Novel Indications for Fecal Microbial Transplantation: Update and Review of the Literature. Dig Dis Sci. 2017;62:1131-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 54. | Gong S, Yan Z, Liu Z, Niu M, Fang H, Li N, Huang C, Li L, Chen G, Luo H, Chen X, Zhou H, Hu J, Yang W, Huang Q, Schnabl B, Chang P, Billiar TR, Jiang Y, Chen P. Intestinal Microbiota Mediates the Susceptibility to Polymicrobial Sepsis-Induced Liver Injury by Granisetron Generation in Mice. Hepatology. 2019;69:1751-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 55. | Quaranta G, Sanguinetti M, Masucci L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front Immunol. 2019;10:2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 56. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 57. | Leustean AM, Ciocoiu M, Sava A, Costea CF, Floria M, Tarniceriu CC, Tanase DM. Implications of the Intestinal Microbiota in Diagnosing the Progression of Diabetes and the Presence of Cardiovascular Complications. J Diabetes Res. 2018;2018:5205126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 58. | Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol Behav. 2016;164:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. Challenges in fecal donor selection and screening for fecal microbiota transplantation: A review. Gut Microbes. 2017;8:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 60. | Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J, Holst JJ, Lenaerts K, Kootte RS, Nieuwdorp M, Groen AK, Olde Damink SW, Boekschoten MV, Smidt H, Zoetendal EG, Dejong CH, Blaak EE. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016;24:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 61. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 62. | Singh R, Zogg H, Wei L, Bartlett A, Ghoshal UC, Rajender S, Ro S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J Neurogastroenterol Motil. 2021;27:19-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 63. | Matsuoka K, Mizuno S, Hayashi A, Hisamatsu T, Naganuma M, Kanai T. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014;63:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 936] [Article Influence: 85.1] [Reference Citation Analysis (1)] |
| 66. | Yu F, Han W, Zhan G, Li S, Jiang X, Wang L, Xiang S, Zhu B, Yang L, Luo A, Hua F, Yang C. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging (Albany NY). 2019;11:10454-10467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 67. | Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J, Guan Y, Zhang J, Guo J, He Y, Wang J, Li J, Lin J, Su M, Li G, Wu M, Qiu L, Xiang J, Xie L, Jia W, Zhou W. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 68. | Zou M, Jie Z, Cui B, Wang H, Feng Q, Zou Y, Zhang X, Yang H, Wang J, Zhang F, Jia H. Fecal microbiota transplantation results in bacterial strain displacement in patients with inflammatory bowel diseases. FEBS Open Bio. 2020;10:41-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med. 2019;70:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 70. | Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 71. | Vezza T, Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, Romero M, Sánchez M, Toral M, Martín-García B, Gómez-Caravaca AM, Arráez-Román D, Segura-Carretero A, Micol V, García F, Utrilla MP, Duarte J, Rodríguez-Cabezas ME, Gálvez J. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol Res. 2019;150:104487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Yiu JHC, Chan KS, Cheung J, Li J, Liu Y, Wang Y, Fung WWL, Cai J, Cheung SWM, Dorweiler B, Wan EYF, Tso P, Xu A, Woo CW. Gut Microbiota-Associated Activation of TLR5 Induces Apolipoprotein A1 Production in the Liver. Circ Res. 2020;127:1236-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 73. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 621] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 74. | Huang Z, Chen J, Li B, Zeng B, Chou CH, Zheng X, Xie J, Li H, Hao Y, Chen G, Pei F, Shen B, Kraus VB, Wei H, Zhou X, Cheng L. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis. 2020;79:646-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 75. | Landman C, Quévrain E. [Gut microbiota: Description, role and pathophysiologic implications]. Rev Med Interne. 2016;37:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, Kahn CR. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018;23:2287-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 77. | West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL; in-FLAME Microbiome Interest Group. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135:3-13; quiz 14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 78. | Staley C, Khoruts A, Sadowsky MJ. Contemporary Applications of Fecal Microbiota Transplantation to Treat Intestinal Diseases in Humans. Arch Med Res. 2017;48:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020;503:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 80. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 834] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 81. | Zheng L, Wen XL, Dai YC. Mechanism of Jianpi Qingchang Huashi Recipe in treating ulcerative colitis: A study based on network pharmacology and molecular docking. World J Clin Cases. 2021;9:7653-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Zhang YL, Cai LT, Qi JY, Lin YZ, Dai YC, Jiao N, Chen YL, Zheng L, Wang BB, Zhu LX, Tang ZP, Zhu RX. Gut microbiota contributes to the distinction between two traditional Chinese medicine syndromes of ulcerative colitis. World J Gastroenterol. 2019;25:3242-3255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 83. | Zheng L, Zhang YL, Dai YC, Chen X, Chen DL, Dai YT, Tang ZP. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol. 2017;23:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Chen DL, Dai YC, Zheng L, Chen YL, Zhang YL, Tang ZP. Features of the gut microbiota in ulcerative colitis patients with depression: A pilot study. Medicine (Baltimore). 2021;100:e24845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Zheng L, Zhang YL, Chen X, Chen DL, Dai YC, Tang ZP. Astragalus Polysaccharides Protects Thapsigargin-induced Endoplasmic Reticulum Stress in HT29 Cells. Open Life Sci. 2019;14:494-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 701] [Article Influence: 140.2] [Reference Citation Analysis (4)] |
| 87. | Aron-Wisnewsky J, Clément K, Nieuwdorp M. Fecal Microbiota Transplantation: a Future Therapeutic Option for Obesity/Diabetes? Curr Diab Rep. 2019;19:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 88. | Bibbò S, Ianiro G, Gasbarrini A, Cammarota G. Fecal microbiota transplantation: past, present and future perspectives. Minerva Gastroenterol Dietol. 2017;63:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y, Jiang C, Qiao J. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 90. | Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020;72:1003-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 91. | Green JE, Davis JA, Berk M, Hair C, Loughman A, Castle D, Athan E, Nierenberg AA, Cryan JF, Jacka F, Marx W. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes. 2020;12:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 92. | Dagenais M, Douglas T, Saleh M. Role of programmed necrosis and cell death in intestinal inflammation. Curr Opin Gastroenterol. 2014;30:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Glick LR, Sossenheimer PH, Ollech JE, Cohen RD, Hyman NH, Hurst RD, Rubin DT. Low-Dose Metronidazole is Associated With a Decreased Rate of Endoscopic Recurrence of Crohn's Disease After Ileal Resection: A Retrospective Cohort Study. J Crohns Colitis. 2019;13:1158-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer. 2019;145:2021-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 95. | Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, Dreyfuss JM, Glickman JN, Vlamakis H, Ananthakrishnan A, Kostic A, Garrett WS, Xavier RJ. The Crohn's disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 96. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1867] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 97. | Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, Cleland T, Xavier RJ. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe. 2017;21:603-610.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 98. | Shaw SY, Blanchard JF, Bernstein CN. Association between early childhood otitis media and pediatric inflammatory bowel disease: an exploratory population-based analysis. J Pediatr. 2013;162:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 100. | Park S, Chun J, Han KD, Soh H, Choi K, Kim JH, Lee J, Lee C, Im JP, Kim JS. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol. 2018;24:4798-4808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 101. | Small CL, Xing L, McPhee JB, Law HT, Coombes BK. Acute Infectious Gastroenteritis Potentiates a Crohn's Disease Pathobiont to Fuel Ongoing Inflammation in the Post-Infectious Period. PLoS Pathog. 2016;12:e1005907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Celiberto LS, Graef FA, Healey GR, Bosman ES, Jacobson K, Sly LM, Vallance BA. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155:36-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 103. | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 633] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 104. | van der Sloot KWJ, Amini M, Peters V, Dijkstra G, Alizadeh BZ. Inflammatory Bowel Diseases: Review of Known Environmental Protective and Risk Factors Involved. Inflamm Bowel Dis. 2017;23:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 105. | van der Sloot KWJ, Weersma RK, Dijkstra G, Alizadeh BZ. Development and validation of a web-based questionnaire to identify environmental risk factors for inflammatory bowel disease: the Groningen IBD Environmental Questionnaire (GIEQ). J Gastroenterol. 2019;54:238-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Xu S, Zou H, Zhang H, Zhu S, Zhou R, Li J. Investigation of inflammatory bowel disease risk factors in 4 families in central China. Exp Ther Med. 2018;15:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Allegretti J, Eysenbach LM, El-Nachef N, Fischer M, Kelly C, Kassam Z. The Current Landscape and Lessons from Fecal Microbiota Transplantation for Inflammatory Bowel Disease: Past, Present, and Future. Inflamm Bowel Dis. 2017;23:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Miyoshi J, Bobe AM, Miyoshi S, Huang Y, Hubert N, Delmont TO, Eren AM, Leone V, Chang EB. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep. 2017;20:491-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 109. | Moossavi S, Miliku K, Sepehri S, Khafipour E, Azad MB. The Prebiotic and Probiotic Properties of Human Milk: Implications for Infant Immune Development and Pediatric Asthma. Front Pediatr. 2018;6:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 110. | Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 1151] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 111. | Huang F, Zheng X, Ma X, Jiang R, Zhou W, Zhou S, Zhang Y, Lei S, Wang S, Kuang J, Han X, Wei M, You Y, Li M, Li Y, Liang D, Liu J, Chen T, Yan C, Wei R, Rajani C, Shen C, Xie G, Bian Z, Li H, Zhao A, Jia W. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun. 2019;10:4971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 517] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 112. | Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 113. | Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z, Bai Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol. 2019;9:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 114. | Liu Y, Wang Y, Ni Y, Cheung CKY, Lam KSL, Xia Z, Ye D, Guo J, Tse MA, Panagiotou G, Xu A. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020;31:77-91.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 115. | Gupta A, Khanna S. Fecal Microbiota Transplantation. JAMA. 2017;318:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 116. | Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, Hansen R, Schwerd T, Maspons A, Shamir R, Szajewska H, Thapar N, de Meij T, Mosca A, Vandenplas Y, Kahn SA, Kellermayer R; FMT Special Interest Group of the North American Society of Pediatric Gastroenterology Hepatology, Nutrition, the European Society for Pediatric Gastroenterology Hepatology, Nutrition. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection and Other Conditions in Children: A Joint Position Paper From the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2019;68:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 117. | Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y, Wang A, Chen W, Sun Z, Lu Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10:10665-10679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 118. | Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, Serban IL. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 119. | Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 120. | Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci. 2017;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 121. | Lübbert C, Salzberger B, Mössner J. Fäkaler Mikrobiomtransfer [Fecal microbiota transplantation]. Internist (Berl). 2017;58:456-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 122. | Borsom EM, Lee K, Cope EK. Do the Bugs in Your Gut Eat Your Memories? Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 123. | Coman V, Vodnar DC. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Exp Gerontol. 2020;141:111095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 124. | Zeng SL, Li SZ, Xiao PT, Cai YY, Chu C, Chen BZ, Li P, Li J, Liu EH. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv. 2020;6:eaax6208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 125. | Kc D, Sumner R, Lippmann S. Gut microbiota and health. Postgrad Med. 2020;132:274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Yu F, Jiang R, Han W, Zhan G, Xu X, Jiang X, Wang L, Xiang S, Zhou Q, Liu C, Zhu B, Hua F, Yang C. Gut microbiota transplantation from db/db mice induces diabetes-like phenotypes and alterations in Hippo signaling in pseudo germ-free mice. Aging (Albany NY). 2020;12:24156-24167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Woldeamlak B, Yirdaw K, Biadgo B. Role of Gut Microbiota in Type 2 Diabetes Mellitus and Its Complications: Novel Insights and Potential Intervention Strategies. Korean J Gastroenterol. 2019;74:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 128. | Jia Q, Li H, Zhou H, Zhang X, Zhang A, Xie Y, Li Y, Lv S, Zhang J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc Ther. 2019;2019:5164298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 129. | Barba C, Soulage CO, Caggiano G, Glorieux G, Fouque D, Koppe L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 130. | Gilbert B, Schrenzel J. [Fecal microbiota transplantation : current status and prospects]. Rev Med Suisse. 2019;15:976-983. [PubMed] |
| 131. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 132. | Napolitano M, Covasa M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front Microbiol. 2020;11:590370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Rasmussen TS, Koefoed AK, Jakobsen RR, Deng L, Castro-Mejía JL, Brunse A, Neve H, Vogensen FK, Nielsen DS. Bacteriophage-mediated manipulation of the gut microbiome - promises and presents limitations. FEMS Microbiol Rev. 2020;44:507-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 134. | Smillie CS, Sauk J, Gevers D, Friedman J, Sung J, Youngster I, Hohmann EL, Staley C, Khoruts A, Sadowsky MJ, Allegretti JR, Smith MB, Xavier RJ, Alm EJ. Strain Tracking Reveals the Determinants of Bacterial Engraftment in the Human Gut Following Fecal Microbiota Transplantation. Cell Host Microbe. 2018;23:229-240.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 266] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 135. | Gurram B, Sue PK. Fecal microbiota transplantation in children: current concepts. Curr Opin Pediatr. 2019;31:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Caesar R. Pharmacologic and Nonpharmacologic Therapies for the Gut Microbiota in Type 2 Diabetes. Can J Diabetes. 2019;43:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 137. | Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, Raghuvanshi R, Quinn RA, Britton R, Parameswaran N, McCabe LR. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J Bone Miner Res. 2020;35:801-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 138. | Fuhri Snethlage CM, Nieuwdorp M, Hanssen NMJ. Faecal microbiota transplantation in endocrine diseases and obesity. Best Pract Res Clin Endocrinol Metab. 2021;35:101483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |