Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2527
Peer-review started: December 10, 2021
First decision: March 11, 2022
Revised: March 11, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: June 21, 2022
Processing time: 188 Days and 7.4 Hours
Systemic rheumatic diseases (SRDs) are chronic, inflammatory, autoimmune disorders with the presence of autoantibodies that may affect any organ or system. Liver dysfunction in SRDs can be associated with prescribed drugs, viral hepatitis, alternative hepatic comorbidities and coexisting autoimmune liver diseases (AILDs), requiring an exclusion of secondary conditions before consider
Core Tip: Liver dysfunction in systemic rheumatic diseases (SRDs) can be associated with prescribed drugs, viral hepatitis, alternative hepatic comorbidities and coexisting autoimmune liver diseases (AILDs), requiring an exclusion of secondary conditions before considering liver involvement. In AILDs, it is imperative to identify the overlapping SRDs at an early stage since such a coexistence may influence the disease course and prognosis. Commonly co-occurring SRDs in AILDs are Sjögren syndrome (SS), rheumatoid arthritis (RA) or systemic lupus erythematosus in autoimmune hepatitis, and SS, RA or systemic sclerosis in primary biliary cholangitis. Therapeutic options can be personalized to control coexisting conditions of liver autoimmunity and rheumatic manifestations in AILD-SRD overlap diseases.
- Citation: Wang CR, Tsai HW. Autoimmune liver diseases in systemic rheumatic diseases. World J Gastroenterol 2022; 28(23): 2527-2545
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2527
Systemic rheumatic diseases (SRDs) are chronic, inflammatory, autoimmune disorders with the presence of autoantibodies that may affect any organ or system; they include systemic lupus erythematosus (SLE), Sjögren syndrome (SS), systemic sclerosis (SSc), rheumatoid arthritis (RA), idiopathic inflammatory myopathies (IIM), mixed connective tissue disease (MCTD), systemic vasculitis (SV), etc.[1]. Although SRDs can have liver involvement, most patients only have abnormal liver enzymes without significant changes in histopathology[2,3]. Hepatic dysfunction can be a secondary phenome
The major cause of abnormal liver function test (LFT) in patients with SRDs is associated with medications, i.e. drug-induced liver injury (DILI)[3]. Given that a variety of medications are used in the management of SRDs, it is frequently encountered in clinical practice. High occurrences of DILI in SRDs are due to the chronic or high-dose prescription of medications, the existence of susceptible factors that makes patients prone to hepatotoxicity, and/or the use of herbal or ayurvedic compounds[2,3]. Elevated liver enzymes with predominant cholestatic or hepatocellular damage pattern can be observed in SRDs treated with non-steroidal anti-inflammatory drugs (NSAIDs), synthetic disease modifying anti-rheumatic drugs (SDMARDs), corticosteroids (CS), immunosuppressants, biologic agents or oral small molecules[2]. Most medications only cause a mild elevation in liver enzymes, which reverses with drug cessation. On rare occasions, severely irreversible hepatic damage may occur and progress into chronic liver disease or fulminant hepatic failure. Despite the relative safety with a low-dose prescription, methotrexate, a SDMARD frequently used in SRD-related arthritis, has been reported to cause acute liver dysfunction with confounding factors like concomitant NSAIDs use, and progressive liver fibrosis and cirrhosis can occur when used chronically[4]. It usually occurs after a prolong use for no less than 2 years and with a total accumulated dose of 1.5 g[5]. Notably, there is a risk of hepatitis B virus (HBV) reactivation depending on the dose and duration of CS use and the status of hepatitis B surface antigen and hepatitis B core antibody in SRDs[6]. Furthermore, acute or progressing liver dysfunction can be related to coexisting VH, requiring screen tests for HBV and hepatitis C virus (HCV) infection to provide early antiviral treatment and avoid reactivating or worsening VH after immunosuppressive therapy[3]. Table 1 summaries the hepatic abnormalities associated with the common medications used in SRDs[2,7,8]. Although immune checkpoint inhibitors have altered the therapeutic paradigm in oncological patients, there is undesirable off-target autoimmune reaction causing adverse effects like musculoskeletal manifestations and immune hepatitis, a pan-lobular active hepatitis resembling AIH[9].
| Medications | Hepatic abnormalities | 2Likelihood score category in DILI |
| NSAIDs | LEE, cholestasis, acute liver failure, VBDS | A for diclofenac, ibuprofen, sulindac |
| Glucocorticoids | LEE, NAFLD, acute liver failure, HBV reactivation | A in high dosages |
| Immunosuppressive agents | ||
| Azathioprine | LEE, cholestasis, NRH, peliosis hepatis, VOD | A |
| Mycophenolate mofetil | LEE | D |
| Cyclophosphamide | LEE, VOD | B |
| Cyclosporine | LEE, cholelithiasis | C |
| Tacrolimus | LEE | C |
| Conventional SDMARDs | ||
| Hydroxychloroquine | LEE | C |
| Leflunomide | LEE, acute liver failure, HBV reactivation | B |
| Methotrexate | LEE, NAFLD, HBV reactivation, fibrosis, cirrhosis | A |
| Penicillamine | LEE, cholestasis | A |
| Sulfasalazine | LEE, cholestasis, DRESS | A |
| Biologic/targeted SDMARDs | ||
| Abatacept | LEE, HBV reactivation | C |
| Anakinra | LEE | C |
| Apremilast | Unlikely liver injury | E |
| Belimumab | Unlikely liver injury | E |
| Mepolizumab | Unlikely liver injury | E |
| Rituximab | LEE, HBV reactivation | A |
| TNF blockers1 | LEE, cholestasis, HBV reactivation, AIH | A for infliximab |
| Tocilizumab | LEE, HBV reactivation | C |
| Tofacitinib | Suspected liver injury, potential HBV reactivation | E’ |
| Ustekinumab | Suspected liver injury, possible HBV reactivation | E’ |
Although the liver is the largest lymphoid organ involved in the immune response against invading pathogens and in the maintenance of tolerance to self-molecules, it can also be a target of autoimmune diseases[10]. AILDs are attributed to a complex interplay of socioeconomic, environmental and genetic factors, all of which may participate in their pathogenesis[11]. Most common AILDs are autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), which may occur individually or in combination[12]. These disorders are characterized by hepatic lymphocyte infiltration, elevated liver enzymes, generation of autoantibodies, and associated HLA loci. Coexisting extra-hepatic autoimmune diseases such as SRDs, have been well described in the literature[13]. AIH often goes into disease remission with first-line therapy, including CS alone or plus AZA[14]. PBC has a normal life expectancy if treated early with ursodeoxycholic acid (UDCA) in responsive patients, while no effective therapy has been found to alter the natural course of PSC, except liver transplantation (LT)[15,16]. Some patients with AILDs may eventually progress into end-stage liver disease requiring LT, and with an increased risk of recurrent activities and acute or chronic rejection[17,18]. Currently, AILD research has focused on obtaining a better understanding of the pathogenetic process for identification of new therapeutic targets to reduce morbidity and improve survival[15]. Table 2 demonstrates the demographic, clinical, laboratory, pathological, therapeutic and prognostic characteristics of three common AILDs[11-20].
| Category | AIH | PBC | PSC |
| Demographic | |||
| Sex | Predominant F, 4:1 | Predominant F, 10:1 | Predominant M, 2:1 |
| Age | Any, median 45 yr | Common above 40 yr | Any, typical 30-50 yr |
| Prevalence | Rare, 4-25 per 100000 | Rare, 2-40 per 100000 | Rare, 4-16 per 100000 |
| Laboratory | |||
| Abnormal LFT | Majorly AST/ALT | Majorly ALP/GGT | Majorly ALP/GGT |
| Serum Ig | Elevated IgG | Elevated IgM | Elevated IgG, IgM |
| Autoantibody | I: ANA, ASMA; II: anti-LKM, -LC | ANA, AMA | ANCA |
| HLA-DR | DR3, DR4 | DR8 | DR52 |
| Liver biopsy | |||
| Interface HA | Typical finding | Occasional | Occasional |
| Portal infiltrate | Lymphoplasmacytic | Lymphocytic | Lymphocytic |
| Bile duct lesion | Occasional | Florid duct lesion | Obliterative duct |
| Granuloma | Rare | Typical finding | Rare |
| Diagnosis | AIH score for definite diagnosis | AMA, liver biopsy, Cholestatic LFT | Cholangiography, Cholestatic LFT |
| Coexistent SRD | |||
| SLE | 0.7%-2.8% | 1.3%-3.7% | 1.70% |
| SS | 1.4%-35% | 3.5%-38% | CR |
| SSc | 0.80% | 2.3%-12% | CR |
| RA | 1.6%-5.4% | 1.8%-13% | 1.2%-3.4% |
| IIM | CR | 0.6%-3.1% | CR |
| MCTD | CR | 0.60% | NA |
| SV | 1.60% | 2.20% | CR |
| Sarcoidosis | 0.60% | 2.70% | 0.80% |
| First-line Tx | CS or CS plus AZA | UDCA | No effective therapy |
| Prognosis | Generally responsive to IS, poor prognosis if untreated | Excellent prognosis if responsive to UDCA | Median survival without LT 12-16 yr after diagnosis |
In AILDs, it is imperative to identify the co-occurring SRDs at an early stage by using autoantibody screening, since such a coexistence may influence their natural course and disease prognosis[21]. The patterns of overlap diseases depend predominantly on genetic determinants, with common susceptible loci widely distributing in both disorders[20]. The similar epidemiological links between AILDs and SRDs are further reflected in their shared pathogenesis, best exemplified by the concept of autoimmune epithelitis, i.e., concomitant PBC and SS[22,23]. Furthermore, AILDs and SRDs have common serologic profiles with the presence of particular autoantibodies and hyper-gammaglobulinemia[21,24]. Progressive liver damage can be identified in overlap diseases despite rare complications with liver cirrhosis and hepatic failure[3]. Table 3 shows the reported prevalence of coexisting AILDs in different SRDs.
| Category | AIH | PBC | PSC | AIH/PBC OS |
| SLE | 1.6%-15% | 2.2%-7.5% | CR | CR |
| SS | 0.4%-4.4% | 3.4%-8.9% | CR | CR |
| SSc | CR | 0.8%-3.3% | CR | CR |
| RA | 1.3% | 3.8%-6.3% | CR | CR |
| IIM | CR | 0.7% | CR | CR |
| MCTD | 1.6% | CR | NA | NA |
The therapeutic strategies in AILDs and SRDs are also overlapping, with CS as first-line treatment in most cases, followed by administration of immunosuppressants, and potential application of targeted therapy[21]. Nevertheless, therapeutic options can be personalized to control coexisting conditions of liver autoimmunity and rheumatic manifestations[24]. A collaboration between hepatologists and rheumatologists in clinical practice can lead to significant advances in managing such a complex scenario. Herein, we provide a comprehensive overview on coexisting AILDs in different SRDs and the therapeutic approach in managing these overlap diseases.
SLE is a less common SRD, occurring mostly in women of childbearing age and having heterogenous clinical manifestations affecting any organ or system as well as presenting antinuclear antibody (ANA) and a variety of autoantibodies[25]. The liver is generally not a target organ in SLE and hepatic involvement is not included in the classification or diagnostic criteria. Abnormal LFT is common in SLE, usually with subtle changes, in up to 60% of cases during the disease course, while elevated liver enzymes occur during disease flares in less than 20% of patients[3,26,27]. Hepatic dysfunction in SLE can be classified into primary form due to disease itself or secondary form including DILI, VH, vascular disorders, alternative liver comorbidities and coexisting AILDs[28]. Before considering the liver involvement in SLE, it is necessary to exclude other secondary conditions.
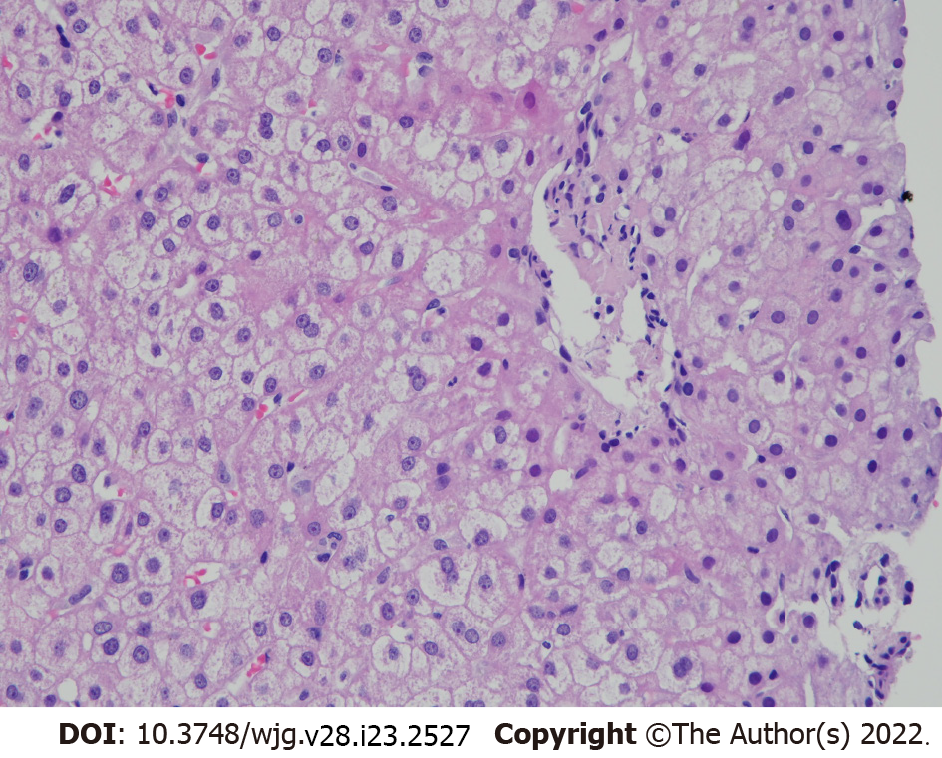
Lupus hepatitis (LH) is reactive liver damage caused by immune-complex deposition, in contrast to lupoid hepatitis, a term used in the 1950s to define what was later known as AIH[29,30]. This manifestation is usually synchronous with disease activity and affects less than 10% of patients[31-33]. It is characterized by asymptomatic transaminasemia with the presence of anti-ribosomal P antibody (commonly known as ARPA) and non-specific histopathological changes. Although CS may help to improve impaired LFT, there is a risk of flare up upon cessation of its use[34]. Figure 1 shows the liver biopsy finding from a patient with LH demonstrating non-specific histopathological changes.
The main cause of liver dysfunction in SLE was salicylate toxicity in the 1950s[35]. Later on, owing to a rare prescription, another common finding of liver biopsy was steatohepatitis, an alternative liver comorbidity. Nowadays, the known risk factors for development of non-alcoholic fatty liver disease (NAFLD) include obesity, physical inactivity and sedentary lifestyle[36], which are also shared by SLE. Furthermore, patients with SLE have been shown to have higher incidences of metabolic syndrome and insulin resistance[37], especially with the use of CS[38]. Increased frequencies of NAFLD have been found in liver biopsy specimens from patients with SLE[39].
The presence of antiphospholipid antibody (aPL) in SLE underlies an increased probability of thrombophilia, leading to antiphospholipid syndrome (APS) with vascular thrombosis[40]. APS can affect the hepatic circulation, causing hepatic arterial thrombosis, portal vein thrombosis and Budd-Chiari syndrome (BCS) as well as the rarely-observed liver infarction and hepatic veno-occlusive disease[40-42]. Notably, BCS resulting from the obstruction of hepatic venous outflow[43] can be an initial manifestation of patients with SLE-associated APS[44]. In particular, aPL has been reported to be involved in the pathogenesis of hepatic nodular regenerative hyperplasia (referred to herein as NRH), small-nodule transformation of hyperplastic hepatocytes with a later development of non-cirrhotic portal hypertension[3,45]. Although higher frequencies of aPL could be detected in AILDs, there was no definite clinical or histological correlation with their presence in such patients[3,46].
Autoimmune gastrointestinal diseases have been linked to SLE with shared pathogenic mechanisms responsible for the development of both disorders[47]. Although AIH and PBC are rare AILDs, the coexistence with either of these diseases is not uncommon among SLE patients with liver enzyme abnormalities, suggesting a causal relationship between their overlap[28,48,49]. Since SLE-PSC overlap disease rarely occurs (but has been described in case reports[28,48]), it remains to be ascertained whether they are casual associations. A review on individual AILD coexisting with SLE is depicted as follows.
AIH is a rare AILD characterized by interface hepatitis as the most specific histological change, and the presence of autoantibodies including anti-liver kidney microsomal-1 (LKM-1)/liver cytosol-1 (LC-1) in type II, a rare subgroup affecting female pediatric patients, and ANA/anti-smooth muscle antibody (ASMA) in type I[50]. Clinical manifestations vary from asymptomatic to nonspecific symptoms of varying severity, including fatigue, malaise, nausea, anorexia and abdominal pain. The criteria established by the International Autoimmune Hepatitis Group (commonly known as the IAHG) are usually used for the diagnosis of AIH[51]. Due to different disease complications and therapeutic regimens between AIH and LH, it is imperative to differentiate between two disease entities[28,34]. AIH may lead to end stage liver disease, and its immunosuppressive therapy needs to be continued for at least 2 years of hepatic biochemical remission before attempting withdrawal[50]. Liver biopsy is highly recommended for their distinguishment[28,34]. LH usually demonstrates lobular infiltrates or occasionally mild periportal infiltrates, whereas AIH is characterized by portal mononuclear infiltrates invading nearby lobules to induce interface hepatitis and form hepatocyte rosettes, followed by confluent lytic necrosis and finally cirrhosis[30].
SLE-AIH overlap disease is defined by fulfilling American College of Rheumatology (commonly known as the ACR) criteria for the classification of SLE in patients who also meet IAIHG criteria for the diagnosis of AIH[34,51,52]. The prevalence of AIH in SLE ranges from 1.6% to 15%, lower in general cohorts and higher in patients with abnormal LFT[39,53-58]. Immunosuppressive treatment for AIH is also effective for SLE, and has been demonstrated to successfully apply to their overlap cases[28]. Most cases with coexisting SLE and AIH responded well to CS or plus immunosuppressants[48]. The long-term outcome for SLE-AIH overlap disease has been observed to be better than AIH alone[34]. Nevertheless, there are sporadic cases of acute liver failure or end-stage liver disease requiring LT[59,60].
PBC is the most common AILD affecting women predominantly. It is characterized by destructive lymphocytic cholangitis involving small bile ducts, and leading to progressive ductopenia, hepatic cholestasis and biliary fibrosis[61]. Clinical manifestations vary from asymptomatic to non-specific symptoms with jaundice and pruritus. According to the guidance from American Association for the Study of Liver Diseases (commonly known as the AASLD), the diagnosis of PBC is established when two of three items are met, including biochemical cholestasis based on alkaline phosphatase (ALP) elevation, presence of antimitochondrial autoantibody (AMA), and histological evidences of nonsuppurative destructive cholangitis and interlobular bile ducts destruction[62]. The nomenclature of PBC has already shifted from cirrhosis to cholangitis, reflecting the dramatically improved prognosis upon first-line UDCA therapy without the development of cirrhosis[63,64].
SLE-PBC overlap is defined by fulfilling the diagnostic criteria for both diseases[34,52,62]. SLE usually affects younger females of childbearing age, whereas PBC is more common in middle-aged women. By genome-wide studies, both diseases have been reported to share the IRF5-TNPO3 gene-spanning haplotype loci[65]. The prevalence of PBC in SLE patients with liver dysfunction ranges from 2.2% to 7.5%, usually lower than that of AIH[39,53-55,57]. In a review of SLE overlapping with PBC, 69% were diagnosed first by PBC, 24% had coexisting SS, and 2 deaths were due to PBC-related hepatic failure[66]. For patients with concomitant SLE and PBC, regardless of the SLE treatment, UDCA is effective first-line therapy for PBC[49].
The diagnosis of PBC-AIH overlap is established with coexisting features of both diseases[67]. Two commonly used criteria for the diagnosis of PBC-AIH overlap syndrome are the IAIHG and Paris criteria[51,68]. Patients with overlapping PBC and AIH have been described to exhibit significantly higher rates of LC, portal hypertension and mortality as compared with those with AIH or PBC alone[69]. PBC with features of AIH should be considered for immunosuppressive therapy[49], while PBC-AIH overlap disease can benefit from combination treatment with UDCA and CS or plus AZA[69]. There is a rare association between SLE and PBC-AIH overlap disease[70]. In a large case series with 71 overlap patients, EHAIDs were identified in 31 (44%), while only 2 (3%) had concurrent SLE[71].
In contrast to western countries, AIH had been considered a rare etiology in the Asia-Pacific region, where VH is a major diagnosis in patients with chronic liver diseases[72]. A very low prevalence of AIH was found in Taiwan in earlier years, raising concerns about under-recognition in an area with a high prevalence of HBV infection and associated liver cirrhosis and hepatocellular carcinoma complications[73], where clinicians would have been more familiar with VH and might have tended to overlook AIH[74]. Recent findings, however, have shown increasing annual incidences of AIH, indicating improved recognition of AIH in this region[72].
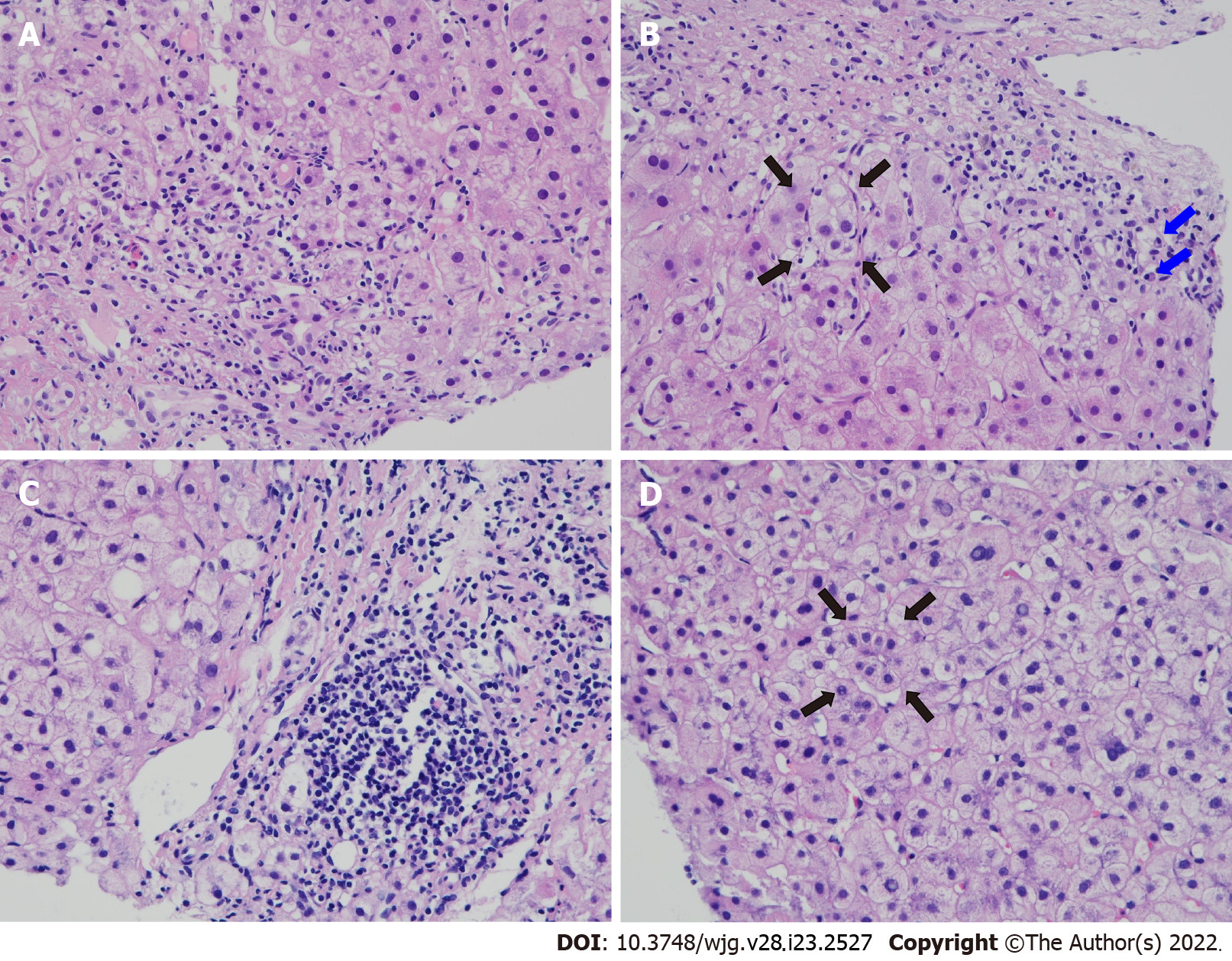
The clinical, laboratory, therapeutic and outcome profiles in 3 patients with SLE-AIH overlap disease diagnosed by ourselves are shown in Table 4. All met IAIHG diagnostic criteria for AIH[51], and case 2 also fulfilled AASLD diagnostic criteria for PBC[62]. Despite CS plus AZA therapy, case 1 progressed into advanced LC, and received LDLT with stabilized LFTs and low SLE activity. Case 2 had the initial diagnosis of AIH with transaminasemia, followed by the development of hepatic cholestasis and sicca symptoms, and finally full-blown manifestations of SLE. Under the diagnosis of coexistent AIH, PBC, SLE and SS, in addition to CS/AZA and UDCA, the patient received B-cell depleting therapy with anti-CD20 monoclonal antibody, with low-dose CS for maintenance, resulting in normalized LFT and low SLE activity. Figure 2 demonstrates histopathological findings in liver biopsy specimens from cases 1 and 2.
| Patient number | 1 | 2 | 3 |
| Sex | Female | Female | Female |
| SLE Dx age | 19 | 50 | 20 |
| ACR criteria | 8/11 | 7/11 | 8/11 |
| AIH Dx age | 26 | 37 | 22 |
| IAIHG score | Definite | Definite | Definite |
| Clinical | |||
| SLE | Skin, joint, renal, hematology, neurology | Skin, joint, renal, hematology, serositis | Skin, joint, renal, hematology, serositis |
| AILD complication | Jaundice, malaise LC with PH | Jaundice, pruritus hepatosplenomegaly | Jaundice, anorexia |
| Coexistent AID | Nil | PBC, SS | Nil |
| Laboratory | |||
| Hemogram | HA, TP | HA, TP, leukopenia | TP, leukopenia |
| Proteinuria autoantibody | 2 g/d | 2.5 g/d | 1 g/d |
| SLE-related | ANA, anti-dsDNA/Sm | ANA, anti-dsDNA/Sm | ANA, anti-dsDNA |
| AILD-related | ASMA | AMA, ASMA | ASMA |
| Others | ARPA, ANCA | ARPA, anti-Ro/La | ARPA |
| 2IgG (mg/dL) | 2130 | 2520 | 1615 |
| 2AST (IU/L) | 1563 | 116 | 97 |
| 2ALT (IU/L) | 1093 | 217 | 177 |
| 2Bil (mg/dL) | 23.8 | 3.7 | 2.4 |
| 2ALP (IU/L) | 432 | 621 | 344 |
| HLA-DR | DR8, DR15 | DR4, DR15 | DR4, DR7 |
| VH | No 3HHV/CMV/EBV | No HHV/CMV/EBV | No HHV/CMV/EBV |
| Treatment | CS/AZA, LDLT and low-dose CS/FK506 after OP | CS/AZA, UDCA RTX and low-dose CS for maintenance | CS/AZA, AZA for maintenance |
| Outcome | Stabilized LFT and low SLEDAI | Normalized LFT and low SLEDAI | Normalized LFT and low SLEDAI |
PSC is a rare cholestatic AILD characterized by persistent, progressive inflammation, fibrosis and stricture of the intrahepatic and extrahepatic bile ducts, leading to cirrhosis[75]. About half of the patients are asymptomatic. The diagnosis is made by cholestasis with ALP elevation and imaging of bile duct strictures, excluding secondary causes. Liver biopsy is indicated only when suspecting overlapping with other AILDs or small-duct PSC, a variant with normal cholangiogram. UDCA is the subject of debate with conflicting data to support its use in PSC[49], and end-stage liver disease requiring LT may develop in affected patients.
Although the association of SLE with PSC is considered to be extremely unusual[34,48], there are several published cases with SLE-PSC overlap disease[76-80]. Furthermore, a 1.7% occurrence of SLE was observed in a Swedish PSC cohort[81]. Whether such a coexistence indicates that both diseases might share common pathogenic pathways remains to be elucidated.
SS is a common SRD affecting the exocrine glands with typical symptoms of dryness of eyes and mouth, histological evidence of focal lymphocytic sialadenitis and the presence of anti-Ro and -La antibodies[82]. The treatment of SS-related dry eyes and mouth is symptomatic with the use of artificial tear and saliva preparation. LFT abnormalities can be identified in nearly half of patients, either persistent or intermittent, and usually mild with cholestatic or hepatocellular pattern[3]. Liver involvement is considered as the most common extra-glandular feature, correlating with the disease activities of SS involving other organs[83]. In a large-scale investigation of 475 cases, after excluding DILI and alternative hepatic comorbidities, the main causes of liver dysfunction were VH in 50% and AILDs in around 20% of patients[84]. Several studies have confirmed a higher prevalence of AILDs among SS, mainly PBC (3.4% to 8.9%), followed by AIH (0.4% to 4.4%)[84-87].
The most frequently associated SRDs in PBC is SS with a prevalence ranging from 3.5% to 38%[88-94]. PBC can be considered a SS of the liver, whereas SS has been equally regarded as a PBC of the exocrine glands[21]. In addition to frequent clinical coexistence and comparable epidemiological features, SS and PBC have similar pathogenic mechanisms and genetic susceptibility backgrounds[95]. Pyruvate dehydrogenase complex E2 subunit, a PBC autoantigen, is also present on the surface of salivary epithelial cells in SS, while HLA-DR2 and -DR3 have been reported as the common susceptibility genes in both disorders[20].
Despite a higher frequency of ASMA than AMA in SS[96], the prevalence of coexisting AIH is lower than PBC[84,85,97]. Owing to a much higher prevalence of SS than SLE in the general population, the occurrence of concomitant SLE and SS in patients with AIH are 0.7% to 2.8% and 0.8% to 7.2%, respectively, lower in SLE than in SS[98-102].
There are published cases and case series describing SS-PSC overlap disease as well as a higher prevalence in small-scale PSC studies[84,103,104], implicating a causative association rather than sporadic occurrence. Notably, almost all reported patients with overlapping SS and PSC have chronic pancreatitis, demonstrating a triad syndrome complex[103]. A possibility of co-occurring IgG4-related disease (IgG4-RD) should be considered in SS-PSC overlap disease with the presentation of autoim
SSc is an uncommon SRD characterized by vasculopathy and fibrosis of the skin and internal organs, with the presence of anti-topoisomerase I and anti-centromere antibodies (ACA) in diffuse and limited cutaneous subsets, respectively[105]. It has a higher mortality rate than other SRDs. The gastrointestinal tract is affected in up to 90% of patients[106], and hepatic fibrosis has been identified at autopsy[107]. Since liver involvement is rarely observed in SSc[3], abnormal LFT should exclude other possibilities first before considering disease per se. There are diverse autoimmune diseases like AILDs co-occurring within SSc patients and their family members[108], suggesting common pathophysiological mechanisms between these disorders.
Increased prevalence of PBC has been observed in SSc, varying from 0.8% to 3.3%[108-112], and there is a 2.3% to 12.4% occurrence of SSc in PBC[90-94,111]. SSc-PBC overlap disease has the presence of both ACA and AMA[113], and tends to occur in older females with the limited cutaneous subset[114]. This overlap disorder has a slower disease progression in comparison with PBC alone; however, survival is similar due to an increase in SSc-related non-liver death. The use of UDCA has been observed to reduce skin lesions in addition to improved hepatic cholestasis in overlap patients[115].
A 0.8% prevalence of SSc has been reported from a AIH cohort[98], and patients with SSc-AIH overlap disease can be found in the literature[116,117]. In a review with 11 cases[117], all had positive ACA and a later presentation of AIH, 9 with limited cutaneous subtype and 3 with AIH-PBC overlap. Despite a risk of scleroderma renal crisis under the higher dosages of CS use, there were normalized or improved LFT without the occurrence of scleroderma renal crisis in overlap patients receiving such a treatment[116].
Overlap condition with large- or small-duct PSC has been observed in patients with SSc[118,119], suggesting that the extensive disturbance of connective tissues in SSc can lead to abnormal collagen deposition in the bile duct epithelium of PSC[120].
RA is a common SRD primarily affecting the joints and causing cartilage and bone damage, with extra-articular presentations and the presence of rheumatoid factor (commonly referred to as RF) and anti-cyclic citrullinated peptide (commonly referred to as CCP) autoantibodies[121]. Among patients with chronic inflammatory joint diseases, liver involvement has been recognized in RA, despite not showing a significant extra-articular manifestation[122]. Elevated liver enzymes have been identified in up to 50% of patients with RA[2]. DILI is not uncommonly observed in RA, especially under the treatment of NSAIDs and SDMARDs including leflunomide, methotrexate, penicillamine and sulfasalazine, all with potential hepatotoxicity[2,7,123]. Patients are at the hazard of developing NAFLD with the risk factors of chronic inflammation and CS use[2]. Prior to the widespread use of methotrexate in RA, the hepatic histopathological findings at autopsy were most commonly mild portal tract inflammation, rarely diffuse fibrosis of advanced grades[124]. Two rare extra-articular manifestations, rheumatoid vasculitis and Felty syndrome, have been reported to cause necrotizing hepatic arteritis with liver rupture and NRH with portal hypertension, respectively[125,126].
There were no differences in the prevalence of HBV and HCV infection in RA as compared with the general population[127]. Nevertheless, immunosuppressive therapy for RA may significantly worsen underlying VH, and further affect the clinical course and disease prognosis, requiring the survey of viral markers and their antibodies before its initiation[123]. Since the use of tumor necrosis factor (TNF) blockades in RA can cause inactive HBV reactivation[7,128], HBsAg-positive individuals should receive anti-viral prophylactic treatment[129]. Although the TNF pathway is involved in perpetuation of hepatic inflammation and fibrosis progression in HCV infection[130], further studies are needed to verify the safety of anti-TNF therapy in HCV-infected patients[131]. Notably, the use of TNF antagonists has been reported to be associated with the development of AIH in RA[7,132].
The most common coexisting AILDs in RA is PBC with a prevalence of 3.8% to 6.3%[53,97,123], while the occurrence of RA in PBC has been reported to be 1.8% to 13%[90-94]. Around 50% of patients with PBC were shown to be positive for RF[133]. Since RA is usually diagnosed before PBC in patients with the overlap disease, AMA should be screened in RA with elevated cholestatic liver enzymes[134]. Genetic studies have shown that RA has HLA-DQB1, STAT4, IRF5, MMEL1 and CTLA4 genes in common with PBC, predisposing to develop PBC in RA with the overlapping genetic trait[135]. Potentially hepatotoxic drugs used in RA can be avoided in patients with RA-PBC overlap disease[123].
AIH is rarely observed in RA with a 1.3% prevalence reported from patients with liver dysfunction[97]. Furthermore, in patients with AIH, there is a 1.6% to 5.4% prevalence of RA[98,100-102]. AIH can be diagnosed during the RA progression as acute or chronic hepatitis, but rarely fulminant hepatic failure[123]. In addition, in patients with AIH-PBC overlap disease, RA is accounting for an occurrence of 4.2%[71].
High circulating levels of TNF were found in AIH, while a TNF antagonist etanercept has been demonstrated to improve the AIH histological lesions in RA[136]. Nevertheless, anti-TNF therapy can induce the production of autoantibodies, including ANA and ASMA, leading to the development of distinct autoimmune diseases[137]. Notably, anti-TNF-inhibitor-associated AIH (also known as ATIAIH), a serious idiosyncratic DILI, has been well documented in a large-scale analysis of 389 cases[138]. ATIAIH has a female predominance, a period of 3-14 mo between starting therapy and AIH occurrence, and improvement upon medication stoppage and CS use. Infliximab is the most frequently administrated medication, and RA is the most commonly reported indication.
There was a 1.2% and a 3.4% prevalence of RA in two large-scale PSC cohorts[81,139]. In patients with RA-PSC overlap disease, the presence of HLA-DR4 has been reported to have unusual progression to cirrhosis, 14-48 mo after the diagnosis of PSC[140], implicating a clinical marker at a high risk of cirrhosis development.
Psoriatic arthritis (PsA) is a less common SRD with psoriasis (PsO) and inflammatory arthritis, associating with extra-articular manifestations which have an impact on their therapeutic regimens[141]. Similar to RA, liver enzyme abnormalities in PsA and PsO can be caused by comorbid NAFLD and used medications including NSAIDs and conventional or biologic/targeted SDMARDs. Despite an increased association of AIH in PsA and PsO[142], these patients might be under anti-TNF therapy, and both diseases are commonly observed complications in ATIAIH[138].
IIM including polymyositis (PM) and dermatomyositis (DM), an uncommon group of SRDs with the presence of myositis-specific/associated antibodies, have weakness due to skeletal muscle inflammation and extra-muscular involvement[143]. Since transaminases are also muscle-derived enzymes with increased levels during IIM disease activity, an increase of aspartate aminotransferase and alanine aminotransferase more than creatine kinase or an alteration of cholestatic enzymes should consider a possibility of hepatic dysfunction[3]. During the first 3 years to 5 years after the onset of DM, the risk of cancer is increased, rarely hepatocellular carcinoma. Since DM can be associated with malignancy as a paraneoplastic syndrome[144], sporadic cases had HBV-associated hepatocellular carcinoma with a concurrent or later diagnosis of DM[145,146].
Although IIM usually occur alone, these SRDs may associate with other extra-muscular autoimmune diseases including AILDs, more frequently in patients with PM than DM[147]. Positive AMA could be identified in 2.5% of patients with IIM[148], and there were sibling cases of familial clustering with PBC-PM overlap disease[149]. PBC can be identified in IIM with a prevalence of 0.7%[148], while the occurrence of PM in PBC ranges from 0.6% to 3.1%[90,92,93]. There are sporadic cases with PM coexisting with AIH, AIH-PBC overlap disease or PSC[150-152].
In addition to the presence of anti-U1 small nuclear ribonucleoprotein (known as snRNP) antibody in high titers, MCTD has distinct features including Raynaud's phenomenon and puffy hands as well as mixed findings from PM, SLE and SSc[153]. It is a rare SRD with a strong HLA linkage, distinctly differing from ethnically matched healthy controls and other SRDs. Hepatic dysfunction occurs in MCTD usually caused by DILI and pulmonary hypertension-related liver congestion[97,153]. Coexistent AILDs are rarely observed in patients with MCTD[153]. In addition to published case reports, a 1.6% prevalence of AIH was found in MCTD[154], while a 0.6% prevalence of MCTD could be identified in PBC[90]. There was no observed association with MCTD in two PSC case series[81,141].
SV is a rare SRD characterized by inflammation of vascular walls, resulting in a broad spectrum of clinical manifestations dependent on the site, type, and size of involved vessels[155]. Although the diagnosis relies on clinical presentations confirmed by histopathological findings, large/medium and small vessel involvement can be supported by angiographical examinations and laboratory tests (e.g., ANCA), respectively[155,156]. Owing to hepatic vascular involvement[2,53], polyarteritis nodosa (referred to herein as PAN), a medium-vessel SV associated with HBV infection[157], may have elevated liver enzymes. A 2.2% prevalence of SV has been reported from a large-scale PBC series with 361 cases[94], while a 1.6% occurrence of SV was identified in a 122-patient AIH series[98]. There were sporadic cases of AIH coexisting with PAN[158].
Testing for ANCA can support the diagnosis of ANCA-associated vasculitis including eosinophilic granulomatosis with polyangiitis (also referred to as EGPA), granulomatosis with polyangiitis and microscopic polyangiitis (also referred to as MPA) in spite of seropositivity in only one-third of EGPA cases[159]. Notably, ANCA has a diagnostic relevance beyond SV, justifying its occurrence in suspected type I AIH which is lacking conventional autoantibodies[160]. AILDs usually develop atypical perinuclear-ANCA not targeting the classical myeloperoxidase with the positive frequencies highest in patients with PSC[21,156]. There is no clinical nor prognostic value of ANCA testing in patients with AILDs. This atypical autoantibody, referred to as peripheral anti-nuclear neutrophil antibody, can react with beta-tubulin isotype 5 and shares structural homology with the intestinal bacterial protein FtsZ[161]. Nevertheless, it is not specific for AILDs, and it is also present in VH and alcoholic liver disease[162]. Interestingly, ANCA was detected in the bile of PSC patients and correlated with the severity of bile duct stricture[163]. Sixteen cases of ANCA-associated vasculitis-AILD overlap disease have been reported, with twelve involving women, PBC in eleven, and MPA in eight[164-166].
Adult-onset Still’s disease (AOSD) is a rare SRD usually affecting young adults, with spiking fever, polyarthritis, evanescent rash and marked hyperferritinemia as well as uncommon life-threatening macrophage activation syndrome[167]. In medical practice, hyperferritinemia is a non-specific finding related to iron overload in only 10% of cases such as hereditary hemochromatosis, while underlying causes attributing to a reactive increase in the rest 90% patients such as AOSD[168]. Hepatic dysfunction is commonly observed in AOSD, mostly due to the disease itself and without any specific histological finding[97,167]. Coexisting AILDs have rarely been observed, and there are sporadic cases of AIH-AOSD overlap disease[169].
Behçet’s disease (BD) is a SRD with a variable worldwide prevalence, characterized by vasculitis affecting the small/Large venous and arterial vessels, and presenting with orogenital ulcers, ocular lesions and systemic involvement[170]. The liver is rarely involved, and the commonest hepatic complication is BCS with thrombosis of the inferior vena cava and hepatic vein[171]. Elevated ALP levels of liver origin has been reported in 10% of patients, with a correlation to disease activity[172]. Case reports of Behçet’s disease concomitant with AIH or PBC can be found in the literature[173,174].
IgG4-RD is a rare SRD, characterized by elevated serum IgG4 concentrations and fibroinflammation in the affected tissues, with dense lymphoplasmacytic infiltrates rich in IgG4-positive plasma cells and storiform fibrosis[175]. Cases of type I AIP and IgG4-related sclerosing cholangitis (commonly referred to as IgG4-SC), two common forms of IgG4-RD usually occurring in combination, have painless jaundice and cholestatic LFT abnormalities due to liver involvement[176,177]. Although CS has favorable therapeutic efficacy[49], AIP and IgG4-SC are associated with significant morbidity and mortality due to extra-pancreatic organ failure and malignancy[176]. AIP has been reported to be associated with PBC and PSC[178,179]. Infiltrating IgG4-positive plasma cells can be observed in the AIH liver, suggesting involvement of IgG4 in its pathogenesis[180]. Nevertheless, the disease concept of IgG4-AIH remains to be established[181].
Sarcoidosis is an uncommon SRD, characterized by the formation of noncaseating granulomas in various organs, predominantly the lungs, lymphatic system, skin, and eyes, or a different combination of these sites[182]. Abnormal LFT has been observed in one-fourth of patients with chronic sarcoidosis; among which, 15% are suspected of having liver involvement with cholestatic pattern of injury[183]. Although hepatic sarcoidosis is mainly asymptomatic, it can progress to LC, while such cases are rare[184]. AILDs coexisting with sarcoidosis have been reported, having a prevalence of 0.6% in AIH and 0.8% in PSC[81,99]. Several case reports have described the association of sarcoidosis with PBC[88]. A 2.7% prevalence of sarcoidosis was found in a PBC cohort from Greece[185], whereas an epidemiological study with 1510 patients from the United Kingdom failed to show an association between the two disorders[186].
Relapsing polychondritis is a rare SRD, characterized by cartilaginous inflammation throughout the body, especially involving the hyaline cartilage of the ears, nose and joints, and the respiratory tract[187]. Liver involvement with cholestatic hepatic dysfunction has been observed scarcely in such patients[188]. The association of relapsing polychondritis with AILDs has been reported with PBC or PSC overlap diseases[189,190].
SRDs are chronic, inflammatory, autoimmune disorders with the presence of autoantibodies that may affect any organ or system. Liver dysfunction in SRDs can be associated with prescribed drugs, VH, alternative hepatic comorbidities and coexisting AILDs, requiring an exclusion of secondary conditions before considering liver involvement. The patterns of overlap diseases depend predominantly on genetic determinants with common susceptible loci widely distributed in both disorders. In AILDs, it is important to identify the overlapping SRDs at an early stage, since such a coexistence may influence the disease course and prognosis. Commonly co-occurring SRDs in AILDs are SS, RA or SLE in AIH, and SS, RA or SSc in PBC. Owing to different disease complications and therapies, it is imperative to differentiate between SLE liver involvement and SLE-AIH overlap disease. Therapeutic options can be personalized to control coexisting conditions of liver autoimmunity and rheumatic manifestations in AILD-SRD overlap diseases. The collaboration between hepatologists and rheumatologists in clinical practice can lead to significant advances in managing such a complex scenario.
The authors are indebted to Dr. IC Wu (Division of Gastroenterology and Hepatology) for his valuable comments and to other doctors at the National Cheng Kung University Hospital involved in the diagnosis and management of reported patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan X, China; Jagdish RK, India; Malnick SDH, Israel S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Bossuyt X, De Langhe E, Borghi MO, Meroni PL. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol. 2020;16:715-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 2. | Gebreselassie A, Aduli F, Howell CD. Rheumatologic Diseases and the Liver. Clin Liver Dis. 2019;23:247-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | De Santis M, Crotti C, Selmi C. Liver abnormalities in connective tissue diseases. Best Pract Res Clin Gastroenterol. 2013;27:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, Bombardier C, Carmona L, van der Heijde D, Bijlsma JW, Boumpas DT, Canhao H, Edwards CJ, Hamuryudan V, Kvien TK, Leeb BF, Martín-Mola EM, Mielants H, Müller-Ladner U, Murphy G, Østergaard M, Pereira IA, Ramos-Remus C, Valentini G, Zochling J, Dougados M. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Conway R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J Hepatol. 2017;9:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (3)] |
| 6. | Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (1)] |
| 7. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012– . [PubMed] |
| 8. | Hayashi PH. Drug-Induced Liver Injury Network Causality Assessment: Criteria and Experience in the United States. Int J Mol Sci. 2016;17:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Sanjeevaiah A, Kerr T, Beg MS. Approach and management of checkpoint inhibitor-related immune hepatitis. J Gastrointest Oncol. 2018;9:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Gao B. Basic liver immunology. Cell Mol Immunol. 2016;13:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Lee BT, Tana MM, Kahn JA, Dara L. We Are Not Immune: Racial and Ethnic Disparities in Autoimmune Liver Diseases. Hepatology. 2021;74:2876-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20 Suppl 1:S15-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Wong GW, Heneghan MA. Association of Extrahepatic Manifestations with Autoimmune Hepatitis. Dig Dis. 2015;33 Suppl 2:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 15. | Tanaka A. Emerging novel treatments for autoimmune liver diseases. Hepatol Res. 2019;49:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Engel B, Taubert R, Jaeckel E, Manns MP. The future of autoimmune liver diseases - Understanding pathogenesis and improving morbidity and mortality. Liver Int. 2020;40 Suppl 1:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Chen C, Ke R, Yang F, Cai Q, Liu J, Huang X, Chen J, Xu F, Jiang Y. Risk factors for recurrent autoimmune liver diseases after liver transplantation: A meta-analysis. Medicine (Baltimore). 2020;99:e20205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Heinemann M, Liwinski T, Adam R, Berenguer M, Mirza D, Malek-Hosseini SA, Heneghan MA, Lodge P, Pratschke J, Boudjema K, Paul A, Zieniewicz K, Fronek J, Mehrabi A, Acarli K, Tokat Y, Coker A, Yilmaz S, Karam V, Duvoux C, Lohse AW, Schramm C; all the other contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Long-term outcome after living donor liver transplantation compared to donation after brain death in autoimmune liver diseases: Experience from the European Liver Transplant Registry. Am J Transplant. 2022;22:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Trivedi PJ, Hirschfield GM. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut. 2021;70:1989-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 20. | Guo L, Zhou L, Zhang N, Deng B, Wang B. Extrahepatic Autoimmune Diseases in Patients with Autoimmune Liver Diseases: A Phenomenon Neglected by Gastroenterologists. Gastroenterol Res Pract. 2017;2017:2376231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Selmi C, Generali E, Gershwin ME. Rheumatic Manifestations in Autoimmune Liver Disease. Rheum Dis Clin North Am. 2018;44:65-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren's syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Parisis D, Chivasso C, Perret J, Soyfoo MS, Delporte C. Current State of Knowledge on Primary Sjögren's Syndrome, an Autoimmune Exocrinopathy. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Sirotti S, Generali E, Ceribelli A, Isailovic N, De Santis M, Selmi C. Personalized medicine in rheumatology: the paradigm of serum autoantibodies. Auto Immun Highlights. 2017;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 25. | Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1845] [Cited by in RCA: 2066] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 26. | Runyon BA, LaBrecque DR, Anuras S. The spectrum of liver disease in systemic lupus erythematosus. Report of 33 histologically-proved cases and review of the literature. Am J Med. 1980;69:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 138] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J Clin Gastroenterol. 2011;45:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | González-Regueiro JA, Cruz-Contreras M, Merayo-Chalico J, Barrera-Vargas A, Ruiz-Margáin A, Campos-Murguía A, Espin-Nasser M, Martínez-Benítez B, Méndez-Cano VH, Macías-Rodríguez RU. Hepatic manifestations in systemic lupus erythematosus. Lupus. 2020;29:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Mackay IR, Taft LI, Cowling DC. Lupoid hepatitis and the hepatic lesions of systemic lupus erythematosus. Lancet. 1959;1:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Adiga A, Nugent K. Lupus Hepatitis and Autoimmune Hepatitis (Lupoid Hepatitis). Am J Med Sci. 2017;353:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Piga M, Vacca A, Porru G, Cauli A, Mathieu A. Liver involvement in systemic lupus erythematosus: incidence, clinical course and outcome of lupus hepatitis. Clin Exp Rheumatol. 2010;28:504-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Zheng RH, Wang JH, Wang SB, Chen J, Guan WM, Chen MH. Clinical and immunopathological features of patients with lupus hepatitis. Chin Med J (Engl). 2013;126:260-266. [PubMed] |
| 33. | Ohira H, Takiguchi J, Rai T, Abe K, Yokokawa J, Sato Y, Takeda I, Kanno T. High frequency of anti-ribosomal P antibody in patients with systemic lupus erythematosus-associated hepatitis. Hepatol Res. 2004;28:137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Afzal W, Haghi M, Hasni SA, Newman KA. Lupus hepatitis, more than just elevated liver enzymes. Scand J Rheumatol. 2020;49:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Horizon AA, Wallace DJ. Risk:benefit ratio of nonsteroidal anti-inflammatory drugs in systemic lupus erythematosus. Expert Opin Drug Saf. 2004;3:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Tanaka N, Kimura T, Fujimori N, Nagaya T, Komatsu M, Tanaka E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J Gastroenterol. 2019;25:163-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (4)] |
| 37. | Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, Stein CM. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. 2007;66:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Wang CR, Tsai HW. Anti- and non-tumor necrosis factor-α-targeted therapies effects on insulin resistance in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. World J Diabetes. 2021;12:238-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Chowdhary VR, Crowson CS, Poterucha JJ, Moder KG. Liver involvement in systemic lupus erythematosus: case review of 40 patients. J Rheumatol. 2008;35:2159-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 545] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 41. | Uthman I, Khamashta M. The abdominal manifestations of the antiphospholipid syndrome. Rheumatology (Oxford). 2007;46:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Guiu B, Loffroy R, Cercueil JP, Sagot P, Krausé D, Tixier H. MRI diagnosis and follow-up of hepatic infarction in a patient with antiphospholipid syndrome in early pregnancy. Arch Gynecol Obstet. 2011;283:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol. 2007;13:2693-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Pandiaraja J, Sathyaseelan A. Budd- Chiari Syndrome as an Initial Manifestation of Systemic Lupus Erythematosus. J Clin Diagn Res. 2016;10:OD01-OD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 46. | Branger S, Schleinitz N, Veit V, Martaresche C, Bourlière M, Roblin X, Garcia S, San Marco M, Camoin L, Durand JM, Harlé JR. [Auto-immune hepatitis and antiphospholipids]. Rev Med Interne. 2007;28:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Alves SC, Fasano S, Isenberg DA. Autoimmune gastrointestinal complications in patients with systemic lupus erythematosus: case series and literature review. Lupus. 2016;25:1509-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Bessone F, Poles N, Roma MG. Challenge of liver disease in systemic lupus erythematosus: Clues for diagnosis and hints for pathogenesis. World J Hepatol. 2014;6:394-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Ali AH, Carey EJ, Lindor KD. The management of autoimmunity in patients with cholestatic liver diseases. Expert Rev Gastroenterol Hepatol. 2016;10:73-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet. 2013;382:1433-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 51. | Wiegard C, Schramm C, Lohse AW. Scoring systems for the diagnosis of autoimmune hepatitis: past, present, and future. Semin Liver Dis. 2009;29:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7731] [Cited by in RCA: 8653] [Article Influence: 309.0] [Reference Citation Analysis (0)] |
| 53. | Matsumoto T, Kobayashi S, Shimizu H, Nakajima M, Watanabe S, Kitami N, Sato N, Abe H, Aoki Y, Hoshi T, Hashimoto H. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Efe C, Purnak T, Ozaslan E, Ozbalkan Z, Karaaslan Y, Altiparmak E, Muratori P, Wahlin S. Autoimmune liver disease in patients with systemic lupus erythematosus: a retrospective analysis of 147 cases. Scand J Gastroenterol. 2011;46:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Takahashi A, Abe K, Saito R, Iwadate H, Okai K, Katsushima F, Monoe K, Kanno Y, Saito H, Kobayashi H, Watanabe H, Ohira H. Liver dysfunction in patients with systemic lupus erythematosus. Intern Med. 2013;52:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Oka H. The survey of autoimmune hepatitis in Japan. In: Annual Report of the Study Group on Severe Hepatitis. Tokyo: Japanese Ministry of Health and Welfare, 1988: 235-241. |
| 57. | Heijke R, Ahmad A, Frodlund M, Wirestam L, Dahlström Ö, Dahle C, Kechagias S, Sjöwall C. Usefulness of Clinical and Laboratory Criteria for Diagnosing Autoimmune Liver Disease among Patients with Systemic Lupus Erythematosus: An Observational Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Tamai Y, Ito K, Kin F, Fukase M. American rheumatism association (ARA) preliminary criteria for the classification of systemic lupus erythematosus and autoimmune hepatitis. Rheumachi. 1974;14:88-94. |
| 59. | Barthel HR, Wallace DJ, Klinenberg JR. Liver transplantation in patients with systemic lupus erythematosus. Lupus. 1995;4:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Wang CR, Wu IC, Tsai HW. An overlap syndrome involving systemic lupus erythematosus and autoimmune hepatitis in a patient receiving a living-donor liver transplantation. Lupus. 2020;29:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396:1915-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 62. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 63. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A, Vierling JM, Poupon R. Changing nomenclature for PBC: From 'cirrhosis' to 'cholangitis'. J Hepatol. 2015;63:1285-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Floreani A, Tanaka A, Bowlus C, Gershwin ME. Geoepidemiology and changing mortality in primary biliary cholangitis. J Gastroenterol. 2017;52:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Kottyan LC, Zoller EE, Bene J, Lu X, Kelly JA, Rupert AM, Lessard CJ, Vaughn SE, Marion M, Weirauch MT, Namjou B, Adler A, Rasmussen A, Glenn S, Montgomery CG, Hirschfield GM, Xie G, Coltescu C, Amos C, Li H, Ice JA, Nath SK, Mariette X, Bowman S; UK Primary Sjögren's Syndrome Registry, Rischmueller M, Lester S, Brun JG, Gøransson LG, Harboe E, Omdal R, Cunninghame-Graham DS, Vyse T, Miceli-Richard C, Brennan MT, Lessard JA, Wahren-Herlenius M, Kvarnström M, Illei GG, Witte T, Jonsson R, Eriksson P, Nordmark G, Ng WF; UK Primary Sjögren's Syndrome Registry, Anaya JM, Rhodus NL, Segal BM, Merrill JT, James JA, Guthridge JM, Scofield RH, Alarcon-Riquelme M, Bae SC, Boackle SA, Criswell LA, Gilkeson G, Kamen DL, Jacob CO, Kimberly R, Brown E, Edberg J, Alarcón GS, Reveille JD, Vilá LM, Petri M, Ramsey-Goldman R, Freedman BI, Niewold T, Stevens AM, Tsao BP, Ying J, Mayes MD, Gorlova OY, Wakeland W, Radstake T, Martin E, Martin J, Siminovitch K, Moser Sivils KL, Gaffney PM, Langefeld CD, Harley JB, Kaufman KM. The IRF5-TNPO3 association with systemic lupus erythematosus has two components that other autoimmune disorders variably share. Hum Mol Genet. 2015;24:582-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Shizuma T. Clinical Characteristics of Concomitant Systemic Lupus Erythematosus and Primary Biliary Cirrhosis: A Literature Review. J Immunol Res. 2015;2015:713728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E; International Autoimmune Hepatitis Group. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 68. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 480] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 69. | To U, Silveira M. Overlap Syndrome of Autoimmune Hepatitis and Primary Biliary Cholangitis. Clin Liver Dis. 2018;22:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | González LA, Orrego M, Ramírez LA, Vásquez G. Primary biliary cirrhosis/autoimmune hepatitis overlap syndrome developing in a patient with systemic lupus erythematosus: a case report and review of the literature. Lupus. 2011;20:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Efe C, Wahlin S, Ozaslan E, Berlot AH, Purnak T, Muratori L, Quarneti C, Yüksel O, Thiéfin G, Muratori P. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Tanaka A, Ma X, Yokosuka O, Weltman M, You H, Amarapurkar DN, Kim YJ, Abbas Z, Payawal DA, Chang ML, Efe C, Ozaslan E, Abe M, Mitchell-Thain R, Zeniya M, Han KH, Vierling JM, Takikawa H. Autoimmune liver diseases in the Asia-Pacific region: Proceedings of APASL symposium on AIH and PBC 2016. Hepatol Int. 2016;10:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Lin CL, Kao JH. Perspectives and control of hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2015;114:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Koay LB, Lin CY, Tsai SL, Lee C, Lin CN, Sheu MJ, Kuo HT, Sun CS. Type 1 autoimmune hepatitis in Taiwan: diagnosis using the revised criteria of the International Autoimmune Hepatitis Group. Dig Dis Sci. 2006;51:1978-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 76. | Alberti-Flor JJ, Jeffers L, Schiff ER. Primary sclerosing cholangitis occurring in a patient with systemic lupus erythematosus and diabetes mellitus. Am J Gastroenterol. 1984;79:889-891. [PubMed] |
| 77. | Lamy P, Valla D, Bourgeois P, Rueff B, Benhamou JP. [Primary sclerosing cholangitis and systemic lupus erythematosus]. Gastroenterol Clin Biol. 1988;12:962-964. [PubMed] |
| 78. | Audan A, Bruley Des Varannes S, Georgelin T, Sagan C, Cloarec D, Serraz H, Le Bodic L. [Primary sclerosing cholangitis and systemic lupus erythematosus]. Gastroenterol Clin Biol. 1995;19:123-126. [PubMed] |
| 79. | Kadokawa Y, Omagari K, Matsuo I, Otsu Y, Yamamoto U, Nishino T, Ohba K, Miyazaki M, Harada T, Taguchi T, Kohno S. Primary sclerosing cholangitis associated with lupus nephritis: a rare association. Dig Dis Sci. 2003;48:911-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Oh DC, Ng TM, Ho J, Leong KP. Systemic lupus erythematosus with concurrent protein-losing enteropathy and primary sclerosing cholangitis: a unique association. Lupus. 2006;15:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Saarinen S, Olerup O, Broomé U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:3195-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Mariette X, Criswell LA. Primary Sjögren's Syndrome. N Engl J Med. 2018;378:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 83. | Kaplan MJ, Ike RW. The liver is a common non-exocrine target in primary Sjögren's syndrome: a retrospective review. BMC Gastroenterol. 2002;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Ramos-Casals M, Sánchez-Tapias JM, Parés A, Forns X, Brito-Zerón P, Nardi N, Vazquez P, Vélez D, Arias I, Bové A, Plaza J, Rodés J, Font J. Characterization and differentiation of autoimmune vs viral liver involvement in patients with Sjögren's syndrome. J Rheumatol. 2006;33:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Lindgren S, Manthorpe R, Eriksson S. Autoimmune liver disease in patients with primary Sjögren's syndrome. J Hepatol. 1994;20:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Hatzis GS, Fragoulis GE, Karatzaferis A, Delladetsima I, Barbatis C, Moutsopoulos HM. Prevalence and longterm course of primary biliary cirrhosis in primary Sjögren's syndrome. J Rheumatol. 2008;35:2012-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 87. | Amador-Patarroyo MJ, Arbelaez JG, Mantilla RD, Rodriguez-Rodriguez A, Cárdenas-Roldán J, Pineda-Tamayo R, Guarin MR, Kleine LL, Rojas-Villarraga A, Anaya JM. Sjögren's syndrome at the crossroad of polyautoimmunity. J Autoimmun. 2012;39:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Floreani A, Cazzagon N. PBC and related extrahepatic diseases. Best Pract Res Clin Gastroenterol. 2018;34-35:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Tsianos EV, Hoofnagle JH, Fox PC, Alspaugh M, Jones EA, Schafer DF, Moutsopoulos HM. Sjögren's syndrome in patients with primary biliary cirrhosis. Hepatology. 1990;11:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Marasini B, Gagetta M, Rossi V, Ferrari P. Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis. 2001;60:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Watt FE, James OF, Jones DE. Patterns of autoimmunity in primary biliary cirrhosis patients and their families: a population-based cohort study. QJM. 2004;97:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM; USA PBC Epidemiology Group. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 93. | Wang L, Zhang FC, Chen H, Zhang X, Xu D, Li YZ, Wang Q, Gao LX, Yang YJ, Kong F, Wang K. Connective tissue diseases in primary biliary cirrhosis: a population-based cohort study. World J Gastroenterol. 2013;19:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Floreani A, Franceschet I, Cazzagon N, Spinazzè A, Buja A, Furlan P, Baldo V, Gershwin ME. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 95. | Selmi C, Gershwin ME. Chronic Autoimmune Epithelitis in Sjögren's Syndrome and Primary Biliary Cholangitis: A Comprehensive Review. Rheumatol Ther. 2017;4:263-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Bournia VK, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun. 2012;39:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Takahashi A, Abe K, Yokokawa J, Iwadate H, Kobayashi H, Watanabe H, Irisawa A, Ohira H. Clinical features of liver dysfunction in collagen diseases. Hepatol Res. 2010;40:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Genetic predispositions for the immunological features of chronic active hepatitis. Hepatology. 1993;18:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H, Hultcrantz R, Sangfelt P, Weiland O, Danielsson A. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 100. | Teufel A, Weinmann A, Kahaly GJ, Centner C, Piendl A, Wörns M, Lohse AW, Galle PR, Kanzler S. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J Clin Gastroenterol. 2010;44:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 101. | Abe M, Mashiba T, Zeniya M, Yamamoto K, Onji M, Tsubouchi H; Autoimmune Hepatitis Study Group-Subgroup of the Intractable Hepato-Biliary Disease Study Group in Japan. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol. 2011;46:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | Wong GW, Yeong T, Lawrence D, Yeoman AD, Verma S, Heneghan MA. Concurrent extrahepatic autoimmunity in autoimmune hepatitis: implications for diagnosis, clinical course and long-term outcomes. Liver Int. 2017;37:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 103. | Montefusco PP, Geiss AC, Bronzo RL, Randall S, Kahn E, McKinley MJ. Sclerosing cholangitis, chronic pancreatitis, and Sjogren's syndrome: a syndrome complex. Am J Surg. 1984;147:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 104] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Zeron PB, Retamozo S, Bové A, Kostov BA, Sisó A, Ramos-Casals M. Diagnosis of Liver Involvement in Primary Sjögren Syndrome. J Clin Transl Hepatol. 2013;1:94-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1497] [Article Influence: 187.1] [Reference Citation Analysis (0)] |
| 106. | Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford). 2009;48 Suppl 3:iii36-iii39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 107. | D'Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 751] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 108. | Hudson M, Rojas-Villarraga A, Coral-Alvarado P, López-Guzmán S, Mantilla RD, Chalem P; Canadian Scleroderma Research Group; Colombian Scleroderma Research Group, Baron M, Anaya JM. Polyautoimmunity and familial autoimmunity in systemic sclerosis. J Autoimmun. 2008;31:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Semin Arthritis Rheum. 1994;24:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Pope JE, Thompson A. Antimitochondrial antibodies and their significance in diffuse and limited scleroderma. J Clin Rheumatol. 1999;5:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 111. | Rigamonti C, Shand LM, Feudjo M, Bunn CC, Black CM, Denton CP, Burroughs AK. Clinical features and prognosis of primary biliary cirrhosis associated with systemic sclerosis. Gut. 2006;55:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 112. | Assassi S, Fritzler MJ, Arnett FC, Norman GL, Shah KR, Gourh P, Manek N, Perry M, Ganesh D, Rahbar MH, Mayes MD. Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol. 2009;36:2250-2256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Liberal R, Grant CR, Sakkas L, Bizzaro N, Bogdanos DP. Diagnostic and clinical significance of anti-centromere antibodies in primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:572-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | McFarlane IM, Bhamra MS, Kreps A, Iqbal S, Al-Ani F, Saladini-Aponte C, Grant C, Singh S, Awwal K, Koci K, Saperstein Y, Arroyo-Mercado FM, Laskar DB, Atluri P. Gastrointestinal Manifestations of Systemic Sclerosis. Rheumatology (Sunnyvale). 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 115. | Göring HD, Panzner M, Lakotta W, Ziemer A. [Coincidence of scleroderma and primary biliary cirrhosis. Results of a systematic study of a dermatologic patient sample]. Hautarzt. 1998;49:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 116. | You BC, Jeong SW, Jang JY, Goo SM, Kim SG, Kim YS, Jeon CH, Jeen YM. Liver cirrhosis due to autoimmune hepatitis combined with systemic sclerosis. Korean J Gastroenterol. 2012;59:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Assandri R, Monari M, Montanelli A. Development of systemic sclerosis in patients with autoimmune hepatitis: an emerging overlap syndrome. Gastroenterol Hepatol Bed Bench. 2016;9:211-219. [PubMed] |
| 118. | Fraile G, Rodríguez-García JL, Moreno A. Primary sclerosing cholangitis associated with systemic sclerosis. Postgrad Med J. 1991;67:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Zampetti A, Rinninella E, Manna R, Franceschi F. Scleroderma and liver disease: a case of an association with primary sclerosing cholangitis. Scand J Rheumatol. 2016;45:334-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 120. | Savarino E, Furnari M, de Bortoli N, Martinucci I, Bodini G, Ghio M, Savarino V. Gastrointestinal involvement in systemic sclerosis. Presse Med. 2014;43:e279-e291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 121. | Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 3148] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 122. | Selmi C, De Santis M, Gershwin ME. Liver involvement in subjects with rheumatic disease. Arthritis Res Ther. 2011;13:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Radovanović-Dinić B, Tešić-Rajković S, Zivkovic V, Grgov S. Clinical connection between rheumatoid arthritis and liver damage. Rheumatol Int. 2018;38:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Ruderman EM, Crawford JM, Maier A, Liu JJ, Gravallese EM, Weinblatt ME. Histologic liver abnormalities in an autopsy series of patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Hocking WG, Lasser K, Ungerer R, Bersohn M, Palos M, Spiegel T. Spontaneous hepatic rupture in rheumatoid arthritis. Arch Intern Med. 1981;141:792-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Thorne C, Urowitz MB, Wanless I, Roberts E, Blendis LM. Liver disease in Felty's syndrome. Am J Med. 1982;73:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Yılmaz N, Karadağ Ö, Kimyon G, Yazıcı A, Yılmaz S, Kalyoncu U, Kaşifoğlu T, Temiz H, Baysal B, Tözün N. Prevalence of hepatitis B and C infections in rheumatoid arthritis and ankylosing spondylitis: A multicenter countrywide study. Eur J Rheumatol. 2014;1:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: Risk and prophylaxis recommendations. World J Gastroenterol. 2015;21:10274-10289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 129. | Pauly MP, Tucker LY, Szpakowski JL, Ready JB, Baer D, Hwang J, Lok AS. Incidence of Hepatitis B Virus Reactivation and Hepatotoxicity in Patients Receiving Long-term Treatment With Tumor Necrosis Factor Antagonists. Clin Gastroenterol Hepatol. 2018;16:1964-1973.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 130. | Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology (Oxford). 2011;50:1700-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 131. | Viganò M, Degasperi E, Aghemo A, Lampertico P, Colombo M. Anti-TNF drugs in patients with hepatitis B or C virus infection: safety and clinical management. Expert Opin Biol Ther. 2012;12:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 132. | Ramos-Casals M, Brito-Zerón P, Soto MJ, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol. 2008;22:847-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 133. | Sherlock S, Scheuer PJ. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med. 1973;289:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 327] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 134. | Siegel JL, Luthra H, Donlinger J, Angulo P, Lindor K. Association of primary biliary cirrhosis and rheumatoid arthritis. J Clin Rheumatol. 2003;9:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 135. | Smyk DS, Bogdanos DP, Mytilinaiou MG, Burroughs AK, Rigopoulou EI. Rheumatoid arthritis and primary biliary cirrhosis: cause, consequence, or coincidence? Arthritis. 2012;2012:391567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Toulemonde G, Scoazec JY, Miossec P. Treatment with etanercept of autoimmune hepatitis associated with rheumatoid arthritis: an open label proof of concept study. Ann Rheum Dis. 2012;71:1423-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Perez-Alvarez R, Pérez-de-Lis M, Ramos-Casals M; BIOGEAS study group. Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013;25:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 138. | Vollmer O, Felten R, Mertz P, Lebrun-Vignes B, Salem JE, Arnaud L. Characterization of auto-immune hepatitis associated with the use of anti-TNFα agents: An analysis of 389 cases in VigiBase. Autoimmun Rev. 2020;19:102460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 139. | Lamberts LE, Janse M, Haagsma EB, van den Berg AP, Weersma RK. Immune-mediated diseases in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 140. | Gow PJ, Fleming KA, Chapman RW. Primary sclerosing cholangitis associated with rheumatoid arthritis and HLA DR4: is the association a marker of patients with progressive liver disease? J Hepatol. 2001;34:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 141. | Ogdie A, Schwartzman S, Eder L, Maharaj AB, Zisman D, Raychaudhuri SP, Reddy SM, Husni E. Comprehensive treatment of psoriatic arthritis: managing comorbidities and extraarticular manifestations. J Rheumatol. 2014;41:2315-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 142. | Yousaf A, Raiker R, Davis SM, Gayam S, Zinn Z. Association between psoriasis, psoriatic arthritis and gastrointestinal disease : An exploratory nationwide inpatient sample analysis. Wien Klin Wochenschr. 2021;133:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 144. | Khan F, Kleppel H, Meara A. Paraneoplastic Musculoskeletal Syndromes. Rheum Dis Clin North Am. 2020;46:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Chou JW, Lin YL, Cheng KS, Wu PY, Reanne Ju T. Dermatomyositis Induced by Hepatitis B Virus-related Hepatocellular Carcinoma: A Case Report and Review of the Literature. Intern Med. 2017;56:1831-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 146. | Han J, Wang S, Kwong TNY, Liu J. Dermatomyositis as an extrahepatic manifestation of hepatitis B virus-related hepatocellular carcinoma: A case report and literature review. Medicine (Baltimore). 2018;97:e11586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325:1487-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 657] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 148. | Zhang L, Yang H, Lei J, Peng Q, Wang G, Lu X. Muscle pathological features and extra-muscle involvement in idiopathic inflammatory myopathies with anti-mitochondrial antibody. Semin Arthritis Rheum. 2021;51:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 149. | Harada N, Dohmen K, Itoh H, Ohshima T, Yamamoto H, Nagano M, Iwata Y, Hachisuka K, Ishibashi H. Sibling cases of primary biliary cirrhosis associated with polymyositis, vasculitis and Hashimoto's thyroiditis. Intern Med. 1992;31:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Ko KF, Ho T, Chan KW. Autoimmune chronic active hepatitis and polymyositis in a patient with myasthenia gravis and thymoma. J Neurol Neurosurg Psychiatry. 1995;59:558-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 151. | Kurihara Y, Shishido T, Oku K, Takamatsu M, Ishiguro H, Suzuki A, Sekita T, Shinagawa T, Ishihara T, Nakashima R, Fujii T, Okano Y. Polymyositis associated with autoimmune hepatitis, primary biliary cirrhosis, and autoimmune thrombocytopenic purpura. Mod Rheumatol. 2011;21:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Seibold F, Klein R, Jakob F. Polymyositis, alopecia universalis, and primary sclerosing cholangitis in a patient with Crohn's disease. J Clin Gastroenterol. 1996;23:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 153. | Gunnarsson R, Hetlevik SO, Lilleby V, Molberg Ø. Mixed connective tissue disease. Best Pract Res Clin Rheumatol. 2016;30:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 154. | Marshall JB, Ravendhran N, Sharp GC. Liver disease in mixed connective tissue disease. Arch Intern Med. 1983;143:1817-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 155. | Chung SW. Vasculitis: From Target Molecules to Novel Therapeutic Approaches. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 156. | Moiseev S, Cohen Tervaert JW, Arimura Y, Bogdanos DP, Csernok E, Damoiseaux J, Ferrante M, Flores-Suárez LF, Fritzler MJ, Invernizzi P, Jayne D, Jennette JC, Little MA, McAdoo SP, Novikov P, Pusey CD, Radice A, Salama AD, Savige JA, Segelmark M, Shoenfeld Y, Sinico RA, Sousa MJ, Specks U, Terrier B, Tzioufas AG, Vermeire S, Zhao MH, Bossuyt X. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev. 2020;19:102618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 157. | Wang CR, Tsai HW. Human hepatitis viruses-associated cutaneous and systemic vasculitis. World J Gastroenterol. 2021;27:19-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 158. | Kennedy F, Kapelow R, Kalyon BD, Roth NC, Rishi A, Barilla-LaBarca ML. A rare case of Polyarteritis Nodosa associated with autoimmune hepatitis: a case report. BMC Rheumatol. 2021;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Wang CR, Tsai YS, Tsai HW, Lee CH. B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 160. | Terjung B, Bogsch F, Klein R, Söhne J, Reichel C, Wasmuth JC, Beuers U, Sauerbruch T, Spengler U. Diagnostic accuracy of atypical p-ANCA in autoimmune hepatitis using ROC- and multivariate regression analysis. Eur J Med Res. 2004;9:439-448. [PubMed] |
| 161. | Terjung B, Söhne J, Lechtenberg B, Gottwein J, Muennich M, Herzog V, Mähler M, Sauerbruch T, Spengler U. p-ANCAs in autoimmune liver disorders recognise human beta-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut. 2010;59:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 162. | De Riva V, Celadin M, Pittoni M, Plebani M, Angeli P. What is behind the presence of anti-neutrophil cytoplasmatic antibodies in chronic liver disease? Liver Int. 2009;29:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 163. | Lenzen H, Weismüller TJ, Negm AA, Wlecke J, Loges S, Strassburg CP, Manns MP, Lankisch TO. Antineutrophil cytoplasmic antibodies in bile are associated with disease activity in primary sclerosing cholangitis. Scand J Gastroenterol. 2013;48:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 164. | Tovoli F, Vannini A, Fusconi M, Frisoni M, Zauli D. Autoimmune liver disorders and small-vessel vasculitis: four case reports and review of the literature. Ann Hepatol. 2013;13:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 165. | Yamashita H, Suzuki A, Takahashi Y, Kaneko H, Kano T, Mimori A. Anti-neutrophil Cytoplasmic Antibody (ANCA)-associated Vasculitis Associated with Primary Biliary Cirrhosis: A Case Report and Literature Review. Intern Med. 2015;54:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 166. | Lohani S, Nazir S, Tachamo N, Pagolu P. Autoimmune hepatitis and eosinophilic granulomatosis with polyangiitis: a rare association. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 167. | Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun. 2018;93:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 168. | Sandnes M, Ulvik RJ, Vorland M, Reikvam H. Hyperferritinemia-A Clinical Overview. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 169. | Fujii K, Rokutanda R, Osugi Y, Koyama Y, Ota T. Adult-onset Still's disease complicated by autoimmune hepatitis: successful treatment with infliximab. Intern Med. 2012;51:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 170. | Yazici Y, Hatemi G, Bodaghi B, Cheon JH, Suzuki N, Ambrose N, Yazici H. Behçet syndrome. Nat Rev Dis Primers. 2021;7:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 171. | Bayraktar Y, Balkanci F, Bayraktar M, Calguneri M. Budd-Chiari syndrome: a common complication of Behçet's disease. Am J Gastroenterol. 1997;92:858-862. [PubMed] |
| 172. | Takeuchi A, Haraoka H, Hashimoto T. Increased serum alkaline phosphatase activity in Behçet's disease. Clin Exp Rheumatol. 1989;7:619-621. [PubMed] |
| 173. | Manna R, Ghirlanda G, Bochicchio GB, Papa G, Annese V, Greco AV, Taranto CA, Magaro M. Chronic active hepatitis and Behçet's syndrome. Clin Rheumatol. 1985;4:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 174. | Iwadate H, Ohira H, Saito H, Takahashi A, Rai T, Takiguchi J, Sasajima T, Kobayashi H, Watanabe H, Sato Y. A case of primary biliary cirrhosis complicated by Behçet's disease and palmoplantar pustulosis. World J Gastroenterol. 2006;12:2136-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 175. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1870] [Article Influence: 143.8] [Reference Citation Analysis (83)] |
| 176. | Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJM, Barnes E. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 177. | Chen JH, Deshpande V. IgG4-related Disease and the Liver. Gastroenterol Clin North Am. 2017;46:195-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 178. | Ichimura T, Kondo S, Ambo Y, Hirano S, Ohmi M, Okushiba S, Morikawa T, Shimizu M, Katoh H. Primary sclerosing cholangitis associated with autoimmune pancreatitis. Hepatogastroenterology. 2002;49:1221-1224. [PubMed] |
| 179. | Li A, Wang Y, Deng Z. Concurrent autoimmune pancreatitis and primary biliary cirrhosis: a rare case report and literature review. BMC Gastroenterol. 2014;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 180. | Yada N, Kudo M, Chung H, Watanabe T. Autoimmune hepatitis and immunoglobulin G4-associated autoimmune hepatitis. Dig Dis. 2013;31:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 181. | Tanaka A, Notohara K. Immunoglobulin G4 (IgG4)-related autoimmune hepatitis and IgG4-hepatopathy: A histopathological and clinical perspective. Hepatol Res. 2021;51:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 182. | Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 766] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 183. | Cremers J, Drent M, Driessen A, Nieman F, Wijnen P, Baughman R, Koek G. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 2012;24:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 184. | Shah N, Mitra A. Gastrointestinal and Hepatic Sarcoidosis: A Review Article. Clin Liver Dis (Hoboken). 2021;17:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 185. | Mantaka A, Koulentaki M, Chlouverakis G, Enele-Melono JM, Darivianaki A, Tzardi M, Kouroumalis EA. Primary biliary cirrhosis in a genetically homogeneous population: disease associations and familial occurrence rates. BMC Gastroenterol. 2012;12:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 186. | Rajoriya N, Wotton CJ, Yeates DG, Travis SP, Goldacre MJ. Immune-mediated and chronic inflammatory disease in people with sarcoidosis: disease associations in a large UK database. Postgrad Med J. 2009;85:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 187. | Lahmer T, Treiber M, von Werder A, Foerger F, Knopf A, Heemann U, Thuermel K. Relapsing polychondritis: An autoimmune disease with many faces. Autoimmun Rev. 2010;9:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 188. | da Graça Ferronato M, Staub LJ, Teixeira Pinto Viana RC, da Rosa L, Cacese Shiozawa MB, Narciso-Schiavon JL, Dantas-Correa EB, de Lucca Schiavon L. Cholestasis as the initial presentation of relapsing polychondritis. Ann Hepatol. 2011;10:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 189. | Conn DL, Dickson ER, Carpenter HA. The association of Churg-Strauss vasculitis with temporal artery involvement, primary biliary cirrhosis, and polychondritis in a single patient. J Rheumatol. 1982;9:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 190. | Mydlak A, Sołdacki D, Foroncewicz B, Stopa Z, Powała A, Budlewski T, Pączek L, Mucha K. Relapsing polychondritis in a liver transplant recipient: A case report. Medicine (Baltimore). 2017;96:e8360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |