Published online Jun 7, 2022. doi: 10.3748/wjg.v28.i21.2361
Peer-review started: October 18, 2021
First decision: December 27, 2021
Revised: January 7, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: June 7, 2022
Processing time: 227 Days and 5.4 Hours
Primary liver cancer (PLC) is a major contributor to cancer-related deaths. Data on global and country-specific levels and trends of PLC are essential for under
To investigate the association between the burden of PLC and socioeconomic development status.
Cancer mortality and incidence rates were obtained from the Global Burden of Disease (GBD) 2019, and the data were stratified by country and territory, sex, and the Socio-demographic Index (SDI) level. The association between the attributable etiology of PLC and socioeconomic development status, represented using the SDI, was described. The attributable etiology of PLC included hepatitis B, hepatitis C, alcohol use, and nonalcoholic steatohepatitis. The association between the attributable etiology of PLC and SDI was further stratified by sex and geographical location. A confidence analysis was also performed based on bootstrap draw.
The age-standardized incidence rate of PLC was 6.5 [95% confidence intervals (CI): 5.9-7.2] per 100000 person-years, which decreased by -27.5% (-37.0 to -16.6) from 1990 to 2019. Several countries located in East Asia, South Asia, West Africa, and North Africa shouldered the heaviest burden of PLC in 2019. In terms of incidence rates, the first leading underlying cause of PLC identified was hepatitis B, followed by hepatitis C, alcohol use, and nonalcoholic steatohepatitis. Regarding stratification using the SDI, the incidence rate of PLC was the highest for high and middle SDI locations. Further, the leading attributable etiologies of PLC were hepatitis B for the middle and high middle SDI locations while hepatitis C and nonalcoholic steatohepatitis for the high SDI locations.
The pronounced association between socioeconomic development status and PLC burden indicates socioeconomic development status affects attributable etiologies for PLC. GBD 2019 data are valuable for policymakers implementing PLC cost-effective interventions.
Core Tip: Primary liver cancer (PLC) is a common cancer with high morbidity and mortality rates. PLC usually occurs as a preventable disease. An association was identified between socioeconomic development status and PLC burden. The leading attributable etiologies of PLC were hepatitis B for the middle and high middle Socio-demographic Index (SDI) locations, and hepatitis C and nonalcoholic steatohepatitis for the high SDI locations. Our findings are valuable to implement tailored prevention strategies for PLC.
- Citation: Xing QQ, Li JM, Dong X, Zeng DY, Chen ZJ, Lin XY, Pan JS. Socioeconomics and attributable etiology of primary liver cancer, 1990-2019. World J Gastroenterol 2022; 28(21): 2361-2382
- URL: https://www.wjgnet.com/1007-9327/full/v28/i21/2361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i21.2361
Primary liver cancer (PLC) was the third leading cause of cancer deaths in 2020 following lung and colorectal cancer[1]. In terms of cancer-related mortality, PLC was the third leading cause in China[2] and the fifth leading cause in the United States[3]. The burden of PLC varies significantly in terms of sex and geographic region due to different risk-factor exposure. The major risk factors include chronic viral infections [hepatitis B virus (HBV), hepatitis C virus (HCV)], alcohol use, and nonalcoholic steatohepatitis (NASH), and they have been widely studied in recent years[4]. PLC is caused by chronic hepatitis B (CHB) (60%) in Africa and East Asia, whereas chronic hepatitis C (CHC) appears to be the major risk factor in the Western world[5]. Thus, it is expected that the appropriate handling of risk factors can significantly contribute to the overall reduction of PLC-related deaths in the near future.
The Global Burden of Disease (GBD) database has been constructed to improve health systems and eliminate disparities; this database comprises a comprehensive catalog of censuses, vital statistics, surveys, and other health-related data. Policymakers can benefit from the GBD database as it enables them to understand the true nature of the health challenges of a country and the shifting challenges over time. In recent years, the prevention of PLC has been eclipsed by substantial improvements in PLC treatment. Given the marked lag between risk factor exposure and the development of PLC, even the well-proven prevention approaches would take decades to reduce of the PLC burden. Although several prior studies have focused on the global prevalence of PLC[4,6], few studies focus on the tailored prevention of PLC. In this study, we focused on identifying the effect of socioeconomic development status on the attributable etiologies of PLC from a global perspective. We hope our findings will be helpful contributions for developing specialized prevention strategies for PLC. Considering the heavy burden of PLC, characterizing this association will help health workers to design tailored prevention strategies and policymakers to allocate research and clinical resources for implementing cost-effective interventions for PLC.
For this study, the incidence and death rates of PLC were acquired from the GBD 2019 (http://ghdx.healthdata.org/gbd-2019) that covered 204 countries and territories[7]. The incidence and death rates were age-standardized according to the GBD 2019 world population recorded per 100000 person-years. The International Classification of Diseases, Tenth Revision (ICD-10) was adopted. The ICD-10 codes for PLC are C22-C22.4, C22.7-C22.8, and Z85.05 (Supplementary material, page 17). Mortality and non-fatal estimates have been described in detail in previous studies[8,9]. Additional information is provided in the Supplementary material.
We assumed that the incidence or death rates in each year followed a log-normal distribution and that the rates in different years were independent of each other. Based on these assumptions, in each bootstrap draw, we measured the increase rates and 95%CIs based on the 25th and 975th ranked values across all 1000 draws. The 2.5% and 97.5% quantiles from the 1000 draws of the posterior distribution were used to generate 95%CIs.
The Socio-demographic Index (SDI) incorporates the mean education level for individuals aged 15 years and older, the total fertility rate in women under the age of 25 years, and lag-distributed income per person. The method of generating the SDI is described in the report by the GBD 2016 Mortality Collaborators[10]. Further, the SDI was used to evaluate the effect of the development levels of a country or region on the burden of PLC based on data obtained from the GBD 2019 (Supplementary material, pages 1-15). The values of the SDI range from 0 to 1, which correspond to the development level of a country or region from the worst to the best. The SDI was categorized based on the references bound as low SDI, low middle SDI, middle SDI, high middle SDI, and high SDI, as shown in the Supplementary material, page 16.
The study was reviewed and approved by the Ethics Committee of First Affiliated Hospital of Fujian Medical University (MTCA, ECFAH of FMU[2015]084-1).
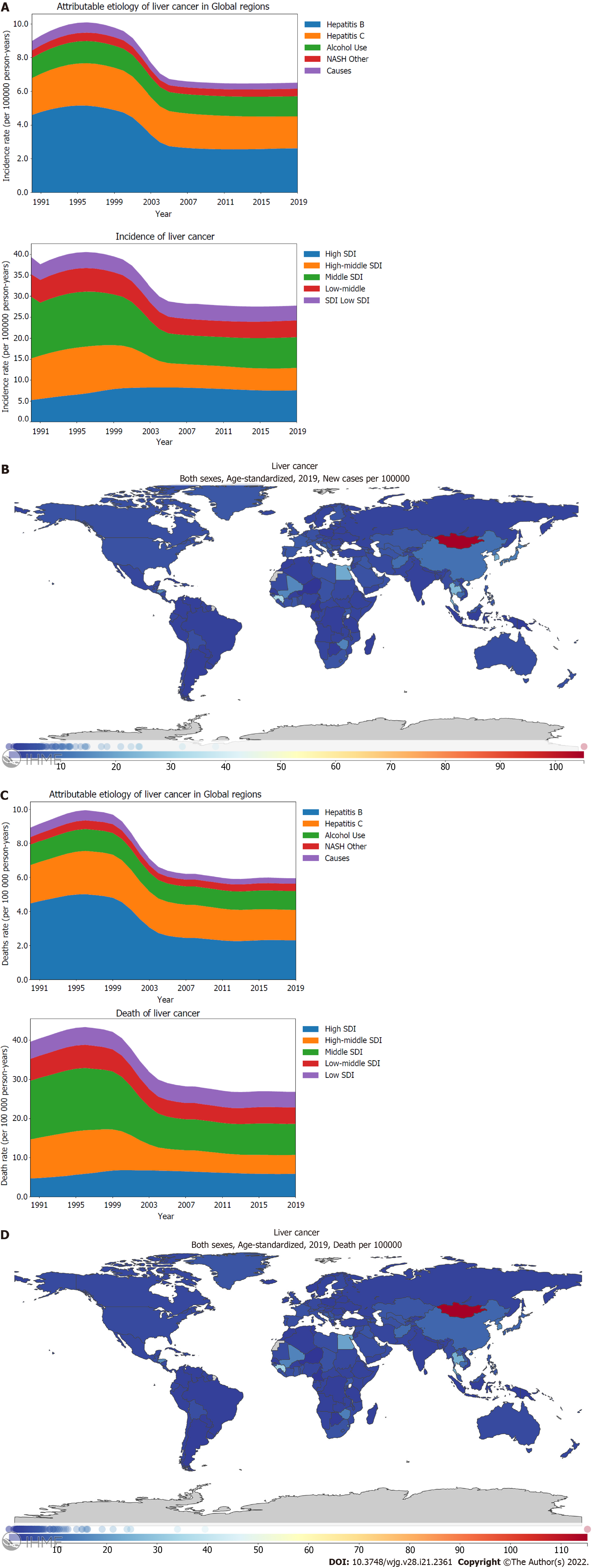
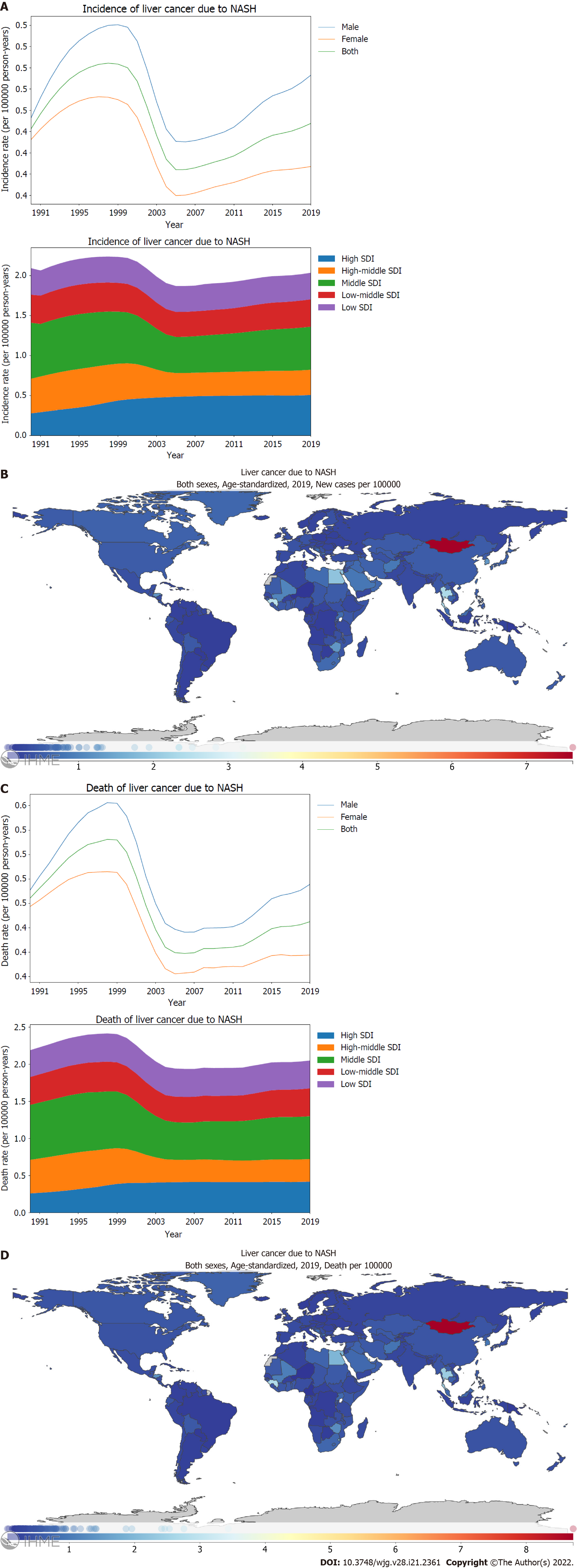
Liver cancer is one the most common cancers. In 2019, the global age-standardized incidence rate of PLC was 6.5 (95%CI: 5.9-7.2) per 100000 person-years (Supplementary material, page 25). Fortunately, the incidence rate of PLC has declined significantly by -27.5% (-37.0 to -16.6) from 1990 to 2019 (Figure 1A; Supplementary material, page 18). The main contributor for this drop was the decreasing burden of PLC caused by hepatitis B and the declining burden of PLC in the middle SDI locations. Between 1990 and 2019, the global incidence rate of PLC peaked in 1995-1996, and then, it decreased gradually. However, the incidence rate of PLC has not declined further since 2010 (Figure 1A; Supplementary material, pages 23-25). Before 2004, the incidence rate of PLC for middle SDI locations surpassed that for high SDI locations whereas high SDI locations exceeded middle SDI locations in terms of the burden of PLC after 2004 (Figure 1A; Supplementary Figure 1B; Supplementary material, pages 29-32). In terms of the incidence rates, the leading underlying cause of PLC was HBV, followed by HCV, alcohol use, and NASH (Figure 1A). Hepatitis B manifested the most drastic decline between 1990 and 2019 as the underlying causes of PLC [57.0% (45.3-71.4)] (Figure 1A; Supplementary material, pages 19-20). Stratified using the SDI, the age-standardized incidence rate of PLC was found to be the highest for high and middle SDI locations compared to those for high middle, low middle, and low SDI locations (Figure 1A; Supplementary Figure 1B; Supplementary material, pages 29-32). Further, a declining pattern was observed for the age-standardized incidence rate of PLC in the high middle [53.8% (45.1-64.5)] and middle SDI locations [49.7% (41.1-59.9)] compared with the increasing trend in the high SDI locations [144.5% (130.3-159.6)] (Figure 1A; Supplementary Figure 1B; Supplementary material, page 18). Between 1990 and 2019, PLC caused by hepatitis B and hepatitis C showed a decreasing trend in the death rate (Figure 1C; Supplementary material, pages 21-22). Stratified using the SDI, the high middle, middle, and low middle SDI locations showed decreasing trends in the age-standardized death rate of PLC. In contrast, the high SDI location showed an increasing trend in the age-standardized death rate of PLC (Figure 1C; Supplementary material, page 18). Several countries located in East Asia, South Asia, West Africa, and North Africa shouldered the heaviest burden of the PLC incidence and death rates. For the age-standardized incidence rate of PLC, Mongolia demonstrated the highest burden [105.2 (82.6-131.5)] per 100000 person-years), followed by Gambia and Guinea (Figure 1B; Supplementary material, pages 33-35). Countries that possessed the highest burden PLC incidence rate also had the highest burden PLC death rate (Figure 1D; Supplementary material, pages 46-48).
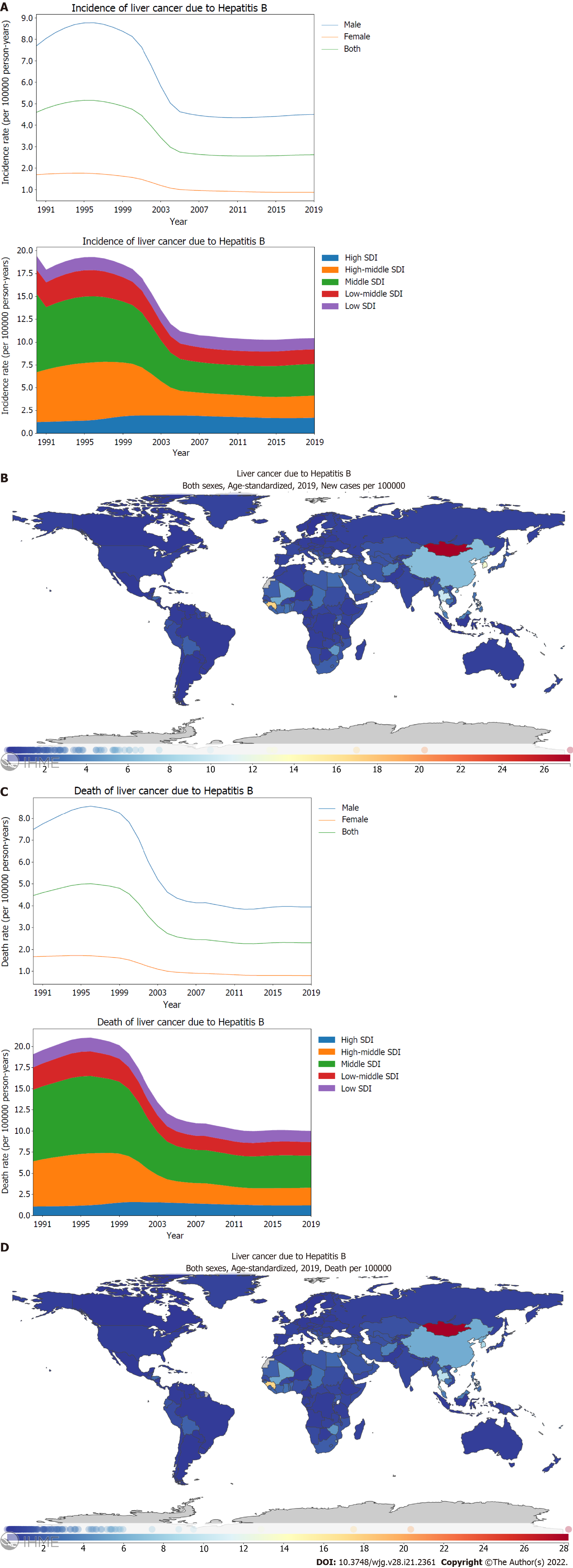
The global age-standardized incidence rate of PLC caused by hepatitis B reached its peak in 1995-1996, and then decreased gradually. However, the burden of the incidence rate has remained stable and has not declined further since 2005. By stratification using sex, the age-standardized incidence rate of PLC caused by hepatitis B was found to be four times higher in males than that in females (Figure 2A; Supplementary material, pages 49-50). Moreover, the age-standardized incidence rate of PLC caused by hepatitis B was found to be higher for middle and high middle SDI locations than for high, low middle, and low SDI locations (Figure 2A; Supplementary Figure 2A; Supplementary material, pages 51-57). Between 1990 and 2019, the decreasing trend in the age-standardized incidence rate of PLC caused by hepatitis B differed significantly based on SDI regions, with the highest declines in the middle [40.3% (31.1-51.8)] and high middle SDI locations [44.8% (34.2-58.8)]. In contrast, high SDI locations showed an increasing trend [139.3% (112.1-173.3)] (Figure 2A; Supplementary Figure 2A; Supplementary material, pages 19-20). In 2019, the incidence rate of PLC caused by hepatitis B differed dramatically between countries or regions. In particular, the highest age-standardized incidence rate was recorded in Mongolia with 27.3 (18.0-39.1) per 100000 person-years, followed by Gambia and Guinea (Figure 2B; Supplementary material, pages 58-61). Similar to the age-standardized incidence rate of PLC caused by hepatitis B, the burden of the PLC death rate caused by hepatitis B was higher for males than that for females (Figure 2C; Supplementary material, pages 62-63). Between 1990 and 2019, the age-standardized death rate of PLC caused by hepatitis B decreased significantly in the high middle [39.0% (30.2-50.6)] and middle SDI locations [44.7% (34.7-57.4)]. However, the high SDI locations showed an increasing trend [113.4% (90.6-141.6)] (Figure 2C; Supplementary Figure 2B; Supplementary material, pages 21-22). In 2019, Mongolia had the highest age-standardized death rate with 28.2 (18.9-40.8) per 100000 person-years, followed by Gambia and Guinea (Figure 2D; Supplementary material, pages 67-70).
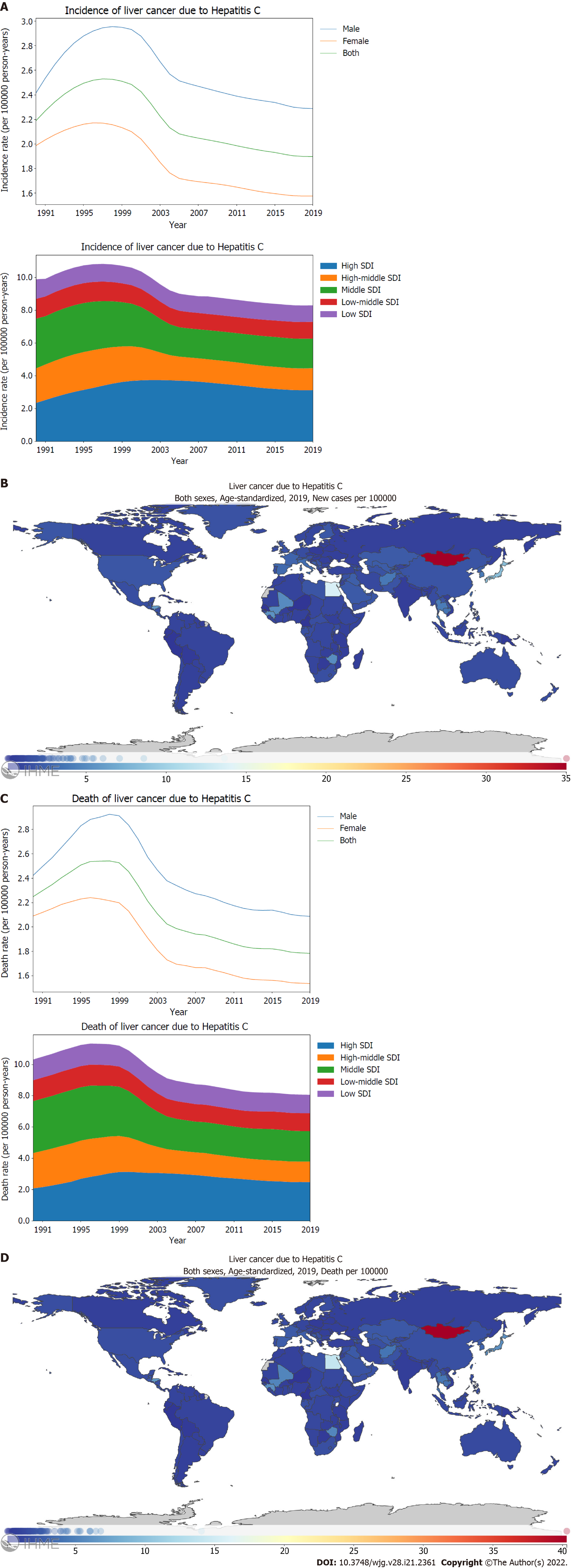
Hepatitis C is the second leading cause of PLC. By stratification using sex, the age-standardized incidence rate and mortality rate of PLC caused by hepatitis C in males was found to be higher than those in females (Figure 3A and C; Supplementary material, pages 71-72 and 80-81). Further, the age-standardized incidence rate of PLC caused by hepatitis C was higher for high and middle SDI locations than for high middle, low middle, and low SDI locations (Figure 3A; Supplementary Figure 3A; Supplementary material, pages 73-75). From 1990 through 2019, the age-standardized incidence rate of PLC caused by hepatitis C differed significantly between the SDI regions, with the middle [59.5% (46.5-76.3)] and high middle SDI locations [63.3% (51.3-78.0)] exhibiting declining trends whereas the high SDI location [133.4% (112.5-158.2)] showed increasing trends (Figure 3A; Supplementary Figure 3A; Supplementary material, pages 19-20). In 2019, the incidence rate of PLC caused by hepatitis C manifested a substantial variance between countries or regions. The highest age-standardized incidence rate was recorded in Mongolia with 35.0 (24.7-46.8) per 100000 person-years, followed by Egypt and Japan (Figure 3B; Supplementary material, pages 76-79). Between 1990 and 2019, the age-standardized death rate of PLC caused by hepatitis C decreased significantly in high middle [57.9% (47.5-71.1)] and middle SDI locations [58.7% (46.2-74.4)]. However, the high SDI locations showed an increasing trend [119.5% (101.8-139.8)] (Figure 3C; Supplementary Figure 3B; Supplementary material, pages 21-22). In 2019, Mongolia had the highest age-standardized death rate with 40.3 (28.6-53.3) per 100000 person-years, followed by Egypt (Figure 3D; Supplementary material, pages 85-88).
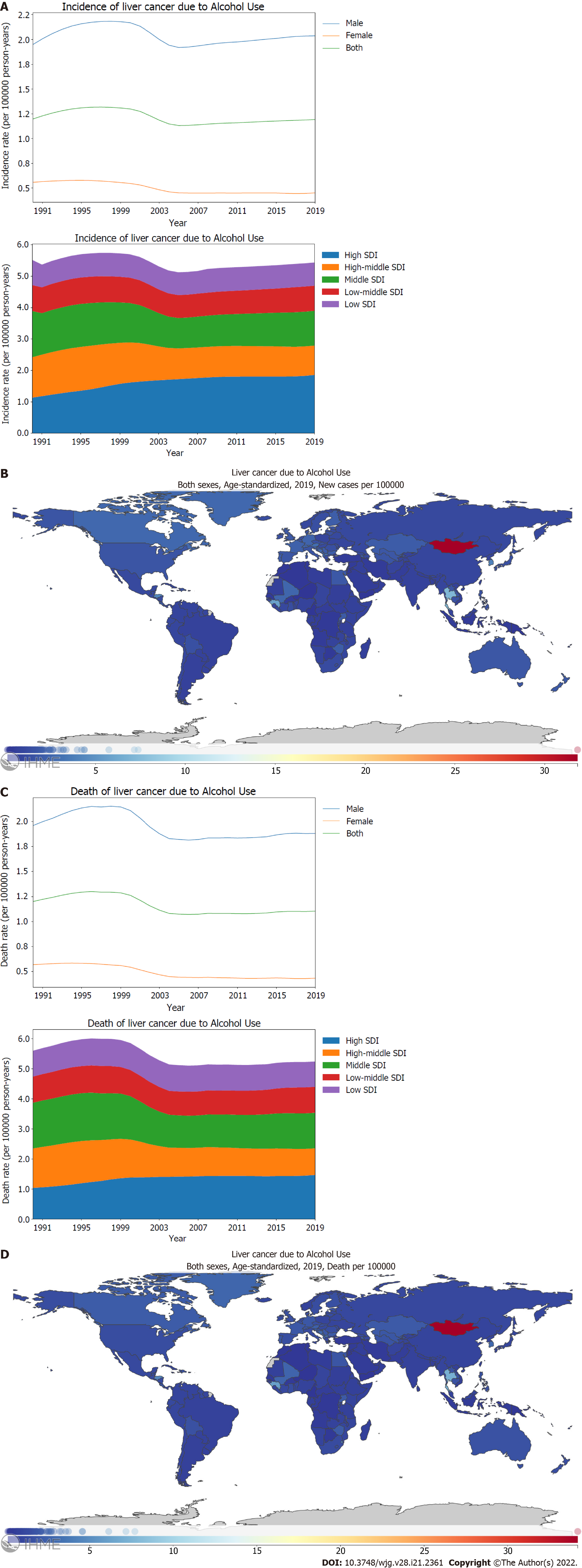
For PLC caused by alcohol use, the age-standardized incidence rate in males was four times higher than that in females (Figure 4A; Supplementary material, pages 89-90). Similar to PLC caused by hepatitis C, the age-standardized incidence rate of PLC caused by alcohol use was found to be higher for high SDI locations than other SDI locations when stratified using the SDI (Figure 4A; Supplementary Figure 4A; Supplementary material, pages 91-93). From 1990 through 2019, there was a notable difference in the trends for age-standardized incidence rates of PLC caused by alcohol use between SDI regions; high middle SDI locations [72.7% (54.3-96.4)] showed a significant decline. In contrast, high SDI locations showed a significant increase [163.5% (126.4-209.8)] (Figure 4A; Supplementary Figure 4A; Supplementary material, pages 19-20). In 2019, the highest incidence rate of PLC caused by alcohol use was recorded in Mongolia with 31.8 (21.3-44.7) per 100000 person-years, followed by Gambia and Thailand (Figure 4B; Supplementary material, pages 94-97). Males showed a higher burden of death rate of PLC caused by alcohol use than females, which corresponds with the higher incidence rate of PLC caused by alcohol use in males (Figure 4C; Supplementary material, pages 98-99). Between 1990 and 2019, the age-standardized death rate of PLC caused by alcohol use decreased significantly in high middle SDI locations [67.6% (50.9-88.9)]. However, high SDI locations showed an increasing trend [141.2% (111.0-179.2)] (Figure 4C; Supplementary Figure 4B; Supplementary material, pages 21-22). In 2019, Mongolia had the highest age-standardized death rate with 34.2 (23.1-47.8) per 100000 person-years, followed by Gambia and Thailand (Figure 4D; Supplementary material, pages 103-106).
By stratification using sex, the age-standardized incidence and mortality rates of PLC attributed to NASH in males was found to be higher than in females (Figure 5A and C; Supplementary material, pages 107-108 and 116-117). Similar to the geographical variance observed in PLC caused by alcohol use, the highest age-standardized incidence and death rates of PLC attributed to NASH were reported in the high and middle SDI locations (Figure 5A and C). Between 1990 and 2019, a remarkable difference was observed in the trends of age-standardized incidence rates of PLC attributed to NASH between SDI regions, with the high middle SDI locations [72.9% (55.1-96.0)] showing a declining trend and the high SDI locations showing an increasing trend [182.9% (135.4-248.6)] (Figure 5A; Supplementary Figure 5A; Supplementary material, pages 19-20). The changing pattern for the age-standardized death rate across SDI locations was comparable to that observed in the incidence rate of the same period. In 2019, Mongolia [7.6 (4.9-11.4)] depicted the highest age-standardized incidence rate, followed by Gambia and Qatar (Figure 5B; Supplementary material, pages 112-115). Similar to the order of age-standardized incidence rate, Mongolia [8.7 (5.6-12.9)], Gambia, and Guinea had the highest age-standardized death rate (Figure 5D; Supplementary material, pages 121-124).
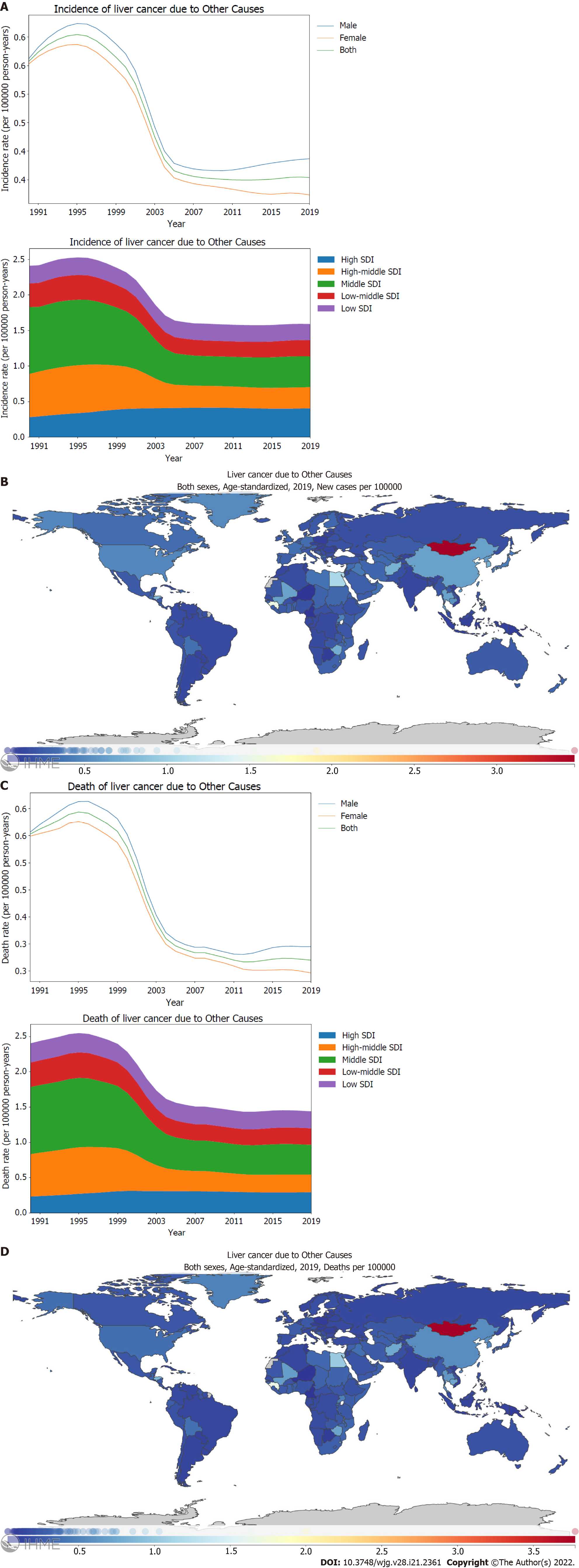
In terms of sex variance, the age-standardized incidence and mortality rates of PLC attributed to other causes were found to be higher in males (Figure 6A and C). When stratified using the SDI, higher incidence and mortality rates of PLC attributed to other causes were observed for high and middle SDI locations than for low middle and low SDI locations (Figure 6A and C). Between 1990 and 2019, there were remarkable geographical differences in the changing trend of age-standardized incidence rates of PLC attributed to other causes across the SDI regions; the high middle, middle, and low middle SDI locations showed a declining trend, whereas the high SDI locations showed an increasing trend [144.8% (112.8-186.3)] (Figure 6A; Supplementary Figure 6A; Supplementary material, pages 19-20). The geographical differences observed in the age-standardized death rate of PLC attributed to other causes across the SDI regions were comparable to the incidence rate (Figure 6C; Supplementary Figure 6B; Supplementary material, pages 21-22). The highest incidence and death rates of PLC attributed to other causes were observed for Mongolia, Gambia, and Guinea (Figure 6B and D; Supplementary material, pages 130-133 and 139-142).
Based on the data from the GBD 2019, we explored the global burden of PLC and focused on the relationship between socioeconomics and the attributable etiologies of PLC. Our main findings are listed below: (1) Global incidence and mortality rates of PLC declined between 1990 and 2019. The decreasing burden of PLC caused by hepatitis B and the declining PLC burden in middle SDI locations was considered the main driver for this favorable trend; (2) PLC had higher prevalence in males; (3) The highest attributable etiology of PLC was hepatitis B, followed by hepatitis C, and alcohol use; (4) The leading attributable etiology of PLC in the middle SDI locations was hepatitis B; and hepatitis C and alcohol use in the high SDI locations; (5) Before 2004, the middle SDI locations surpassed high SDI locations in terms of PLC burden. However, the high SDI locations exceeded the middle SDI locations in terms of PLC burden after 2004; (6) Between 1990 and 2019, the incidence rate of PLC decreased for the high middle SDI locations; it increased for the high SDI locations, according to the stratified causes of PLC including hepatitis B, hepatitis C, alcohol use, and NASH; and (7) In 2019, several countries located in East Asia, South Asia, West Africa, and North Africa shouldered the heaviest burden for incidence and death rates of PLC.
The risk factors for liver cancer include HBV, HCV, alcohol consumption, metabolic syndrome, diabetes[11]. Although there are substantial variations between countries in the underlying etiologies; globally, HBV accounts for 33%; alcohol, 30%; HCV, 21%, and other causes, 16% of liver cancer deaths[4]. Similar to these findings, we found that the leading attributable etiology of PLC was hepatitis B, followed by hepatitis C, alcohol use, NASH, and other causes, based on the GBD 2019. CHB and CHC cause sustained or repeated inflammatory damage, followed by liver fibrosis and cirrhosis. After liver cirrhosis is established, the risk of hepatocellular carcinoma (HCC) increases substantially. Further, liver cirrhosis caused by NASH substantially increases the risk for HCC[12]. A superimposed condition can enhance the possibility of PLC. For example, alcohol use can contribute to the occurrence of PLC in the setting of CHC.
We observed an impressive association between socioeconomic status and the attributable etiologies of PLC. For high middle and middle SDI regions, hepatitis B was the main etiology of PLC whereas hepatitis C was the main etiology of PLC for high SDI regions; this was in accordance with another similar study[6]. In addition to the heavier burden of PLC caused by hepatitis C, the high SDI locations had a higher prevalence of PLC attributed to alcohol use. Given that the prevalence of drinking is greatest for high SDI locations and the least in low middle SDI locations[13], this finding was expected. Although viral hepatitis including CHB and CHC remains the most common cause of liver deaths, nonalcoholic fatty liver disease (NAFLD) is a rapidly growing contributor to liver mortality and morbidity. A similar phenomenon has been observed in China. In 2016, NAFLD cases requiring inpatient care in China outnumbered their counterparts for chronic viral hepatitis[14]. In the United States, the attributable population factors for HCC were greatest for metabolic disorders[15]. Interestingly, the global age-standardized incidence rate of PLC due to hepatitis B reached its peak in 1995-1996, then decreased gradually, as was shown in the GBD 2019. Wide HBV vaccine coverage may have been the potential cause of this beneficial phenomenon. A genetically engineered hepatitis B vaccine was available in 1986. In China, vaccination against HBV began in 1985 using a plasma-derived hepatitis B vaccine. In 1992, a genetically engineered hepatitis B vaccine was licensed in China and managed nationally. The integration of the HBV vaccination into the Expanded Program on Immunization in China has reduced chronic HBV infection by 90% among children < 15 years of age[16]. One of our studies found that the global incidence of acute hepatitis B has decreased gradually since 1990[17]. Usually, a declining trend for HBV incidence precedes a decreasing trend of PLC incidence due to hepatitis B by 10 to 20 years. Similarly, wide HBV vaccine coverage may have contributed to the declining PLC burden in high middle SDI locations since hepatitis B was the most important attributable etiology of PLC in these regions.
According to the GBD 2019 data, PLC is more prevalent in males. In fact, this is in line with several other observations[4,11]. MyD88-dependent IL-6 production, Foxa1, and Foxa2 play a role in the gender disparity in PLC[18,19]. Furthermore, according to the GBD 2019, there were 534000 (487000-589000) incident cases, and 485000 (444000-526000) deaths attributed to liver cancer globally in 2019; these were significantly lower than those reported in the GBD 2017 and GLOBOCAN 2020[1,6]. In the GBD 2019, the mapping of ICD-10 C22.9 was changed to a garbage code because this would have included both primary and secondary or metastatic cancers (see also https://www.thelancet.com/pb-assets/Lancet/gbd/summaries/diseases/Liver-cancer.pdf). In clinical practice, liver metastasis originating from colorectal cancer or stomach cancer is rather common. Therefore, fewer deaths were mapped for liver cancer in the GBD 2019 than in the GBD 2017. That is, the data of PLC from the databases of the GBD 2016 and GBD 2017 may have unintentionally included cases of metastatic liver cancer.
In recent years, incidence and mortality rates of PLC have declined in middle SDI locations, such as China and other Eastern and Southeastern Asian countries[20-22]. In line with these findings, the PLC burden has been declining in the high middle and middle SDI locations from 1990 to 2019 according to the GBD 2019; this decline has benefited from the decreasing trend of viral hepatitis, such as CHB. However, the incidence and mortality rates of PLC increased in high SDI locations during the same period, which is in line with several other studies[4,23,24]. After 2004, the PLC burden in high SDI locations surpassed that in middle SDI locations. Several factors contributed to this reversal. First, hepatitis B was the leading attributable etiology of PLC in middle SDI locations. However, vaccination coverage for hepatitis B contributed to the declining trend of PLC in the middle SDI locations. Second, the increasing trend of PLC burden in high SDI locations was attributed to the increasing prevalence of alcohol use and metabolic risk factors for HCC, including metabolic syndrome, obesity, type II diabetes, and NASH[11,13]. As shown in Figures 4A, 4C, 5A and 5C, the gradually increasing burdens of alcohol use and NASH aggravated the burden of PLC in high SDI locations. The epidemiology of HCC has been shifting away from a disease predominated by viral hepatitis to NASH. A similar phenomenon was observed in the United States[25]. Thus, maintaining adequate surveillance of alcohol abuse and NASH is vital to develop strategies against the burden of PLC caused by these conditions.
Although PLC causes a heavy burden of cancer incidence and mortality, it (to be precise, HCC) can be prevented by avoiding the risk factors. Compared with the cohort without vaccination, universal HBV vaccination reduced the relative prevalence of HBsAg to 0.24 (0.16-0.35)[26]. Similarly, escalating vaccination policy in China has significantly reduced the prevalence of HBsAg in the recent three decades[16]. Given the heavy burden of PLC caused by hepatitis B in middle and high middle SDI locations, universal HBV vaccination in these areas is considered a practical and principal strategy to minimize the liver cancer burden. Data have indicated that universal HBV vaccination has contributed to a dramatic decline in the PLC burden in several countries and regions[27,28]. For CHB or HBV-related liver cirrhosis, effective antiviral treatment should be provided based on the relative guidelines[29]. Treatment with > 5 years of oral antiviral therapy effectively decreases the HCC incidence regardless of whether patients have baseline cirrhosis[30]. The early diagnosis of CHB and CHC before liver cirrhosis is important considering that liver cirrhosis substantially contributes to the risk of PLC. In areas where conditions permit, performing non-invasive examinations, such as liver stiffness measure, for individuals with high risk may be potentially beneficial. Unfortunately, there is no effective vaccine for HCV available now; however, DAA have made the eradication of HCV a reality. The achievement of an HCV cure before HCC diagnosis is associated with improved survival[31].
Globally, alcohol use was the seventh leading risk factor for deaths in 2016[13]. As shown in the GBD 2019, alcohol use is a major cause of PLC, especially in high SDI locations. This highlights the need for developing strategies to decrease alcohol use. NAFLD is the third-most common cause of cancer-related deaths worldwide and the seventh most common cause in the United States[32]. Considering the increasing trend of PLC due to NASH, especially in high SDI and middle SDI locations, the control or even reversal of NASH is of critical importance, and this can be attained with lifestyle changes comprising diet, exercise, and weight loss.
There are several limitations to this study: (1) There is a possibility of the underestimation of the PLC burden in low middle and low SDI locations because of inadequate cancer screening. However, underestimation of the PLC burden is an inevitable problem, especially in low middle and low SDI locations owing to inadequate cancer screening and lack of registration. Similar limitations have been reported in cervical cancer screenings in low- and middle-income countries[33]. Additionally, in one of our previous studies, the underestimation of acute hepatitis in low-income countries was evident[17]; (2) Insufficient disclosure of geographical variances in large countries such as China and the United States. The GBD reports cancer burden by country or region; however, a large country has significant geographical variances in cancer burden for the urban or rural regions; (3) The lack of finer data for complex cancer, as PLC can be further divided into HCC and cholangiocarcinoma. These subgroups of cancer tend to have different etiologies and exhibit different features in terms of incidence and mortality rates; and (4) The inclusion of undefined etiologies in “other causes” can be leading causes in certain locations.
Despite these limitations, the GBD 2019 data are valuable for policymakers to implement cost-effective interventions, address modifiable risk factors, and prevent PLC efficiently.
The pronounced association between socioeconomic development status and PLC burden indicates socioeconomic development status affects attributable etiologies for PLC. GBD 2019 data are valuable for policymakers implementing PLC cost-effective interventions.
Primary liver cancer (PLC) is a common cancer with high morbidity and mortality rates. PLC usually occurs as a preventable disease. Data on global and country-specific levels and trends of PLC are essential for understanding the effects of this disease and helping policymakers to allocate resources.
The association between socioeconomic development status and attributable etiologies for PLC is still unclear.
To investigate the association between the burden of PLC and socioeconomic development status.
Cancer mortality and incidence rates of PLC were obtained from the Global Burden of Disease (GBD) 2019, and the data were stratified by the Socio-demographic Index (SDI) level. The association between the attributable etiology of PLC and SDI was described.
Several countries located in East Asia, South Asia, West Africa, and North Africa shouldered the heaviest burden of PLC in 2019. In terms of incidence rates, the first leading underlying cause of PLC identified was hepatitis B. The incidence rate of PLC was the highest for high and middle SDI locations. The leading attributable etiologies of PLC were hepatitis B for the middle and high middle SDI locations and hepatitis C and nonalcoholic steatohepatitis for the high SDI locations.
Socioeconomic development status significantly affects attributable etiologies for PLC.
Our findings are valuable to implement tailored prevention strategies for PLC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karatza AA, Greece; Muhammad Irham L, Indonesia; Naseri M, Iran; Salama II, Egypt S-Editor: Yan JP L-Editor: A P-Editor: Guo X
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11942] [Article Influence: 2985.5] [Reference Citation Analysis (4)] |
| 4. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1500] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 6. | Lin L, Yan L, Liu Y, Qu C, Ni J, Li H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer. 2020;9:563-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 7. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11327] [Cited by in RCA: 9637] [Article Influence: 1927.4] [Reference Citation Analysis (35)] |
| 8. | GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 9. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 10. | GBD 2016 Mortality Collaborators. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 11. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1346] [Article Influence: 336.5] [Reference Citation Analysis (1)] |
| 12. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 571] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 13. | GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2194] [Cited by in RCA: 2096] [Article Influence: 299.4] [Reference Citation Analysis (0)] |
| 14. | Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (1)] |
| 15. | Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 16. | Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Zheng H, Sun X, Miao N, Yin Z, Feng Z, Liang X, Wang Y. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg Infect Dis. 2017;23:765-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 17. | Zeng DY, Li JM, Lin S, Dong X, You J, Xing QQ, Ren YD, Chen WM, Cai YY, Fang K, Hong MZ, Zhu Y, Pan JS. Global burden of acute viral hepatitis and its association with socioeconomic development status, 1990-2019. J Hepatol. 2021;75:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 18. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1490] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 19. | Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 20. | Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, Chen W, He J. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 21. | Bai L, Liu Z, Fang Q, Yan Q, Shi O, Bao P, Mu L, Chen X, Zhang T. The trends and projections in the incidence and mortality of liver cancer in urban Shanghai: a population-based study from 1973 to 2020. Clin Epidemiol. 2018;10:277-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Wu J, Yang S, Xu K, Ding C, Zhou Y, Fu X, Li Y, Deng M, Wang C, Liu X, Li L. Patterns and Trends of Liver Cancer Incidence Rates in Eastern and Southeastern Asian Countries (1983-2007) and Predictions to 2030. Gastroenterology. 2018;154:1719-1728.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Pinheiro PS, Callahan KE, Jones PD, Morris C, Ransdell JM, Kwon D, Brown CP, Kobetz EN. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945-1965 birth cohort by ethnicity. JHEP Rep. 2019;1:162-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 25. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 26. | Whitford K, Liu B, Micallef J, Yin JK, Macartney K, Van Damme P, Kaldor JM. Long-term impact of infant immunization on hepatitis B prevalence: a systematic review and meta-analysis. Bull World Health Organ. 2018;96:484-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310:974-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 653] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 29. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1961] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 30. | Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S, Loglio A, Siakavellas S, Keskın O, Gatselis N, Hansen BE, Lehretz M, de la Revilla J, Savvidou S, Kourikou A, Vlachogiannakos I, Galanis K, Yurdaydin C, Berg T, Colombo M, Esteban R, Janssen HLA, Lampertico P. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 31. | Lockart I, Hajarizadeh B, Alavi M, Davison S, Prakoso E, Levy MT, George J, Dore GJ, Danta M. Hepatitis C virus cure before hepatocellular carcinoma diagnosis is associated with improved survival. J Viral Hepat. 2021;28:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1317] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 33. | Lemp JM, De Neve JW, Bussmann H, Chen S, Manne-Goehler J, Theilmann M, Marcus ME, Ebert C, Probst C, Tsabedze-Sibanyoni L, Sturua L, Kibachio JM, Moghaddam SS, Martins JS, Houinato D, Houehanou C, Gurung MS, Gathecha G, Farzadfar F, Dryden-Peterson S, Davies JI, Atun R, Vollmer S, Bärnighausen T, Geldsetzer P. Lifetime Prevalence of Cervical Cancer Screening in 55 Low- and Middle-Income Countries. JAMA. 2020;324:1532-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |