Published online Jun 7, 2022. doi: 10.3748/wjg.v28.i21.2350
Peer-review started: December 10, 2021
First decision: January 27, 2022
Revised: February 8, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 7, 2022
Processing time: 173 Days and 21.9 Hours
Contrast-enhanced ultrasound (CEUS) can be used to diagnose focal liver lesions (FLLs) in children. The America College of Radiology developed the CEUS liver imaging reporting and data system (LI-RADS) for standardizing CEUS diagnosis of FLLs in adult patients. Until now, no similar consensus or guidelines have existed for pediatric patients to improve imaging interpretation as adults.
To evaluate the performance of CEUS LI-RADS combined with alpha-fetoprotein (AFP) in differentiating benign and malignant FLLs in pediatric patients.
Between January 2011 and January 2021, patients ≤ 18 years old who underwent CEUS for FLLs were retrospectively evaluated. The following criteria for diagnosing malignancy were proposed: Criterion I considered LR-4, LR-5, or LR-M lesions as malignancies; criterion II regarded LR-4, LR-5 or LR-M lesions with simultaneously elevated AFP (≥ 20 ng/mL) as malignancies; criterion III took LR-4 Lesions with elevated AFP or LR-5 or LR-M lesions as malignancies. The sensitivity, specificity, accuracy and area under the receiver operating characteristic curve (AUC) were calculated to determine the diagnostic value of the aforementioned criteria.
The study included 63 nodules in 60 patients (mean age, 11.0 ± 5.2 years; 26 male). There were no statistically significant differences between the specificity, accuracy, or AUC of criterion II and criterion III (95.1% vs 80.5%, 84.1% vs 87.3%, and 0.794 vs 0.902; all P > 0.017). Notably, criterion III showed a higher diagnostic sensitivity than criterion II (100% vs 63.6%; P < 0.017). However, both the specificity and accuracy of criterion I was inferior to those of criterion II and criterion III (all P < 0.017). For pediatric patients more than 5 years old, the performance of the three criteria was overall similar when patients were subcategorized by age when compared to all patients in aggregate.
CEUS LI-RADS combined with AFP may be a powerful diagnostic tool in pediatric patients. LR-4 with elevated AFP, LR-5 or LR-M lesions is highly suggestive of malignant tumors.
Core Tip: Contrast-enhanced ultrasound liver imaging reporting and data system (CEUS LI-RADS) is used for the diagnosis of focal liver lesions (FLLs) in adult patients at high risk of hepatocellular carcinoma. CEUS has recently been approved to be used in characterization of FLLs in children. Our study investigated the diagnostic value of CEUS LI-RADS in association with serum alpha-fetoprotein (AFP) in differentiating malignant from benign FLLs in pediatric patients. Our study demonstrated that CEUS LI-RADS combined with AFP may be a powerful diagnostic tool for pediatric patients. LR-4 with elevated AFP, LR-5 or LR-M lesions are highly indicative of malignant tumors.
- Citation: Jiang ZP, Zeng KY, Huang JY, Yang J, Yang R, Li JW, Qiu TT, Luo Y, Lu Q. Differentiating malignant and benign focal liver lesions in children using CEUS LI-RADS combined with serum alpha-fetoprotein. World J Gastroenterol 2022; 28(21): 2350-2360
- URL: https://www.wjgnet.com/1007-9327/full/v28/i21/2350.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i21.2350
Pediatric patients have different treatment strategies regarding benign and malignant focal liver lesions (FLLs)[1]. Hepatoblastoma (HB) constitutes the most common malignant tumor, accounting for 67% of all pediatric malignant FLLs, followed by hepatocellular carcinoma (HCC), at 28%[2-4]. Thanks to the development of surgical techniques and chemotherapy, the overall 5-year survival rates of HB exceed 80% with timely treatment, and those of nonmetastatic HCC patients who can be treated surgically are 70%-80%[5]. In comparison, the survival rate of children with inoperable hepatic malignancies was less than 20%[6].
Although computed tomography (CT) and magnetic resonance imaging (MRI) are usually recommended for the differential diagnosis of pediatric FLLs[7], both have some limitations. CT increases children’s radiation exposure, while MRI requires a long imaging time and high cost[8-10]. Furthermore, children are exposed to the risk of contrast-induced nephrotoxicity and potential use of sedation[11]. Serum alpha-fetoprotein (AFP) is the most widely used tumor biomarker for the screening of HCC and HB in high-risk pediatric populations[12]. However, AFP levels remained in the normal range in 30%-40% of HCC patients and 10% of HB patients. Moreover, the positive predictive value of AFP is poor, making the value of AFP alone as a diagnostic tool very limited[13,14]. Therefore, developing a potent diagnostic method for differentiating benign from malignant FLLs in children is urgently needed.
The American College of Radiology developed the Liver Imaging Reporting and Data System (LI-RADS) to standardize the diagnosis of HCC and assist in the diagnosis of other hepatic malignant tumors[7]. In addition, CEUS can overcome the shortcomings of the aforementioned imaging modalities[15]. Moreover, CEUS has been approved for use in the diagnosis of FLL in the pediatric population[16]. Therefore, this study aimed to evaluate the diagnostic performance of the combination of CEUS LI-RADS and AFP in differentiating benign and malignant FLLs in a pediatric population.
This retrospective study was approved by the institutional review board of our hospital, and informed consent was waived.
From January 2011 to January 2021, hepatic CEUS examinations performed in a tertiary academic medical center were retrospectively collected.
The inclusion criteria were: (1) Age ≤ 18 years at the time of examination; (2) visible liver nodules at baseline US; and (3) sufficient images of the arterial phase, portal phase, and parenchymal phase.
The exclusion criteria were: (1) Lesions previously treated; (2) known or strongly suspected active extrahepatic primary malignancy; (3) poor image quality; and (4) no accepted reference standard (see more detail in a later section).
Conventional and contrast-enhanced US examinations were performed using a Philips IU22 system (Philips Medical Solutions; Mountain View, CA, United States) with a C5-1 convex or an L9-3 Linear probe. After routine ultrasound examinations, all pediatric patients underwent CEUS examinations using the pulse inversion harmonic imaging technique with a mechanical index less than 0.1. A bolus injection of 1.2 mL of ultrasound contrast agent (SonoVue; Bracco, Milan, Italy) was administered through a vascular catheter needle placed in the anterior cubital vein. The imaging timer was started immediately upon completion of the contrast agent injection. A 5 mL flush of 0.9% sodium chloride solution followed the ultrasound contrast agent injection. The target area was continuously scanned in the first 60 s, followed by intermittent scans and records until the examiner confidently observed washout or faded liver parenchymal enhancement, typically 5 min or longer. CEUS imaging was digitally stored for further evaluation.
Pathological diagnosis from surgical resection or percutaneous biopsy was taken as the reference standard. In addition, lesions without pathological diagnosis were considered benign if their size increased less than 50% at the 12-mo imaging follow-up. Meanwhile, serum AFP ≥ 20 ng/mL was regarded as elevated[13].
In a previous study, lesions with categories of LR-1, LR-2 or LR-3 were considered benign, while LR-4, LR-5 or LR-M was defined as malignancy[16]. Moreover, the meta–analysis conducted by Christian et al[17], including 17 studies (2760 patients, 3556 lesions), showed that 80% of LR-4, 97% of LR-5, and 93% of LR-M lesions were malignant. Therefore, we proposed the following criteria for the diagnosis of malignancy in the pediatric population: Criterion I considered LR-4, LR-5, or LR-M lesions as malignancies; criterion II regarded LR-4, LR-5 or LR-M lesions with simultaneously elevated AFP (≥ 20 ng/mL) as malignancies; and criterion III took LR-4 lesions with elevated AFP or LR-5 or LR-M lesions as malignancies.
Two certified radiologists (Qiu TT and LI JW, with more than 3 years and 5 years of experience in hepatic CEUS, respectively) who were blinded to the reference standard and other clinical data reviewed the CEUS examinations of all cases independently and assigned a category according to the CEUS LI-RADS (2017 version). When there was an inconformity, arbitration from a blinded expert radiologist (Lu Q, with 17 years of experience) was performed. Briefly, the main diagnostic criteria of CEUS LI-RADS are nodule size, enhancement degree and pattern in the arterial phase, timing and degree of washout. Moreover, the ancillary features for category adjustment are nodule-in-nodule architecture and mosaic architecture.
Qualitative data are presented as the numbers and percentages. Quantitative data are presented as a combination of the mean values and standard deviations. The comparison of numeric variables was performed using t tests. Differences in categorical variables were analyzed using χ2 tests or Fisher’s exact tests. The unit of analysis is each FLL rather than each patient. The accuracy, sensitivity, specificity, area under the curve (AUC) and 95% confidence intervals (CI) were calculated to evaluate the diagnostic power of CEUS LI-RADS in association with AFP in distinguishing benign and malignant FLLs. The performance of the diagnostic criteria was further assessed by the fourfold table and compared by using the McNemar test. A P value less than 0.05 was considered statistically significant. The P values were corrected for multiple comparisons through the Bonferroni method (Bonferroni-adjusted P values < 0.017). Given that HCC more commonly occurs in children over 5 years old among pediatric patients[18], subgroup analysis was also conducted. Based on the value of κ, the strength of agreement is defined as follows: κ < 0.20 suggests poor agreement, 0.21-0.40 suggests fair agreement, 0.41-0.60 suggests moderate agreement, 0.61-0.80 suggests good agreement, and 0.80-1.00 suggests almost perfect agreement. Statistical analyses were performed using statistical software (MedCalc10.4.7.0; MedCalc Software, Ostend, Belgium).
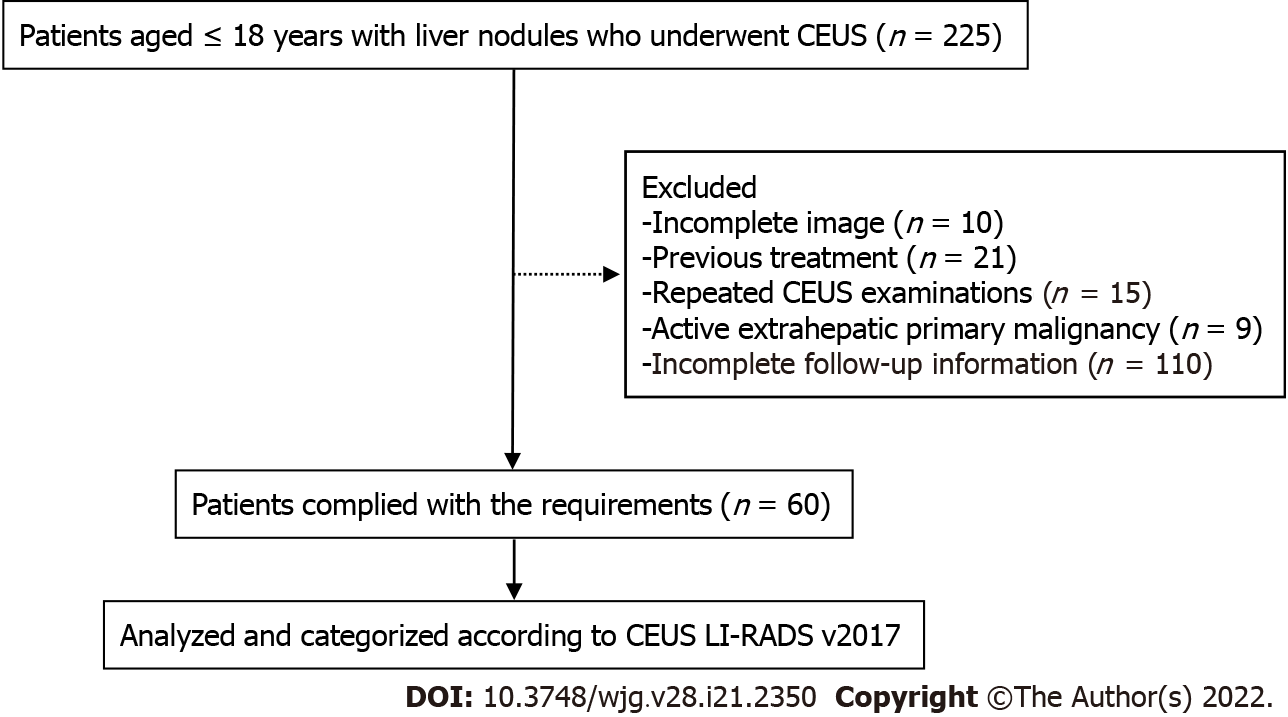
According to the inclusion and exclusion criteria, 63 lesions from 60 patients were enrolled in this study (Figure 1), among which 3 patients had 2 FLLs. The main clinical characteristics of the patients, including age, sex, serum AFP, tumor size, and high-risk factors for HCC, are shown in Table 1. The average size of the 63 lesions was 68 ± 39 mm, ranging from 11 to 163 mm. Males accounted for 43.3% of the included patients, and AFP levels exceeding 20 ng/mL were present in 14 patients. The AFP level of malignant lesions (Figure 2) was higher than that of benign lesions [63.6% (14/22) vs 4.9% (2/41), P < 0.0001]. In our study, 14 patients had high-risk factors for HCC, including 8 chronic hepatitis B and 6 cirrhosis.
| Characteristics | All Patients (n = 60) | Patients with malignant lesions (n = 20) | Patients with benign lesions (n = 40) | P value2 |
| Age, yr; mean ± SD, (range) | 11.0 ± 5.2 (0-18) | 9.7 ± 5.4 (0-18) | 11.7 ± 5.1 (0-18) | 0.98 |
| Gender, n (%) | 0.54 | |||
| Male | 26 (43.3) | 10 (50.0) | 16 (40.0) | |
| Female | 34 (56.7) | 10 (50.0) | 24 (60.0) | |
| AFP level (ng/mL), n (%) | < 0.05 | |||
| AFP > 20 | 14 (23.3) | 12 (60.0) | 2 (5.0) | |
| AFP < 20 | 46 (76.7) | 8 (40.0) | 38 (95.0) | |
| High-risk factors1 | 0.24 | |||
| High risk for HCC1 | 14 (23.3) | 7 (35.0) | 7 (17.5) | |
| No high risk for HCC1 | 46 (76.7) | 13 (75.0) | 33 (82.5) |
Histopathological results and follow-up results of the lesions are summarized in Table 2. Histopathological results of 52 (82.5%) lesions were obtained by surgical resections or US-guided core needle biopsies. The 11 (17.5%) lesions were regarded as benign through the one-year follow-up.
| Diagnosis | All flls (n = 63) | Flls from Patients > 5 yr (n = 53) |
| Pathologic analysis | 2 | 42 |
| Malignant liver lesions | 22 | 17 |
| HCC | 10 | 10 |
| HB | 6 | 2 |
| Undifferentiated sarcoma | 2 | 1 |
| Non-Hodgkin’s lymphoma | 1 | 1 |
| Neuroendocrine carcinoma | 1 | 1 |
| Desmoplastic small round cell tumor | 1 | 1 |
| Perivascular epithelioid cell tumor | 1 | 1 |
| Benign liver lesions | 30 | 25 |
| FNH | 14 | 12 |
| RN/DN | 3 | 3 |
| Area of granulomatous inflammation | 3 | 3 |
| Adenomatoid hyperplasia | 3 | 3 |
| Infantile hemangioendothelioma | 2 | 0 |
| Liver abscess | 1 | 0 |
| Other benign tumors | 3 | 3 |
| Follow-up < 50% size increase in 12 mo | 11 | 11 |
| Hemangioma | 3 | 3 |
| FNH | 3 | 1 |
| RN/DN | 2 | 2 |
| Other benign tumors | 3 | 3 |
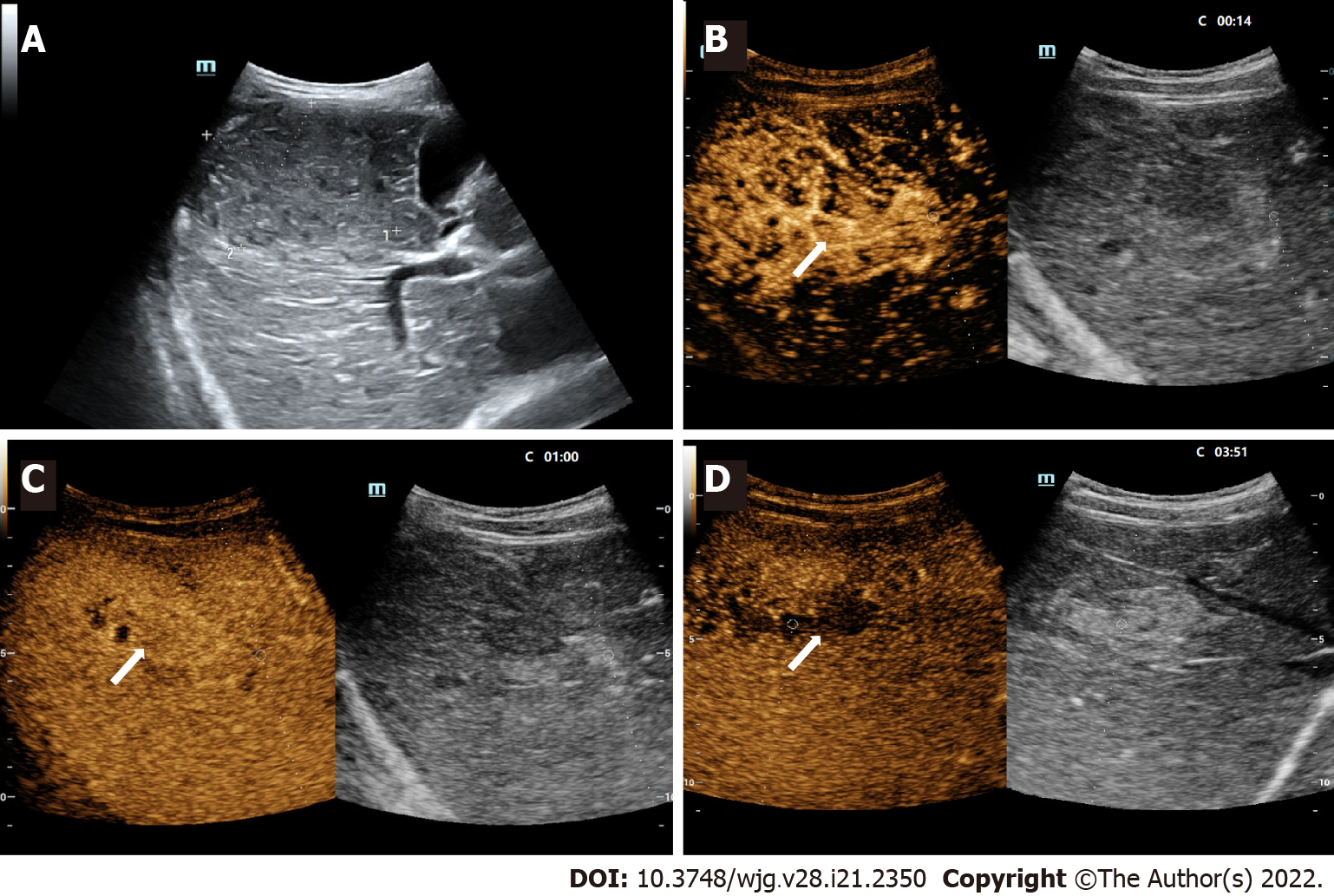
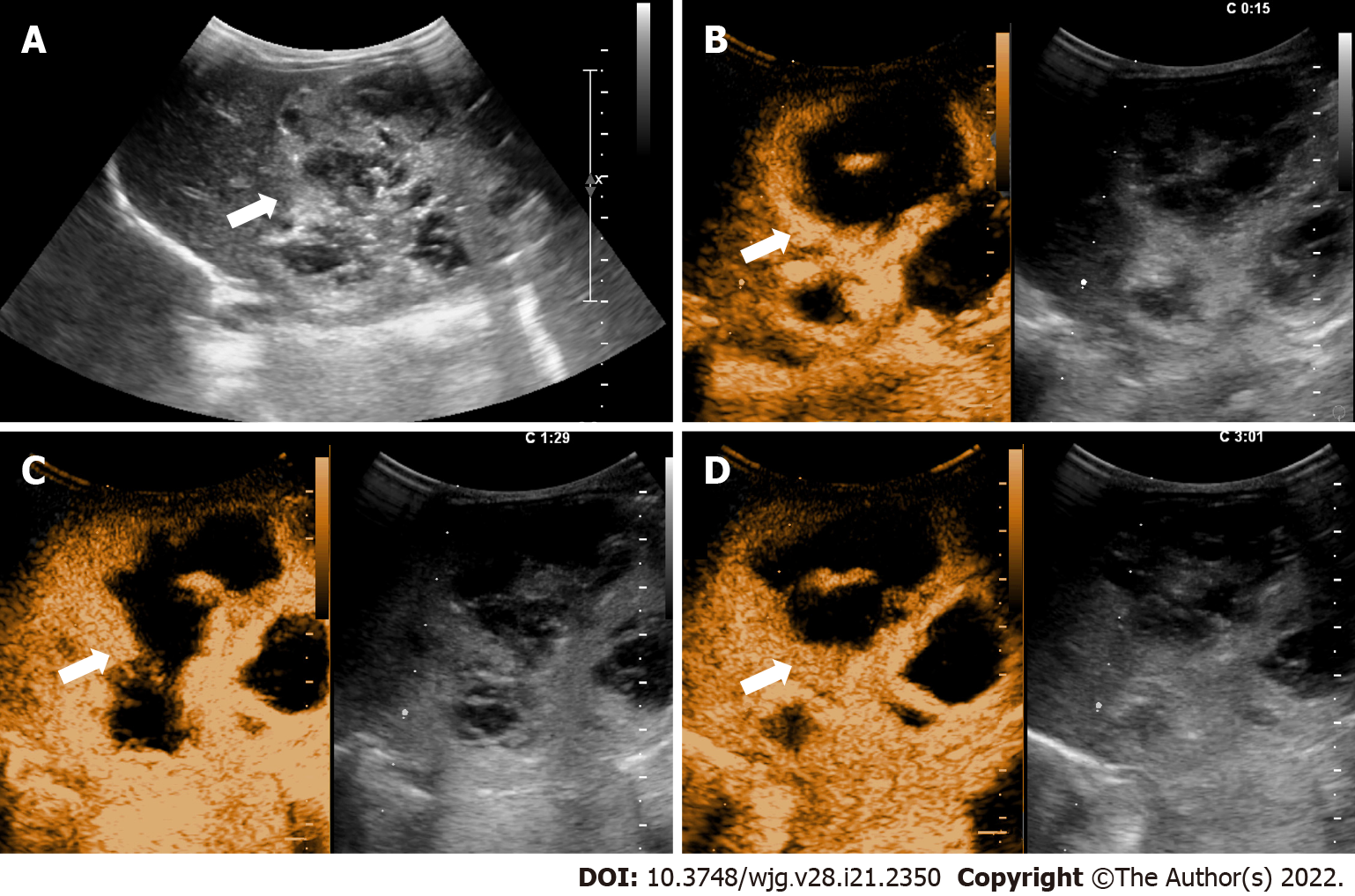
The distribution of FLLs in CEUS LI-RADS categories and lesions with elevated AFP levels are displayed in Table 3. In this study, 2 benign lesions were classified as LR-M, including one granulomatous inflammation and one abscess. Furthermore, 4 benign lesions in LR-5 included one adenomatoid hyperplasia, one abscess, and 2 focal nodular hyperplasia (FNH). Among the lesions defined as LR-4, there were only two lesions with elevated AFP. Postoperative pathology confirmed them as a regenerative nodule and an infantile hemangioendothelioma (Figure 3). The CEUS characteristics of various FLLs are presented in Table 4.
| CEUS LI-RADS | No. of nodules (n = 63) | No. of malignant lesions (n = 22) | No. of benign lesions (n = 41) | AFP > 20 ng/mL (n = 16) |
| LR-1 | 4 | 0 | 4 | 0 |
| LR-2 | 0 | 0 | 0 | 0 |
| LR-3 | 8 | 0 | 8 | 0 |
| LR-4 | 23 | 0 | 23 | 2 |
| LR-5 | 22 | 18 | 4 | 13 |
| LR-M | 6 | 4 | 2 | 1 |
| Image features | Malignant lesions | Benign lesions | ||||
| HCC (n = 10) | HB (n = 6) | Other malignant lesions (n = 6) | FNH (n = 17) | RN/DN (n = 5) | Other benign tumors (n = 18) | |
| Gray-scale echogenicity | ||||||
| Hyperechoic | 3 | 4 | 5 | 4 | 2 | 9 |
| Hypoechoic | 7 | 2 | 1 | 13 | 3 | 9 |
| Arterial phase, hyperenhancement | ||||||
| Homogeneous | 4 | 2 | 9 | 1 | 4 | |
| Inhomogenous | 6 | 4 | 5 | 8 | 5 | |
| Rim | 1 | 2 | ||||
| Peripheral nodular | 3 | |||||
| Isoenhancement | 2 | 2 | ||||
| Hypoenhancement | 2 | 2 | ||||
| Late phase | ||||||
| Hyperenhancement | 10 | 5 | ||||
| Isoenhancement | 5 | 5 | 8 | |||
| Hypoenhancement | 10 | 6 | 6 | 2 | 5 | |
| Washout | ||||||
| < 60 s | 1 | 3 | 1 | |||
| Marked, ≤ 120 s | 1 | |||||
The rating of liver nodules according to CEUS LI-RADS of the two readers indicated good agreement, with a κ value of 0.76 (95%CI: 0.62-0.90).
Table 5 summarizes the diagnostic performances of different diagnostic criteria in differentiating benign and malignant FLLs in children. Table 6 shows a comparison of different criteria on indicators of diagnostic performance. Notably, there was no statistically significant difference between the specificity, accuracy, or AUC of criterion II and criterion III (95.1% vs 80.5%, 84.1% vs 87.3%, and 0.794 vs 0.902; all P > 0.017). Notably, criterion III showed a higher diagnostic sensitivity than criterion II (100% vs 63.6%; P < 0.017). However, both the specificity and accuracy of criterion I was inferior to those of criterion II and criterion III (all P values < 0.017).
| Diagnostic criteria | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC |
| Criterion I | 100.0 (84.6-100.0) | 29.3 (16.1-45.5) | 54.0 (40.9-66.6) | 0.646 (0.516-0.763) |
| Criterion II | 63.6 (40.7-82.8) | 95.1 (83.5-99.4) | 84.1 (72.7-92.1) | 0.794 (0.673-0.885) |
| Criterion III | 100.0 (84.6-100.0) | 80.5 (65.1-91.2) | 87.3 (76.5-94.4) | 0.902 (0.801-0.963) |
| P value | Sensitivity | Specificity | Accuracy | AUC |
| Criterion I vs criterion II | < 0.017 | < 0.0001 | < 0.017 | > 0.017 |
| Criterion I vs criterion III | - | < 0.0001 | < 0.0001 | < 0.0001 |
| Criterion II vs criterion III | < 0.017 | > 0.017 | > 0.05 | > 0.05 |
In total, 53 FLLs were included in this subgroup analysis. The diagnostic performance of CEUS LI-RADS in association with AFP for predicting overall hepatic malignancy and HCC among patients older than 5 years is shown in Supplementary Table 1. Moreover, a comparison of indicators for diagnostic power among the three criteria is shown in Supplementary Table 2. The performance of the three criteria was similar overall when patients were subcategorized by age when compared to all patients in aggregate. In short, there was no statistically significant difference between the specificity, accuracy, or AUC of criterion II and criterion III (97.2% vs 86.1%, 83.3% vs 87.0%, and 0.780 vs 0.931; all P > 0.017). Notably, criterion III showed a higher diagnostic sensitivity than criterion II (100% vs58.8%; P < 0.017). However, both the specificity and accuracy of criterion I was inferior to those of criterion II and criterion III (all P < 0.017). Interestingly, if LR-5 lesions with elevated AFP were regarded as HCC in this subgroup, the sensitivity, specificity, accuracy, and AUC of diagnosing HCC were 80.0% (95%CI: 44.4%-97.5%), 95.4% (95%CI: 84.2%-99.4%), 94.4% (95%CI: 84.6%-98.8%) and 0.877 (95%CI: 0.757-0.951), respectively.
Proper differentiation between benign and malignant FLLs is essential in the treatment of pediatric liver disease. We found that CEUS LI-RADS in association with AFP presented an effective way to differentiate benign tumors from malignancies in pediatric patients. The sensitivity and specificity of criterion III (LR-4 with elevated AFP or LR-5 or LR-M lesions) reached 100.0% and 80.5%, respectively.
The specificity (29.3%) of diagnostic criterion I (LR-4, LR-5, or LR-M lesions) was significantly reduced compared to criteria II and III. This may be because there were a considerable number of benign lesions in LR-4. Notably, differentiation between benign and malignant FLLs in pediatric patients by CEUS LI-RADS alone had an accuracy of 54.0% and specificity of 29.3%, suggesting that CEUS LI-RADS alone is not suitable for this scenario. CEUS LI-RADS was mainly used as a diagnostic tool for HCC in adults at high risk. This study explored the possibility of expanding the application of this diagnostic algorithm in pediatric patients. However, only a few pediatric patients have high-risk factors for HCC, and the disease spectrum of FLLs between adults and children is different. HB and HCC account for a majority of pediatric hepatic malignancies, while hemangioma and FNH account for a majority of pediatric hepatic benign lesions. Because a significant difference in AFP was found between benign and malignant FLLs[19], CEUS LI-RADS combined with serum AFP is proposed for better characterization of FLLs in pediatric patients.
Compared with criterion III, the sensitivity (63.6%) of criterion II decreased significantly. A possible explanation was that 2 HCC patients and 6 patients with other hepatic malignancies presented normal serum AFP values (< 20 ng/mL), resulting in false negatives of the aforementioned lesions according to criterion II.
In this study, 13 FNHs were assigned to LR-4, and 2 FNHs were assigned to LR-5. A retrospective study by Kong et al[20] found that 42.9% of FNHs displayed global homogeneous hyperenhancement, and 42.9% of FNHs showed centrifugal enhancement in the arterial phase. Centrifugal arterial enhancement was often present in FNH < 3 cm. This is probably because the blood supply of larger lesions is more abundant[21]. Moreover, atypical FNHs could demonstrate washout in the portal and late phases[22]. Due to the above reasons, FNHs could be classified as LR-4 or LR-5 Lesions. However, AFP in patients with FNH is generally within the normal range[23]. Therefore, the combination of CEUS LI-RADS and AFP may potentially avoid diagnosing FNH as a malignancy.
We also performed subgroup analysis by the age of 5 to explore whether those patients could use CEUS LI-RADS combined with AFP to identify malignant FLLs or even HCC. For differentiating malignant from benign FLLs, the results of subgroup analysis were similar to the overall analysis. LR-5 in adult patients had a high diagnostic specificity for HCC. In this study, LR-5 Lesions with elevated AFP for diagnosing HCC presented high specificity (95.4%) in pediatric patients over 5 years old. Consequently, we speculate that CEUS LI-RADS combined with AFP has the potential to diagnose HCC in children older than 5 years. Nevertheless, the number (n = 10) of pediatric HCC patients included in this study was too small. Further study with a larger sample is needed to validate this hypothesis.
In this study, a 19-hour-old newborn patient with infantile hemangioendothelioma presented a significant increase in AFP levels (AFP > 1210 ng/mL). Regarding the features of CEUS, the patient showed inhomogeneous hyperenhancement in the arterial phase and isoenhancement in the portal and delayed phases, and there were areas of nonenhancement within the lesion. The aforementioned feature indicated that the lesion was likely a benign lesion. However, because the lesion was diagnosed as malignant by contrast-enhanced CT, the patient underwent surgical resection of the hepatic mass. Postoperative pathology confirmed that the lesion was an infantile hemangioendothelioma. Within 60 ± 24 h after birth, the serum AFP of newborns can range from 9700 to 11190 ng/mL and drop rapidly to a level close to the normal level of adults within one year[24]. Therefore, we should be meticulous with elevated AFP in differentiating FLLs of newborns. In addition, infantile hemangioendothelioma is a common benign tumor in newborns, most of which do not require surgical treatment[25]. Therefore, the diagnosis of benign and malignant FLLs in newborns should be made with caution, and the diagnostic method needs to be further explored.
This study had several limitations. First, this was a retrospective study with a relatively small sample size, which may inevitably lead to selection bias. Second, CEUS LI-RADS was mainly used for patients at risk of HCC, while only 14 patients in this study met the prerequisites for risk factors. Moreover, the risk factors for HCC in children do not exactly correspond to those in adults. Lastly, there were a considerable number of benign lesions confirmed by histopathology results, which might have led to the selection of benign lesions with atypical imaging findings. Thus, the specificity of the diagnostic criteria may have been underestimated.
We propose a novel method that might be a powerful diagnostic tool to differentiate malignant from benign FLLs in pediatric patients. LR-4 with elevated AFP, LR-5 or LR-M lesions could effectively differentiate benign and malignant tumors in pediatric patients.
Contrast-enhanced ultrasound (CEUS) has recently been approved to be used in characterization of focal liver lesions (FLLs) in children. The America College of Radiology developed the CEUS liver imaging reporting and data system (LI-RADS) for standardizing CEUS diagnosis of FLLs in adult patients. However, it is not suitable for pediatric patients.
To explore a method for differentiating benign and malignant FLLs in pediatric patients.
To evaluate the performance of CEUS LI-RADS combined with alpha-fetoprotein (AFP) in differentiating benign and malignant FLLs in pediatric patients.
The following criteria for diagnosing malignancy were proposed: Criterion I considered LR-4, LR-5, or LR-M lesions as malignancies; criterion II regarded LR-4, LR-5 or LR-M lesions with simultaneously elevated AFP (≥ 20 ng/mL) as malignancies; criterion III took LR-4 Lesions with elevated AFP or LR-5 or LR-M lesions as malignancies. The sensitivity, specificity, accuracy and area under the receiver operating characteristic curve (AUC) were calculated to determine the diagnostic value of the aforementioned criteria.
There were no statistically significant differences between the specificity, accuracy, or AUC of criterion II and criterion III. Notably, criterion III showed a higher diagnostic sensitivity than criterion II. However, both the specificity and accuracy of criterion I was inferior to those of criterion II and criterion III. For pediatric patients more than 5 years old, the performance of the three criteria was overall similar when patients were subcategorized by age when compared to all patients in aggregate.
We propose a novel method that might be a powerful diagnostic tool to differentiate malignant from benign FLLs in pediatric patients. LR-4 with elevated AFP, LR-5 or LR-M lesions could effectively differentiate benign and malignant tumors in pediatric patients.
CEUS LI-RADS combined with AFP might be a powerful diagnostic tool to differentiate malignant from benign FLLs in pediatric patients.
We thank all medical staff and technicians who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bianchi F, Spain; Boscarelli A, Italy S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Kelgeri C, Renz D, McGuirk S, Schmid I, Sharif K, Baumann U. Liver Tumours in Children: The Hepatologist's View. J Pediatr Gastroenterol Nutr. 2021;72:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and Pediatric Hepatocellular Carcinoma: An Update. Pediatr Dev Pathol. 2020;23:79-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Varol Fİ. Pediatric Hepatocellular Carcinoma. J Gastrointest Cancer. 2020;51:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Chavhan GB, Siddiqui I, Ingley KM, Gupta AA. Rare malignant liver tumors in children. Pediatr Radiol. 2019;49:1404-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Di Giuseppe G, Youlden DR, Aitken JF, Pole JD. Pediatric hepatic cancer incidence and survival: 30-year trends in Ontario, Canada; the United States; and Australia. Cancer. 2021;127:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Angelico R, Grimaldi C, Saffioti MC, Castellano A, Spada M. Hepatocellular carcinoma in children: hepatic resection and liver transplantation. Transl Gastroenterol Hepatol. 2018;3:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Schooler GR, Squires JH, Alazraki A, Chavhan GB, Chernyak V, Davis JT, Khanna G, Krishnamurthy R, Lungren MP, Masand PM, Podberesky DJ, Sirlin CB, Towbin AJ. Pediatric Hepatoblastoma, Hepatocellular Carcinoma, and Other Hepatic Neoplasms: Consensus Imaging Recommendations from American College of Radiology Pediatric Liver Reporting and Data System (LI-RADS) Working Group. Radiology. 2020;296:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Li S, Zhou L, Chen R, Chen Y, Niu Z, Qian L, Fang Y, Xu L, Xu H, Zhang L. Diagnostic efficacy of contrast-enhanced ultrasound versus MRI Liver Imaging Reporting and Data System (LI-RADS) for categorising hepatic observations in patients at risk of hepatocellular carcinoma. Clin Radiol. 2021;76:161.e1-161.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Ma L, Liang WZ, Zhu YP, Zhu YQ, Zhang DZ. Differences in CEUS and CE-MRI appearance of HCC: a case report. AUDT. 2019;3 (4):197-199. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | He MN, Xu L, Jiang TA. Time-intensity curve analysis of hepatocellular carcinoma using two contrast-enhanced ultrasound methods: contrast pulse sequencing and contrast harmonic imaging. AUDT. 2020;4 (3):217-222. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Schreiber-Dietrich DG, Cui XW, Piscaglia F, Gilja OH, Dietrich CF. Contrast enhanced ultrasound in pediatric patients: a real challenge. Z Gastroenterol. 2014;52:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Ferraro S, Panzeri A, Braga F, Panteghini M. Serum α-fetoprotein in pediatric oncology: not a children's tale. Clin Chem Lab Med. 2019;57:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 397] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 14. | Rojas Y, Guillerman RP, Zhang W, Vasudevan SA, Nuchtern JG, Thompson PA. Relapse surveillance in AFP-positive hepatoblastoma: re-evaluating the role of imaging. Pediatr Radiol. 2014;44:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | El-Ali AM, Davis JC, Cickelli JM, Squires JH. Contrast-enhanced ultrasound of liver lesions in children. Pediatr Radiol. 2019;49:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Khanna G, Chavhan GB, Schooler GR, Fraum TJ, Alazraki AL, Squires JH, Salter A, Podberesky DJ, Towbin AJ. Diagnostic Performance of LI-RADS Version 2018 for Evaluation of Pediatric Hepatocellular Carcinoma. Radiology. 2021;299:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, Tang A, Singal AG, Costa AF, Fowler K, McInnes MDF. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. 2019;156:976-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 18. | Ludwig DR, Romberg EK, Fraum TJ, Rohe E, Fowler KJ, Khanna G. Diagnostic performance of Liver Imaging Reporting and Data System (LI-RADS) v2017 in predicting malignant liver lesions in pediatric patients: a preliminary study. Pediatr Radiol. 2019;49:746-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Rozell JM, Catanzano T, Polansky SM, Rakita D, Fox L. Primary liver tumors in pediatric patients: proper imaging technique for diagnosis and staging. Semin Ultrasound CT MR. 2014;35:382-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kong WT, Wang WP, Huang BJ, Ding H, Mao F, Si Q. Contrast-enhanced ultrasound in combination with color Doppler ultrasound can improve the diagnostic performance of focal nodular hyperplasia and hepatocellular adenoma. Ultrasound Med Biol. 2015;41:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Wang W, Chen LD, Lu MD, Liu GJ, Shen SL, Xu ZF, Xie XY, Wang Y, Zhou LY. Contrast-enhanced ultrasound features of histologically proven focal nodular hyperplasia: diagnostic performance compared with contrast-enhanced CT. Eur Radiol. 2013;23:2546-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign liver lesions. Abdom Radiol (NY). 2018;43:848-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 23. | Mneimneh W, Farges O, Bedossa P, Belghiti J, Paradis V. High serum level of alpha-fetoprotein in focal nodular hyperplasia of the liver. Pathol Int. 2011;61:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Koklu E, Gunes T, Akcakus M, Ozturk MA, Kurtoglu S. Alpha-fetoprotein levels in the neonatal period. Eur J Pediatr. 2008;167:961-2; author reply 963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Kupeli S. Evolving strategy in treatment of infantile hemangiomas: from steroids to propranolol. Cukurova Med J. 2016;41 (2):354-359. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |