Published online Jun 7, 2022. doi: 10.3748/wjg.v28.i21.2334
Peer-review started: July 19, 2021
First decision: August 9, 2021
Revised: August 21, 2021
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 7, 2022
Processing time: 317 Days and 16.5 Hours
Single-nucleotide polymorphisms (SNPs) of the serotonin type 3 receptor subunit (HTR3) genes have been associated with psychosomatic symptoms, but it is not clear whether these associations exist in irritable bowel syndrome (IBS).
To assess the association of HTR3 polymorphisms with depressive, anxiety, and somatization symptoms in individuals with IBS.
In this retrospective study, 623 participants with IBS were recruited from five specialty centers in Germany, Sweden, the United States, the United Kingdom, and Ireland. Depressive, anxiety, and somatization symptoms and sociodemographic characteristics were collected. Four functional SNPs — HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A — were genotyped and analyzed using the dominant and recessive models. We also performed separate analyses for sex and IBS subtypes. SNP scores were calculated as the number of minor alleles of the SNPs above. The impact of HTR3C c.489C>A was tested by radioligand-binding and calcium influx assays.
Depressive and anxiety symptoms significantly worsened with increasing numbers of minor HTR3C c.489C>A alleles in the dominant model (Fdepressive = 7.475, Pdepressive = 0.006; Fanxiety = 6.535,
We have provided the first evidence that HTR3C c.489C>A is involved in depressive and anxiety symptoms in individuals with IBS. The SNP score indicated that an increasing number of minor alleles is linked to the worsening of depressive symptoms in IBS.
Core Tip: Bringing together high quality data as well as methodological expertise, our results show that: In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in irritable bowel syndrome (IBS); a higher number of minor alleles, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A, was linked to more severe depressive symptoms in IBS; and the potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors. These results will contribute towards standardization and harmonization of genetic research strategies in IBS.
- Citation: Berens S, Dong Y, Fritz N, Walstab J, D'Amato M, Zheng T, Wahl V, Boekstegers F, Bermejo JL, Martinez C, Schmitteckert S, Clevers E, Engel F, Gauss A, Herzog W, Spiller R, Goebel-Stengel M, Mönnikes H, Andresen V, Thomas F, Keller J, Pehl C, Stein-Thöringer C, Clarke G, Dinan TG, Quigley EM, Sayuk G, Simrén M, Tesarz J, Rappold G, van Oudenhove L, Schaefert R, Niesler B. Serotonin type 3 receptor subunit gene polymorphisms associated with psychosomatic symptoms in irritable bowel syndrome: A multicenter retrospective study. World J Gastroenterol 2022; 28(21): 2334-2349
- URL: https://www.wjgnet.com/1007-9327/full/v28/i21/2334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i21.2334
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits[1-4]. The pathophysiology of IBS has not entirely been resolved, but is understood to be biopsychosocial and affected by an impaired function of the central and enteric nervous systems and their crosstalk via the brain-gut axis[5,6]. IBS patients often present with increased comorbid depressive and anxiety symptoms[7-11], highlighting the complex relationship between visceral sensitivity and subjective psychological perceptions[12,13]. Nevertheless, about 50% of IBS patients report GI symptoms but show no comorbid affective symptoms[14].
There is evidence that disturbances of the serotonergic system are important in GI disorders such as IBS and in mental disorders, both of which interact via the brain-gut axis[15,16]. The serotonin type 3 receptors (5-HT3R) modulate key functions in the GI tract[17,18]. In line with such functions, 5-HT3R antagonists are beneficial in the treatment of diarrhea-predominant IBS (IBS-D)[19-22]. 5-HT3Rs are also involved in emotional processing, mood regulation, and visceral perception and have been associated with depressive and anxiety symptoms that represent comorbid phenotypes in IBS[23]. Single-nucleotide polymorphisms (SNPs) in the 5-HT3R subunit genes (HTR3), namely HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847), are associated with IBS according to studies investigating the effects of sex or IBS subtypes[12,24-30]. However, whether HTR3 polymorphisms are associated with IBS and comorbid depressive and anxiety symptoms has not been determined because existing studies have missing phenotypic data on comorbidities and small sample sizes. These studies had case-control designs and investigated associations between these polymorphisms in individuals with IBS phenotypes or mental behavioral conditions and controls rather than combining genetic data with specific psychosocial characteristics of IBS patients.
This multicenter observational study focused on a large IBS patient cohort comprising 768 participants from centers in Germany, Sweden, the United States, the United Kingdom, and Ireland with the aim of meeting three objectives: (1) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden (i.e., depressive, anxiety, and somatization symptoms) within an IBS population; (2) To investigate the impact of the HTR3 SNP score (computed as the number of minor alleles) on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3) To perform a functional analysis of variant 5-HT3AC receptors.
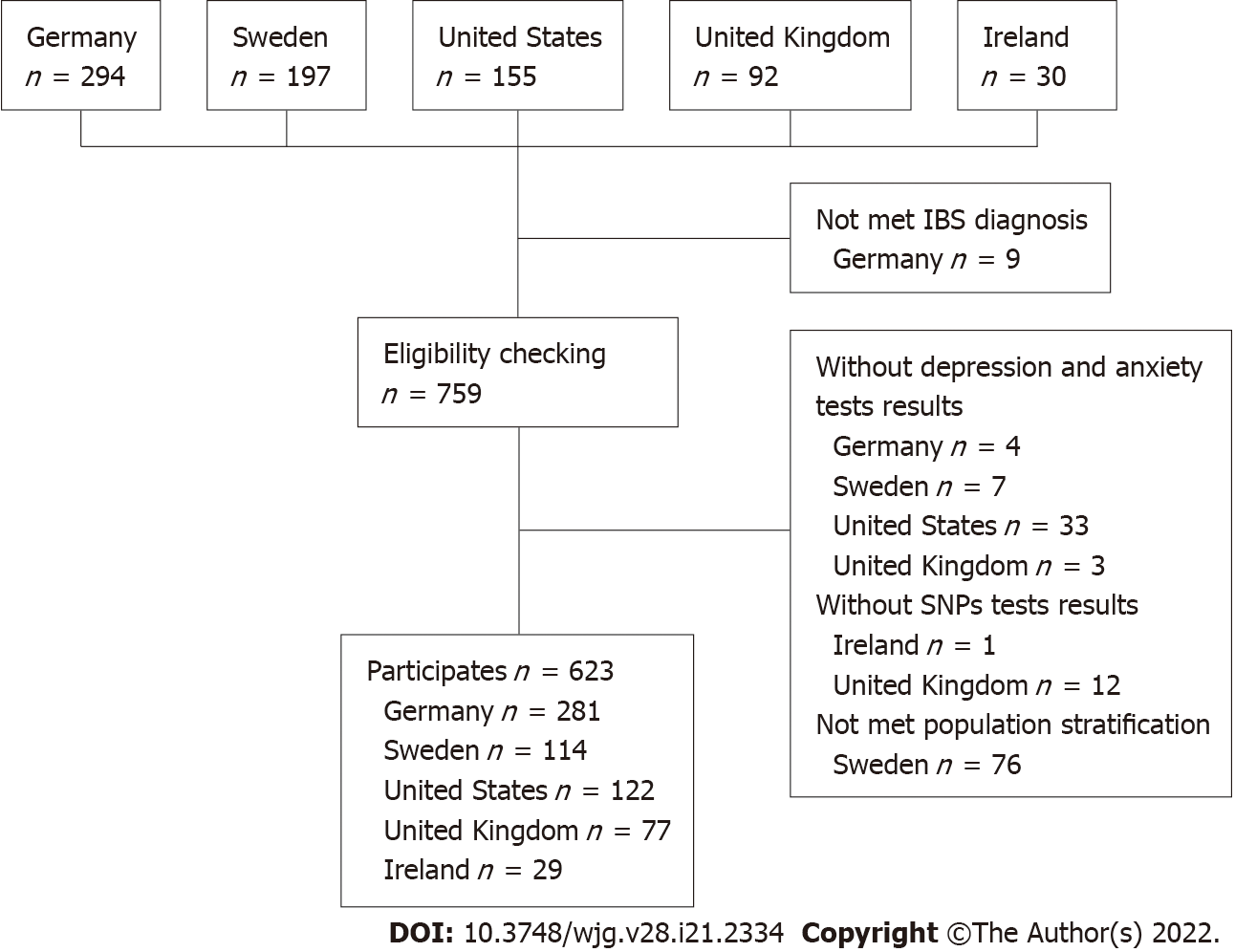
The study population was pooled from five different tertiary care expert centers. German participants were recruited from the Specialty Clinic for Functional GI Disorders at the Department of General Internal Medicine and Psychosomatics of Heidelberg University Hospital[31] and from our clinical partners in the IBS-Net in Hamburg, Krefeld, Berlin, Vilsbiburg, and Munich (www.ibs.uni-hd.de). Swedish participants were recruited at the specialized unit for patients with functional GI disorders at Sahlgrenska University Hospital in Gothenburg. United States participants were recruited at Washington University, Barnes-Jewish Hospital in St. Louis, Missouri. United Kingdom participants were recruited at the Nottingham Digestive Diseases Center and participants from Ireland from a specialty clinic at Cork University Hospital. Participant recruitment is shown in Figure 1.
Written informed consent was obtained from all participants and the experiments were in accordance with the principles of the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. All studies were approved by the following local Ethics Committees: Heidelberg, Germany: Ethical Committee, Medical Faculty of the Heidelberg University Hospital (S067/2010); Cork, Ireland: Clinical Research Ethics Committee (APC024); Gothenburg, Sweden: Regional Ethical Review Board in Gothenburg (S489-02 and 731-09); Nottingham, United Kingdom: registered at clinical trial clinicaltrials.gov (identifier NCT00745004) and approved by Nottingham Research Ethics Committee 2 (REC reference number 08/H0408/134)[21]; and St-Louis, United States: Washington University St. Louis, Human Research Protection Office (IRB ID #: 201103220).
Only patients diagnosed with IBS according to the ROME III criteria were included in the analysis. All participants were of Caucasian ancestry and had comparable population stratification. Patients under 18 years of age or without SNP test results were excluded.
Genomic DNA was isolated from IBS patient blood samples using ethylenediaminetetraacetic acid according to standard protocols[32]. Four polymorphic HTR3 loci, namely HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847) were selected as target SNPs for this study. The corresponding primers were designed and synthesized using AssayDesigner 3.1 software. Genotyping was performed at the Department of Human Molecular Genetics at Heidelberg University Hospital using the KASPar® SNP Genotyping System (KBiosciences, Ltd, Hoddesdon, United Kingdom). To analyze HTR3 SNPs, the fluorescence plate reader of the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, California, United States) was used as recommended. About 10% of the samples were repeat tested to ensure genotyping accuracy.
In addition to sociodemographic characteristics and IBS diagnosis, we also collected data on depressive, anxiety, and somatization symptoms[33] and genetic markers of the serotonergic system.
IBS diagnosis: The diagnostic classification of IBS was based on the Scoring Algorithm for Rome III Diagnostic Questionnaire for Adult Functional GI Disorders (SA for Rome III-DQ)[34,35] in all five centers. Percentages of the different IBS subtypes, i.e., constipation-predominant IBS, IBS-D, IBS with mixed bowel habits, and unclassified IBS, were also calculated.
Depressive symptoms: The nine-item depression module from the Patient Health Questionnaire (PHQ-9)[36,37] was used to measure depressive symptoms in the German cohort. The Hospital Anxiety and Depression Scale depression subscale[38] was used to identify depressive symptoms in participants from Sweden, the United Kingdom, and Ireland and the Beck Depression Inventory[39] was used to measure the severity of depressive symptoms in United States participants.
Anxiety symptoms: In the German cohort, symptoms of generalized anxiety were assessed using the brief measurement for generalized anxiety disorder (GAD-7)[40]. In the cohorts from Sweden, the United Kingdom, and Ireland, the HADS anxiety subscale was used to identify anxiety symptoms. The Beck Anxiety Inventory[41] was used to assess anxiety in the United States cohort.
Somatization symptoms: In the cohorts from Germany, Sweden, and the United States, the 15-item somatization module from the PHQ-15[42] was used to identify somatization symptoms.
Genetic markers of the serotonergic system: The four functional SNPs HTR3A c.-42C>T (rs1062613), HTR3B c.386A>C (rs1176744), HTR3C c.489C>A (rs6766410), and HTR3E c.*76G>A (rs56109847) were selected for validation based on previous reports as outlined above[25].
These procedures are described in the Supplementary material.
All statistical procedures were carried out using IBM SPSS Statistics 22.0 for Windows. Variables with a skewed distribution were log-transformed prior to further analysis. If different measurements had been collected in the five centers, z values were calculated to enable pooling. The Hardy-Weinberg equilibrium (HWE) of genotype frequency distribution was tested using SHEsis[43]. Genome-wide SNP data were generated by the Bellygenes team of D’Amato M using the Illumina Global Screening array and platform[44,45]. We used the multidimensional scaling approach to correct for population stratification in PLINK[46]. Following the guidance provided at https://github.com/MareesAT/GWA_tutorial/, our data were anchored by data of the 1000 Genomes project (http://www.1000genomes.org/). The 10 main components were used as covariates in the association tests to correct for population stratification[46] and exclude outliers. Polymorphisms were analyzed separately using the dominant and the recessive models. Also, stratified analyses based on sex and IBS subtypes were carried out. ANOVA was used to analyze group differences and to check for linear trends in depressive, anxiety, and somatization symptoms. For the independent variable, a SNP score was computed based on the number of minor alleles (i.e., continuous from 0 to 8 for the four SNPs). Based on the first human HTR3 locus-specific variant database (www.htr3.uni-hd.de)[25], scoring criteria were as follows: major allele homozygous variant gene = 0; heterozygous variant gene = 1; and minor allele homozygous variant gene = 2 (for details see Table 1). Statistical comparisons were made between the two groups using the χ2 test or Fisher’s exact test for frequencies and t tests or Mann-Whitney U tests for metric variables. Normal distribution and variance homogeneity were checked as conditions. Statistical tests were two-sided based on an alpha error of 0.05%. All analyses were explorative and not confirmatory. False discovery rates (FDRs) were calculated based on overall P values using the Benjamini-Hochberg method[47]. Significant values that were no longer significant after FDR multiple testing correction were named “nominally significant”.
| Major allele homozygous genotype | Heterozygous variant genotype | Minor allele homozygous genotype | |
| HTR3A c.-42C>T (rs1062613) | CC | CT | TT |
| HTR3B c.386A>C (rs1176744) | AA | AC | CC |
| HTR3C c.489C>A (rs6766410) | CC | CA | AA |
| HTR3E c.*76G>A (rs56109847) | GG | GA | AA |
In total, 623 participants from five independent expert centers were included in this study (45.1% from Germany, 18.3% from Sweden, 19.6% from the United States, 12.4% from the United Kingdom, and 4.7% from Ireland). We excluded 76 Swedish participants who did not meet the population stratification criteria (Supplementary Figure 1). Participants from the United Kingdom and Ireland were excluded from the main analysis because the sample size was small. Table 2 presents the sociodemographic characteristics, IBS subtypes, and psychosomatic symptoms of the included participants. Participants had a mean ± SD age of 41.7 ± 16.1 years and 69.5% were female. Overall, IBS patients showed minimal to mild levels of depressive and anxiety symptoms, moderate levels of IBS symptoms, and moderate levels of somatization symptoms.
| Total | Centers | |||||
| Germany | Sweden | United States | United Kingdom | Ireland | ||
| n | 100.0 (623) | 45.1 (281) | 18.3 (114) | 19.6 (122) | 12.4 (77) | 4.7 (29) |
| Age | 41.7 ± 16.1 (18, 91) | 40.7 ± 16.4 (18, 88) | 33.7 ± 11.8 (18, 60) | 54.9 ± 14.6 (25, 91) | 40.8 ± 11.6 (18, 70) | 30.3 ± 8.6 (19, 51) |
| Sex | ||||||
| Female | 69.5 (433) | 63.3 (178) | 69.3 (79) | 77.9 (95) | 72.7 (56) | 86.2 (25) |
| IBS subtypes | ||||||
| IBS-C | 14.1 (87) | 8.9 (25) | 12.3 (14) | 36.7 (44) | 0 | 13.8 (4) |
| IBS-D | 41.7 (258) | 43.4 (122) | 19.6 (22) | 28.3 (34) | 100.0 (77) | 10.3 (3) |
| IBS-M | 42.5 (263) | 45.6 (128) | 67.0 (75) | 31.7 (38) | 0 | 75.9 (22) |
| IBS-U1 | 1.8 (11) | 2.1 (6) | 0.9 (1) | 3.3 (4) | 0 | 0 |
| Depressive symptoms | - | 9.4 ± 5.92 | 5.2 ± 3.54 | 13.2 ± 9.65 | 5.9 ± 4.04 | 4.3 ± 3.24 |
| Anxiety symptoms | - | 7.7 ± 4.83 | 8.3 ± 4.54 | 14.6 ± 9.76 | 9.6 ± 4.64 | 7.8 ± 4.54 |
| Symptom severity | - | 280.3 ± 90.77 | 297.3 ± 100.57 | 43.2 ± 16.28 | - | - |
| Somatization symptoms9 | 14.8±6.3 | 16.2 ± 7.3 | 13.3 ± 4.7 | 13.8 ± 5.0 | - | - |
Genotype and allele frequencies of the functional HTR3A c.-42C>T, HTR3B c.386A>, HTR3C c.489C>A, and HTR3E c.*76G>A polymorphisms were calculated. No significant differences in genotype frequency were observed between sexes or IBS subtypes. For HTR3A c.-42C>T, the frequency of the minor T allele was 21.5%; for HTR3B c.386A>C, the frequency of the minor C allele was 29.6%; for HTR3C c.489C>A, the frequency of the minor A allele was 41.6%; and for HTR3E c.*76G>A, the frequency of minor A allele was 6.1%. The genotypic distribution of the four polymorphic loci of rs1062613, rs1176744, rs6766410, and rs56109847 were in accordance with HWE (all P > 0.05). The results are shown in SupplementaryTables 1-3.
HTR3 SNPs were separately analyzed using the dominant model and the recessive model stratified for sex and IBS subtypes. Depressive and anxiety symptoms worsened significantly with increasing numbers of minor alleles of HTR3C c.489C>A in the dominant model (Fdepressive = 7.475, Pdepressive = 0.006;
| Models | Mental symptoms | SNPs | Total | Sex | IBS subtypes | |||
| Male | Female | IBS-C | IBS-D | IBS-M | ||||
| Dominant model | Depressive symptoms | HTR3A c.-42C>T (rs1062613) | 2.541 | 4.149 ↑ | 0286 | 0.998 | 0.376 | 0.694 |
| HTR3B c.386A>C (rs1176744) | 0.067 | 0.005 | 0.198 | 0.282 | 0.124 | 0.232 | ||
| HTR3C c.489C>A (rs6766410) | 7.475 ↑ | 0.672 | 7.040 ↑ | 0.261 | 5.670 ↑ | 2.328 | ||
| HTR3E c.*76G>A (rs62625044) | 0.054 | 0.013 | 0.180 | 0.208 | 0.461 | 0.010 | ||
| Anxiety symptoms | HTR3A c.-42C>T (rs1062613) | 0.511 | 0.210 | 0.280 | 0.604 | 0.672 | 0.162 | |
| HTR3B c.386A>C (rs1176744) | 0.926 | 0.406 | 0.801 | 0.468 | 1.369 | 0.770 | ||
| HTR3C c.489C>A (rs6766410) | 6.535 ↑ | 0.158 | 7.550 ↑ | 0.186 | 13.444 ↑ | 0.045 | ||
| HTR3E c.*76G>A (rs62625044) | 1.055 | 0.429 | 0.853 | 0.000 | 0.495 | 1.109 | ||
| Somatization symptoms | HTR3A c.-42C>T (rs1062613) | 0.016 | 0.141 | 0.106 | 0.086 | 0.017 | 0.203 | |
| HTR3B c.386A>C (rs1176744) | 0.217 | 0.332 | 0.326 | 0.407 | 0.415 | 2.125 | ||
| HTR3C c.489C>A (rs6766410) | 0.784 | 0.035 | 1.085 | 0.269 | 0.000 | 1.962 | ||
| HTR3E c.*76G>A (rs62625044) | 0.002 | 1.554 | 0.263 | 0.128 | 0.729 | 0.354 | ||
| Recessive model | Depressive symptoms | HTR3A c.-42C>T (rs1062613) | 0.002 | 0.421 | 0.132 | 0.093 | 1.320 | 1.064 |
| HTR3B c.386A>C (rs1176744) | 1.118 | 2.027 | 0.107 | 1.741 | 0.197 | 0.744 | ||
| HTR3C c.489C>A (rs6766410) | 0.047 | 0.821 | 0.401 | 2.306 | 6.190 ↑ | 0.676 | ||
| HTR3E c.*76G>A (rs62625044) | 0.677 | - | 0.574 | 0.541 | - | - | ||
| Anxiety symptoms | HTR3A c.-42C>T (rs1062613) | 0.479 | 0.027 | 0.549 | 0.057 | 0.315 | 0.350 | |
| HTR3B c.386A>C (rs1176744) | 0.872 | 0.081 | 1.691 | 0.476 | 0.361 | 1.923 | ||
| HTR3C c.489C>A (rs6766410) | 0.028 | 2.562 | 0.344 | 0.570 | 3.509 | 2.093 | ||
| HTR3E c.*76G>A (rs62625044) | 0.216 | - | 0.179 | 0.242 | - | - | ||
| Somatization symptoms | HTR3A c.-42C>T (rs1062613) | 0.217 | 0.018 | 0.257 | 0.138 | 0.092 | 0.431 | |
| HTR3B c.386A>C (rs1176744) | 6.482 ↑ | 2.245 | 3.575 | 0.142 | 14.033 ↑ | 0.341 | ||
| HTR3C c.489C>A (rs6766410) | 0.468 | 1.813 | 0.128 | 4.715 ↓ | 0.002 | 0.113 | ||
| HTR3E c.*76G>A (rs62625044) | 0.001 | - | 0.011 | 0.000 | - | - | ||
SNP scores ranged from 0 to 6; 37.1% had one or zero minor alleles of the analyzed HTR3 SNPs and 30.8% had three or more minor alleles of the analyzed HTR3 SNPs. No significant differences in SNP scores were observed between sexes (F = 3.550, P = 0.060) or IBS subtypes (F = 1.485, P = 0.227). ANOVAs were conducted and linear trends were checked to analyze the effect of SNP scores on depressive, anxiety, and somatization symptoms. Overall, an increasing number of minor alleles was linked to worsening depressive symptoms (F = 7.710, P-linear trend = 0.006). However, material trends did not reveal a link between more minor alleles and worsening anxiety or somatization symptoms. By stratifying analyses for sex, an increasing number of minor alleles was linked to worsened depressive symptoms in female participants (F = 5.770, P-linear trend = 0.017). There was no significant association between SNP score and depressive, anxiety, or somatization symptoms when looking at IBS subtypes separately (Table 4). As mentioned above, the analyses of participants from the United Kingdom and Ireland are presented separately in the Supplementary Table 5.
| Total | Sex | IBS subtypes | ||||
| Male | Female | IBS-C | IBS-D | IBS-M | ||
| Depressive symptoms | ||||||
| F value | 7.710 | 1.336 | 5.770 | 1.551 | 2.159 | 0.196 |
| P-linear trend value | 0.006 | 0.250 | 0.017 | 0.184 | 0.144 | 0.659 |
| Anxiety symptoms | ||||||
| F value | 2.150 | 0.028 | 2.514 | 1.372 | 2.400 | 1.412 |
| P-linear trend value | 0.143 | 0.866 | 0.114 | 0.245 | 0.123 | 0.236 |
| Somatization symptoms | ||||||
| F value | 1.15 | 0.354 | 1.028 | 0.716 | 2.718 | 0.628 |
| P-linear trend value | 0.283 | 0.553 | 0.311 | 0.614 | 0.102 | 0.429 |
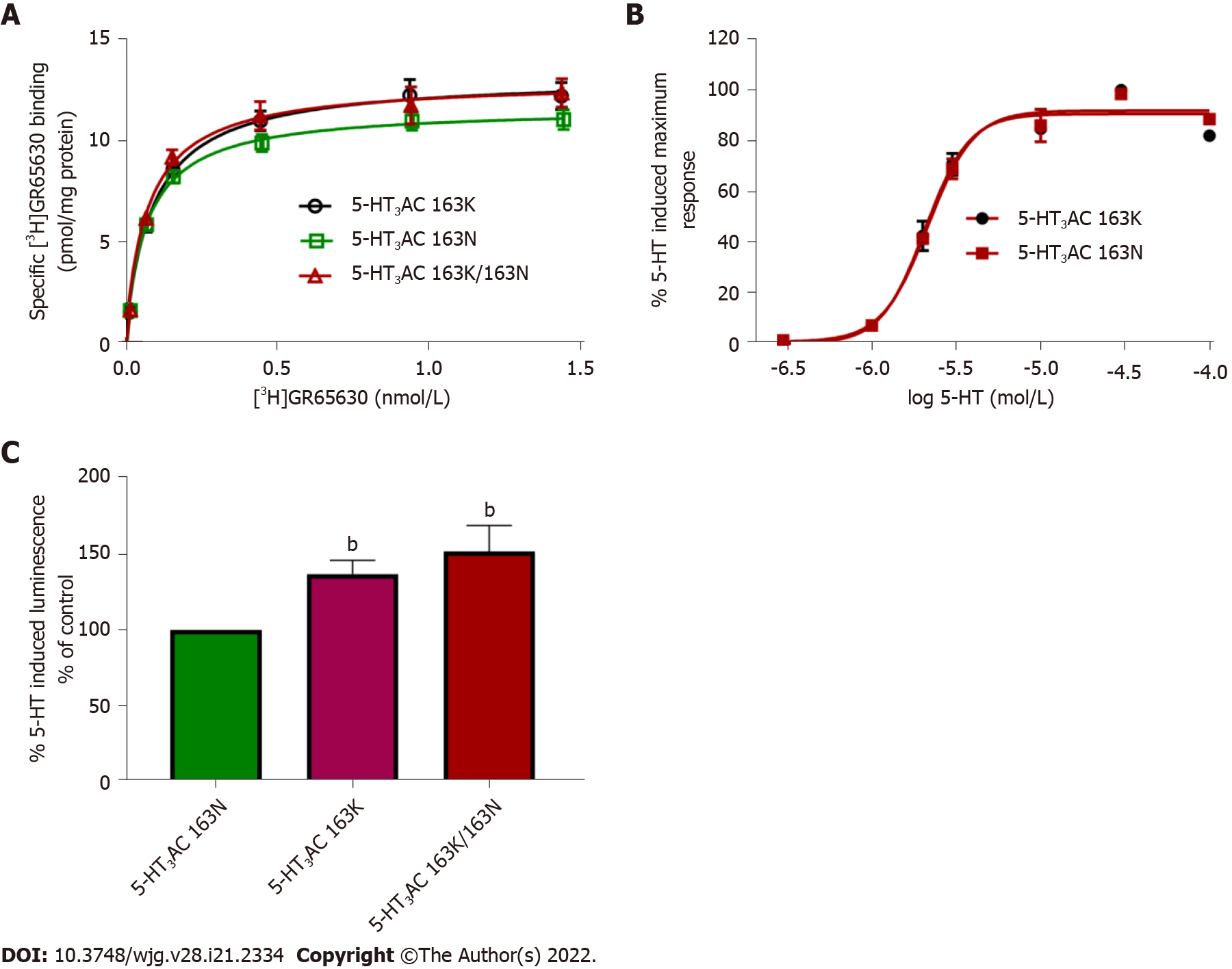
The HTR3C SNP encodes the amino acid exchange p.Asn163Lys (p.N163K), and recombinantly expressed 5-HT3AC receptors harboring variant 5-HT3C subunits that mimic the homozygous minor allele and the heterozygous state presented with increased cell surface expression and enhanced 5-HT maximum response. Radioligand-binding assays revealed higher Bmax values of 117.1% ± 4.38% and 111.9% ± 1.79%. Calcium influx assays showed increased 5-HT-induced maximum effects of 137.2% ± 9.0% and 151.9% ± 17.3% for the minor allele 5-HT3AC 163K or combined 5-HT3AC 163N/5-HT3AC 163K receptors compared with the major allele representing 5-HT3AC 163N receptors, respectively. The affinity of the specific 5-HT3 receptor antagonist [3H]GR65630, as reflected by the Kd values, and the potency of 5-HT, as reflected by the EC50 values, did not differ between the receptor variants (Figure 2).
The 5-HT3 receptors modulate essential functions in the GI tract such as GI motility as well as mood and emotions[19], and HTR3 SNPs have been associated with depression, anxiety, and IBS[25]. In this study, we showed that: (1) In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in IBS; (2) A higher number of minor alleles (i.e., a higher SNP score, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) was linked to more severe depressive symptoms in IBS; and (3) The potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors. These findings are discussed in more detail below.
Participants with IBS were more frequently female than male, in line with previous findings[48,49] that IBS is more prevalent in young and middle-aged females. Most participants with IBS were only mildly affected by depression and anxiety symptoms. Of note, German participants only visited the Specialty Clinic for Functional GI Disorders at Heidelberg University Hospital after a long history of dealing with IBS[31]; therefore, these participants reported more severe depressive symptoms. However, causal relationships between IBS symptoms, depression, and anxiety are still controversial.
The investigated SNPs rs1062613, rs1176744, rs6766410, and rs56109847 were in accordance with HWE. There was no population stratification, and the sample was representative and excluded genotyping errors. Although participants were recruited from various centers in different countries, there were no obvious selective differences.
HTR3A c.-42C>T (rs1062613): Depressive symptoms were “nominally significantly” more severe with increasing numbers of minor HTR3A c.-42C>T alleles in male participants according to the dominant model. This SNP has been associated with bipolar affective disorder[50], IBS symptom severity, amygdaloid activity[29,51], early life trauma[52], altered emotional networks in the human brain, and the onset of depression[53]. However, these studies only included single sex participants and did not include subgroup analyses.
HTR3B c.386A>C (rs1176744): Somatization symptoms worsened significantly with increasing numbers of minor HTR3B c.386A>C alleles in the dominant model. The HTR3B variant p.Tyr129Ser (rs1176744) has been associated with bipolar affective disorder in males and with major depression in females as well as with pain catastrophizing, a coping style characterized by excessively negative thoughts and emotions related to pain[30,54-56]. This discrepancy may be due to an enhancement or weakening of this association by polymorphic interactions in the serotonin pathways[56].
HTR3C c.489C>A (rs6766410): Depressive and anxiety symptoms worsened significantly with increasing numbers of minor HTR3C c.489C>A alleles in the dominant model. This effect seemed to be driven by female sex and IBS-D. HTR3C c.489C>A was previously associated with IBS-D in female patients[57], but the proportion of male patients was small in this study, which may limit the applicability of these findings. In the recessive model, depressive and anxiety symptoms “nominally significantly” worsened with increasing numbers of minor alleles of HTR3C c.489C>A in Irish participants. However, these results should be interpreted with caution because the Irish sample size was low. As far as we are aware, HTR3C c.489C>A has not been analyzed in individuals with affective disorders before.
HTR3E c.*76G>A (rs56109847): HTR3E is restrictedly and robustly expressed in the GI tract[58,59], suggesting that it plays a special role in 5-HT3 receptor function in the gut. In this study, we did not find a relationship between functional polymorphisms of HTR3E and depressive and anxiety symptoms in IBS patients. This may be attributed to a floor effect because depressive and anxiety symptoms were minimal to mild in our sample[60].
A single gene variant is not sufficient to explain all symptoms shaping the clinical phenotype of a complex disorder like IBS[61]. By computing SNP scores based on the number of minor alleles of rs1062613, rs1176744, rs6766410, and rs56109847, our study revealed that an increasing number of minor alleles is linked to increasing severity of depressive symptoms. However, there was no obvious association between an increasing number of minor alleles and the severity of anxiety or somatization symptoms. Stratification for sex revealed a correlation between increasing numbers of minor alleles and worsening depressive symptoms in female participants.
HTR3 genes encode different 5-HT3 subunits to make up heteromeric receptors. The 5-HT3A subunits play a major role in these receptors because they can form functional receptors on their own. The other subunits can only form functional receptors with 5-HT3A and seem to modulate the function and properties of the receptors[62]. How these native receptors might contribute to the pathogenesis of IBS, particularly regarding co-expression patterns of HTR3, has not been established yet. The HTR3A and HTR3E variants reside within untranslated regions and the respective SNPs correlate with increased expression levels, whereas the HTR3B variant changes the channel properties[25]. To gain insight into the pathophysiological relevance of the associated HTR3C variant c.489C>A (rs6766410), we characterized the pharmacological and functional properties of those 5-HT3AC receptors that altered the 5-HT-mediated maximum response and expression of variant 5-HT3AC receptors. However, how structural modifications in these receptors affect their function in vivo and how they modulate the serotonergic system to influence mood, emotional processing, and the manifestation of IBS and comorbid conditions remains to be determined.
Our study has some limitations. First, different instruments were used by different centers to assess phenotypic features. To correct for this, scale scores were converted into z standard scores. However, given that the participants reported no severe psychosomatic symptoms, the discovery chances might be limited. Second, there is no sufficient evidence to show the relationship between risk alleles and respective major/minor alleles as patients and healthy controls were not compared in this study. Similarly, the relative strength of the cumulative effect represented by the SNP score was also affected to a certain extent. Third, our participants were all Caucasian, so the results may not apply to other ethnic groups.
Despite these limitations, our study has some strengths. First, this was a multicenter study so had a large sample size. Large, well-characterized samples like ours are necessary to identify molecular causes of IBS and comorbid conditions[12]. Second, this study investigated the association between polymorphisms in HTR3 genes and comorbid psychosomatic symptoms for the first time. We conducted population stratification tests to ensure that the included populations were comparable. We also performed stratified analyses of sex and IBS subtypes and a more stringent multiple testing correction by FDR. Third, SNP scores have higher power and are better suited to testing multiple instead of single variants. This is useful because the pathogenesis of IBS is complex with multiple factors contributing to the manifestation of various subtypes. Also, individual genes may only play a minor role[12].
IBS is a complex condition. The continuous improvement of the allelic variation database for HTR3[25] and deep phenotyping combined with gene information (also in other datasets) may help to identify disease subgroups accurately and consistently, thereby facilitating future treatment[33,63]. This will be an important step towards standardization and unification of IBS genetic research strategies.
Our results provide the first evidence that the accumulation of HTR3 SNPs (reflected by the SNP score computed by HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS. This study has revealed that depressive and anxiety symptoms significantly worsened from the major to the minor allele of HTR3C c.489C>A in the dominant model and an increasing number of minor alleles are linked to more severe depressive symptoms in IBS.
Over the past decades, genetic evidence on the key players within the serotonergic system including the serotonin type 3 (5-HT3) receptor subunit genes (HTR3) accumulated showing association with irritable bowel syndrome (IBS) as well as mental illnesses. However, it has never been explored whether associations of the single-nucleotide polymorphisms (SNPs) of HTR3 genes to depressive and anxiety symptoms can be replicated within IBS.
In order to address this knowledge gap, This multicenter observational study focused on a large IBS patient cohort comprising 768 participants from centers in Germany, Sweden, the United States, the United Kingdom, and Ireland.
The objectives are: (1) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden within an IBS population; (2) To investigate the impact of the HTR3 SNP score on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3) To perform a functional analysis of variant 5-HT3AC receptors.
In this retrospective study, 623 participants with IBS were recruited from five specialty centers in Germany, Sweden, the United States, the United Kingdom, and Ireland. Depressive, anxiety, and somatization symptoms and sociodemographic characteristics were collected. Four functional SNPs — HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A — were genotyped and analyzed using the dominant and recessive models. We also performed separate analyses for sex and IBS subtypes. SNP scores were calculated as the number of minor alleles of the SNPs above. The impact of HTR3C c.489C>A was tested by radioligand-binding and calcium influx assays.
Bringing together high quality data as well as methodological expertise, our results show that: (1) In the dominant model, HTR3C c.489C>A was correlated with depressive and anxiety symptoms in IBS; (2) A higher number of minor alleles (i.e., the higher the SNP score, which was computed by combining the individual SNP status of HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) was linked to more severe depressive symptoms in IBS; and (3) The potential relevance of the HTR3C SNP was corroborated in functional assays showing changes in the expression level of 5-HT3AC variant receptors.
Our results provide the first evidence that the accumulation of HTR3 SNPs (reflected by the SNP score computed by HTR3A c.-42C>T, HTR3B c.386A>C, HTR3C c.489C>A, and HTR3E c.*76G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS.
We are confident that these results are of interest to your readership, as they contribute substantially to update current knowledge regarding the role of accumulation of HTR3 SNPs in depressive and anxiety symptoms in IBS patients. In turn, our data will contribute towards standardization and harmonization of genetic research strategies in IBS.
We would like to thank all patients for their participation in this study and the supporting staff at each site. We acknowledge the kind support of Bartram CR and Hinderhofer K. We would also thank Startt B for proofreading and Bacon C for editing the manuscript. This manuscript results in part from collaboration and network activities promoted under the frame of the international network GENIEUR (Genes in Irritable Bowel Syndrome Research Network Europe), which has been funded by the COST program (BM1106, www.GENIEUR.eu) and is currently supported by the European Society of Neurogastroenterology and Motility (ESNM, www.ESNM.eu).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yin ZT, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Layer P, Andresen V, Pehl C, Allescher H, Bischoff SC, Classen M, Enck P, Frieling T, Haag S, Holtmann G, Karaus M, Kathemann S, Keller J, Kuhlbusch-Zicklam R, Kruis W, Langhorst J, Matthes H, Mönnikes H, Müller-Lissner S, Musial F, Otto B, Rosenberger C, Schemann M, van der Voort I, Dathe K, Preiss JC; Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten; Deutschen Gesellschaft für Neurogastroenterologie und Motilität. [Irritable bowel syndrome: German consensus guidelines on definition, pathophysiology and management]. Z Gastroenterol. 2011;49:237-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Dong Y, Baumeister D, Berens S, Eich W, Tesarz J. Stressful Life Events Moderate the Relationship Between Changes in Symptom Severity and Health-related Quality of Life in Patients With Irritable Bowel Syndrome. J Clin Gastroenterol. 2020;54:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262-1273.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 4. | Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin Gastroenterol Hepatol. 2020;18:392-398.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 6. | Stasi C, Caserta A, Nisita C, Cortopassi S, Fani B, Salvadori S, Pancetti A, Bertani L, Gambaccini D, de Bortoli N, Dell'Osso L, Blandizzi C, Marchi S, Bellini M. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: A longitudinal assessment. J Gastroenterol Hepatol. 2019;34:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Melchior C, Desprez C, Riachi G, Leroi AM, Déchelotte P, Achamrah N, Ducrotté P, Tavolacci MP, Gourcerol G. Anxiety and Depression Profile Is Associated With Eating Disorders in Patients With Irritable Bowel Syndrome. Front Psychiatry. 2019;10:928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Weinberg DS, Smalley W, Heidelbaugh JJ, Sultan S; Amercian Gastroenterological Association. American Gastroenterological Association Institute Guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1146-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG. Feeling down? J Affect Disord. 2020;266:429-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Gros DF, Antony MM, McCabe RE, Swinson RP. Frequency and severity of the symptoms of irritable bowel syndrome across the anxiety disorders and depression. J Anxiety Disord. 2009;23:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Black CJ, Yiannakou Y, Houghton LA, Shuweihdi F, West R, Guthrie E, Ford AC. Anxiety-related factors associated with symptom severity in irritable bowel syndrome. Neurogastroenterol Motil. 2020;32:e13872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Gazouli M, Wouters MM, Kapur-Pojskić L, Bengtson MB, Friedman E, Nikčević G, Demetriou CA, Mulak A, Santos J, Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat Rev Gastroenterol Hepatol. 2016;13:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Zhang WX, Zhang Y, Qin G, Li KM, Wei W, Li SY, Yao SK. Altered profiles of fecal metabolites correlate with visceral hypersensitivity and may contribute to symptom severity of diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:6416-6429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (1)] |
| 16. | Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Painsipp E, Shahbazian A, Holzer P. Alosetron, cilansetron and tegaserod modify mesenteric but not colonic blood flow in rats. Br J Pharmacol. 2009;158:1210-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Trump D. Commentary on: "Ipilimumab vs placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial." Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR, CA184-043 Investigators. Departments of Urology and Immunology and Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN, USA, Electronic address: kwon.eugene@mayo.edu; Johns Hopkins Sidney Kimmel Comprehensive Cancer Center and Brady Urological Institute, Baltimore, MD, USA; Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA; Institut Gustave Roussy, University of Paris-Sud, Villejuif, France; Institut Gustave Roussy, Villejuif, France; VU University Medical Centre, Amsterdam, Netherlands; Vienna General Hospital, Medical University Vienna, Vienna, Austria; Institut Bergonié, Bordeaux, France; CHU Caremeau, Nimes, France; Centro Médico Austral, Buenos Aires, Argentina; Centre Jean Perrin, Clermont-Ferrand, France; St John of God Hospital, Subiaco, WA, Australia; University Hospital of Siena, Istituto Toscano Tumori, Siena, Italy; Hospital de Caridade de Ijuí, Ijuí, Brazil; Nottingham University Hospital, Nottingham, UK; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Netherlands Cancer Institute and Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands; Institute of Oncology Ion Chiricuta and University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania; Hospital Británico de Buenos Aires, Buenos Aires, Argentina; Herlev Hospital, Herlev, Denmark; Odense University Hospital, Odense, Denmark; University of Texas MD Anderson Cancer Center, Houston,. Urol Oncol. 2016;34:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2014;12:953-9.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Spiller RC. Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol. 2011;11:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Grzesiak M, Beszłej JA, Waszczuk E, Szechiński M, Szewczuk-Bogusławska M, Frydecka D, Dobosz T, Jonkisz A, Lebioda A, Małodobra M, Mulak A. Serotonin-Related Gene Variants in Patients with Irritable Bowel Syndrome and Depressive or Anxiety Disorders. Gastroenterol Res Pract. 2017;2017:4290430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Niesler B, Walstab J, Combrink S, Möller D, Kapeller J, Rietdorf J, Bönisch H, Göthert M, Rappold G, Brüss M. Characterization of the novel human serotonin receptor subunits 5-HT3C,5-HT3D, and 5-HT3E. Mol Pharmacol. 2007;72:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Celli J, Rappold G, Niesler B. The Human Serotonin Type 3 Receptor Gene (HTR3A-E) Allelic Variant Database. Hum Mutat. 2017;38:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Guan T, Li T, Cai W, Huang D, Ouyang P, Wang Y, Chen H, Wu K, Ma X. HTR3A and HTR3E gene polymorphisms and diarrhea predominant irritable bowel syndrome risk: evidence from a meta-analysis. Oncotarget. 2017;8:100459-100468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Melke J, Westberg L, Nilsson S, Landen M, Soderstrom H, Baghaei F, Rosmond R, Holm G, Björntorp P, Nilsson LG, Adolfsson R, Eriksson E. A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry. 2003;60:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Niesler B, Weiss B, Fischer C, Nöthen MM, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Franzek E, Rappold GA. Serotonin receptor gene HTR3A variants in schizophrenic and bipolar affective patients. Pharmacogenetics. 2001;11:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, Bueller JA, Suyenobu B, Jarcho JM, McRoberts JA, Niesler B, Mayer EA. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Takao H, Minabe Y, Nakatani N, Higuchi T, Detera-Wadleigh SD, Yoshikawa T. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol Psychiatry. 2006;60:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Berens S, Kraus F, Gauss A, Tesarz J, Herzog W, Niesler B, Stroe-Kunold E, Schaefert R. A Specialty Clinic for Functional Gastrointestinal Disorders in Tertiary Care: Concept and Patient Population. Clin Gastroenterol Hepatol. 2017;15:1127-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Boeckxstaens GE, Drug V, Dumitrascu D, Farmer AD, Hammer J, Hausken T, Niesler B, Pohl D, Pojskic L, Polster A, Simren M, Goebel-Stengel M, Van Oudenhove L, Vassallo M, Wensaas KA, Aziz Q, Houghton LA; COST Action BM1106 GENIEUR members. Phenotyping of subjects for large scale studies on patients with IBS. Neurogastroenterol Motil. 2016;28:1134-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Dong Y, Baumeister D, Berens S, Eich W, Tesarz J. High Rates of Non-Response Across Treatment Attempts in Chronic Irritable Bowel Syndrome: Results From a Follow-Up Study in Tertiary Care. Front Psychiatry. 2019;10:714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1479] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 36. | Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3285] [Cited by in RCA: 3652] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 37. | Berens S, Schaefert R, Baumeister D, Gauss A, Eich W, Tesarz J. Does symptom activity explain psychological differences in patients with irritable bowel syndrome and inflammatory bowel disease? J Psychosom Res. 2019;126:109836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 39. | Wong KM, Mak ADP, Yuen SY, Leung ONW, Ma DY, Chan Y, Cheong PK, Lui R, Wong SH, Wu JC. Nature and specificity of altered cognitive functioning in IBS. Neurogastroenterol Motil. 2019;31:e13696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 18879] [Article Influence: 993.6] [Reference Citation Analysis (0)] |
| 41. | Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1093] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 42. | Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 2248] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 43. | Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1774] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 44. | Nelson SC, Romm JM, Doheny KF, Pugh EW, Laurie CC. Imputation-based genomic coverage assessments of current genotyping arrays: Illumina HumanCore, OmniExpress, Multi-Ethnic global array and sub-arrays, Global Screening Array, Omni2. 5M, Omni5M, and Affymetrix UK Biobank. 2017 Preprint. Available from: bioRxiv:150219. [DOI] [Full Text] |
| 45. | Bonfiglio F, Henström M, Nag A, Hadizadeh F, Zheng T, Cenit MC, Tigchelaar E, Williams F, Reznichenko A, Ek WE, Rivera NV, Homuth G, Aghdassi AA, Kacprowski T, Männikkö M, Karhunen V, Bujanda L, Rafter J, Wijmenga C, Ronkainen J, Hysi P, Zhernakova A, D'Amato M. A GWAS meta-analysis from 5 population-based cohorts implicates ion channel genes in the pathogenesis of irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie-Claire C, Derks EM. A tutorial on conducting genome-wide association studies: Quality control and statistical analysis. Int J Methods Psychiatr Res. 2018;27:e1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 47. | Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol. 1995;57:289-300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17030] [Cited by in RCA: 23320] [Article Influence: 3331.4] [Reference Citation Analysis (0)] |
| 48. | Zhu L, Huang D, Shi L, Liang L, Xu T, Chang M, Chen W, Wu D, Zhang F, Fang X. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2015;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Simrén M, Ringström G, Björnsson ES, Abrahamsson H. Treatment with hypnotherapy reduces the sensory and motor component of the gastrocolonic response in irritable bowel syndrome. Psychosom Med. 2004;66:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Niesler B, Flohr T, Nöthen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. Association between the 5' UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics. 2001;11:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Iidaka T, Ozaki N, Matsumoto A, Nogawa J, Kinoshita Y, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J Neurosci. 2005;25:6460-6466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Gatt JM, Williams LM, Schofield PR, Dobson-Stone C, Paul RH, Grieve SM, Clark CR, Gordon E, Nemeroff CB. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depress Anxiety. 2010;27:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Gatt JM, Nemeroff CB, Schofield PR, Paul RH, Clark CR, Gordon E, Williams LM. Early life stress combined with serotonin 3A receptor and brain-derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biol Psychiatry. 2010;68:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Hammer C, Cichon S, Mühleisen TW, Haenisch B, Degenhardt F, Mattheisen M, Breuer R, Witt SH, Strohmaier J, Oruc L, Rivas F, Babadjanova G, Grigoroiu-Serbanescu M, Hauser J, Röth R, Rappold G, Rietschel M, Nöthen MM, Niesler B. Replication of functional serotonin receptor type 3A and B variants in bipolar affective disorder: a European multicenter study. Transl Psychiatry. 2012;2:e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Frank B, Niesler B, Nöthen MM, Neidt H, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Rappold G. Investigation of the human serotonin receptor gene HTR3B in bipolar affective and schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Horjales-Araujo E, Demontis D, Lund EK, Finnerup NB, Børglum AD, Jensen TS, Svensson P, Vase L. Polymorphism in serotonin receptor 3B is associated with pain catastrophizing. PLoS One. 2013;8:e78889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Kapeller J, Houghton LA, Walstab J, Boenisch H, Rappold G, Niesler B. 1003 A Coding Variant in the Serotonin Receptor 3C Subunit Is Associated with Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology. 2009;136:A-155. [DOI] [Full Text] |
| 58. | Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Karnovsky AM, Gotow LF, McKinley DD, Piechan JL, Ruble CL, Mills CJ, Schellin KA, Slightom JL, Fitzgerald LR, Benjamin CW, Roberds SL. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene. 2003;319:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Maxwell SE, Delaney HD. Measurement and Statistics - an Examination of Construct-Validity. Psychol Bull. 1985;97:85-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 62. | Yaakob NS, Nguyen DT, Exintaris B, Irving HR. The C and E subunits of the serotonin 5-HT3 receptor subtly modulate electrical properties of the receptor. Biomed Pharmacother. 2018;97:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Katsnelson A. Diagnostics: Filling in the missing pieces. Nature. 2016;533:S110-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |