Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2201
Peer-review started: January 2, 2022
First decision: March 10, 2022
Revised: March 18, 2022
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: May 28, 2022
Processing time: 144 Days and 14.3 Hours
Patients with primary sclerosing cholangitis (PSC) are at a high risk of developing cholestatic liver disease and biliary cancer, and endoscopy is crucial for the complex management of these patients.
To clarify the utility of recently introduced digital single-operator video cholangioscopy (SOVC) for the endoscopic management of PSC patients.
In this observational study, all patients with a history of PSC and in whom digital SOVC (using the SpyGlass DS System) was performed between 2015 and 2019 were included and retrospectively analysed. Examinations were performed at a tertiary referral centre in Germany. In total, 46 SOVCs performed in 38 patients with a history of PSC were identified. The primary endpoint was the evaluation of dominant biliary strictures using digital SOVC, and the secondary endpoints were the performance of selective guidewire passage across biliary strictures and the diagnosis and treatment of biliary stone disease in PSC patients.
The 22 of 38 patients had a dominant biliary stricture (57.9%). In 4 of these 22 patients, a cholangiocellular carcinoma was diagnosed within the stricture (18.2%). Diagnostic evaluation of dominant biliary strictures using optical signs showed a sensitivity of 75% and a specificity of 94.4% to detect malignant strictures, whereas SOVC-guided biopsies to gain tissue for histopathological analysis showed a sensitivity of 50% and a specificity of 100%. In 13% of examinations, SOVC was helpful for guidewire passage across biliary strictures that could not be passed by conventional methods (technical success rate 100%). Biliary stone disease was observed in 17.4% of examinations; of these, in 37.5% of examinations, biliary stones could only be visualized by SOVC and not by standard fluoroscopy. Biliary stone treatment was successful in all cases (100%); 25% required SOVC-assisted electrohydraulic lithotripsy. Complications, such as postinterventional cholangitis and pancreatitis, occurred in 13% of examinations; however, no procedure-associated mortality occurred.
Digital SOVC is effective and safe for the endoscopic management of PSC patients and may be regularly considered an additive tool for the complex endoscopic management of these patients.
Core Tip: Endoscopic management of patients with primary sclerosing cholangitis (PSC) is complex; our study is the first to evaluate the utility of single-operator video cholangioscopy (SOVC) with digital imaging quality in these patients. Our data indicate that the use of digital SOVC in PSC patients substantially improves the evaluation of biliary strictures and that SOVC effectively supports interventions, such as stricture dilation and biliary stone treatment, in PSC patients; mild to moderate complications occurred in a minority of cases. Concluding digital SOVC may be effective and safe as an additive tool for the complex endoscopic management of PSC patients.
- Citation: Bokemeyer A, Lenze F, Stoica V, Sensoy TS, Kabar I, Schmidt H, Ullerich H. Digital single-operator video cholangioscopy improves endoscopic management in patients with primary sclerosing cholangitis-a retrospective observational study. World J Gastroenterol 2022; 28(20): 2201-2213
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2201
Primary sclerosing cholangitis (PSC) is an immune-mediated chronic liver disease characterized by inflammatory, fibrotic, and destructive changes of the bile ducts, leading to cholestasis, biliary stricture development and hepatic fibrosis. Because of the chronic disease course, PSC patients are at a high risk of developing liver cirrhosis and cholangiocellular carcinoma (CCC)[1,2]. Although PSC patients do not regularly show clinical symptoms in early disease, those with advanced disease often develop typical clinical symptoms including right upper quadrant pain (20%), pruritus (10%), jaundice (6%) and fatigue (6%)[1]. Endoscopy is crucial for the diagnostic and therapeutic management of PSC patients, as documented by recent guidelines[2,3]. Standard endoscopic management of PSC patients includes endoscopic retrograde cholangiography (ERC) and is often challenging. In particular, standard endoscopic management is required in patients with biliary strictures: Diagnostic assessment of strictures may become necessary to exclude malignancy, and therapeutic interventions, including stricture dilation to improve cholestatic disease, may be needed[2,3]. Despite endoscopic treatment, PSC patients may develop advanced liver cirrhosis requiring organ transplantation[4], revealing the unmet need for additional therapeutic options.
Cholangioscopic techniques have progressed in recent years. In 2015, the first digital single-operator video cholangioscope (SpyGlassTM DS System, Boston Scientific, Marlborough, MA, United States) was released[5]. Compared with the previous fibre-optic system, this digital single-operator video cholangioscopy (SOVC) instrument is armed with digital imaging, enabling up to four-times higher resolution, a 60% wider field of view, improved manoeuvrability, and irrigation capacities to clean the field of view[5-9]. Furthermore, forceps biopsies are available, allowing SOVC-guided tissue sampling[5,6,8,9], guidewires can be selectively passed across biliary strictures to allow subsequent interventions[8], and SOVC-assisted lithotripsy devices are ready to treat biliary stone disease[10]. Recently, digital SOVC was technically updated, leading to further advances in lighting and image resolution (SpyGlassDS 2.0; Boston Scientific, Marlborough, MA, United States).
The latest guidelines state that intraductal cholangioscopy can help to diagnose indeterminate biliary strictures in PSC patients and that cholangioscopy may be useful for tissue sampling[2,3]. However, the data are rare, and no study thus far has reported the use of newly introduced digital SOVC in PSC patients. Considering the superior imaging quality and manoeuvrability of digital SOVC instruments, further research is required to address the question of whether digital SOVC may offer an effective additive endoscopic treatment in these patients.
Therefore, this study aimed to evaluate the efficacy and safety of digital SOVC for the diagnostic and interventional endoscopic management of patients with PSC.
This retrospective, monocentre study was performed at the Department of Medicine B for Gastroenterology, Hepatology, Endocrinology and Clinical Infectiology of the University Hospital Muenster, Germany. The data from all patients ≥ 18 years of age and with a previously diagnosed PSC who had undergone digital SOVC using the SpyGlass DS System (Boston Scientific, Marlborough, MA, United States) between December 2015 and November 2019 were retrieved from the clinical data systems. PSC diagnosis was previously known and not initially established during performed SOVC examinations. Biliary tract cancer was not previously diagnosed in these patients; likewise, IgG4-related sclerosing cholangitis was not known in our patient cohort. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Board of the Westphalian Wilhelms-University of Muenster and Medical Council of Westphalia-Lippe, Germany. To minimize known sources of bias, this trial was reported according to the STROBE statement, wherever appropriate and applicable[11].
Cholangioscopies were performed by highly experienced endoscopists according to the generally accepted guidelines using an ERC case volume exceeding 200/year and performing ERC procedures for at least five years[12]. Before examination, all the patients received prophylactic antibiotic treatment; nonsteroidal anti-inflammatory drugs (NSAIDs; e.g., indomethacin) were not regularly administered before the procedure. CO2 insufflation was used during all examinations. Before cholangioscopy, an endoscopic papillotomy was performed, or one had been previously performed. The cholangioscope (digital SOVC; Boston Scientific, Marlborough, MA, United States) was inserted into the biliary duct in a guidewire-assisted method; targeted biopsies were acquired using SpyBite forceps (Boston Scientific, Marlborough, MA, United States). For biliary stone treatment, electrohydraulic lithotripsy (EHL) was performed using a bipolar lithotripsy 2.4 F catheter probe (Walz Elektrotechnik GmbH, Rohrdorf, Germany) with saline solution irrigation (SSI) controlled over a dedicated irrigation pump. The probe produces high-frequency hydraulic pressure waves, resulting in the fragmentation of biliary stones[10,13].
The primary endpoint of this study was to evaluate the efficacy and safety of digital SOVC to detect malignancy in dominant biliary strictures in patients with PSC, depending on the visual inspection and histological evaluation of SOVC-acquired biopsies. According to the European guidelines, strictures were defined as dominant if they had a diameter smaller than 1.5 mm in the common bile duct and smaller than 1 mm in the right and left hepatic ducts[14]. Visual signs suggesting malignancy were documented if the performing endoscopists classified visual findings as suspicious for malignancy in the presence of irregular vessels, easy bleeding, irregular surfaces and elevated masses protruding into the duct lumen[15,16]. Acquired biopsy material was analysed by an experienced pathologist and classified as suspicious for malignancy if cancer cells or high-grade cell dysplasia were detected. The final diagnosis (reference standard) of biliary stricture dignity was based on a detailed evaluation of all the available data, including clinical information, cross-sectional imaging reports and histopathological analyses, which could be found in the electronic patient chart. The median follow-up time was 12 mo [interquartile range (IQR) 7-27 mo]; during this time, the patients were followed up by repeated checks of the available electronic medical records.
For secondary endpoint analysis, the use of digital SOVC for the diagnosis and treatment of biliary stone disease in PSC patients was documented. Furthermore, the use of digital SOVC for selective guidewire insertion across biliary strictures in cases that were solely performed because of a previous failure of conventional endoscopic methods to treat a biliary stricture via selective guidewire placement was evaluated.
Adverse events following examination were documented as follows: (1) Postinterventional pancreatitis was defined if patients developed abdominal pain and a threefold increase in the serum lipase levels within 48 h of the examination[17]; (2) Postinterventional cholangitis was documented as the presence of new fever (> 38.0 °C) and newly or significantly higher cholestatic and inflammatory markers requiring antibiotics within three days of the examination[17]; and (3) Severe bleeding was diagnosed if bleeding was observed during intervention that required immediate endoscopic therapy or if haemoglobin level decreased by two points or more[17]. Adverse events were graded as mild, moderate, or severe, depending on the length of additional hospital stay (mild = 1-3 d, moderate = 4-10 d, severe = > 10 d)[17].
The data were analysed using IBM SPSS Statistics 27.0 (IBM Corp., Armonk, United States). Additionally, contingency table-derived data were determined using StatPages[18]. Frequencies and percentages were recorded for categorical variables; means and standard errors (SEs) were reported for continuous variables. Missing data are indicated and reported in the text and tables. The statistical methods of this study were reviewed by Arne Bokemeyer.
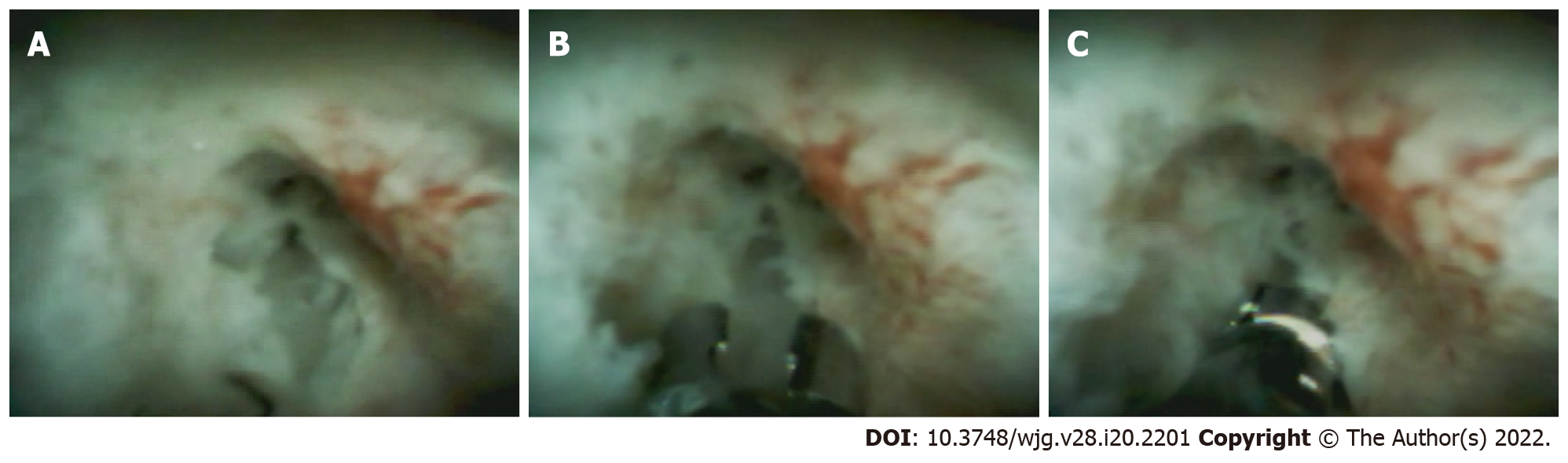
During the study period, 151 ERCs were performed in 72 patients with PSC, and in 30.5% of these ERCs, digital SOVC was additionally carried out (46/151). These 46 cholangioscopies, conducted in 38 PSC patients, were included in the final dataset (Figures 1 and 2). The main indication for SOVC use was the assessment of biliary strictures (80.4%), followed by selective guidewire placement across biliary strictures (13%) and treatment of biliary stone disease (4.3%). A total of 68.4% of the patients were male, whereas 31.6% of the patients were female. The mean age was 44.8 years (SE: ± 2.1 years). Considering all patients, the mean period from the initial ERC performed for PSC diagnosis to the performance of the first SOVC was 99.9 mo (SE ± 16.6). A total of 52.6% of the patients had liver cirrhosis, and 29% were enrolled for liver transplantation. In 10.5% of patients, a final diagnosis of a malignant biliary tumour was established (Table 1). In these patients, the mean time from initial ERC performed for PSC diagnosis to the digital SOVC, which was sufficient to establish bile duct cancer diagnosis, was 71.3 mo (standard error: ± 16.6) with a range of at least 11 mo up to 150 mo.
| Variable | Patients (n = 38) |
| Primary sclerosing cholangitis, n (%) | 38 (100) |
| Age (in years) | 44.8 (± 2.1) |
| Male, n (%) | 26 (68.4) |
| Female, n (%) | 12 (31.6) |
| Liver cirrhosis, n (%) | 20 (52.6) |
| Enlisted for liver transplantation, n (%) | 11 (28.9) |
| Diagnosis of a cholangiocellular carcinoma, n (%) | 4 (10.5) |
Of the cholangioscopies, 38 examinations were initial, and 8 were repeated examinations (Table 2). All the examinations were ERC-based (100%). The median total examination time was 73 min (± 5.2 min; missing data in 6/46 examinations). In one case, the digital SOVC system technically failed during examination and could not be relaunched (2.2%; Table 2).
| Variable | Digital SOVC (n = 46) |
| Type of digital SOVC | |
| Initial examinations, n (%) | 38 (82.6) |
| Repeated examinations, n (%) | 8 (17.4) |
| Main indication for using SOVC | |
| Stricture assessment, n (%) | 37 (80.4) |
| Selective guidewire placement, n (%) | 6 (13) |
| Cholangiolithiasis, n (%) | 2 (4.3) |
| Others, n (%) | 1 (2.2) |
| Clinical patient data before SOVC (multiple items permitted) | |
| Prior papillotomy, n (%) | 41 (89.1) |
| Elevated serum bilirubin level (> 1.2 mg/dl), n (%) | 30 (65.2) |
| Prior post-ERC-pancreatitis, n (%) | 10 (21.7) |
| Type of digital SOVC | |
| ERC-based digital SOVC, n (%) | 46 (100) |
| Total examination time (ERC + digital SOVC; min) | 73 (± 5.2); n = 40/46 |
| Dysfunction of the SOVC-system, n (%) | 1 (2.2) |
| Procedures during SOVC-examination (multiple items permitted) | |
| SOVC-assisted guidewire insertion, n (%) | 39 (84.7) |
| SOVC-assisted forceps biopsies, n (%) | 25 (54.3) |
| SOVC-assisted EHL, n (%) | 2 (4.3) |
| Additive procedures during ERC-examination (multiple items permitted) | |
| Balloon dilation of the biliary tract, n (%) | 35 (76.1) |
| New papillotomy, n (%) | 7 (15.2) |
| Conventional transpapillary biopsy, n (%) | 6 (13.0) |
| Endoprosthesis placement, n (%) | 5 (10.9) |
| Periinterventional application of drugs to prevent AE | |
| Antibiotics, n (%) | 46 (100) |
| NSAID (Diclofenac/Indomethacin), n (%) | 6 (13) |
During SOVC, the main procedures were selective SOVC-assisted guidewire insertions to support diagnostic assessment and therapeutic interventions of the biliary tract in 84.7% of examinations, SOVC-assisted forceps biopsy acquisition in 54.3% of examinations, and the performance of SOVC-assisted EHL for refractory biliary stone disease in 4.3% of examinations. Biliary strictures were dilated in 76.1% of examinations, and endoprostheses were placed in 10.9% of examinations (Table 2).
Dominant biliary strictures were present in 22 of 38 patients (57.9%; Table 3). Dominant strictures were mainly localized intrahepatically (59.1%), followed by strictures at the intra- and extrahepatic passages (27.3%) and extrahepatic strictures (13.6%). In 4 of 22 patients, dominant strictures were of a malignant entity (18.2%). The malignant strictures were localized at the intra- and extrahepatic crossing in three patients, and in one patient the stricture was localized intrahepatically at the left hepatic duct. Using SOVC, visual signs of malignancy could be observed in 18.2% of patients. In 13 of 22 patients, SOVC-assisted forceps biopsies were obtained (59.1%; Figure 1). In 2 of 13 biopsies, histopathological analysis revealed signs of malignancy (carcinoma or high-grade dysplasia; 15.4%). In 1 of 13 patients, insufficient tissue was obtained using forceps biopsies, making an accurate histopathological analysis impossible (7.7%; Table 3). The visual examination of dominant strictures had an accuracy of 90.9% (CI: 72.8%-99.2%), a sensitivity of 75% (CI: 25.2%-97.8%), a specificity of 94.4% (83.4%-99.5%), a positive predictive value of 75% (25.2%-97.8%), and a negative predictive value of 94.4% (83.4%-99.5%; Table 4). Histopathological analysis of SOVC-assisted biopsy acquisition had an accuracy of 83.3% (CI: 57.2%-83.3%), a sensitivity of 50% (10.8%-50%), a specificity of 100% (80.4%-100%), a positive predictive value of 100% (21.7%-100%), and a negative predictive value of 80% (64.3%-80%) (Table 4).
| Variable | Dominant strictures (n = 22) |
| Entity of dominant stricture, n (%) | |
| Benign | 18 (81.8) |
| Malignant | 4 (18.2) |
| Localization of dominant stricture, n (%) | |
| Intrahepatic | 13 (59.1) |
| Extrahepatic | 3 (13.6) |
| Intra- and extrahepatic crossing | 6 (27.3) |
| Visual evaluation of stricture by endoscopists, n (%) | |
| Suspicious for malignancy | 4 (18.2) |
| Suspicious for benignancy | 18 (81.8) |
| SOVC-guided forceps biopsies, n (%) | |
| Carcinoma/high-grade dysplasia | 2 (9.1) |
| Benign findings | 10 (45.5) |
| Inadequate material | 1 (4.5) |
| Variable | Accuracy (%) | Sensitivity (%) | Specificity (%) | Pos. pred. value (%) | Neg. pred. value (%) |
| Visual evaluation (95%CI) | 90.9 (72.8-99.2) | 75 (25.2-97.8) | 94.4 (83.4-99.5) | 75 (25.2-97.8) | 94.4 (83.4-99.5) |
| Histological evaluation (95%CI) | 83.3 (57.2-83.3) | 50 (10.8-50.0) | 100 (80.4-100) | 100 (21.7-100) | 80 (64.3-80) |
In 8 of 46 examinations (17.3%), biliary stones were found (Table 5). Stones were localized intrahepatically (37.5%), extrahepatically (37.5%) and both intra- and extrahepatically (25%). The stone size ranged between 3 and 20 mm, and the number of stones ranged between 1 and 5 per examination. In 3 of 8 cases (37.5%), biliary stones were only visualized using SOVC, and standard fluoroscopy failed to detect biliary stones. In all 8 examinations, biliary stone treatment was finally successful; however, in 2 of 8 examinations (25%), biliary stone disease was refractory to standard ERC methods, including stone extraction with baskets and/or balloon catheters, which was why EHL was applied for stone fragmentation. In both cases, EHL successfully led to complete stone fragmentation (100%; Table 5).
| Variable | Examinations (n = 46) |
| Cholangiolithiasis, n (%) | 8/46 (17.3) |
| Localization | |
| Extrahepatic, n (%) | 3/8 (37.5) |
| Intrahepatic, n (%) | 3/8 (37.5) |
| Intra- and extrahepatic, n (%) | 2/8 (25) |
| Stone size (range) | 3-20 mm |
| Stone number (range) | 1-5 |
| Treatment | |
| Complete success (conventional ± EHL), n (%) | 8/8 (100) |
| Success only via use of EHL, n (%) | 2/8 (25) |
| Stone identification only via SOVC, n (%) | 3/8 (37.5) |
| Selective guidewire insertion across biliary strictures, n (%) | 6/46 (13) |
| Kind of procedures | |
| Initial examinations, n (%) | 5/6 (83.3) |
| Repeated examinations, n (%) | 1/6 (16.7) |
| Technical success, n (%) | 6/6 (100) |
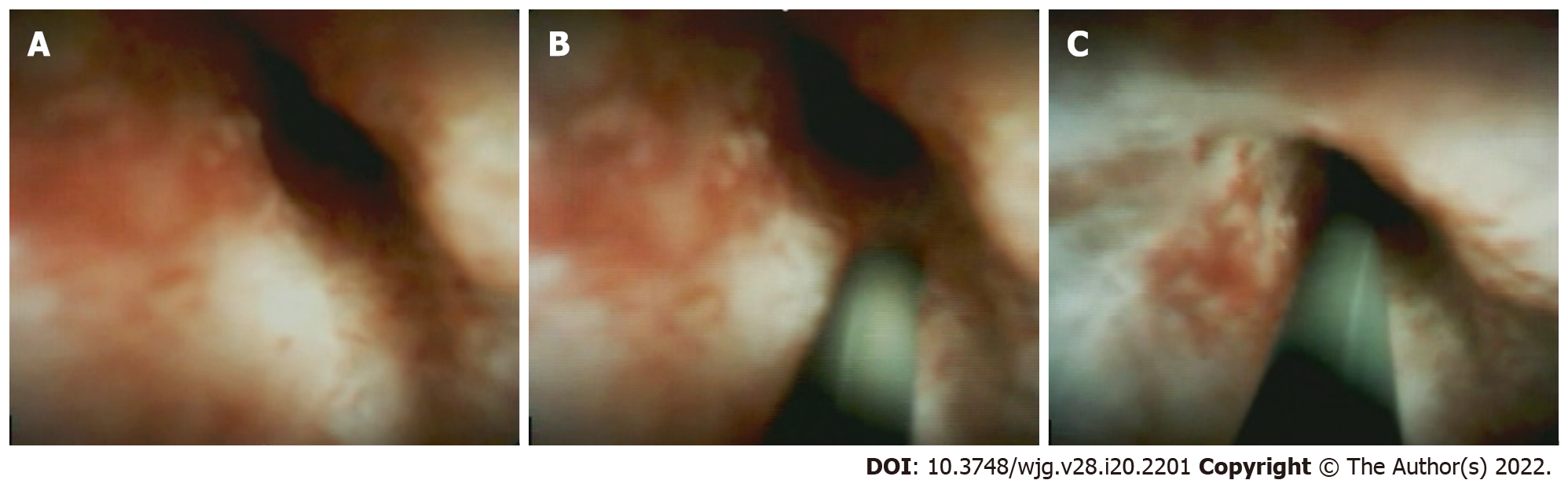
The 6 of 46 examinations were solely performed because of a previous failure of conventional endoscopic methods to treat a biliary stricture via selective guidewire placement (13%; Figure 2). Of these, 5 were initial SOVC procedures, and one was a repeated procedure. The technical success rate of SOVC-assisted guidewire insertions across biliary strictures was 100%, enabling subsequent dilation of the stricture (Table 5).
In 13% of the procedures, adverse events were documented (Table 6). More specifically, postinterventional pancreatitis was observed in 6.5% of cases, of which 67% had a moderate and 33% a severe disease course. Postinterventional cholangitis occurred in 6.5% of cases, of which all had a moderate disease course (100%). Other procedure-related adverse events, including severe bleeding or organ perforations, did not occur. All procedure-related complications could be successfully managed by conservative therapeutic approaches. No mortality due to procedure-related adverse events occurred. Because of side effects, patients needed to stay in the hospital for 6.5 more days (SE ± 1.5 d) (Table 6).
| Variable | Digital SOVCs (n = 46) |
| Overall complications, n (%) | 6 (13) |
| Pancreatitis, n (%) | 3 (6.5) |
| Grade 1 | 0 (0) |
| Grade 2 | 2 (4.3) |
| Grade 3 | 1 (2.2) |
| Cholangitis, n (%) | 3 (6.5) |
| Grade 1 | 0 (0) |
| Grade 2 | 3 (6.5) |
| Grade 3 | 0 (0) |
| Others (bleeding/perforation), n (%) | 0 (0) |
| Procedure-related mortality, n (%) | 0 (0) |
| Suspected prolonged hospital stay due to complications (in days) | 6.5 (± 1.5) |
Although a few previous reports evaluated the utility of cholangioscopy in PSC patients in general[19,20], our study is the first to evaluate the efficacy and safety of SOVC with digital imaging quality in patients with PSC. Digital SOVC is effective and safe as an additive tool for the complex endoscopic management of these patients. In addition to evaluating biliary strictures, digital SOVC facilitates interventions to the biliary tract because of selective guidewire placements across biliary strictures and helps to diagnose and treat biliary stone disease.
Stricture assessment of biliary strictures in PSC patients is critical to excluding malignancy: PSC patients have a lifetime risk of developing CCC of up to 20%[2]. Clinical judgement, laboratory markers, and cross-sectional imaging are insufficient to exclude malignancy, explaining why endoscopic evaluation, including tissue sampling, becomes necessary[2,3,21]. If a standard work-up including ERC with transpapillary tissue sampling fails to determine stricture aetiology, the performance of peroral cholangioscopy is suggested[21]. Visual interpretation of biliary strictures using cholangioscopy may help diagnose indeterminate biliary strictures: A recent meta-analysis including 283 procedures with digital SOVC in unselected patients found a sensitivity of 94% and a specificity of 95% in detecting malignancy in biliary strictures[22]. In addition to optical evaluation, cholangioscopic-guided biopsies can be obtained: Another recent meta-analysis in unselected patients found a sensitivity of 72% and a specificity of 99% in diagnosing biliary malignancy using cholangioscopy-guided biopsies[23]. Despite these promising results in unselected patients, the results in selected PSC patients might be different: A prospective trial using legacy fibreoptic SOVC in 47 patients with PSC evaluated the use of SOVC-assisted forceps biopsies and found a sensitivity of only 33% and a specificity of 100% in detecting malignant biliary strictures[20]. In our study, visual evaluation of indeterminate biliary strictures identified malignancy with a sensitivity of 75% and a specificity of 94% and histopathological analysis of SOVC-guided biopsies showed a sensitivity of 50% and specificity of 100%. In comparison, our study showed a lower sensitivity and specificity of visual and bioptical stricture assessment than those in previous studies in unselected patients using SOVC. However, comparing our results to previous studies with fibreoptic SOVC including only selected PSC patients, our sensitivity and specificity rates for the diagnostic evaluation of biliary strictures might be improved. As a limitation, digital SOVCs might only be advanced with difficulties to all intrahepatic strictures due to the decreasing lumen of the proximal bile ducts making proximal intrahepatic ducts partially inaccessible for cholangioscopic assessment. To guide cholangioscopy intrahepatically, the use of guidewires can help to advance the cholangioscope to more proximal localized strictures.
In conclusion, this study shows that using SOVC with digital imaging quality may significantly improve the diagnostic evaluation of indeterminate strictures in PSC patients. However, validated criteria for optical evaluation of strictures are missing and may be particularly needed in stricture evaluation of PSC patients because inflammatory tissue alterations of the bile ducts hinder easy evaluation of biliary stricture aetiology. Furthermore, no consensus exists concerning the number of biopsies that should be obtained to ensure adequate biopsy material[24]. Speculatively, the sensitivity rates of histopathological evaluation may be improved by a higher number of SOVC-guided biopsies[25]; furthermore, larger forceps biopsies for digital SOVC were recently introduced, promising to further improve cholangioscopic diagnostics in the future. Histopathological analysis is essential for excluding differential diagnoses including Ig4-related sclerosing cholangitis, which may mimic a PSC-like disease. In addition to radiologic and serological assessment, tissue acquisition for histopathological analysis is important for diagnostic assessment[26], and SOVCs might help to gain sufficient histopathological material for correct assessment.
Endoscopic interventions, including stricture dilation, can be performed to improve cholestatic disease in PSC patients and are part of current guideline recommendations[2,3]. Technically, biliary dilation should be preferred to inserting biliary stents[2,3]: Notably, in our cohort, stricture dilation was regularly performed in most patients (76%), whereas only a few received biliary stenting (10.9%). To facilitate biliary dilation or stenting, a guidewire must be placed across the biliary stricture[8,27,28]; however, this selective guidewire placement might fail using standard ERC techniques. A previous trial using fiberoptic SOVCs in 15 patients after liver transplantation showed a technical success rate of 60% of placing a guidewire across a stricture[28]. Another study, which was published by our group, using digital SOVC for selective guidewire insertion in 23 unselected patients showed an overall technical success rate of 70%; notably, the technical success rate was significantly higher in benign strictures than in malignant strictures (88% vs 46%; P = 0.02)[8]. In the current study, in 6 examinations, conventional ERC techniques failed to pass a guidewire across a biliary stricture, and digital SOVC helped in all cases to perform selective guidewire placement, enabling subsequent stricture dilation (technical success rate: 100%). In conclusion, digital SOVC with improved imaging quality is highly successful in facilitating selective guidewire placement across biliary strictures, even in PSC patients in whom previous attempts to pass a stricture with a guidewire failed using standard ERC techniques.
Patients with PSC may have a high incidence of biliary stone disease[29,30]. In two previous studies, PSC patients had an incidence of biliary stone disease of up to 50%[29,30]. In our cohort, we found a slightly lower incidence of biliary stone disease; however, stones were still frequently found in 17.3% of examinations. A previous trial with 41 PSC patients undergoing fibreoptic cholangioscopy using the mother-baby-technique suggested that 30% of biliary stones were missed by standard fluoroscopy and could only be visualized using cholangioscopy[29]. In our cohort, nearly 40% of biliary stones were missed on fluoroscopy and could only be detected using digital SOVC likely confirming that digital SOVC with improved imaging quality substantially helps detect biliary stones in PSC patients. Although the utility of cholangioscopy for stone detection in PSC patients might be superior using digital SOVC, it might be less likely that a routine use of digital SOVCs for stone detection in PSC patients is cost-effective, which might especially be true for MRCP-negative cases. Sometimes the extraction of biliary stones proximal to biliary strictures might be challenging. Dilation of the distal biliary stricture might substantially help extract stones. Furthermore, EHL might be used for stone fragmentation. In 25% of our cases, SOVC-assisted EHL was used for refractory biliary stone disease and showed complete treatment success (100%). This high technical success rate of biliary stone treatment was similar to that in previous trials, varying from 86% to 100%[9,10,15,16,31] supporting the role of digital SOVC as an effective treatment for refractory biliary stone disease, even in PSC patients.
Additionally, we evaluated the safety of using digital SOVC in PSC patients. In ERC, adverse events occurred in approximately 7% of examinations[32]. Concerning digital SOVC, earlier studies observed complication rates ranging from 0 to 16.4%[9,10,15,16,31], and a recent meta-analysis applying digital SOVC to evaluate biliary strictures found a complication rate of 7%[33]. We found a complication rate of 13%, which is in the upper range of previous trials, although only fully trained endoscopists performed procedures in our cohort. Our cholangitis rate (6.5%) was slightly higher than that of unselected patients (4%), likely because of the complexity of our cases: Only PSC patients were included in our cohort; among these, more than 50% had cirrhotic liver disease, and nearly 30% were enlisted for liver transplantation. The risk of cholangioscopy in PSC patients is controversial: Considering that the stricturing disease course hampers adequate biliary drainage post contrast injection, PSC patients may be at special risk of developing post-ERC cholangitis[34]. Furthermore, our pancreatitis rate (6.5%) was slightly higher than the post-ERC pancreatitis rates observed in unselected patients (2%-4%)[32,35]. Fortunately, all cases of pancreatitis could be managed conservatively, and no surgical management was necessary. In our cohort, rectal NSAIDs were not routinely applied at the start of the study; however, considering our results, rectal NSAIDs should be regularly dispensed in all patients with PSC undergoing digital SOVC, which is the current standard of care in our department and part of recent guideline recommendations[17]. In summary, considering this moderate rate of complications, digital SOVC should be performed in selected cases and by experienced endoscopists at tertiary referral centres.
Our study has several limitations. First, our study was retrospective and is limited by a small sample size comprising only 46 procedures; however, it is the first to exclusively evaluate digital SOVC use in PSC patients; furthermore, PSC is a rare disease, making our number of procedures noteworthy. Second, we included cases at only one hospital in our analysis; however, our centre is a large tertiary referral centre offering special endoscopic experience to perform cholangioscopic procedures, and we could ensure that all endoscopists were fully trained, improving the reliability of our results. Nevertheless, our study results are limited by a lack of validation, making future prospective multicentre studies necessary. Third, our endoscopists were not blinded to patient history, likely biasing their visual impression to determine the biliary stricture dignity; however, digital SOVC was performed because strictures were still indeterminate despite previously performed diagnostics. Fourth, in all our patients, a previous traditional cholangiography was performed before the use of digital SOVC, which might have confounded the rate of cholangitis described in our study. However, this setting was our routine clinical practice. Initially, endoscopists performed traditional cholangiography, which revealed findings making further cholangioscopic assessment instantly necessary.
In summary, our data indicate that using digital SOVC in patients with PSC is efficient and safe. In addition to evaluating biliary strictures, which may be substantially improved because of superior image quality, SOVC supports interventions due to selective guidewire placements across biliary strictures and helps diagnose and treat biliary stone disease, explaining why digital SOVC might be frequently used as an additive tool for the complex endoscopic management of PSC patients.
Patients with primary sclerosing cholangitis (PSC) have a high risk of developing cholestatic liver disease, biliary strictures, and biliary cancer, which frequently require endoscopy for diagnostic and therapeutic management.
Recently, digital single-operator video cholangioscopy (SOVC) was introduced, offering superior image quality and manoeuvrability. However, no study thus far has reported the use of newly introduced digital SOVC in PSC patients.
To clarify the efficacy and safety of the recently introduced SOVC for the endoscopic management of patients with PSC.
This observational study retrospectively included all patients with a known PSC and in whom digital SOVC (with the SpyGlass DS System) was performed between 2015 and 2019 at a tertiary referral centre. In total, 46 SOVCs performed in 38 patients with PSC were identified. The primary endpoint was the evaluation of dominant biliary strictures using digital SOVC.
The 22 of 38 patients had a dominant biliary stricture (57.9%), and in 18.2% of these cases, a cholangiocellular carcinoma was diagnosed within the stricture. Diagnostic evaluation of dominant biliary strictures using optical signs showed a sensitivity of 75% and a specificity of 94.4% in detecting malignant strictures, whereas SOVC-guided biopsies to obtain tissue for histopathological analysis showed a sensitivity of 50% and a specificity of 100%. In 13% of examinations, SOVC was helpful for guidewire passage across biliary strictures that could not be passed by conventional methods (technical success rate 100%) and furthermore, in 8 examinations, SOVC helped visualize and treat biliary stone disease (100% success rate). Mild to moderate complications occurred in 13% of examinations.
Digital SOVC is effective and safe for the complex endoscopic management of PSC patients.
In the future, digital SOVC might be regularly considered as an additive tool for the endoscopic management of patients with PSC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fujimori N, Japan; Gerussi A, Italy; Kitamura K, Japan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:2501-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, Collier J, Dyson JK, Jones DE, Patanwala I, Thain C, Walmsley M, Pereira SP. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68:1356-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 3. | Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, Färkkilä M, Fickert P, Hirschfield GM, Laghi A, Marzioni M, Fernandez M, Pereira SP, Pohl J, Poley JW, Ponsioen CY, Schramm C, Swahn F, Tringali A, Hassan C. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49:588-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Bjøro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | SpyGlass-DS-System-ebrochure. SpyGlass™ DS II Direct Visualization System. [cited 10 January 2022]. Available from: http://www.bostonscientific.com/content/dam/bostonscientific/endo/portfolio-group/SpyGlass%20DS/SpyGlass-DS-System-ebrochure.pdf. |
| 6. | Pereira P, Peixoto A, Andrade P, Macedo G. Peroral cholangiopancreatoscopy with the SpyGlass® system: what do we know 10 years later. J Gastrointestin Liver Dis. 2017;26:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Shah RJ, Neuhaus H, Parsi M, Reddy DN, Pleskow DK. Randomized study of digital single-operator cholangioscope compared to fiberoptic single-operator cholangioscope in a novel cholangioscopy bench model. Endosc Int Open. 2018;6:E851-E856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Bokemeyer A, Gross D, Brückner M, Nowacki T, Bettenworth D, Schmidt H, Heinzow H, Kabar I, Ullerich H, Lenze F. Digital single-operator cholangioscopy: a useful tool for selective guidewire placements across complex biliary strictures. Surg Endosc. 2019;33:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Lenze F, Bokemeyer A, Gross D, Nowacki T, Bettenworth D, Ullerich H. Safety, diagnostic accuracy and therapeutic efficacy of digital single-operator cholangioscopy. United European Gastroenterol J. 2018;6:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Brewer Gutierrez OI, Bekkali NLH, Raijman I, Sturgess R, Sejpal DV, Aridi HD, Sherman S, Shah RJ, Kwon RS, Buxbaum JL, Zulli C, Wassef W, Adler DG, Kushnir V, Wang AY, Krishnan K, Kaul V, Tzimas D, DiMaio CJ, Ho S, Petersen B, Moon JH, Elmunzer BJ, Webster GJM, Chen YI, Dwyer LK, Inamdar S, Patrick VB, Attwell A, Hosmer A, Ko C, Maurano A, Sarkar A, Taylor LJ, Gregory MH, Strand DS, Raza A, Kothari S, Harris JP, Kumta NA, Manvar A, Topazian MD, Lee YN, Spiceland CM, Trindade AJ, Bukhari MA, Sanaei O, Ngamruengphong S, Khashab MA. Efficacy and Safety of Digital Single-Operator Cholangioscopy for Difficult Biliary Stones. Clin Gastroenterol Hepatol. 2018;16:918-926.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | STROBE. What is STROBE? [cited 10 January 2022]. Available from: https://www.strobe-statement.org/. |
| 12. | Baron TH, Petersen BT, Mergener K, Chak A, Cohen J, Deal SE, Hoffinan B, Jacobson BC, Petrini JL, Safdi MA, Faigel DO, Pike IM; ASGE/ACG Taskforce on Quality in Endoscopy. Quality indicators for endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 2006;101:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | ASGE Technology Committee; Watson RR, Parsi MA, Aslanian HR, Goodman AJ, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Sethi A, Sullivan SA, Thosani NC, Trikudanathan G, Trindade AJ, Maple JT. Biliary and pancreatic lithotripsy devices. VideoGIE. 2018;3:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | European Society of Gastrointestinal Endoscopy; European Association for the Study of the Liver. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol. 2017;66:1265-1281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Navaneethan U, Hasan MK, Kommaraju K, Zhu X, Hebert-Magee S, Hawes RH, Vargo JJ, Varadarajulu S, Parsi MA. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc. 2016;84:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Ogura T, Imanishi M, Kurisu Y, Onda S, Sano T, Takagi W, Okuda A, Miyano A, Amano M, Nishioka N, Yamada T, Masuda D, Takenaka M, Kitano M, Higuchi K. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Dig Endosc. 2017;29:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | ASGE Standards of Practice Committee, Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 18. | 2-way Contingency. 2-way Contingency Table Analysis. 2018. [cited 10 January 2022]. Available from: http://statpages.info/ctab2x2.html. |
| 19. | Siiki A, Rinta-Kiikka I, Koivisto T, Vasama K, Sand J, Laukkarinen J. Spyglass single-operator peroral cholangioscopy seems promising in the evaluation of primary sclerosing cholangitis-related biliary strictures. Scand J Gastroenterol. 2014;49:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Arnelo U, von Seth E, Bergquist A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy. 2015;47:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Pouw RE, Barret M, Biermann K, Bisschops R, Czakó L, Gecse KB, de Hertogh G, Hucl T, Iacucci M, Jansen M, Rutter M, Savarino E, Spaander MCW, Schmidt PT, Vieth M, Dinis-Ribeiro M, van Hooft JE. Endoscopic tissue sampling - Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1174-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | de Oliveira PVAG, de Moura DTH, Ribeiro IB, Bazarbashi AN, Franzini TAP, Dos Santos MEL, Bernardo WM, de Moura EGH. Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis. Surg Endosc. 2020;34:3321-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Badshah MB, Vanar V, Kandula M, Kalva N, Badshah MB, Revenur V, Bechtold ML, Forcione DG, Donthireddy K, Puli SR. Peroral cholangioscopy with cholangioscopy-directed biopsies in the diagnosis of biliary malignancies: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Martinez NS, Trindade AJ, Sejpal DV. Determining the Indeterminate Biliary Stricture: Cholangioscopy and Beyond. Curr Gastroenterol Rep. 2020;22:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Kawashima H, Itoh A, Ohno E, Goto H, Hirooka Y. Transpapillary biliary forceps biopsy to distinguish benign biliary stricture from malignancy: how many tissue samples should be obtained? Dig Endosc. 2012;24 Suppl 1:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 27. | Lee YY, Gwak GY, Lee KH, Lee JK, Lee KT, Kwon CH, Joh JW, Lee SK. Predictors of the feasibility of primary endoscopic management of biliary strictures after adult living donor liver transplantation. Liver Transpl. 2011;17:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Woo YS, Lee JK, Noh DH, Park JK, Lee KH, Lee KT. SpyGlass cholangioscopy-assisted guidewire placement for post-LDLT biliary strictures: a case series. Surg Endosc. 2016;30:3897-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Gluck M, Cantone NR, Brandabur JJ, Patterson DJ, Bredfeldt JE, Kozarek RA. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Imanishi M, Ogura T, Kurisu Y, Onda S, Takagi W, Okuda A, Miyano A, Amano M, Nishioka N, Masuda D, Higuchi K. A feasibility study of digital single-operator cholangioscopy for diagnostic and therapeutic procedure (with videos). Medicine (Baltimore). 2017;96:e6619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 33. | Wen LJ, Chen JH, Xu HJ, Yu Q, Liu K. Efficacy and Safety of Digital Single-Operator Cholangioscopy in the Diagnosis of Indeterminate Biliary Strictures by Targeted Biopsies: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Navaneethan U, Jegadeesan R, Nayak S, Lourdusamy V, Sanaka MR, Vargo JJ, Parsi MA. ERCP-related adverse events in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2015;81:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Korrapati P, Ciolino J, Wani S, Shah J, Watson R, Muthusamy VR, Klapman J, Komanduri S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E263-E275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |