Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2176
Peer-review started: November 16, 2021
First decision: December 26, 2021
Revised: January 9, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: May 28, 2022
Processing time: 192 Days and 2.4 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for about 90% of liver cancer cases. It is currently the fifth most common cancer in the world and the third leading cause of cancer-related mortality. Moreover, recurrence of HCC is common. Microvascular invasion (MVI) is a major factor associated with recurrence in postoperative HCC. It is difficult to evaluate MVI using traditional imaging modalities. Currently, MVI is assessed primarily through pathological and immunohistochemical analyses of postoperative tissue samples. Needle biopsy is the primary method used to confirm MVI diagnosis before surgery. As the puncture specimens represent just a small part of the tumor, and given the heterogeneity of HCC, biopsy samples may yield false-negative results. Radiomics, an emerging, powerful, and non-invasive tool based on various imaging modalities, such as computed tomography, magnetic resonance imaging, ultrasound, and positron emission tomography, can predict the HCC-MVI status preoperatively by delineating the tumor and/or the regions at a certain distance from the surface of the tumor to extract the image features. Although positive results have been reported for radiomics, its drawbacks have limited its clinical translation. This article reviews the application of radiomics, based on various imaging modalities, in preoperative evaluation of HCC-MVI and explores future research directions that facilitate its clinical translation.
Core Tip: Hepatocellular carcinoma-microvascular invasion (HCC-MVI) is closely related to the prognosis of patients, so accurate and individualized prediction of MVI status before treatment is very important. Radiomics is a non-invasive method for predicting HCC-MVI status preoperatively. The standardization of relevant implementation processes of radiomics, such as the delineation of the region of interest, the improvement of algorithms, and the combination of liver imaging reporting and data system, will all contribute to the accurate prediction of MVI. In addition, the introduction of the biological significance of the disease can make up for the shortcomings of the clinical transformation of radiomics to a certain extent.
- Citation: Lv K, Cao X, Du P, Fu JY, Geng DY, Zhang J. Radiomics for the detection of microvascular invasion in hepatocellular carcinoma. World J Gastroenterol 2022; 28(20): 2176-2183
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2176.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2176
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for about 90% of liver cancers cases[1]. According to the World Health Organization, HCC is currently the fifth most common cancer in the world and the third leading cause of cancer-related mortality[2]. The rate of HCC occurrence varies with geographic regions. It is more commonly observed in the underdeveloped regions of the world; for example, its annual incidence rate in East Asia and Sub-Saharan Africa is higher, exceeding 15 per 100000 individuals. Nonetheless, the incidence of HCC is rising rapidly in Europe and the United States, and is expected to continue to rise over the next 10 years[3-5]. Although various treatment modalities are available for different stages of HCC, recurrence is common in the following types of HCC patients: patients with single masses with preserved liver function, no portal hypertension, and treated with resection; patients with multiple intrahepatic tumors or poor liver function, no major comorbidities, and listed for transplantation; patients with up to three tumors (each 4 cm or smaller), not eligible for transplantation, and treated with ablation; patients not eligible for ablation and receiving embolization; and some patients subjected to systemic and radiation treatments[6-8]. According to a previous study, about 70% of HCC patients relapse within 5 years after surgical resection and 35% relapse within 5 years after liver transplantation[9]. Microvascular invasion (MVI) is defined as the invasion of tumor cells into the vascular endothelial cell space, including microvessels of the portal vein, hepatic artery, and lymphatic vessels[10]. Previous studies[11,12] demonstrated that MVI is the strongest independent predictor of early HCC recurrence. It is primarily detected through immunohistochemical and pathological analyses of postoperative tissue specimens[13]. The inability to identify MVI preoperatively leads to incomplete surgical resection and increases the risk of postoperative recurrence. This limits the effectiveness of treatment and affects the long-term survival of liver cancer patients. Although conventional imaging examinations, such as computed tomography (CT)-based quantitative image analysis and dynamic contrast enhanced ultrasound (CEUS)[14,15] can predict MVI preoperatively, routine analyses of these images provide insufficient information, such as two-dimensional analysis of lesion morphology, size, etc based on conventional images.
Radiomics is an emerging, powerful, and non-invasive tool that uses a series of data mining algorithms and statistical analysis tools for the high-throughput analysis of image features. It can detect delicate features from conventional radiological images that are invisible to the naked eye and has been increasingly adopted to predict MVI. This enables extraction of many features at the whole lesion level. These features may provide information regarding heterogeneity and invasiveness of the disease that can be of predictive or prognostic value, thus leading to the selection of the best possible treatment[16]. HCC shows a large amount of intra- and inter-tumor heterogeneity at the biological level[17-20]. Biopsy, which is the main method for diagnosing HCC, usually involves removal of only a small part of the tumor, thus excluding the possibility of assessing intratumoral heterogeneity. Hence, in recent years, research pertaining to HCC using radiomics has attracted increasing attention[21]. Quantitative radiomics data, along with models or nomograms based on multiple imaging modalities including CT, magnetic resonance imaging (MRI), ultrasound (US), and positron emission tomography (PET), demonstrate high accuracy in predicting HCC-MVI preoperatively. In this review, we discuss the applications of radiomics based on various imaging modalities in the preoperative detection of HCC-MVI (Table 1). In addition, future research directions are explored.
| Ref. | Modality/images/patients/ROI | Software | Predictors of MVI |
| Peng et al[23] | CT/AP and PVP CT images/304/tumor | IBEX software package | Radiomics signature, AFP level, hypoattenuating halo, internal arteries, and nonsmooth tumor margin |
| Ma et al[24] | CT/AP, PVP and DP CT images/157/tumor | ITK-Snap | Age, MTD, AFP, Radiomics signature, hepatitis B |
| Xu et al[9] | CT/AP and PVP CT images/495/VOIentire, VOI50%, and VOIpenumbra | In-house software written in Python 3.6.1 | AST, AFP, tumor margin, growth pattern, capsule, peritumoral enhance, RVI, R-score of VOIentire on PP |
| Feng et al[27] | MRI/HBP of Gd-EOB-DTPA/160/tumoural and peritumoural (1cm) regions | ITK-Snap | NA |
| Yang et al[28] | MRI/T1, T2, DWI, Gd-EOB-DTPA MRI AP, PVP, DP, and HBP/208/tumor | ITK-Snap | AFP, nonsmooth tumor margin, arterial peritumoral enhancement, radiomics signatures of HBP T1WI and HBP T1 maps |
| Nebbia et al[29] | MRI/T1, T2, DWI, Gd-DTPA MRI AP and PVP/99/tumoural and peritumoural (1 cm) regions | NA | NA |
| Hu et al[30] | US/Grayscale US/482/tumor | A.K. software | radiomics score, AFP, and tumor size |
| Dong et al[31] | US/Grayscale US/322/tumor and peri-tumor (half of the tumor radius) | MITK | NA |
| Li et al[35] | PET-CT/[18F]FDG PET-CT/80/areas with abnormal uptake | Lifex software | SUVmax, TLR, Rad-score |
Liver cirrhosis or hepatitis B-related HCC can be diagnosed highly accurately through non-invasive approaches based on multi-phase CT/MRI imaging features, such as hyperenhancement in the arterial phase (AP) and hypoenhancement in the portal venous phase (PVP)[22]. For example, Peng et al[23] constructed a radiomics model based on hepatic AP and PVP images to delineate tumor regions and extract radiomic features to predict the preoperative MVI status of HCC related to hepatitis B. They found that radiomics signature, the alpha-fetoprotein (AFP) level, hypoattenuating halo, internal arteries, and non-smooth tumor margins are independent predictors of MVI. This model showed good correction and discrimination capabilities in the training and validation cohorts. The C indices for the training and validation cohorts were 0.846 and 0.844, respectively, and the area under the curve (AUC) of the radiomics nomogram in the MVI risk analysis was 0.845. In a similar study, Ma et al[24] found that the radiomics features of PVP were better than those of the AP and delayed phase (DP); the C index and AUC values of the model, combined with radiomic features and clinical factors, were close to those reported by Peng et al[23]. In a recent study based on AP and PVP images, Xu et al[9] marked the tumor regions and regions 5 mm away from the tumor surface, as regions of interest (ROI) to extract radiomic features to predict the HCC-MVI status and long-term clinical outcomes of patients with HCC. It was found that aspartate aminotransferase (AST) and AFP levels, tumor margin, growth pattern, capsule, peritumoral enhancement, radio-genomic venous invasion (RVI), and the radiomic score (R-score) were all predictors of MVI. The AUC of this model was 0.909, and the progression-free survival and overall survival of the MVI group were significantly lower than those of the non-MVI group. This showed that combining large-scale clinical radiology and radiomics analyses could reliably predict MVI and clinical outcomes.
Although both conventional multi-phase contrast enhanced CT and MRI can be used to obtain the unique image features of HCC, MRI provides many additional imaging sequences that are helpful in diagnosing HCC without radiation damage; the additional sequences include T2-weighted imaging (T2WI), diffusion weighted imaging (DWI), and enhanced scanning by combined use of some extracellular and hepatocyte contrast agents, such as gadoxetic acid, that have the ability to distinguish relatively small and subtle lesions through the hypointensity received in the hepatobiliary phase (HBP)[25]. Moreover, a combination of MRI parameters can facilitate early diagnosis of small HCC. For example, double hypointensity in the portal/venous phase and HBP can be considered an MRI pattern that is highly suggestive of hypovascular HCC[26]. Thus, MRI-based radiomics provides more possibilities for HCC-MVI assessment. Feng et al[27], based on a gadolinium-ethoxybenzyl-diethylenetriamine (Gd-EOB-DTPA) MRI HBP image, delineated the tumor and designated a 1-cm area around it as the ROI. The combined intratumoral and peritumoral radiomics model presented in that study predicted the AUC value of MVI to be 0.85. In another study, Yang et al[28] used gadoxetic acid-enhanced MRI to delineate the tumor, which was then used as the ROI. They found that the AFP level, non-smooth tumor margins, arterial peritumoral enhancement, and radiomics signatures of HBP T1WI and HBP T1 maps could all be used as predictors of MVI. The prediction model that combined clinical radiation factors and fusion radiomic features from HBP images predicted the AUC of MVI to be 0.943, which was higher than that reported by Feng et al[27]. Meanwhile, the C indices of the generated nomograms in the training and validation groups were 0.936 and 0.864, respectively. This may stem from the varied delineation of the ROIs and the construction of models based on different sequences. Recently, Nebbia et al[29] devised a method based on radiomics through multi-sequence and traditional Gd-DTPA MRI for preoperative detection of MVI. It was found that the T2 and PVP sequences were superior to other MRI sequences in single-sequence models (i.e. T1, DWI, and late AP). The combination of these two sequences obtained the highest AUC value of 0.867 in predicting MVI, indicating that preoperative liver MRI scans are promising for predicting MVI and that the information obtained from a multi-parameter MRI sequence is crucial in identifying MVI.
Compared with CT/MR, US is radiation-free, easy to implement, and simple to use for liver examinations. If potential predictors can be identified, US/CEUS may become an alternative technique to provide additional information for the detection of MVI. Hu et al[30], based on US images, outlined the tumor region as the ROI to extract radiomic features and constructed a US-based radiomics score for preoperative prediction of HCC-MVI. The study showed that an AFP level > 400 ng/mL and tumor size > 5 cm were significantly related to MVI. The AFP level, gray-scale US-based tumor size, and radiomics score were identified to be independent predictors of MVI. The radiomics-based nomogram (AUC: 0.731) showed better performance in MVI detection than the clinical nomogram (AUC: 0.634). However, there was no significant difference between the MVI-positive and -negative groups in terms of other clinical and pathological features, as well as CEUS features. Similarly, Dong et al[31] envisaged that radiomic algorithms based on US images might have potential predictive value in the detection of MVI in patients with HCC. Unlike Hu et al[30], Dong et al[31] delineated the gross-tumor region (GTR) and the peri-tumoral region (PTR, the uniform dilated half of the tumor radius) and showed that the AUC of the radiomic nomogram, combined with the AFP level, characteristics of the tumor, and the area around the tumor, was 0.744, which was slightly higher than that reported by Hu et al[30]. This also supports the idea that the peritumoral area is an onset area of MVI. It is thought to be the main blood transmission route of portal vein thrombosis and the main route for intra- and extra-hepatic metastases[32]. This signifies that, when evaluating HCC-MVI based on radiomics, the area around the tumor also needs to be evaluated.
High glucose consumption by the tumor cell microenvironment, including that in HCC, can be reflected in 18-fluorodeoxyglucose PET (18FDG-PET). Although the tumor detection efficiency is affected by liver cirrhosis and a high background signal, studies have confirmed that the maximum standard uptake value (SUVmax) of 18FDG-PET is related to the recurrence rate of HCC and patient survival, and that the combination of MRI and 18FDG-PET can improve the accuracy of HCC histopathological grading[33,34]. In a recent study[35], the PET/CT model, after integrating the radiomic features composed of five PET- and six CT-derived texture features, showed an AUC of 0.891 in the training cohort. The prediction of disease-free survival (DFS) was also more accurate (C index of 0.831). This study indicated that the newly developed [18F]FDG PET/CT radiomics feature is an independent biomarker for evaluating MVI and DFS in patients with ultra-early and early HCC.
Although radiomics has achieved some exciting results in evaluating the HCC-MVI status, many limitations still exist. The current lack of standardization in image acquisition schemes, segmentation methods, and radiomics tools used for analysis may lead to differences in the measurement of radiomic features that do not stem from biological differences[16]. Existing studies[36] have shown varied reproducibility in extracting quantitative imaging features from tumor regions segmented by different methods. Fortunately, the radiomics quality score (RQS) has been proposed to assess whether radiomics research meets best practices[37]. In addition, there are huge challenges in interpreting the correlation between radiomics features and their biological significance, especially that of the texture features obtained from high-level analysis. This leads to restrictions on the use of radiomics in clinical applications[38].
Every step involved in radiomics-based analyses needs to be optimized and standardized, including the adjustment of imaging schemes and parameters, development of (semi-) automated segmentation methods, and refinement of algorithms and high-throughput analysis modeling methods. In addition, in the process of establishing a radiomics model, potential independent variables should be included in the multivariate analysis (such as protein induced by vitamin K absence or antagonist-II), which will help improve the sensitivity, specificity, and predictive ability of the model[39]. In addition, the imaging characteristics of certain liver nodules in patients with chronic liver disease are complex and diverse. For example, the imaging manifestations of primary and recurrent cirrhosis nodules are different[40], and tuberculosis nodules are easily misdiagnosed as HCC[41]. Through targeted collection of a large number of these different liver nodules, corresponding predictions can also be made using radiomics. Furthermore, due to the complexity and similarity in imaging characteristics of certain hepatic nodules in high-risk HCC, the American College of Radiology (ACR) has issued a diagnostic program, namely the liver imaging reporting and data system (LI-RADS, https://www.acr.org). Several studies[42-44] have indicated that ACR CT/MRI LI-RADS and CEUS LI-RADS possess high application value in identifying primary hepatic nodules in patients with high-risk HCC, and especially display high diagnostic performance with LR-5 and LR-M nodules. In a recent study, Zhou et al[45] observed that the model incorporating CEUS LR-M and clinical features was able to predict the MVI status of HCC. Therefore, undoubtedly, LI-RADS combined with radiomics using various imaging modalities shows promise in the prediction of HCC-MVI.
Although radiomics can predict MVI and determine related predictors, there are differences between groups. With the exponential growth of interest in the development of artificial intelligence (AI)-based applications, there is now an opportunity to apply AI in radiomics analyses. Deep learning, especially convolutional neural networks (CNNs), can capture texture information in the initial convolution layer. CNNs may replace several current radiomics-based analysis methods[46]. Recently, a new concept of “deep radiomics” was proposed. This technique combines radiomics and deep learning analysis by creating feature images based on texture features and then using them as input for CNNs to classify the images. Therefore, along with the prediction of MVI, the use of AI-based technology is also expected to identify individual MVI.
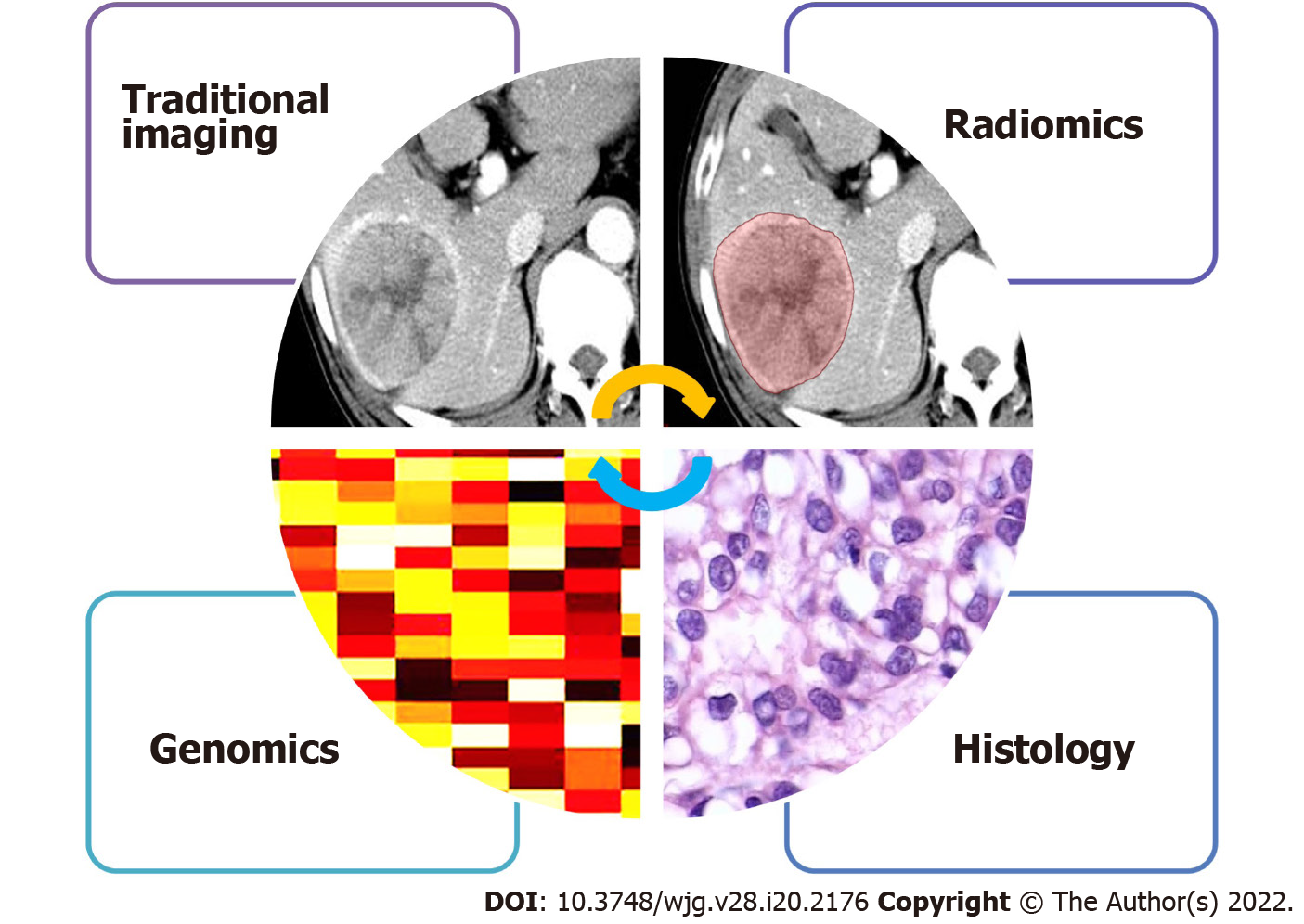
Data-driven radiomics is inherently unable to provide insights into the biological basis of the observed relationship. It is important to note that HCC shows large intra- and inter-tumor heterogeneity at the biological level. With the rapid development and popularization of machine learning, researchers are paying increasing attention to the improvement of prediction ability; however, they are far from understanding the biological significance of the observed research results. This disconnect between the predictive models and their biological significance inherently limits their widespread clinical translation. Therefore, it is necessary to introduce biological significance into the radiomics field through various available biological methods, such as genome association, immunohistochemical analyses, local microscopic pathological image texture analyses, and evaluation of macroscopic histopathological marker expression (Figure 1)[47].
Radiomics based on multiple imaging modalities has performed remarkably well in predicting the HCC-MVI status. In future, researchers need to focus on standardizing image segmentation and radiomics processing processes and the optimization of algorithms to further improve the accuracy of individualized prediction. Importantly, it may be possible to provide some new insights through combining deep learning and LI-RADS with radiomics and introducing biological significance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Granito A, Italy; Zhou X, China S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1643] [Article Influence: 205.4] [Reference Citation Analysis (0)] |
| 2. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3238] [Article Influence: 462.6] [Reference Citation Analysis (1)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6057] [Article Influence: 865.3] [Reference Citation Analysis (3)] |
| 4. | Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 6. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 658] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 7. | Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Witt JS, Rosenberg SA, Bassetti MF. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21:e74-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 10. | Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17 Suppl 2:S72-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 12. | Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 414] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 14. | Zheng J, Chakraborty J, Chapman WC, Gerst S, Gonen M, Pak LM, Jarnagin WR, DeMatteo RP, Do RKG, Simpson AL; Hepatopancreatobiliary Service in the Department of Surgery of the Memorial Sloan Kettering Cancer Center; Research Staff in the Department of Surgery at Washington University School of Medicine. Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma Using Quantitative Image Analysis. J Am Coll Surg. 2017;225:778-788.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Dong Y, Qiu Y, Yang D, Yu L, Zuo D, Zhang Q, Tian X, Wang WP, Jung EM. Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma. Clin Hemorheol Microcirc. 2021;77:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5550] [Article Influence: 616.7] [Reference Citation Analysis (3)] |
| 17. | Fransvea E, Paradiso A, Antonaci S, Giannelli G. HCC heterogeneity: molecular pathogenesis and clinical implications. Cell Oncol. 2009;31:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Friemel J, Rechsteiner M, Frick L, Böhm F, Struckmann K, Egger M, Moch H, Heikenwalder M, Weber A. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21:1951-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 19. | Lin DC, Mayakonda A, Dinh HQ, Huang P, Lin L, Liu X, Ding LW, Wang J, Berman BP, Song EW, Yin D, Koeffler HP. Genomic and Epigenomic Heterogeneity of Hepatocellular Carcinoma. Cancer Res. 2017;77:2255-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 20. | Zhu S, Hoshida Y. Molecular heterogeneity in hepatocellular carcinoma. Hepat Oncol. 2018;5:HEP10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150-R166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 816] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 22. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3026] [Article Influence: 432.3] [Reference Citation Analysis (3)] |
| 23. | Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 25. | Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 26. | Granito A, Galassi M, Piscaglia F, Romanini L, Lucidi V, Renzulli M, Borghi A, Grazioli L, Golfieri R, Bolondi L. Impact of gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance on the non-invasive diagnosis of small hepatocellular carcinoma: a prospective study. Aliment Pharmacol Ther. 2013;37:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Feng ST, Jia Y, Liao B, Huang B, Zhou Q, Li X, Wei K, Chen L, Li B, Wang W, Chen S, He X, Wang H, Peng S, Chen ZB, Tang M, Chen Z, Hou Y, Peng Z, Kuang M. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 28. | Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 29. | Nebbia G, Zhang Q, Arefan D, Zhao X, Wu S. Pre-operative Microvascular Invasion Prediction Using Multi-parametric Liver MRI Radiomics. J Digit Imaging. 2020;33:1376-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Hu HT, Wang Z, Huang XW, Chen SL, Zheng X, Ruan SM, Xie XY, Lu MD, Yu J, Tian J, Liang P, Wang W, Kuang M. Ultrasound-based radiomics score: a potential biomarker for the prediction of microvascular invasion in hepatocellular carcinoma. Eur Radiol. 2019;29:2890-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 31. | Dong Y, Zhou L, Xia W, Zhao XY, Zhang Q, Jian JM, Gao X, Wang WP. Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma: Initial Application of a Radiomic Algorithm Based on Grayscale Ultrasound Images. Front Oncol. 2020;10:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Hu HT, Shen SL, Wang Z, Shan QY, Huang XW, Zheng Q, Xie XY, Lu MD, Wang W, Kuang M. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY). 2018;43:3324-3330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Cho KJ, Choi NK, Shin MH, Chong AR. Clinical usefulness of FDG-PET in patients with hepatocellular carcinoma undergoing surgical resection. Ann Hepatobiliary Pancreat Surg. 2017;21:194-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Kaissis GA, Lohöfer FK, Hörl M, Heid I, Steiger K, Munoz-Alvarez KA, Schwaiger M, Rummeny EJ, Weichert W, Paprottka P, Braren R. Combined DCE-MRI- and FDG-PET enable histopathological grading prediction in a rat model of hepatocellular carcinoma. Eur J Radiol. 2020;124:108848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Li Y, Zhang Y, Fang Q, Zhang X, Hou P, Wu H, Wang X. Radiomics analysis of [18F]FDG PET/CT for microvascular invasion and prognosis prediction in very-early- and early-stage hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2021;48:2599-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Qiu Q, Duan J, Duan Z, Meng X, Ma C, Zhu J, Lu J, Liu T, Yin Y. Reproducibility and non-redundancy of radiomic features extracted from arterial phase CT scans in hepatocellular carcinoma patients: impact of tumor segmentation variability. Quant Imaging Med Surg. 2019;9:453-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3561] [Article Influence: 445.1] [Reference Citation Analysis (0)] |
| 38. | Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY). 2021;46:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Xu XF, Yu JJ, Xing H, Shen F, Yang T. How to better predict microvascular invasion and recurrence of hepatocellular carcinoma. J Hepatol. 2017;67:1119-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Leoni S, Piscaglia F, Granito A, Borghi A, Galassi M, Marinelli S, Terzi E, Bolondi L. Characterization of primary and recurrent nodules in liver cirrhosis using contrast-enhanced ultrasound: which vascular criteria should be adopted? Ultraschall Med. 2013;34:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Yang C, Liu X, Ling W, Song B, Liu F. Primary isolated hepatic tuberculosis mimicking small hepatocellular carcinoma: A case report. Medicine (Baltimore). 2020;99:e22580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH. LI-RADS Version 2017 vs Version 2018: Diagnosis of Hepatocellular Carcinoma on Gadoxetate Disodium-enhanced MRI. Radiology. 2019;292:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Lv K, Cao X, Dong Y, Geng D, Zhang J. CT/MRI LI-RADS version 2018 vs CEUS LI-RADS version 2017 in the diagnosis of primary hepatic nodules in patients with high-risk hepatocellular carcinoma. Ann Transl Med. 2021;9:1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Zhang T, Huang ZX, Wei Y, Jiang HY, Chen J, Liu XJ, Cao LK, Duan T, He XP, Xia CC, Song B. Hepatocellular carcinoma: Can LI-RADS v2017 with gadoxetic-acid enhancement magnetic resonance and diffusion-weighted imaging improve diagnostic accuracy? World J Gastroenterol. 2019;25:622-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Zhou H, Sun J, Jiang T, Wu J, Li Q, Zhang C, Zhang Y, Cao J, Sun Y, Jiang Y, Liu Y, Zhou X, Huang P. A Nomogram Based on Combining Clinical Features and Contrast Enhanced Ultrasound LI-RADS Improves Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Front Oncol. 2021;11:699290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Parekh VS, Jacobs MA. Deep learning and radiomics in precision medicine. Expert Rev Precis Med Drug Dev. 2019;4:59-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 47. | Tomaszewski MR, Gillies RJ. The Biological Meaning of Radiomic Features. Radiology. 2021;298:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 350] [Article Influence: 87.5] [Reference Citation Analysis (0)] |