Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2163
Peer-review started: October 27, 2021
First decision: December 27, 2021
Revised: December 31, 2021
Accepted: April 15, 2022
Article in press: April 15, 2022
Published online: May 28, 2022
Processing time: 212 Days and 0.3 Hours
Pancreatic neuroendocrine neoplasms (PanNENs) are rare neoplasms with strong heterogeneity that have experienced an increasing incidence rate in recent years. For patients with locally advanced or distant metastatic PanNENs, systemic treatment options vary due to the different differentiations, grades and stages. The available options for systemic therapy include somatostatin analogs, mole-cularly targeted agents, cytotoxic chemotherapeutic agents, immune checkpoint inhibitors, and peptide receptor radionuclide therapy. In addition, the development of novel molecularly targeted agents is currently in progress. The sequence of selection between different chemotherapy regimens has been of great interest, and resistance to chemotherapeutic agents is the major limitation in their clinical application. Novel agents and high-level clinical evidence continue to emerge in the field of antiangiogenic agents. Peptide receptor radionuclide therapy is increasingly employed for the treatment of advanced neuroendocrine tumors, and greater therapeutic efficacy may be achieved by emerging radio-labeled peptides. Since immune checkpoint inhibitor monotherapies for PanNENs appear to have limited antitumor activity, dual immune checkpoint inhibitor therapies or combinations of antiangiogenic therapies and immune checkpoint inhibitors have been applied in the clinic to improve clinical efficacy. Combining the use of a variety of agents with different mechanisms of action provides new possibilities for clinical treatments. In the future, the study of systemic therapies will continue to focus on the screening of the optimal benefit population and the selection of the best treatment sequence strategy with the aim of truly achieving individualized precise treatment of PanNENs.
Core Tip: Pancreatic neuroendocrine neoplasms (PanNENs) are rare neoplasms with strong heterogeneity. The systemic treatment options of advanced PanNENs vary due to the different differentiations, grades and stages and include somatostatin analogs, molecularly targeted agents, cytotoxic chemotherapeutic agents, immune checkpoint inhibitors, and peptide receptor radionuclide therapy. Despite the multiple systemic therapeutic options for PanNENs, problems such as drug resistance, adverse side effects and limited scope of application still exist. Thus, we review the clinical development of medical treatment options, focusing on ongoing clinical studies and the development of novel targeted drugs, to provide a reference for clinicians and researchers.
- Citation: Li YL, Cheng ZX, Yu FH, Tian C, Tan HY. Advances in medical treatment for pancreatic neuroendocrine neoplasms. World J Gastroenterol 2022; 28(20): 2163-2175
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2163
Neuroendocrine neoplasms (NENs) are a group of rare neoplasms originating from neuroendocrine cells. However, with the development of diagnostic techniques and improvements in the clinical understanding of these tumors, the incidence is increasing yearly. Based on the United States Surveillance, Epidemiology, and End Results database, the age-adjusted incidence of NENs has increased nearly 6.4-fold from 1.09 per 100000 persons in 1973 to 6.98 per 100000 in 2012. The pancreas (0.84 per 100000 persons) is one of the most common primary sites of NENs, ranked after the lung, small intestine, and rectum[1]. In countries such as China and India, pancreatic NENs (PanNETs) are the most common gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and have the highest morbidity[2].
PanNENs are highly heterogeneous neoplasms that appear as various clinical manifestations and biological behaviors. PanNENs can be classified as nonfunctional PanNENs (60%-90%) or functional PanNETs based on the absence or presence of symptoms associated with the overproduction of specific hormones, respectively[3]. The most common functional PanNETs are gastrinomas and insulinomas; less frequent are glucagonomas, somatostatinomas, vasoactive intestinal polypeptide-secreting tumors, and adrenocorticotropic hormone-secreting tumors[4-7]. The majority of PanNETs are sporadic, although PanNETs can occur as part of hereditary multiple tumor syndromes, such as multiple endocrine neoplasia type 1 and von Hippel-Lindau (VHL) disease[8]. PanNENs are classified into three main histological categories according to the 2019 World Health Organization Classification of Tumors of the Digestive System (5th edition): well-differentiated neuroendocrine tumors (NETs), poorly differentiated neuroendocrine carcinomas (NECs), and mixed neuroendocrine-non-neuroendocrine neoplasms. NETs are further classified into three grades (G1, G2, and G3) according to their mitotic rate (mitoses/2 mm2) and Ki-67 proliferation index[9]. PanNENs clinical staging is currently based on the eighth edition of the American Joint Committee on Cancer tumor node metastasis (TNM) staging system. Well-differentiated PanNETs are staged by PanNENs TNMs, whereas poorly differentiated PanNECs are staged according to the TNM staging of pancreatic cancer[10].
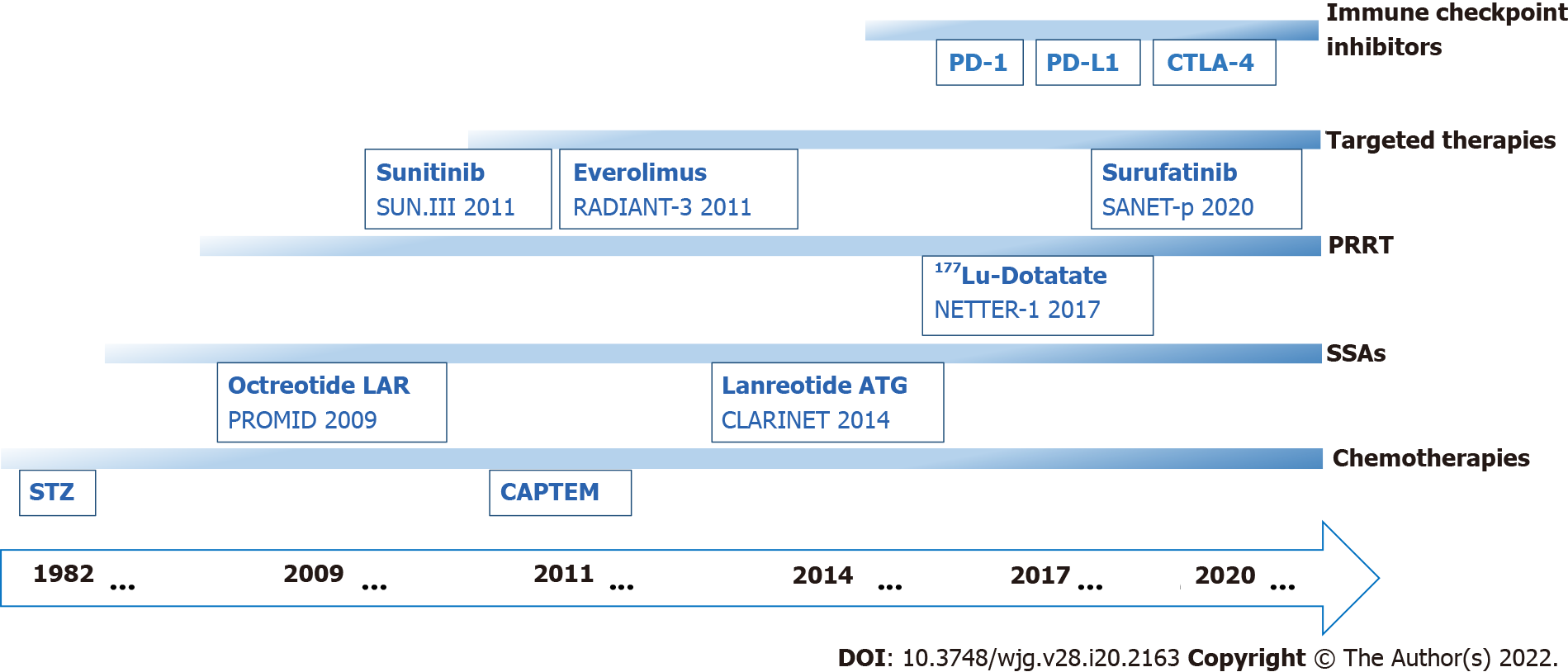
Therapeutic strategies vary due to the different grades and stages of PanNENs. Radical surgery is the primary treatment option for patients with locally resectable PanNENs; however, 40%-50% of NENs present with distant metastases at the time of initial diagnosis, limiting the opportunity for surgical resection. Moreover, many patients with resected PanNENs will develop recurrence with distant metastases[11,12]. Systemic therapeutic options for patients with locally advanced or distant metastatic PanNENs involve somatostatin analogs (SSAs), molecularly targeted agents, cytotoxic chemotherapeutic agents, immune checkpoint inhibitors, and peptide receptor radionuclide therapy (PRRT) (Figure 1).
The therapeutic goals for patients with locally unresectable and metastatic PanNENs include both hormone control and anti-tumor therapy. The scope of this article does not include medical treatments for the control of functional PanNENs hormone-related symptoms, but rather focuses on anti-tumor therapy. Despite the multiple systemic therapeutic options for PanNENs, problems such as drug resistance, adverse side effects and limited scope of application still exist, and clinical needs have not been met. Thus, conquering drug resistance, expanding the scope of application and developing new clinical drugs have been the main focus of researchers in recent decades. This article reviews the clinical development of existing drugs, focusing on ongoing clinical studies and the development of novel targeted drugs, to provide a reference for addressing the current clinical treatment dilemma and the future direction of PanNENs drug research.
Systemic treatment with chemotherapy is one of the main therapies for advanced PanNENs. Appropriate chemotherapy regimens are delivered according to pathological classification and grades, mainly including temozolomide-based combination regimens and platinum-based regimens. Among them, temozolomide-based combination chemotherapy (CAPTEM and STEM) regimens can be used for the first-line treatment of patients with advanced PanNETs G2/G3[19-21] and for the second-line treatment of patients with PanNECs[22,23]. The synergistic effect of capecitabine in combination with temozolomide chemotherapy may be due to its ability to deplete O6-methylguanine DNA methyltransferase (MGMT) levels in tumor cells, thereby enhancing the alkylating effect of temozolomide[24]. The association between MGMT expression status and temozolomide efficacy has been demonstrated in other tumor types, and several studies in NENs have suggested that MGMT promoter methylation or low protein expression correlates better with a favorable therapeutic response to temozolomide and that MGMT status can be used as a biological indicator of the response to alkylating agent treatment in NENs[25,26]. However, because most of the current studies on the relationship between MGMT status and temozolomide efficacy are small-sample, retrospective studies, there is some controversy. A prospective study (NCT03217097) of the relationship between MGMT status and temozolomide efficacy in NETs is underway in which patients with advanced NETs have been divided into two groups according to whether they have MGMT methylation and are receiving temozolomide or oxaliplatin-based chemotherapy at a 1:1 (unmethylated group) and 2:1 (methylated group) ratio, respectively. ORR was used as the primary outcome indicator[27]. Additional prospective studies (NCT02698410, NCT01824875, NCT01525082) have evaluated MGMT status as a secondary outcome.
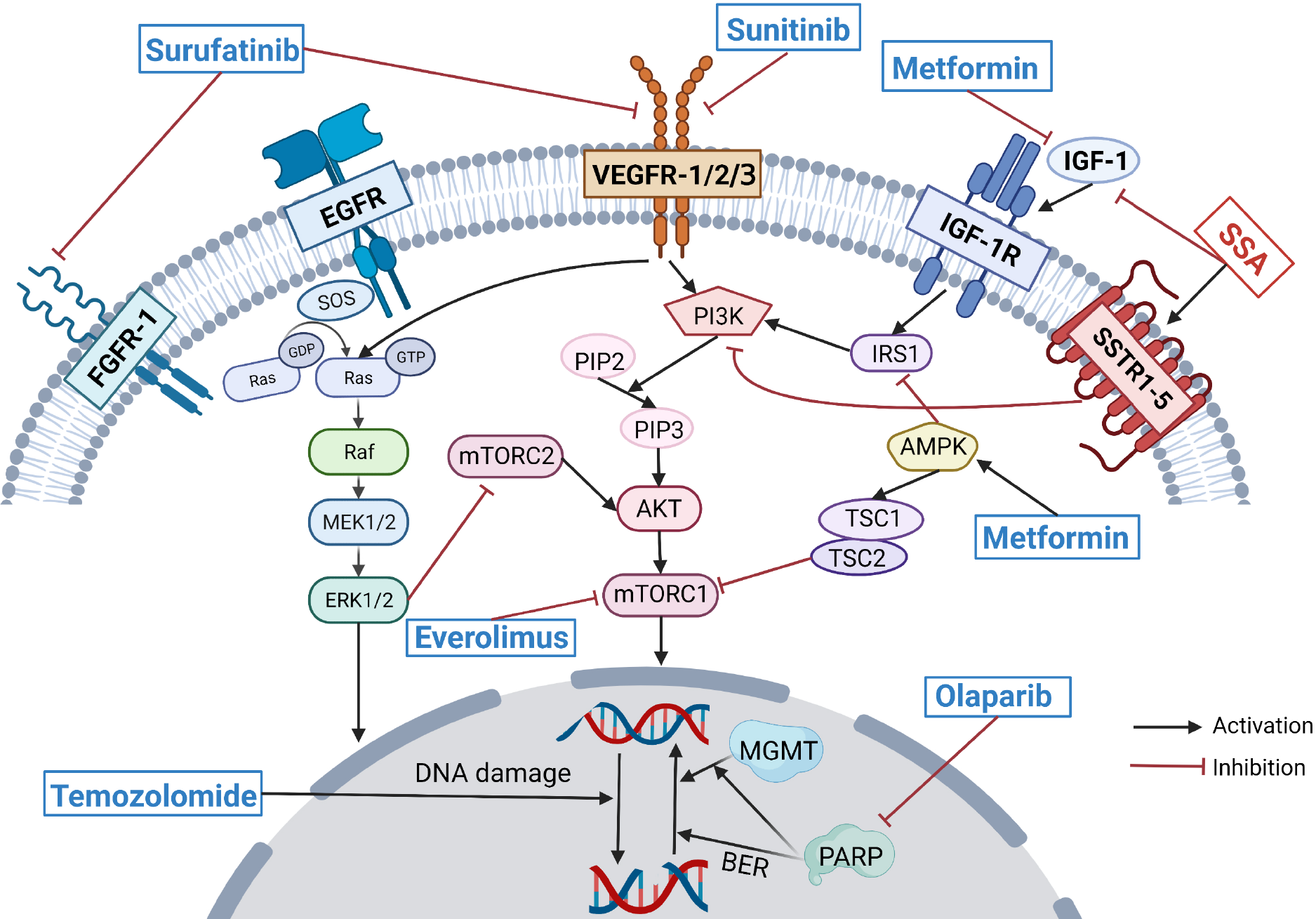
In clinical practice, drug resistance is one of the major obstacles to the effective treatment of temozolomide-based chemotherapy in PanNENs. Current studies on temozolomide resistance mechanisms are mainly conducted in the field of glioma. In addition to the overexpression of MGMT, the overexpression of the base excision repair (BER) pathway, alteration of autophagy, and activation of the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway contribute to acquired temozolomide resistance[28]. Wu et al[29] found enhanced temozolomide sensitivity in a preclinical study of glioblastoma multiforme (GBM) that poly (ADP-ribose) polymerase (PARP) inhibitors, particularly in MGMT-unmethylated GBM, and this effect may be mediated by inhibition of MGMT-mediated repair by abolishing MGMT function and inhibition of BER-mediated repair (Figure 2). Therefore, the combination of PARP inhibitors and temozolomide may be one solution for overcoming temozolomide resistance, and clinical trials applying this combination regimen in patients with small-cell lung cancer have yielded good clinical results[30]. A phase II clinical trial (NCT04394858) is currently evaluating the efficacy of single-agent temozolomide vs the combination of temozolomide and olaparib in patients with advanced pheochromocytoma and paraganglioma; however, no clinical trials of temozolomide in combination with PARP inhibitors for PanNENs have been conducted. Temozolomide resistance has also been observed to be associated with activation of the PI3K/AKT/mTOR pathway, and inhibition of the PI3K/AKT/mTOR pathway sensitizes tumor cells to apoptosis upon temozolomide treatment[31]. In a prospective phase II clinical trial, temozolomide in combination with everolimus as second-line therapy for advanced PanNETs resulted in an ORR of 40% and a median PFS of 15.4 mo, with no synergistic toxicity observed[32].
The most frequent chemotherapy schemes in advanced PanNECs are platinum-based (EP, EC) regimens for first-line therapy and FOLFOX, FOLFIRI, or CAPTEM regimens for second-line therapy[19,33,34]. For other options of first-line treatment regimens for PanNECs, a prospective study (NCT04325425) is evaluating the mFOLFIRINOX regimen vs the EP/EC regimen for advanced gastroenteropancreatic neuroendocrine carcinomas (GEP-NECs) as first-line therapy. Another study (NCT03387592) is evaluating the efficiency of the CAPTEM regimen vs the FOLFIRI regimen as second-line therapy for advanced NECs. These prospective clinical studies provide additional treatment options for patients with advanced PanNECs and high-level evidence for clinical decision-making.
SSAs, with their antisecretory and antiproliferative effects, are some of the most common first-line treatments for patients with advanced PanNENs and can be used to control hormonal symptoms in patients with functional PanNETs[35] and for patients with advanced nonfunctional PanNENs to play an antiproliferative role. SSAs are primary treatment options for patients with PanNETs-G1/G2, Ki-67 < 10%, SSTR-positive, and slow-growing tumors[11-14,19,36]. The most widely used clinical SSAs include octreotide LAR and lanreotide autogel, both of which bind primarily to SSTR2 and SSTR5.
Several investigators have also evaluated the efficacy of SSAs in exerting antitumor effects in PanNENs with Ki-67 ≥ 10%. A multicenter retrospective study included advanced, well-differentiated PanNETs with Ki-67 of 10%-35% receiving first-line, long-acting SSAs and observed that SSAs still exert antiproliferative activity against PanNETs with Ki-67 ≥ 10% but have limited effect in PanNETs with high grade (G3) and hepatic tumor load > 25%[37].
In clinical practice, using SSAs at nonconventional high doses (increased administered dose or reduced administration interval) is a common empirical option in patients with progression PanNETs on a standard SSA first-line treatment[38]. A multicenter retrospective study of nonconventional doses of SSAs for GEP-NETs showed that in well-differentiated G1/G2 GEP-NETs, the overall median PFS was 31 mo with high-dose SSA (HD-SSA) therapy after progression on a standard SSA therapy, suggesting that HD-SSAs are active and safe treatment options in patients with progressive well-differentiated GEP-NETs[39]. A prospective single-arm phase II study (NCT02651987) assessed the efficacy of increasing the dose frequency of 120 mg lanreotide autogel (LAN) in 48 patients with progressive G1/G2 PanNETs and showed that 120 mg LAN every 14 d in PanNETs (progressive on standard 120 mg LAN every 28 d) produced promising PFS and DCR, especially in patients with Ki-67 ≤ 10%[40].
In addition, SSAs in combination with other agents (chemotherapy agents, antiangiogenic therapies, everolimus, immune checkpoint inhibitors, etc.) are also frequent second-line treatment options in real-world studies[41]. SSA combination therapy may have better efficacy in well-differentiated PanNETs with Ki-67 > 10% or high tumor burden, and a prospective multicenter phase II clinical study (NCT02231762) evaluating the efficacy of the combination of LAN and temozolomide in patients with advanced GEP-NETs is being conducted.
The efficacy of SSAs is correlated with the expression of somatostatin receptors on the tumor cell surface[38]. DNA methyltransferase inhibitors and histone deacetylase inhibitors were observed to upregulate SSTR2 expression in NET cell lines in some preclinical studies and could potentially be an option for overcoming SSA resistance, but the lack of progress in clinical studies requires further validation[42,43]. In clinical management, it is essential to identify the population favorable for SSA therapy, and SSAs in combination with other agents are expected to be synergistic in patients with higher grade, higher tumor load, and SSTR-positive PanNETs.
The targeted therapies currently used in PanNETs mainly include mammalian target of rapamycin (mTOR) inhibitors and antiangiogenic agents. FDA-approved target agents for the treatment of PanNENs include everolimus (mTOR inhibitor) and sunitinib (tyrosine kinase inhibitor)[12,19], and the recommendation of surufatinib (tyrosine kinase inhibitor) has been added to the Chinese guidelines for the diagnosis and treatment of pancreatic neuroendocrine neoplasms (2020)[36].
Everolimus, a mTOR inhibitor, has been shown to inhibit tumor cell growth through suppression of the PI3K/AKT/mTOR pathway. The primary results of the RADIANT-3 trial reported that patients with advanced PanNETs in the everolimus group had a median PFS of 11 mo, with a 6.4-mo increase vs the placebo group, and the safety profile of everolimus was good[16]. Everolimus resistance is thought to be related to its ability to inhibit only partial mTORC1 but not mTORC2, thus causing over-activation of upstream signaling, including PI3K/AKT[44]. Thus, both the dual PI3K/mTOR inhibitor BEZ235 and the dual inhibitor of mTORC1 and mTORC2, CC-223, are thought to have the potential to overcome everolimus resistance, but the phase II clinical trial of BEZ235 failed due to severe adverse effects[45]. The results of a phase II clinical trial of CC-233 in nonpancreatic NETs patients who failed first-line therapy suggested a DCR of 90% (95%CI: 76.9-97.3%) and a median PFS of 19.5 mo (95%CI: 10.4-28.5 mo), with a safety profile comparable with that of everolimus, but its use in PanNET patients still needs to be prospectively explored[46].
Several preclinical studies suggest that SSAs can inhibit insulin-like growth factor-1 (IGF-1), an upstream signal of the PI3K/AKT/mTOR pathway (Figure 2), and LAN was observed to reduce the survival rate of everolimus-resistant cell lines. SSAs may be able to overcome resistance to everolimus[38,47]. Unfortunately, in the COOPERATE-2 study of everolimus in combination with pasireotide LAR vs everolimus monotherapy in patients with advanced PanNETs, no prolongation of PFS was observed in the combination group vs the monotherapy group[48]. However, the trial extension results of the LUNA study of everolimus in combination with pasireotide LAR for advanced pulmonary and thymic NETs suggested that the everolimus combined with pasireotide group had a significantly longer median PFS than the pasireotide monotherapy group and the everolimus monotherapy group (8.51 mo vs 12.48 mo vs 16.53 mo)[49]. The Japan Clinical Oncology Group is also conducting a multicenter, randomized, controlled, phase III trial (jRCT1031200023) to confirm the superiority of combined everolimus plus lanreotide therapy over everolimus monotherapy for advanced GEP-NETs[50].
Resistance to everolimus may also be overcome by combined metformin therapy. Pusceddu et al[51] conducted a retrospective analysis of 445 patients with advanced PanNETs treated with everolimus and/or SSAs in 24 medical centers in Italy and observed that the median PFS of 44.2 mo was significantly longer in patients treated with metformin glucose-lowering therapy than in nondiabetic patients (15.1 mo) and longer than that in diabetic patients receiving other glucose-lowering treatments (20.8 mo). Metformin is associated with increased PFS in patients treated with SSA and in patients treated with everolimus (with or without SSAs). A preclinical study showed that metformin may induce more effective mTOR blockade through its effects on IGF-1 and adenosine 5’-monophosphate-activated protein kinase and may counteract the resistance mechanism triggered by everolimus (Figure 2)[52]. Because both metformin and SSA were observed to have the potential to overcome everolimus resistance in preclinical studies, a prospective study evaluating the efficacy of the triple-drug combination of everolimus, octreotide, and metformin for the treatment of advanced PanNENs (NCT02294006) is underway.
Antiangiogenic agents include tyrosine kinase inhibitors (TKIs) and non-TKI agents such as bevacizumab [anti-vascular endothelial growth factor (VEGF) monoclonal antibody]. PanNETs are highly vascularized with overexpression of proangiogenic factors such as VEGF, so antitumor angiogenesis is an effective therapeutic approach[53]. Antiangiogenic agents have evolved rapidly in the field of PanNET therapy in recent years, with new agents and high-grade clinical evidence emerging. Sunitinib, a polytyrosine kinase inhibitor, became the first antiangiogenic agent approved by the FDA for the treatment of patients with advanced PanNETs based on favorable results from a randomized double-blind phase III clinical trial[15]. Surufatinib is a new multitargeted TKI that blocks tumor angiogenesis by inhibiting both vascular endothelial growth factor receptor-1/2/3 (VEGFR-1/2/3) and fibroblast growth factor receptor-1 (FGFR-1) (Figure 2) and regulates tumor-associated macrophages to promote the immune response of the body to tumor cells by inhibiting colony-stimulating factor-1 receptor[54]. The SANET-p study, a phase III clinical trial of surufatinib in patients with advanced PanNETs, suggested a significant prolongation of median PFS (10.9 mo vs 3.7 mo) and improvement of ORR (19% vs 2%) in the surufatinib group compared with the placebo group[17]. The results from the PanNET cohort of the phase II cohort study (NCT01466036) of cabozantinib, a multitargeted tyrosine kinase inhibitor targeting VEGFR2 and cellular-mesenchymal epithelial transition factor, suggested a median PFS of 21.8 mo (95%CI: 8.5-32.0 mo) and an ORR of 15% (95%CI: 5%-36%)[55]. This result has raised expectations for the publication of the results of the ongoing randomized, double-blind phase III clinical trial of cabozantinib (NCT03375320). The TALENT study (GETNE1509) is a phase II clinical study of the VEGFR1-3 and FGFR1-4 inhibitors lenvatinib in which good clinical efficacy was observed in the PanNET cohort with a tolerable safety profile[56]. In addition, the TALENT study quantified a series of proangiogenic factors, such as VEGF-A, angiopoietin-2 (Ang2), and VEGFR-2. The results suggested that high Ang2 Levels and low FGF2 Levels were significantly associated with ORR, and Ang2 and VEGFR-2 Levels in patients treated with sunitinib could predict the efficacy of lenvatinib, confirming that biomarkers can not only predict drug efficacy but also provide a reference for patient sequential therapy selection[57]. Axitinib[58] and pazopanib[59] have also demonstrated efficacy in phase II clinical trials for the treatment of NETs. Clinical advances in antiangiogenic agents have brought more new options for the treatment of advanced PanNETs.
With the advent of the immunotherapy era, phase I/II clinical trials of various immune checkpoint inhibitors such as programmed cell death-ligand 1 (PD-L1) inhibitors, programmed cell death-1 (PD-1) inhibitors and cytotoxic T-lymphocyte antigen 4 inhibitors have been widely conducted and rapidly developed in the PanNEN field. The results of a phase Ib study of toripalimab as a second-line regimen for the treatment of patients with advanced NENs suggested an ORR of 22.2% and a DCR of 55.5% in the PanNEN subgroup and observed that patients with positive PD-L1 expression, TMB-H (top 10%) and/or MSI-H positivity may preferentially benefit from the treatment[60]. The results of some current clinical trials have shown no significant benefit observed in patients with NENs treated with immune checkpoint inhibitors alone[61,62], and dual immunotherapy with PD-L1/PD-1 inhibitors in combination with CLTA-4 inhibitors is starting to gain interest. The DUNE study (GETNE 1601) is a phase II multicohort clinical study assessing the efficacy of durvalumab in combination with tremelimumab for the treatment of advanced GEP-NENs and pulmonary NETs, with recent results suggesting an ORR of 6.3% for the G1/G2 grade PanNET cohort, 9.1% for the G3 grade GEP-NEN cohort, and 7.4% and 0% for the pulmonary NET and giNET cohorts respectively[63]. A multicohort phase II clinical trial (NCT02923934) assessed the efficacy of ipilimumab in combination with nivolumab in NETs, with the latest results suggesting an ORR of 25% for the NET cohort (20 pts), and the study is still ongoing[64]. Clinical trials of immune checkpoint inhibitors in combination with other agents (antiangiogenic agents, chemotherapy, PRRT, and SSAs) continue to emerge, and some current clinical studies of immunotherapy in combination with antiangiogenic agents suggest better efficacy. An ORR of 20% and a median PFS of 3.94 mo were observed in a phase II clinical trial of toripalimab in combination with surufatinib in patients with advanced NECs after the failure of first-line chemotherapy. Surufatinib in combination with toripalimab has been suggested as a second-line treatment option for patients with advanced NECs[65], and a phase III clinical trial (NCT05015621) is evaluating the efficacy of the combination of surufatinib and toripalimab versus FOLFIRI regimen as a second-line treatment option for patients with advanced NECs. Clinical trials of immunotherapy in combination with other therapies being conducted in patients with NENs are shown in Table 1.
| Drugs | Population | n | Phase | Primary outcomes | NCT number |
| Surufatinib + toripalimab vs FOLFIRI | NEC | 194 | III | OS | NCT05015621 |
| Penpulimab + anlotinib | NET | 150 | II | ORR | NCT04207463 |
| Pembrolizumab + liver-directed/PRRT | NET | 32 | ORR | NCT03457948 | |
| Nivolumab + chemotherapy | NEN G3 | 38 | II | OS | NCT03980925 |
| Toripalimab + FOLFSIM vs EP/EC | Advanced NEC | 336 | II/III | OS | NCT03992911 |
| Nivolumab + ipilimumab + cabozantinib | PD-NET | 30 | II | ORR | NCT04079712 |
| Pembrolizumab + lanreotide depot | GEP-NET | 22 | Ib/II | ORR | NCT03043664 |
PRRT has been widely used in the treatment of NETs in Europe, the USA and Asia[66]. 177Lu-DOTATATE has been used for more than a decade, but clinical studies of PRRT are still dominated by phase I/II clinical trials, and there is a lack of phase III clinical trials with large samples for NETs. The NETTER-1 study, a pivotal phase III clinical study of PRRT, treated two groups of midgut NETs with
In addition to common targets such as SSTR2, mTOR, and VEGFR, researchers are actively exploring new targeted agents for clinical application in the field of PanNENs. Cyclin-dependent kinases (CDKs), which regulate cell cycle progression, have been considered promising new targets for tumor therapy. Palbociclib, a small molecule compound that specifically inhibits CDK4 and CDK6, induced G1 phase blockade in Rb-positive CDK4-overexpressing PanNET cell lines in an in vitro assay, blocking and inhibiting the growth of PanNET cell lines[71,72]. However, the results of a phase II clinical trial of palbociclib for G1/G2 grade PanNETs suggested a median PFS of only 2.6 mo (95%CI: 0-14.4)[71]. ONC201, an agent with specific targeting of the dopamine-like DRD2 receptor and the mitochondrial protease ClpP, inhibits the growth of PanNET cell lines by TRAIL/DR5 upregulation, dual AKT/ERK pathway inhibition and promotion of a comprehensive stress response to exerting anticancer effects. The phase II study of ONC201 for NETs (NCT03034200) included 10 patients with metastatic PC-PGs (Group A) and 12 patients with other neuroendocrine tumors (Group B), and the latest results of this study suggested 5 PRs and 2 SDs in Group A and 1 PR and 2 SDs in Group B. A full outcome report is still pending[73]. Antibody-coupled drugs developed against SSTR targets have received much attention. PEN-221, an antibody-drug conjugate (ADC) targeting SSTR2, connects the cytotoxic microtubule protein inhibitor DM1 to Tyr3-octreotate and can rapidly internalize DM1 into SSTR2-expressing cells and exert cytotoxic effects after binding to SSTR2[74]. A phase II clinical study of PEN221 in SSTR2-positive gastrointestinal NETs is ongoing, and the latest results suggested that 23 (88.5%) of 26 evaluable patients were assessed as SD, with a median PFS of 9 mo[75]. Belzutifan is a second-generation small-molecule hypoxia-inducible factor (HIF)-2α inhibitor, which is recommended in the latest NCCN guidelines for advanced PanNET patients with germline VHL alteration[19]. In a phase II, open-label, single-group trial of belzutifan in patients with renal cell carcinoma associated with VHL disease, 22 patients with PanNETs were included, among them 20 patients (91%) had a confirmed response (including 3 patients (14%) who had a complete response)[76]. Phase I/II clinical trials of new targeted agents such as ATR inhibitors, DLL3 inhibitors, HDAC inhibitors, and PARP inhibitors applied to NETs are currently underway (Table 2), but there is still a long way to go before clinical application.
| Drugs | Targets | Population | n | Phase | Primary outcomes | NCT number |
| Ribociclib + Everolimus | CDK4/6 Inhibitor | Advanced NET | 21 | II | PFS | NCT03070301 |
| Abemaciclib | CDK4/6 Inhibitor | Advanced GEP-NET | 37 | II | ORR | NCT03891784 |
| BAY 1895344 | ATR Kinase Inhibitor | SCLC/PD-NEC/PDA | 87 | I | MTD, AEs | NCT04514497 |
| Lurbinectedin, berzosertib | ATR Kinase Inhibitor | SCLC/HGNEC | 75 | I/II | MTD, ORR | NCT04802174 |
| BI 764532 | DLL3 Inhibitor | SCLC/NEN Expressing DLL3 | 110 | I | MTD | NCT04429087 |
| Entinostat | HDAC Inhibitor | Abdominal NET | 40 | II | ORR | NCT03211988 |
| Niraparib + dostarlimab | PARP Inhibitor | SCLC/HGNEC | 48 | PFS, ORR | NCT04701307 |
Although there are various treatments for PanNENs, choosing appropriate therapeutic schemes for different patients in terms of pathological classification, grades and stages is still difficult. The selection of a cytotoxic chemotherapy regimen is a concern of researchers, and the results of prospective studies may help to address it. First, the problem of drug resistance facing CAPTEM regimen, the most widely used plan, will probably be solved by the combination of PARP inhibitors and mTOR inhibitors, although more evidence is needed. Due to the limited effect of SSAs on inhibiting cancer cell proliferation, progress has mainly been made in the selection of appropriate patients and the synergistic effect of combination therapy. Many new antiangiogenic agents and high-level clinical evidence have emerged, and the reversal of drug resistance to everolimus has also advanced. The use of PRRT in the treatment of NETs is increasingly common, and new radiolabeled peptides appear to be more effective. Immunotherapy faces challenges, and the better curative effect of dual immune checkpoint inhibitor therapies and the combinations of immune checkpoint inhibitors plus other agents need further investigation. Although there are limited choices in the treatment of PanNENs, the combination of common medicines such as SSAs, cytotoxic chemotherapy, everolimus, sunitinib and immune checkpoint inhibitors can have synergistic effects or alleviate drug resistance, thus bringing new vitality to the treatment of PanNENs. The development of biomarkers in clinical research provides a reference for the prediction of curative effects and the selection of sequential therapy, and biomarker-directed therapy helps in choosing appropriate medicines for different people. Advances in the search for new targets and the use of new medicines on common targets will provide more choices for the treatment of PanNENs, but applying them in clinical practice will still take time. Further investigation of PRRT and immunotherapy and the development of new targeted agents will be the focus of future research, and the progress made in the reversal of drug resistance will help clinical practice. In addition, the selection of appropriate therapeutic schemes for different patients and the execution of individualized and precise therapy will be a continuous concern for researchers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujimori N, Japan; Maurea S, Italy S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 2. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 4. | Wu W, Jin G, Li H, Miao Y, Wang C, Liang T, Ou J, Zhao Y, Yuan C, Li Y, Lou W, Wu Z, Qin R, Wang H, Hao J, Yu X, Huang H, Tan G, Liu X, Xu K, Wang L, Yang Y, Hao C, Wang W, Guo K, Wei J, Wang Y, Peng C, Wang X, Cai S, Jiang J, Wu X, Li F, Pancreatic SSGO. The current surgical treatment of pancreatic neuroendocrine neoplasms in China: a national wide cross-sectional study. J Pancreatol. 2019;2:35-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez A, Llanos-Muñoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jiménez-Fonseca P, Teulé A, Sastre-Valera J, Benavent-Viñuelas M, Monleon A, Salazar R. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010;21:1794-1803. [PubMed] [DOI] [Full Text] |
| 8. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 10. | Doescher J, Veit JA, Hoffmann TK. [The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery]. HNO. 2017;65:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O'Dorisio TM, Halperin DM, Fishbein L, Eads J, Hope TA, Singh S, Salem R, Metz DC, Naraev BG, Reidy-Lagunes DL, Howe JR, Pommier RF, Menda Y, Chan JA. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:863-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 12. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 743] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 13. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1742] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 14. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 15. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 16. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 17. | Xu J, Shen L, Bai C, Wang W, Li J, Yu X, Li Z, Li E, Yuan X, Chi Y, Yin Y, Lou W, Xu N, Bai Y, Zhang T, Xiu D, Wang X, Yuan Y, Chen J, Qin S, Jia R, Lu M, Cheng Y, Zhou Z, He J, Su W. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2239] [Article Influence: 279.9] [Reference Citation Analysis (0)] |
| 19. | Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, Giordano T, Halfdanarson TR, Halperin D, He J, Heaney A, Heslin M J, Kandeel F, Kardan A, Khan SA, Kuvshinoff BW, Lieu C, Miller K, Pillarisetty VG, Reidy D, Salgado SA, Shaheen S, Soares HP, Soulen MC, Strosberg JR, Sussman CR, Trikalinos NA, Uboha NA, Vijayvergia N, Wong T, Lynn B, Hochstetler C. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors Version 4; 2021. Available from: https://www.nccn.org/Login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. |
| 20. | Zhao J, Zhao H, Chi Y. Safety and Efficacy of the S-1/Temozolomide Regimen in Patients with Metastatic Neuroendocrine Tumors. Neuroendocrinology. 2018;106:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 549] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 22. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 23. | Rogowski W, Wachuła E, Gorzelak A, Lebiedzińska A, Sulżyc-Bielicka V, Iżycka-Świeszewska E, Żołnierek J, Kos-Kudła B. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol. 2019;70:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Chatzellis E, Angelousi A, Daskalakis K, Tsoli M, Alexandraki KI, Wachuła E, Meirovitz A, Maimon O, Grozinsky-Glasberg S, Gross D, Kos-Kudła B, Koumarianou A, Kaltsas G. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology. 2019;109:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Trillo Aliaga P, Spada F, Peveri G, Bagnardi V, Fumagalli C, Laffi A, Rubino M, Gervaso L, Guerini Rocco E, Pisa E, Curigliano G, Fazio N. Should temozolomide be used on the basis of O6-methylguanine DNA methyltransferase status in patients with advanced neuroendocrine tumors? Cancer Treat Rev. 2021;99:102261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Qi Z, Tan H. Association between MGMT status and response to alkylating agents in patients with neuroendocrine neoplasms: a systematic review and meta-analysis. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Lemelin A, Barritault M, Hervieu V, Payen L, Péron J, Couvelard A, Cros J, Scoazec JY, Bin S, Villeneuve L, Lombard-Bohas C, Walter T; MGMT-NET investigators. O6-methylguanine-DNA methyltransferase (MGMT) status in neuroendocrine tumors: a randomized phase II study (MGMT-NET). Dig Liver Dis. 2019;51:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Yan Y, Xu Z, Dai S, Qian L, Sun L, Gong Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res. 2016;35:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 29. | Wu S, Li X, Gao F, de Groot JF, Koul D, Yung WKA. PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma. Neuro Oncol. 2021;23:920-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 30. | Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, Myers DT, Morris R, Kem M, Dubash TD, Kennedy EA, Digumarthy SR, Sequist LV, Hata AN, Maheswaran S, Haber DA, Lawrence MS, Shaw AT, Mino-Kenudson M, Dyson NJ, Drapkin BJ. Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer. Cancer Discov. 2019;9:1372-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 31. | Zając A, Sumorek-Wiadro J, Langner E, Wertel I, Maciejczyk A, Pawlikowska-Pawlęga B, Pawelec J, Wasiak M, Hułas-Stasiak M, Bądziul D, Rzeski W, Reichert M, Jakubowicz-Gil J. Involvement of PI3K Pathway in Glioma Cell Resistance to Temozolomide Treatment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Chan JA, Blaszkowsky L, Stuart K, Zhu AX, Allen J, Wadlow R, Ryan DP, Meyerhardt J, Gonzalez M, Regan E, Zheng H, Kulke MH. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. 2013;119:3212-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Bardasi C, Spallanzani A, Benatti S, Spada F, Laffi A, Antonuzzo L, Lavacchi D, Marconcini R, Ferrari M, Rimini M, Caputo F, Santini C, Cerma K, Casadei-Gardini A, Andrikou K, Salati M, Bertolini F, Fontana A, Dominici M, Luppi G, Gelsomino F. Irinotecan-based chemotherapy in extrapulmonary neuroendocrine carcinomas: survival and safety data from a multicentric Italian experience. Endocrine. 2021;74:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, Loriot Y, Duvillard P, Chougnet CN, Déandréis D, Schlumberger M, Borget I, Ducreux M, Baudin E. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Stueven AK, Kayser A, Wetz C, Amthauer H, Wree A, Tacke F, Wiedenmann B, Roderburg C, Jann H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 36. | Wu WM, Chen J, Bai CM, Chi Y, Du YQ, Feng ST, Huo L, Jiang YX, Li JN, Lou WH, Luo J, Shao CH, Shen L, Wang F, Wang LW, Wang O, Wang Y, Wu HW, Xing XP, Xu JM, Xue HD, Xue L, Yang Y, Yu XJ, Yuan CH, Zhao H, Zhu XZ, Zhao YP; Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. [The Chinese guidelines for the diagnosis and treatment of pancreatic neuroendocrine neoplasms (2020)]. Zhonghua Wai Ke Za Zhi. 2021;59:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Merola E, Alonso Gordoa T, Zhang P, Al-Toubah T, Pellè E, Kolasińska-Ćwikła A, Zandee W, Laskaratos F, de Mestier L, Lamarca A, Hernando J, Cwikla J, Strosberg J, de Herder W, Caplin M, Cives M, van Leeuwaarde R. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is ≥10%? Oncologist. 2021;26:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Carmona-Bayonas A, Jiménez-Fonseca P, Custodio A, Grande E, Capdevila J, López C, Teule A, Garcia-Carbonero R; Spanish Neuroendocrine Tumor Group (GETNE). Optimizing Somatostatin Analog Use in Well or Moderately Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Curr Oncol Rep. 2017;19:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Lamberti G, Faggiano A, Brighi N, Tafuto S, Ibrahim T, Brizzi MP, Pusceddu S, Albertelli M, Massironi S, Panzuto F, Badalamenti G, Riccardi F, Butturini G, Gelsomino F, De Divitiis C, Modica R, Bongiovanni A, La Salvia A, Torchio M, Colao A, Ferone D, Campana D. Nonconventional Doses of Somatostatin Analogs in Patients With Progressing Well-Differentiated Neuroendocrine Tumor. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Pavel M, Ćwikła JB, Lombard-Bohas C, Borbath I, Shah T, Pape UF, Capdevila J, Panzuto F, Truong Thanh XM, Houchard A, Ruszniewski P. Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur J Cancer. 2021;157:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Jalbert JJ, Casciano R, Meng J, Brais LK, Pulgar SJ, Berthon A, Dinet J, Kulke MH. Treatment Patterns and Health Resource Use Among Patients with Metastatic Gastroenteropancreatic Neuroendocrine Tumors Treated at a Tertiary Referral Center. Oncologist. 2020;25:e644-e650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Guenter RE, Aweda T, Carmona Matos DM, Whitt J, Chang AW, Cheng EY, Liu XM, Chen H, Lapi SE, Jaskula-Sztul R. Pulmonary Carcinoid Surface Receptor Modulation Using Histone Deacetylase Inhibitors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Jin XF, Auernhammer CJ, Ilhan H, Lindner S, Nölting S, Maurer J, Spöttl G, Orth M. Combination of 5-Fluorouracil with Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression, and Radioligand Binding in Neuroendocrine Tumor Cells In Vitro. J Nucl Med. 2019;60:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Lee L, Ito T, Jensen RT. Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin Pharmacother. 2018;19:909-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Fazio N, Buzzoni R, Baudin E, Antonuzzo L, Hubner RA, Lahner H, DE Herder WW, Raderer M, Teulé A, Capdevila J, Libutti SK, Kulke MH, Shah M, Dey D, Turri S, Aimone P, Massacesi C, Verslype C. A Phase II Study of BEZ235 in Patients with Everolimus-resistant, Advanced Pancreatic Neuroendocrine Tumours. Anticancer Res. 2016;36:713-719. [PubMed] |
| 46. | Wolin E, Mita A, Mahipal A, Meyer T, Bendell J, Nemunaitis J, Munster PN, Paz-Ares L, Filvaroff EH, Li S, Hege K, de Haan H, Mita M. A phase 2 study of an oral mTORC1/mTORC2 kinase inhibitor (CC-223) for non-pancreatic neuroendocrine tumors with or without carcinoid symptoms. PLoS One. 2019;14:e0221994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Sciammarella C, Luce A, Riccardi F, Mocerino C, Modica R, Berretta M, Misso G, Cossu AM, Colao A, Vitale G, Necas A, Fedacko J, Galdiero M, Correale P, Faggiano A, Caraglia M, Capasso A, Grimaldi A. Lanreotide Induces Cytokine Modulation in Intestinal Neuroendocrine Tumors and Overcomes Resistance to Everolimus. Front Oncol. 2020;10:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Kulke MH, Ruszniewski P, Van Cutsem E, Lombard-Bohas C, Valle JW, De Herder WW, Pavel M, Degtyarev E, Brase JC, Bubuteishvili-Pacaud L, Voi M, Salazar R, Borbath I, Fazio N, Smith D, Capdevila J, Riechelmann RP, Yao JC. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol. 2019;30:1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Baudin E, Berruti A, Giuliano M, Mansoor W, Bobirca C, Houtsma E, Fagan N, Oberg K E, Ferolla P. First long-term results on efficacy and safety of long-acting pasireotide in combination with everolimus in patients with advanced carcinoids (NET) of the lung/thymus: Phase II LUNA trial. J Clin Oncol. 2021;39:8574-8574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Hijioka S, Morizane C, Ikeda M, Ishii H, Okusaka T, Furuse J. Current status of medical treatment for gastroenteropancreatic neuroendocrine neoplasms and future perspectives. Jpn J Clin Oncol. 2021;51:1185-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Pusceddu S, Vernieri C, Di Maio M, Marconcini R, Spada F, Massironi S, Ibrahim T, Brizzi MP, Campana D, Faggiano A, Giuffrida D, Rinzivillo M, Cingarlini S, Aroldi F, Antonuzzo L, Berardi R, Catena L, De Divitiis C, Ermacora P, Perfetti V, Fontana A, Razzore P, Carnaghi C, Davì MV, Cauchi C, Duro M, Ricci S, Fazio N, Cavalcoli F, Bongiovanni A, La Salvia A, Brighi N, Colao A, Puliafito I, Panzuto F, Ortolani S, Zaniboni A, Di Costanzo F, Torniai M, Bajetta E, Tafuto S, Garattini SK, Femia D, Prinzi N, Concas L, Lo Russo G, Milione M, Giacomelli L, Buzzoni R, Delle Fave G, Mazzaferro V, de Braud F. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology. 2018;155:479-489.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Vitali E, Boemi I, Tarantola G, Piccini S, Zerbi A, Veronesi G, Baldelli R, Mazziotti G, Smiroldo V, Lavezzi E, Spada A, Mantovani G, Lania AG. Metformin and Everolimus: A Promising Combination for Neuroendocrine Tumors Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Couvelard A, O'Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Xu JM, Wang Y, Chen YL, Jia R, Li J, Gong JF, Qi C, Hua Y, Tan CR, Wang J, Li K, Sai Y, Zhou F, Ren YX, Qing WG, Jia H, Su WG, Shen L. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: results from a phase I study. Oncotarget. 2017;8:42076-42086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Chan JA, Faris JE, Murphy JE, Blaszkowsky LS, Kwak EL, McCleary NJ, Fuchs CS, Meyerhardt JA, Ng K, Zhu AX, Abrams TA, Wolpin BM, Zhang S, Reardon A, Fitzpatrick B, Kulke MH, Ryan DP. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol. 2017;35:228-228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Capdevila J, Fazio N, Lopez Lopez C, Teule A, Valle J W, Tafuto S, Custodio A B, Reed N, Raderer M, Grande E, Garcia-Carbonero R, Jiménez-Fonseca P, Alonso V, Antonuzzo L, Spallanzani A, Berruti A, Sevilla I, La Casta Munoa A, Hernando-Cubero J, Ibrahim T. Final results of the TALENT trial (GETNE1509): a prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs). J Clin Oncol. 2019;37:4106-4106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Capdevila J, Jimenez-Valerio G, Martinez A, Hernando J, Ibrahim T, Fazio N, Lopez C, Teule A, Valle W, Tafuto S, Custodio B, Reed NS, Raderer M, Grande E, Garcia-Carbonero R, Jiménez-Fonseca P, Alonso V, Casanovas O. Plasma biomarker study of lenvatinib in gastroenteropancreatic neuroendocrine tumors reveals Ang2 and FGF2 as predictors of treatment response: Results from the international phase II TALENT trial (GETNE 1509). J Clin Oncol. 2021;39:4113-4113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Strosberg JR, Cives M, Hwang J, Weber T, Nickerson M, Atreya CE, Venook A, Kelley RK, Valone T, Morse B, Coppola D, Bergsland EK. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr Relat Cancer. 2016;23:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Grande E, Capdevila J, Castellano D, Teulé A, Durán I, Fuster J, Sevilla I, Escudero P, Sastre J, García-Donas J, Casanovas O, Earl J, Ortega L, Apellaniz-Ruiz M, Rodriguez-Antona C, Alonso-Gordoa T, Díez JJ, Carrato A, García-Carbonero R. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol. 2015;26:1987-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Lu M, Zhang P, Zhang Y, Li Z, Gong J, Li J, Li Y, Zhang X, Lu Z, Wang X, Zhou J, Peng Z, Wang W, Feng H, Wu H, Yao S, Shen L. Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin Cancer Res. 2020;26:2337-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 61. | Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res. 2020;26:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 62. | Mulvey C, Raj NP, Chan JA, Aggarwal RR, Cinar P, Hope T A, Kolli K, Zhang L, Calabrese S, Grabowsky JA, Modarresi L, Kelly V, Stonely D, Munster PN, Reidy DL, Fong L, Bergsland EK. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part A (pembrolizumab alone). J Clin Oncol. 2019;37:363-363. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Matos Garcia I, Grande E, Garcia-Carbonero R, Lopez C, Teule A, Capdevila J. A multicohort phase II study of durvalumab plus tremelimumab for the treatment of patients (PTS) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic (GEP) or lung origin (the DUNE trial-GETNE1601-). J Clin Oncol. 2017;35:TPS4146-TPS4146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Klein O, Kee D, Markman B, Chang Lee R, Michael M, Mileshkin LR, Scott CL, Linklater R, Menon S, Tebbutt NC, Palmer J, Behren A, Cebon JS. A phase II clinical trial of ipilimumab/nivolumab combination immunotherapy in patients with rare upper gastrointestinal, neuroendocrine, and gynecological malignancies. J Clin Oncol. 2019;37:2570-2570. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Shen L, Yu X, Lu M, Zhang X, Cheng Y, Zhang Y, Li Z, Xu J, Weng D, Wu C, Ma Y, Cheng K, WANG W, Gao H, Li Y, Zhou J, Shi H, Tan P, Su W. Surufatinib in combination with toripalimab in patients with advanced neuroendocrine carcinoma: Results from a multicenter, open-label, single-arm, phase II trial. J Clin Oncol. 2021;39:e16199-e16199. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Ito T, Masui T, Komoto I, Doi R, Osamura RY, Sakurai A, Ikeda M, Takano K, Igarashi H, Shimatsu A, Nakamura K, Nakamoto Y, Hijioka S, Morita K, Ishikawa Y, Ohike N, Kasajima A, Kushima R, Kojima M, Sasano H, Hirano S, Mizuno N, Aoki T, Ohtsuka T, Okumura T, Kimura Y, Kudo A, Konishi T, Matsumoto I, Kobayashi N, Fujimori N, Honma Y, Morizane C, Uchino S, Horiuchi K, Yamasaki M, Matsubayashi J, Sato Y, Sekiguchi M, Abe S, Okusaka T, Kida M, Kimura W, Tanaka M, Majima Y, Jensen RT, Hirata K, Imamura M, Uemoto S. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol. 2021;56:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 67. | Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar AE, Mittra E, Wolin E M, Yao JC, Pavel ME, Grande E, Van Cutsem E, Seregni E, Duarte H, Gericke G, Bartalotta A, Demange A, Mutevelic S, Krenning E. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J Clin Oncol. 2021;39:4112-4112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Clement D, Navalkissoor S, Srirajaskanthan R, Courbon F, Dierickx L, Eccles A, Lewington V, Mitjavila M, Percovich J C, Lequoy B, He B, Folitar I, Ramage J. Efficacy and safety of 177Lu-DOTATATE in patients (pts) with advanced pancreatic neuroendocrine tumors (PanNETs): Data from the NETTER-R international, retrospective registry. J Clin Oncol. 2021;39:4116-4116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Nicolas GP, Ansquer C, Lenzo NP, Grønbæk H, Haug A, Navalkissoor S, Beauregard J, Germann N, McEwan S, Wild D, Hicks RJ. An international open-label study on safety and efficacy of 177Lu-satoreotide tetraxetan in somatostatin receptor positive neuroendocrine tumours (NETs): An interim analysis. Ann Oncol. 2020;31:S711-S724. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Cives M, Pelle' E, Strosberg J. Emerging Treatment Options for Gastroenteropancreatic Neuroendocrine Tumors. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Grande E, Teulé A, Alonso-Gordoa T, Jiménez-Fonseca P, Benavent M, Capdevila J, Custodio A, Vera R, Munarriz J, La Casta A, Díez JJ, Gajate P, Molina-Cerrillo J, Matos I, Cristóbal EM, Ruffinelli JC, Palacios J, García-Carbonero R. The PALBONET Trial: A Phase II Study of Palbociclib in Metastatic Grade 1 and 2 Pancreatic Neuroendocrine Tumors (GETNE-1407). Oncologist. 2020;25:745-e1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Tang LH, Contractor T, Clausen R, Klimstra DS, Du YC, Allen PJ, Brennan MF, Levine AJ, Harris CR. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res. 2012;18:4612-4620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Anderson PM, Gortz J. Phase 2 study of DRD2 antagonist/ClpP agonist ONC201 in neuroendocrine tumors. J Clin Oncol. 2021;39:3002-3002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | White BH, Whalen K, Kriksciukaite K, Alargova R, Au Yeung T, Bazinet P, Brockman A, DuPont M, Oller H, Lemelin CA, Lim Soo P, Moreau B, Perino S, Quinn JM, Sharma G, Shinde R, Sweryda-Krawiec B, Wooster R, Bilodeau MT. Discovery of an SSTR2-Targeting Maytansinoid Conjugate (PEN-221) with Potent Activity in Vitro and in Vivo. J Med Chem. 2019;62:2708-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Halperin DM, Johnson ML, Chan JA, Hart LL, Cook N, Patel VM, Schlechter BL, Cave J, Dowlati A, Blaszkowsky LS, Meyer T, Eads JR, Culp D, Kriksciukaite K, Mei L, Bilodeau M, Bloss J, Kulke MH. The safety and efficacy of PEN-221 somatostatin analog (SSA)-DM1 conjugate in patients (PTS) with advanced GI mid-gut neuroendocrine tumor (NET): Phase 2 results. J Clin Oncol. 2021;39:4110-4110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, Thamake S, Park EK, Perini RF, Linehan WM, Srinivasan R; MK-6482-004 Investigators. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N Engl J Med. 2021;385:2036-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 414] [Article Influence: 103.5] [Reference Citation Analysis (0)] |