Published online May 21, 2022. doi: 10.3748/wjg.v28.i19.2137
Peer-review started: January 2, 2022
First decision: March 10, 2022
Revised: March 21, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: May 21, 2022
Processing time: 134 Days and 19.5 Hours
Post-polypectomy surveillance intervals are currently determined based on pathology results.
To evaluate a polyp-based resect and discard model that assigns surveillance intervals based solely on polyp number and size.
Patients undergoing elective colonoscopies at the Montreal University Medical Center were enrolled prospectively. The polyp-based strategy was used to assign the next surveillance interval using polyp size and number. Surveillance intervals were also assigned using optical diagnosis for small polyps (< 10 mm). The primary outcome was surveillance interval agreement between the polyp-based model, optical diagnosis, and the pathology-based reference standard using the 2020 United States Multi-Society Task Force guidelines. Secondary outcomes included the proportion of reduction in required histopathology evaluations and proportion of immediate post-colonoscopy recommendations provided to patients.
Of 944 patients (mean age 62.6 years, 49.3% male, 933 polyps) were enrolled. The surveillance interval agreement for the polyp-based strategy was 98.0% [95% confidence interval (CI): 0.97–0.99] compared with pathology-based assignment. Optical diagnosis-based intervals achieved 95.8% (95%CI: 0.94–0.97) agreement with pathology. When using the polyp-based strategy and optical diagnosis, the need for pathology assessment was reduced by 87.8% and 70.6%, respectively. The polyp-based strategy provided 93.7% of patients with immediate surveillance interval recommendations vs 76.1% for optical diagnosis.
The polyp-based strategy achieved almost perfect surveillance interval agreement compared with pathology-based assignments, significantly reduced the number of required pathology evaluations, and provided most patients with immediate surveillance interval recommendations.
Core Tip: Background current post-polypectomy surveillance intervals are based on pathology outcomes. Our aim was to test a novel polyp-based resect and discard model that assigns surveillance interval based on number and size of polyps. Findings Surveillance interval based on a polyp-based strategy achieved 98.0% (95% confidence interval: 0.97–0.99) agreement with pathology-based intervals when applied according to the current surveillance guideline. Implications for patient care the polyp-based strategy can easily be implemented without any requirement for specialist devices and training. The majority of patients can be provided with immediate surveillance interval recommendations, without having to wait for results of pathology analysis.
- Citation: Taghiakbari M, Hammar C, Frenn M, Djinbachian R, Pohl H, Deslandres E, Bouchard S, Bouin M, von Renteln D. Non-optical polyp-based resect and discard strategy: A prospective clinical study. World J Gastroenterol 2022; 28(19): 2137-2147
- URL: https://www.wjgnet.com/1007-9327/full/v28/i19/2137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i19.2137
Screening colonoscopy and removal of detected polyps has been utilized to reduce colorectal cancer (CRC) morbidity and mortality[1]. The majority (90%) of polyps found during colonoscopies are less than 10 mm in size, with diminutive polyps (< 5 mm) accounting for about 70%–80%[2]. Most of these small polyps have been shown to be at very low risk for progression towards CRC. Advanced histology is found in only 0.5% of diminutive polyps and 1.5% of small polyps[2]. Histopathologic evaluation of small polyps can incur significant costs, therefore alternative modalities have been proposed, such as image-enhanced endoscopy-assisted optical polyp diagnosis (the "resect and discard" strategy)[3-5].
While optical diagnosis has achieved high level of accuracy in academic settings[6,7], reports from general clinical practice have not been able to reproduce these results, with accuracies ranging between 75% and 85%, and surveillance interval assignment agreement with pathology of only 81%[8,9]. In a recent survey study, 59.9% of endoscopists reported that optical diagnosis was not feasible for clinical implementation, and 84.2% were not using the strategy in their current clinical practice[10]. Limitations of the resect and discard strategy included fear of making an incorrect optical diagnosis, assigning incorrect surveillance intervals, and training requirements[10]. Therefore, we aimed to develop a resect and discard model that did not require optical diagnosis to assign colonoscopy surveillance intervals. A retrospective study using this model, named the polyp-based resect and discard (PBRD) strategy, showed an 89.3% agreement with pathology-based surveillance recommendations[11]. This current study aimed to evaluate the PBRD model in a prospective clinical study comparing the strategy with optical polyp diagnosis using pathology-based surveillance interval recommendations as the reference standard.
Patients (aged 45–80 years) undergoing elective screening, surveillance, or diagnostic colonoscopies between May 2017 and December 2018 at the Centre Hospitalier de l'Université de Montréal (CHUM) were included. Exclusion criteria were known inflammatory bowel disease, active colitis, coagulopathy, familial polyposis syndromes, American Society of Anesthesiologists classification score of > 3, emergency colonoscopies, personal history of CRC, hospitalized patients, and presence of CRC during colonoscopy. The study was planned and conducted as a sub-study in patients enrolled in two prospective clinical studies (NCT04032912 and NCT03515343, respectively). The study protocol and data collection were approved by the local institutional research board as an amendment to the two prospective clinical studies (17.135 and 16.367, respectively).
Colonoscopy procedures were performed as per the standard of care. Adequate bowel preparation was determined by a Boston Bowel Preparation Score (BBPS) of ≥ 6. Location, size, and morphology according to the Paris classification were documented for each polyp. All polyps were removed and sent for histopathology evaluation. Polyps 1–10 mm were optically diagnosed using either i-Scan or Optivista image-enhanced endoscopy (Pentax, Montvale, NJ, United States) and classified using the Narrow-band imaging international colorectal endoscopic (NICE) classification system[4,12]. Endoscopist level of confidence (low or high) in optical histology prediction was documented. Endoscopists then used the PBRD strategy to assign the next surveillance interval immediately after colonoscopy (real-time application). Then, a research assistant (MT) used the PBRD strategy (post hoc) to determine whether endoscopists had deviated from the intended PBRD strategy and assessed the PBRD model results when used without deviations.
The PBRD strategy was developed by the research group and previously tested in a pilot study[11]. There was no overlap between the cohort enrolled in the pilot study and the cohort presented herein. The PBRD uses number and size of polyps, and first-degree family history of CRC to predict the next surveillance interval. At the time of the study, the 2012 United States Multi-Society Task Force (USMSTF) guidelines[13] were the most current guidelines used to develop the PBRD strategy (Table 1). With the publication of the updated 2020 USMSTF guidelines[14] during the course of the study, we adapted the PBRD model to reflect those changes through consensus between two researchers (RD and DvR), and tested its performance post hoc (Table 1). Since the 2020 guidelines are the most contemporaneous, the 2020-based analysis was used as the primary outcome of the study. Pathology-based surveillance intervals were therefore determined using 2012 or 2020 USMSTF guidelines as appropriate[13,14]. In cases of multiple recommended intervals (for example, 7–10 years), the longest interval was chosen to compare the strategies.
| Scenario | Rule | If family history of CRC (first-degree relative) | |
| Surveillance interval recommendation based on 2012 guidelines, yr | |||
| 1 | No polyp | 10 | 5 |
| 2 | 1–2 diminutive polyps (largest polyp max 5 mm) | 10 | 5 |
| 3 | 1–3 small polyps (all polyps 1–9 mm and the largest polyp max < 10 mm) | 5 | 5 |
| 4 | ≥ 4 polyps, any size | Follow-up pathology results | - |
| 5 | At least 1 polyp ≥ 10 mm | Follow-up pathology results | - |
| 6 | Insufficient or inadequate bowel preparation | 1 | - |
| 7 | Unclear | - | - |
| Surveillance interval recommendation based on 2020 guidelines, yr | |||
| 1 | No polyp | 10 | 5 |
| 2 | 1–3 diminutive polyps; or 2 diminutive polyps and 1 small polyp | 10 | 5 |
| 3 | 1–2 small polyps exclusively | 10 | 5 |
| 4 | > 3 polyps, any size, or > 2 polyps 6–9 mm | Follow-up pathology results | – |
| 5 | ≥ 1 polyp ≥ 10 mm | Follow-up pathology results | – |
| 6 | Insufficient or inadequate bowel preparation | 1 | 1 |
The primary outcome was the agreement between PBRD-based surveillance intervals and pathology-based surveillance intervals and agreement between updated PBRD-based surveillance intervals and pathology-based surveillance intervals. Secondary endpoints were: Agreement between optical diagnosis-based surveillance intervals and pathology-based intervals; agreement between real-time endoscopist allocation of intervals based on PBRD compared with pathology-based intervals; proportion of immediate post-colonoscopy surveillance recommendations provided to patients based on both PBRD (real-time and post hoc) and optical diagnosis; proportion of required histopathology evaluations when using PBRD and optical diagnosis. Other secondary outcomes included the proportion of patients with findings that could have been provided with immediate surveillance interval recommendations: no polyps detected; inadequate bowel preparation; polyps sized 1–10 mm that were all optically diagnosed with high confidence (for the optical diagnosis strategy); patients fitting scenarios 1, 2, 3, and 6 (for the PBRD strategy) (Table 1). Polyps undergoing low-confidence optical diagnosis, polyps > 10 mm in size, and all polyps in patients fitting scenarios 4 and 5 (for the PBRD strategy) required histopathology evaluation.
Patient, procedure, and polyp characteristics were presented as crude numbers and frequency for categorical variables, and mean ± SD or median (range) for continuous variables. Agreements between the PBRD model, optical diagnosis, and pathology-based surveillance recommendations were presented as proportions with two-tailed 95% confidence interval (CI). For secondary outcomes, proportional estimates with two-tailed 95%CI were presented. A chi-squared test or a two-tailed Fisher's exact test was used to compare proportions. SPSS version 26.0 (IBM Corp., Armonk, New York, United States) and MedCalc version 19.4 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org) were used for analyses.
A total of 1157 patients were screened, and 944 patients with 933 polyps were included in the final analysis (mean ± SD age 62.6 ± 8.6 years; 49.3% male) (Supplementary Figure 1). Most colonoscopies were performed for screening and surveillance. Among all detected polyps, 819 (87.8%) were either diminutive or small (1–9 mm). Table 2 shows the details of patient, procedure, and polyp characteristics.
| Characteristics | |
| Patients, n | 944 |
| Age, yr | 62.6 (8.6) |
| Male sex, n (%) | 465 (49.3) |
| Family history of CRC2, n (%) | |
| No | 682 (72.2) |
| Yes | 259 (27.4) |
| Colonoscopy indication, n (%) | |
| Screening | 299 (31.7) |
| FIT+ | 39 (4.1) |
| Adenoma surveillance | 206 (21.8) |
| Anemia/bleeding | 158 (16.7) |
| Diarrhea | 28 (3.0) |
| Other | 214 (22.7) |
| Procedures | |
| Bowel preparation quality, n (%) | |
| Adequate | 851 (90.1) |
| Inadequate3 | 93 (9.9) |
| Cecal intubation rate, n (%) | 902 (95.6) |
| Polyp detection rate4, % | 53.7% |
| Adenoma detection rate4, % | 36.4% |
| Polyps, n | 933 |
| Polyp size, mm | 5.8 (8.3) |
| Polyp size, n (%) | |
| ≤ 5 mm | 689 (73.8) |
| 6–9 mm | 130 (13.9) |
| ≥ 10 mm | 114 (12.2) |
| Histopathology, n (%) | |
| Hyperplastic polyp | 274 (29.4) |
| Tubular adenoma | 468 (50.2) |
| Villous adenoma | 36 (3.9) |
| Traditional serrated adenoma | 1 (0.1) |
| Sessile serrated adenoma/polyp | 38 (4.1) |
| Other | 103 (11.0) |
| High-grade dysplasia | 13 (1.4) |
| Tubular adenoma with HGD | 3/13 (23.1) |
| Villous adenoma with HGD | 10/13 (76.9) |
| Optical histology prediction based on NICE classification | 842/933 (90.2)5 |
| Non-neoplastic | 345 (41.0) |
| Neoplastic | 497 (59.0) |
| High-confidence optical diagnosis | 648 (69.5) |
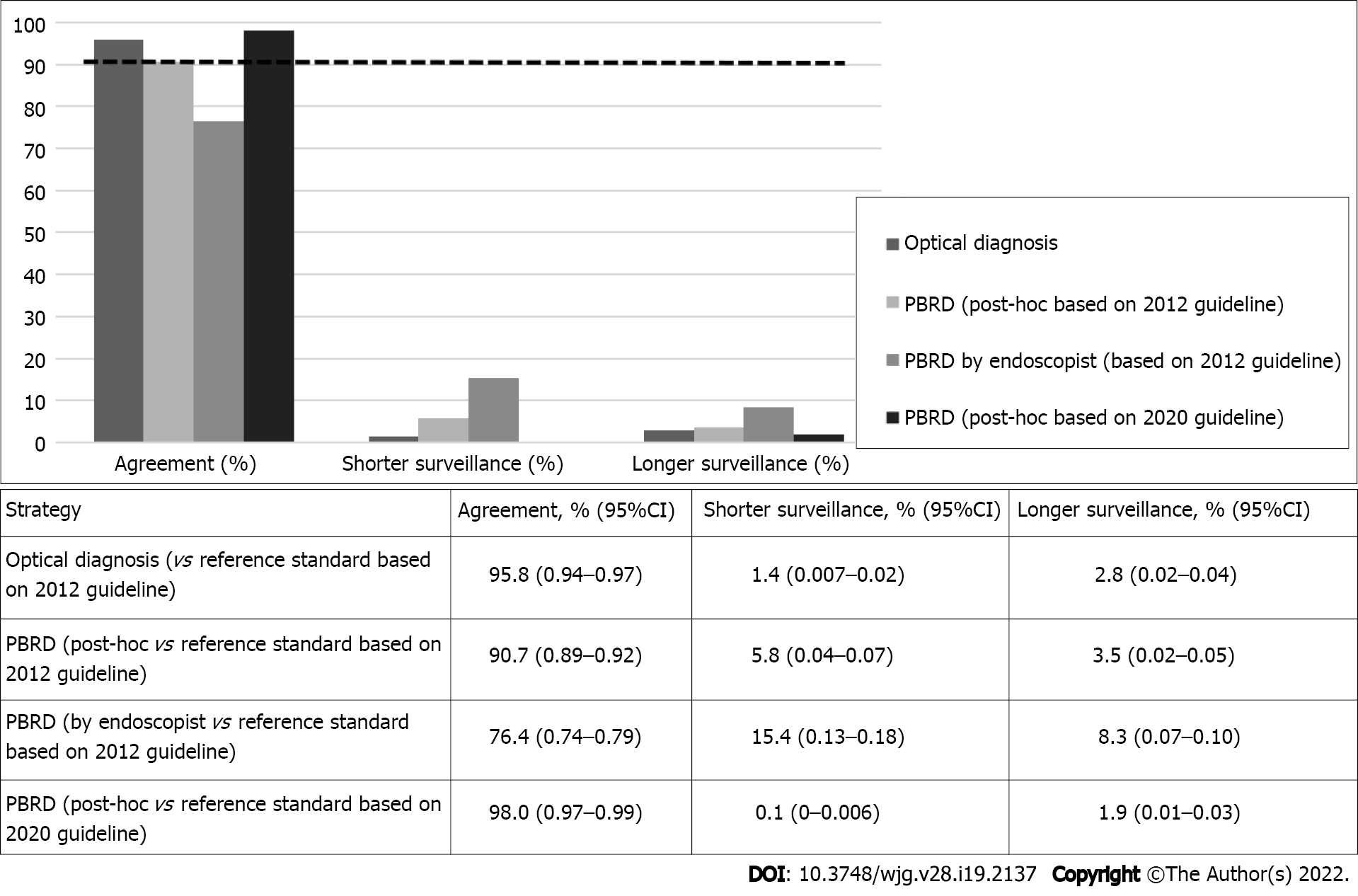
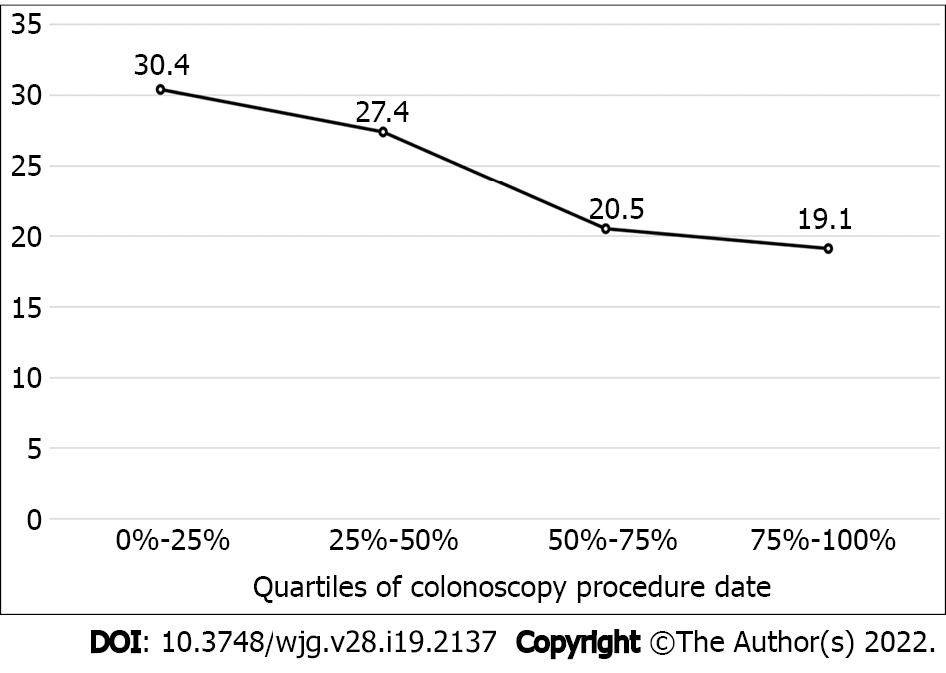
The PBRD strategy based on the 2020 guidelines reached 98.0% (95%CI: 0.97–0.99) agreement with pathology-based surveillance intervals (Figure 1). Based on the 2012 guidelines, surveillance interval agreement between real-time PBRD strategy and pathology was 76.4% (95%CI: 0.74–0.79). Endoscopists using the PBRD strategy assigned shorter and longer surveillance intervals in 15.4% and 8.3% of patients, respectively. When applied post hoc, the PBRD strategy based on the 2012 guidelines reached 90.7% (95%CI: 0.89–0.92) agreement with pathology-based recommendations, with shorter and longer intervals assigned in 5.8% and 3.5% of patients, respectively. The proportion of endoscopist assigned surveillance intervals that adhered to pathology-based intervals was significantly lower than those assigned post-hoc using the same strategy (P < 0.0001) (Table 3). None of the patients that should have received shorter surveillance intervals through the post-hoc PBRD model had a polyp with advanced histology. Only 3/145 patients that should have been assigned to shorter surveillance intervals by endoscopists had polyps with advanced histology. Deviations from the strategy decreased as the study progressed (Figure 2).
A total of 842 (90.2%) polyps ≤ 10 mm were optically diagnosed using NICE; of those, 648 (69.5%) were classified with high confidence (Table 2). The agreement between surveillance intervals assigned by optical diagnosis and pathology-based intervals using 2012 guidelines was 95.8% (95%CI: 0.94–0.97) (Figure 1). The agreement with pathology for surveillance intervals assigned by optical diagnosis was significantly higher than that for both the PBRD strategy used by endoscopists (P < 0.0001) and the PBRD strategy calculated post-hoc based on 2012 (P < 0.0001). Supplementary Table 1 shows allocation of surveillance intervals between optical diagnosis and pathology.
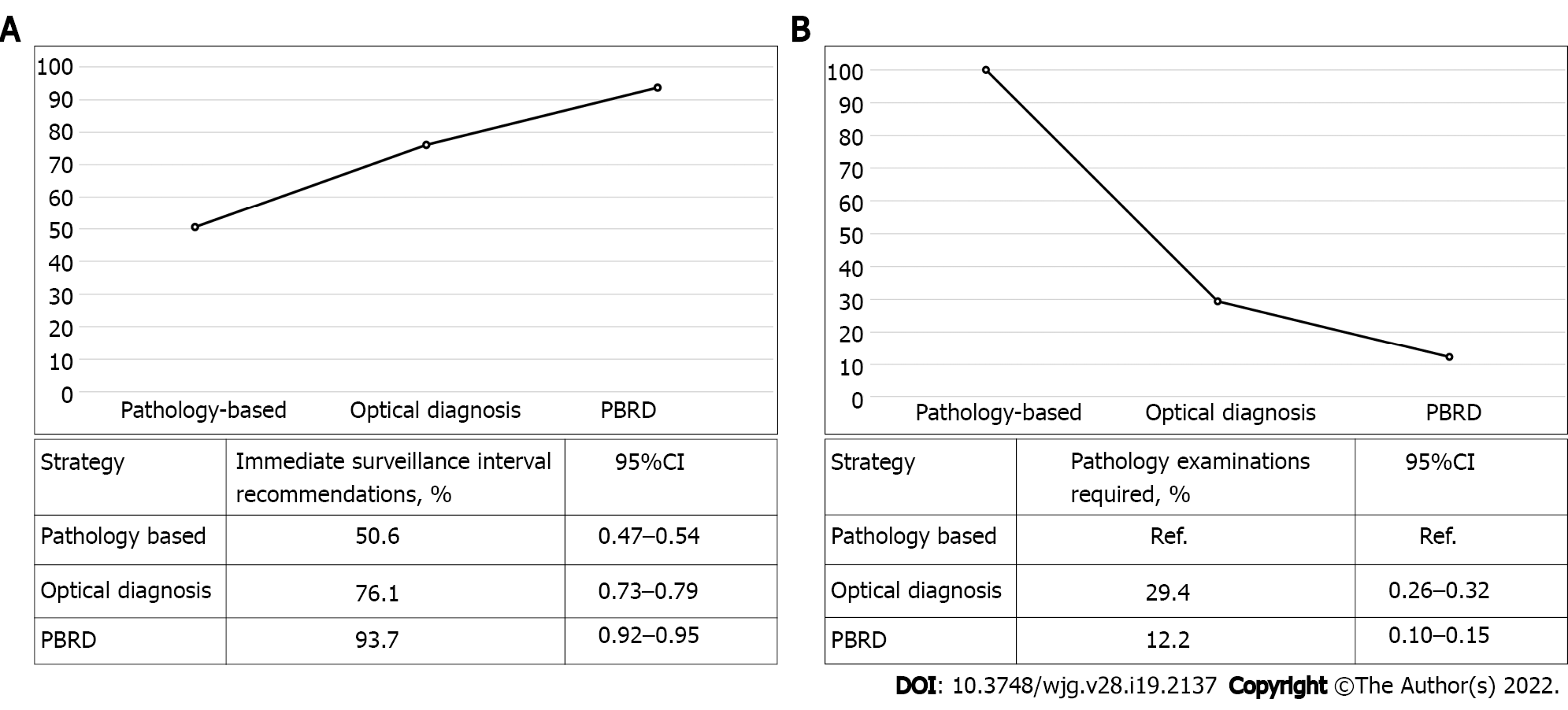
When using the standard clinical approach, 50.6% of patients could have been given an immediate surveillance recommendation post-colonoscopy. The PBRD strategy (based on 2020 guidelines) and optical diagnosis would have allowed for immediate surveillance interval recommendation in 93.7% (95%CI: 0.92–0.95, and 76.1% (95%CI: 0.73-0.79) of patients, respectively (Figure 3A). The PBRD strategy reduced 87.8% of histopathology evaluations compared with 70.6% for optical diagnosis (Figure 3B).
Our study found that surveillance interval assignment using the PBRD strategy based on the 2020 USMSTF guidelines reached 98.0% agreement with pathology. This agreement was significantly higher compared to optical diagnosis-based strategies. In contrast to optical diagnosis, the use of the PBRD strategy is independent of operator skill, leading to increased reproducibility in routine endoscopic practice.
Interestingly, we found that when the PBRD strategy was used in real-time by endoscopists, adherence to guideline recommendations was lower; endoscopists chose a different surveillance interval than the PBRD strategy in 20% of patients, possibly due to clinical information not reflected in the strategy, such as second-degree relatives with CRC or other individual factors. Our findings also reflect previously described practice patterns where endoscopists often assigned shorter surveillance intervals for low-risk lesions and normal colonoscopies, with highly variable but often low (< 50%) adherence to guidelines. Reasons for non-adherence stated in the literature included disagreement with guidelines, inadequate or suboptimal bowel preparation, and concern for missed polyps[15,16]. These factors potentially played a role in endoscopist deviations from the PBRD strategy in our study. Another explanation to these deviations could be the learning curve for PBRD implementation. We found that as the study progressed, the percentage of deviations from the PBRD strategy decreased (Figure 2).
Agreement between pathology-based and post-hoc allocation of surveillance intervals using the PBRD strategy based on 2020 USMSTF guidelines was significantly higher than the agreement between pathology-based and optical-based allocation of surveillance intervals [98.0% (95%CI: 0.97–0.99) vs 95.8% (95%CI: 0.94–0.97); P = 0.005]. Additionally, the agreement between optical diagnosis-based surveillance intervals and pathology surpassed the American Society for Gastrointestinal Endoscopy Preservation and Incorporation of Valuable endoscopic Innovations 90% benchmark[3]. However, agreement between surveillance interval assignments using real-time application of PBRD by endoscopists, and pathology was significantly lower than for optical diagnosis. In our study, 70% of polyps were optically classified with high confidence, similar to the rates reported by other studies[17]. Increasing the rate of high-confidence optical diagnosis would contribute to the acceptance of this technique in routine endoscopic practice, particularly for non-academic endoscopists. However, endoscopists are often reluctant to use optical diagnosis due to concerns of incorrect diagnosis, inappropriate surveillance interval assignment, and fear of potential medicolegal repercussions[10]. As our adaptation of the PBRD strategy to reflect the updated 2020 USMSTF guideline resulted in a significantly higher agreement compared with the 2012-based PBRD model [98.0% (95%CI: 0.97–0.99) vs 90.7% (95%CI: 0.89–0.92); P < 0.0001], we believe that the PBRD strategy may be a safe alternative that can be easily applied by endoscopists pending further research confirming efficacy in real-time endoscopic practice, and gastroenterology society endorsements. The PBRD strategy and optical diagnosis resulted in significant reductions in required histopathology evaluations, and increased the percentage of patients with same-day surveillance interval assignment. A significant proportion of post-colonoscopy colorectal cancers (PCCRCs) are due to administrative or decision-making errors[18]. Fail-safe mechanisms are therefore needed to ensure the assignment of an appropriate surveillance interval during the index session for follow-up examination. For instance, histopathology might not be followed up adequately, or patients might fail to receive their surveillance interval after pathology results are available. This would exacerbate loss to follow-up and increase the chance of PCCRC. The PBRD strategy could offer a simple solution for endoscopists to communicate the appropriate time for the next surveillance colonoscopy without requiring histopathology evaluation.
Another advantage of the PBRD strategy is that very high agreement with pathology-based surveillance intervals can be achieved without any specialized training, skill, or dedicated equipment. The PBRD strategy might be easier to implement and may address fears cited by endoscopists. As the fear of discarding polyps with advanced histology remains a significant concern and could limit the widespread adoption of resect and discard strategies, revised versions of PBRD could exclude polyps with morphology of potentially advanced lesions (e.g., Paris IIc or III). Furthermore, it might be beneficial to limit the use of the PBRD strategy to diminutive polyps only, which would reduce the risk of assigning polyps with high-grade dysplasia or serrated adenomas to longer surveillance intervals, as advanced pathology occurs more frequently in polyps of 6–9 mm. Notably, the post hoc application of the PBRD strategy did not result in discarding any polyp with advanced histology in our study. Limiting this strategy to 1–3 mm polyps could also be feasible, especially when optical diagnosis is not possible and pathology examination to determine the histology of these polyps not reliable. Approaches to replace pathology for these polyps are likely safe as a recent study showed that advanced histologic features in diminutive polyps did not contribute towards metachronous CRC[19].
The emergence of new modalities such as artificial intelligence (AI)-assisted classification could provide an alternative to the proposed approach in the future. However, the accuracy of AI-based optical diagnosis in broader clinical practice with different endoscopists, platforms, and settings remains to be evaluated, with widespread clinical implementation far from reality. Furthermore, it is unlikely that every endoscopy unit could implement this cutting-edge technology immediately or at all once available. Therefore, the PRBD strategy could be used as a bridge or complementary system to AI. The current strategy of resection and histopathologic analysis of all polyps is associated with high costs. Previous studies estimated that the annual cost saving in the United States population following the adoption of a resect and discard policy for diminutive polyps ranges from United States $33 million to $1 billion annually[20]. By reducing such costs, healthcare systems could increase efficiency and reallocate savings to other resources in CRC prevention, such as screening in younger age groups.
Several limitations should be discussed. Patient recruitment was at a single academic center, limiting the generalizability of our results. Future research should assess PBRD in multicentered studies and community-based practices. The PBRD strategy could be improved by considering other important clinical factors, such as in-depth family and personal history of CRC and/or polyps, suboptimal bowel preparation score (e.g., BBPS of 5 or 6), or offering more granular choices to clinicians. Furthermore, results of this study may have been improved if PBRD was limited to diminutive or 1–3 mm polyps only, due to the low prevalence of advanced histology in such polyps at the expense of lower proportion of patients with same day surveillance interval assignment and higher proportion of required pathology examinations[2].
In conclusion, the PBRD strategy reached 98.0% agreement with surveillance intervals assigned through pathology using the 2020 USMSTF guidelines. Performance with the 2012 guidelines was lower when implemented correctly but still surpassed the 90% benchmark. Optical diagnosis also performed above the 90% benchmark in our study. Therefore, the PBRD strategy may be a feasible alternative to resect and discard that can be used without specialized equipment, training, or optical diagnosis skills.
The majority (90%) of polyps found during colonoscopies are less than 10 mm in size, with diminutive polyps (< 5 mm) accounting for about 70%–80%. Advanced histology is found in only 1.7% of diminutive polyps and 10.1% of small polyps. Current post-polypectomy surveillance intervals are based on pathology outcomes. However, histopathologic evaluation of small polyps can incur significant costs.
Alternative modalities have been proposed, such as image-enhanced endoscopy-assisted optical polyp diagnosis. Limitations of the optical diagnosis included fear of making an incorrect optical diagnosis, assigning incorrect surveillance intervals, and training requirements.
We aimed to develop a novel resect and discard model that did not require optical diagnosis to assign colonoscopy surveillance intervals, and assigned surveillance interval based on number and size of polyps, so-called the polyp-based resect and discard (PBRD) strategy.
In a clinical prospective study, all patients undergoing elective colonoscopies were enrolled. The polyp-based strategy was used to assign the next surveillance interval using polyp size and number. Surveillance intervals were also assigned using optical diagnosis for small polyps (< 10 mm). The primary outcome was surveillance interval agreement between the polyp-based model and the pathology-based reference standard using the 2020 United States Multi-Society Task Force guidelines.
Surveillance interval based on a polyp-based strategy achieved 98.0% (95% confidence interval: 0.97–0.99) agreement with pathology-based intervals when applied according to the current surveillance guideline.
The polyp-based strategy can easily be implemented without any requirement for specialist devices and training. The majority of patients can be provided with immediate surveillance interval recommendations, without having to wait for results of pathology analysis.
Future research should assess PBRD in multicentered studies and community-based practices.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pausawasdi N, Thailand; Yeniova A, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Zhang R, Zheng Y, Mak TW, Yu R, Wong SH, Lau JY, Poon CC. Automatic Detection and Classification of Colorectal Polyps by Transferring Low-Level CNN Features From Nonmedical Domain. IEEE J Biomed Health Inform. 2017;21:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, Wani SB, Edmundowicz SA, Sharma P, Rastogi A. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, Lieberman DA. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 4. |
|
| 5. | Schachschal G, Mayr M, Treszl A, Balzer K, Wegscheider K, Aschenbeck J, Aminalai A, Drossel R, Schröder A, Scheel M, Bothe CH, Bruhn JP, Burmeister W, Stange G, Bähr C, Kießlich R, Rösch T. Endoscopic vs histological characterisation of polyps during screening colonoscopy. Gut. 2014;63:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kaltenbach T, Rex DK, Wilson A, Hewett DG, Sanduleanu S, Rastogi A, Wallace M, Soetikno R. Implementation of optical diagnosis for colorectal polyps: standardization of studies is needed. Clin Gastroenterol Hepatol. 2015;13:6-10.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Glover B, Patel N, Ashrafian H, Teare J. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol. 2018;11:1756284818814948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | van den Broek FJ, van Soest EJ, Naber AH, van Oijen AH, Mallant-Hent RCh, Böhmer CJ, Scholten P, Stokkers PC, Marsman WA, Mathus-Vliegen EM, Curvers WL, Bergman JJ, van Eeden S, Hardwick JC, Fockens P, Reitsma JB, Dekker E. Combining autofluorescence imaging and narrow-band imaging for the differentiation of adenomas from non-neoplastic colonic polyps among experienced and non-experienced endoscopists. Am J Gastroenterol. 2009;104:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, Fockens P, Dekker E. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012;10:1016-20; quiz e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Willems P, Djinbachian R, Ditisheim S, Orkut S, Pohl H, Barkun A, Bouin M, Faulques B, von Renteln D. Uptake and barriers for implementation of the resect and discard strategy: an international survey. Endosc Int Open. 2020;8:E684-E692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Duong A, Bouin M, Leduc R, Deslandres E, Deshêtres A, Weber A, Pohl H, Barkun AN, von Renteln D. A7 the polyp-based resect-and-discard strategy. J Can Assoc Gastroenterol. 2019;2 (Suppl 2):15-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Patrun J, Okreša L, Iveković H, Rustemović N. Diagnostic Accuracy of NICE Classification System for Optical Recognition of Predictive Morphology of Colorectal Polyps. Gastroenterol Res Pract. 2018;2018:7531368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1439] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 14. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the us multi-society task force on colorectal cancer. Gastroenterology. 2020;158:1131-1153.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 15. | Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW, Goede SL, Dekker E, Lesterhuis W, ter Borg F, Vecht J, Spoelstra P, Engels L, Bolwerk CJ, Timmer R, Kleibeuker JH, Koornstra JJ, de Koning HJ, Kuipers EJ, van Ballegooijen M. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015;64:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Iwatate M, Sano Y, Hattori S, Sano W, Hasuike N, Ikumoto T, Kotaka M, Murakami Y, Hewett DG, Soetikno R, Kaltenbach T, Fujimori T. The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps. Endosc Int Open. 2015;3:E140-E145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Kaminski MF. Avoiding a plane crash with colonoscopy. Endoscopy. 2022;54:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Tang Y, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2021;15:2580-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Kandel P, Wallace MB. Should We Resect and Discard Low Risk Diminutive Colon Polyps. Clin Endosc. 2019;52:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |