Published online May 21, 2022. doi: 10.3748/wjg.v28.i19.2100
Peer-review started: May 19, 2021
First decision: June 12, 2021
Revised: July 17, 2021
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 21, 2022
Processing time: 362 Days and 17.3 Hours
The machine perfusion (MP) preservation including hypothermic MP (HMP) and midthermic MP (MMP) has been considered as a promising strategy to preserve the functions of liver donated after cardiac death. The importance of under
To investigate the ultrastructural changes in the LSEC and sinusoids around them after MP.
Porcine liver grafts undergo a warm ischemia time of 60 minutes perfused for 4 h with modified University of Wisconsin gluconate solution. Group A grafts were preserved with HMP at 8 °C constantly for 4 h. Group B grafts were preserved with a rewarming solution at 22 °C by MMP for 4 h. Then the ultrastructural changes in the LSEC and sinusoids in Group A and B were comparatively analyzed by using osmium-maceration scanning electron microscopy with complementary transmission electron microscopy methods.
An analysis of the LSEC after warm ischemia revealed that mitochondria with condensed-shaped cristae, abnormal vesicles, reduction of ribosomes and the endoplasmic reticulum (ER) surround the mitochondria appeared. The MP subsequent after warm ischemia alleviate the abnormal vesicles and reduction of ribosomes in LSEC, which indicated the reduction of the ER damage. However, MMP could restore the tubular mitochondrial cristae, while after HMP the condensed and narrow mitochondrial cristae remained. In addition, the volume of the sinusoidal space in the liver grafts after MMP were restored, which indicated a lower risk of pressure injury than HMP.
MMP alleviates the ER damage of LSEC by warm ischemia, additionally restore the metabolism of LSEC via the normalization of mitochondria and prevent the share stress damage of liver grafts.
Core Tip: The importance of understanding liver sinusoidal endothelial cells (LSEC) damage in regulating liver injury during machine perfusion (MP) preservation has been emphasized. Here, we comparatively analyzed the ultrastructural changes in the LSEC and sinusoids around them at four hours after hypothermic MP (HMP) or midthermic MP (MMP). MP alleviated the ER damage of LSEC caused by warm ischemia. Moreover, MMP temperature conditions restore the metabolism of LSEC via the normalization of cristae of mitochondria and prevent the damage of the liver graft by share stress.
- Citation: Bochimoto H, Ishihara Y, Mohd Zin NK, Iwata H, Kondoh D, Obara H, Matsuno N. Ultrastructural changes in porcine liver sinusoidal endothelial cells of machine perfused liver donated after cardiac death. World J Gastroenterol 2022; 28(19): 2100-2111
- URL: https://www.wjgnet.com/1007-9327/full/v28/i19/2100.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i19.2100
The shortage of brain-dead donors for liver grafts is a serious problem worldwide[1]. One way to expand the donor organ liver pool is by using grafts with extended criteria including liver donated after cardiac death (DCD)[2-4]. However, there is a high risk of acute and chronic liver injury of primary nonfunction caused by ischemia-reperfusion injury after transplantation of DCD liver grafts[5]. Thus, the development of the preservation methods of the liver grafts after cardiac death is required to overcome these problems[1].
The superiority of machine perfusion (MP) preservation to simple cold storage was reported in clinical kidney preservation[6]. Similarly, strategies as MP with oxygen and nutrition-containing solution have also been reported to have numerous advantages to liver transplantation[7,8]. On the other hand, the MP of the DCD grafts has been discussed about the optimal conditions including perfusion temperature, oxygenation, flow rate and pressure, steady or pulsatile flow. Recently, hypothermic machine perfusion (HMP) has been established to preserve the functions of liver grafts, and its application in clinical practice has begun[9].
In addition, the warm MP including midthermic MP (MMP) has also been reported as a promising strategy to maintain the liver graft function to avoid cold ischemic injury and possibly repair the metabolic activity compared to HMP[10,11]. Our previous study also indicated the utilization of MMP reduces the hepatocellular enzyme release to perfusate[12]. We further confirmed that hepatocytes and bile canaliculi of DCD liver grafts after MMP retain a functional ultrastructure compared to HMP, by using scanning electron microscopy after osmium-maceration (OM-SEM) with complementary transmission electron microscopy (TEM)[9,13]. These results showed the practicality of OM-SEM with complementary TEM not only for determining 3D detailed ultrastructure in porcine hepatocytes but in evaluating the function of the transplanted liver which is reflected by ultrastructural characteristics of hepatocytes.
In addition to hepatocyte and bile canaliculi, the importance of liver sinusoidal endothelial cells (LSEC) damage in regulating liver injury during MP has been affirmed[14]. The inferior outcomes following extended criteria donor liver transplantation are thought to be endothelial dysfunction-related, however, it ultimately culminates in early graft dysfunction[14]. Thus, in this study, we comparatively analyzed the ultrastructural changes in the LSEC and sinusoids around them at four hours after HMP or MMP by using OM-SEM with complementary TEM methods. As a consequence, the LSEC that regressed one hour after warm ischemia showed a tendency to recover after MP, especially in MMP, suggesting the preventative effects of HMP and MMP on LSEC-related functions in liver grafts.
Rabbit anti-ERG monoclonal antibody was purchased from Nichirei Bioscience Corporation (Tokyo, Japan; clone EP111) for chromogenic visualization of viable LSEC in liver grafts and observation by bright field microscopy.
Domestic female porcine (cross-bred Large White, Landrace, and Duroc pigs; age, 2-3 mo; body weight, approximately 25 kg) from Taisetsusanroku-sya Co., Ltd. (Asahikawa, Japan). The animals were kept in a well-ventilated and temperature/humidity controlled room (in which a light on 12-h cycle), and ad libitum access to food and water. All animal work was performed according to the Guide for the Care and Use of Laboratory Animals at Asahikawa Medical University. All animal studies and procedures were approved by the Institutional Animal Ethics Committee of the Clinical Research Center, Asahikawa Medical University (permit No. 14172).
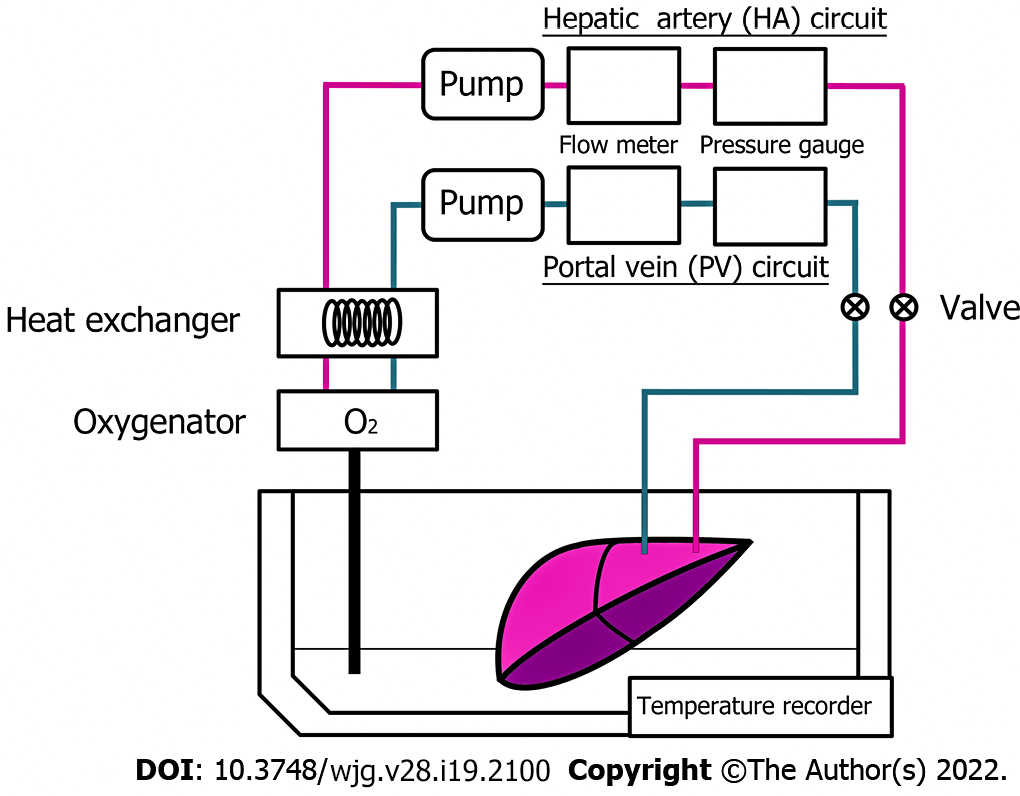
The harvested porcine liver was perfused with MP systems (Figure 1), as described previously[9,13]. This system consists of 2 separate circulating perfusion circuits for the hepatic artery (HA) and portal vein (PV), where each has a roller pump, a pressure sensor, and a flow meter connected to a separate circulation system via plastic connectors, allowing pulsatile and non-pulsatile flow, respectively. A gas blender attached to the oxygenator mixes air with oxygen is installed to the PV and HA circuit. Both circuits were connected to the hepatic vessels via plastic connectors and oxygen concentration was perfused and maintained at PO2 200-300 mmHg, where they are monitored by using dissolved oxygen meter installed. Waterproof thermocouples were installed in the perfusion systems to measure the solution temperature, and the heat exchanger was installed in the systems where ice-cold water controls the temperature in the organ chamber. As described previously, the flow rate was controlled as 0.22 mL/min/g for the PV and 0.06 mL/min/g for the HA[9,13].
Pigs weighing approximately 25 kg were used as liver graft donors. In this study, the animals were intubated and ventilated under inhalation anesthesia with isoflurane (Forane; Abbott, Japan), and laparotomized. Immediately after laparotomy, the tissue samples were biopsied from the liver surface as a control. Then the animals were injected with potassium chloride intravenously to induce cardiac arrest followed by the removal of ventilation, as described previously[9,13]. The time of the induction of cardiac arrest was set as the point of 0 min of warm ischemia. During warm ischemia, the HA and PV were isolated from the surrounded tissues to connect with organ flush lines. After 60 min of warm ischemia, the tissue samples of the liver were biopsied from distinct regions of the liver surface. Immediately after tissue sampling, the liver grafts that were procured were initially flushed with a Euro-Collins solution that is less viscous via the HA and PV routes at 8 °C as a back table operation. After the operation, the organ flush lines were connected to the perfusion preservation machine systems, and the liver was continuously perfused for 4 h with a modified University of Wisconsin gluconate solution, described previously[9]. The liver grafts were conserved as two groups, A and B. The grafts in group A were perfused at a constant temperature of 8 °C as HMP (n = 3), on the other hand, the grafts in group B were gradually warmed from 8 °C to 22 °C during perfusion as MMP (n = 3), as described previously[13]. After 4 h of MP, liver tissue samples in each group were collected from the well-perfused area of the surface of liver grafts. All the blocks of liver samples were immediately fixed with appropriate fixative for the analysis as described below.
The viability of LSEC in liver grafts preserved for 4 h was evaluated by hyaluronic acid (HyA) levels in perfusate collected from the suprahepatic vena cava as described previously[15]. These results in the text and figures are expressed by the means ± SEM. Comparisons of the significance of differences between each MP group A and B were performed by using unpaired two-tailed t-tests.
The tissue samples biopsied from the liver of porcine were immediately immersed into 10% buffered formalin subsequently embedded in paraffin. The paraffin-embedded samples were sectioned into 4 μm slices and mounted on microscope glass slides. After the paraffin was removed by xylene, these tissue slices were rehydrated with series of graded ethanol, subsequently transferred into EDTA (pH 9.0) for antigen retrieval. After washing with PBS, the tissue slices were incubated in 0.3% hydrogen peroxide in methanol for 15 min, subsequently transferred into SuperBlock Blocking Buffer for 20 min as blocking. After these treatments, the tissue slices were incubated in a dilution (1:10) of primary antibody for 1 h at room temperature. Then the tissue slices were visualized by the Envision system (DAKO; Glostrup, Denmark), counterstained by using the hematoxylin. After the dehydration with a series of graded ethanol and xylene, the tissue slices were mounted with Malinol. The sections were observed by light microscopy.
The biopsied tissue samples from the liver of porcine were cut into small pieces and immersed in the fixative mixture of 2% glutaraldehyde (GA)/2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4) for 2 h at 4 °C. After washing with 0.1 M PB containing 7.5% sucrose, the samples were post-fixed with 1% osmium tetroxide (OsO4) in 0.1 M PB for 2 h at 4 °C. After washing with 0.1 M PB containing 7.5% sucrose, the samples were dehydrated in a series of graded ethanol solutions (starting with 50% and increasing to 100% ethanol). Then the samples were embedded in epoxy resin (Epon 812). Ultrathin sections (80 nm thick) from the embedded samples in Epoxy resin were cut using a diamond knife. The sections were observed by using TEM (HT7700; Hitachi High Technologies Inc., Tokyo, Japan) without staining by uranyl acetate and lead citrate.
The osmium maceration method was described previously[9,13]. In brief, the biopsied liver tissue samples of porcine were incised into small pieces and fixed with a fixative mixture of 0.5% GA/0.5% PFA in 0.1 M PB (pH 7.4), for 30 min at 4 °C. Then the tissue samples were fixed with 1% OsO4 in 0.1 M PB for 6 h at 4 °C. After washing thoroughly with 0.1 M PB, the samples were immersed into 25% dimethyl sulfoxide (DMSO) for 30 min and subsequently 50% DMSO for 30 min as cryoprotection. Cryoprotected samples were frozen on a precooled aluminum plate chilled with liquid nitrogen. The frozen tissue samples were fractured into two pieces by using a 2.0 mm flathead screwdriver and a hammer. After freeze-fracture, the samples were transferred into 50% DMSO again for thawing, washed with 0.1 M PB, and then immersed into 0.1% OsO4 in 0.1 M PB solution for 96 h at 20 °C under the light for osmium maceration. After osmium maceration, the tissue samples were further fixed with 1% OsO4 in 0.1 M PB for 1 h at 4 °C. The samples were then washed thoroughly with 0.1 M PB before being conductive stained with 1% tannic acid in 0.1 M PB and subsequently with 1% OsO4 in 0.1 M PB. Then the samples were dehydrated in a series of graded ethanol solutions (starting 70% and increasing to 100% ethanol) and immersed in t-butyl alcohol and dried in a freeze dryer (ES2030; Hitachi High Technologies Inc., Tokyo, Japan). The dried specimens were then mounted onto an aluminum plate with silver paste and received the surface coating with platinum-palladium in an ion sputter coating equipment (E1010; Hitachi High Technologies Inc., Tokyo, Japan). After all the processes said were completed, the specimens were evaluated by using a field emission scanning electron microscopy (FE-SEM, S4100; Hitachi High Technologies Inc., Tokyo, Japan).
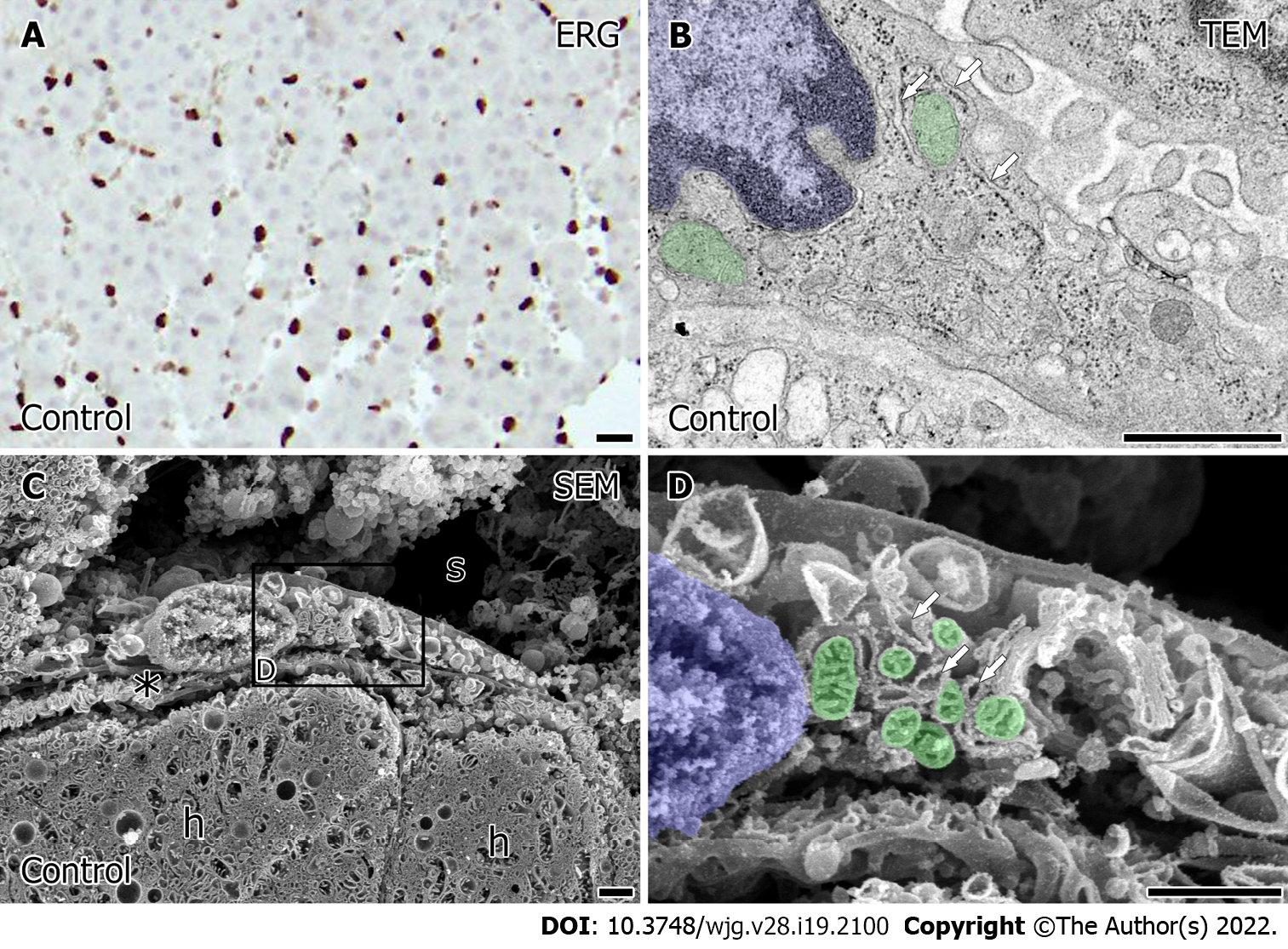
In the porcine liver, the interspersed ERG positive sinusoidal endothelial cells were observed (Figure 2A). Observation of the intercellular ultrastructure of the sinusoidal endothelial cells by TEM showed that the rough endoplasmic reticulum formed narrow networks of lumens (Figure 2B, arrow), and cytoplasmic vesicles or caveolae occasionally open to the plasma membrane. LSEC had abundant free-ribosome in their cytoplasm and the small sausage-shaped mitochondria (Figure 2B, green) localized around the nucleus (Figure 2B, blue). Observation utilized by SEM revealed more detailed ultrastructural characteristics of LSEC. Low magnified observation showed the flat-shaped LSEC lined the sinusoids (Figure 2C). Also, the space of Disse presented the normal appearance in which apical microvilli of the hepatocytes protruded. In high magnification by SEM, LSEC had the network of rough endoplasmic reticulum and vesicles correspond to TEM observation. In LSEC, mitochondria (Figure 2D, green) had tubular or plate-formed cristae sparsely arranged identified around the nucleus (Figure 2D, blue).
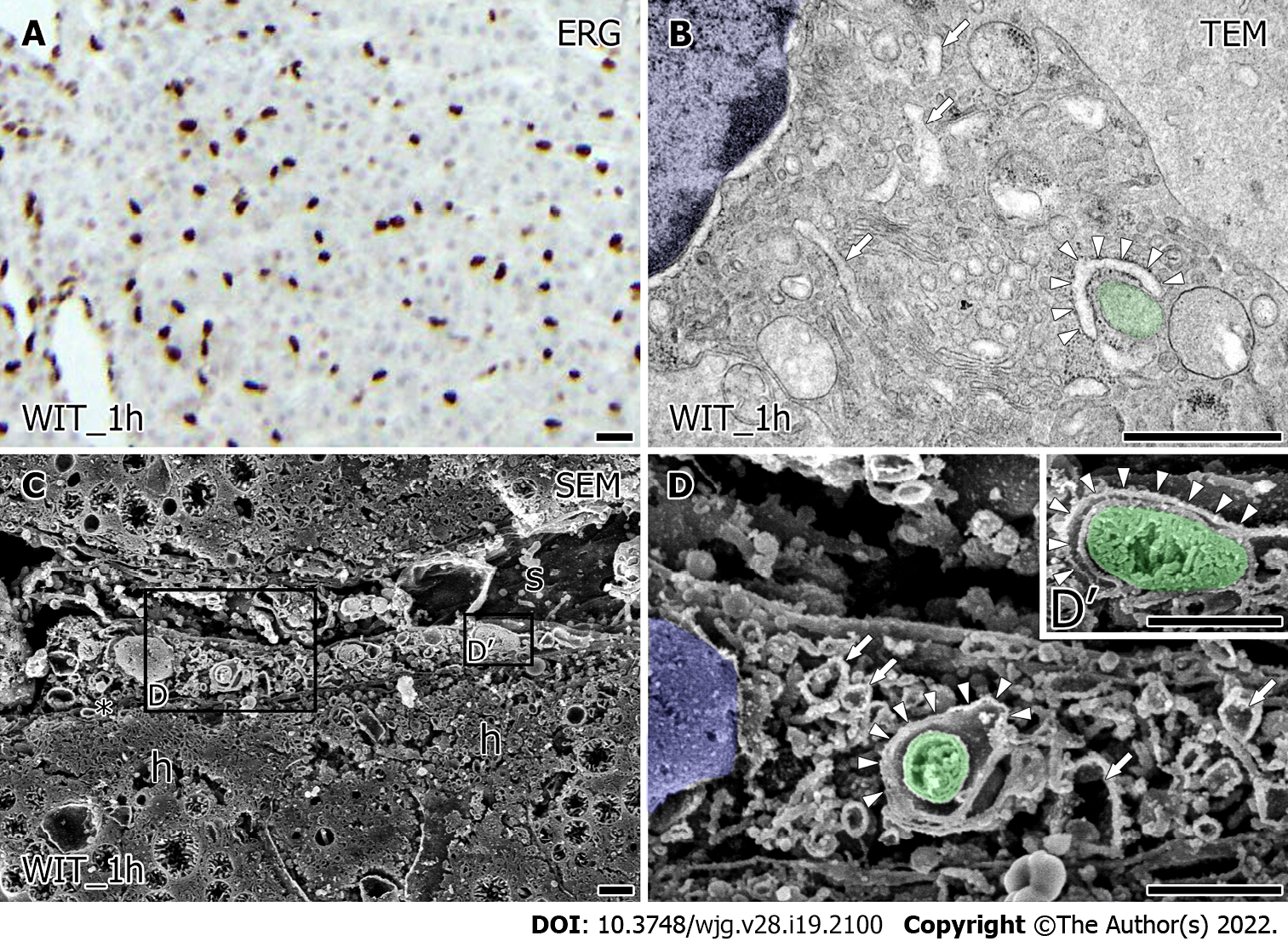
Even after warm ischemia, ERG-positive viable LSEC was detected in liver grafts similar to the samples before warm ischemia (Figure 3A). However, the ultrastructural characteristics of LSEC were substantially changed after warm ischemia. TEM observation revealed that LSEC had a slightly expanded rER, lysosome-like structure, and numerous vesicles. Furthermore, it appears to have a decrease of free ribosomes from the cytoplasm of LSEC (Figure 3B) and membranous structure (Figure 3B, arrowhead) surrounding mitochondria (Figure 3B, green) at the peripheral region from the nucleus (Figure 3B, blue). Low magnified SEM observation showed the LSEC-covered sinusoid in liver grafts after warm ischemia; however, its lumen was reduced reflecting the loss of blood flow (Figure 3C). Additionally, the space of Disse between LSEC and hepatocytes (Figure 3C) was reduced and microvilli were regressed. High magnified SEM observation revealed that a slightly expanded network of rER (Figure 3D, arrow) and numerous vesicles in LSEC. Around the nucleus (Figure 3D, blue), small mitochondria with densely distributed tubular cristae (Figure 3D, green) were surrounded by rER (Figure 3D and D’, arrowhead).
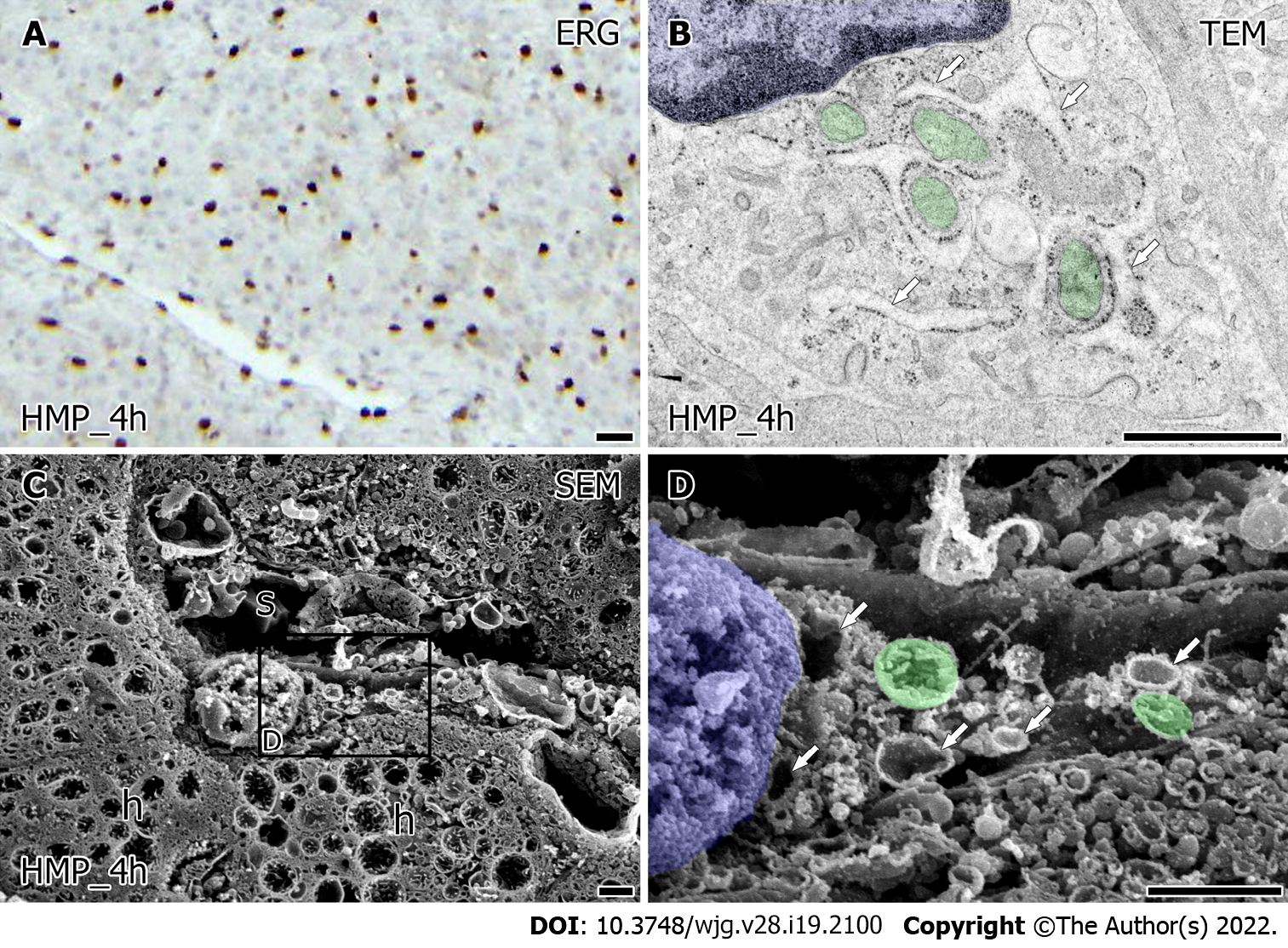
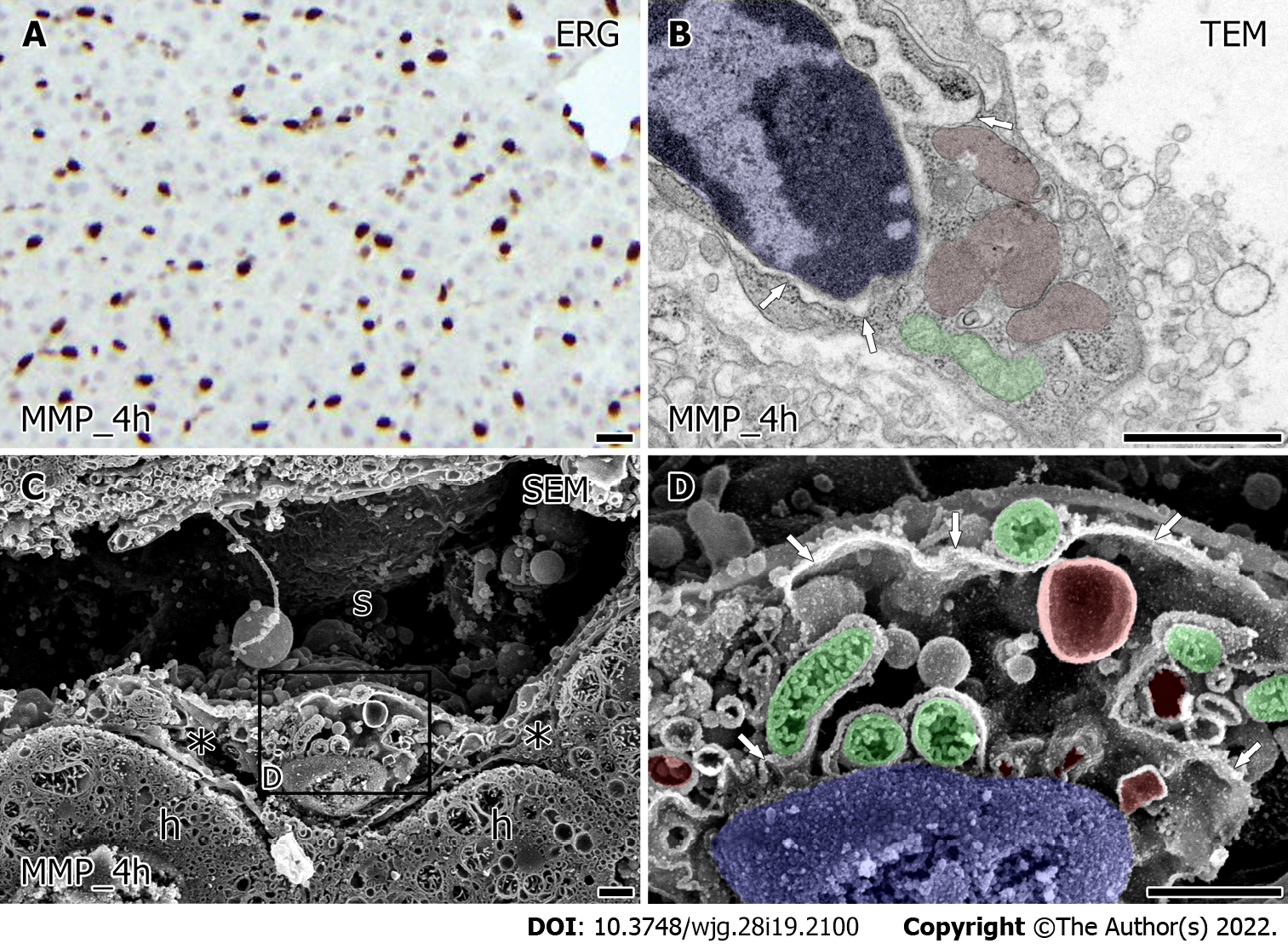
An immunohistochemical study for ERG showed similarly viable LSEC in liver grafts after 4 h for both HMP and MMP (Figures 4A and 5A). The value of HyA in the perfusate did not indicate a significant difference between HMP and MMP. However, there was a trend of improvement in MMP (Supplementary Figure 1, 38.33 ± 8.88 HMP_4h vs 26.00 ± 3.46 ng/mL MMP_4h, P = 0.27). Furthermore, the ultrastructural characteristics of LSEC after each MP also showed a difference (Figures 4 and 5). TEM observation of LSEC after 4 h of HMP showed that small globular mitochondria and the slightly expanded network formation of rER localized in the cytoplasm (Figure 4B, arrow). Besides, intracellular vesicles and free ribosomes in LSEC after HMP were decreased from the point of warm ischemia 1 h. Low magnified SEM observation showed that the lumen of the sinusoids (Figure 4C) were lined by LSEC, although there were scarcely spaced cavities of Disse between the LSEC and hepatocytes (Figure 4C). At higher magnification, intracellular expanded rER networks were observed in LSEC. Besides, the spherical mitochondria (Figure 4D, green) had densely but shrinking tubular cristae and remained localized around the nucleus (Figure 4D, blue). On the other hand, after 4 h of MMP, expanded rER (Figure 5B, arrow) and the network of endomembranous structures that had a smooth surface and expanded lumen with high electron density simultaneously appeared in LSEC by TEM observation (Figure 5B, red). Moreover, LSEC had the relatively abundant interspersed free-ribosomes in their cytoplasm and mitochondria with sausage-like formation neighboring to the nucleus (Figure 5B). Observation by SEM revealed more detailed intrahepatic ultrastructure containing LSEC. In low magnification, sinusoids of liver grafts after 4 h of MMP tend to enlarge and form the cavity of Disse than that of HMP (Figure 5C). High-magnified SEM observation revealed that LSEC had the expanded rER attached to the surface-smoothed vacuolar structure in the cytoplasm (Figure 5D). Furthermore, the mitochondria with regularly arranged cristae localized around the nucleus in LSEC take a form of sausage-like structure again (Figure 5D, green).
In this present study, we confirmed the usefulness of the OM-SEM with complementary TEM for the description of the ultrastructure of organelles in LSEC. Based on this, we revealed the intracellular ultrastructural characteristics of porcine LSEC post warm ischemia after HMP and MMP preservation by utilizing osmium maceration for SEM and complementary TEM methods.
OM-SEM observation could demonstrate the 3-dimensional architecture of mitochondria cristae in porcine LSEC (Figure 1D). Previous studies using OM-SEM analyzed the intracellular ultrastructure of hepatocytes including bile canaliculi but did not describe the porcine LSEC[9,13,16]. Our data revealed for the first time that the mitochondria in porcine LSEC have tubular cristae with a relative expansion of matrix. The orthodox-shaped mitochondrial cristae are thought to reflect the state of Low ADP[17].
The distribution of anti-ERG antibody-positive cells which indicate viable LSEC in the liver after 60 min of warm ischemia does not differ from normal, which is consistent with our previous report[18]. Nevertheless, the intracellular ultrastructural characteristics of LSEC were altered after warm ischemia. Our data revealed that mitochondria with condensed-shaped cristae, vesicles, and the ER surround the mitochondria appeared in the LSEC after warm ischemia (Figure 3D and D’). It is known that LSECs are specifically sensitive to energy deficiency[19]. The condensed-shaped cristae of mitochondria reflect an energy substrate deficiency[17]. During warm ischemia, the lack of energetic substrate interferes with active transmembrane transport in LSEC[20]. ER injury of LSEC also precedes mitochondrial disintegration in the setting of warm ischemia[4]. Damaged ER may cause the appearance of vesicles and vacuoles in LSEC after warm ischemia[21,22]. These suggest that warm ischemia impaired the membrane transport between the ER and mitochondria consequently caused morphological distortion of ER including vesiculation and membranes surrounding mitochondria.
The MP after warm ischemia alleviates the abnormal vesicles and promotes the reduction of ribosomes in LSEC (Figures 4 and 5). In previous studies, it was indicated that LSEC injury reduces liver viability, and MP protects against and alleviates the effects of endothelial injury at the ultrastructural level[23-25]. One of the potential protective factors of our machine perfusion systems is the early hypothermic period. The lowest degree of damage on LSEC using electron microscopy was seen at the 4 and 25 °C of machine perfusion groups[26]. Hypothermic state of machine perfusion rescue DCD livers subjected to prolonged warm ischemia via downregulation of LSEC activation[27]. The flow of machine perfusion is also another potential protective factor. The flow stasis leads to acute endothelial dysfunction and apoptosis via rapid loss of shear-stress-dependent KLF2 in LSEC[28]. Machine perfusion which triggers protection of LSEC via upregulation of shear stress-sensitive protective genes[29], consequently may alleviate the ultrastructural abnormalities shown by the present study. Furthermore, the high oxygen availability during machine perfusion also maintains the structural integrity of LSEC[30]. Short-term oxygenated perfusion potentially reduces the risk of a possible release of reactive oxygen species and endothelial shear stress[4]. Consistent with these studies, our present study showed that MP alleviates the ultrastructural abnormalities that appeared in LSEC after warm ischemia.
After warm ischemia, MMP restores the tubular mitochondrial cristae, while after HMP the condensed and narrow mitochondrial cristae remained (Figures 4 and 5). This is consistent with the finding of the previous study that the hepatocyte preserved by HMP has strongly swollen mitochondria, in contrast, MMP could preserve the functional appearance of mitochondria in hepatocytes[13]. These differences of findings rely on the temperature condition during MP. The ultrastructural feature of mitochondria with narrow cristae which appeared after HMP is similar to mitochondria observed in LSEC after cold incubation in UW solution in vitro[31]. During hypothermia, LSEC undergoes a cold-induced decrease in mitochondrial membrane potential and finally loses viability and induces apoptosis[31-33]. Similarly, in vivo, the HMP compromises liver grafts by prompting cold-induced damage to LSEC[34]. HMP led to a significant slowdown of mitochondrial respiration rate[33], therefore, interrupting the balance of oxygen supply/demand of LSEC. Furthermore, the reduction of membrane fluidity induced by continuous cool perfusion temperatures of HMP was examined in a previous study[35]. Extended times of HMP may also be subject to disadvantages and limitations concerning endoplasmic stress and alteration of LSEC[16].
On the other hand, the controlled oxygenated rewarming, similar to MMP, was reported to significantly increase gene expression and protein levels of the autophagy-related beclin-1 in liver grafts[11]. Electron-dense endomembranous structure that appears in LSEC after MMP resembles autophagolysosome which suggests that the LSEC is protected by autophagy[36]. The restored tubular orthodox-shaped mitochondrial cristae in LSEC after MMP (Figures 5B and D), could supply more appropriate energy to match the demand of intracellular function of LSEC. MMP perfusate tends to have lower HyA levels compared to HMP (Supplementary Figure 1) supports the justification of LSEC has more functional integrity after MMP[15].
In addition to hypothermia, fluid shear stress can further aggravate the damage in LSEC after HMP[7,37]. It was observed that the reduction of the space of Disse in the liver grafts after HMP may be the cause of the pressure injury (Figures 4 and 5). Liver sinusoids are also known to be very sensitive to endothelial shear stress because LSEC is easily damaged by high pressure[38,39]. Endothelial injury by shear stress is the main risk of HMP[4,24,37,40]. Vascular perfusion of the liver at 4 °C induces vasoconstriction of the hepatic vasculature[41] and alters LSEC, which is vulnerable to hypothermia[31]. Morphological changes in LSEC structures and increased vascular resistance in the liver graft occurred during HMP, which obstruct the sinusoidal flow[37]. In the present study, the LSEC after HMP was observed as flat-shaped, which consistent with the findings reported by Chai et al[42].
In contrast to the HMP, our previous study showed that the pressure transitions of the HA decrease in the initial stage of MMP[12], which suggests the lower risk of shear stress in LSEC after MMP. In the present study, we showed that the volume of the sinusoidal space in the liver grafts after MMP was restored as control liver, which suggests that the decreased vascular resistance via sinusoidal space enlargement led to reducing the risk of pressure injury in MMP.
This present study has several limitations. First, the condition of MP including temperature during perfusion should be more refined for clinical application. Similar to the previous study of allogenic transplantation of porcine liver grafts under similar conditions as this study[18], the present MMP condition is not suitable for clinical transplantation. Our previous studies suggested that the liver grafts preserved by MMP suffered damage to some extent[13]. The MP with rewarming gave a more promising alternative, as many results preferring these temperature settings were reported in previous studies[43]. Furthermore, previous studies suggest that oxidative stress and endothelial shear stress can be reduced by modulating the perfusion temperature and oxygenation[44]. However, the rewarming velocity of liver grafts, the critical temperature, and the appropriate period of warm temperature needed to adjust metabolic parameters are yet to be sufficiently defined[43]. Therefore, the ultrastructural characteristics of the LSEC preserved by a better-modified MP method should be analyzed in future studies.
However, this study did not evaluate the ultrastructural characteristics of the LSEC in the liver monitored after MP for evaluation of the ischemic reperfusion injury. The most marked destructive changes of ultrastructure in LSEC are seen during reperfusion[45]. Thus, the results of the present study should be further investigated by future studies utilized normothermic reperfusion ex-situ.
In conclusion, MP preservation alleviated the ER damage of LSEC caused by warm ischemia. Moreover, MMP temperature conditions restore the metabolism of LSEC via the normalization of cristae of mitochondria and prevent the damage of the liver graft by share stress. In the future, more appropriate MP conditions clinically to preserve the LSEC functions of the liver grafts should be established by using normothermic reperfusion machine systems. Further analysis of detailed mechanisms of the ultrastructural changes in LSEC under various conditions, including different levels of oxygenation and different temperatures, during perfusion storage, is required for the application of MP to clinical transplantation.
Using liver grafts donated after cardiac death (DCD) is one way to solve the shortage of donors for liver transplantation. Machine perfusion (MP) preservation has been considered as a promising, and the optimal conditions of perfusion temperature of MP of the DCD grafts, including hypothermic MP (HMP) and midthermic MP (MMP) has been discussed.
Recent research showed the practicality of scanning electron microscopy after osmium-maceration (OM-SEM) with complementary transmission electron microscopy (TEM) in evaluating the function of the liver grafts which is reflected by ultrastructural characteristics of hepatocytes. This has prompted the application of this novel strategy for the evaluation of liver sinusoidal endothelial cells (LSEC) damage in regulating liver injury during MP.
The present study aimed to establish the usefulness of the OM-SEM with complementary TEM for the evaluation of LSEC damage, and comparatively investigate the ultrastructural changes associated with LSEC at 4 h after HMP or MMP.
Female pigs were intubated and ventilated under anesthesia, and their liver tissues were biopsied immediately after laparotomy, as control, and 60 min of warm ischemia. The liver grafts which had warm ischemia of 60 min were perfused for 4 h with modified University of Wisconsin gluconate solution by HMP at 8 °C constantly or MMP rewarming up to 22 °C and biopsied at the endpoint of MP. the LSEC in all biopsied liver samples were analyzed by immunohistochemistry and OM-SEM with complementary TEM. The viability of LSEC in liver grafts preserved for 4 h was evaluated by hyaluronic acid levels in the perfusate.
Immunohistochemistry showed the interspersed viable ERG positive sinusoidal endothelial cells in all biopsied liver samples. After warm ischemia, the LSEC showed the mitochondria with condensed-shaped cristae, abnormal vesicles, reduction of ribosomes and the ER surrounding the mitochondria. Both the HMP and MMP after warm ischemia alleviate the ER damage in LSEC indicated by the abnormal vesicles and reduction of ribosomes. The value of HyA in the perfusate did not indicate a significant difference between HMP and MMP, although there was a trend of improvement in MMP, moreover, only MMP, not HMP, could restore the tubular cristae of mitochondria.
This research confirmed the usefulness of the OM-SEM with complementary TEM for the evaluation of LSEC damage reflected on the ultrastructure of organelles. MP alleviate the ultrastructural abnormalities indicating ER damage of LSEC caused by warm ischemia. Moreover, MMP temperature conditions restore the metabolism of LSEC via the normalization of ultrastructural characteristics of cristae of mitochondria and prevent the damage of the liver graft by share stress.
The OM-SEM with complementary TEM is applicable for the detailed evaluation of LSEC damage reflected on the ultrastructure of the MP of various conditions, including different temperatures during perfusion storage, for clinically application of the MP for liver transplantation.
We thank all of the lab members and colleagues for their helpful suggestions and assistance in the experiments. We are extremely grateful to Dr. Hiroyuki Kanazawa for assistance with immunohistochemistry and Mr. Yoshiyasu Satake for assistance with carrying out all of the research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Carlis R, Italy; Zhu C, China S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Dutkowski P, Linecker M, DeOliveira ML, Müllhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 2. | Okamura Y, Hata K, Tanaka H, Hirao H, Kubota T, Inamoto O, Kageyama S, Tamaki I, Yermek N, Yoshikawa J, Uemoto S. Impact of Subnormothermic Machine Perfusion Preservation in Severely Steatotic Rat Livers: A Detailed Assessment in an Isolated Setting. Am J Transplant. 2017;17:1204-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Goldaracena N, Barbas AS, Selzner M. Normothermic and subnormothermic ex-vivo liver perfusion in liver transplantation. Curr Opin Organ Transplant. 2016;21:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Dutkowski P, de Rougemont O, Clavien PA. Machine perfusion for 'marginal' liver grafts. Am J Transplant. 2008;8:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20:6159-6169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Moers C, Pirenne J, Paul A, Ploeg RJ; Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2012;366:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Burra P, Zanetto A, Russo FP, Germani G. Organ Preservation in Liver Transplantation. Semin Liver Dis. 2018;38:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Westerkamp AC, Mahboub P, Meyer SL, Hottenrott M, Ottens PJ, Wiersema-Buist J, Gouw AS, Lisman T, Leuvenink HG, Porte RJ. End-ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transpl. 2015;21:1300-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Ishihara Y, Bochimoto H, Kondoh D, Obara H, Matsuno N. The ultrastructural characteristics of bile canaliculus in porcine liver donated after cardiac death and machine perfusion preservation. PLoS One. 2020;15:e0233917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Hoyer DP, Mathé Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, Paul A, Minor T. Controlled Oxygenated Rewarming of Cold Stored Livers Prior to Transplantation: First Clinical Application of a New Concept. Transplantation. 2016;100:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Minor T, Efferz P, Fox M, Wohlschlaeger J, Lüer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Obara H, Matsuno N, Shigeta T, Hirano T, Enosawa S, Mizunuma H. Temperature controlled machine perfusion system for liver. Transplant Proc. 2013;45:1690-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bochimoto H, Matsuno N, Ishihara Y, Shonaka T, Koga D, Hira Y, Nishikawa Y, Furukawa H, Watanabe T. The ultrastructural characteristics of porcine hepatocytes donated after cardiac death and preserved with warm machine perfusion preservation. PLoS One. 2017;12:e0186352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Bhogal RH, Mergental H, Mirza DF, Afford SC. The Emerging Importance of Liver Sinusoidal Endothelial Cells in Regulating Injury during Machine Perfusion of Deceased Liver Donors. Semin Liver Dis. 2018;38:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Tamaki I, Hata K, Okamura Y, Nigmet Y, Hirao H, Kubota T, Inamoto O, Kusakabe J, Goto T, Tajima T, Yoshikawa J, Tanaka H, Tsuruyama T, Tolba RH, Uemoto S. Hydrogen Flush After Cold Storage as a New End-Ischemic Ex Vivo Treatment for Liver Grafts Against Ischemia/Reperfusion Injury. Liver Transpl. 2018;24:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Okouchi Y, Sasaki K, Tamaki T. Ultrastructural changes in hepatocytes, sinusoidal endothelial cells and macrophages in hypothermic preservation of the rat liver with University of Wisconsin solution. Virchows Arch. 1994;424:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Kanazawa H, Obara H, Yoshikawa R, Meng L, Hirano T, Okada Y, Nishikawa Y, Matsuno N. Impact of Machine Perfusion on Sinusoid Microcirculation of Liver Graft Donated After Cardiac Death. J Surg Res. 2020;245:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Minor T, Hachenberg A, Tolba R, Pauleit D, Akbar S. Fibrinolytic preflush upon liver retrieval from non-heart beating donors to enhance postpreservation viability and energetic recovery upon reperfusion. Transplantation. 2001;71:1792-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 21. | Li X, Elwell MR, Ryan AM, Ochoa R. Morphogenesis of postmortem hepatocyte vacuolation and liver weight increases in Sprague-Dawley rats. Toxicol Pathol. 2003;31:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Jurado F, Buján J, Mora NP, Jiménez M, Arahuetes R, Bellón JM. A histopathological study of anoxic-resuscitated liver allografts. Histol Histopathol. 1997;12:123-133. [PubMed] |
| 23. | de Vries RJ, Pendexter CA, Cronin SEJ, Marques B, Hafiz EOA, Muzikansky A, van Gulik TM, Markmann JF, Stott SL, Yeh H, Toner M, Uygun K, Tessier SN. Cell release during perfusion reflects cold ischemic injury in rat livers. Sci Rep. 2020;10:1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Schlegel A, Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int. 2015;28:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | van der Plaats A, Maathuis MH, 'T Hart NA, Bellekom AA, Hofker HS, van der Houwen EB, Verkerke GJ, Leuvenink HG, Verdonck P, Ploeg RJ, Rakhorst G. The Groningen hypothermic liver perfusion pump: functional evaluation of a new machine perfusion system. Ann Biomed Eng. 2006;34:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Nostedt JJ, Churchill T, Ghosh S, Thiesen A, Hopkins J, Lees MC, Adam B, Freed DH, Shapiro AMJ, Bigam DL. Avoiding initial hypothermia does not improve liver graft quality in a porcine donation after circulatory death (DCD) model of normothermic perfusion. PLoS One. 2019;14:e0220786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Schlegel A, Kron P, Graf R, Dutkowski P, Clavien PA. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Selten J, Schlegel A, de Jonge J, Dutkowski P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract Res Clin Gastroenterol. 2017;31:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Russo L, Gracia-Sancho J, García-Calderó H, Marrone G, García-Pagán JC, García-Cardeña G, Bosch J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology. 2012;55:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Minor T, Akbar S, Tolba R, Dombrowski F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J Hepatol. 2000;32:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Kerkweg U, Jacob M, De Groot H, Mannherz HG, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: contribution of mitochondrial alterations. Transplantation. 2003;76:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Rauen U, Polzar B, Stephan H, Mannherz HG, de Groot H. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 1999;13:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 253] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Schlegel A, de Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 34. | Olthof PB, Reiniers MJ, Dirkes MC, Gulik TMV, Golen RFV. Protective Mechanisms of Hypothermia in Liver Surgery and Transplantation. Mol Med. 2016;21:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Czigany Z, Lurje I, Schmelzle M, Schöning W, Öllinger R, Raschzok N, Sauer IM, Tacke F, Strnad P, Trautwein C, Neumann UP, Fronek J, Mehrabi A, Pratschke J, Schlegel A, Lurje G. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Boteon YL, Laing R, Mergental H, Reynolds GM, Mirza DF, Afford SC, Bhogal RH. Mechanisms of autophagy activation in endothelial cell and their targeting during normothermic machine liver perfusion. World J Gastroenterol. 2017;23:8443-8451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 37. | Jain S, Xu H, Duncan H, Jones JW Jr, Zhang JX, Clemens MG, Lee CY. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology. 2004;48:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Schlegel A, Kron P, Dutkowski P. Hypothermic Oxygenated Liver Perfusion: Basic Mechanisms and Clinical Application. Curr Transplant Rep. 2015;2:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Jomaa A, Gurusamy K, Siriwardana PN, Claworthy I, Collier S, de Muylder P, Fuller B, Davidson B. Does hypothermic machine perfusion of human donor livers affect risks of sinusoidal endothelial injury and microbial infection? Transplant Proc. 2013;45:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant. 2016;21:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | van der Plaats A, 't Hart NA, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Hypothermic machine preservation in liver transplantation revisited: concepts and criteria in the new millennium. Ann Biomed Eng. 2004;32:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Chai YC, Dang GX, He HQ, Shi JH, Zhang HK, Zhang RT, Wang B, Hu LS, Lv Y. Hypothermic machine perfusion with metformin-University of Wisconsin solution for ex vivo preservation of standard and marginal liver grafts in a rat model. World J Gastroenterol. 2017;23:7221-7231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Marecki H, Bozorgzadeh A, Porte RJ, Leuvenink HG, Uygun K, Martins PN. Liver ex situ machine perfusion preservation: A review of the methodology and results of large animal studies and clinical trials. Liver Transpl. 2017;23:679-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Ishii D, Matsuno N, Gochi M, Otani M, Shonaka T, Takahashi H, Nishikawa Y, Yoshikawa R, Obara H, Miyamoto K, Furukawa H. Applicability of Hypothermic Oxygenate Machine Perfusion Preservation for Split-Liver Transplantation in a Porcine Model: An Experimental Study. Ann Transplant. 2020;25:e919920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Perri RE, Shah V. Hepatic Sinusoidal Endothelial Cells [Internet]. In: Signaling Pathways in Liver Diseases. Berlin/Heidelberg: Springer-Verlag 1996: 53-62. Available from: http://Link.springer.com/10.1007/3-540-27194-5_5. |