Published online May 21, 2022. doi: 10.3748/wjg.v28.i19.2034
Peer-review started: January 4, 2022
First decision: March 10, 2022
Revised: March 20, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: May 21, 2022
Processing time: 139 Days and 10.4 Hours
The coronavirus disease 2019 (COVID-19) is known to cause gastrointestinal symptoms. Recent studies have revealed COVID-19-attributed acute pancreatitis (AP). However, clinical characteristics of COVID-19-attributed AP remain unclear. We performed a narrative review to elucidate relation between COVID-19 and AP using the PubMed database. Some basic and pathological reports revealed expression of angiotensin-converting enzyme 2 and transmembrane protease serine 2, key proteins that aid in the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the pancreas. The experimental and pathological evaluation suggested that SARS-CoV-2 infects human endocrine and exocrine pancreas cells, and thus, SARS-CoV-2 may have a direct involvement in pancreatic disorders. Additionally, systemic inflammation, especially in children, may cause AP. Levels of immune mediators associated with AP, including interleukin (IL)-1β, IL-10, interferon-γ, monocyte chemotactic protein 1, and tumor necrosis factor-α are higher in the plasma of patients with COVID-19, that suggests an indirect involvement of the pancreas. In real-world settings, some clinical features of AP complicate COVID-19, such as a high complication rate of pancreatic necrosis, severe AP, and high mortality. However, clinical features of COVID-19-attributed AP remain uncertain due to insufficient research on etiologies of AP. Therefore, high-quality clinical studies and case reports that specify methods for differential diagnoses of other etiologies of AP are needed.
Core Tip: Several review articles have explored the relationship between coronavirus disease 2019 (COVID-19) and acute pancreatitis (AP). However, due to various etiologies associated with AP, COVID-19-attributed AP is controversially defined. Therefore, this narrative review attempted to reveal clinical features of COVID-19-attributed AP focused on surveillance of the other etiologies of AP. The clinical features of COVID-19-attributed AP remain uncertain due to insufficient data on etiologies of AP. Therefore, prospective cohort studies focused on patients with COVID-19 with idiopathic AP are required, especially to clearly exclude other etiologies of AP.
- Citation: Onoyama T, Koda H, Hamamoto W, Kawahara S, Sakamoto Y, Yamashita T, Kurumi H, Kawata S, Takeda Y, Matsumoto K, Isomoto H. Review on acute pancreatitis attributed to COVID-19 infection. World J Gastroenterol 2022; 28(19): 2034-2056
- URL: https://www.wjgnet.com/1007-9327/full/v28/i19/2034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i19.2034
The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was declared a pandemic by the World Health Organization (WHO) on March 11, 2020. COVID-19 causes respiratory symptoms, such as cough, fever, sputum production, and shortness of breath, and also leads to gastrointestinal symptoms, such as nausea, vomiting, and diarrhea[1,2]. Some studies revealed that SARS-CoV-2 RNA can be detected in the gastrointestinal tract[3,4]. SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) protein that serves as an entry point for the virus into epithelial cells[5]. SARS-CoV-2 also invades the gastrointestinal tract via ACE2, allowing development of gastrointestinal symptoms[2,6-9]. Recent studies suggest that SARS-CoV-2 infection might induce pancreatic injury or acute pancreatitis (AP)[10,11]. Schepis et al[12] identified SARS-CoV-2 RNA in a pancreatic pseudocyst sample collected from a patient with COVID-19[12]. Moreover, ACE2 expressed in the pancreas is associated with pancreatic injury[13]. In experimental system, SARS-CoV-2 infects human endocrine and exocrine cells of the pancreas, ex vivo and in vivo[14]. However, clinical features of COVID-19-attributed AP remain uncertain. Some systematic reviews have reported COVID-19-attributed pancreatic injury[15,16], but it remains uncertain whether the pancreatic injury is truly caused by SARS-CoV-2 due to insufficient search for the etiology of AP. Gallstones and alcoholism are two common etiological factors of AP[17-19]. Certain medications (valproic acid, azathioprine, and sulfonamides), metabolic disturbances (hypercalcemia and hypertriglyceridemia), and infections are also rare etiologies[19,20]. Trauma, iatrogenic considerations [e.g., endoscopic retrograde cholangiopancreatography (ERCP)], anatomy (e.g., pancreatic tumor or pancreatic divisum), ischemia/reperfusion, and genetic mutation are also reported as etiologies of AP[19,21-23]. Many studies have reported that bacterial, mycobacterial, helminthic, protozoan, and fungal infections are etiologies of AP[24,25]. Furthermore, hepatotropic virus, Coxsackie virus, Echovirus, Cytomegalovirus, human immunodeficiency virus, Herpes simplex virus, mumps virus, measles virus, varicella-zoster virus, and other viruses may cause infectious AP[24-26]. Therefore, the COVID-19-attributed AP should be diagnosed by sufficient exclusion of other etiologies of AP. Additionally, the diagnostic criteria for COVID-19 is reverse transcription-polymerase chain reaction (RT-PCR) or serological test for SARS-CoV-2, and also clinical examinations, such as chest computed tomography (CT) and clinical history. Moreover, the diagnostic criteria for AP and severity of COVID-19 and AP are not unified across reports. Thus, it is required to sufficiently evaluate etiologies of AP, and clearly define diagnostic and severity criteria of COVID-19 and AP for reviewing COVID-19-attributed AP. A sufficient evaluation of etiologies of AP should be performed, especially in case studies.
This study aimed to perform a review of literature to reveal recent findings on the association between AP and SARS-CoV-2. The review also focusses on the real-world data of COVID-19-attributed AP, with surveillance for other etiologies of AP, and reveals some clinical features of COVID-19-attributed AP.
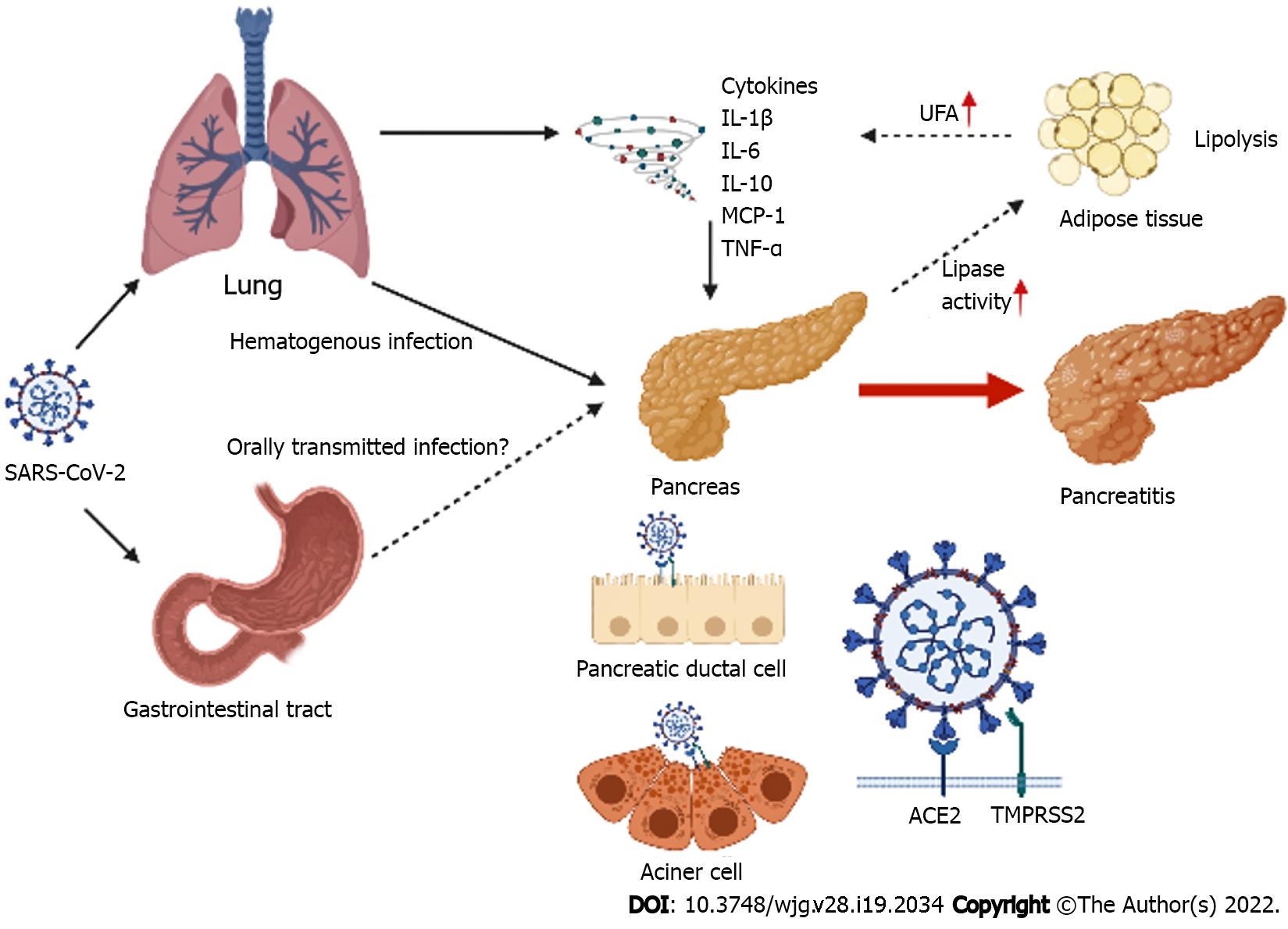
Coronaviruses constantly circulate in human populations and usually cause mild respiratory symptoms. The gastrointestinal symptoms, although not as common as respiratory symptoms, have been observed in some patients with COVID-19[2]. The SARS-CoV-2 depends on ACE2, a protein that binds to viral spike (S) protein, to enter epithelial cells[5]. SARS-CoV-2 also invades the gastrointestinal tract via ACE2 and leads to development of gastrointestinal symptoms[2,6-9]. The pancreas also expresses ACE2, with mRNA of ACE2 being expressed in the exocrine glands and islets (Figure 1)[13,27]. The transmembrane protease serine 2 (TMPRSS2), co-expressed with ACE2, is known to be required for virus entry[5,28]. TMPRSS2 is associated with proteolytic cleavage of the viral S protein, and mediates membrane insertion of S protein and viral membrane fusion. TMPRSS2 is also expressed in the pancreas, including the pancreatic ductal epithelial cells, acinar cells, and islet cells[29,30]. According to single cell analysis of human pluripotent stem cells, ACE2 and TMPRSS2 are co-expressed in the acinar cells, ductal cells, alpha cells, and beta cells[31]. Furthermore, ACE2 protein is expressed in the islet and exocrine tissue microvasculature and in a subset of pancreatic ducts, whereas TMPRSS2 is restricted to ductal cells[30]. In tissue sections derived from the pancreas of five healthy humans, the expression of ACE2 was detected in endothelial cells, a subpopulation of ductal cells, and endocrine cells, while TMPRSS2 was detected in beta cells. However, both ACE2 and TMPRSS2 were poorly expressed in acinar cells[14]. Pathological evaluation of patients deceased due to COVID-19 identified SARS-CoV-2 positivity in some ductal cells, a few acinar cells, and endocrine cells. Therefore, SARS-CoV-2 was confirmed to infect human endocrine and exocrine cells of the pancreas, in vivo[14]. Moreover, infection with SARS-CoV-2 increases expression of C-X-C motif chemokine ligand 12 (CXCL12), nuclear factor kappa β subunit 1 (NFKβ1), and signal transducer and activator of transcription 3 (STAT3) that are known to be associated with pancreatitis-related inflammation[32]. Therefore, SARS-CoV-2 may directly injure the pancreas. SARS-CoV-2 is considered to primarily disseminate via the bloodstream, but there are no data on how SARS-CoV-2 is transported to the pancreas[15]. Some patients with COVID-19 develop AP without any respiratory symptoms at onset[33]. However, it is uncertain whether SARS-CoV-2 is transported to the pancreas via the gastrointestinal tract, where SARS-CoV-2 is detected in patients with COVID-19[3].
Recent studies reported that the rate of pancreatic enzyme elevation ranged between 12.1% and 17.3% in patients with COVID-19[10,34,35]. Wang et al[10] reported the first case series of COVID-19-attributed pancreatic injury and suggested two pathophysiological theories of how pancreatic injury was caused by SARS-CoV-2[10]. First, viral infection causes direct pancreatic injury, as described above. Second, an indirect pancreatic injury is caused by systemic inflammatory responses to respiratory failure or by harmful systemic immune response induced by SARS-CoV-2 infection (Figure 1). Severe COVID-19, including acute respiratory distress syndrome (ARDS) and multiorgan failure is also known to be associated with cytokine storms in the host[36,37]. Levels of proinflammatory immune mediators associated with pancreatitis, including interleukin (IL)-1β, IL-6, IL-10, interferon-γ (IFN-γ), monocyte chemotactic protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) are higher in the plasma of patients with COVID-19 than those in healthy controls. Furthermore, in infected patients, levels of MCP-1 and TNF-α are significantly higher requiring admission to intensive care units (ICUs) than those in patients not being treated in ICUs[2,38,39]. Another study suggested that levels of IL-6, IL-10, and TNF-α were increased in patients with severe COVID-19 than in those with non-severe COVID-19[40]. Several points remain unclear in the relationship between proinflammatory immune mediators regulated by the viral effect and AP, but serum amylase and lipase elevation in patients with COVID-19 are associated with severity of COVID-19[41-44]. Additionally, AP is also more complicated in cases with severe COVID-19 than in patients with non-severe COVID-19[45]. These facts may support that cytokine storm caused by SARS-CoV-2 induces AP. In contrast, recent research suggests that the release of pancreatic lipase is associated with an increase in levels of unsaturated fatty acids[46]. It is hypothesized that the intestinal release of pancreatic lipase increases lipolysis and plasma levels of unsaturated fatty acids that may damage mitochondria and cause an increase in proinflammatory immune mediators[47]. This increase in cytokines can accelerate disease pathogenesis and lead to severe COVID-19[41,48]. Additional research and analyses are needed to validate this hypothesis, but it is uncertain whether severe COVID-19 causes AP or complicated AP is associated with increased severity of COVID-19 or both. Moreover, severe COVID-19 may complicate AP with other etiologies, including ischemia, hypercalcemia, and drugs[48]. Corticosteroid is known to induce pancreatitis; but, drug-induced AP is observed in < 5% of total AP cases, and corticosteroid accounts for only 2.8% of the drug-induced AP cases[19,49]. Tocilizumab, an antibody for IL-6 indicated for COVID-19, is also reported as an etiology for AP[50,51].
Further, multisystemic inflammatory syndrome in children (MIS-C)/pediatric inflammatory multisystem syndrome (PIMS), novel multisystem inflammatory conditions with some features similar to those of Kawasaki disease, and toxic shock syndrome leading to multiorgan failure and shock cause gastrointestinal symptoms in children[52-54]. Recent case studies also revealed that MIS-C/PIMS may complicate AP[55-58]. An international survey on children with co-occurrence of COVID-19 and AP showed that 2 of 22 patients had MIS-C/PIMS. The mechanisms of MIS-C/PIMS are unclear, but SARS-CoV-2 may cause AP via inflammatory immune mediators.
In a prospective, cohort study in the Netherlands, Bulthuis et al[59] confirmed COVID-19 in 433 patients with RT-PCR and/or chest CT scores[59]. Eight of the 433 patients met the Revised Atlanta Classification of AP and all were teetotalers. Three of eight patients had other etiologies (two biliary and one post-ERCP); thus, five (1.2%) were suspected with COVID-19-attributed AP. The median age of the five cases was 60 (range, 47–71) years, and 80% were men. Necrotic changes were not observed in the pancreas of the five patients. All five had organ failures, and three (60%) succumbed to non-pancreatitis-related complications of COVID-19 although their AP was not severe. Vatansev et al[60] reported a retrospective cohort study comprising 150 patients, of which 29 had AP, and COVID-19 was confirmed with RT-PCR[60]. The mean age of 29 patients was 64.07 years, and 18 were men. In this study, AP was defined as abdominal pain, increased serum amylase and lipase levels (values not disclosed), and contrast-enhanced abdominal CT findings. Patients with some complications and history of habits, including gallstones, hypercalcemia, hypertriglyceridemia, alcohol consumption, and chronic pancreatitis were excluded from the study. All 29 patients had respiratory failures when diagnosed with AP. According to the Revised Atlanta Classification, the severity of AP was mild and moderate in 19 and 10 cases, respectively. The mortality was 8 of 29 patients (28%) died due to respiratory failure and multiple organ failure. These findings suggest a high mortality rate in patients with COVID-19-attributed AP. However, despite a strict investigation of etiologies of AP in the latter study, the number of patients suspected with COVID-19-attributed AP was higher, although a simple comparison was not possible due to differences in COVID-19 diagnostic criteria. Nevertheless, these studies failed to exclude other etiologies of AP, such as drugs, infections except SARS-CoV-2, and ischemia/reperfusion.
Akarsu et al[45] investigated the impact of AP on prognosis of COVID-19 in a prospective study[45]. They included 316 patients with COVID-19, of which 40 had complicated AP with various etiologies. AP was defined according to the Revised Atlanta Classification. The study showed a positive correlation between the severity of pneumonia and AP, and indicated that the frequency of AP increased with severity of pneumonia. Moreover, the mortality rate in patients with COVID-19-attributed AP was higher than that in patients with COVID-19 without AP (32.5% vs 7.97%, P < 0.0001). These studies showed that the incidence of COVID-19-attributed AP was rare, whereas comorbid COVID-19 was severe and had poor prognosis regardless of the severity of AP. This tendency was identified in suspected COVID-19-attributed AP and also AP with other etiologies. Furthermore, as described above, it is possible that severe conditions that needed treatment at ICU, induced AP in patients with COVID-19. Interestingly, Kumar et al[33] focused on the difference in the onset of AP in patients with COVID-19 in a retrospective study[33]. Lipase levels were measured and COVID-19 was confirmed with RT-PCR in 985 patients; of these, 17 cases were diagnosed with AP according to the Revised Atlanta Classification. Eight of these 17 presented with typical symptoms of AP on admission. The others developed AP after the onset of COVID-19 pneumonia and treatment with mechanical ventilation for ARDS. The number of patients were less, but the mortality rate in patients who were primarily admitted for AP was higher than that in patients who developed AP later (12.5% vs 33.3%). Several different clinical backgrounds should be considered as the reasons, etiologies of AP also seemed to be one of the causes, and it was not clear in the study. Ischemia/reperfusion and drugs were considered as etiologies of AP in patients who developed AP later, as they were treated with mechanical ventilation for ARDS.
There are several studies on AP and SARS-CoV-2 during the COVID-19 pandemic, and some of them focused on differences in the clinical course of AP, with or without comorbid COVID-19. Pandanaboyana et al[61] conducted a prospective, international, multicenter, large cohort study on patients with AP and coexistent SARS-CoV-2 infection[61]. The study, called the COVID PAN collaborative study, comprised 1777 patients with AP with various etiologies, of which 149 were SARS-CoV-2 positive. The study had some limitations, such as the diagnostic criteria of AP was uncertain, although the severity of AP was based on the Revised Atlanta Classification. The most important limitation was that the criteria for diagnosis of COVID-19 was RT-PCR for SARS-CoV-2 and also chest CT images and/or clinical course. The authors performed subgroup analysis to compare outcomes between patients negative for SARS-CoV-2 and those positive for SARS-CoV-2 confirmed by RT-PCR. After exclusion of patients from the subgroup analysis due to missing values, 82 of 909 patients with AP were positive for SARS-CoV-2. The 30-d mortality, rate of persistent organ failure, and acute pancreatic fluid collection were higher, and the length of hospital stay was longer in patients positive for SARS-CoV-2 than in those negative for SARS-CoV-2. Three retrospective cohort studies also reported results similar to those of the COVID PAN collaborative study[62-64]. Thus, concurrent AP and SARS-CoV-2 infection may lead to worse clinical outcomes, such as prolonged hospital stay, requirement of mechanical ventilation, high incidence of multiple organ failure, and high mortality than that with AP-alone. In contrast, two of three studies also revealed that the incidence of idiopathic AP in patients positive for SARS-CoV-2 was higher than that in patients negative for SARS-CoV-2[62,63]. Interestingly, the COVID PAN collaborative study also revealed that[65] SARS-CoV-2 infection may cause AP similar to other infections. Miró et al[66] conducted a retrospective case-control study, called the Unusual Manifestations of COVID-19 (UMC-19) study, comprising emergency units in Spain[66]. The diagnostic criteria for AP were according to the Revised Atlanta Classification. The diagnosis of COVID-19 was based on RT-PCR or antigen detection test, and also on chest image findings and clinical conditions. In 62 emergency departments, of the 1463693 patients tested for COVID-19, 74814 cases tested positive, and 54 of them (0.072%) developed AP. Furthermore, the frequency of non-COVID-19 patients with AP was 0.161% (2231/13888879). To compare outcomes between AP and COVID-19 groups, patients were randomly distributed into two groups-162 patients with AP without COVID-19 and 162 patients without AP with COVID-19. Patients with AP with COVID-19 showed severe clinical courses with high mortality than patients without AP with COVID-19. Moreover, there were no differences in the etiologies of AP with or without concurrent SARS-CoV-2 infections. Additionally, the incidence of AP in patients with COVID-19 was lower than that in patients with AP without COVID-19, consistent with results discussed above (0.072% vs 0.161%). In contrast, there were no differences between patients with COVID-19 with AP and those with COVID-19 without AP, except for the length of hospitalization. These results suggest that COVID-19 affects the prognosis of patients with concurrent AP and COVID-19 than those with AP-alone. A recent meta-analysis suggested that patients with AP and COVID-19 were frequently men, had idiopathic etiology of AP, a high rate of pancreatic necrosis, higher severity of AP, and serious clinical courses, such as requirement of ICU admission and mechanical ventilation, and high mortality than patients with AP without COVID-19[67]. The prognoses in patients with COVID-19 with AP and those with COVID-19 without AP were different. It remains unclear whether SARS-CoV-2 infections increase AP, as the incidence of AP is rare, but its severity is high when concurrent with COVID-19. An online survey[68] including 22 children with AP and COVID-19 was reported. They were diagnosed as COVID-19 with RT-PCR or detection of SARS-CoV-2 IgG antibodies, while the diagnostic criteria for AP was unavailable. Children aged 10–14 years accounted for 54.5% of all participants in the study. Their clinical courses were serious-60% of them required treatment in the ICU, 45% had multi-organ involvement, 11% had complicated pancreatic necrosis, and 24% developed shock.
The association of AP with SARS-CoV-2 remains unclear, but patients with concurrent AP and COVID-19 show worse prognoses. The fact that some studies reported idiopathic AP during the COVID-19 pandemic indicates existence of COVID-19-attributed AP. Therefore, prospective cohort studies focused on patients with COVID-19 with idiopathic AP, especially on how to exclude other etiologies of AP, are needed to clarify the COVID-19-attributed AP.
We identified relevant studies in the literature by searching the PubMed database. The review was restricted to articles published between December 2019 and October 2021, and selected case reports published in English. The search terms were as follows: COVID-19 pancreatitis AND "2019/01/01" (Date-Publication) to "2021/10/31" (Date-Publication) OR SARS-CoV-2 pancreatitis AND "2019/01/01" (Date-Publication) to "2021/10/31" (Date-Publication). We also screened reference lists of selected studies to manually identify relevant studies and include them in the narrative review.
AP was defined according to diagnostic criteria of the Revised Atlanta Classification[17]. The presence of two out of three features, including abdominal pain consistent with AP, serum lipase activity (or amylase activity) at least three-times greater than the upper normal limit, and characteristic findings of AP on abdominal imaging, was required for the diagnosis of AP.
The severity (mild, moderately severe, and severe) of AP was also defined according to the Revised Atlanta Classification[17].
The diagnosis of COVID-19 was defined as a positive RT-PCR result or serological test for SARS-CoV-2. The other methods, such as medical history, or chest CT findings, were excluded.
The severity of COVID-19 (mild, moderate, severe, and critical), as classified by the WHO guidelines, was used to stratify patients in the study (https://www.who.int/publications/i/item/clinical-management-of-covid-19). Critical COVID-19 was defined based on the criteria for ARDS, sepsis, septic shock, or other conditions that would normally require life-sustaining therapies, such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy. Severe COVID-19 was defined based on any of the following criteria: (1) Oxygen saturation < 90% on room air; (2) Respiratory rate > 30 breaths/min in adults and children aged > 5 years, ≥ 60 breaths/min in children aged < 2 mo, ≥ 50 in children aged 2–11 mo, and ≥ 40 in children aged 1–5 years; and (3) Signs of severe respiratory distress (accessory muscle use, inability to complete full sentences, and, in children, very severe chest-wall indrawing, grunting, central cyanosis, or presence of any other general danger signs). Non-severe COVID-19 was defined as the absence of any criteria for severe or critical COVID-19.
The data for some cases was insufficient to confirm the severity of COVID-19, but patients who received oxygen therapy were considered to have severe condition. Nevertheless, the indication for oxygen administration varied with institution. However, oxygen saturation < 90%, a criteria for severe COVID-19, was generally used as an indication for oxygen therapy. Additionally, patients who received oxygen therapy generally required hospitalization. Therefore, patients who received oxygen therapy were considered to have severe condition.
The literature search of the PubMed database identified 735 studies that met the criteria. We identified eight additional relevant articles in the references of these studies. We excluded 580 non-case study articles, a study in non-English language, three preprints, and an article that could not be reviewed. Of the remaining 158 case studies, 61 were without pancreatic injury or pancreatitis, and thus, were excluded. Moreover, 16 case reports on AP caused by other etiologies, such as biliary disease, alcohol, acute on chronic pancreatitis, hypertriglyceridemia, cytomegalovirus infection, methanol, lymphoma, and vaccination were excluded. In the remaining 81 case studies, six studies comprised eight cases that were not confirmed for AP based on the Revised Atlanta Classification. Two case studies did not include objective data for COVID-19 diagnosis. After removing these studies, 73 studies that included eight case series and 65 case reports were finally assessed in this review. In three of eight case series, one patient without pancreatitis (but with acute cholecystitis), two with negative RT-PCR test, and eight without RT-PCR test or serological IgG for SARS-CoV-2 were excluded. Eighty-two cases of suspected COVID-19-attributed AP were evaluated. Four of 82 cases were RT-PCR negative, but were serological IgG positive for SARS-CoV-2. However, these studies had a limitation that the etiological search for AP was insufficient in almost all cases. Therefore, we classified the potential of other etiologies for AP as-probable, improbable, and uncertain-for each case. The definition of “probable” for each etiology was as follows: Alcoholic AP, consuming ≥ 60 g of ethanol every day before AP onset; biliary AP, gallstones or biliary tract dilation on the abdominal image findings at AP onset (regarded as biliary AP when any serological hepatobiliary test results exceeded more than three-times the upper normal limit unless endoscopic ultrasonography or ERCP excluded any biliary diseases); hypertriglyceridemia, fasting triglycerides > 1000 mg/dL (11.3 mmol/L) at AP onset[19]; hypercalcemia, serological calcium > 10.4 mg/dL (2.60 mmol/L) at AP onset; drug-induced AP, new medication within one month before AP onset; acute aggravation on chronic pancreatitis, chronic pancreatitis existed, such as pancreatic calcification, before AP onset; infections, positive serological diagnosis for pathogens at AP onset; ischemia/reperfusion, episodes of hypoxic or hypovolemic status, i.e., cardiopulmonary arrest, shock, or mechanical ventilation, before pancreatitis onset; and trauma/anatomy, history of abdominal trauma or upper abdominal surgery with reconstruction of the gastrointestinal tract. In children, genetic AP was defined as existence of a family history of AP, i.e., ≥ 2 first-degree relatives (or ≥ 3 s-degree relatives) to have unexplained recurrent acute or chronic pancreatitis in ≥ 2 generations[69]. Moreover, in cases aged 0–19 years, MIS-C/PIMS-induced AP was also evaluated. The MIS-C/PIMS was defined according to the WHO definition (https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19). In case of insufficient etiological information, the probability of AP was regarded as uncertain.
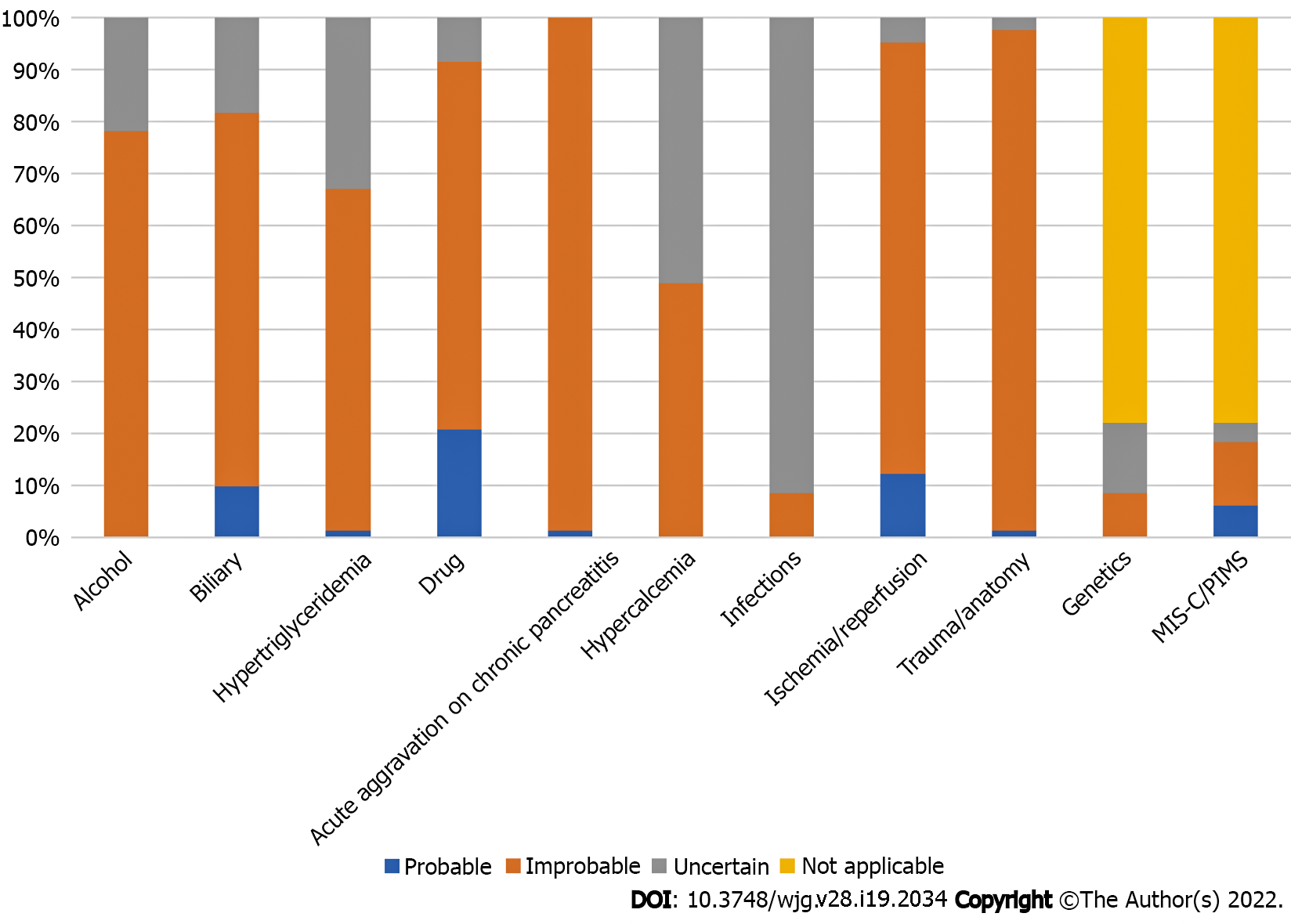
The characteristics of patients with COVID-19-attributed AP are shown in Tables 1–3. The probability of other etiologies for AP in included cases were as follows-alcohol: 0 probable, 64 improbable, and 18 uncertain; biliary AP: 8, 59, and 15; hypertriglyceridemia: 1, 54, and 27; hypercalcemia: 0, 40, and 42; drug-induced AP: 17, 58, and 17; acute aggravation on chronic pancreatitis: 1, 81, and 0; infections: 0, 7, and 75; ischemia/reperfusion: 10, 68, and 4; trauma/anatomy: 1, 79, and 2; genetic AP: 0, 7, and 11; and MIS-C/PIMS: 5, 10, and 3, respectively (Table 3 and Figure 2).
| Patient characteristics | Value |
| Age, median (range, yr) | 42.0 (6-87) |
| Gender, male/female/NA | 37/44/1 |
| Comorbidities | |
| Hypertension | 22 |
| Diabetes | 15 |
| Dyslipidemia | 4 |
| Heart disease | 3 |
| Cerebrovascular | 0 |
| Respiratory disease | 4 |
| Renal dysfunction | 5 |
| Obesity | 16 |
| Pregnancy | 2 |
| Malignancy | 2 |
| History of abdominal surgery | 13 |
| Others | 10 |
| Alcohol consumption, none/low/heavy/NA | 53/9/0/20 |
| History of smoking, never/experience/current/NA | 32/2/0/48 |
| Severity of COVID-19 | |
| Non-severe/severe/critical/NA | 35/23/20/4 |
| Symptoms | |
| Fever | 49 |
| Breath shortness | 16 |
| Cough | 28 |
| Dyspnea | 18 |
| Sore throat | 18 |
| Fatigue | 9 |
| Headache | 7 |
| Myalgia | 10 |
| Anorexia | 13 |
| Diarrhea | 16 |
| Abdominal pain | 75 |
| Nausea | 39 |
| Vomiting | 48 |
| Others | 23 |
| Blood test | |
| WBC, median (range, × 103/mm3) | 13.10 (3.40-230.00) |
| PLT, median (range, × 103/mm3) | 235.5 (52.0-502.0) |
| D-Dimer, median (range, μg/mL) | 4.9 (0.3-17.7) |
| Amylase, median (range, U/L) | 635 (47-4530) |
| Lipase, median (range, U/L) | 895.5 (35.6-11920.0) |
| LDH, median (range, U/L) | 366.0 (170-3553) |
| CRP, median (range, mg/dL) | 8.5 (0.3-59.7) |
| Imaging findings (n = 75) | |
| Pancreatic enlargement | 42 |
| Peripancreatic fluid collection | 33 |
| Peripancreatic inflammatory change | 48 |
| Pancreatic ischemic change | 12 |
| No change of pancreas | 6 |
| Not visualized | 2 |
| Severity of acute pancreatitis | |
| Mild/moderate/severe/NA | 28/20/28/6 |
| Therapy for COVID-19 | |
| Lopinavir/ritonavir | 4 |
| Favipiravir | 4 |
| Umifenovir | 2 |
| Remdesivir | 8 |
| Hydroxychloroquine | 2 |
| Tocilizumab | 2 |
| Corticosteroid (n = 53) | 21 |
| Oxygen therapy (n = 69) | 42 |
| Mechanical ventilation | 19 |
| Therapy for acute pancreatitis | |
| Conservative therapy/surgical drainage/NA | 72/4/6 |
| Period of hospitalization, median (range, day) | 7.5 (2-76) |
| Prognosis | |
| Alive/death/NA | 69/10/3 |
| Case No. | Ref. | Age | Gender | Diagnostic evidence for SARS-CoV-2 | Severity of COVID-19 | Abdominal pain | Amylase/lipase (IU/L) | Abdominal image findings | Modality of image | Severity of acute pancreatitis | Treatment for pancreatitis | Prognosis |
| 1 | Anand et al[70] | 59 | Female | RT-PCR | Non-severe | Presence | NA/NA | Diffuse pancreatic enlargement | CT | Mild | Conservative | Alive |
| 2 | Hadi et al[71] | 47 | Female | RT-PCR | Critical | Absence | 1500/NA | Diffuse pancreatic enlargement | AUS | Severe | NA | NA |
| 3 | Hadi et al[71] | 68 | Female | RT-PCR | Critical | Presence | 934/NA | NA | NA | Severe | NA | NA |
| 4 | Aloysius et al[72] | 36 | Female | RT-PCR | Critical | Presence | 325/627 | No change | CT | Severe | Conservative | Alive |
| 5 | Miao et al[73] | 26 | Female | RT-PCR | Non-severe | Presence | NA/430 | Diffuse pancreatic enlargement | CT | NA | NA | Alive |
| 6 | Szatmary et al[74] | 29 | Male | RT-PCR | Non-severe | Presence | 77/NA | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 7 | Szatmary et al[74] | 47 | Male | RT-PCR | Non-severe | Presence | 211/NA | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 8 | Rabice et al[75] | 36 | Female | RT-PCR | Severe | Presence | 88/875 | Not visualized | AUS | Mild | Conservative | Alive |
| 9 | Alloway et al[76] | 7 | Female | RT-PCR | Non-severe | Presence | NA/1672 | Diffuse pancreatic enlargement | AUS and CT | Mild | Conservative | Alive |
| 10 | Karimzadeh et al[77] | 65 | Female | RT-PCR | Severe | Presence | 285/294 | No change | CT | Mild | Conservative | Alive |
| 11 | Gonzalo-Voltas et al[78] | 76 | Male | RT-PCR | Non-severe | Presence | 3568/NA | Diffuse pancreatic enlargement | CT | Moderate | Conservative | Alive |
| 12 | Bokhari et al[79] | 32 | Male | RT-PCR | Non-severe | Presence | 672/721 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Mild | Conservative | Alive |
| 13 | Mazrouei et al[80] | 24 | Male | RT-PCR | Non-severe | Presence | 391/578 | Enlargement of pancreatic tail, peripancreatic fluid collection | CT | Mild | Conservative | Alive |
| 14 | Ahmed et al[81] | 47 | Male | RT-PCR | Severe | Presence | 349/NA | Peripancreatic inflammatory change | CT | Severe | NA | Alive |
| 15 | Brikman et al[82] | 61 | Male | RT-PCR | Severe | Presence | 142/203 | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 16 | Kataria et al[83] | 49 | Female | RT-PCR | Severe | Presence | 501/1541 | Diffuse pancreatic enlargement, peripancreatic fluid collection | CT | Mild | Conservative | Alive |
| 17 | Cerda-Contreras et al[84] | 72 | Female | RT-PCR | Severe | Absence | 1789/1247 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Severe | Conservative | Dead |
| 18 | Cheung et al[85] | 38 | Male | RT-PCR | Non-severe | Presence | NA/338.7 | Acute pancreatitis | CT | NA | Conservative | Alive |
| 19 | Kumaran et al[86] | 67 | Female | RT-PCR | Severe | Presence | 1483/NA | Peripancreatic inflammatory change and fluid collection, non-enhancement of most of the head and proximal body | CT | Severe | Conservative | Alive |
| 20 | Purayil et al[87] | 58 | Male | RT-PCR | Non-severe | Presence | 249/NA | Not visualized | AUS | Mild | Conservative | Alive |
| 21 | Dietrich et al[88] | 72 | Male | RT-PCR | Critical | Presence | NA/185 | Normal pancreas | CT | Mild | NA | Alive |
| 22 | Patnaik et al[89] | 29 | Male | RT-PCR | Non-severe | Presence | 2861/1650 | Diffuse pancreatic enlargement, peripancreatic fluid collection | CT | Mild | Conservative | Alive |
| 23 | Wang et al[90] | 42 | Male | RT-PCR | Critical | Presence | 132/382 | Pancreatic enlargement, peripancreatic fluid collection | CT | Moderate | Conservative | Dead |
| 24 | Wang et al[90] | 35 | Male | RT-PCR | Non-severe | Presence | NA/1042 | Pancreatic enlargement, peripancreatic fluid collection | CT | Moderate | Conservative | Alive |
| 25 | Alves et al[91] | 56 | Female | RT-PCR | Critical | Presence | 544/2993 | Dffuse pancreatic enlargement, peripancreatic inflammatory change | CT and MRI | Severe | Conservative | Alive |
| 26 | Kurihara et al[92] | 55 | Male | RT-PCR | Critical | Absence | 252/263 | Pancreatic enlargement, peripancreatic inflammatory change | CT | Severe | Conservative | Alive |
| 27 | Lakshmanan and Malik[93] | 68 | Male | RT-PCR | Non-severe | Absence | 1030/2035 | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 28 | Samies et al[94] | 15 | Male | RT-PCR | Non-severe | Presence | NA/233 | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 29 | Samies et al[94] | 11 | Male | RT-PCR | Non-severe | Presence | 215/953 | No change | CT | Mild | Conservative | Alive |
| 30 | Samies et al[94] | 16 | Female | RT-PCR | Non-severe | Presence | NA/1909 | Pancreatic enlargement | CT | Mild | Conservative | Alive |
| 31 | Fernandes et al[95] | 36 | Female | RT-PCR | NA | Presence | 710/640 | Pancreatic enlargement, peripancreatic fluid collection | CT | Moderate | Conservative | Alive |
| 32 | Stevens et al[55] | 10 | Female | Serological IgG | Severe | Presence | NA/1371 | Peripancreatic inflammatory change | CT | Severe | Conservative | Alive |
| 33 | Shinohara et al[96] | 58 | Male | RT-PCR | Critical | Presence | 795/NA | Diffuse pancreatic enlargement peripancreatic inflammatory change | CT | Moderate | Conservative | Alive |
| 34 | Meyers et al[97] | 67 | Male | RT-PCR | NA | Presence | NA/5295 | Interstitial edematous pancreatitis peripancreatic inflammatory change | CT | Moderate | NA | NA |
| 35 | Ghosh et al[98] | 63 | Male | RT-PCR | Severe | Absence | 58/412 | Focal pancreatic enlargement, peripancreatic fluid collection | CT | NA | Conservative | Alive |
| 36 | Tollard et al[99] | 32 | Female | RT-PCR | Critical | Presence | NA/321 | Diffuse pancreatic enlargement peripancreatic inflammatory change | CT | Severe | Conservative | Dead |
| 37 | Kandasamy et al[100] | 45 | Female | RT-PCR | Severe | Presence | 364/293 | Diffuse pancreatic enlargement, peripancreatic inflammatory change and fluid collection | CT | Moderate | Conservative | Alive |
| 38 | Hassani et al[101] | 78 | Female | RT-PCR | Critical | Presence | 1200/1450 | Pancreatic enlargement necrotizing pancreatitis | AUS and CT | Severe | Conservative | Dead |
| 39 | Suchman et al[56] | 10 | Female | RT-PCR | Non-severe | Presence | NA/365.7 | NA | NA | Moderate | Conservative | Alive |
| 40 | Suchman et al[56] | 16 | Male | RT-PCR | Critical | Presence | NA/233.3 | NA | NA | Severe | Conservative | Alive |
| 41 | Narang et al[102] | 20 | Female | RT-PCR | Severe | Presence | 1168/916 | Acute pancreatitis | MRI | Severe | Conservative | Alive |
| 42 | Acherjya et al[103] | 57 | Female | RT-PCR | Severe | Presence | 80/8352 | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Moderate | Conservative | Alive |
| 43 | Alwaeli et al[104] | 30 | Male | RT-PCR | Critical | Presence | 151/1022 | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Severe | Conservative | Alive |
| 44 | Simou et al[105] | 67 | NA | RT-PCR | Severe | Absence | NA/576 | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Severe | Conservative | Dead |
| 45 | Jespersen Nizamic et al[106] | 49 | Female | RT-PCR | Non-severe | Presence | NA/2864 | Peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 46 | Abraham et al[107] | 61 | Female | RT-PCR | Non-severe | Presence | NA/1018 | NA | NA | Mild | Conservative | Alive |
| 47 | Abhinay et al[108] | 13 | Female | RT-PCR | NA | Presence | 217/365 | Peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 48 | Bouali et al[109] | 60 | Female | RT-PCR | Critical | Presence | NA/627 | Peripancreatic fluid collection | CT | Severe | Drainage of abdominal cavity, total gasterectomy | Dead |
| 49 | Alharmi et al[110] | 52 | Female | RT-PCR | Non-severe | Presence | 47/NA | Atrophic pancreas, peripancreatic inflammatory change, peripancreatic fluid collection | CT | Moderate | Conservative | Alive |
| 50 | Abbas et al[57] | 13 | Female | RT-PCR | Critical | Presence | 217.8/NA | Fluid collection | CT | Severe | Conservative | Alive |
| 51 | Bineshfar et al[111] | 14 | Male | RT-PCR | Non-severe | Presence | 1914/NA | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 52 | Paz et al[112] | 14 | Male | RT-PCR | Non-severe | Presence | 196/247 | Peripancreatic inflammatory change, fluid collection | MRI | Mild | Conservative | Alive |
| 53 | Wifi et al[113] | 72 | Female | RT-PCR | Non-severe | Presence | 1667/710 | No change | CT | Mild | Conservative | Alive |
| 54 | Sandhu et al[114] | 25 | Female | RT-PCR | Critical | Presence | 350/35.6 | Diffuse pancreatic enlargement | CT | Severe | Conservative | Dead |
| 55 | Mohammadi Arbati et al[115] | 28 | Male | RT-PCR | Critical | Presence | 1273/758 | Peripancreatic inflammatory change, fluid collection, acute necrotic pancreatitis | CT | Severe | Conservative | Alive |
| 56 | Amé et al[116] | 42 | Female | RT-PCR | NA | Presence | 2263/2799 | Pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | NA | Conservative | Alive |
| 57 | Gupta et al[117] | 25 | Female | RT-PCR | Severe | Presence | 1814/11920 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Severe | Conservative | Alive |
| 58 | Muhammad Abrar Jeelani et al[118] | 24 | Male | RT-PCR | Non-severe | Presence | NA/4174 | Peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 59 | Maalouf et al[119] | 62 | Male | RT-PCR | Non-severe | Presence | NA/4361 | Peripancreatic inflammatory change, necrotic collection | MRI | Moderate | Conservative | Alive |
| 60 | Sanchez et al[120] | 16 | Male | RT-PCR | Severe | Presence | NA/961 | Peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 61 | Ehsan et al[121] | 13 | Female | RT-PCR | Severe | Presence | 598/2331 | Peripancreatic inflammatory change, fluid collection | CT | Moderate | Conservative | Alive |
| 62 | Hanif et al[122] | 30 | Female | RT-PCR | Severe | Presence | 820/626 | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Severe | Conservative | Alive |
| 63 | Chandra et al[123] | 53 | Male | RT-PCR | Critical | Presence | NA/1200 | Peripancreatic inflammatory change | CT | Severe | Conservative | Alive |
| 64 | Alfishawy et al[124] | 17 | Male | RT-PCR | Severe | Absence | 285/273 | Pancreatic loculations | CT | Moderate | Conservative | Alive |
| 65 | Berrichi et al[125] | 36 | Female | RT-PCR | Critical | Presence | NA/2570 | Diffuse pancreatic enlargement, peripancreatic inflammatory change | CT | Severe | Conservative | Dead |
| 66 | Berrichi et al[125] | 51 | Female | RT-PCR | Severe | Presence | NA/676 | Diffuse pancreatic enlargement | CT | Mild | Conservative | Alive |
| 67 | Kripalani et al[126] | 79 | Female | RT-PCR | Critical | Presence | 1075/6178 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, acute necrotic pancreatitis | CT | Severe | Cycto-jejunostomy | Alive |
| 68 | Narayan et al[127] | 28 | Male | RT-PCR | Critical | Presence | Elevated/elevated | Diffuse pancreatic enlargement, peripancreatic inflammatory change and acute necrotic pancreatitis | CT | Severe | Conservative | Dead |
| 69 | Narayan et al[127] | 45 | Female | RT-PCR | Severe | Presence | Elevated/elevated | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 70 | Eldaly et al[128] | 44 | Male | RT-PCR | Non-severe | Presence | 773/286 | Diffuse pancreatic enlargement | CT | Mild | Conservative | Alive |
| 71 | Basukala et al[129] | 49 | Female | RT-PCR | Non-severe | Presence | 1563/568 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection, necrotic pancreatitis | CT | Severe | Gastrocolic ligament and followed by necrotic debridement, and drainage placement | Dead |
| 72 | Schembri Higgans et al[130] | 63 | Female | RT-PCR | Non-severe | Presence | 1079/NA | After Whipple surgery | CT | Mild | Conservative | Alive |
| 73 | Schembri Higgans et al[130] | 87 | Female | RT-PCR | Non-severe | Presence | 499/NA | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 74 | Schembri Higgans et al[130] | 64 | Female | RT-PCR | Non-severe | Presence | 2141/NA | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| 75 | Kopiczko et al[131] | 6 | Female | RT-PCR | Non-severe | Presence | 1124/4250 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Mild | Conservative | Alive |
| 76 | Kareva et al[58] | 11 | Male | Serological IgG | Non-severe | Presence | 489/576 | Edematous appendix no change of pancreas | AUS | NA | Conservative | Alive |
| 77 | da Costa Ferreira et al[132] | 35 | Male | RT-PCR | Severe | Presence | 1669/NA | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection normal gallbladder and biliary tract | CT | Severe | Conservative | Alive |
| 78 | Sánchez-Gollarte et al[133] | 60 | Male | Serological IgG | Severe | Presence | 4530/2220 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection, necrotic pancreatitis | CT | Severe | Drainage of abdominal cavity | Alive |
| 79 | Sudarsanam et al[134] | 35 | Male | RT-PCR | Non-severe | Presence | Normal/normal | Peripancreatic inflammatory change, fluid collection, necrotic pancreatitis of tail | CT | NA | Conservative | Alive |
| 80 | Faghih Dinevari et al[135] | 18 | Female | RT-PCR | Non-severe | Presence | 1288/1541 | Diffuse pancreatic enlargement, peripancreatic inflammatory change, fluid collection | CT | Mild | Conservative | Alive |
| 81 | Goldstein et al[136] | 11 | Male | Serological IgG | Severe | Presence | 1607/2434 | Peripancreatic inflammatory change, fluid collection, necrotic pancreatitis | MRI | Moderate | Conservative | Alive |
| 82 | Gadiparthi et al[137] | 74 | Female | RT-PCR | Non-severe | Presence | 229/7550 | Peripancreatic inflammatory change | CT | Mild | Conservative | Alive |
| Case No. | Alcohol | Biliary | Hypertrig | Drug | Acute aggravati | Hypercal | Infection | Ischemia/reperfusi | Trauma/ anatomy | Genetics | MIS-C/PIMS |
| 1 | N | ? | ? | Y | N | ? | ? | N | N | - | - |
| 2 | N | N1 | N | ? | N | N | ? | Y | N | - | - |
| 3 | ? | ? | N | ? | N | N | ? | Y | N | - | - |
| 4 | N | N | N | N | N | ? | ? | N | N | - | - |
| 5 | N | N | N | N | N | N | N2 | N | N | - | - |
| 6 | N | N | N | N | N | ? | ? | ? | N | - | - |
| 7 | N | N | N | N | N | ? | ? | ? | N | - | - |
| 8 | N | N | N | N | N | ? | ? | N | N | - | - |
| 9 | N | ? | ? | N | N | ? | ? | N | N | ? | N |
| 10 | ? | N | N | N | N | N | ? | N | N | - | - |
| 11 | N | N | ? | Y | N | ? | ? | N | N | - | - |
| 12 | N | N | N | N | N | N | ? | N | N | - | - |
| 13 | N | N | ? | N | N | ? | ? | N | N | - | - |
| 14 | ? | Y | ? | Y | N | ? | ? | N | N | - | - |
| 15 | N | N | N | Y | N | N | ? | N | N | - | - |
| 16 | N | N | N | Y | N | N | ? | N | N | - | - |
| 17 | ? | ? | N | Y | N | N | ? | Y | N | - | - |
| 18 | N | N | N | N | N | N | ? | N | N | - | - |
| 19 | N | N | N | N | N | N | ? | N | N | - | - |
| 20 | N | N | ? | N | N | ? | ? | N | N | - | - |
| 21 | N | N | ? | N | N | ? | ? | N | N | - | - |
| 22 | N | N | N | N | N | N | ? | N | N | - | - |
| 23 | N | N | N | N | N | N | ? | N | N | - | - |
| 24 | N | N | N | N | N | N | ? | N | N | - | - |
| 25 | N | N | N | N | N | N | ? | N | N | - | - |
| 26 | ? | N | N | Y | N | N | ? | Y | N | - | - |
| 27 | N | N | N | N | N | N | ? | N | N | - | - |
| 28 | N | ? | N | N | N | ? | ? | N | N | N | N |
| 29 | N | N | N | N | N | ? | ? | N | N | N | ? |
| 30 | N | Y | N | N | Y | ? | ? | N | N | N | N |
| 31 | N | N1 | ? | N | N | ? | ? | N | N | - | - |
| 32 | N | Y | N | N | N | ? | ? | N | N | ? | Y |
| 33 | N | N | N | Y | N | N | ? | Y | N | - | - |
| 34 | N | N | N | N | N | ? | ? | N | N | - | - |
| 35 | N | ? | ? | ? | N | ? | ? | N | N | - | - |
| 36 | N | Y | N | N | N | N | ? | Y | N | - | - |
| 37 | N | N | ? | N | N | ? | ? | N | N | - | - |
| 38 | N | N | N | N | N | N | ? | N | N | - | - |
| 39 | N | ? | ? | ? | N | ? | ? | ? | ? | ? | Y |
| 40 | N | ? | ? | ? | N | ? | ? | ? | ? | ? | N |
| 41 | N | N | N | Y | N | ? | ? | Y | N | - | - |
| 42 | N | N | N | Y | N | N | ? | N | N | - | - |
| 43 | N | N | N | N | N | N | N2 | N | N | - | - |
| 44 | N | N | N | Y | N | N | N2 | Y | N | - | - |
| 45 | N | ? | ? | N | N | ? | ? | N | N | - | - |
| 46 | ? | ? | ? | N | N | N | ? | N | N | - | - |
| 47 | ? | ? | ? | N | N | ? | ? | N | N | ? | N |
| 48 | ? | ? | ? | N | N | ? | ? | N | N | - | - |
| 49 | N | N | N | Y | N | N | ? | N | N | - | - |
| 50 | N | N | N | Y | N | ? | N2 | N | N | N | Y |
| 51 | N | N | ? | N | N | ? | ? | N | N | ? | N |
| 52 | ? | N | N | N | N | ? | ? | N | N | N | N |
| 53 | ? | N | N | N | N | N | ? | N | N | - | - |
| 54 | N | Y | N | N | N | N | ? | N | N | - | - |
| 55 | N | N | N | N | N | N | ? | N | N | - | - |
| 56 | N | N | N | N | N | N | ? | N | N | - | - |
| 57 | N | N | N | ? | N | N | ? | N | N | - | - |
| 58 | N | N | N | N | N | N | ? | N | N | - | - |
| 59 | N | N | N | N | N | ? | ? | N | N | - | - |
| 60 | N | N | ? | N | N | N | ? | N | N | N | N |
| 61 | N | N | N | N | N | ? | ? | N | N | ? | ? |
| 62 | N | ? | N | Y | N | N | N | Y | N | - | - |
| 63 | N | N | N | N | N | ? | ? | N | N | - | - |
| 64 | N | Y | Y | N | N | ? | ? | N | N | ? | ? |
| 65 | ? | N | ? | N | N | ? | ? | N | N | - | - |
| 66 | ? | N | ? | N | N | ? | ? | N | N | - | - |
| 67 | N | N | ? | N | N | ? | ? | N | N | - | - |
| 68 | ? | N | N | Y | N | ? | ? | N | N | - | - |
| 69 | ? | ? | ? | Y | N | ? | ? | Y | N | - | - |
| 70 | N | N | N | N | N | ? | ? | N | N | - | - |
| 71 | N | N | N | N | N | N | ? | N | N | - | - |
| 72 | N | N | ? | N | N | ? | ? | N | Y | - | - |
| 73 | ? | N | N | N | N | ? | ? | N | N | - | - |
| 74 | ? | Y | ? | N | N | ? | ? | N | N | - | - |
| 75 | N | N | N | N | N | N | N | N | N | ? | N |
| 76 | N | N | ? | N | N | N | ? | N | N | ? | Y |
| 77 | N | Y | N | N | N | N | ? | N | N | - | - |
| 78 | ? | N | ? | ? | N | N | ? | N | N | - | - |
| 79 | N | N | N | N | N | N | ? | N | N | - | - |
| 80 | N | N | N | N | N | N | ? | N | N | N | N |
| 81 | ? | ? | ? | Y | N | ? | N2 | N | N | ? | Y |
| 82 | N | N | N | N | N | N | ? | N | N | - | - |
The median age at onset of COVID-19-attributed AP was 42.0 (range, 6–87) years. The men-to-women ratio of COVID-19-attributed AP was 37:44. The patient comorbidities were: Hypertension (22 cases), diabetes (15 cases), heart disease (3 cases), respiratory disease (4 cases), obesity (16 cases), renal dysfunction (5 cases), dyslipidemia (4 cases), hyper/hypothyroidism (4 cases), gastroesophageal reflux disease (2 cases), thrombophilia (1 case), thrombosis (1 case), osteoporosis (1 case), anxiety (2 cases), and malignant disease (2 cases). There were 13 patients with histories of abdominal surgeries, including cholecystectomy, hysterectomy, cesarean section, appendectomy, Whipple procedure, small bowel resection, and renal transplantation. Two pregnant women were also included. Almost all patients had no history of alcohol and cigarette abuse. Abdominal pain was the most frequent symptom (91.5%, 75/82), followed by fever (59.8%, 49/78), vomiting (58.5%, 48/79), nausea (47.6%, 39/66), and cough (34.1%, 28/78). None of the patients had any symptoms. The median levels of white blood cells, platelets, D-Dimer, amylase, lipase, lactate dehydrogenase, and C-reactive protein were 13100/μL (range, 3400–230000), 235500/μL (range, 52000–502000), 4.9 μg/mL (range, 0.3–17.7), 635 U/L (range, 47–4530), 895.5 U/L (range, 35.6–11920.0), 366 U/L (range, 170–3553), and 8.5 mg/dL (range, 0.3–59.7), respectively.
The study included 43 patients (55.8%, 43/77) with severe or critical COVID-19. Oxygen therapy for COVID-19 was required in 42 patients (51.2%, 42/69); of those, 19 were treated with mechanical ventilation. Seventeen patients received antiviral therapy with lopinavir/ritonavir, favipiravir, umifenovir, or remdesivir. One of them was treated with both lopinavir/ritonavir and favipiravir. Moreover, two patients received hydroxychloroquine, an antimalarial drug, for treatment of COVID-19. Two cases were treated with tocilizumab, an anti-IL-6 monoclonal antibody, for COVID-19. Corticosteroids were also administered for the treatment of COVID-19 in 21 patients (39.6%, 21/53).
Abdominal images were evaluated in 75 patients, except in two patients as the pancreases could not be visualized via abdominal ultrasonography. Two other patients underwent abdominal CT or magnetic resonance imaging, and the findings were consistent with those of pancreatitis-alone, without details. Of the remaining 71 patients, 48 showed peripancreatic inflammatory changes and 33 had peripancreatic fluid collections. Pancreatic enlargement occurred in 42 patients. Pancreatic ischemic changes, such as decreased contrast enhancement in pancreatic parenchyma on abdominal computed tomography was observed in 12 patients (16.9%, 12/71). However, no change was observed in the abdominal image findings in six patients.
In this review, 28 patients were classified as having severe AP (36.8%, 28/76). Almost all patients with AP received conservative therapy, except for four cases. These four patients underwent invasive treatment; of which, one patient with AP and gastric necrosis underwent drainage of the peripancreatic necrotic collections and total gastrectomy, but failed to recover. The other patient received gastrocolic ligament, necrotic debridement, and drainage placement for acute hemorrhagic necrotizing pancreatitis, and died two days after surgery.
The median period of hospitalization for recovering from COVID-19-attributed AP was 8.0 d (range, 2–76). Ten patients (12.7%, 10/79) died due to critical COVID-19 (median hospitalization, 7.0 d; range, 2–22 d).
In summary, patients suspected with COVID-19-attributed AP were relatively young (median age, 42 years), 36.8% of them had severe conditions, and had a high mortality rate (12.7%) similar to that reported in cohort studies and meta-analyses. However, it cannot be ignored that many AP cases reported in these studies may have occurred due to etiologies other than SARS-CoV-2.
It remains controversial whether SARS-CoV-2 infections increase AP, but some basic and pathological approaches suggest mechanisms of direct and indirect involvement of the pancreas caused by SARS-CoV-2. Moreover, there are several clinical data to support existence of COVID-19-attributed AP. First, the incidence of idiopathic AP in patients positive for SARS-CoV-2 is higher than that in patients negative for SARS-CoV-2 in some cohorts with concurrent COVID-19 and AP. Second, SARS-CoV-2 infects pancreatic exocrine and endocrine cells as per the pathological evaluation of patients deceased due to COVID-19. Moreover, some clinical features of COVID-19-attributed AP, including various etiologies of AP, are revealed, such as a high rate of pancreatic necrosis, higher severity of AP, and serious clinical courses. However, clinical features of COVID-19-attributed AP remain uncertain. A sufficient investigation on etiologies of AP would improve understanding of the clinical features of COVID-19-attributed AP. High-quality clinical studies and case reports that specify the method for differential diagnoses of the other etiologies of AP, including alcohol, biliary, hypertriglyceridemia, hypercalcemia, drugs, ischemia/reperfusion, trauma, infections, and genetic associations need to be evaluated. The present review of case studies suggests criteria to classify the possibility of other etiologies for AP. The criteria may not be appropriate, but the review highlights the insufficient etiological workup of AP, especially for other infections and hypercalcemia. Moreover, in some cases, it is difficult to completely exclude drug-induced and biliary AP from the etiology of AP. These details may be informative for designing future clinical studies.
Several unsolved questions remain, such as the risk factors of AP in patients with COVID-19. It is also uncertain why some patients with COVID-19-attributed AP become severe while others do not. AP may be a rare complication of COVID-19, but some cases develop severe AP attributed to SARS-CoV-2 infection. Thus, the potential of COVID-19-attributed AP should be thoroughly investigated.
We thank our colleagues in the Division of Gastroenterology and Nephrology, Department of Multidisciplinary Internal Medicine, Faculty of Medicine, Tottori University (Tottori, Japan) for their support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Omar BJ, India; Sivanand N, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18835] [Article Influence: 3767.0] [Reference Citation Analysis (7)] |
| 2. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30045] [Article Influence: 6009.0] [Reference Citation Analysis (3)] |
| 3. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1126] [Article Influence: 225.2] [Reference Citation Analysis (1)] |
| 4. | Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:355-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14207] [Article Influence: 2841.4] [Reference Citation Analysis (0)] |
| 6. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2906] [Article Influence: 581.2] [Reference Citation Analysis (0)] |
| 7. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1992] [Article Influence: 398.4] [Reference Citation Analysis (1)] |
| 8. | Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 9. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3274] [Cited by in RCA: 3661] [Article Influence: 732.2] [Reference Citation Analysis (0)] |
| 10. | Wang F, Wang H, Fan J, Zhang Y, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159:367-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 11. | Bruno G, Fabrizio C, Santoro CR, Buccoliero GB. Pancreatic injury in the course of coronavirus disease 2019: A not-so-rare occurrence. J Med Virol. 2021;93:74-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Schepis T, Larghi A, Papa A, Miele L, Panzuto F, De Biase L, Annibale B, Cattani P, Rapaccini GL. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020;20:1011-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18:2128-2130.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 472] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 14. | Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C, Fois G, Eiseler T, Gehrmann J, van Vuuren J, Wessbecher IM, Frick M, Costa IG, Breunig M, Grüner B, Peters L, Schuster M, Liebau S, Seufferlein T, Stenger S, Stenzinger A, MacDonald PE, Kirchhoff F, Sparrer KMJ, Walther P, Lickert H, Barth TFE, Wagner M, Münch J, Heller S, Kleger A. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 363] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 15. | Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Pribadi RR, Simadibrata M. Increased serum amylase and/or lipase in coronavirus disease 2019 (COVID-19) patients: Is it really pancreatic injury? JGH Open. 2021;5:190-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4290] [Article Influence: 357.5] [Reference Citation Analysis (44)] |
| 18. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 570] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 19. | Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 549] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 20. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1375] [Article Influence: 114.6] [Reference Citation Analysis (3)] |
| 21. | Lucidi V, Alghisi F, Dall'Oglio L, D'Apice MR, Monti L, De Angelis P, Gambardella S, Angioni A, Novelli G. The etiology of acute recurrent pancreatitis in children: a challenge for pediatricians. Pancreas. 2011;40:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Vue PM, McFann K, Narkewicz MR. Genetic Mutations in Pediatric Pancreatitis. Pancreas. 2016;45:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/Reperfusion-Induced pancreatitis. Dig Surg. 2000;17:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Imam Z, Simons-Linares CR, Chahal P. Infectious causes of acute pancreatitis: A systematic review. Pancreatology. 2020;20:1312-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Rawla P, Bandaru SS, Vellipuram AR. Review of Infectious Etiology of Acute Pancreatitis. Gastroenterology Res. 2017;10:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Simons-Linares CR, Imam Z, Chahal P. Viral-Attributed Acute Pancreatitis: A Systematic Review. Dig Dis Sci. 2021;66:2162-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, Xin Y, Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 28. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 736] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 29. | Kerslake R, Hall M, Randeva HS, Spandidos DA, Chatha K, Kyrou I, Karteris E. Coexpression of peripheral olfactory receptors with SARSCoV2 infection mediators: Potential implications beyond loss of smell as a COVID19 symptom. Int J Mol Med. 2020;46:949-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G; HPAP Consortium, Brissova M, Powers AC. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020;32:1028-1040.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 31. | Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125-136.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 508] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 32. | Shaharuddin SH, Wang V, Santos RS, Gross A, Wang Y, Jawanda H, Zhang Y, Hasan W, Garcia G Jr, Arumugaswami V, Sareen D. Deleterious Effects of SARS-CoV-2 Infection on Human Pancreatic Cells. Front Cell Infect Microbiol. 2021;11:678482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Kumar V, Barkoudah E, Souza DAT, Jin DX, McNabb-Baltar J. Clinical course and outcome among patients with acute pancreatitis and COVID-19. Eur J Gastroenterol Hepatol. 2021;33:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Barlass U, Wiliams B, Dhana K, Adnan D, Khan SR, Mahdavinia M, Bishehsari F. Marked Elevation of Lipase in COVID-19 Disease: A Cohort Study. Clin Transl Gastroenterol. 2020;11:e00215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | McNabb-Baltar J, Jin DX, Grover AS, Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Shen L, Chan WW. Lipase Elevation in Patients With COVID-19. Am J Gastroenterol. 2020;115:1286-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036-1045.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3510] [Cited by in RCA: 3152] [Article Influence: 630.4] [Reference Citation Analysis (0)] |
| 37. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 938] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 38. | Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 39. | Ding L, Yang Y, Li H, Wang H, Gao P. Circulating Lymphocyte Subsets Induce Secondary Infection in Acute Pancreatitis. Front Cell Infect Microbiol. 2020;10:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3408] [Article Influence: 681.6] [Reference Citation Analysis (0)] |
| 41. | Ramsey ML, Elmunzer BJ, Krishna SG. Serum Lipase Elevations in COVID-19 Patients Reflect Critical Illness and not Acute Pancreatitis. Clin Gastroenterol Hepatol. 2021;19:1982-1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Li G, Liu T, Jin G, Li T, Liang J, Chen Q, Chen L, Wang W, Wang Y, Song J, Liang H, Zhang C, Zhu P, Zhang W, Ding Z, Chen X, Zhang B. Multidisciplinary T. Serum amylase elevation is associated with adverse clinical outcomes in patients with coronavirus disease 2019. [cited 10 December 2021]. Available from: http://www.aging-us.com. |
| 43. | Ahmed A, Fisher JC, Pochapin MB, Freedman SD, Kothari DJ, Shah PC, Sheth SG. Hyperlipasemia in absence of acute pancreatitis is associated with elevated D-dimer and adverse outcomes in COVID 19 disease. Pancreatology. 2021;21:698-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Troncone E, Salvatori S, Sena G, De Cristofaro E, Alfieri N, Marafini I, Paganelli C, Argirò R, Giannarelli D, Monteleone G, Del Vecchio Blanco G. Low Frequency of Acute Pancreatitis in Hospitalized COVID-19 Patients. Pancreas. 2021;50:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Akarsu C, Karabulut M, Aydin H, Sahbaz NA, Dural AC, Yegul D, Peker KD, Ferahman S, Bulut S, Dönmez T, Asar S, Yasar KK, Adas GT. Association between Acute Pancreatitis and COVID-19: Could Pancreatitis Be the Missing Piece of the Puzzle about Increased Mortality Rates? J Invest Surg. 2022;35:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | El-Kurdi B, Khatua B, Rood C, Snozek C, Cartin-Ceba R, Singh VP; Lipotoxicity in COVID-19 Study Group. Mortality From Coronavirus Disease 2019 Increases With Unsaturated Fat and May Be Reduced by Early Calcium and Albumin Supplementation. Gastroenterology. 2020;159:1015-1018.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Hegyi P, Szakács Z, Sahin-Tóth M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology. 2020;159:824-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Muniraj T, Dang S, Pitchumoni CS. PANCREATITIS OR NOT? J Crit Care. 2015;30:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Meczker Á, Hanák L, Párniczky A, Szentesi A, Erőss B, Hegyi P; Hungarian Pancreatic Study Group. Analysis of 1060 Cases of Drug-Induced Acute Pancreatitis. Gastroenterology. 2020;159:1958-1961.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, Cameron ML, Garcia-Diaz J, Chávez V, Mekebeb-Reuter M, Lima de Menezes F, Shah R, González-Lara MF, Assman B, Freedman J, Mohan SV. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384:20-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 919] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 51. | Flaig T, Douros A, Bronder E, Klimpel A, Kreutz R, Garbe E. Tocilizumab-induced pancreatitis: case report and review of data from the FDA Adverse Event Reporting System. J Clin Pharm Ther. 2016;41:718-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1565] [Cited by in RCA: 1761] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 53. | DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. J Pediatr. 2020;223:199-203.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 54. | Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1324] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 55. | Stevens JP, Brownell JN, Freeman AJ, Bashaw H. COVID-19-associated Multisystem Inflammatory Syndrome in Children Presenting as Acute Pancreatitis. J Pediatr Gastroenterol Nutr. 2020;71:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Suchman K, Raphael KL, Liu Y, Wee D, Trindade AJ; Northwell COVID-19 Research Consortium. Acute pancreatitis in children hospitalized with COVID-19. Pancreatology. 2021;21:31-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Abbas M, Törnhage CJ. Family Transmission of COVID-19 Including a Child with MIS-C and Acute Pancreatitis. Int Med Case Rep J. 2021;14:55-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Kareva L, Stavrik K, Mironska K, Hasani A, Bojadzieva S, Shuntov NC. A Case of Multisystem Inflammatory Syndrome in Children Presenting as Acute Appendicitis and Pancreatitis. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2021;42:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Bulthuis MC, Boxhoorn L, Beudel M, Elbers PWG, Kop MPM, van Wanrooij RLJ, Besselink MG, Voermans RP. Acute pancreatitis in COVID-19 patients: true risk? Scand J Gastroenterol. 2021;56:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Vatansev H, Yıldırım MA, Kuccukturk S, Karaselek MA, Kadiyoran C. Clinical Evaluation of Acute Pancreatitis Caused by SARS-CoV-2 Virus Infection. Gastroenterol Res Pract. 2021;2021:5579795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 61. | Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, Belgaumkar A, Gupta A, Siriwardena AK, Haque AR, Awan A, Balakrishnan A, Rawashdeh A, Ivanov B, Parmar C, M Halloran C, Caruana C, Borg CM, Gomez D, Damaskos D, Karavias D, Finch G, Ebied H, K Pine J, R A Skipworth J, Milburn J, Latif J, Ratnam Apollos J, El Kafsi J, Windsor JA, Roberts K, Wang K, Ravi K, V Coats M, Hollyman M, Phillips M, Okocha M, Sj Wilson M, A Ameer N, Kumar N, Shah N, Lapolla P, Magee C, Al-Sarireh B, Lunevicius R, Benhmida R, Singhal R, Balachandra S, Demirli Atıcı S, Jaunoo S, Dwerryhouse S, Boyce T, Charalampakis V, Kanakala V, Abbas Z, Nayar M; COVID PAN collaborative group. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut. 2021;70:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 62. | Dirweesh A, Li Y, Trikudanathan G, Mallery JS, Freeman ML, Amateau SK. Clinical Outcomes of Acute Pancreatitis in Patients With Coronavirus Disease 2019. Gastroenterology. 2020;159:1972-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ; Northwell COVID-19 Research Consortium. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients With Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology. 2020;159:2226-2228.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 64. | Karaali R, Topal F. Evaluating the effect of SARS-Cov-2 infection on prognosis and mortality in patients with acute pancreatitis. Am J Emerg Med. 2021;49:378-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Nayar M, Varghese C, Kanwar A, Siriwardena AK, Haque AR, Awan A, Balakrishnan A, Rawashdeh A, Ivanov B, Parmar C, Halloran CM, Caruana C, Borg CM, Gomez D, Damaskos D, Karavias D, Finch G, Ebied H, Pine JK, Skipworth JRA, Milburn J, Latif J, Apollos J, El Kafsi J, Windsor JA, Roberts K, Wang K, Ravi K, Coats MV, Hollyman M, Phillips M, Okocha M, Wilson MS, Ameer NA, Kumar N, Shah N, Lapolla P, Magee C, Al-Sarireh B, Lunevicius R, Benhmida R, Singhal R, Balachandra S, Demirli Atıcı S, Jaunoo S, Dwerryhouse S, Boyce T, Charalampakis V, Kanakala V, Abbas Z, Tewari N, Pandanaboyana S; COVIDPAN Collborative Group; COVID Pain Collborative Group. SARS-CoV-2 infection is associated with an increased risk of idiopathic acute pancreatitis but not pancreatic exocrine insufficiency or diabetes: long-term results of the COVIDPAN study. Gut. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Miró Ò, Llorens P, Jiménez S, Piñera P, Burillo-Putze G, Martín A, Martín-Sánchez FJ, Lamberechts J, Alquézar-Arbé A, Jacob J, Noceda J, Cano Cano MJ, Fortuny Bayarri MJ, Marín Porrino JM, Meléndez N, Pérez García C, Brasó Aznar JV, Ponce MC, Díaz Fernández E, Ejarque Martínez L, Peiró Gómez A, Tost J, Domínguez MJ, Teigell Muñoz FJ, González Del Castillo J; Spanish Investigators on Emergency Situations TeAm (SIESTA) network. A case-control emergency department-based analysis of acute pancreatitis in Covid-19: Results of the UMC-19-S6. J Hepatobiliary Pancreat Sci. 2021;28:953-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Yang F, Huang Y, Li T, Fu Y, Sun C, Xu Y, Windsor J, Fu D. Prevalence and outcomes of acute pancreatitis in COVID-19: a meta-analysis. Gut. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 68. | Slae M, Wilschanski M, Sanjines E, Abu-El-Haija M, Sellers ZM. International Survey on Severe Acute Respiratory Syndrome Coronavirus 2 and Acute Pancreatitis Co-occurrence in Children. Pancreas. 2021;50:1305-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM, Uomo G, Delhaye M, Spicak J, Drumm B, Jansen J, Mountford R, Whitcomb DC, Neoptolemos JP; European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC). Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 70. | Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107:e182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20:665-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 72. | Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 73. | Miao Y, Lidove O, Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107:e270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Szatmary P, Arora A, Thomas Raraty MG, Joseph Dunne DF, Baron RD, Halloran CM. Emerging Phenotype of Severe Acute Respiratory Syndrome-Coronavirus 2-associated Pancreatitis. Gastroenterology. 2020;159:1551-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Rabice SR, Altshuler PC, Bovet C, Sullivan C, Gagnon AJ. COVID-19 infection presenting as pancreatitis in a pregnant woman: A case report. Case Rep Womens Health. 2020;27:e00228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Alloway BC, Yaeger SK, Mazzaccaro RJ, Villalobos T, Hardy SG. Suspected case of COVID-19-associated pancreatitis in a child. Radiol Case Rep. 2020;15:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Karimzadeh S, Manzuri A, Ebrahimi M, Huy NT. COVID-19 presenting as acute pancreatitis: Lessons from a patient in Iran. Pancreatology. 2020;20:1024-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Gonzalo-Voltas A, Uxia Fernández-Pérez-Torres C, Baena-Díez JM. Acute pancreatitis in a patient with COVID-19 infection. Med Clin (Engl Ed). 2020;155:183-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Bokhari SMMA, Mahmood F. Case Report: Novel Coronavirus-A Potential Cause of Acute Pancreatitis? Am J Trop Med Hyg. 2020;103:1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Mazrouei SSA, Saeed GA, Al Helali AA. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Ahmed AOE, Mohamed SF, Saleh AO, Al-Shokri SD, Ahmed K, Mohamed MFH. Acute abdomen -like-presentation associated with SARS-CoV-2 infection. IDCases. 2020;21:e00895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ. 2020;192:E858-E859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Kataria S, Sharif A, Ur Rehman A, Ahmed Z, Hanan A. COVID-19 Induced Acute Pancreatitis: A Case Report and Literature Review. Cureus. 2020;12:e9169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 84. | Cerda-Contreras C, Nuzzolo-Shihadeh L, Camacho-Ortiz A, Perez-Alba E. Baricitinib as Treatment for Coronavirus Disease 2019 (COVID-19): Friend or Foe of the Pancreas? Clin Infect Dis. 2021;73:e3977-e3978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent Acute Pancreatitis in a Patient with COVID-19 Infection. Am J Case Rep. 2020;21:e927076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 87. | Purayil N, Sirajudeen J, Va N, Mathew J. COVID-19 Presenting as Acute Abdominal Pain: A Case Report. Cureus. 2020;12:e9659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Dietrich CG, Hübner D, Marx G, Bickenbach J, Bootsveld A. Primary presentation of COVID-19 solely with gastrointestinal symptoms: a problem for the containment of the disease. Eur J Gastroenterol Hepatol. 2020;32:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Patnaik RNK, Gogia A, Kakar A. Acute pancreatic injury induced by COVID-19. IDCases. 2020;22:e00959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Wang K, Luo J, Tan F, Liu J, Ni Z, Liu D, Tian P, Li W. Acute Pancreatitis as the Initial Manifestation in 2 Cases of COVID-19 in Wuhan, China. Open Forum Infect Dis. 2020;7:ofaa324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Alves AM, Yvamoto EY, Marzinotto MAN, Teixeira ACS, Carrilho FJ. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis. 2020;24:561-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Kurihara Y, Maruhashi T, Wada T, Osada M, Oi M, Yamaoka K, Asari Y. Pancreatitis in a Patient with Severe Coronavirus Disease Pneumonia Treated with Veno-venous Extracorporeal Membrane Oxygenation. Intern Med. 2020;59:2903-2906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 93. | Lakshmanan S, Malik A. Acute Pancreatitis in Mild COVID-19 Infection. Cureus. 2020;12:e9886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Samies NL, Yarbrough A, Boppana S. Pancreatitis in Pediatric Patients With COVID-19. J Pediatric Infect Dis Soc. 2021;10:57-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Fernandes DA, Yumioka AS, Filho HRM. SARS-CoV-2 and acute pancreatitis: a new etiological agent? Rev Esp Enferm Dig. 2020;112:890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Shinohara T, Otani A, Yamashita M, Wakimoto Y, Jubishi D, Okamoto K, Kanno Y, Ikeda M, Ishigaki K, Nakai Y, Harada S, Okugawa S, Koike K, Moriya K. Acute Pancreatitis During COVID-19 Pneumonia. Pancreas. 2020;49:e106-e108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Meyers MH, Main MJ, Orr JK, Obstein KL. A Case of COVID-19-Induced Acute Pancreatitis. Pancreas. 2020;49:e108-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Ghosh A, Gupta V, Misra A. COVID19 induced acute pancreatitis and pancreatic necrosis in a patient with type 2 diabetes. Diabetes Metab Syndr. 2020;14:2097-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Tollard C, Champenois V, Delemer B, Carsin-Vu A, Barraud S. An inaugural diabetic ketoacidosis with acute pancreatitis during COVID-19. Acta Diabetol. 2021;58:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Kandasamy S. An unusual presentation of COVID-19: Acute pancreatitis. Ann Hepatobiliary Pancreat Surg. 2020;24:539-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 101. | Hassani AH, Beheshti A, Almasi F, Ketabi Moghaddam P, Azizi M, Shahrokh S. Unusual gastrointestinal manifestations of COVID-19: two case reports. Gastroenterol Hepatol Bed Bench. 2020;13:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Narang K, Szymanski LM, Kane SV, Rose CH. Acute Pancreatitis in a Pregnant Patient With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. 2021;137:431-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Acherjya GK, Rahman MM, Islam MT, Alam AS, Tarafder K, Ali M, Deb SR. Acute pancreatitis in a COVID-19 patient: An unusual presentation. Clin Case Rep. 2020;8:3400-3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Alwaeli H, Shabbir M, Khamissi Sobi M, Alwaeli K. A Case of Severe Acute Pancreatitis Secondary to COVID-19 Infection in a 30-Year-Old Male Patient. Cureus. 2020;12:e11718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Simou EM, Louardi M, Khaoury I, Abidi MA, Mansour A, Louadghiri AE, Fahmaoui K, Ezzouine H, Charra B. Coronavirus disease-19 (COVID-19) associated with acute pancreatitis: case report. Pan Afr Med J. 2020;37:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Jespersen Nizamic T, Huang Y, Alnimri M, Cheng M, Chen LX, Jen KY. COVID-19 Manifesting as Renal Allograft Dysfunction, Acute Pancreatitis, and Thrombotic Microangiopathy: A Case Report. Transplant Proc. 2021;53:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Abraham G, Rohit A, Mathew M, Parthasarathy R. Successful Automated Peritoneal Dialysis (APD) in a COVID-19 patient with acalculous pancreatitis with no detectable virus in the dialysate effluent. Indian J Med Microbiol. 2021;39:128-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 108. | Abhinay A, Pradhap K, Singh A, Rao SK, Prasad R, Mishra OP. Corona Virus Disease-19 Presented with Acute Pancreatitis. Indian J Pediatr. 2021;88:482-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Bouali M, Ouchane M, Elbakouri A, Bensardi F, Elhattabi K, Fadil A. Total gastric necrosis following acute pancreatitis in a patient with COVID -19: Case report and literature review. Ann Med Surg (Lond). 2021;62:362-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | AlHarmi RAR, Fateel T, Sayed Adnan J, AlAwadhi K. Acute pancreatitis in a patient with COVID-19. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 111. | Bineshfar N, Mirahmadi A, Karbasian F, Pourbakhtyaran E, Karimi A, Sarafi M. Acute Pancreatitis as a Possible Unusual Manifestation of COVID-19 in Children. Case Rep Pediatr. 2021;2021:6616211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Paz L, Eslava E, Ribes M, Mayer EF. Acute Pancreatitis in a Teenager With SARS-CoV-2 Infection. Pediatr Infect Dis J. 2021;40:e161-e162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Wifi MN, Nabil A, Awad A, Eltatawy R. COVID-induced pancreatitis: case report. Egypt J Intern Med. 2021;33:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Sandhu H, Mallik D, Lokavarapu MJ, Huda F, Basu S. Acute Recurrent Pancreatitis and COVID-19 Infection: A Case Report with Literature Review. Cureus. 2021;13:e13490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Mohammadi Arbati M, Molseghi MH. COVID-19 Presenting as Acute Necrotizing Pancreatitis. J Investig Med High Impact Case Rep. 2021;9:23247096211009393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Amé RM, Balderramo D. Is necessary to rule out Severe Acute Respiratory Syndrome Coronavirus 2 infection in every patient admitted for acute pancreatitis? Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Gupta A, Bansal DP, Rijhwani P, Singh V. A Case Report on Acute Pancreatitis in a Patient With Coronavirus Disease 2019 (COVID-19) Pneumonia. Cureus. 2021;13:e14628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Muhammad Abrar Jeelani H, Sheikh MM, Samuel SS, Omotosho YB, Sharko A, Albetar R. Acute Pancreatitis in a Patient With COVID-19 After the Resolution of Respiratory Symptoms. J Investig Med High Impact Case Rep. 2021;9:23247096211024773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Maalouf RG, Kozhaya K, El Zakhem A. SARS-CoV-2 induced necrotizing pancreatitis. Med Clin (Barc). 2021;156:629-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Sanchez RE, Flahive CB, Mezoff EA, Gariepy C, Hunt WG, Vaz KKH. Case Report: Acute Abdominal Pain as Presentation of Pneumonia and Acute Pancreatitis in a Pediatric Patient With COVID-19. JPGN Rep. 2021;2:e011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 121. | Ehsan P, Haseeb M, Khan Z, Rehan A, Singh R. Coronavirus Disease 2019 Pneumonia and Acute Pancreatitis in a Young Girl. Cureus. 2021;13:e15374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Hanif M, Khan AW, Ullah S, Sundas F, Khan SJ. Can COVID-19 Cause Pancreatitis? J Coll Physicians Surg Pak. 2021;31:S120-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Chandra R, Lazar NJ, Goldman S, Imam Z, Mansour R. Novel Coronavirus (COVID-19) Infection-Attributed Acute Pancreatitis: A Case Report and Literature Review. Cureus. 2021;13:e15725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Alfishawy M, Nassar M, Mohamed M, Fatthy M, Elmessiery RM. New-onset Type 1 Diabetes Mellitus with Diabetic Ketoacidosis and Pancreatitis in a Patient with COVID-19. Sci Afr. 2021;13:e00915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Berrichi S, Bouayed Z, Jebar K, Zaid I, Nasri S, Bkiyar H, Skiker I, Housni B. Acute pancreatitis as an atypical manifestation of COVID-19: A report of 2 cases. Ann Med Surg (Lond). 2021;68:102693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Kripalani Y, Houssein K, Shaikh A. Acute Necrotizing Pancreatitis with Cystic Lesion Concomitant with SARS-CoV-2. Eur J Case Rep Intern Med. 2021;8:002712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Narayan VSG, Datley AY, Upadhyay R. Acute Pancreatitis in Severe COVID Pneumonia. J Assoc Physicians India. 2021;69:11-12. [PubMed] |
| 128. | Eldaly AS, Fath AR, Mashaly SM, Elhadi M. Acute pancreatitis associated with severe acute respiratory syndrome coronavirus-2 infection: a case report and review of the literature. J Med Case Rep. 2021;15:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Basukala S, Shah KB, Karki B, Thapa N, Basukala B, Karki S, Pathak BD. Acute hemorrhagic necrotizing pancreatitis in patient with COVID-19: a case report and review of literature. J Surg Case Rep. 2021;2021:rjab401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Schembri Higgans J, Bowman S, Abela JE. COVID-19 associated pancreatitis: A mini case-series. Int J Surg Case Rep. 2021;87:106429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Kopiczko N, Kwiatek-Średzińska K, Uścinowicz M, Kowalczuk-Krystoń M, Lebensztejn DM. SARS-CoV-2 Infection as a Cause of Acute Pancreatitis in a Child-A Case Report. Pediatr Rep. 2021;13:552-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | da Costa Ferreira CP, Marques KR, de Mattos GHF, de Campos T. Acute pancreatitis in a COVID-19 patient in Brazil: a case report. J Med Case Rep. 2021;15:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 133. | Sánchez-Gollarte A, Jiménez-Álvarez L, Pérez-González M, Vera-Mansilla C, Blázquez-Martín A, Díez-Alonso M. Clostridium perfringens necrotizing pancreatitis: an unusual pathogen in pancreatic necrosis infection. Access Microbiol. 2021;3:000261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Sudarsanam H, Ethiraj D, Govarthanan NK, Kalyanasundaram S, Chitra SA, Mohan S. COVID-19 Associated Acute Necrotizing Pancreatitis with Normal Serum Amylase and Lipase Levels: Report of an Unusual Finding. Oman Med J. 2021;36:e304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Faghih Dinevari M, Rasoolimanesh M, Tarverdizadeh M, Riazi A, Abbasian S, Zeinolabedini A, Hassannezhad S. Acute pancreatitis in a young woman with COVID-19 infection: A case-report. Caspian J Intern Med. 2021;12:S474-S478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 136. | Goldstein R, Kogan D, Fiorito TM, Glasser CL. Suspected Case of Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 Infection Presenting as Acute Pancreatitis in a Child With Leukemia. Infect Dis Clin Pract (Baltim Md). 2021;29:e465-e467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 137. | Gadiparthi C, Mohapatra S, Kanna S, Vykuntam V, Chen W. Acute pancreatitis in a patient with COVID-19: a case report. Transl Gastroenterol Hepatol. 2021;6:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |