Published online May 14, 2022. doi: 10.3748/wjg.v28.i18.2021
Peer-review started: October 13, 2021
First decision: December 3, 2021
Revised: December 16, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: May 14, 2022
Processing time: 211 Days and 1 Hours
Autoimmune hepatitis-primary biliary cholangitis (AIH-PBC) overlap syndrome has a worse prognosis than AIH or PBC alone. Therefore, accurately staging liver fibrosis and dynamically monitoring disease progression are essential.
To investigate the performance of two-dimensional shear-wave elastography (2D-SWE) for noninvasively staging liver fibrosis and assessing the clinical utility of repeated 2D-SWE for monitoring treatment response in AIH-PBC overlap syndrome.
A total of 148 patients diagnosed with AIH-PBC overlap syndrome were retro
LS value was strongly correlated with liver fibrosis stage (Spearman r = 0.84, P < 0.0001). The areas under the receiver operating characteristic curves of LS for diagnosing significant fibrosis (≥ S2), severe fibrosis (≥ S3), and cirrhosis (S4) were 0.91, 0.97, and 0.96, respectively. Patients with complete biochemical remission had a considerable decrease in LS values (P < 0.0001). More importantly, the declined LS in patients with S0-S2 was significantly lower than that in patients with S3-S4 (P = 0.0002). In contrast, patients who failed to achieve biochemical remission had a slight but not significant decrease in LS (P = 0.37).
LS measured by 2D-SWE is an accurate and reliable method in assessing liver fibrosis, especially for diagnosing severe fibrosis (≥ 3) and monitoring treatment response in patients with AIH-PBC overlap syndrome.
Core Tip: Two-dimensional shear-wave elastography is an accurate and reliable method in monitoring treatment response in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome.
- Citation: Yan YL, Xing X, Wang Y, Wang XZ, Wang Z, Yang L. Clinical utility of two-dimensional shear-wave elastography in monitoring disease course in autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol 2022; 28(18): 2021-2033
- URL: https://www.wjgnet.com/1007-9327/full/v28/i18/2021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i18.2021
Autoimmune hepatitis-primary biliary cholangitis (AIH-PBC) overlap syndrome is defined as presenting features of AIH and PBC simultaneously or sequentially. AIH is a progressive inflammatory liver disorder characterized histologically by interface hepatitis, serologically by high levels of transaminases and immunoglobulin G (IgG) and the presence of autoantibodies. PBC is progressive autoimmune liver disease with nonsuppurative destructive cholangitis and interlobular bile duct destruction[1]. AIH-PBC overlap syndrome has a worse prognosis than AIH or PBC alone[2]. Therefore, accurately assessing stage of liver disease and dynamically monitoring the progression of disease are essential for some AIH-PBC overlap syndromes with rapid disease progression.
Liver biopsy still remains the gold standard for establishing the diagnosis, assessing disease severity and determining treatment. According to the most recent guidelines, long-term treatment with immunosuppressants in combination with ursodeoxycholic acid is recommended when AIH-PBC overlap syndrome is established[3,4]. Frequent biochemical markers measurement during the follow-up was necessary to monitor the therapeutic response. However, they cannot monitor the histologically progression and reflect the severity of liver fibrosis. Furthermore, hepatic histological remission usually lagged behind biochemical remission by several months[5]. Therefore, detecting the severity of histology at the beginning of treatment and frequent monitoring during long-term follow-up are important for management of AIH-PBC overlap syndrome. Liver biopsy is still the gold standard for diagnosing AIH-PBC overlap syndrome and staging liver fibrosis, and can be accepted by most patients, but the second or repeated liver biopsy cannot be easily performed due to its costly and invasive procedure during the long-term follow-up.
During the past decades, several noninvasive markers are found to have high accuracy for detecting liver fibrosis and disease progression. 2D-SWE by using the supersonic shear imaging technique is an ultrasound-based real-time imaging method with the following advantages for assessing liver disease: (1) nontarget structure and elastic artifacts can be effectively avoided to improve reliability by integrating B-mode imaging and color-coded tissue stiffness maps in real time; (2) it is widely applied to measure liver stiffness in the whole world; and (3) there is no limitation in patients with ascites[6]. Our previous studies showed that 2D-SWE has a great performance for staging liver fibrosis in AIH[7] and PBC patients[8]. However, the utility of 2D-SWE in monitoring treatment response remains blank.
The aim of this study was to evaluate the clinical utility of 2D-SWE in evaluating liver fibrosis and to assess the usefulness of repeated 2D-SWE for monitoring treatment response in AIH-PBC overlap syndrome.
This is a single-center retrospective study, which was approved by the ethics committee of our hospital, and due to the retrospective feature, the patients informed consent was waived. Patients was strictly diagnosed according to the Paris criteria[9,10]. The presence of at least two of the three accepted criteria was required for the diagnosis of AIH and PBC. The AIH criteria were as follows: (1) Alanine aminotransferase (ALT) levels at least five times higher than the upper limit of normal (ULN); (2) Serum IgG levels at least two times higher than the ULN or a positive test for anti-smooth muscle antibodies; and (3) Moderate or severe periportal or periseptal piecemeal lymphocytic necrosis in liver biopsy. PBC criteria were as follows: (1) Alkaline phosphatase (ALP) levels at least two times higher than the ULN or γ-glutamyltranspeptidase (GGT) levels at least five times higher than the ULN; (2) A positive test for anti-mitochondrial antibodies; and (3) Florid bile duct lesions in liver biopsy. Among them, moderate interfacial inflammation of the liver was necessary for diagnosis of AIH-PBC overlap syndrome. The patients between September, 2016 and April 2021 in West China hospital were enrolled. The inclusion criteria were as follows: (1) Patients met the diagnosis criteria of PBC-AIH overlap syndrome with liver biopsy; and (2) Age was adults with lower than 75 years old. The exclusion criteria were as follows: (1) Patients with overlapping etiologies of liver disease, such as virus, alcohol liver disease[11], non-alcoholic fatty liver disease[12], drug-induced liver disease[13], hereditary metabolic liver disease or other causes of liver damage; (2) Patients with primary hepatic carcinoma or other malignant tumors; (3) 2D-SWE measurement after liver biopsy for more than 1 mo and with treatment; (4) Patients without 2D-SWE results or serological data; (5) Patients with ascites, varices bleeding, esophageal variceal ligation (EVL) or splenectomy; and (6) Patients with severe systemic disease. Among them, the follow-up time with 2D-SWE greater than 1 year was subsequently analyzed to evaluate the changes of LS values after standard treatment. Complete biochemical remission was defined by normalization of serum AST, ALT, ALP and IgG levels[14].
2D-SWE measurements were obtained by using the Aixplorer system (SuperSonic Imagine, Aix-en-Provence) with a SC6-1 (frequency of 1-6 MHz) convex probe. Two experience radiologists independently performed the procedures according to the guidelines developed by the World Federation for Ultrasound in Medicine and Biology[15]. The 2D-SWE measurements were performed following our previous study[8], Briefly, the elasticity image box, approximately 4 cm × 3 cm, was placed 1-2 cm under the liver capsule in the parenchyma area of the right hepatic lobe. At least three valid measurements were obtained in each patient. The median LS value was recorded (in kPa)[16]. When less than two-thirds of the signal filled in the 2D SWE region of interest or the large vessels and biliary tracts were not avoided, measurements were considered failed or unqualified[17].
Ultrasound-guided liver biopsy was performed in the right liver lobe. Quality of the liver specimen was evaluated. All specimens were analyzed by one experienced pathologist. The Scheuer scoring system, a common scoring system in clinical practice, evaluates liver fibrosis and necroinflammatory activity. Liver fibrosis was scored as follows the previous studies[7]: the liver fibrosis was staged from stage 0 (S0) to stage 4 (S4) cirrhosis (S0-S4). Necroinflammative was also scored from grade 0 (G0), no portal or periportal and lobular necroinflammatory activity to grade 4 (G4), severe piecemeal portal or periportal necrosis and severe or diffuse hepatocellular damage inside the lobule (G0-G4). Significant fibrosis was liver fibrosis stage ≥ S2; severe fibrosis was liver fibrosis stage ≥ S3 and cirrhosis was liver fibrosis stage = S4. Mild liver necroinflammation was defined as necroinflammatory activity lower than G2 (G0–G2), and significant liver necroinflammation was defined as necroinflammatory activity higher than G2 (G3-G4).
Statistical analyses were performed in SPSS Statistics version 24.0 (IBM Corporation, Armonk, NY), MedCalc software version 19.1.0 (MedCalc Software, Mariakerke, Belgium). A P value lower than 0.05 was considered statistically significant. Descriptive statistics were summarized as the median and interquartile (25%-75%). One-way ANOVA analysis or the Mann-Whitney test was used to compare quantitative variables. Correlations between LS values and biochemical biomarkers and histological features were evaluated with the Spearman correlation test, and those with P < 0.05 were subsequently included in multiple regression analysis. The performance of noninvasive methods in the assessment of liver fibrosis stages was determined by using the area under the receiver operating characteristic curve (AUC). Optimal cutoff values to predict different fibrosis stages were identified with the highest Youden’s index. The corresponding sensitivity, specificity, negative predictive value, positive predictive value and accuracy were calculated. The cutoff values of LS for ruling out and ruling in were defined with sensitivity > 95% and specificity > 95%, respectively. The AUCs of noninvasive biomarkers were compared with the DeLong test. The Z test was used to compare the AUCs of LS for predicting liver fibrosis in the G0-G2 subgroups and G3-G4 subgroups. Two-way ANOVA was used to evaluate the changes in LS. Wilcoxon matched pairs test was used to compare the LS values before treatment and each follow-up.
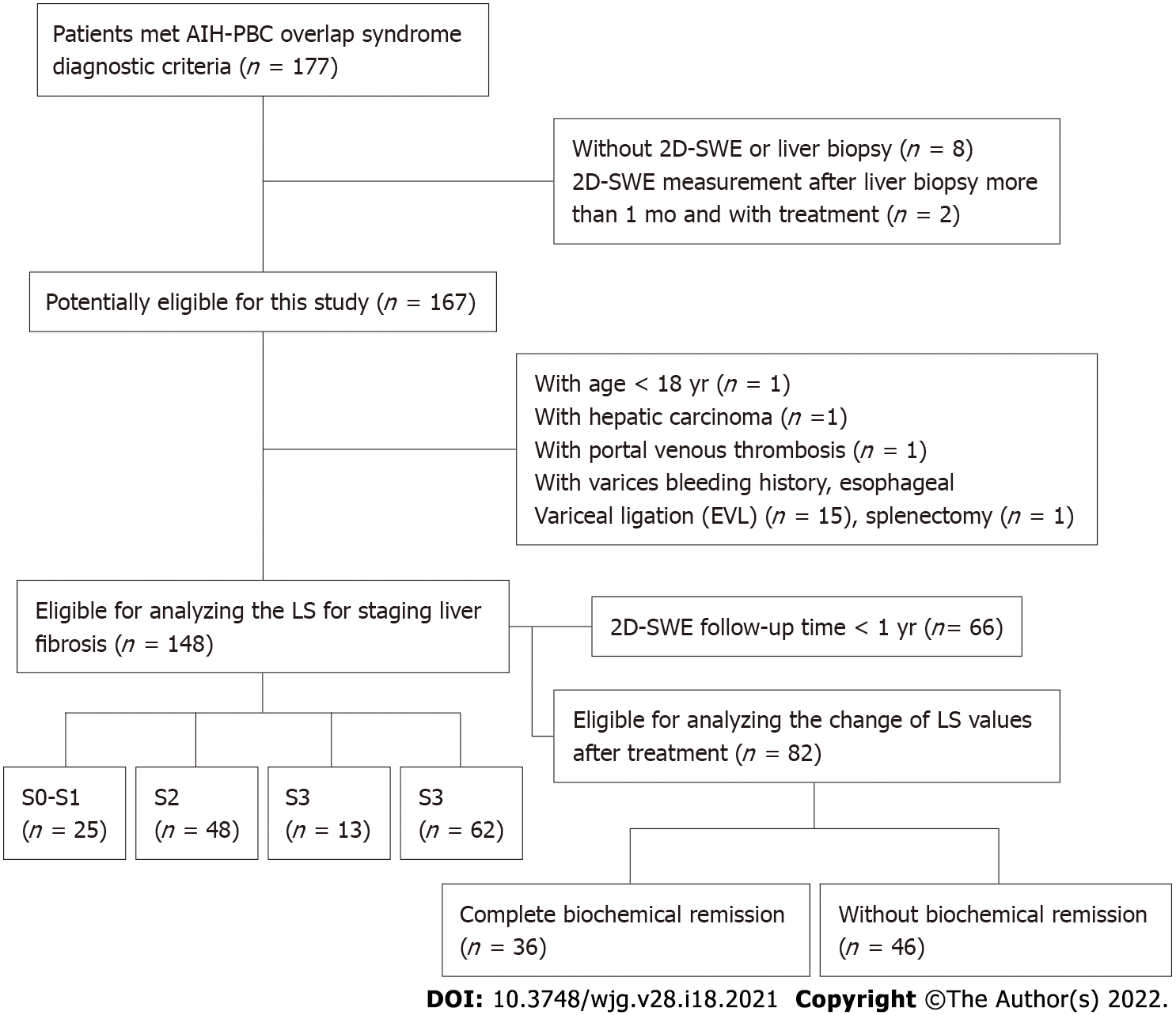
A total of 177 patients considered AIH-PBC patients were enrolled. Eight patients without 2D-SWE results or liver biopsy, 2 patients of with the 2D-SWE measurements after liver biopsy for more than 1 mo and with treatment, 15 patients with varices bleeding or EVL, 1 patient with splenectomy, 1 patient with portal venous thrombosis, 1 patient with age < 18 years old, and 1 patient with hepatic carcinoma were excluded. Finally, 148 patients were included to analyze the LS for staging liver fibrosis in this study (Figure 1). Among them, 82 patients had a 2D-SWE follow-up of more than 1 year, 36 patients had complete biochemical remission, and 46 patients had no biochemical remission. The characteristics of the patients are described in Table 1. The numbers of patients with S0–S1, S2, S3 and S4 were 25 (15.4%), 48 (30.2%), 13 (9.9%) and 62 (44.4%), respectively.
| Characteristic | Value |
| Gender, female, n (%) | 132 (90) |
| Age, yr | 49 (44-55) |
| BMI (kg/m2) | 21.5 (19.6-22.9) |
| Total bilirubin, μmol/L | 36.6 (19.3-72.7) |
| ALB, g/L | 38.7 (34.1-43.7) |
| PLT, × 109/L | 127 (79-185) |
| ALT, IU/L | 100 (63-185) |
| AST, IU/L | 114 (74-177) |
| ALP, IU/L | 342 (194-518) |
| GGT, IU/L | 298 (176-499) |
| IgG, g/L | 20.5 (16.1-24.7) |
| IgM, g/L | 3250 (2023-5095) |
| LKM, positive, n (%) | 0 |
| ANA, positive, n (%) | 144/148 (97.3) |
| AMA, positive, n (%) | 89/148 (60.1) |
| Fibrosis stage, n (%) | |
| S0-S1 | 25 (15.4) |
| S2 | 48 (30.2) |
| S3 | 13 (9.9) |
| S4 | 62 (44.4) |
| Activity grade, n (%) | |
| G0-G1 | 10 (6.8) |
| G2 | 53 (35.2) |
| G3 | 78 (53.1) |
| G4 | 7 (4.9) |
| LS, kPa | 13.6 (9.8-20.6) |
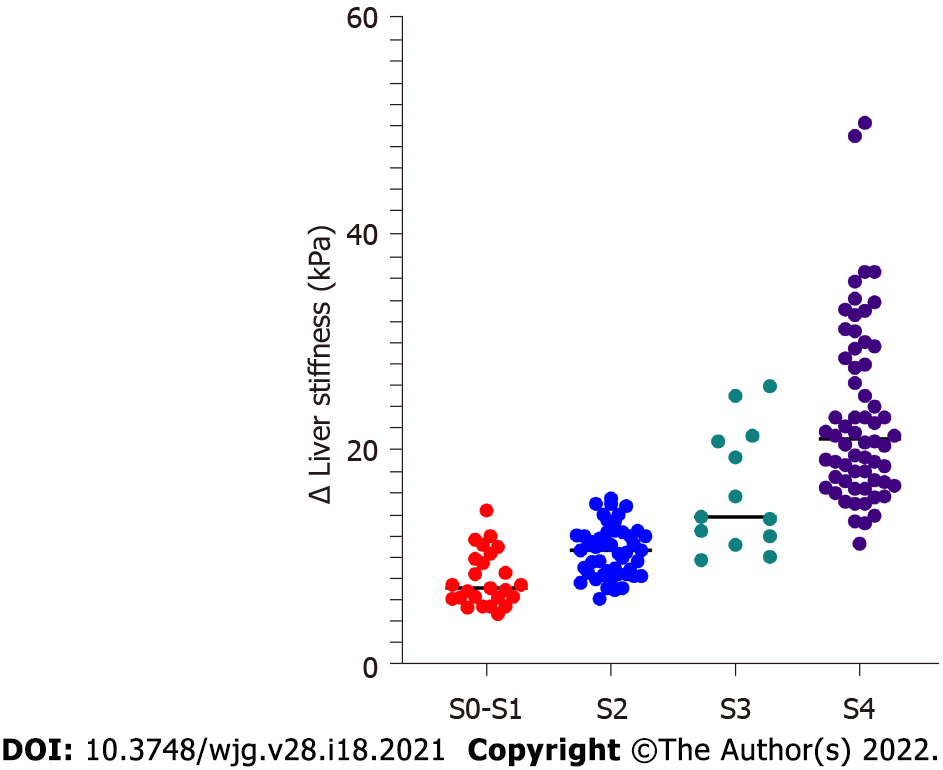
LS had a strong correlation with serum biomarkers and histological features (Table 2). PLT (Spearman r = -0.43, P < 0.0001), total bilirubin (Spearman r = 0.60, P < 0.0001), AST (Spearman r = 0.33, P < 0.0001), albumin (Spearman r = -0.67, P < 0.0001), and IgG (Spearman r = 0.38, P < 0.0001) had significant correlations with LS values. The necroinflammatory activity grades (Spearman r = 0.32, P < 0.0001) and fibrosis stages (Spearman r = 0.84, P < 0.0001) also had a significant correlation with LS. In multiple regression analysis, only total bilirubin (P = 0.005) and fibrosis stages (P < 0.0001) had strong correlations with LS values (Supplementary Table 1). In addition, LS values in patients were increased with fibrosis stage S0-S1 [7.2 (6.3-9.9) kPa], S2 [10.7 (8.6-12.2) kPa], S3 [13.8 (12.0-20.8)], S4 [21.1 (17.1-28.4) kPa] (P < 0.0001) (Figure 2).
| Spearman r | P value | |
| Necro-inflammatory activity grades | 0.32 | 0.0006 |
| Fibrosis stages | 0.84 | < 0.0001 |
| PLT, × 109/L | -0.43 | < 0.0001 |
| TB, μmol/L | 0.60 | < 0.0001 |
| ALT, IU/L | -0.04 | 0.63 |
| AST, IU/L | 0.33 | < 0.0001 |
| ALB, g/L | -0.67 | < 0.0001 |
| ALP, IU/L | 0.02 | 0.77 |
| IgG, IU/L | 0.38 | < 0.0001 |
The utility of LS measured by 2D-SWE for staging liver fibrosis is shown in Table 3. For diagnosing significant fibrosis (≥ S2), the AUC of LS was 0.91 (95%CI: 0.85-0.96). The optimal cutoff value of LS was 12.1 kPa with the highest combined sensitivity (67.5%) and specificity (96.0%). The cutoff values of LS for ruling out and ruling in significant fibrosis (≥ S2) were 7.9 kPa and 12.1 kPa, respectively. For assessing severe fibrosis (≥ S3), the AUC of LS was 0.97 (95%CI: 0.94-0.99). The optimal cutoff value of LS was 15.0 kPa with the highest combined sensitivity (85.3%) and specificity (95.9%). The cutoff values of LS for ruling out and ruling in severe fibrosis (≥ S3) were 11.9 kPa and 15.0 kPa, respectively. For diagnosing cirrhosis (S4), the AUC of 2D-SWE was 0.96 (95%CI: 0.92-0.99). The optimal cutoff value of 2D-SWE was 18.0 kPa with the highest combined sensitivity (90.3%) and specificity (91.9%). The cutoff values of LS for ruling out and ruling in liver cirrhosis were 13.9 kPa and 19.4 kPa, respectively.
| Cutoff | AUC (95%CI) | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Significant fibrosis (≥ S2) | |||||||
| LS, kPa | 12.1 | 0.91 (0.85-0.96) | 67.5% | 96.0% | 98.8% | 37.5% | 72.3% |
| Rule out | 7.9 | 95.1% | 60.0% | 92.1% | 71.3% | 89.2% | |
| Rule in | 12.1 | 67.5% | 96.0% | 98.8% | 37.5% | 72.3% | |
| Severe fibrosis (≥ S3) | |||||||
| LS, kPa | 15.0 | 0.97 (0.94-0.99) | 85.3% | 95.9% | 95.5% | 86.4% | 90.5% |
| Rule out | 11.9 | 94.7% | 75.3% | 79.8% | 93.3% | 85.1% | |
| Rule in | 15.0 | 85.3% | 95.9% | 95.5% | 86.4% | 90.5% | |
| Cirrhosis (S4) | |||||||
| LS, kPa | 15.1 | 0.96 (0.92-0.99) | 90.3% | 91.9% | 88.9% | 92.9% | 91.2% |
| Rule out | 13.9 | 95.2% | 84.9% | 82.0% | 96.1% | 89.2% | |
| Rule in | 19.4 | 58.1% | 95.3% | 89.9% | 75.9% | 79.7% | |
The effects of necroinflammatory activity on LS measured by 2D-SWE were evaluated. LS values in the subgroup of patients with G0-G2 and the subgroup of patients with G3-G4 showed no significant difference for staging liver fibrosis S0-S1 (P = 0.57), S2 (P = 0.51), S3 (P = 0.62) and S4 (P = 0.77) (Supplementary Table 2). The effect of necroinflammatory activity on the accuracy of LS values for staging liver fibrosis is shown in Supplementary Table 3. The AUCs of LS for diagnosing significant fibrosis (≥ S2) (P = 0.57), severe fibrosis (≥ S3) (P = 0.89), and cirrhosis (S4) (P = 0.06) were not significantly different between subgroups of patients with G0-G2 and G3-G4 necroinflammatory activity.
The cutoff values for ruling out significant fibrosis (≥ S2), severe fibrosis (≥ S3) and cirrhosis (S4) were 7.9 kPa, 11.9 kPa and 13.9 kPa, respectively. The accuracy of these cutoff values for predicting each stage of liver fibrosis is listed in Supplementary Table 4. In patients with S0–S1, S2, S3 and S4, 15/25 (60.0%), 26/48 (54.2%), 4/13 (30.8%) and 58/62 (93.5%) of them were correctly classified, respectively. The accuracy of 2D-SWE for predicting severe fibrosis (≥ S3) was 71/75 (94.7%).
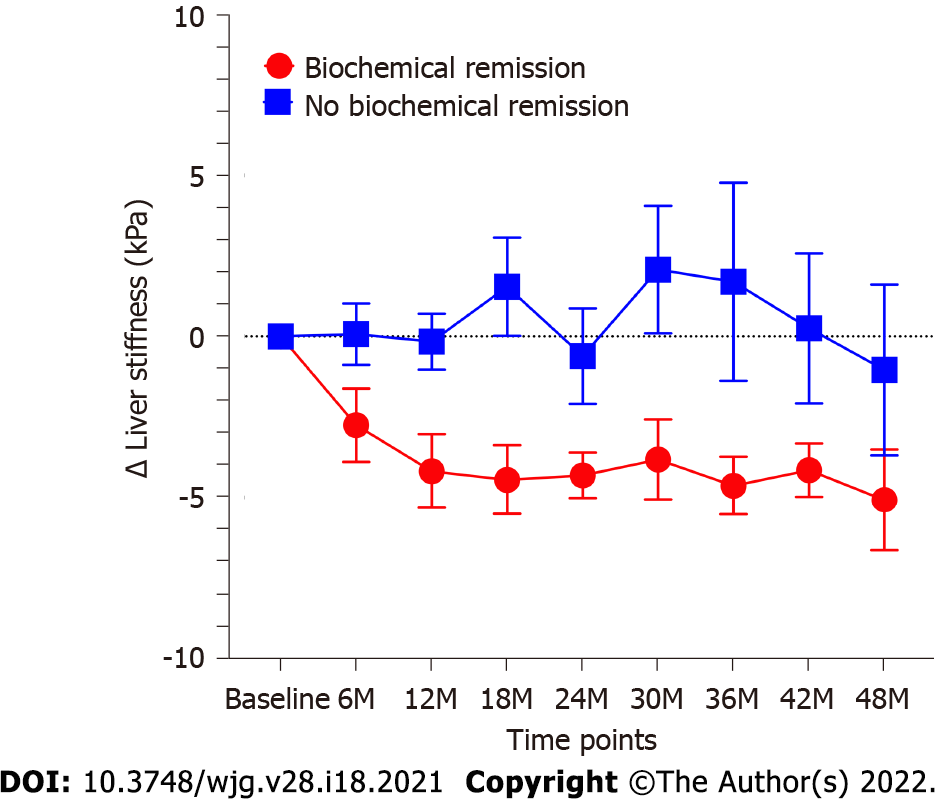
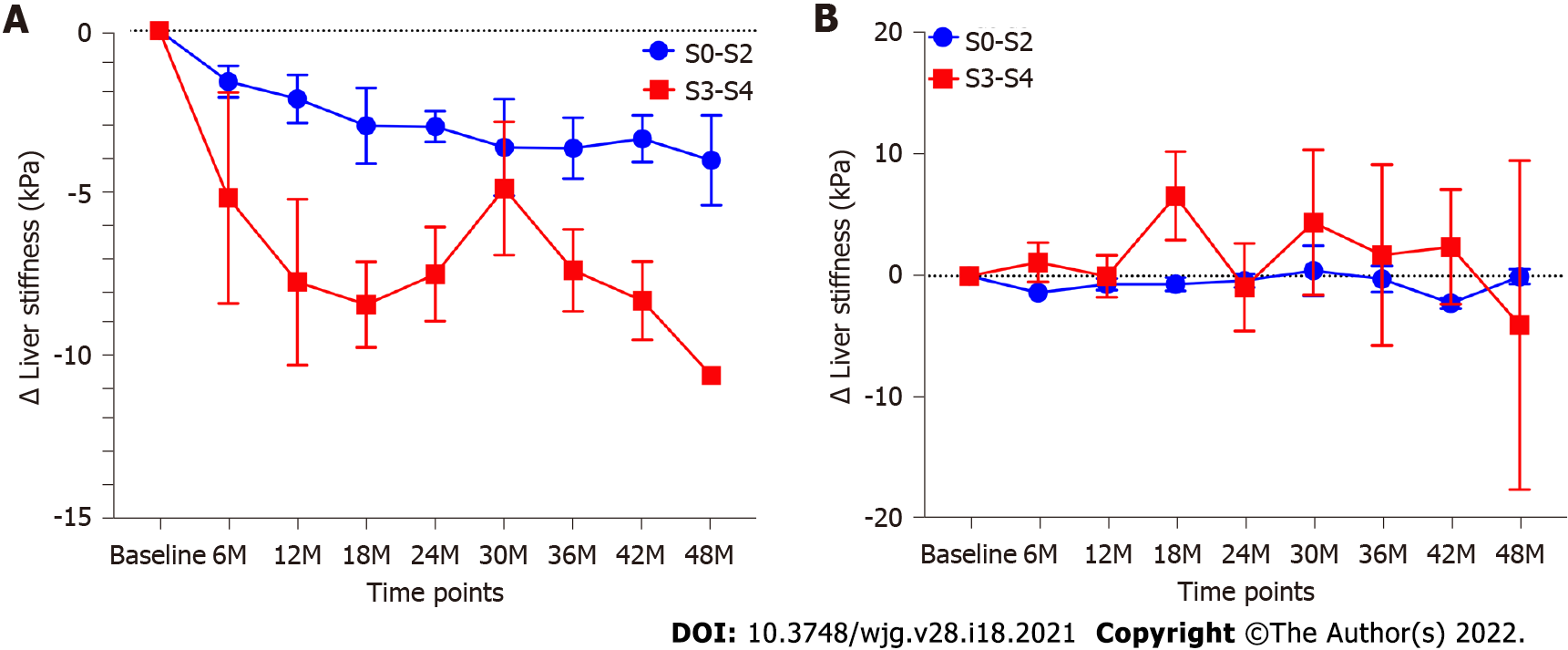
Eighty-two patients with 2D-SWE follow-up time of more than 1 year were evaluated. The total median follow-up time of these patients was 3.3 (2.2-4.1) years. The median follow-up time of 2D-SWE in these patients was 2.5 (1.5-3.5) years. Among these patients, 36 patients had complete biochemical remission, and 46 patients had no biochemical remission. The baseline characteristics of patients with or without complete biochemical remission were shown in Table 4. Total bilirubin (P = 0.009), ALP (P = 0.0005) and GGT (P = 0.005) in patients with biochemical remission were lower than those in patients without biochemical remission. The change of LS values in patients with biochemical remission was significantly more than that in patients without biochemical remission (P < 0.0001). LS values in patients with complete biochemical remission present a considerable decrease (P < 0.0001). The LS values in all follow-up time points were lower than control point (all P < 0.01). In contrast, LS values in patients who failed to achieve complete biochemical remission showed a slight but not significant decrease (P = 0.37) (Figure 3). In patients with complete biochemical remission, the changes in LS in patients with S0-S2 fibrosis stages were significantly lower than those in patients with S3-S4 fibrosis stages (P = 0.0002) (Figure 4A). However, there was no similar change in patients without biochemical remission (Figure 4B).
| Characteristic | Without biochemical remission (n = 46) | Complete biochemical remission (n = 36) | P value |
| Sex, female, n (%) | 39 (90) | 35 (97.2) | 0.07 |
| Age, yr | 50 (43-54) | 49 (44-55) | 0.99 |
| BMI (kg/m2) | 21.1 (19.4-22.4) | 21.9 (20.0-23.0) | 0.27 |
| TB, μmol/L | 38.7 (27.4-61.2) | 19.6 (15.5-43.6) | 0.009 |
| ALB, g/L | 39.7 (34.8-45.3) | 41 (35.5-44.8) | 0.56 |
| PLT, × 109/L | 126 (90-170) | 150 (85-215) | 0.18 |
| ALT, IU/L | 98 (71-136) | 146 (50-215.3) | 0.17 |
| AST, IU/L | 111 (82-153) | 126 (68-247) | 0.63 |
| ALP, IU/L | 417 (297-533) | 217 (143-412) | 0.0005 |
| GGT, IU/L | 419 (269-558) | 289 (111-451) | 0.005 |
| IgG, g/L | 19.2 (15.3-22.3) | 21.9 (16.5-26.2) | 0.06 |
| IgM, g/L | 3500 (2400-4830) | 3290 (2245-5925) | 0.86 |
| Fibrosis stage, n | 0.19 | ||
| S0-S1 | 4 | 9 | |
| S2 | 19 | 14 | |
| S3 | 6 | 2 | |
| S4 | 17 | 11 | |
| Activity grad, n | 0.29 | ||
| G0-G1 | 1 | 1 | |
| G2 | 19 | 8 | |
| G3 | 24 | 26 | |
| G4 | 2 | 1 | |
| LS, kPa | 12.9 (10.0-17.0) | 11.3 (7.7-15.4) | 0.14 |
AIH-PBC overlap syndrome is a special clinical subgroup of chronic liver disease with poor clinical prognosis and higher risk of liver related complications than AIH or PBC alone. Therefore, disease progression needs intensive follow-up and dynamic monitoring in a timely manner. Noninvasive methods for staging liver fibrosis and monitoring disease progression are worthy of more concern. In this study, we assessed the clinical utility of 2D-SWE for staging liver fibrosis and evaluated the usefulness of repeated 2D-SWE to monitor disease progression in patients with AIH-PBC overlap syndrome.
2D-SWE is equal or superior to TE in staging liver fibrosis[18-21]. A meta-analysis study reported 2D-SWE had an excellent performance for staging fibrosis in common causes of liver disease, such as hepatitis C, hepatitis B, and nonalcoholic fatty liver disease, the AUCs were ranged from 86% to 96%. Autoimmune liver disease (AILD) is a rare group of liver diseases in the whole world, especially AIH-PBC overlap syndrome. Few studies have assessed the performance of 2D-SWE to evaluate severity of AILD. Our studies reported that the AUCs of LS measured by 2D-SWE for staging liver fibrosis were ranged from 0.88-0.99 in patients with PBC and 0.84-0.94 in patients with AIH, respectively. Recently, Janik et al[22] showed that the AUC of 2D-SWE for diagnosing cirrhosis was 0.93 in patients with AIH. In addition, Wu et al[23] reported that the AUCs of LS measured by TE for diagnosing F ≥ 2, F ≥ 3, and F4 were 0.84, 0.91, and 0.97, respectively, in patients with AIH-PBC overlap syndrome. In this study, the AUCs of LS measured by 2D-SWE in detecting significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3), and cirrhosis (S4) were 0.91, 0.97, and 0.96, respectively, which showed a broadly consistent accuracy with previous studies. 61/114 (53.5%) patients with AILD, 68/103 (66.02%) patients with AIH, 116/157 (73.9%) patients with PBC, respectively, can be correctly classified[7,8,24]. 105/148 (69.6%) patients with AIH-PBC overlap syndrome can be correctly classified in this study. These results suggest that LS measured by 2D-SWE is reliable method for staging liver fibrosis in patients with AIH-PBC over syndrome, even in patients with AILD.
The cutoff value of LS may vary among different etiologies and should be considered in diagnosing liver fibrosis[18,25,26]. The LS values ranged from 7.0 kPa to 8.3 kPa, 8.2 kPa to 9.2 kPa, and 9.9 kPa to 13.3 kPa for detecting significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3) and cirrhosis (S = 4), respectively. Patients with hepatitis B or C viruses have lower LS values for detecting corresponding fibrosis stages[25]. A study including 114 patients with AILD concluded that the optimal LS values for detecting significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3) and cirrhosis (S = 4) were 9.7 kPa, 13.2 kPa, and 16.3 kPa, respectively[24]. Our previous study showed that the optimal LS values for diagnosing significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3) and cirrhosis (S4) were 10.0 kPa, 15.8 kPa and 19.3 kPa, respectively in patients with AIH and 10.7 kPa, 12.2 kPa and 14.1 kPa, respectively in patients with PBC[7,8]. In this study, the optimal cutoff values of LS for staging significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3) and cirrhosis (S4) were 12.1 kPa, 15.0 kPa and 15.1 kPa, respectively in patients with AIH-PBC overlap syndrome. The cutoff values of LS for ruling out significant fibrosis (S ≥ 2), severe fibrosis (S ≥ 3) and cirrhosis (S4) were 7.9 kPa, 11.9 kPa and 13.9 kPa, respectively. According to these current studies, we try to draw a conclusion that the causes of liver disease might affect the LS values and that AIH-PBC over syndrome might have higher cutoff values than other causes of liver disease for diagnosing liver fibrosis.
As discussed in the current European Association for the Study of the Liver Clinical Practice Guidelines, the active liver inflammation may affect the LS values. The increased transaminase and total bilirubin might lead to overestimation of LS measurements[27,28]. In this study, LS showed a strong correlation with several serum biomarkers, such as PLT, total bilirubin, AST, albumin and IgG (P < 0.0001). However, in the multiple regression analysis, only liver fibrosis stages and total bilirubin were associated with the LS values. These findings were consistent with previous studies[28-31], and the LS values might be influenced by elevated total bilirubin. Therefore, considering the feature of AIH-PBC over syndrome, the cutoff values for diagnosing liver fibrosis could be higher than other causes of liver disease. The treatment history may affect the diagnostic accuracy of 2D-SWE for staging fibrosis. ALT could affect the LS values measured by TE, which are mainly reported in patients with chronic hepatitis B[32-34]. However, the ALT did not associate with LS in this study. Xu et al[35] also reported that in patients with AIH, ALT cannot affect the LS values measured by transient elastography. Therefore, whether the level of ALT can represent liver histological inflammatory activity needs more study to validate it.
Hartl et al[29] reported a significant decrease in LS measured by TE in AIH patients with complete biochemical remission, especially in subgroups of patients with S3-S4 fibrosis stages. The declines in LS measured by TE may reflect the remission of both liver inflammation and fibrosis[36,37]. In this study, the LS values were significantly decreased in patients with complete biochemical remission. In addition, the changes in LS values in patients with S3-S4 were more than those in patients with S0-S2. However, there was no significant change in LS values in patients without biochemical remission. These results demonstrate that LS measured by 2D-SWE can monitor the therapeutic effect. Due to the lack of liver biopsy after treatment, there was no definite correlation between the decline in LS values and remission of liver inflammation or fibrosis.
This study has some limitations that warrant discussion: (1) The patient cohort was small, which can be understood that the prevalence of AIH-PBC overlap syndrome also very low; (2) There is no detailed information on the failure rate of 2D-SWE; (3) In this cohort, we did not acquire the TE data, therefore, we cannot compare the performance of 2D-SWE with TE for diagnosing fibrosis and monitoring treatment due to the retrospective features of this study; and (4) There was no liver biopsy after treatment with complete biochemical remission.
In conclusion, LS measured by 2D-SWE is a potential noninvasive method for staging liver fibrosis, especially for diagnosing severe fibrosis (≥ 3) in patients with AIH-PBC overlap syndrome. The diagnostic performance of 2D-SWE is superior to that of serum fibrosis models for staging liver fibrosis. More importantly, 2D-SWE can monitor the treatment response in patients with complete biochemical remission with a significant decline in LS values.
Autoimmune hepatitis-primary biliary cholangitis (AIH-PBC) overlap syndrome has a worse prognosis than AIH or PBC alone. Therefore, accurately diagnosing liver fibrosis and dynamically monitoring disease progression are essential.
The evaluation of two-dimensional shear-wave elastography (2D-SWE) in detecting liver fibrosis and monitoring treatment response in patients with AIH-PBC overlap syndrome remains blank.
To evaluate the diagnostic utility of 2D-SWE in staging liver fibrosis and assess the usefulness of repeated 2D-SWE for monitoring treatment response in AIH-PBC overlap syndrome.
Patients with biopsy-proven AIH-PBC overlap syndrome were retrospectively enrolled. The performances of 2D-SWE and serum indexes for staging liver fibrosis were evaluated. The Scheuer scoring system was used to evaluate hepatic inflammation and liver fibrosis. Changes in liver stiffness (LS) measured by 2D-SWE in patients with or without complete biochemical remission were measured.
LS was strongly correlated with liver fibrosis stage (Spearman r = 0.84, P < 0.0001). The areas under the receiver operating characteristic curves of LS for significant fibrosis, severe fibrosis, and cirrhosis were 0.91, 0.97, and 0.96, respectively. Patients with complete biochemical remission showed a considerable decrease in LS values (P < 0.0001). More importantly, the decline in LS in patients with S0-S2 was significantly lower than that in patients with S3-S4 (P = 0.0002). In contrast, patients who failed to achieve biochemical remission showed a slight but not significant decrease in LS (P = 0.37).
2D-SWE is an accurate and reliable method in assessing liver fibrosis and monitoring treatment response in patients with AIH-PBC overlap syndrome.
2D-SWE can monitor the treatment effect in patients with AIH-PBC overlap syndrome.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed GA, Egypt; Morozov S, Russia; Zharikov YO, Russia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Purohit T, Cappell MS. Primary biliary cirrhosis: Pathophysiology, clinical presentation and therapy. World J Hepatol. 2015;7:926-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 2. | Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol. 2007;102:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Bonder A, Retana A, Winston DM, Leung J, Kaplan MM. Prevalence of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2011;9:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 572] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 5. | Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, Hübener S, Pannicke N, Weiler-Normann C, Quaas A, Lohse AW, Schramm C. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 7. | Xing X, Yan Y, Shen Y, Xue M, Wang X, Luo X, Yang L. Liver fibrosis with two-dimensional shear-wave elastography in patients with autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2020;14:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Yan Y, Xing X, Lu Q, Wang X, Luo X, Yang L. Assessment of biopsy proven liver fibrosis by two-dimensional shear wave elastography in patients with primary biliary cholangitis. Dig Liver Dis. 2020;52:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1203] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 10. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 480] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G; NIAAA Alcoholic Hepatitis Consortia. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 12. | Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28 Suppl 4:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, Ding Y, Duan ZP, Fu QC, Guo XY, Hu P, Hu XQ, Jia JD, Lai RT, Li DL, Liu YX, Lu LG, Ma SW, Ma X, Nan YM, Ren H, Shen T, Wang H, Wang JY, Wang TL, Wang XJ, Wei L, Xie Q, Xie W, Yang CQ, Yang DL, Yu YY, Zeng MD, Zhang L, Zhao XY, Zhuang H; Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 15. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC, Barr R, Chou YH, Ding H, Farrokh A, Friedrich-Rust M, Hall TJ, Nakashima K, Nightingale KR, Palmeri ML, Schafer F, Shiina T, Suzuki S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 16. | Paisant A, Lemoine S, Cassinotto C, de Lédinghen V, Ronot M, Irlès-Depé M, Vilgrain V, Le Bail B, Paradis V, Canivet CM, Michalak S, Rousselet MC, Rautou PE, Lebigot J, Hunault G, Crouan A, Aubé C, Boursier J. Reliability Criteria of Two-Dimensional Shear Wave Elastography: Analysis of 4277 Measurements in 788 Patients. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Gao Y, Zheng J, Liang P, Tong M, Wang J, Wu C, He X, Liu C, Zhang S, Huang L, Jiang T, Cheng C, Meng F, Mu X, Lu Y, Li Y, Ai H, Qiao X, Xie XY, Wang W, Yin LP, Wu YY, Zheng R. Liver Fibrosis with Two-dimensional US Shear-Wave Elastography in Participants with Chronic Hepatitis B: A Prospective Multicenter Study. Radiology. 2018;289:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2020;214:W20-W26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Imajo K, Honda Y, Kobayashi T, Nagai K, Ozaki A, Iwaki M, Kessoku T, Ogawa Y, Takahashi H, Saigusa Y, Yoneda M, Kirikoshi H, Utsunomiya D, Aishima S, Saito S, Nakajima A. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Cassinotto C, Boursier J, Paisant A, Guiu B, Irles-Depe M, Canivet C, Aube C, de Ledinghen V. Transient Versus Two-Dimensional Shear-Wave Elastography in a Multistep Strategy to Detect Advanced Fibrosis in NAFLD. Hepatology. 2021;73:2196-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Cassinotto C, Lapuyade B, Guiu B, Marraud des Grottes H, Piron L, Merrouche W, Irles-Depe M, Molinari N, De Ledinghen V. Agreement Between 2-Dimensional Shear Wave and Transient Elastography Values for Diagnosis of Advanced Chronic Liver Disease. Clin Gastroenterol Hepatol. 2020;18:2971-2979.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Janik MK, Kruk B, Szczepankiewicz B, Kostrzewa K, Raszeja-Wyszomirska J, Górnicka B, Lammert F, Milkiewicz P, Krawczyk M. Measurement of liver and spleen stiffness as complementary methods for assessment of liver fibrosis in autoimmune hepatitis. Liver Int. 2021;41:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Wu HM, Sheng L, Wang Q, Bao H, Miao Q, Xiao X, Guo CJ, Li H, Ma X, Qiu DK, Hua J. Performance of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol. 2018;24:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Zeng J, Huang ZP, Zheng J, Wu T, Zheng RQ. Non-invasive assessment of liver fibrosis using two-dimensional shear wave elastography in patients with autoimmune liver diseases. World J Gastroenterol. 2017;23:4839-4846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 26. | Serra C, Grasso V, Conti F, Felicani C, Mazzotta E, Lenzi M, Verucchi G, D'errico A, Andreone P. A New Two-Dimensional Shear Wave Elastography for Noninvasive Assessment of Liver Fibrosis in Healthy Subjects and in Patients with Chronic Liver Disease. Ultraschall Med. 2018;39:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Liang XE, Chen YP, Zhang Q, Dai L, Zhu YF, Hou JL. Dynamic evaluation of liver stiffness measurement to improve diagnostic accuracy of liver cirrhosis in patients with chronic hepatitis B acute exacerbation. J Viral Hepat. 2011;18:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Kim SU, Han KH, Park JY, Ahn SH, Chung MJ, Chon CY, Choi EH, Kim DY. Liver stiffness measurement using FibroScan is influenced by serum total bilirubin in acute hepatitis. Liver Int. 2009;29:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Hartl J, Ehlken H, Sebode M, Peiseler M, Krech T, Zenouzi R, von Felden J, Weiler-Normann C, Schramm C, Lohse AW. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Seo YS, Um SH, Jung ES, Yim HJ, Kim CD, Ryu HS. Serum alanine aminotransferase level alone may not accurately represent necro-inflammation in patients with acute hepatitis. Hepatology. 2009;49:1053-4; author reply 1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Das K, Sarkar R, Ahmed SM, Mridha AR, Mukherjee PS, Das K, Dhali GK, Santra A, Chowdhury A. "Normal" liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology. 2012;55:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Fung J, Lai CL, Cheng C, Wu R, Wong DK, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol. 2011;106:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, Zhao J, Wang T, Hu X, Schuppan D. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Yang X, Chang X, Wu S, Sun X, Zhu X, Wang L, Xu Y, Yao X, Rao S, Hu X, Xia M, Bian H, Yan H, Gao X. Performance of liver stiffness measurements obtained with FibroScan is affected by glucose metabolism in patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2021;20:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Xu Q, Sheng L, Bao H, Chen X, Guo C, Li H, Ma X, Qiu D, Hua J. Evaluation of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2017;32:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Liang X, Xie Q, Tan D, Ning Q, Niu J, Bai X, Chen S, Cheng J, Yu Y, Wang H, Xu M, Shi G, Wan M, Chen X, Tang H, Sheng J, Dou X, Shi J, Ren H, Wang M, Zhang H, Gao Z, Chen C, Ma H, Chen Y, Fan R, Sun J, Jia J, Hou J. Interpretation of liver stiffness measurement-based approach for the monitoring of hepatitis B patients with antiviral therapy: A 2-year prospective study. J Viral Hepat. 2018;25:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Vinikoor MJ, Sinkala E, Chilengi R, Mulenga LB, Chi BH, Zyambo Z, Hoffmann CJ, Saag MS, Davies MA, Egger M, Wandeler G; IeDEA- Southern Africa. Impact of Antiretroviral Therapy on Liver Fibrosis Among Human Immunodeficiency Virus-Infected Adults With and Without HBV Coinfection in Zambia. Clin Infect Dis. 2017;64:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |