Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1845
Peer-review started: September 25, 2021
First decision: November 16, 2021
Revised: November 26, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 7, 2022
Processing time: 215 Days and 23.7 Hours
Ampullary adenoma is a rare premalignant lesion, but its incidence is increasing. Endoscopic papillectomy has become the first treatment of choice for ampullary adenomas due to its safety and effectiveness, thereby replacing surgical resection. However, recurrence rates and adverse events after endoscopic papillectomy were reported in up to 30% of cases.
To review the long-term outcomes of endoscopic papillectomy and investigate the factors that affect these outcomes.
We retrospectively analyzed the data of patients who underwent endoscopic papillectomy for ampullary adenoma at five tertiary hospitals between 2013 and 2020. We evaluated clinical outcomes and their risk factors. The definitions of outcomes were as follow: (1) curative resection: complete endoscopic resection without recurrence; (2) endoscopic success: treatment of ampullary adenoma with endoscopy without surgical intervention; (3) early recurrence: reconfirmed adenoma at the first endoscopic surveillance; and (4) late recurrence: reconfirmed adenoma after the first endoscopic surveillance.
A total of 106 patients were included for analysis. Of the included patients, 81 (76.4%) underwent curative resection, 99 (93.4%) had endoscopic success, showing that most patients with non-curative resection were successfully managed with endoscopy. Sixteen patients (15.1%) had piecemeal resection, 22 patients (20.8%) had shown positive/uncertain resection margin, 11 patients (16.1%) had an early recurrence, 13 patients (10.4%) had a late recurrence, and 6 patients (5.7%) had a re-recurrence. In multivariate analysis, a positive/uncertain margin [Odds ratio (OR) = 4.023, P = 0.048] and piecemeal resection (OR = 6.610, P = 0.005) were significant risk factors for early and late recurrence, respectively. Piecemeal resection was also a significant risk factor for non-curative resection (OR = 5.424, P = 0.007). Twenty-six patients experienced adverse events (24.5%).
Endoscopic papillectomy is a safe and effective treatment for ampullary adenomas. Careful selection and follow-up of patients is mandatory, particularly in cases with positive/uncertain margin and piecemeal resection.
Core Tip: This is a multi-center study evaluating the clinical outcomes of 106 patients who underwent endoscopic papillectomy for ampullary adenoma. In our results, margin-positive/uncertain pathologic reports and piecemeal resection were significant factors for the curative resection and recurrences. Unexpectedly, many recurrences were observed in margin-negative resection, but in most cases, they were successfully managed with minimally invasive endoscopic therapies. Since there is no definite factor for predicting and preventing recurrence and re-recurrence, regular follow-up with endoscopy should be performed in every patient regardless of resection margin or resection type, especially in patients with margin-positive/uncertain and piecemeal resection.
- Citation: Choi SJ, Lee HS, Kim J, Choe JW, Lee JM, Hyun JJ, Yoon JH, Kim HJ, Kim JS, Choi HS. Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study. World J Gastroenterol 2022; 28(17): 1845-1859
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1845.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1845
Ampullary adenomas (AAs) are rare lesions, with a prevalence of 0.04%-0.12% in autopsy, and account for 0.2%-5% of newly diagnosed intestinal neoplasms[1-3]. As the number of endoscopic surveillance or computed tomography (CT) scans has increased, the number of AAs detected has also increased. Patients with AA are often asymptomatic, and other complaints are related to biliary or pancreatic obstruction, such as jaundice, biliary colic, or pancreatitis. Even in asymptomatic patients, an AA needs to be removed because of its malignant potential[4]. Furthermore, complete excision of AAs is necessary because of poor diagnostic accuracy with false-negative rates of up to 30% and diagnostic discrepancy of pathologic results, reported as 25%-60%, with forceps biopsy[5,6].
Endoscopic papillectomy (EP) was first introduced for the treatment of AA by Suzuki et al[7] in 1983, and both endoscopic and surgical approaches have been considered for the treatment of AA. EP is now considered as the first treatment of choice for benign AA due to the high recurrence, mortality, and morbidity of surgery[8-11]. Nevertheless, there remain concerns regarding EP. The reported EP adverse event rate is over 20% and while most cases are not severe, this cannot be neglected[12]. The recurrence rate after EP is high at 58.3%, and re-recurrence or persistence of AA has often been reported, requiring patients to undergo additional procedures or surgery[13,14]. Despite recent guidelines, there is no consensus on outcome parameters, and there are no established indication for EP or guidelines for EP technique, and no guidelines for the management of recurrence and re-recurrence[12,15,16].
Here, we aimed to evaluate the clinical outcomes of patients who underwent EP and investigate the factors that affect recurrence and adverse events to assist in improving the outcomes of EP and establishing the guidelines for EP.
We retrospectively reviewed the medical charts of patients who underwent EP for AA between January 2013 and December 2019 and their follow-up data until December 2020 at five tertiary hospitals: Korea University Anam Hospital, Korea University Guro Hospital, Korea University Ansan Hospital, Hanyang University Seoul Hospital, and Hanyang University Guri Hospital. We excluded patients who underwent EP or surgical ampullectomy prior to enrollment and those who were followed up for less than a year after EP. Patients with non-adenomatous lesions were also excluded.
Patient baseline characteristics including age, sex, body mass index, clinical presentations, and initial pathologic reports of the lesion were recorded. Patients were screened for familial adenomatous polyposis (FAP), and mean follow-up periods were calculated. Parameters for EP techniques were recorded using written reports of EP, endoscopic images, and fluoroscopic images. These parameters included endoscopic ultrasound (EUS), cholangiogram, pancreatogram, submucosal lifting, type of resection(en-bloc/piecemeal), thermal ablation after resection, complete endoscopic resection, bile duct stent insertion (BDS), and pancreatic duct stent insertion (PDS).
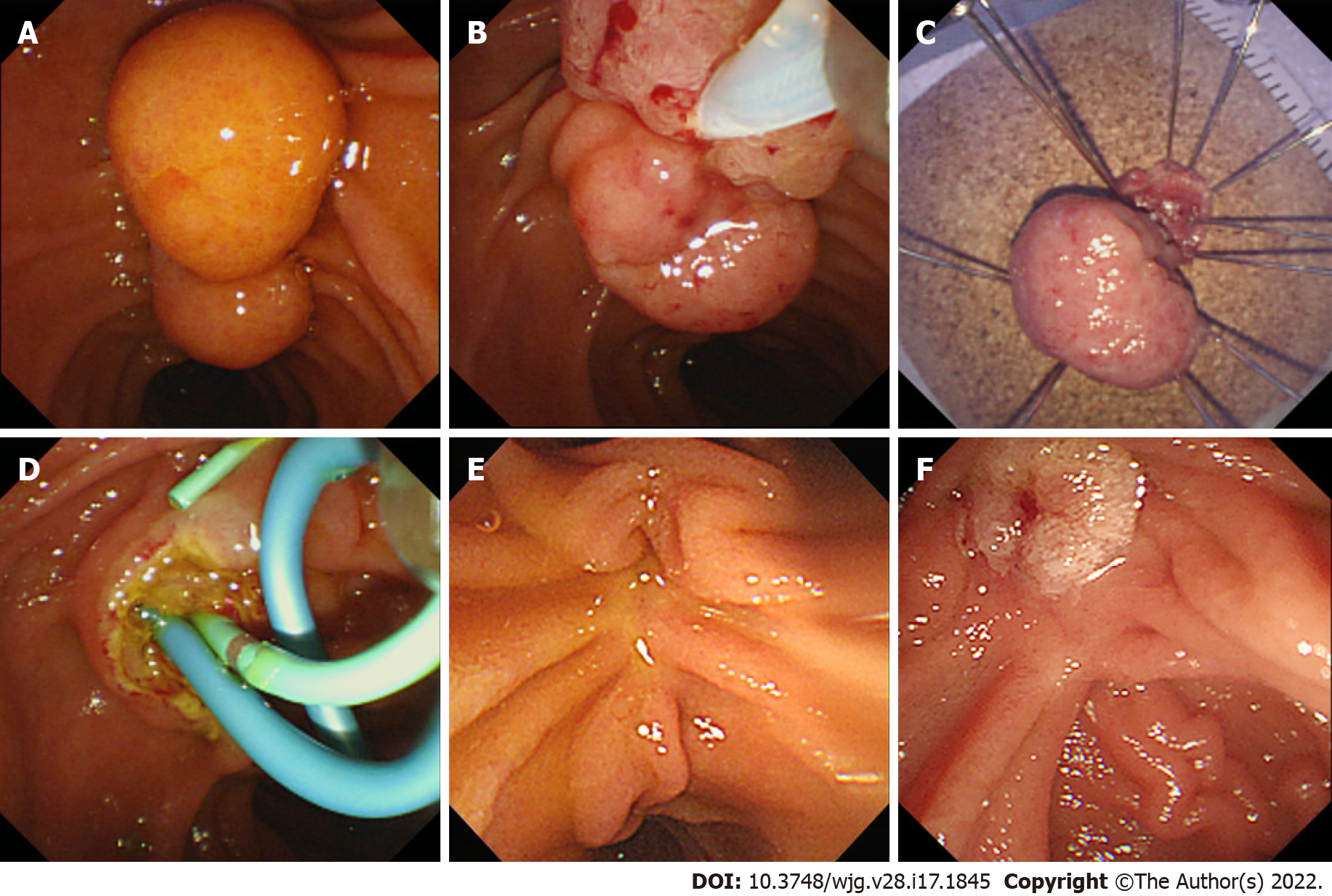
EP was performed at five tertiary hospitals with over 500 endoscopic retrograde cholangiography (ERCP) annual cases by seven experts with over five years of ERCP experience. Before EP, EUS was performed at the endoscopist’s discretion. After adequate sedation, the ampulla of Vater was carefully inspected for its size, extent, and signs of malignancy (Figure 1A). Following the inspection, a cholangiogram and pancreatogram were obtained in cases requiring evaluation of a possible intraductal invasion. Then, snare polypectomy was performed (Figure 1B). Mucosal lifting using saline was performed if needed. With a standard polypectomy snare, the adenoma was tightly grasped, and the electrical current was applied until complete resection of the lesion was achieved.
En-bloc resection of AA was first attempted, and a piecemeal resection was performed if en-bloc resection was not possible. The resected specimen was removed and sent for pathologic evaluation (Figure 1C). The specimen was reviewed by a gastrointestinal pathologist and one or more residents in each hospital.
The EP site was observed for possible remnant lesions and immediate adverse events. Where remnant tissue was suspected, removal was performed with repeated biopsy, snaring, or thermal ablation with argon plasma coagulation (APC). In the event of immediate bleeding, epinephrine was sprayed with additional APC if bleeding persisted. In the event of duodenal perforation, endoscopic hemoclips were applied for the primary closure and surgery was subsequently performed. Sphinterotomies, BDS, and PDS were performed if needed (Figure 1D). The procedure was terminated if there was no more residual tissue or in the absence of immediate adverse events. Subsequently, the patient was observed on the ward with physical examination, monitoring of vital signs, laboratory tests, and X-rays for early adverse events. The details of each endoscopic procedure were determined by the endoscopist.
All patients underwent routine follow-up after EP. Within 3 mo of the procedure, patients underwent endoscopic surveillance for assessment of remnant tissue and recurrence, and stent removal (Figure 1E). Biopsy was performed if any remnant lesion was suspected. If the biopsy result showed remnants or early recurrence, additional therapeutic plans were decided by the endoscopist with the patient (Figure 1F). If no abnormal lesion was identified, the patient underwent further surveillance at six-monthly intervals for the first two years and annually thereafter.
EP results included the resection specimen size, pathologic findings, accuracy of endoscopic biopsy, resection margin, curative resection, early and late recurrence, re-recurrence, endoscopic success, mean hospital stay, and mean adenoma-free period. EP outcomes were obtained from pathologic reports and medical charts.
Curative resection was defined as complete endoscopic resection without recurrence during follow-up. Early recurrence was defined as reconfirmed adenoma following biopsy at the first surveillance endoscopy. Late recurrence was defined as reconfirmed adenoma following biopsy after the first surveillance endoscopy. Re-recurrence was defined as recurrence of adenoma at the follow-up biopsy after the treatment of early or late recurrence. Endoscopic success was defined as treatment of AA with endoscopy, including cases with residual tissue, recurrence or complications, without surgical intervention. Resection margins were categorized into 3 groups, negative, positive, and uncertain, and they were analyzed as positive/uncertain group and negative group[17,18].
Adverse events of EP were categorized into early events (pancreatitis, delayed bleeding, cholangitis, and perforation) occurring within 30 d of the procedure and late events (papillary stenosis and death) occurring after 30 d following the procedure. Endoscopic adverse events and their severity were graded according to the American Society for Gastrointestinal Endoscopy criteria[19]. We set the minimum follow-up duration to one year to avoid underestimation of recurrence and adverse events. Univariate analysis and multivariate analysis were performed to evaluate the risk factors of early and late recurrence, non-curative resection, and adverse events.
Continuous variables were expressed as mean and standard deviation, and Categorical variables were expressed as a number and percentage. Univariate logistic regression analysis was performed to analyze the risk factors for early and late recurrences, non-curative resection, and adverse events. Variables that were significant in the univariate analysis were included in the multivariate logistic regression analysis. A P value < 0.05 was considered significant. The probability of adenoma-free after EP was analyzed using the Kaplan–Meier method. The statistical analyses were performed using the SPSS version 22.0 software (IBM Corp., Armonk, N.Y., USA).
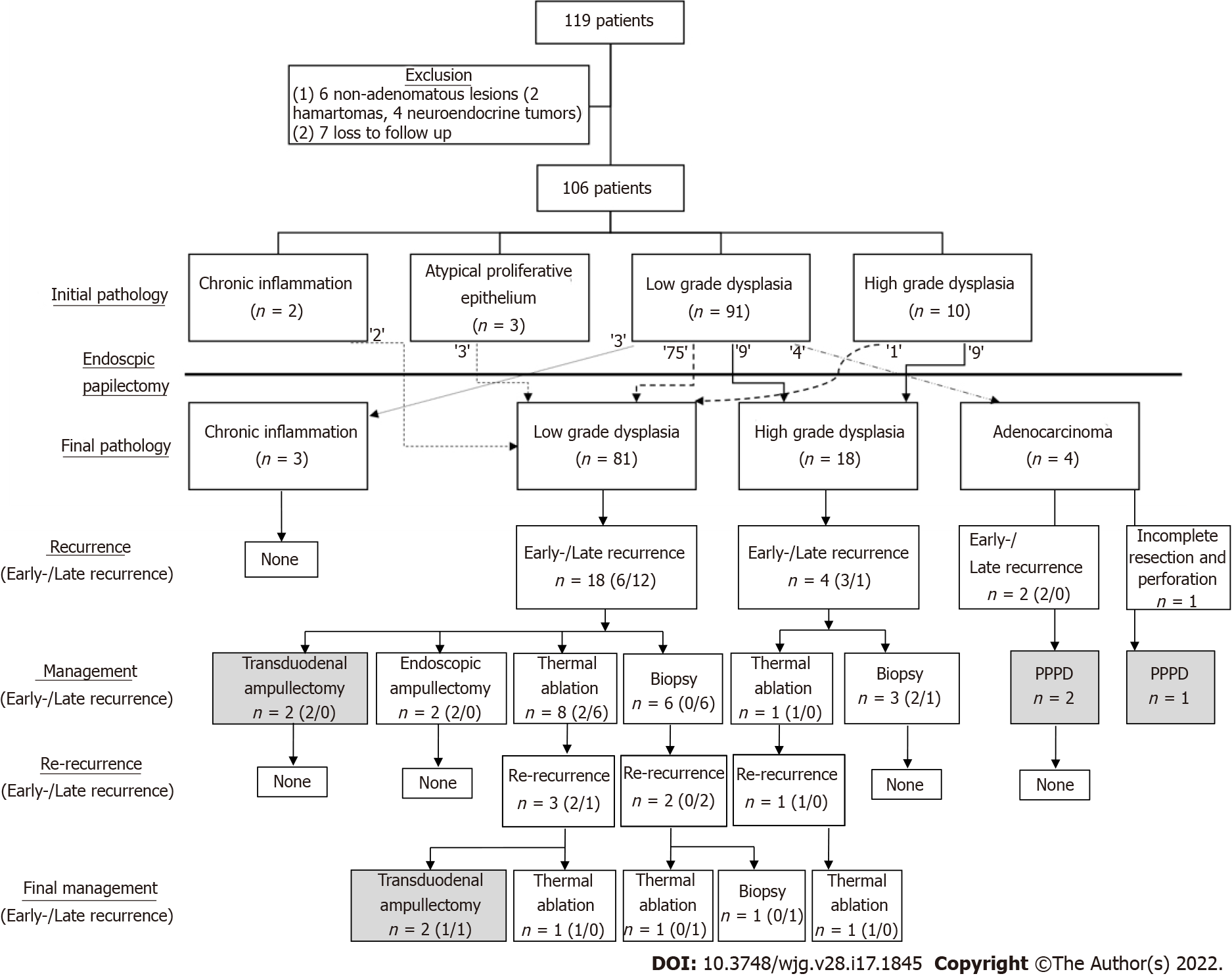
We retrospectively collected the medical records of 119 patients and their follow-up data (Figure 2). We excluded seven patients who failed to meet the follow-up criteria or were lost to follow-up within a year of the procedure, and six patients because of non-adenomatous lesions. After the exclusion criteria were applied, 106 patients were finally included for analysis.
Patient baseline characteristics are shown in Table 1. Seventy-three patients (68.9%) were asymptomatic, and AA was diagnosed incidentally from screening endoscopy or CT scan. The most frequent symptoms associated with AA were jaundice in 16 patients (15.1%) and abdominal discomfort in 13 patients (12.3%). All patients underwent biopsy before the EP procedure, and their pathology reports were as follows: chronic inflammation in 2 patients (1.9%), atypical proliferative epithelium in 3 patients (2.8%), adenoma with low-grade dysplasia in 91 patients (85.8%), and adenoma with high-grade dysplasia in 10 patients (9.4%).
| Characteristics | n (%)/mean ± SD (n = 106) |
| Age, yr | 61.4 ± 12.8 |
| ≤ 65 | 57.5) |
| > 65 | 45 (42.5) |
| Sex | |
| Male | 62 (58.5) |
| Female | 44 (41.5) |
| Body mass index (kg/m2) | 23.3 ± 2.2 |
| ≤ 25 | 90 (84.9) |
| > 25 | 16 (15.1) |
| Clinical presentation | |
| Asymptomatic | 73 (68.9) |
| Jaundice | 16 (15.1) |
| Abdominal discomfort | 13 (12.3) |
| Other | 4 (3.8) |
| Familial adenomatous polyposis | 3 (2.8) |
| Initial pathology | |
| Chronic inflammation | 2 (1.9) |
| Atypical proliferative epithelium | 3 (2.8) |
| Low-grade dysplasia | 91 (85.8) |
| High-grade dysplasia | 10 (9.4) |
| Mean follow-up, mo | 36.2 ± 18.3 |
The EP techniques used for the patients are listed in Table 2. EUS was performed in 37 patients (34.9%), and a cholangiogram and pancreatogram were obtained in 70 patients (66.0%) and 87 patients (82.1%), respectively. Four patients (3.8%) underwent submucosal lifting with normal saline. En-bloc resection was performed in 90 patients (84.9%), and piecemeal resection was performed in 16 patients (15.1%). After the resection, thermal ablation was performed in 24 patients (22.6%) because of remnant tissue or immediate bleeding. Complete endoscopic resection was successfully performed in 105 patients (99.1%), and in one patient the lesion could not be completely removed due to underlying fibrosis and a diagnosis of adenocarcinoma was finally made. BDS and PDS were performed in 25 patients (23.6%) and 78 patients (73.6%), respectively.
| n (%)/mean ± SD (n = 106) | |
| EUS | 37 (34.9) |
| ERCP | |
| Cholangiogram | 70 (66.0) |
| Pancreatogram | 87 (82.1) |
| Submucosal lifting | 4 (3.8) |
| Type of resection | |
| En-bloc | 90 (84.9) |
| Piecemeal | 16 (15.1) |
| Thermal ablation after resection | 24 (22.6) |
| Complete endoscopic resection | 105 (99.1) |
| Stent implantation | |
| Bile duct | 25 (23.6) |
| Pancreatic duct | 78 (73.6) |
| Resection specimen size, mm | 13.6 ± 5.5 |
| ≤ 15 | 77 (72.6) |
| > 15 | 29 (27.4) |
| Final pathology | |
| Chronic inflammation | 3 (2.8) |
| Adenoma | |
| Low grade | 81 (76.4) |
| High grade | 18 (17.0) |
| Adenocarcinoma | 4 (3.8) |
| Accuracy of endoscopic biopsy | |
| Underestimate | 18 (17.0) |
| Overestimate | 4 (3.8) |
| Resection margin | |
| Negative | 84 (79.2) |
| Positive/Uncertain | 22 (20.8) |
| Positive | 19 (17.9) |
| Uncertain | 3 (2.8) |
| Curative resection | 81 (76.4) |
| Early recurrence | 11 (10.4) |
| Late recurrence | 13 (12.3) |
| Re-recurrence | 6 (5.7) |
| Endoscopic success | 99 (93.4) |
| Mean hospital stay, d | 5.7 ± 3.0 |
| Mean adenoma-free period, mo | 29.6 ± 21.3 |
Table 2 also summarizes the results of the EP. The mean size of the resected specimen was 13.6 ± 5.5 mm, and the final pathologic results were as follows: chronic inflammation in 3 cases (2.8%), low-grade dysplasia in 81 cases (76.4%), high-grade dysplasia in 18 cases (17.0%), and adenocarcinoma in 4 cases (3.8%). Figure 2 shows the diagnostic discrepancies between the initial and final pathologies. Lesions that showed chronic inflammation or atypical proliferative epithelium on initial biopsy were all low-grade dysplasia on final diagnosis. Out of 91 cases of low-grade dysplasia on the initial biopsy, the final pathologic results were chronic inflammation in three cases (2.8%), low-grade dysplasia in 75 cases (82.4%), high-grade dysplasia in 9 cases (9.9%), and adenocarcinoma in four cases (3.8%). Out of 10 cases of high-grade dysplasia on the initial biopsy, the final pathologic results were low-grade dysplasia in one case and high-grade dysplasia in nine cases. Endoscopic biopsy was accurate in 84 patients (79.2%), with underestimation in 18 patients (17.0%) and overestimation in 4 patients (3.8%).
R0 resection was achieved in 84 patients (79.2%), and curative resection was achieved in 81 patients (76.4%). Early recurrence was found in 11 patients (10.4%), late recurrence was found in 13 patients (12.3%), and all recurrences were local lesions. Re-recurrence occurred in six patients (5.7%), and patient characteristics are summarized in Supplementary Table 1. Figure 2 also shows the number of early and late recurrences from final pathologic results, how these cases were managed, how many re-recurrences occurred after the initial management, and final management of re-recurrences.
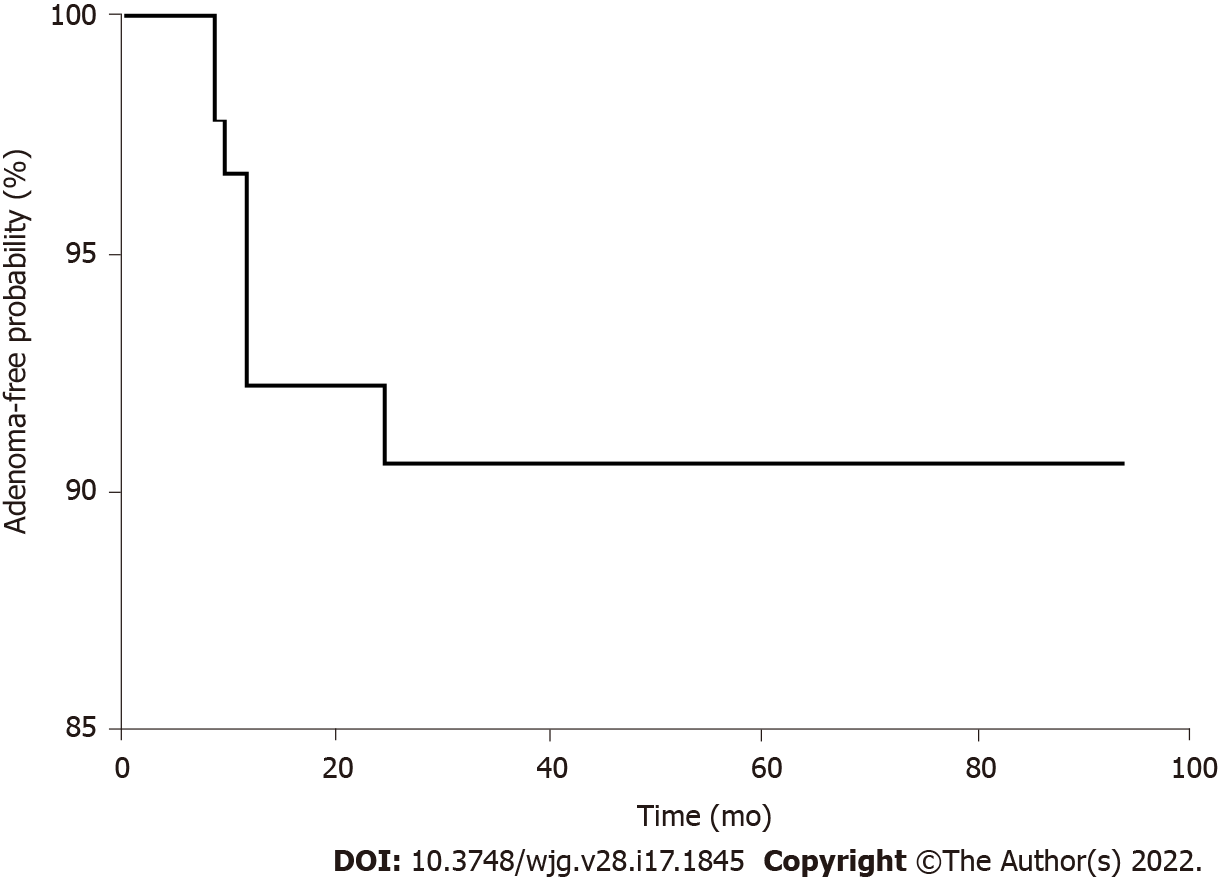
Initial management of the 11 patients with early recurrence involved endoscopic therapy in 7 cases (two EPs, two biopsies, and three thermal ablations) and surgery in four cases [two transduodenal ampullectomies (TA) and two pylorus-preserving pancreaticoduodenectomies (PPPD)]. Three patients with re-recurrence were managed with thermal ablation (two cases) and TA (one case). The 13 patients with late recurrence were initially managed endoscopically (six biopsies and seven ablations), and three patients with re-recurrence underwent thermal ablation, biopsy, and TA, respectively. Altogether, 99 patients (93.4%) were managed by endoscopy alone, and seven patients (6.6%) underwent additional surgical management: four patients due to a remnant lesion, two patients due to re-recurrence, and one patient due to incomplete resection and perforation (Figure 2). The mean adenoma-free period was 29.6 ± 21.3 mo, and the adenoma-free survival is shown in Figure 3. Except for a patient who showed recurrence after 27 mo of the EP procedure, 12 patients (92.3%) experienced recurrence within a year of the EP procedure.
Table 3, Table 4, and Table 5 show the univariate and multivariate analysis of the risk factors for early recurrence, late recurrence and non-curative resection, respectively. Age over 65, EUS, size > 1.5 cm and positive/uncertain resection margin were statistically significant risk factors for early recurrence in univariate analysis, and positive/uncertain resection margin [Odds ratio (OR) = 4.023; 95%CI: 1.088-16.387; P = 0.048] was the significant factor for early recurrence in multivariate analysis. Presence of symptom (OR = 4.659; 95%CI: 1.292-16.797; P = 0.019) and piecemeal resection (OR = 7.114; 95%CI: 1.993-25.398; P = 0.003) were significant risk factors for late recurrence in univariate analysis, and piecemeal resection (OR = 6.610; 95%CI: 1.760-24.820; P = 0.005) was the only significant factor for late recurrence in multivariate analysis. Body mass index over 25, presence of symptom, and piecemeal resection were significant risk factors for non-curative resection, and multivariate analysis showed that piecemeal resection (OR = 5.424; 95%CI: 1.582-18.600; P = 0.007) was a significant risk factor for non-curative resection.
| Simple logistic regression (Univariate analysis) | Multiple logistic regression (Multivariate analysis) | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, > 65 yr | 4.18 (1.042-16.774) | 0.044 | 3.441 (0.807-14.672) | 0.095 |
| Sex, Male | 1.8 (0.513-6.319) | 0.359 | ||
| Body mass index > 25 kg/m2 | 1.286 (0.251-6.587) | 0.763 | ||
| Symptomatic | 3.022 (0.851-10.738) | 0.087 | ||
| EUS | 3.792 (1.031-13.946) | 0.045 | 1.622 (0.290-9.073) | 0.582 |
| Cholangiogram | 1.125 (0.307-4.128) | 0.859 | ||
| Pancreatogram | 2.338(0.281-19.450) | 0.432 | ||
| Submucosal lifting | 3.067 (0.291-32.329) | 0.351 | ||
| Piecemeal resection | 1.286 (0.251-6.587) | 0.763 | ||
| Thermal ablation | 0.313 (0.038-2.578) | 0.280 | ||
| BDS | 1.244 (0.304-5.096) | 0.761 | ||
| PDS | 0.901 (0.221-3.675) | 0.885 | ||
| Size, > 15 mm | 3.757 (1.048-13.461) | 0.042 | 1.811 (0.344-9.521) | 0.483 |
| Initial pathology | ||||
| Benign | Ref | |||
| LGD | 0.683 (0.074-6.272) | 0.736 | ||
| HGD | 2.333 (0.167-32.584) | 0.529 | ||
| Positive/uncertain resection margin | 5.925 (1.610-21.801) | 0.007 | 4.023 (1.088-16.387) | 0.048 |
| Complication | 2.937 (0.815-10.582) | 0.100 | ||
| Simple logistic regression (Univariate analysis) | Multiple logistic regression (Multivariate analysis) | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, > 65 yr | 0.564 (0.162-1.961) | 0.368 | ||
| Sex, Male | 0.865 (0.263-2.847) | 0.812 | ||
| Body mass index > 25 kg/m2 | 2.095 (0.645-6.810) | 0.219 | ||
| Symptomatic | 4.659 (1.292-16.797) | 0.019 | 4.213 (0.091-16.728) | 0.061 |
| EUS | 0.521 (0.134-2.023) | 0.346 | ||
| Cholangiogram | 1.250 (0.377-4.140) | 0.715 | ||
| Pancreatogram | 2.880 (0.351-23.609) | 0.324 | ||
| Submucosal lifting | 2.500 (0.240-26.004) | 0.443 | ||
| Piecemeal resection | 7.114 (1.993-25.398) | 0.003 | 6.610 (1.760-24.820) | 0.005 |
| Thermal ablation | 2.434 (0.715-8.293) | 0.155 | ||
| BDS | 1.524 (0.426-5.449) | 0.517 | ||
| PDS | 4.657 (0.576-37.636) | 0.149 | ||
| Size, > 15 mm | 0.444 (0.092-2.140) | 0.312 | ||
| Positive/uncertain resection margin | 0.286 (0.035-2.326) | 0.242 | ||
| Complication | 0.913 (0.231-3.606) | 0.897 | ||
| Simple logistic regression (Univariate analysis) | Multiple logistic regression (Multivariate analysis) | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, > 65 yr | 1.485 (0.595-3.703) | 0.397 | ||
| Sex, Male | 1.256 (0.503-3.141) | 0.625 | ||
| Body mass index > 25 kg/m2 | 3.340 (1.090-10.235) | 0.035 | 2.942 (0.855-10.117) | 0.087 |
| Symptomatic | 2.905 (1.133-7.466) | 0.026 | 3.509 (0.942-9.914) | 0.058 |
| EUS | 1.455 (0.572-3.699) | 0.431 | ||
| Cholangiogram | 1.222 (0.475-3.148) | 0.678 | ||
| Pancreatogram | 2.877 (0.615-13.458) | 0.179 | ||
| Submucosal lifting | 3.636 (0.484-27.302) | 0.209 | ||
| Piecemeal resection | 4.625 (1.510-14.162) | 0.007 | 5.424 (1.582-18.600) | 0.007 |
| Thermal ablation | 1.185 (0.410-3.427) | 0.754 | ||
| BDS | 1.464 (0.526-4.076) | 0.466 | ||
| PDS | 1.949 (0.601-6.322) | 0.266 | ||
| Size, > 15 mm | 1.452 (0.543-3.881) | 0.457 | ||
| Initial pathology | ||||
| Benign | Ref | |||
| LGD | 0.446 (0.098-2.036) | 0.297 | ||
| HGD | 0.556 (0.065-4.755) | 0.592 | ||
| Positive/uncertain resection margin | 1.839 (0.648-5.220) | 0.252 | ||
| Complication | 1.778 (0.656-4.817) | 0.258 | ||
Altogether, adverse events occurred in 26 patients as shown in Table 6. Early adverse events were as follows: pancreatitis in 14 patients (13.2%), delayed bleeding in 11 patients (10.4%), cholangitis in six patients (5.7%), and perforation in one patient (0.9%). No late adverse events were reported. In most cases, the severity of adverse events was classified as either mild or moderate, except for one case with perforation. Table 7 shows the univariate and multivariate analysis of risk factors for adverse events, including pancreatitis and delayed bleeding. FAP, pancreatogram, thermal ablation and PDS were significant risk factors for pancreatitis in univariate analysis, and in multivariate analysis, thermal ablation (OR = 4.128; 95%CI: 1.005-17.128; P = 0.048) was a positive risk factor, while PDS (OR = 0.205; 95%CI: 0.044-0.945; P = 0.042) was a negative risk factor for pancreatitis. Cholangiogram, piecemeal resection, and BDS were significant risk factors for delayed bleeding in univariate analysis, and piecemeal resection (OR = 6.698; 95%CI: 1.1.599-28.057; P = 0.009) was the only significant risk factor in multivariate analysis. No significant risk factor for cholangitis or perforation was identified.
| n (%) (n = 106) | |
| Early | |
| Pancreatitis | 14 (13.2) |
| Delayed bleeding | 11 (10.4) |
| Cholangitis | 6 (5.7) |
| Perforation | 1 (0.9) |
| Late | |
| Papillary stenosis | 0 (0.0) |
| Mortality | 0 (0.0) |
| Total | 26 (24.5) |
| Pancreatitis | Bleeding | |||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, > 65 yr | 0.722 (0.225-2.323) | 0.585 | 0.753 (0.206-2.745) | 0.667 | ||||
| Sex, Male | 1.486 (0.481-4.590) | 0.491 | 1.800 (0.513-6.319) | 0.359 | ||||
| Body mass index > 25 kg/m2 | 0.412 (0.071-3.902) | 0.635 | 0.533 (0.063-4.480) | 0.563 | ||||
| Symptomatic | 0.144 (0.018-1.153) | 0.068 | 1.994 (0.562-7.073) | 0.285 | ||||
| EUS | 2.067 (0.664-6.428) | 0.210 | 1.641 (0.465-5.788) | 0.442 | ||||
| FAP | 15.167 (1.277-180.166) | 0.031 | 9.363 (0.429-204.392) | 0.155 | 4.650 (0.386-55.945) | 0.226 | ||
| Cholangiogram | 1.550 (0.493-4.870) | 0.453 | 3.983 (1.081-14.669) | 0.038 | 2.235 (0.323-15.460) | 0.415 | ||
| Pancreatogram | 0.278 (0.087-0.885) | 0.030 | 0.534 (0.106-2.678) | 0.446 | 2.338 (0.281-19.450) | 0.432 | ||
| Piecemeal resection | 0.929 (0.187-4.606) | 0.928 | 6.364 (1.661-24.375) | 0.007 | 6.698 (1.599-28.057) | 0.009 | ||
| Thermal ablation | 4.412 (1.366-14.250) | 0.013 | 4.128 (1.005-17.128) | 0.048 | 2.143 (0.570-8.052) | 0.259 | ||
| BDS | 0.868 (0.222-3.393) | 0.838 | 4.800 (1.323-17.418) | 0.017 | 2.647 (0.398-17.619) | 0.314 | ||
| PDS | 0.102 (0.030-0.349) | 0.000 | 0.205 (0.044-0.945) | 0.042 | 1.607 (0.325-7.951) | 0.561 | ||
| Size, > 15 mm | 1.072 (0.308-3.731) | 0.913 | 2.465 (0.690-8.812) | 0.165 | ||||
| Initial pathology | ||||||||
| Benign | Ref | Ref | ||||||
| LGD | 0.975 (0.109-8.693) | 0.982 | 0.293 (0.050-1.697) | 0.171 | ||||
| HGD | 2.333 (0.167-32.584) | 0.529 | 0.429 (0.031-5.985) | 0.529 | ||||
| Positive/uncertain resection margin | 3.562 (1.086-11.685) | 0.036 | 0.833 (0.167-4.167) | 0.824 | ||||
Of the 106 patients, curative resection was performed in 81 patients (76.4%) with 26 cases of adverse events (24.5%), 11 early recurrences (10.4%), 13 Late recurrences (12.3%), and 6 re-recurrences (5.7%). Our results were consistent with those of previous studies showing curative resection rates of 73.0%-82.7%, adverse events rates of 15.0%-43.6%, early recurrence rates of 2.7%-19.0%, and late recurrence rates of 0-23.9%[14,20-23]. There are large variations in the reported outcomes, particularly among studies involving small numbers of cases because there is no consensus on which parameter best represents the performance of EP. The parameters used in previous studies are inconsistent, and inclusion criteria for EP vary.
Factors used for the evaluation of outcomes in previous studies include visual resection margin, histologic resection margin, recurrence, adverse events, need for surgery, and combinations of these factors. We suggest that curative resection (negative visual resection margin and no recurrence), adverse events, and endoscopic success (negative visual resection margin and no need for surgery) best represent the outcomes of EP. An ideal outcome for EP is the achievement of complete removal of the AA, without adverse events, and without recurrence, which is curative resection with no adverse event. Moreover, even in the event of recurrence, most of these patients can be and are managed endoscopically, representing cases of endoscopic success. We attempted to identify the factors that could predict and improve these outcomes.
In 84 patients (79.2%) the initial and final pathologic results were consistent, which is comparable to previously reported studies[20,23]. The initial biopsy result for the four patients with adenocarcinoma was reported as low-grade dysplasia. Biopsies of AA can occasionally be insufficient because the biopsy is often performed using a forward-viewing endoscope, making a targeted biopsy difficult. Therefore, even if the initial result is benign, it is important to remove the lesion completely with an adequate margin-free area in case of malignancy.
The ampullary lesions found after EP are often described as remnant/residual or recurrence in the literature[14,20,22-24]. Currently, these two categories are clinically distinguished according to the timing of lesion discovery: most studies define remnant/residual as the part of the previous lesion found at the first or any surveillance endoscopy performed 3-6 mo after EP, and recurrence as the lesion found after the first surveillance endoscopy or 6 mo after EP. Both are confirmed histologically. There is a clear difference between these definitions as remnant/residual refers to the remaining part of the pathologic lesion, while recurrence refers to a pathologic lesion that is newly developed after the procedure[25]. However, it is often difficult to separate these cases clinically. Diagnosis of a remnant could be delayed and the lesion may be found after the first surveillance endoscopy for a number of reasons including small size of remnant tissues and tissue burn from the procedure, and the delay in diagnosis leads to underestimation of remnant/residual lesions and overestimation of recurrence cases[18]. Considering a newly identified lesion at the first surveillance endoscopy as a remnant/residual in R0 resection may also be controversial. Therefore, instead of labeling these two groups differently, it is preferable to refer to both lesions as recurrence and distinguish these cases according to the timing of diagnosis. The recent European Society of Gastrointestinal Endoscopy guideline states that up to two thirds of recurrences are early recurrences[15].
These two groups differ in terms of the timing of the diagnosis and clinical implications, and there may also be differences in patient management. Recurrences were managed at the endoscopist’s discretion using various strategies, including endoscopic and surgical management. Except for early recurrence from adenocarcinoma that was managed with PPPD, it was difficult to identify which factors were considered for a particular treatment. However, early recurrences tend to be managed more aggressively than late recurrences, although the numbers were small for comparisons to be statistically significant (3 vs 1 TA and 2 vs 0 additional EP for early vs late recurrences). This tendency may be explained in that in cases of early recurrence, the initial removal of the lesion has been incomplete, so more invasive treatment may be required compared to the previous treatment method. Conversely, late recurrences are newly developed lesions that are typically small or are early lesions.
It is often difficult to establish which area of the adenoma is responsible for the recurrence because recurrences are typically small, but it can be presumed that they occur from the bile duct, pancreatic orifice, base of ampulla, or resection margin. Reported risk factors for recurrence include age, sex, FAP, intraductal involvement, incomplete resection, piecemeal resection, and final pathology, although the results of these studies are rather inconsistent[13,23,25-27]. Here, a positive/uncertain resection margin in the pathologic report was a significant risk factor for early recurrence, and piecemeal resection was a significant risk factor for late recurrence. This is the first study to analyze the risk factors for both early and late recurrence, considering the different definitions and characteristics of recurrence. A positive resection margin could increase the risk of remnants at the resection margin, but this association was not found to be significant in previous studies[25]. This may be because the positive margin following an EP procedure is occasionally unreliable, as the resected lesions are often too small to be properly manipulated, and cauterization may mask a positive margin[17]. Also, many previous studies do not clearly state how they analyzed the lesion with uncertain margin. More studies are needed to understand the clinical implications of a positive/uncertain resection margin and develop further management strategies for margin-positive/uncertain lesions. Additionally, to reduce recurrence after EP, it is important to check the peripheral and deep margin of the lesion meticulously before the EP, including intraductal involvement, and secure the resection margin properly during the EP. This is because recurrence could be caused by the poor selection of patients or inability to secure the margin during the procedure, which is sometimes inevitable due to the characteristics of the lesion or the procedure itself. A more aggressive procedure could secure an adequate margin but may cause adverse events such as perforation, so proper selection of patients and careful approaches are mandatory. Similar considerations apply to the higher risk of late recurrence in piecemeal resection. In piecemeal resection, the resection margin may be unreliable, and a thorough evaluation and follow-up for recurrence is required. Piecemeal resection was a significant risk factor for non-curative resection, meaning en-bloc resection is a significant protective factor for curative resection, while a pathologic margin was not significant. A positive/uncertain margin and piecemeal resection are important factors for recurrence prediction, although their negative predictive values were 79.8% and 77.4%, respectively; thus, curative resection cannot be assumed in lesions with a negative margin or en-bloc resection.
Interestingly, our study was the first to compare the effects of factors including hospital setting and endoscopist experience on the outcome of EP (Supplementary Table 2), and these were not significant for remnant, recurrence, or adverse events. These findings suggest that there was no significant difference in EP results between hospitals with a certain volume of ERCP cases and endoscopists with a certain level of experience. Further studies with larger number of patients are needed to support our suggestion.
Of the six patients with re-recurrence, two patients experienced re-recurrence even after a further session of endoscopic treatment and underwent surgery. Patients with persistent AA showed no specific features to guide the early prediction of the lesion characteristics and early transition to more invasive therapy. The finding that EUS was performed in both patients with re-recurrence suggests that EUS may not adequately predict recurrence or persistence. Moreover, it is unclear as to what extent a benign, although premalignant, AA lesion should be treated at recurrence, considering the adverse events associated with the available treatments. High-quality recommendations or guidelines are necessary.
Our results showed that most adverse events caused by EP showed mild- to moderate-grade severity. The role of thermal ablation in bleeding remains controversial and studies have shown that the risk of pancreatitis increased with the size of the lesion and when hemostasis was performed[22,23,28]. Here, thermal ablation was not significantly associated with bleeding or recurrence although it increased the risk of pancreatitis. The role of PDS is still under debate, but results of several studies, including ours, advocate the use of PDS for prophylaxis of pancreatitis[29-31]. Moreover, no pancreatic stenosis was observed, and this could be explained by our relatively high PDS rate at 73.6%. Hence, it is expected that PDS will help prevent pancreatitis and pancreatic stenosis, and we recommend routine pancreatic stenting, if possible. Also, our result showed that piecemeal resection was the only significant risk factor for delayed bleeding. Piecemeal resection was performed for lesions where en-bloc resection was impossible, therefore, the lesions with piecemeal resection tend to be larger[32]. A previous study did not show the correlation between piecemeal resection and bleeding, based on the small number of piecemeal resection cases, but colonic lesions with piecemeal resection show significant bleeding during endoscopic mucosal resection[32,33]. Further research is needed to support the role and adverse events of piecemeal resection in endoscopic papillectomy.
Our study has several limitations. As the indications for EP have not been established, selection bias could not be avoided. Moreover, due to the lack of guidelines on the optimal EP technique, several decisions made during the procedure were at the discretion of the endoscopist. Not all hospitals distinguished margin-positive cases as vertical or lateral involvement, therefore, we simplified the involvement of the margin as positive/uncertain or negative. Finally, the study design was retrospective, and factors regarding the procedure and follow-up could not be controlled.
EP is a feasible treatment option for AA with high technical success. However, diagnostic discrepancy, remnant lesions, recurrence, and adverse events cannot be neglected. Unlike gastric or colon adenoma resection, even in cases of complete resection, remnant lesions, recurrence, and re-recurrence were identified, emphasizing the importance of follow-up. For patients with a positive/uncertain resection margin in particular, close follow-up for early recurrence is required, and the possibility of late recurrence should be considered in patients with piecemeal resection. Especially in patients with a positive/uncertain resection margin or piecemeal resection, the possibility of recurrence should be considered, and closer follow-up for recurrence is required.
The incidence of ampullary adenoma (AA) is increasing, partly from increasing number of imaging studies and from true increase in incidence. Because of its malignant potential, AA has to be removed either surgically or endoscopically.
The role of endoscopic papillectomy (EP) in treatment of AA has been growing due to its relatively low invasiveness, but recurrences and side effects are reported in up to 30% of cases.
Our study aimed to evaluate the clinical outcomes of EP in patients with AA, performed at five tertiary hospitals.
We collected the clinical data of patients with AA who underwent EP at five tertiary hospitals between 2013 and 2020 and analyzed the clinical outcomes and adverse events. Clinical outcomes were curative resection, defined as complete endoscopic resection without recurrence, endoscopic success, defined as treatment of ampullary adenoma with endoscopy alone, and recurrence, defined reconfirmed adenoma in endoscopy. Recurrence was divided into early and late, based on an interval of 6 mo.
Among 106 patients included, curative resection was achieved in 81 patients (76.4%), endoscopic success was achieved in 99 patients (93.4%), early recurrence was identified in 11 patients (16.1%), and late recurrence was identified in 13 patients, and re-recurrence was identified in 6 patients (12.3%). In multivariate analysis, the risk of early and late recurrences was significantly increased in a positive/uncertain margin and piecemeal resection, respectively. The risk of non-curative resection was significantly increased in piecemeal resection. Twenty-six patients experienced adverse events (24.5%): 14 pancreatitis, 11 delayed bleeding, 6 cholangitis, and 1 perforation.
EP is a relatively safe procedure with high endoscopic success rate. Due to the diagnostic discrepancy, recurrence, re-recurrence and adverse events, careful selection and follow-up of patients are also needed.
A positive/uncertain margin and piecemeal resection were significant risk factors for poor outcomes; therefore, every effort should be made to ensure adequate free margin and to perform en-bloc resection during EP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Philipose J, United States; Sahin TT, Turkey; Tsuji Y, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Rosenberg J, Welch JP, Pyrtek LJ, Walker M, Trowbridge P. Benign villous adenomas of the ampulla of Vater. Cancer. 1986;58:1563-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Scarpa A, Capelli P, Zamboni G, Oda T, Mukai K, Bonetti F, Martignoni G, Iacono C, Serio G, Hirohashi S. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163-1172. [PubMed] |
| 3. | Ramai D, Ofosu A, Singh J, John F, Reddy M, Adler DG. Demographics, tumor characteristics, treatment, and clinical outcomes of patients with ampullary cancer: a Surveillance, Epidemiology, and End Results (SEER) cohort study. Minerva Gastroenterol Dietol. 2019;65:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Jung S, Kim MH, Seo DW, Lee SK. Endoscopic snare papillectomy of adenocarcinoma of the major duodenal papilla. Gastrointest Endosc. 2001;54:622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Askew J, Connor S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB (Oxford). 2013;15:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Suzuki K. Two cases with ampullary cancer who underwent endoscopic excision. Prog Dig Endosc. 1983;23:236-239. |
| 8. | Hong S, Song KB, Lee YJ, Park KM, Kim SC, Hwang DW, Lee JH, Shin SH, Kwon J, Ma CH, Hwang S, Park G, Park Y, Lee SJ, Kim YW. Transduodenal ampullectomy for ampullary tumors - single center experience of consecutive 26 patients. Ann Surg Treat Res. 2018;95:22-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lee R, Huelsen A, Gupta S, Hourigan LF. Endoscopic ampullectomy for non-invasive ampullary lesions: a single-center 10-year retrospective cohort study. Surg Endosc. 2021;35:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, Branch MS, Pappas TN. Endoscopic versus surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Dubois M, Labgaa I, Dorta G, Halkic N. Endoscopic and surgical ampullectomy for non-invasive ampullary tumors: Short-term outcomes. Biosci Trends. 2017;10:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Spadaccini M, Fugazza A, Frazzoni L, Leo MD, Auriemma F, Carrara S, Maselli R, Galtieri PA, Chandrasekar VT, Fuccio L, Aljahdli E, Hassan C, Sharma P, Anderloni A, Repici A. Endoscopic papillectomy for neoplastic ampullary lesions: A systematic review with pooled analysis. United European Gastroenterol J. 2020;8:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Ma T, Jang EJ, Zukerberg LR, Odze R, Gala MK, Kelsey PB, Forcione DG, Brugge WR, Casey BW, Syngal S, Chung DC. Recurrences are common after endoscopic ampullectomy for adenoma in the familial adenomatous polyposis (FAP) syndrome. Surg Endosc. 2014;28:2349-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Napoleon B, Gincul R, Ponchon T, Berthiller J, Escourrou J, Canard JM, Boyer J, Barthet M, Ponsot P, Laugier R, Helbert T, Coumaros D, Scoazec JY, Mion F, Saurin JC; Sociéte Française d’Endoscopie Digestive (SFED, French Society of Digestive Endoscopy). Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Vanbiervliet G, Strijker M, Arvanitakis M, Aelvoet A, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Moss A, Napoleon B, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Barthet M, van Hooft JE. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 16. | Fritzsche JA, Fockens P, Barthet M, Bruno MJ, Carr-Locke DL, Costamagna G, Coté GA, Deprez PH, Giovannini M, Haber GB, Hawes RH, Hyun JJ, Itoi T, Iwasaki E, Kylänpaä L, Neuhaus H, Park JY, Reddy DN, Sakai A, Bourke MJ, Voermans RP. Expert consensus on endoscopic papillectomy using a Delphi process. Gastrointest Endosc. 2021;94:760-773.e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 17. | Sakai A, Tsujimae M, Masuda A, Iemoto T, Ashina S, Yamakawa K, Tanaka T, Tanaka S, Yamada Y, Nakano R, Sato Y, Kurosawa M, Ikegawa T, Fujigaki S, Kobayashi T, Shiomi H, Arisaka Y, Itoh T, Kodama Y. Clinical outcomes of ampullary neoplasms in resected margin positive or uncertain cases after endoscopic papillectomy. World J Gastroenterol. 2019;25:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Yasuda I, Kobayashi S, Takahashi K, Nanjo S, Mihara H, Kajiura S, Ando T, Tajiri K, Fujinami H. Management of Remnant or Recurrent Lesions after Endoscopic Papillectomy. Clin Endosc. 2020;53:659-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1833] [Article Influence: 122.2] [Reference Citation Analysis (1)] |
| 20. | Kang SH, Kim KH, Kim TN, Jung MK, Cho CM, Cho KB, Han JM, Kim HG, Kim HS. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol. 2017;17:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Bohnacker S, Seitz U, Nguyen D, Thonke F, Seewald S, deWeerth A, Ponnudurai R, Omar S, Soehendra N. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Nam K, Song TJ, Kim RE, Cho DH, Cho MK, Oh D, Park DH, Lee SS, Seo DW, Lee SK, Kim MH, Baek S. Usefulness of argon plasma coagulation ablation subsequent to endoscopic snare papillectomy for ampullary adenoma. Dig Endosc. 2018;30:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Li S, Wang Z, Cai F, Linghu E, Sun G, Wang X, Meng J, Du H, Yang Y, Li W. New experience of endoscopic papillectomy for ampullary neoplasms. Surg Endosc. 2019;33:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Sahar N, Krishnamoorthi R, Kozarek RA, Gluck M, Larsen M, Ross AS, Irani S. Long-Term Outcomes of Endoscopic Papillectomy for Ampullary Adenomas. Dig Dis Sci. 2020;65:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Ridtitid W, Tan D, Schmidt SE, Fogel EL, McHenry L, Watkins JL, Lehman GA, Sherman S, Coté GA. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long-term follow-up. Gastrointest Endosc. 2014;79:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Klein A, Qi Z, Bahin FF, Awadie H, Nayyar D, Ma M, Voermans RP, Williams SJ, Lee E, Bourke MJ. Outcomes after endoscopic resection of large laterally spreading lesions of the papilla and conventional ampullary adenomas are equivalent. Endoscopy. 2018;50:972-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Yang JK, Hyun JJ, Lee TH, Choi JH, Lee YN, Choe JW, Park JS, Kwon CI, Jeong S, Kim HJ, Moon JH, Park SH. Can prophylactic argon plasma coagulation reduce delayed post-papillectomy bleeding? J Gastroenterol Hepatol. 2021;36:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Menees SB, Schoenfeld P, Kim HM, Elta GH. A survey of ampullectomy practices. World J Gastroenterol. 2009;15:3486-3492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Singh P, Das A, Isenberg G, Wong RC, Sivak MV Jr, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? Gastrointest Endosc. 2004;60:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Yamamoto K, Sofuni A, Tsuchiya T, Ishii K, Tsuji S, Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, Asai Y, Matsunami Y, Nagakawa Y, Yamaguchi H, Itoi T. Clinical Impact of Piecemeal Resection Concerning the Lateral Spread of Ampullary Adenomas. Intern Med. 2019;58:901-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kim HH, Kim JH, Park SJ, Park MI, Moon W. Risk factors for incomplete resection and complications in endoscopic mucosal resection for lateral spreading tumors. Dig Endosc. 2012;24:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |