Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1768
Peer-review started: October 19, 2021
First decision: December 3, 2021
Revised: January 7, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 7, 2022
Processing time: 192 Days and 4.2 Hours
Theranostics is the highly targeted molecular imaging and therapy of tumors. Targeted peptide receptor radionuclide therapy has taken the lead in demon
Core Tip: 68Ga and 64Cu DOTATATE positron emission tomography imaging is the most sensitive and accurate method to identify well-differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NETs). The paired therapeutic radiotracer, 177Lu DOTATATE, delivers targeted radiation which can prolong progression free survival. This is now established as the therapeutic best standard of care for patients with progressive, metastatic, or unresectable well-differentiated somatostatin receptors positive GEP-NETs. Ongoing investigations continue to expand the potential indications for DOTATATE theranostics. Additional novel ligands are also currently being developed for targeted imaging and therapy of GEP-NETs.
- Citation: Grey N, Silosky M, Lieu CH, Chin BB. Current status and future of targeted peptide receptor radionuclide positron emission tomography imaging and therapy of gastroenteropancreatic-neuroendocrine tumors. World J Gastroenterol 2022; 28(17): 1768-1780
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1768.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1768
Neuroendocrine tumors (NETs) are a relatively rare and heterogeneous group of tumors arising from neuroendocrine cells throughout the body, which can cause a variety of symptoms based on the location and cell type. Midgut NETs are the most common, with the small bowel being the most frequent site of the primary lesion[1,2]. Although the incidence is relatively low, the majority are slow-growing, well-differentiated tumors which effectively contributes to a high prevalence. Improved clinical, laboratory, and imaging detection also likely contribute to an apparent increasing prevalence. NETs are often detected incidentally or after they metastasize and cause clinical symptoms either from their hormonal release and/or from mass effect.
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) comprise the majority of NETs (over 75%), with lung NETs comprising an additional approximately 15%[1]. GEP-NETs are characterized according to the WHO 2010 classification system based on the Ki-67 index, a widely used marker of cell proliferation. In this classification, GEP-NETs are scored as G1 (Ki-67 ≤ 2%), G2 (Ki-76 = 3%-20%), or G3 (Ki-67 > 20%), with G1 and G2 classified as well-differentiated tumors, and G3 tumors classified as poorly differentiated/de-differentiated carcinomas. Further stratification of G3 tumors can be assigned into well-differentiated (Ki-67 20%-50%) and poorly differentiated carcinomas (Ki-67 > 50%). This classification system, as well as the proposed subdivision of G3 tumors, has prognostic utility and helps direct appropriate diagnostic imaging and therapy.
The majority of well differentiated GEP-NETs (> 90%) express the G-coupled protein somatostatin receptors (SSTR)[3-5]. The WHO classification typically correlates with SSTR expression with well-differentiated tumors (G1 and G2) highly expressing SSTRs, and poorly differentiated tumors (G3) having lower SSTR expression. Diagnostic imaging and targeted radionuclide therapy take advantage of tumor SSTR expression by utilizing radiopharmaceuticals that bind to the same ligand. This elegant duality is emblematic of the expanding field of “theranostics”, a portmanteau of therapy and diagnostics.
In this paper, we review the current approaches to the diagnosis and treatment of GEP-NETs. We focus primarily on DOTATATE (68Ga DOTATATE and 64Cu DOTATATE) positron emission tomogra
Somatostatin is an endogenously produced peptide hormone that binds to various SSTRs. In humans, there are 5 subtypes[6], with GEP-NETs predominantly expressing SSTR-2 both in the primary tumor and in their metastases[7].
The currently approved nuclear medicine DOTATATE PET imaging agents used to bind SSTR-2 are composed of three functional parts: (1) The radioactive PET imaging component (68Ga or 64Cu); (2) The PET radiometal chelator and linker, DOTA (tetraazacyclododecanetetraacetic acid); and (3) The peptide binding part, TATE (tyrosine-3-octreotate)[8]. The earlier imaging agents used to visualize GEP-NETs was a single photon octreotide-based agent, [123I-Tyr3]octreotide[4], followed by 111In pentetreotide (Octreoscan, Mallinkcrodt)[9]. 111In pentetreotide remained the mainstay of GEP-NET imaging until the approval of the PET imaging agents. Both 68Ga DOTATATE and the closely related 68Ga DOTATOC have received approvals in Europe and the United States[10,11], as has the more recently approved 64Cu DOTATATE. Because of the extremely high binding affinity of 68Ga DOTATATE to SSTR-2 (approximately 100 times greater than 111In-pentetreotide)[12], the superior imaging characteristics of PET compared to SPECT, and the lower radiation delivered, DOTATATE PET imaging is now recommended in all cases over 111In-pentetreotide[13], including use in both adults and children[14].
The appropriate use criteria for SSTR imaging in cases of known or suspected well-differentiated NETs with 68Ga- and 64Cu DOTATATE PET/CT imaging and 177Lu DOTATATE peptide receptor radionuclide have been recently summarized[13]. These include the following nine indications: (1) Initial staging after the histologic diagnosis of NET; (2) Evaluation of an unknown primary; (3) Evaluation of a mass suggestive of NET not amenable to endoscopic or percutaneous biopsy; (4) Staging of NET before planned surgery; (5) Monitoring of NET seen predominantly on SSTR PET; (6) Evaluation of patients with biochemical evidence and symptoms of a NET; (7) Evaluation of patients with biochemical evidence of a NET without evidence on conventional imaging or a prior histologic diagnosis; (8) Restaging at time of clinical or laboratory progression without progression on conventional imaging; and (9) New indeterminate lesion on conventional imaging with unclear progression[13].
In addition to its primary use in GEP-NETs, 68Ga DOTATATE PET has been used to image other SSTR positive tumors such as paragangliomas, pheochromocytomas, neuroblastomas, meningiomas, medullary thyroid cancers, Merkel cell carcinomas, small cell carcinomas, esthesioneuroblastomas, and tumor-induced oncogenic osteomalacia[8].
68Ga DOTATATE is readily compounded from generator eluted 68Ga and a sterile vial kit[15]. Before intravenous administration, there is little patient preparation except for good hydration and frequent voiding to minimize radiation dose to the kidneys. An uptake phase between radiotracer administration and PET imaging is typically approximately 60 min, similar to 18F fluorodeoxyglucose (FDG) PET. This allows time for tumor uptake and for background washout clearance via the kidneys, which is analogous to the procedure used in FDG PET. Patients are typically imaged from the mid-thigh to the skull. In cases where tumor is known or suspected outside of this field of view, or in cases of unknown primary, longer imaging times may be needed to include the extremities. Intravenous contrast is not essential for accurate interpretation in most cases, but it can be helpful in some clinical settings. Additional procedural details are listed in the European Association of Nuclear Medicine guidelines[14].
Many patients with GEP-NETs will be on short- or long-acting somatostatin analogue (SSA) therapy which could interfere with 68Ga DOTATATE PET uptake due to competitive binding. Temporary discontinuation of short-acting SSAs is recommended for 24-48 h, and long-acting SSAs should be avoided for approximately 4-6 wk[8,16]. Long-acting release (LAR) SSAs are administered on 4 wk cycles, allowing 68Ga DOTATATE imaging to be scheduled for the end of the monthly cycle prior to redosing. Regardless of the specific approach, subsequent 68Ga DOTATATE scans are preferably performed with the exact timing between SSA injections.
The normal biodistribution of 68Ga DOTATATE differs in several aspects compared to the most commonly used radiotracer, 18F FDG. For both 68Ga DOTATATE and 18F FDG, the genitourinary tract is the normal route of excretion for unbound radiotracer, and thus high radiotracer activity can be seen in the kidneys, ureters, and bladder. In 68Ga DOTATATE, the spleen has the highest normal activity, followed by the adrenal glands[17]. Unlike FDG, the pituitary is the only part of the central nervous system with high physiologic uptake. Salivary glands and the thyroid can have moderate uptake. Activity in the pancreas is variable and occasionally focal in the head and uncinate process[18]. Variable but typically lower-level diffuse uptake is seen in the small and large bowel[8]. Physiologic uptake in the liver can be quite variable[19], and uptake can be low in the spleen and liver if total body tumor burden is high (“sink effect”)[20]. 68Ga DOTATATE uptake is otherwise low throughout the muscles, adipose tissue, and bone.
The recommended dose for 68Ga DOTATATE is 2 MBq/kg of body weight (0.054 mCi/kg) up to 200 MBq (5.4 mCi). The organ with the highest dose delivered is the spleen due to its high uptake. The whole body radiation effective dose equivalent (EDE) from 68Ga DOTATATE PET is 2-3 mSv[21,22], which is approximately only 10% of the 26 mSv received from a typical dose (222 MBq or 6 mCi) of 111In pentetreotide. Despite the addition of an EDE of 1-9 mSv from the CT component, which is needed for attenuation correction and anatomic localization, the total dose of 68Ga DOTATATE PET/CT (3-12 mSv) remains significantly lower than that of 111In pentetreotide.
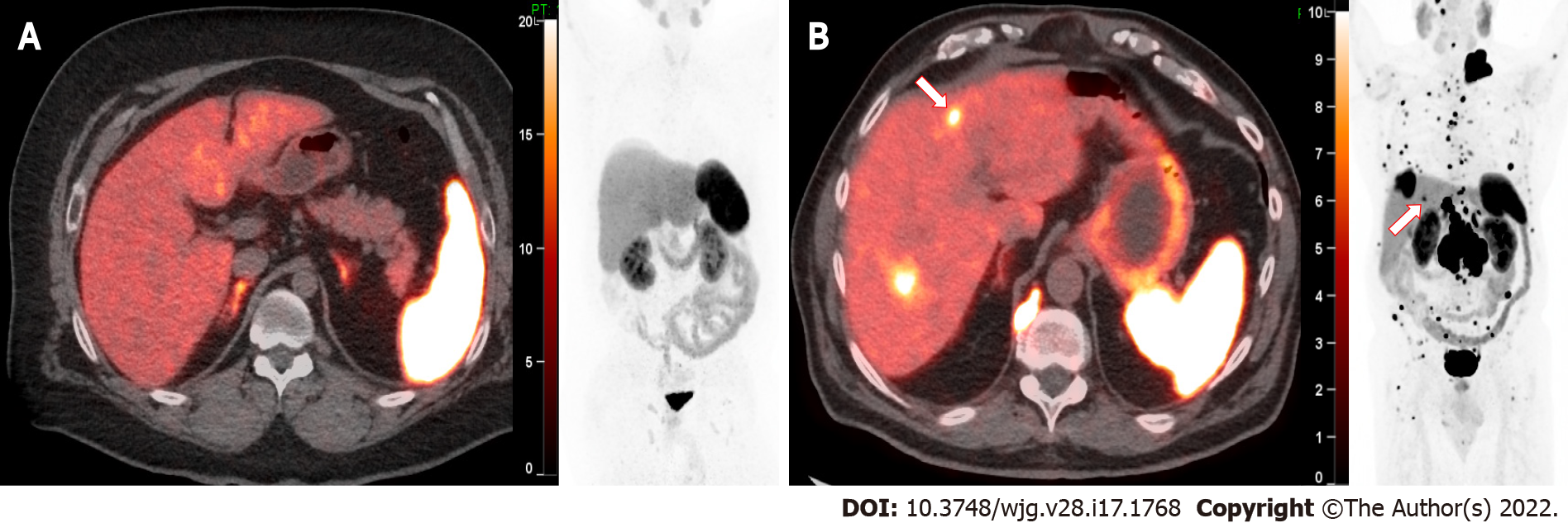
Due to the very high affinity of 68Ga DOTATATE for SSTR-2, target lesions typically show extremely high uptake. With deficient (i.e., very low) background radiotracer uptake throughout most of the body including the thorax, head and neck, bone marrow, muscle, and brain (except the pituitary), the tumor to background ratio can be exceptionally high. In a large prospective study of GEP-NETs comparing accuracy of 68Ga DOTATATE, conventional imaging [CT and magnetic resonance imaging (MRI)], and 111In pentetreotide, 68Ga DOTATATE showed clear superiority in lesion detectability with mean tumor SUVmax values of over 65[23]. Figure 1 shows an example of normal intense uptake in the spleen and adrenals and moderate 68Ga DOTATATE uptake in the liver. Small liver lesions can also be visualized despite relatively high liver background activity. Extremely high radiotracer activity can be seen in larger lesions, as shown in Figure 2.
False positives are uncommon and do not typically present a diagnostic dilemma. The most common causes of false positives are inflammation or infection due to leukocytes and macrophages expressing SSTR-2[24]. For example, inflammatory prostatitis is relatively common and can show intense focal uptake within the prostate[25]. Other potential causes of false positive can arise from osteoblastic activity[26], such as in degenerative changes, fractures, fibrous dysplasia, vertebral hemangiomas, and epiphyses in pediatric patients[27,28].
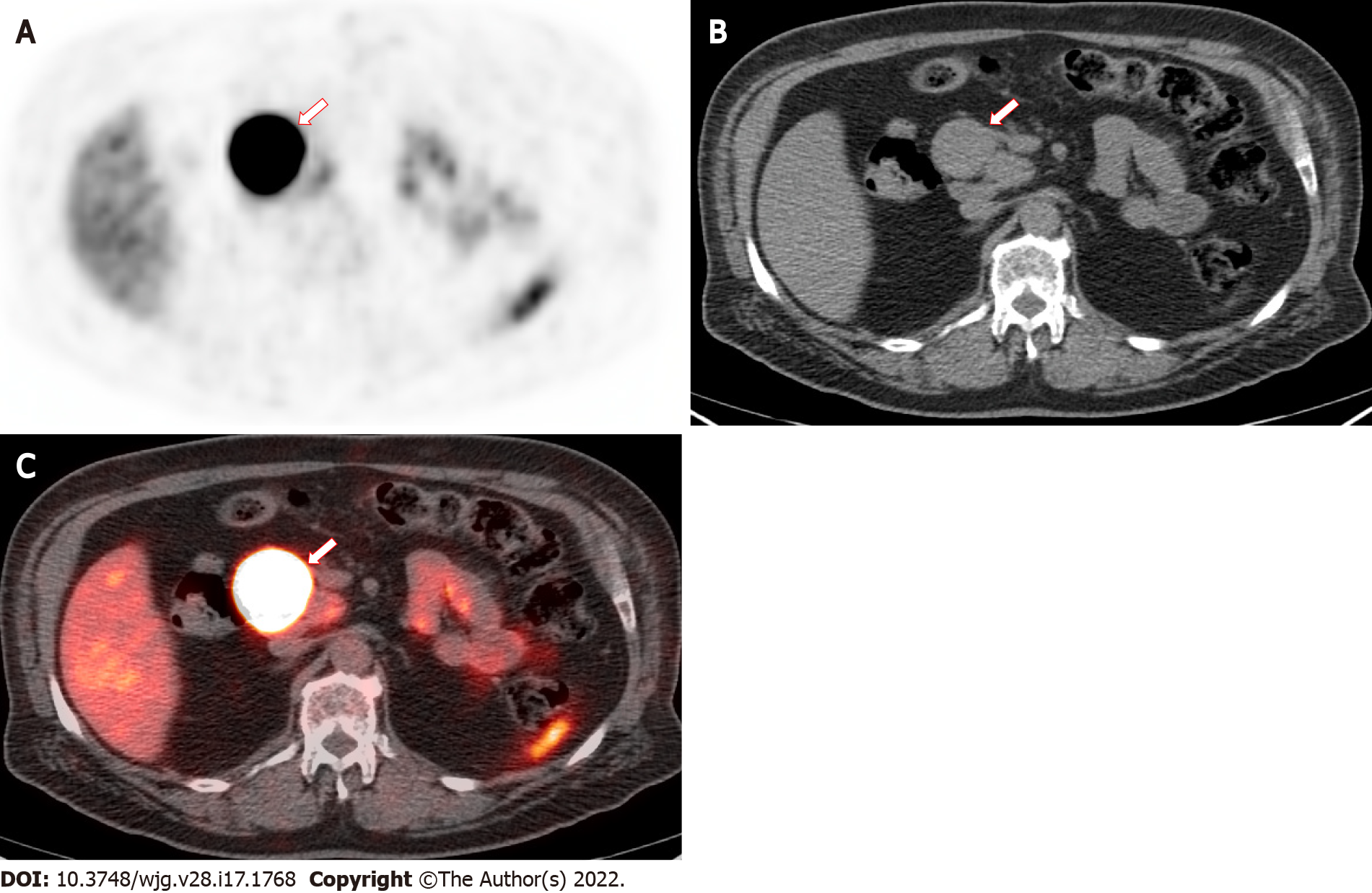
False negative results may be due to loss of receptor expression. GEP-NETs that dedifferentiate and subsequently lose SSTR-expression may show lower 68Ga DOTATATE avidity. Another cause of false negatives, as seen in PET imaging, may arise from small lesions below PET resolution. In our experience, however, the highly avid 68Ga DOTATATE uptake in well differentiated GEP-NETs is commonly adequate to overcome the limitations of partial volume effect. If tumor uptake is sufficiently high, it can also overcome the detrimental effects of a relatively low administered dose and other physical limitations of 68Ga compared to 18F[29-32]. Small lesions can be readily visualized in phantom and patient clinical studies if the background activity is low. The non-contrast portion of the CT frequently does not reveal hepatic lesions; however, hepatic GEP-NETs as small 5 mm may be visualized despite high normal background liver activity[30].
68Ga DOTATATE PET is an excellent study for evaluating GEP-NETs and demonstrates very high sensitivity and specificity of 93% and 95%, respectively[33]. Its superior performance in detecting GEP-NETs compared to 111In pentetreotide and CT or MRI is well established[23]. This can result in a significant change in clinical management in up to a third of patients even when compared to 111In pentetreotide[34-36]. 68Ga DOTATATE has also shown increasing utility in detecting lesions not seen on other modalities. It can confirm suspicion of GEP-NET found on other modalities, determine the true extent of tumor, stage disease, identify otherwise occult tumors such as thoracic ACTH-secreting carcinoids, and guide treatment options. It may be used to confirm a clinical or biochemical suspicion of GEP-NET, identify the primary lesion in known metastatic disease, determine resectability, exclude greater extent of disease prior to resection, and importantly, identify candidates for hormonal therapy or PRRT[8].
The primary practical advantage of 64Cu DOTATATE is its longer physical half-life (64Cu t½ = 12.7 h). This allows for more flexibility in production, shipment to distant locations, and flexibility in the timing of PET imaging. 68Ga DOTATATE (68Ga t½ = 68 minutes) is typically produced at a site close to the PET imaging center due to the short physical half-life. 64Cu DOTATATE allows flexibility for delayed imaging three hours after injection with no significant difference in lesion detectability[37]. Compared to the DOTATOC ligand, the 64Cu DOTATATE showed slightly higher lesion detectability than 68Ga DOTATOC[38]. Other differences between 64Cu compared to 68Ga do not appear to have a large clinical impact on lesion detectability. 64Cu compared to 68Ga has a disadvantage in a lower percentage of positrons emitted per decay (17.5% vs 88.9%). However, 64Cu has the physical advantage of shorter mean positron range prior to annihilation (0.7 mm vs 3.5 mm) which can improve spatial resolution[39]. Data are emerging to support quantitative imaging of 64Cu DOTATATE which correlates with prognosis[40,41]. Finally, pre-clinical studies of new investigational agents, such as 55Co DOTATATE, show the potential for further improvements in tumor uptake and image contrast when compared to currently approved agents[42].
FDG is a glucose analogue that enters cells through glucose transporters, undergoes phosphorylation, and then remains trapped within the cell as FDG-6-phosphate. This imaging metric of glycolysis is frequently upregulated in many cancers and generally associated with more aggressive, rapidly growing malignancies.
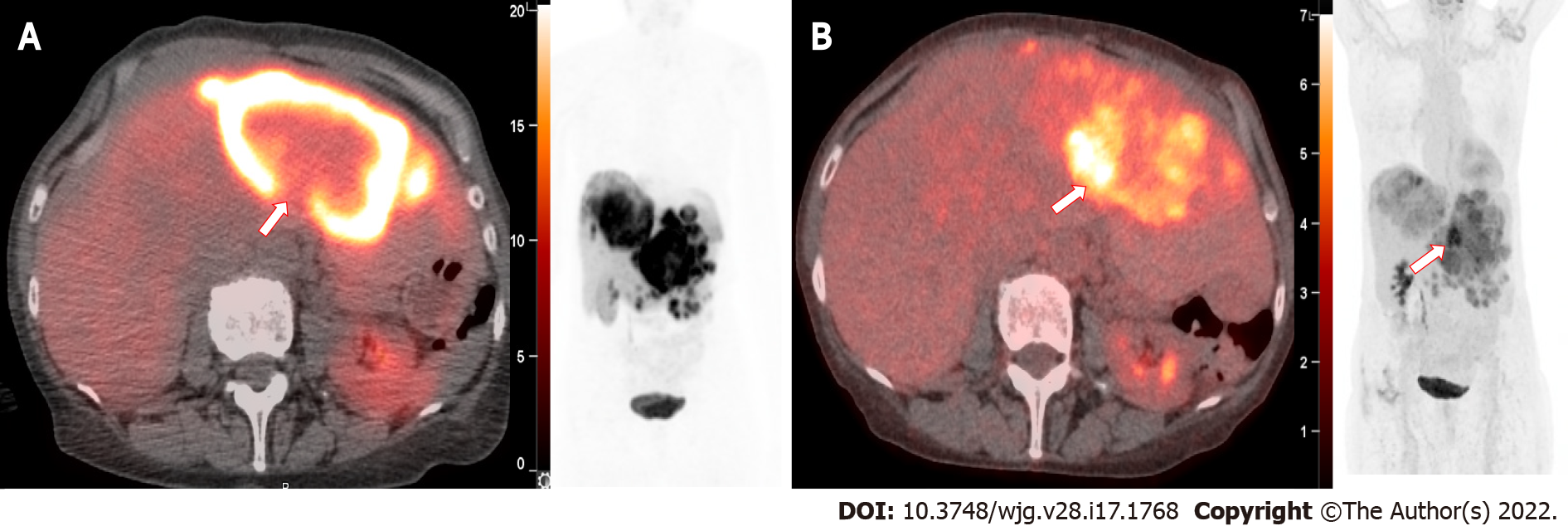
Well differentiated GEP-NETs, however, are typically slower growing, have lower mitotic rates, and have lower rates of proliferation including lower Ki-67 indices. The WHO classification of GEP-NETs relies on the Ki-67 index which reflects cellular proliferation. As GEP-NETs become more dedifferentiated, they tend to lose SSTRs expression and decrease in 68Ga DOTATATE avidity. These often exhibit a more aggressive and faster growing phenotype with increasing proliferation (Ki-67) and increasing glycolytic activity. Clinically, when a known GEP-NET tumor increases in size, yet decreases in 68Ga DOTATATE avidity, an FDG-PET can be useful in identifying tumor which may be exhibiting this dedifferentiating phenotype (“flip-flop” effect), and warrant consideration of a change in therapy. Patients with NETs demonstrating FDG avid disease are at a 10 fold increased risk of death[43]. Other studies have shown similar findings[44,45].
FDG PET may also be useful in identifying heterogeneity of tumors by directing biopsy of tumors suspicious for a more aggressive or higher-grade histology. When both 68Ga DOTATATE PET-CT and FDG PET-CT are performed, the differential imaging features may assist in prognosis and guide therapy options. FDG PET can therefore be complementary and aid in management decisions in which tumor dedifferentiation is suspected. An example of an FDG positive hepatic metastasis is shown in Figure 3.
Partial or complete surgical resection of GEP-NET is the preferred approach when possible[46]. Hormone therapy is another mainstay of GEP-NET treatment. Short- or long-acting SSAs bind to SSRTs and inhibit or slow tumor growth while simultaneously helping with hormone secretion related symptoms. Additional treatment options may include mTOR inhibitors, VEGF inhibitors, chemotherapy, radiation, and liver metastases directed embolization therapies[47]. More recently, 177Lu DOTATATE PRRT has been established as a safe and effective treatment of metastatic GEP-NETs.
PRRT is the logical extension of SSTR imaging into the treatment realm and comprises the therapy component of theranostics. The imaging radionuclide (68Ga or 64Cu) is replaced with a beta emitter, 177Lu, which deposits lethal radiation precisely to the SSTR-2 positive cells, providing targeted radiotherapy to tumors. The resultant 177Lu DOTATATE radionuclide delivers local radiation specifically to tumor visualized on 68Ga DOTATATE imaging. 177Lu is primarily a beta emitter with a mean range of 2 mm in tissue, and a small fraction is gamma radiation (6.6% at 113-keV and 11% at 208-keV). This results in a relatively low exposure to individuals surrounding the patient, allowing therapies to be performed as an outpatient. The relatively long half-life of 6.7 d (160 h) delivers sustained radiotherapy for a prolonged period; however, this requires extended precautions to avoid exposure from urinary contamination. 177Lu DOTATATE (Lutathera) was approved by the EMA in 2017 and by the FDA in January 2018[47].
177Lu DOTATATE was approved specifically for treatment of SSTR-positive GEP-NETs that have progressed on SSA therapy[47]. The most appropriate patients for therapy are based upon guidelines developed by the NETTER-1 trial[48]. There are multiple considerations for patient selection for PRRT; however, patients with progressive metastatic low and intermediate grade GEP-NETs typically have highly positive SSTR scans and are most likely to benefit.
The standard protocol for 177Lu DOTATATE therapy is based upon the NETTER-1 trial[48]. Patients are prescribed four doses of 7.4 GBq (200 mCi) eight weeks apart for a cumulative dose of 29.6 GBq (800 mCi). At least 30 min prior to therapy administration, an amino acid infusion is started for renal protection and lasts four hours. The two amino acids required for renal protection are arginine and lysine. Although other formulations of different amino acids exist, they do not provide any additional benefit and can cause significant nausea and vomiting.
Based on the typical dose of 7.4 GBq of 177Lu DOTATATE, the exposure rate at 1 m is 2 mR/h and decreases by 50% within 24 h[47]. If the patient is able to abide by standard radiation safety precautions, this can be performed as an outpatient. Precautions include bathroom hygiene, similar to radioiodine treatments, and appropriate distancing from others, specifically children and pregnant women, for approximately 3 d after therapy. Individualized safety instructions may be prepared by a radiation safety officer or radiation physicist, depending on the institution, and reviewed with the patient during the consent process.
177Lu DOTATATE therapy in GEP-NETs has demonstrated efficacy in many studies over several years. The most notable large prospective randomized trial is the NETTER-1 trial[48]. This prospective randomized trial in adults with biopsy-proven low- and intermediate grade (G1 or G2, i.e., Ki-67 level ≤ 20%) GEP-NETs evaluated subjects treated with 177Lu DOTATATE and SSA compared to a control group on high dose SSA alone. Inclusion criteria were metastatic disease or locally advanced and inoperable disease which was progressing on SSA[48].
The primary endpoint of the study was progression free survival (PFS) with secondary endpoints of objective response rate (ORR), overall survival (OS), safety, and the side-effect profile. Patients were judged to have failed treatment if there was tumor progression based on follow up imaging by CT or MRI according to RECIST 1.1 criteria[49].
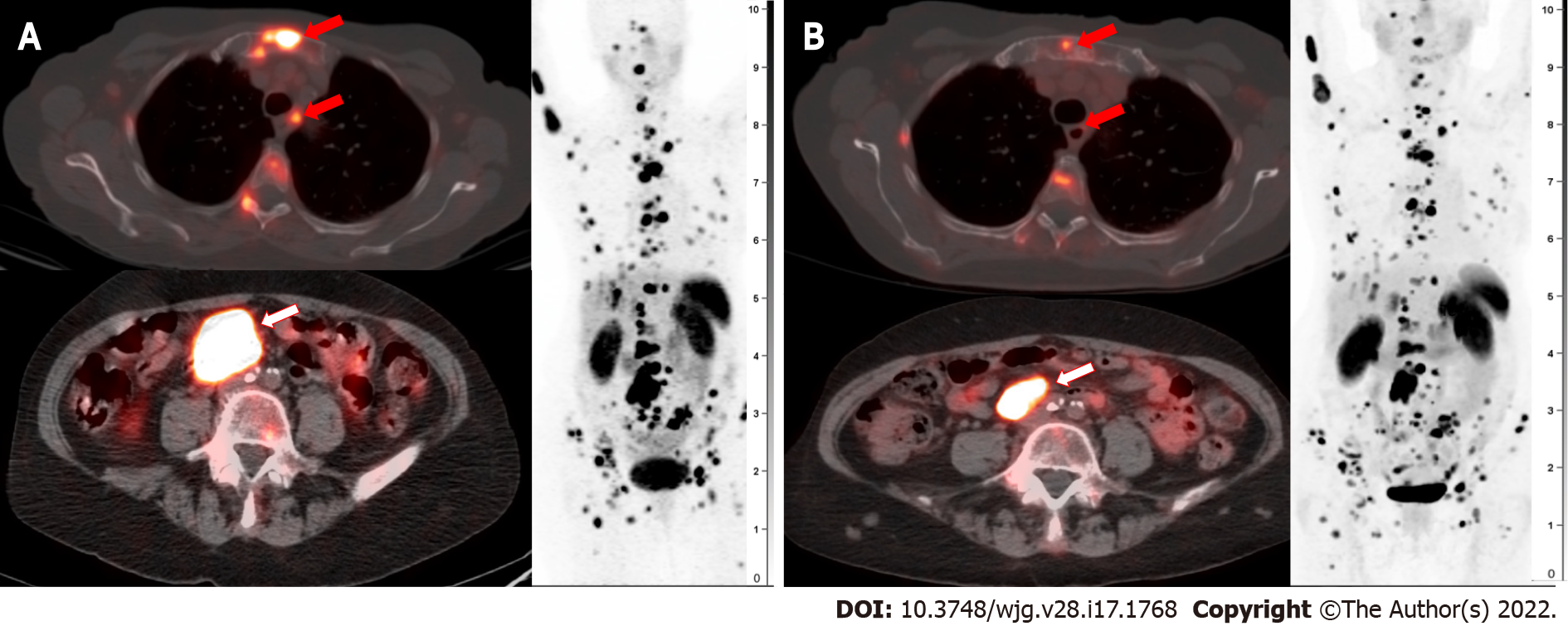
Patients in the treatment arm experienced significantly better PFS at 20 mo of 65.2% (95%CI: 50.0-76.8) compared to 10.8% (95%CI: 3.5-23.0) in the control group. In other words, in the treatment group there was a 79% lower risk of disease progression or death and a 60% lower risk of death alone. The secondary endpoint of ORR was 18% in the treatment group and 3% in the control group. As noted in the NETTER-1 study, multiple large randomized trials with other systemic therapies, such as SSAs alone or in combination with other non-radionuclides, showed response rates of only 5% or less[50-53]. Median overall survival could not be calculated yet at the conclusion of NETTER-1, but there was a trend towards longer overall survival in the treatment group. An example of a patient with partial response to 177Lu DOTATATE is shown in Figure 4.
In NETTER-1, transient WHO grade 3 and 4 hematologic toxicity (thrombocytopenia 2%; lymphopenia 9%; neutropenia 1%) and no renal toxicity were reported after 14 mo of follow up[48]. Rare but serious side effects including acute leukemia and myelodysplastic syndrome have been reported, occurring in < 1% and < 1%-2% of patients, respectively[47,48,54]. Other studies have similarly shown limited side effects with 177Lu DOTATATE therapy[54,55].
Common mild side effects (Grade 1 or 2) include nausea (59%) and vomiting (47%)[48]; however, this has been primarily attributed to the specific amino acid infusion. Use of the simpler arginine and lysine infusion appears to have much lower incidence and severity of side effects. Fatigue (40%), decreased appetite (18%), headache (16%), and alopecia (11%) were significantly higher in the 177Lu DOTATATE treatment group[48]. Although relatively frequent, abdominal pain (26%), and diarrhea (29%) were not statistically different compared to the control group. An uncommon side effect of treatment is hormone crisis, which in one study of 504 patients happened in only 6 (or 1.2%) and can be adequately managed in a brief hospital stay with complete recovery[54].
While 68Ga- and 177Lu DOTATATE have shown remarkable efficacy in imaging and treatment of GEP-NETs, many additional imaging and treatment options are currently under investigation. A comprehensive review is not possible in the context of the rapidly evolving landscape of theranostics. A few representative clinical trials are briefly mentioned to provide a perspective of the breadth of ongoing investigations.
Extending PRRT into higher grade GEP-NETs is an active area of investigation. The COMPOSE trial compares well-differentiated higher grade (G2 or G3, Ki-67 index between 10%-55%) GEP-NETs treated with 177Lu Edotreotide (DOTATOC) compared to best standard of care chemotherapy regimens[56]. The COMPETE trial similarly evaluates advanced GEP-NETs for safety and efficacy of 177Lu DOTATOC compared to Everolimus[57]. The NETTER-2 trial investigates higher proliferation index tumors (G2 or G3) as first line therapy with 177Lu DOTATATE therapy and SSA compared to high dose SSR therapy alone[58].
While 177Lu DOTATATE is now given as a four dose regimen, additional doses have been administered on an investigational basis. If a patient shows continued improvement in tumor burden and symptoms throughout the 177Lu DOTATATE treatment course established by NETTER-1, (four cycles, 8 wk apart), they may benefit from continued treatment with additional doses of 177Lu DOTATATE. A meta-analysis suggests that 177Lu DOTATATE re-treatment in patients with advanced GEP-NETs is well tolerated with a safety profile similar to initial PRRT[59]. This provides an additional treatment strategy potentially to improve PFS, OS, and disease related survival.
Either systemic or arterially-delivered PRRT with 90Y DOTATATE or -DOTATOC is an approach that is under further investigation. 90Y is beta emitter with a higher energy and longer mean free path in soft tissue than 177Lu. This theoretically favors treatment of larger tumors where high intratumor pressure limits blood flow and radiotracer delivery. However, a variety of factors mediate tumor killing including bystander effect[60]. A major limiting drawback of 90Y is its greater toxic effects to surrounding tissues and bone marrow[61]. Renal dose is also higher than 177Lu which poses a higher risk of nephrotoxicity[62]. Arterially-administered 90Y DOTATATE is more focally directed but is operator intensive and requires a prolonged procedure typically performed in the interventional radiology suite.
Antagonists: In contrast to the established SSTR agonists (e.g., DOTATATE), somatostatin antagonists are currently being investigated for imaging and therapy of GEP-NETs. Agonist-ligand complexes are internalized into the cell and entrapped, which is believed to generate higher contrast imaging and prolonged tumor targeted therapy. Antagonists were developed to evaluate the functions of receptors[63] and are typically not internalized, but this may be overcome by binding to a higher number of receptor sites than agonists[64]. The SSTR-2 antagonist JR11 has shown uptake in renal cell cancers, most breast cancers, non-Hodgkin lymphomas, and medullary thyroid cancers with binding comparable to NET targeting with SSTR-2 agonists[65]. This study also showed that peritumoral vessels, lymphocytes, nerves, mucosa, and stroma were more strongly labeled with the antagonist than with the agonist. Antagonists, therefore, may show higher binding leading to improved detection and more avid tumor binding in targeted radiotherapy. 68Ga NODAGA-LM3, 68Ga DOTA-LM3, and 68Ga NODAGA-JR11 (OPS202) are three of the agents showing early promise[66-68] with clinical trials underway[69]. Similar to DOTATATE, therapeutic radionuclides can be attached to these antagonists for PRRT[70,71].
Alpha emitters: PRRT for GEP-NETs is currently performed primarily by beta emitters (177Lu and 90Y), but targeted alpha therapy (TAT) is potentially much more effective[72,73]. Alpha particles travel a much shorter distance in tissue, typically on the order of only a few cell diameters, and deliver a dramatically higher damaging radiation effect to cells compared to beta emitters. In contrast to beta emission, which results primarily in single breaks in DNA, highly energetic alpha particles result in clusters of double stranded DNA breaks which are irreparable and highly lethal[72]. Alpha particles also generate more ionization events and an immunogenic cell death which could generate an immunostimulatory environment and promote an abscopal effect[73]. The dual effect of higher tumor cell death and limited radiation to non-target tissues increases the lethality to tumor cells and decreases the off-target adverse side effects.
The primary systemic alpha emitter under investigation is 225Ac which can be stably bound to DOTATATE or DOTATOC[73]. Early studies have shown promising results[74,75] with avoidance of severe renal and hematologic toxicity[76]. In the future, this could be given as an initial treatment strategy, in sequence with 177Lu DOTATATE, or as a salvage therapy for patients progressing on 177Lu PRRT.
Beyond radionuclides, there are other drugs and regimens being developed for treatment of GEP-NETs. These therapies may provide additional benefits to PRRT, particularly if they can be used to sensitize tumors to PRRT or be sequenced in such a way to deliver synergistic lethality. Alternative methods of administration may also allow higher local dose PRRT via intra-arterial rather than systemic delivery[77,78]. PRRT could also be used in a neoadjuvant fashion prior to surgery to make some patients operative candidates and increase the chances of curative resection[54]. The sorting out of the milieu of therapies, their timing, and indications will require ongoing research.
Currently, the most sensitive and accurate established method to image well-differentiated GEP-NETs is with DOTATATE PET imaging. Due to the favorable uptake properties and biodistribution, targeted PRRT with 177Lu DOTATATE has been established as best standard of care for patients with progressive, metastatic, or unresectable well-differentiated SSTR positive GEP-NETs. 177Lu DOTATATE is well tolerated with a very mild toxicity profile and rare serious adverse events. Ongoing investigations are continuing to expand in both imaging and therapy applications for DOTATATE and novel ligand theranostics for GEP-NETs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Nuclear Medicine, No. 134373.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bergsma H, Netherlands; Yang X, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139:2679-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3245] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 3. | de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, Lameris JS, Reubi JC, Lamberts SW. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 400] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Papotti M, Bongiovanni M, Volante M, Allìa E, Landolfi S, Helboe L, Schindler M, Cole SL, Bussolati G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Reubi JC. Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med. 1995;36:1825-1835. [PubMed] |
| 7. | Reubi JC. Peptide receptor expression in GEP-NET. Virchows Arch. 2007;451 Suppl 1:S47-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 9. | Hicks RJ. Use of molecular targeted agents for the diagnosis, staging and therapy of neuroendocrine malignancy. Cancer Imaging. 2010;10 Spec no A:S83-S91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Wangerin KA, Muzi M, Peterson LM, Linden HM, Novakova A, Mankoff DA, Kinahan PE. A virtual clinical trial comparing static vs dynamic PET imaging in measuring response to breast cancer therapy. Phys Med Biol. 2017;62:3639-3655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hennrich U, Benešová M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Mäcke HR. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 760] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 13. | Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, Howe JR, Kulke MH, Kunz PL, Mailman J, May L, Metz DC, Millo C, O'Dorisio S, Reidy-Lagunes DL, Soulen MC, Strosberg JR. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018;59:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, Papathanasiou ND, Pepe G, Oyen W, De Cristoforo C, Chiti A. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Byrd DW, Doot RK, Allberg KC, MacDonald LR, McDougald WA, Elston BF, Linden HM, Kinahan PE. Evaluation of Cross-Calibrated 68Ge/68Ga Phantoms for Assessing PET/CT Measurement Bias in Oncology Imaging for Single- and Multicenter Trials. Tomography. 2016;2:353-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Bodei L, Ambrosini V, Herrmann K, Modlin I. Current Concepts in 68Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J Nucl Med. 2017;58:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Kuyumcu S, Özkan ZG, Sanli Y, Yilmaz E, Mudun A, Adalet I, Unal S. Physiological and tumoral uptake of (68)Ga-DOTATATE: standardized uptake values and challenges in interpretation. Ann Nucl Med. 2013;27:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Al-Ibraheem A, Bundschuh RA, Notni J, Buck A, Winter A, Wester HJ, Schwaiger M, Scheidhauer K. Focal uptake of 68Ga-DOTATOC in the pancreas: pathological or physiological correlate in patients with neuroendocrine tumours? Eur J Nucl Med Mol Imaging. 2011;38:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Anderson J, Silosky M, Karki R, Morgan R, Chin B. Normal biodistribution and tumor uptake of 68Ga DOTATATE PET / CT in the clinical setting: normal background activity, and organ specific tumor characterization of metastatic lesions. J Nucl Med. 2019;60(supplement 1):3031. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Beauregard JM, Hofman MS, Kong G, Hicks RJ. The tumour sink effect on the biodistribution of 68Ga-DOTA-octreotate: implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2012;39:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, Delbeke D, Walker RC. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J Nucl Med. 2016;57:872-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Sandström M, Velikyan I, Garske-Román U, Sörensen J, Eriksson B, Granberg D, Lundqvist H, Sundin A, Lubberink M. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, Bauer W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Schmidt MQ, Trenbeath Z, Chin BB. Neuroendocrine prostate cancer or prostatitis? World J Nucl Med. 2019;18:304-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Mackie EJ, Trechsel U, Bruns C. Somatostatin receptors are restricted to a subpopulation of osteoblast-like cells during endochondral bone formation. Development. 1990;110:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Brogsitter C, Hofmockel T, Kotzerke J. 68Ga DOTATATE uptake in vertebral hemangioma. Clin Nucl Med. 2014;39:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Klinaki I, Al-Nahhas A, Soneji N, Win Z. 68Ga DOTATATE PET/CT uptake in spinal lesions and MRI correlation on a patient with neuroendocrine tumor: potential pitfalls. Clin Nucl Med. 2013;38:e449-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Silosky MS, Patten L, Chin BB. Target activity repeatability in a clinically relevant phantom: 18F vs 68Ga. J Nucl Med. 2021;62(Suppl 1):1442. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Silosky MS, Karki R, Morgan R, Anderson J, Chin BB. Physical characteristics of 68Ga DOTATATE PET/CT affecting small lesion detectability. Am J Nucl Med Mol Imaging. 2021;11:27-39. [PubMed] |
| 31. | Chin BB, Silosky M, Morgan R, Karki R, Anderson J. 68Ga DOTATATE organ specific tumor signal to noise (S/N) ratios: Comparison of lesion detectability from phantom studies to lesion detectability in clinical practice. J Nucl Med. 2019;60(supplement 1):478. [DOI] [Full Text] |
| 32. | Silosky M, Karki R, Chin BB. 68Ga and 18F quantification, and detectability of hot spots using an ACR Phantom: Contributions of radionuclide physical differences to hot spot detectability. J Nucl Med. 2019;60 (supplement 1):1200. |
| 33. | Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 35. | Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, Lakhani V, Baum RP, Berlin J, Smith GT, Graham M, Sandler MP, Delbeke D, Walker RC. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J Nucl Med. 2016;57:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 36. | Barrio M, Czernin J, Fanti S, Ambrosini V, Binse I, Du L, Eiber M, Herrmann K, Fendler WP. The Impact of Somatostatin Receptor-Directed PET/CT on the Management of Patients with Neuroendocrine Tumor: A Systematic Review and Meta-Analysis. J Nucl Med. 2017;58:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 37. | Loft M, Carlsen EA, Johnbeck CB, Johannesen HH, Binderup T, Pfeifer A, Mortensen J, Oturai P, Loft A, Berthelsen AK, Langer SW, Knigge U, Kjaer A. 64Cu-DOTATATE PET in Patients with Neuroendocrine Neoplasms: Prospective, Head-to-Head Comparison of Imaging at 1 Hour and 3 Hours After Injection. J Nucl Med. 2021;62:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Johnbeck CB, Knigge U, Loft A, Berthelsen AK, Mortensen J, Oturai P, Langer SW, Elema DR, Kjaer A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J Nucl Med. 2017;58:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 39. | Anderson CJ, Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother Radiopharm. 2009;24:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 40. | Carlsen EA, Johnbeck CB, Loft M, Pfeifer A, Oturai P, Langer SW, Knigge U, Ladefoged CN, Kjaer A. Semiautomatic Tumor Delineation for Evaluation of 64Cu-DOTATATE PET/CT in Patients with Neuroendocrine Neoplasms: Prognostication Based on Lowest Lesion Uptake and Total Tumor Volume. J Nucl Med. 2021;62:1564-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Carlsen EA, Johnbeck CB, Binderup T, Loft M, Pfeifer A, Mortensen J, Oturai P, Loft A, Berthelsen AK, Langer SW, Knigge U, Kjaer A. 64Cu-DOTATATE PET/CT and Prediction of Overall and Progression-Free Survival in Patients with Neuroendocrine Neoplasms. J Nucl Med. 2020;61:1491-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Andersen TL, Baun C, Olsen BB, Dam JH, Thisgaard H. Improving Contrast and Detectability: Imaging with [55Co]Co-DOTATATE in Comparison with [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE. J Nucl Med. 2020;61:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 44. | Karfis I, Marin G, Levillain H, Drisis S, Muteganya R, Critchi G, Taraji-Schiltz L, Guix CA, Shaza L, Elbachiri M, Mans L, Machiels G, Hendlisz A, Flamen P. Prognostic value of a three-scale grading system based on combining molecular imaging with 68Ga-DOTATATE and 18F-FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasias. Oncotarget. 2020;11:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Han S, Lee HS, Woo S, Kim TH, Yoo C, Ryoo BY, Ryu JS. Prognostic Value of 18F-FDG PET in Neuroendocrine Neoplasm: A Systematic Review and Meta-analysis. Clin Nucl Med. 2021;46:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB; UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 47. | Mittra ES. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. AJR Am J Roentgenol. 2018;211:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 48. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2236] [Article Influence: 279.5] [Reference Citation Analysis (0)] |
| 49. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13077] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 50. | Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Ducreux M, Ruszniewski P, Chayvialle JA, Blumberg J, Cloarec D, Michel H, Raymond JM, Dupas JL, Gouerou H, Jian R, Genestin E, Hammel P, Rougier P. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol. 2000;95:3276-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 743] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 53. | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K, Van Cutsem E, Yao JC; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 754] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 54. | Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 55. | Yordanova A, Eppard E, Kürpig S, Bundschuh RA, Schönberger S, Gonzalez-Carmona M, Feldmann G, Ahmadzadehfar H, Essler M. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017;10:4821-4828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 56. | ClinicalTrials.gov. Lutetium 177Lu-Edotreotide Versus Best Standard of Care in Well-differentiated Aggressive Grade-2 and Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs)-COMPOSE (COMPOSE) [website]. 2021. [cited 15 August 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04919226. |
| 57. | ClinicalTrials.gov. Efficacy and Safety of 177Lu-edotreotide PRRT in GEP-NET Patients (COMPETE) 2017. [cited 15 August 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03049189. |
| 58. | ClinicalTrials.gov. Study to Evaluate the Efficacy and Safety of Lutathera in Patients With Grade 2 and Grade 3 Advanced GEP-NET (NETTER-2) 2019. [cited 15 August 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03972488. |
| 59. | Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2021;93:102141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 60. | Wang R, Zhou T, Liu W, Zuo L. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget. 2018;9:18637-18647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 61. | Kunikowska J, Królicki L, Hubalewska-Dydejczyk A, Mikołajczak R, Sowa-Staszczak A, Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging. 2011;38:1788-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Konijnenberg M, Melis M, Valkema R, Krenning E, de Jong M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007;48:134-142. [PubMed] |
| 63. | Bass RT, Buckwalter BL, Patel BP, Pausch MH, Price LA, Strnad J, Hadcock JR. Identification and characterization of novel somatostatin antagonists. Mol Pharmacol. 1996;50:709-715. [PubMed] |
| 64. | Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Mäcke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436-16441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 65. | Reubi JC, Waser B, Mäcke H, Rivier J. Highly Increased 125I-JR11 Antagonist Binding In Vitro Reveals Novel Indications for sst2 Targeting in Human Cancers. J Nucl Med. 2017;58:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J, Erlanger TE, Cathomas R, Christ E, Fani M, Wild D. Sensitivity Comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J Nucl Med. 2018;59:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 67. | Nicolas GP, Beykan S, Bouterfa H, Kaufmann J, Bauman A, Lassmann M, Reubi JC, Rivier JEF, Maecke HR, Fani M, Wild D. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med. 2018;59:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Zhu W, Cheng Y, Jia R, Zhao H, Bai C, Xu J, Yao S, Huo L. A Prospective, Randomized, Double-Blind Study to Evaluate the Safety, Biodistribution, and Dosimetry of 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3 in Patients with Well-Differentiated Neuroendocrine Tumors. J Nucl Med. 2021;62:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Zhu W, Jia R, Yang Q, Cheng Y, Zhao H, Bai C, Xu J, Yao S, Huo L. A prospective randomized, double-blind study to evaluate the diagnostic efficacy of 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3 in patients with well-differentiated neuroendocrine tumors: compared with 68Ga-DOTATATE. Eur J Nucl Med Mol Imaging. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Baum RP, Zhang J, Schuchardt C, Mueller D, Maecke H. First-in-human study of novel SSTR antagonist 177Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with metastatic neuroendocrine neoplasms: Dosimetry, safety and efficacy. J Nucl Med. 2021;62:1571-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 71. | Dalm SU, Nonnekens J, Doeswijk GN, de Blois E, van Gent DC, Konijnenberg MW, de Jong M. Comparison of the Therapeutic Response to Treatment with a 177Lu-Labeled Somatostatin Receptor Agonist and Antagonist in Preclinical Models. J Nucl Med. 2016;57:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Targeted Alpha Therapy Working Group; Parker C, Lewington V, Shore N, Kratochwil C, Levy M, Lindén O, Noordzij W, Park J, Saad F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018;4:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 73. | Nelson BJB, Andersson JD, Wuest F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 74. | Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 75. | Zhang J, Kulkarni HR, Baum RP. Peptide Receptor Radionuclide Therapy Using 225Ac-DOTATOC Achieves Partial Remission in a Patient With Progressive Neuroendocrine Liver Metastases After Repeated β-Emitter Peptide Receptor Radionuclide Therapy. Clin Nucl Med. 2020;45:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Kratochwil C, Apostolidis L, Rathke H, Apostolidis C, Bicu F, Bruchertseifer F, Choyke PL, Haberkorn U, Giesel FL, Morgenstern A. Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity. Eur J Nucl Med Mol Imaging. 2021;49:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Kratochwil C, López-Benítez R, Mier W, Haufe S, Isermann B, Kauczor HU, Choyke PL, Haberkorn U, Giesel FL. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocr Relat Cancer. 2011;18:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Zhang J, Kulkarni HR, Singh A, Baum RP. Successful Intra-arterial Peptide Receptor Radionuclide Therapy of DOTATOC-Negative High-Grade Liver Metastases of a Pancreatic Neuroendocrine Neoplasm Using 177Lu-DOTA-LM3: A Somatostatin Receptor Antagonist. Clin Nucl Med. 2020;45:e165-e168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |