Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1681
Peer-review started: August 29, 2021
First decision: October 16, 2021
Revised: October 30, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 28, 2022
Processing time: 237 Days and 18.3 Hours
Childhood obesity and fatty liver are associated with adverse outcomes such as diabetes, metabolic syndrome, and cardiovascular diseases in adulthood. It is very important to identify relevant risk factors and intervene as early as possible. At present, the relationship between maternal and offspring metabolic factors is conflicting.
To estimate the association of maternal obesity and gestational diabetes mellitus (GDM) with overweight/obesity and fatty liver risk in offspring at 8 years of age.
The prospective study included mothers who all had a 75-g oral glucose tolerance test at 24-28 wk of gestation and whose offspring completed follow-up at 8 years of age. Offspring birth weight, sex, height, weight, and body mass index (BMI) were measured and calculated. FibroScan-502 examination with an M probe (Echosens, Paris, France) was prospectively conducted in offspring aged 8 years from the Shanghai Prenatal Cohort Study.
A total of 430 mother-child pairs were included in the analysis. A total of 62 (14.2%) mothers were classified as obese, and 48 (11.1%) were classified as having GDM. The mean age of the offspring at follow-up was 8 years old. Thirty-seven (8.6%) offspring were overweight, 14 (3.3%) had obesity, and 60 (14.0%) had fatty liver. The prevalence of overweight, obesity and fatty liver in offspring increased significantly across maternal BMI quartiles (all P < 0.05). Among offspring of mothers with GDM, 12 (25.0%) were overweight, 4 (8.3%) were obese, and 12 (25.0%) had fatty liver vs. 25 (6.5%), 10 (2.6%) and 48 (12.6%), respectively, for offspring of mothers without GDM (all P < 0.05). In multiple logistic regression, after adjustment for variables, the OR for fatty liver in offspring was 8.26 (95%CI: 2.38-28.75) for maternal obesity and GDM.
This study showed that maternal obesity can increase the odds of overweight/obesity and fatty liver in offspring, and GDM status also increases the odds of overweight/obesity in offspring. Weight management and glycemic control before and during pregnancy need to be highlighted in primary prevention of pediatric obesity and fatty liver.
Core Tip: It is very important to identify relevant risk factors for childhood obesity and fatty liver and intervene as early as possible, considering their adverse outcomes. In this work, we reported the association of maternal obesity and gestational diabetes mellitus (GDM) with overweight/obesity and fatty liver risk in offspring at 8 years of age. This study showed that maternal obesity can increase the odds of overweight/obesity and fatty liver in offspring, and GDM status also increases the odds of overweight/obesity in offspring. Weight management and glycemic control before and during pregnancy need to be highlighted in primary prevention of pediatric obesity and fatty liver.
- Citation: Zeng J, Shen F, Zou ZY, Yang RX, Jin Q, Yang J, Chen GY, Fan JG. Association of maternal obesity and gestational diabetes mellitus with overweight/obesity and fatty liver risk in offspring. World J Gastroenterol 2022; 28(16): 1681-1691
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1681
With economic development and changing living and eating habits, the prevalence of obesity in children has been rapidly increasing in recent decades[1]. It is alarming because it is also associated with health consequences such as metabolic syndrome, diabetes, cardiovascular diseases, and even many types of cancers in adulthood[2,3]. The incidence of fatty liver in children is also rising, due, in part, to the increasing prevalence of childhood obesity. At present, there is still no effective noninvasive means for diagnosing fatty liver in children. Recently, some novel noninvasive techniques for the assessment of liver fat have been developed. Transient elastography (TE) is one of these new techniques based on inducing a shear wave to the liver and measuring the velocity of the wave. The device (FibroScan-502, Echosens, Paris, France) was developed using the TE technique, and controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) can be obtained simultaneously by the device in a rapid, noninvasive, reproducible, and painless way. FibroScan-502 has also been used in the assessment of liver fat and fibrosis in pediatric individuals with liver diseases, and the reference values of CAP have been studied in our previous article[4].
Recent studies have suggested that maternal body mass index (BMI) is associated with the birth weight of offspring and is a risk factor for offspring obesity[5,6]. Gestational diabetes mellitus (GDM) is the occurrence of glucose intolerance during pregnancy and usually resolves after birth[7]. Meanwhile, many studies have shown that GDM can increase the incidence of impaired glucose tolerance in offspring and increase the risk of offspring obesity[8,9].
Therefore, in this article, we aimed to assess whether maternal BMI and in utero exposure to GDM are associated with a long-term risk of overweight/obese and fatty liver among offspring 8 years postpartum.
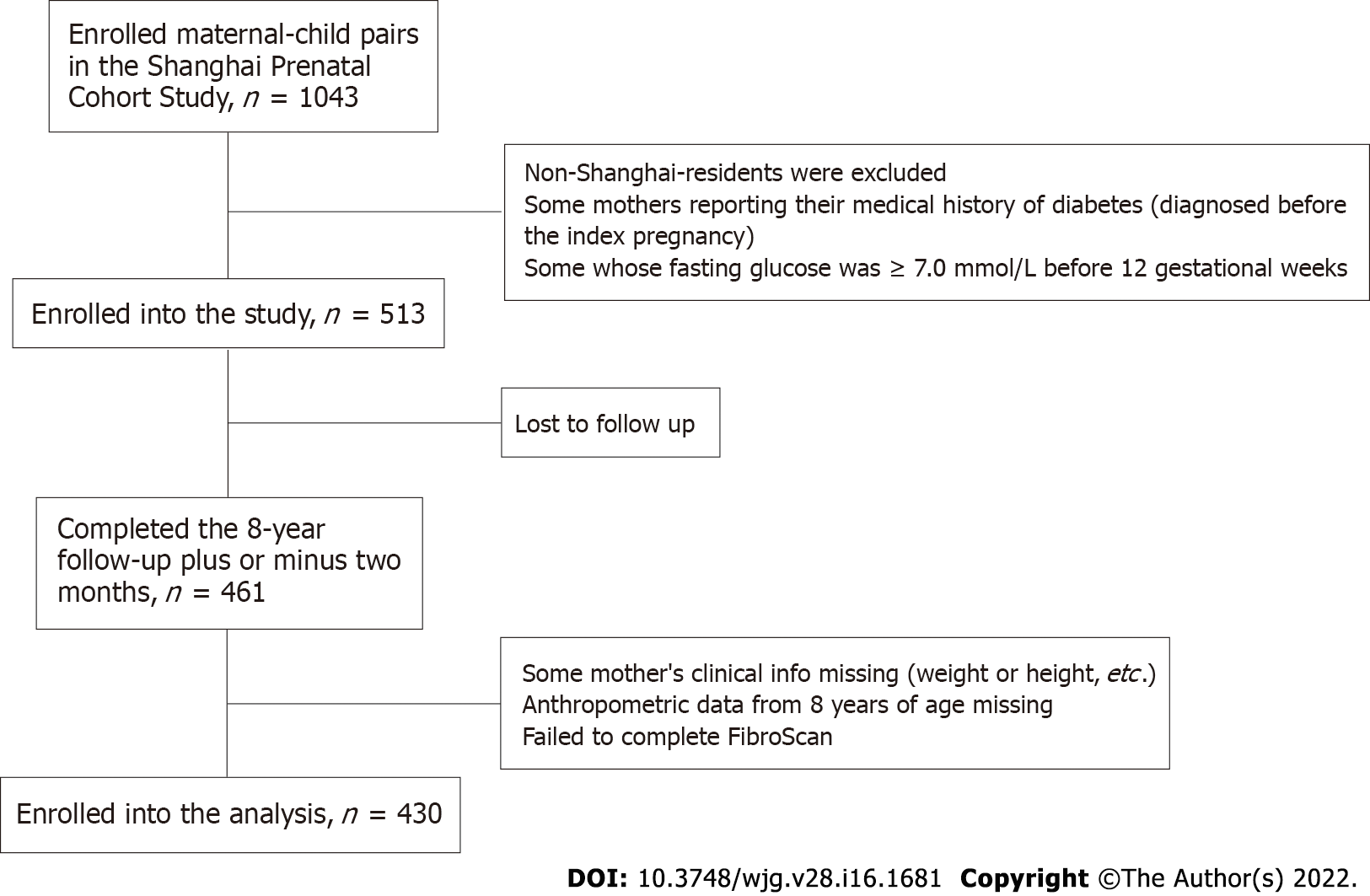
The individuals included in the prospective study were 430 maternal-child pairs from the Shanghai Prenatal Cohort Study, which is a prospective study that enrolled 1043 Han maternal-child pairs between January 2012 and December 2013 at Xinhua Hospital and International Peace Maternity and Child Hospital in Shanghai. The offspring were followed up at the age of 8 years (94 to 98 mo) with medical examinations. The exclusion criteria for the study population were as follows: (1) Non-Shanghai residents; (2) lost to follow-up; (3) missing some of the mothers' clinical information on prepregnancy and the offspring's anthropometric data; (4) mothers' medical history of diabetes (diagnosed before the index pregnancy) and other participants whose fasting glucose was ≥ 7.0 mmol/L before 12 gestational weeks; and (5) failure of FibroScan-502 measurement with an M probe. Ethics approval was obtained by the Ethics Committees (XHEC-C-2012-023). The parents of all the participating children were required to give informed consent for study participation and sign the written documents.
All mothers' heights and weights were measured in light indoor clothing and without shoes during early pregnancy. The oral glucose tolerance test (OGTT) was conducted between 24 and 28 wk gestation among those mothers. All followed-up offspring underwent annual medical examination at the health examination center in Xinhua Hospital. Stadiometers (Seca 416 Infantmeter, United States) were used to measure height to the nearest 0.1 cm. Digital scales (Detector 6745 Baby Scale, United States) were used to measure body weight to the nearest 0.1 kg. Participant characteristics and anthropometric indices, including age, sex, body weight, height, chest circumference, waist circumference, hip circumference and BMI, were obtained.
Following a fast of at least 6 h, all offspring underwent FibroScan-502 examination with an M-probe (3.5 MHz) (Echosens, Paris, France) by the same physician. The device estimates liver stiffness in kilopascals (kPa) and liver steatosis in decibels/meter (dB/m). CAP in dB/m and LSM in kPa were obtained simultaneously by each examination. A TE examination was considered successful when 10 valid measurements with a success rate of at least 60% were conducted and the interquartile range (IQR) was less than 30% of the median LSM value[10]. Subjects with unsuccessful examinations were excluded from the analyses.
Maternal obese, overweight and lean: A BMI during the early pregnancy greater than 25 kg/m2 was used to define the obese population, and a BMI less than 25 kg/m2 was used to define the nonobese population. The nonobese population was further divided into lean (< 23 kg/m2) and overweight (23-25 kg/m2) groups.
GDM: All mothers without diagnosed diabetes were screened for GDM by a one-step approach undergoing a 75g OGTT after fasting overnight between 24 and 28 wk gestation according to the guideline from Obstetrics and Gynecology Branch of Chinese Medical Association[11]. GDM was diagnosed when the glucose level that met or exceeded any of the following standards: a blood glucose value of 92, 180 or 153 mg/dL before or one or two hours after taking a 75 g glucose tolerance test, respectively.
Offspring overweight/obesity were defined by using the International Obesity Task Force age- and sex-specific cutoff points[12].
Offspring fatty liver: The offspring were considered to have fatty liver when the CAP value exceeded the normal value of 214.53 dB/m[4].
Continuous variables are expressed as the mean ± SD for a normal distribution and as the median ± IQR for a skewed distribution. General linear models for continuous variables were used to compare means of characteristics, and the χ2 test for categorical variables was applied to compare offspring proportions across quartiles of maternal BMI during early pregnancy. We further explored the effects of maternal GDM status on such associations by a stratified analysis according to GDM status. Multivariate logistic regression models were used to examine the relationship between maternal BMI during early pregnancy and GDM status and offspring overweight/obesity status and fatty liver prevalence. Multiple logistic regression was used for continuous outcomes, and the results are reported as odds ratios (ORs) with 95%CIs. Three multivariate-adjusted models were included in these analyses. Significance tests were two tailed, and a P value < 0.05 was considered statistically significant. The data analysis for this article was generated using SAS Version 9.4.
A total of 513 maternal-child pairs from the Shanghai Prenatal Cohort Study were prospectively followed for 8 years. Of these individuals, 430 mothers and their offspring were included in the analysis (Figure 1).
The characteristics of participating mothers and offspring are shown in Table 1. The mean maternal age before pregnancy was 29 (4.9) years (20 to 42 years old). The mean maternal BMI was 21.55 (3.59) kg/m2. A total of 62 (14.2%) mothers were classified as obese, and 48 (11.1%) were classified as having GDM. The mean birth weight of the offspring was 3.40 ± 0.48 kg and 210 (48.8%) were boys. A total of 37 (8.6%) offspring were classified as overweight, 14 (3.3%) offspring were classified as obese, and 60 (14.0%) had fatty liver (Table 1).
| Variables | All (n = 430) | Quartiles of maternal BMI (kg/m2) | ||||
| Q1 (n = 108): 15.00-19.13 | Q2 (n = 105): 19.14-20.76 | Q3 (n = 115): 20.77-23.44 | Q4 (n = 102): 23.45-42.00 | P value | ||
| Maternal characteristics | ||||||
| Age, yr | 29.33 ± 4.89 | 29.21 ± 4.59 | 28.97 ± 3.14 | 29.59 ± 3.73 | 29.44 ± 7.36 | 0.805 |
| Height, cm | 162.97 ± 16.46 | 162.77 ± 5.09 | 161.84 ± 4.93 | 161.61 ± 4.29 | 162.26 ± 6.18 | 0.358 |
| Weight, kg | 56.73 ± 9.77 | 47.48 ± 3.45 | 52.21 ± 3.53 | 57.76 ± 3.53 | 69.9 ± 9.1 | 0.000 |
| BMI, kg/m2 | 21.55 ± 3.59 | 17.92 ± 0.97 | 19.91 ± 0.43 | 22.1 ± 0.79 | 26.57 ± 3.3 | 0.000 |
| GDM, n (%) | 48 (11.2) | 6 (5.6) | 8 (7.6) | 11 (9.6) | 23 (22.5) | 0.000 |
| Offspring characteristics | ||||||
| Birth weight, kg | 3.40 ± 0.48 | 3.33 ± 0.42 | 3.34 ± 0.39 | 3.42 ± 0.55 | 3.51 ± 0.51 | 0.021 |
| Boy, n (%) | 210 (48.8) | 47 (43.5) | 53 (50.5) | 53 (46.1) | 57 (55.9) | 0.304 |
| Height, cm | 122.78 ± 29.93 | 121.59 ± 31.31 | 129.03 ± 4.91 | 118.81 ± 36.96 | 123.35 ± 31.01 | 0.242 |
| Weight, kg | 25.58 ± 10.57 | 22.98 ± 8.83 | 26.37 ± 7.8 | 25.18 ± 10.25 | 28.15 ± 13.12 | 0.070 |
| BMI, kg/m2 | 15.89 ± 4.42 | 14.58 ± 3.81 | 15.70 ± 4.00 | 16.20 ± 3.69 | 17.25 ± 5.81 | 0.025 |
| Waist circumference, cm | 58.70 ± 10.79 | 54.33 ± 12.76 | 58.33 ± 7.08 | 59.15 ± 9.32 | 63.13 ± 9.42 | 0.000 |
| Hip circumference, cm | 69.04 ± 13.73 | 65.3 ± 14.55 | 69.17 ± 13.47 | 69.39 ± 7.36 | 72.14 ± 13.39 | 0.041 |
| Chest circumference, cm | 56.19 ± 21.03 | 49.79 ± 22.55 | 53.44 ± 22.62 | 56.72 ± 16.15 | 63.41 ± 14.73 | 0.003 |
| Waist-height ratio | 0.46 ± 0.05 | 0.44 ± 0.05 | 0.45 ± 0.05 | 0.46 ± 0.04 | 0.48 ± 0.06 | 0.000 |
| Waist-hip ratio | 0.84 ± 0.08 | 0.83 ± 0.06 | 0.84 ± 0.07 | 0.84 ± 0.06 | 0.86 ± 0.12 | 0.292 |
| Chest-height ratio | 0.44 ± 0.14 | 0.4 ± 0.16 | 0.44 ± 0.12 | 0.41 ± 0.17 | 0.48 ± 0.11 | 0.023 |
| CAP, dB/m | 167.71 ± 46.09 | 151.76 ± 37.01 | 164.33 ± 41.45 | 169.97 ± 40.76 | 188.38 ± 58.89 | 0.001 |
| LSM, kPa | 3.36 ± 0.75 | 3.31 ± 0.8 | 3.52 ± 0.8 | 3.35 ± 0.73 | 3.4 ± 0.71 | 0.480 |
| Overweight, n (%) | 37 (8.6) | 4 (3.7) | 7 (6.7) | 5 (4.4) | 21 (20.6) | 0.000 |
| Obesity, n (%) | 14 (3.3) | 0 (0) | 0 (0) | 2 (1.7) | 12 (11.8) | 0.000 |
| Fatty liver, n (%) | 60 (14.0) | 2 (1.9) | 10 (9.5) | 19 (16.5) | 29 (28.4) | 0.001 |
Across maternal BMI quartiles, mothers in higher maternal BMI quartiles were more likely to have a greater weight and GDM. The offspring of mothers in higher maternal BMI quartiles were also more likely to have greater birth weight, BMI, waist circumference, hip circumference, chest circumference and CAP values and to be more prone to overweight/obese and fatty liver (Table 1).
The fatty liver risk of these offspring increased progressively from the lowest to the highest quartiles of maternal BMI, with odds ratios (ORs) of 5.84 (95%CI: 0.67-50.67), 9.76 (95%CI: 1.21-78.83), and 26.3 (95%CI: 3.21-215.3), respectively, after controlling for the sex and age of the offspring (Model 1). Further adjusting for maternal age, nulliparity (Model 2), GDM status of the mothers and birth weight (Model 3) did not change the associations (Table 2).
| Quartiles of BMI | Model 1 | P value | Model 2 | P value | Model 3 | P value | ||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |||||
| Offspring fatty liver | 1.21 | 1.1-1.33 | 0.000 | 1.21 | 1.1-1.34 | 0.000 | 1.23 | 1.11-1.36 | 0.000 | |
| Q1 | Reference | Reference | Reference | |||||||
| Q2 | 5.84 | 0.67-50.67 | 0.109 | 5.70 | 0.65-49.77 | 0.115 | 5.52 | 0.63-48.36 | 0.123 | |
| Q3 | 9.76 | 1.21-78.83 | 0.033 | 9.60 | 1.18-77.79 | 0.034 | 9.50 | 1.17-77.33 | 0.035 | |
| Q4 | 26.3 | 3.21-215.3 | 0.002 | 26.95 | 3.27-222.23 | 0.002 | 26.09 | 3.08-220.72 | 0.003 | |
| Offspring overweight/obesity | 1.19 | 1.07-1.33 | 0.002 | 1.19 | 1.07-1.33 | 0.002 | 1.20 | 1.07-1.34 | 0.002 | |
| Q1 | Reference | Reference | Reference | |||||||
| Q2 | 2.09 | 0.37-11.60 | 0.401 | 2.23 | 0.40-12.57 | 0.364 | 2.17 | 0.38-12.5 | 0.385 | |
| Q3 | 1.30 | 0.22-7.62 | 0.770 | 1.43 | 0.24-8.49 | 0.694 | 1.43 | 0.24-8.58 | 0.696 | |
| Q4 | 10.6 | 2.17-51.76 | 0.004 | 11.66 | 2.34-58.14 | 0.003 | 10.75 | 2.05-56.28 | 0.005 | |
The OR of the offspring overweight/obesity risk in the highest quartile of maternal BMI was 10.6 (95%CI: 2.17-51.76) after controlling for the sex and age of the children (Model 1) (Table 2).
As shown in Table 3, mothers with GDM weighed more, had higher maternal BMIs and had a higher prevalence of maternal obesity than mothers without GDM (all P < 0.05). The offspring of mothers with GDM had higher BMI, chest circumference, hip circumference, and CAP values (all P < 0.05). Among offspring whose mothers had GDM, 4 (8.3%) were obese, compared with 10 (2.6%) offspring whose mothers did not have GDM (P < 0.000). However, there were no significant differences in birth weight, sex distribution, weight at follow-up, waist circumference, or LSM values between the offspring of mothers with and without GDM (Table 3).
| Variables | Mother without GDM (n = 382) | Mother with GDM (n = 48) | P value |
| Maternal characteristics | |||
| Age, yr | 29.25 ± 5.1 | 29.98 ± 3.17 | 0.172 |
| Height, cm | 163.12 ± 17.48 | 162.02 ± 4.96 | 0.336 |
| Weight, kg | 56.16 ± 9.65 | 61 ± 9.47 | 0.001 |
| BMI, kg/m2 | 21.33 ± 3.59 | 23.22 ± 3.28 | 0.000 |
| Obesity, n (%) | 42 (11.0) | 20 (41.7) | 0.000 |
| Children characteristics | |||
| Birth weight, kg | 3.41 ± 0.45 | 3.37 ± 0.61 | 0.653 |
| Boy, n (%) | 186 (49.1) | 25 (52.1) | 0.699 |
| Height, cm | 122.19 ± 30.67 | 125.07 ± 25.75 | 0.597 |
| Weight, kg | 25.13 ± 10.28 | 29.15 ± 10.38 | 0.058 |
| BMI, kg/m2 | 15.74 ± 4.43 | 17.72 ± 3.94 | 0.024 |
| Waist circumference, cm | 58.2 ± 10.79 | 61.39 ± 10.23 | 0.148 |
| Hip circumference, cm | 68.15 ± 13.73 | 73.83 ± 7.94 | 0.003 |
| Chest circumference, cm | 54.38 ± 21.03 | 64.48 ± 7.96 | 0.000 |
| Waist-height ratio | 0.46 ± 0.05 | 0.47 ± 0.07 | 0.202 |
| Waist-hip ratio | 0.85 ± 0.08 | 0.83 ± 0.07 | 0.265 |
| Chest-height ratio | 0.43 ± 0.15 | 0.49 ± 0.05 | 0.024 |
| CAP, dB/m | 166.49 ± 45.65 | 187.26 ± 50.59 | 0.029 |
| LSM, kPa | 3.36 ± 0.75 | 3.53 ± 0.77 | 0.303 |
| Overweight, n (%) | 25 (6.5) | 12 (25.0) | 0.000 |
| Obesity, n (%) | 10 (2.6) | 4 (8.3) | 0.000 |
| Fatty liver, n (%) | 48 (12.6) | 12 (25.0) | 0.073 |
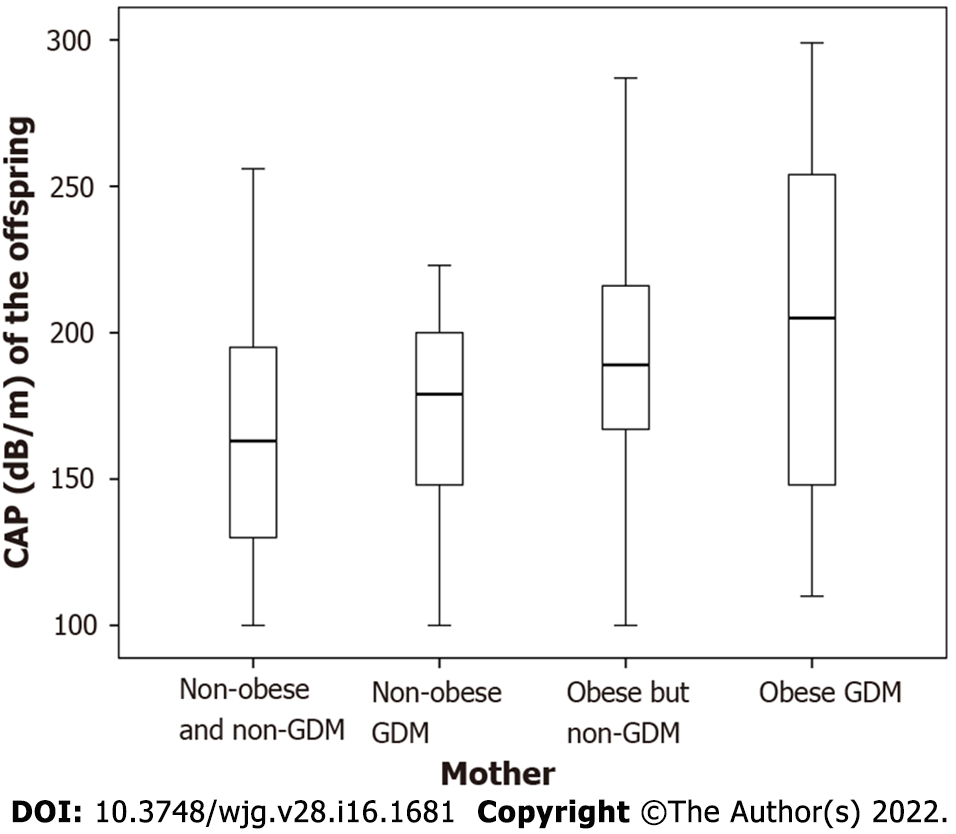
The CAP values of the offspring gradually increased in the mothers with neither obesity nor GDM (163.62 ± 44.41) dB/m, GDM but no obesity (173.43 ± 34.57) dB/m, obesity but no GDM (190.73 ± 49.74) dB/m to both obesity and GDM (202.15 ± 61.55) dB/m (all P < 0.05) (Figure 2).
Maternal obesity was positively associated with childhood fatty liver with OR 4.57 (95%CI: 1.96-10.67) and childhood overweight/obesity with OR 5.73 (95%CI: 2.18-15.10) (Model 1). Further adjustment for maternal age, nulliparity (Model 2), GDM status of the mother and birth weight (Model 3) did not change the associations (Table 4).
| Outcomes | Risk factors | Model 1 | P value | Model 2 | P value | Model 3 | P value | |||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |||||
| Fatty liver | Maternal obesity | 4.57 | 1.96-10.67 | 0.000 | 4.79 | 2.03-11.31 | 0.000 | 4.64 | 1.82-11.87 | 0.001 |
| GDM | 2.39 | 0.91-6.29 | 0.077 | 2.45 | 0.93-6.49 | 0.071 | 2.49 | 0.94-6.61 | 0.068 | |
| Maternal obesity and GDM | 7.19 | 2.15-24.13 | 0.001 | 7.72 | 2.25-26.46 | 0.001 | 8.26 | 2.38-28.75 | 0.001 | |
| Overweight/obesity | Maternal obesity | 5.73 | 2.18-15.10 | 0.000 | 5.69 | 2.14-15.10 | 0.000 | 4.15 | 1.44-11.97 | 0.009 |
| GDM | 4.70 | 1.72-12.81 | 0.003 | 4.85 | 1.76-13.37 | 0.002 | 4.84 | 1.76-13.36 | 0.002 | |
| Maternal obesity and GDM | 16.97 | 4.07-70.88 | 0.000 | 16.97 | 4.07-70.88 | 0.000 | 17.22 | 4.08-72.79 | 0.000 | |
Maternal GDM was also positively associated with childhood overweight/obesity, with an OR of 4.70 (95%CI: 1.72-12.81) (Model 1). Additionally, further adjustment for maternal age, nulliparity (Model 2), and offspring birth weight (Model 3) did not change the associations. In addition, the association of maternal GDM with childhood fatty liver was not statistically significant, with an OR of 2.39 (95%CI: 0.91-6.29) (Model 1) (Table 4).
In this prospective birth cohort, we assessed the causal association of maternal metabolic disorders with offspring overweight/obesity and fatty liver in Han Chinese populations. This study demonstrated that high maternal BMI increased the odds of both childhood overweight/obesity and fatty liver, independent of maternal age, offspring birth weight, and childhood waist circumference at 8 years of age. Furthermore, maternal pregnancy glucose concentrations were positively correlated with offspring CAP values at school age. These two findings corroborated that the negative impacts of maternal obesity and impaired glucose metabolism on offspring livers are long-term and not merely limited to infancy.
More impressively, the negative effects of maternal obesity and impaired glucose metabolism might vary in degree. A recent cohort study based on magnetic resonance imaging found that maternal early-pregnancy glucose levels were associated with a 1.95-fold increase in odds of offspring non-alcoholic fatty liver disease (NAFLD) only among mothers of European ancestry[13]. In our study, maternal blood samples were collected in the second trimester. We observed that maternal mid-pregnancy glucose levels had only a weak relation with offspring fatty liver among Han Chinese populations, while maternal obesity was more strongly associated with offspring fatty liver than GDM.
Maternal obesity and impaired glucose metabolism have lasting impacts on offspring hepatic health through epigenetic, dietary, and metabolic factors[14,15]. A sibling comparison cohort reported that maternal weight gain was aligned with the odds of offspring obesity[16], suggesting that maternal overnutrition may be a predisposing factor for offspring metabolic dysbiosis. This conclusion was also validated in some animal models, such as macaques and mice, and investigators found that reversing the high-fat diet to a low-fat diet during the subsequent pregnancy alleviated offspring hepatic lipid accumulation[17-19]. In terms of mechanisms, one study demonstrated that maternal obesity might render innate immunity dysfunctional, and another study observed that maternal obesity accelerated the progression of offspring NAFLD through activation of lipogenesis and oxidative stress pathways[20,21].
Our observations were mutually verified with previous studies and have several differences as follows. Two studies focused on the relation between maternal factors and infant hepatic fat[22,23]. Modi et al[23] observed that increasing maternal BMI might initiate lipid accumulation in infant livers. Subsequently, Brumbaugh and colleagues reported that infants of GDM mothers had greater hepatic steatosis than infants of non-GDM mothers[22]. In contrast, our study revealed the relatively long-term health outcomes in school-age children to corroborate that such associations might predispose children to fatty liver later in life.
Another study in obese mothers observed a positive relation with offspring ultrasound-diagnosed NAFLD during adolescence. However, ultrasound has limited power to detect mild steatosis and cannot quantify histological characteristics such as hepatic lipid content and liver stiffness. In our studies, we assessed pediatric liver pathology through TE, which is a reliable noninvasive diagnostic tool for fibrosis assessment in NAFLD[24]. Meanwhile, a biopsy-confirmed study reported that an association between parental obesity and offspring liver fibrosis was found in Italians[25]. As maternal impaired glucose metabolism was only related to offspring NAFLD in Europeans[13], the association between maternal obesity and progression of NAFLD in offspring may also differ across ethnic groups and the possible mechanisms need to be explored[26,27].
To the best of our knowledge, the present study is the first prospective birth cohort to assess the causal relationship between maternal metabolic dysbiosis and the odds of fatty liver in offspring. After adjustment for multiple regression models, the results were rigorous and trustworthy. Nonetheless, there are still several limitations that are worthy of discussion. First, to date, there is no widely accepted threshold of TE to detect childhood liver steatosis and fibrosis[28]. We used the 95th percentile cutoff values reported in a large health check-up cohort of preschool children as a surrogate threshold for this study[4]. Second, in this cohort, only 11.1% of mothers developed GDM during pregnancy, which reflected the true prevalence of GDM in the Chinese population. However, the small number of mothers with GDM might lead to type-2 statistical errors. Further nested case-control studies can address this issue and are recommended. Ultimately, single nucleotide polymorphisms of the patatin-like phospholipase domain containing 3, transmembrane 6 superfamily member 2, glucokinase regulatory protein, and several other susceptibility genes were not determined in this cohort. Further studies are needed to explore whether a predisposed genetic background mediates the influence of maternal metabolic dysbiosis on offspring NAFLD.
With the rapid spread of childhood fatty liver, it is urgent to develop preventive strategies against childhood fatty liver. In this regard, the current observations could be applied to the primary prevention of childhood obesity and fatty liver. The earliest timepoints of primary prevention of pediatric fatty liver could be before pregnancy. Weight management and glycemic control before and during pregnancy may help to promote liver and metabolic health status in children. Furthermore, lifestyle intervention before pregnancy is worth further investigation.
In this study, maternal obesity increased the odds of both fatty liver and obesity in offspring, independent of maternal age, GDM status and offspring birth weight at 8 years of age. On another note, the association between maternal GDM and childhood fatty liver trended toward significance in the Chinese population, and this association needs to be confirmed in studies with larger sample sizes. To prevent these intergenerational predisposing factors, weight management and glycemic control before and during pregnancy need to be highlighted for primary prevention of pediatric fatty liver.
Associations were found among childhood obesity, fatty liver and adverse outcomes such as diabetes, metabolic syndrome, and cardiovascular diseases in adulthood. It is important to identify relevant risk factors and intervene as early as possible.
We aimed to discover the possible relationship between metabolic factors in mothers and offspring.
We aimed to estimate the association of maternal obesity and gestational diabetes mellitus (GDM) with overweight/obesity and fatty liver risk in offspring.
The mothers in the study all underwent a 75 g oral glucose tolerance test at 24-28 wk of gestation, and their offspring completed follow-up at 8 years of age. An examination was prospectively conducted in offspring using a FibroScan-502 with an M probe (Echosens, Paris, France).
A total of 430 mother-child pairs were included in the analysis. The prevalence of overweight, obesity and fatty liver in offspring increased significantly across maternal BMI quartiles and among mothers with GDM (all P < 0.05). In the multiple logistic regression analysis, after adjustment for variables, the OR for fatty liver in offspring was 8.26 (95%CI: 2.38-28.75) for participants with maternal obesity and GDM.
Maternal obesity can increase the odds of overweight/obesity and fatty liver in offspring, and GDM status also increases the odds of overweight/obesity in offspring.
To prevent these intergenerational predisposing factors, weight management and glycemic control before and during pregnancy need to be emphasized for primary prevention of pediatric fatty liver.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Popova PV, Russia; Pustozerov EA, Russia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4666] [Article Influence: 583.3] [Reference Citation Analysis (2)] |
| 2. | Pothuraju R, Rachagani S, Junker WM, Chaudhary S, Saraswathi V, Kaur S, Batra SK. Pancreatic cancer associated with obesity and diabetes: an alternative approach for its targeting. J Exp Clin Cancer Res. 2018;37:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Sonntag D. Why Early Prevention of Childhood Obesity Is More Than a Medical Concern: A Health Economic Approach. Ann Nutr Metab. 2017;70:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Zeng J, Zhang X, Sun C, Pan Q, Lu WY, Chen Q, Huang LS, Fan JG. Feasibility study and reference values of FibroScan 502 with M probe in healthy preschool children aged 5 years. BMC Pediatr. 2019;19:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, Cavadino A, Paternoster L, Armstrong LL, De Silva NM, Wood AR, Horikoshi M, Geller F, Myhre R, Bradfield JP, Kreiner-Møller E, Huikari V, Painter JN, Hottenga JJ, Allard C, Berry DJ, Bouchard L, Das S, Evans DM, Hakonarson H, Hayes MG, Heikkinen J, Hofman A, Knight B, Lind PA, McCarthy MI, McMahon G, Medland SE, Melbye M, Morris AP, Nodzenski M, Reichetzeder C, Ring SM, Sebert S, Sengpiel V, Sørensen TI, Willemsen G, de Geus EJ, Martin NG, Spector TD, Power C, Järvelin MR, Bisgaard H, Grant SF, Nohr EA, Jaddoe VW, Jacobsson B, Murray JC, Hocher B, Hattersley AT, Scholtens DM, Davey Smith G, Hivert MF, Felix JF, Hyppönen E, Lowe WL Jr, Frayling TM, Lawlor DA, Freathy RM; Early Growth Genetics (EGG) Consortium. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA. 2016;315:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Kim C. Gestational diabetes: risks, management, and treatment options. Int J Womens Health. 2010;2:339-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wang L, Liu H, Zhang S, Leng J, Li W, Zhang T, Li N, Baccarelli AA, Hou L, Hu G. Maternal gestational diabetes and different indicators of childhood obesity: a large study. Endocr Connect. 2018;7:1464-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 Suppl 2:S169-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Cho Y, Tokuhara D, Morikawa H, Kuwae Y, Hayashi E, Hirose M, Hamazaki T, Tanaka A, Kawamura T, Kawada N, Shintaku H. Transient Elastography-Based Liver Profiles in a Hospital-Based Pediatric Population in Japan. PLoS One. 2015;10:e0137239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Obstetrics and gynecology branch of Chinese medical association; Perinatal Medicine Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of pregnancy complicated with diabetes. honghua Fuchanke Zazhi. 2014;8:561-569. [DOI] [Full Text] |
| 12. | Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10513] [Cited by in RCA: 10502] [Article Influence: 420.1] [Reference Citation Analysis (0)] |
| 13. | Geurtsen ML, Wahab RJ, Felix JF, Gaillard R, Jaddoe VWV. Maternal Early-Pregnancy Glucose Concentrations and Liver Fat Among School-Age Children. Hepatology. 2021;74:1902-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol. 2019;16:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 15. | Goldner D, Lavine JE. Nonalcoholic Fatty Liver Disease in Children: Unique Considerations and Challenges. Gastroenterology. 2020;158:1967-1983.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Lawlor DA, Lichtenstein P, Fraser A, Långström N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? Am J Clin Nutr. 2011;94:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Gregorio BM, Souza-Mello V, Carvalho JJ, Mandarim-de-Lacerda CA, Aguila MB. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am J Obstet Gynecol. 2010;203:495.e1-495.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, Taylor PD. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, Butt A, Saraswati R, Novelli M, Fusai G, Poston L, Taylor PD, Oben JA. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013;58:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, Reynolds R, Alston M, Hoffman C, Pan Z, Friedman JE, Barbour LA. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162:930-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, Santhakumaran S, Doré CJ, Alavi A, Bell JD. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Serra-Burriel M, Graupera I, Torán P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, Fabrellas N, Arslanow A, Expósito C, Hernández R, Lai-Hung Wong G, Harman D, Darwish Murad S, Krag A, Pera G, Angeli P, Galle P, Aithal GP, Caballeria L, Castera L, Ginès P, Lammert F; investigators of the LiverScreen Consortium. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Mosca A, De Cosmi V, Parazzini F, Raponi M, Alisi A, Agostoni C, Nobili V. The Role of Genetic Predisposition, Programing During Fetal Life, Family Conditions, and Post-natal Diet in the Development of Pediatric Fatty Liver Disease. J Pediatr. 2019;211:72-77.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Popova P, Vasilyeva L, Tkachuck A, Puzanov M, Golovkin A, Bolotko Y, Pustozerov E, Vasilyeva E, Li O, Zazerskaya I, Dmitrieva R, Kostareva A, Grineva E. A Randomised, Controlled Study of Different Glycaemic Targets during Gestational Diabetes Treatment: Effect on the Level of Adipokines in Cord Blood and ANGPTL4 Expression in Human Umbilical Vein Endothelial Cells. Int J Endocrinol. 2018;2018:6481658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Popova PV, Vasileva LB, Tkachuk AS, Puzanov MV, Bolotko YA, Pustozerov EA, Gerasimov AS, Zazerskaya IE, Li OA, Vasilyeva EY, Kostareva AA, Dmitrieva RI, Grineva EN. Association of tribbles homologue 1 gene expression in human umbilical vein endothelial cells with duration of intrauterine exposure to hyperglycaemia. Genet Res (Camb). 2018;100:e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 726] [Article Influence: 90.8] [Reference Citation Analysis (0)] |