Published online Apr 14, 2022. doi: 10.3748/wjg.v28.i14.1430
Peer-review started: September 9, 2021
First decision: November 21, 2021
Revised: December 5, 2021
Accepted: March 7, 2022
Article in press: March 7, 2022
Published online: April 14, 2022
Processing time: 208 Days and 22 Hours
Primary biliary cholangitis and primary sclerosing cholangitis (PSC) are the most common cholestatic liver diseases (CLD) in adults and are both characterized by an immune pathogenesis. While primary biliary cholangitis is a model autoim
Core Tip: Cholestatic liver diseases (CLD) in adults are characterized by an immune pathogenesis. Osteoporosis is the most common bone disease in CLD, resulting in frequent fractures and leading to significant morbidity. Sarcopenia is emerging as a frequent complication with a significant prognostic impact and severe implications on the quality of life of patients. The lack of useful preventive measures and efficacious treatment strategies remains one of the largest challenges in the management of patients with CLD.
- Citation: Pugliese N, Arcari I, Aghemo A, Lania AG, Lleo A, Mazziotti G. Osteosarcopenia in autoimmune cholestatic liver diseases: Causes, management, and challenges . World J Gastroenterol 2022; 28(14): 1430-1443
- URL: https://www.wjgnet.com/1007-9327/full/v28/i14/1430.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i14.1430
Cholestatic liver diseases (CLD) are characterized by progressive inflammation, damage, and destruction of bile ducts that lead to liver damage and, eventually, liver cirrhosis and systemic alterations. Accumulation of bile acids within liver cells cause detergent-induced damage of cellular membranes, which ultimately determines the development of apoptosis, inflammation, necrosis, fibrosis, and carcinogenesis[1,2]. Primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) are the most common CLD in adults. Although PBC is considered a classic autoimmune disease with nearly 95% of patients presenting very specific autoantibodies against mitochondrial antigens, PSC is recognized as an immune-mediated disease, with immunogenetic features and a strong association with inflammatory bowel disease (IBD). Both PBC and PSC are associated with a vast group of extrahepatic manifestations, including, but not limited to, fatigue, low bone mass, and other autoimmune diseases such as IBD, systemic sclerosis, and Sjogren syndrome[2,3].
Bone disease, including osteopenia and osteoporosis, is a common complication of CLD. Osteoporosis is characterized by a decreased bone density that leads to an increased risk of fractures. It increases morbidity and mortality in patients, and it is four times more common in patients with PBC compared to gender and age-matched controls. Moreover, sarcopenia and skeletal frailty have recently emerged as frequent complications of CLD, leading to severe morbidity, worse clinical outcome of disease, and lower quality of life of patients. The burden of osteosarcopenia in patients with CLD remains significant and therefore prevention is essential. In recent years, there have been advances in elucidating the risk factors and pathogenetic mechanisms underlying osteoporosis and sarcopenia in CLD but, unfortunately, validated diagnostic and therapeutic guidelines are not yet available. This review focuses on the pathogenic mechanisms and clinical implications of osteosarcopenia in CLD and summarizes expert recommendations for appropriate diagnostic and therapeutic approaches.
The World Health Organization (WHO) defines osteoporosis as a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture. In clinical practice, this condition is diagnosed using dual X-ray absorptiometry (DXA) for measurement of bone mineral density (BMD) at the lumbar spine, femoral neck, and total hip. In individuals older than 50 years of age and post-menopausal women, skeletal demineralization is graded based on comparisons of patient’s BMD with the average for young adults, after adjusting for race and gender. A T-score less than or equal to −2.5 standard deviations (SD) at the hip or spine is defined as osteoporosis, whereas osteopenia is defined as a T-score between −1 and −2.5 SD. These densitometric definitions cannot be applicable for younger subjects in whom the Z-score (i.e., the number of SD from age-matched controls) of 2.0 or lower is used to define a BMD “below the expected range for age”[4]. Although low BMD is consistently correlated with an increased fracture risk in the general population, fragility fractures may develop even in the context of normal BMD especially in subjects with secondary osteoporosis, in whom bone quality is affected more than bone quantity[5]. In these cases, evaluation of trabecular and cortical bone microstructure by high-resolution peripheral computed tomography can provide more reliable information on risk of fractures[6]. In the United States and Europe, the estimated number of osteoporosis-related hip fractures is about 0.3 and 1.7 million per year, respectively. More relevant is the impact of vertebral fractures (VFs) that have been consistently reported as an earlier and frequent complication of osteoporosis in the general population potentially associated with decreased survival and impaired quality of life[7-9].
Osteomalacia, defined by a reduction in bone mineralization with a preserved bone mass, was once thought to play a primary role in increasing fracture risk in patients with CLD[10], but more recent studies have proven that it is rarely associated with liver diseases, as it has been only reported in patients with advanced PBC and severe intestinal malabsorption associated with limited exposure to sunlight[11]. However, skeletal fragility can be associated with a worse outcome in patients with liver disease, as it entails an increased risk of fractures and consequently an overall increased disability prevalence and reduced quality of life[12].
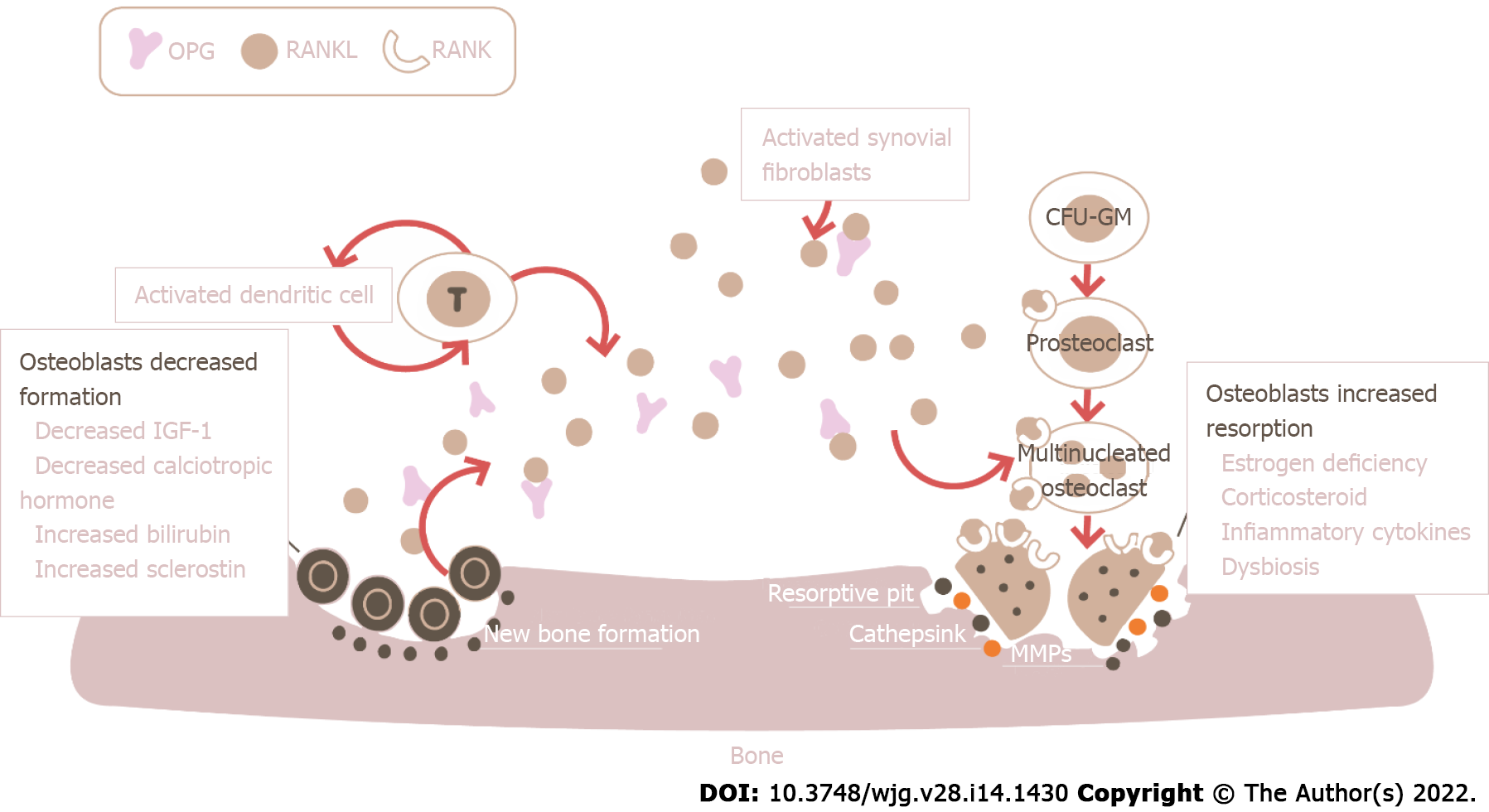
Although osteoporosis is associated with liver diseases including cirrhosis, it is most prevalent in cholestatic disorders[13]. Most of the studies that try to understand the relationship between the bone and liver have focused on PBC, though the pathophysiological mechanisms likely overlap in the case of end-stage liver disease from other etiologies. These mechanisms are numerous and not fully elucidated (Figure 1). The predominant process determining the reduction in bone mass appears to be a reduction in bone formation[11,13], although it seems that in some cases an increase in bone resorption is also involved, such as in post-menopausal women and patients with hypogonadism[14-16]. Bone formation, mediated by osteoblasts, and bone resorption, dependent on osteoclasts, are the two opposite processes that influence bone mass: When resorption exceeds formation, bone mass inevitably decreases and this negative balance leads to bone loss and osteoporosis. Osteoblast dysfunction may be directly linked to elevated serum levels of bile acids and bilirubin[17]. Some in vitro studies showed that lithocholic acid (LCA), a monohydroxylated secondary bile acid, can negatively influence osteoblasts' activity, both directly and indirectly through the ligation to vitamin D receptor (VDR) and the successive modification of expression of VDR-mediated genes, such as receptor activator for nuclear factor kappa B (NF-kB) ligand (RANKL) and bone gamma-carboxyglutamate protein, which serve as a regulator of osteoclast and osteoblast maturation, respectively[18]. Curiously, it appears that albumin, when added to cultures of osteoblasts exposed to LCA, can reduce the toxic effects of the molecule on the osteoblasts. It could be hypothesized that the amount of circulating albumin is one of the critical factors linked to the harmful effects on bone of circulating bile acids[11], but data regarding this association are still lacking. Osteoblast dysfunction can also depend on the reduced circulating levels of osteoblast stimulating factors such as insulin-like growth factor 1 (IGF1) secondary to the lack of hepatic synthesis seen in advanced chronic liver disease (ACLD) and reduced absorption[19,20], respectively. IGF1 acts directly on bone to promote longitudinal growth during the development phase and maintain adequate levels of bone mass once peak bone mass is reached[21]. Liver diseases impair the somatotropic axis and associate with liver growth hormone (GH) resistance; the consequent reductions in serum IGF1 can contribute to impair osteoblast function and cause skeletal fragility in individuals with CLD[21,22]. In this context, the role of paracrine and autocrine actions of locally produced IGF1 in the skeleton under control of parathyroid hormone (PTH) is unknown and in need of future studies[21].
In patients with PBC, low liver tissue and serum levels of RANKL and high levels of osteoprotegerin (OPG) have been previously reported[23-25]. This is at first glance unexpected, since OPG has a bone preserving function while RANKL activates osteoclastogenesis and tends to increase bone loss. However, RANKL can be interpreted both as a marker of bone resorption and as a marker of osteoblasts activity: Low RANKL would therefore indicate low osteoblast activity and a reduced bone turnover, which inevitably leads to increased bone fragility and risk of fractures[26]. High OPG levels on the other hand could indicate the homeostatic response attempting to prevent bone loss. Finally, the importance of osteoblast dysfunction has been proved by a series of studies that evaluated bone histomorphometry in patients with advanced CLD undergoing orthotopic liver transplant (OLT). In these studies, osteoblast numbers and bone formation rates appear to be decreased when compared to controls[27,28]. This data correlates with the low levels of osteocalcin, a non-collagenous marker of bone formation, seen in patients with cholestasis[29]: Two different studies observed that osteocalcin levels were decreased in up to 74% of PBC patients[30,31]. However, a previous study has revealed that increased bone resorption and turnover showed by bone histomorphometry are early characteristics of PBC-related bone disease[32]. More studies are needed to evaluate the variation of bone formation and bone resorption markers and to link them to cholestasis. An exemplary study on PBC patients showed no significant decrease in the levels of bone-specific alkaline phosphatase, a bone formation marker, but up to 95% of patients showed above-normal values; also, no significant variation was observed in the urine levels of type I collagen-cross-linked N-telopeptide, a marker of bone resorption[33]. Although it seems reasonable to think that a defect in the secretion of bile acids leads to reduced intestinal absorption of vitamin D, thus leading to hypocalcemia and secondary hyperparathyroidism, data in this regard are conflicting and not conclusive. Old studies have found decreased calcium absorption and serum vitamin D levels in PBC patients[34], but others have found normal vitamin D, calcium, and PTH levels even among osteoporotic patients with PBC[27,35]. It also appears that vitamin D supplementation in patients with cholestasis and malabsorption is unable to significantly improve BMD[36,37], although some old studies proved otherwise[34].
Since the main risk factors for the development of osteoporosis in OLT patients are pre-transplant bone mass state and pre-transplant fragility fractures, it is not surprising that cirrhotic patients who undergo liver transplant for PBC and PSC are at an extremely high risk of developing osteoporosis and suffering pathological fractures. Osteoporosis is a primary co-morbidity in post-transplant patients and is becoming more and more relevant, as their longtime survival has significantly increased in the last few years. In these patients, there is a rapid bone loss within the first 3 to 6 mo after transplantation and a frequency of fragility fractures of about 21%, most of which happen in the first period after transplant[38]. A study reported that the severity of bone loss was more frequently seen in patients of younger age with PSC, higher pretransplant BMD, no IBD, shorter duration of disease, current smoking, and ongoing cholestasis at 4 mo since OLT[39]. An important contributing cause of rapid bone loss in the immediate postoperative period of these patients is probably the use of high doses of corticosteroids and other immunosuppressive agents, such as tacrolimus and cyclospirn A, as well as immobilization during hospitalization after OLT[40]. After the first 3 to 6 postoperative months, bone gain occurs during the first 2 years with favoring factors for improvement of lower baseline and/or 4-mo BMD, premenopausal status for females, lesser glucocorticoids, no ongoing cholestasis, and higher levels of vitamin D and parathyroid function[39].
Sarcopenia, a progressive and generalized loss of skeletal muscle mass, strength, and function, is the other side of the coin of the metabolic abnormality in patients with liver disease[41]. It can be assessed in numerous ways, such as by bioelectrical impedance analysis or DXA, but the recommended method is by measuring anthropometric parameters, such as skeletal muscle area (SMA) and the Skeletal Muscle Index (SMI), by computed tomography and magnetic resonance imaging[42,43]. SMI is the metric recommended by the International Consensus panel on cachexia. It is the result of SMA (cm²) depicted on a single image slice [usually at the level of the third lumbar vertebra (L3)], adjusted by the height of the patient (m²) (this is often referred to as L3SMI method). This measurement can be easily compared to specific cut-off values based on healthy European young adults[44]. Importantly, sarcopenia is significantly associated with mortality and reduced quality of life in patients with liver cirrhosis[45,46].
Although recent data demonstrate that sarcopenia can be identified in up to 70% of cirrhotic patients[47], no data are available on the prevalence of sarcopenia in non-advanced cholestatic diseases. An outstanding study has recently been published analyzing the relationship between PBC, bone diseases, and sarcopenia[48]. Saeki et al[48] demonstrated that the association between osteoporosis and sarcopenia was stronger than the association among osteoporosis, female gender, and menopause in PBC patients, and vice versa osteoporosis and VFs were important risk factors for sarcopenia, independently of sex and menopause[48]. The increasing evidence of the existing association between bone mass and muscle loss, defined all together as osteosarcopenia, is of incredible relevance in liver diseases, given that it impacts the prognosis and health-related quality of life. The association of the two conditions is particularly hazardous, since it causes both ease of falling (due to sarcopenia) and bone vulnerability (due to osteoporosis)[49]. More data are, however, needed to fully comprehend the real impact of sarcopenia on non-advanced CLD. At the moment, the only considerations that can be made are based on studies on cirrhotic patients, in which, however, it appears that the main mechanism is linked to hyperammonemia, a phenomenon not present in the early stages of cholestatic diseases[50]. The role of cholestasis in sarcopenia in cirrhotic patients is only hinted at and remains anecdotal. Protein synthesis, protein breakdown, and muscle regenerative capacity mediated by satellite cells are the main processes that influence skeletal muscle mass: When their balance is disrupted, there is a loss in lean mass leading to sarcopenia. Skeletal muscle expression and serum levels of myostatin, a member of the TGFβ superfamily, are increased in patients with ACLD[51,52] and this hormone increases autophagy and proteolysis and prevents protein synthesis by inhibiting the mTORC1 pathway. Serum ammonia has been recognized as a stimulus in the synthesis of myostatin, by acting via a NF-kB mediated mechanism[53-55]. Hyperammonemia also impairs the formation of α-ketoglutarate (αKG), a molecule involved in the cycle of tricarboxylic acids (TCA)[53]. This results in several potential consequences including lower flux of the TCA cycle, impaired mitochondrial function, and decreased ATP synthesis. Since protein synthesis is an energy intense process, low ATP concentrations may also cause reduced protein synthesis. Similarly to hyperammonemia, hypoglycemia and low glucose levels in skeletal muscle cells are linked to the lack of glycogenolysis secondary to the reduction of hepatic glycogen stores, and can lead to the consumption of muscle amino acids for the production of energy[56]. Although hyperammonemia does not manifest itself in non-advanced CLD, the mechanisms leading to sarcopenia may be in some way analogous. Liver damage caused by the underlying pathology, malabsorption, and increased energy demands may lead to an energy deficit that would push the skeletal muscles to use amino acids as an alternative source of energy, in a similar way to how it occurs in case of hypoglycemia due to reduced hepatic glycogen reserve[57].
PBC is a chronic inflammatory autoimmune cholestatic liver disease which, if left untreated, could culminate in end-stage biliary cirrhosis[58]. It is the most studied condition as regards metabolic bone disease, both for the high prevalence of osteoporosis in PBC patients and for the possibility of directly studying the pathophysiological mechanisms underlying the interaction between cholestasis and reduction in bone mass.
Osteoporosis is a common complication of PBC, with the most recent studies reporting a prevalence ranging from 20% to 45%, four-fold higher than that in the general population[59,60], with the highest prevalence in patients with cirrhosis on the liver transplant list[39]. Accordingly, the incidence and prevalence of fractures are also increased in PBC patients, ranging from 0% to 14% over a 2-year period and from 9% to 22%, respectively[61]. In a prospective study, Guañabens et al[62] observed a prevalence of vertebral, non-vertebral, and overall fractures of 11.2%, 12.2%, and 20.8%, respectively (Table 1). In that study, more than 20% of fractures occurred without a densitometric diagnosis of osteoporosis consistent with the pathophysiological concept that alteration of osteoblastogenesis and bone formation in CLD may induce impairment of bone quality more than bone quantity as in other forms of secondary osteoporosis. In addition to the common risk factors associated with skeletal fragility, such as age and post-menopausal state, additional independent risk factors have been identified in patients with PBC. The most prominent risk factor for developing osteoporosis is the stage of the disease. A study based on histologic staging observed that patients with more advanced histologic stages (such as stage 3 or 4) had more than a 5-fold increased risk of developing osteoporosis than patients with an earlier stage of the disease and the rate of bone loss over time was significantly greater in the former as compared with the latter[63]. A more recent study observed that liver stiffness measured by FibroScan was directly related with the reduction of both cortical and trabecular bone parameters in the tibia and distal radius[64]. The stage of disease appears to have an effect far stronger than the post-menopausal state in PBC patients[65]. Other less important risk factors that seem to have been identified include female gender, the necessity of transplant and, to some extent, genetic predisposition[58,66]. Indeed, some studies identified some gene loci that positively correlate with osteoporosis[67-69]. Although vitamin D does not seem to play an important role in determining skeletal fragility in CLD, polymorphisms of VDR have been associated with osteoporosis in individuals with PBC. One study in women affected by PBC concluded that VDR genotype is an independent genetic predictor of osteoporosis[67]. In addition, a polymorphism of the gene encoding collagen type I alpha1 (COLIA1), Sp1, is associated with reduced baseline BMD in patients with PBC[69]. Lastly, some polymorphisms of a tight junction membrane gene, the claudin-14 (CLDN-14), suspected to be involved in the pathogenesis of CLD, has been proved to be associated with low BMD in PBC patients[70].
| Ref. | N of patients | Age (yr) mean | Female (%) | Diagnosis | Osteoporosis (%) | Vertebral fractures (%) | Peripheral fractures (%) | Overall fractures (%) | Cirrhosis (%) |
| Guañabens et al[114] | 38 | 51 | 100 | DPA | 45 | 13 | NR | 13 | 94 |
| Parés et al[68] | 61 | 54 | 100 | DXA | 21 | 10 | 10 | 13 | 26 |
| Guañabens et al[65] | 142 | 54 | 100 | DXA | 31 | 14 | 11 | 14 | 26 |
| Guichelaar et al[39] | 156 | 53 | 86 | DXA | 44 | 22 | NR | 22 | 100 |
| Guañabens et al[62] | 185 | 56 | 100 | DXA | 32 | 11 | 12 | 21 | 23 |
| Solaymani-Dodaran et al[115] | 930 | NR | 88 | NR | NR | NR | 7.4 | 14.7 | 31 |
Another important independent risk factor is low body mass index (BMI). Beyond the direct association of reduced muscle mass and low bone mass, it seems that in PBC patients, preponderant hormonal mechanisms associated with BMI come into play: Leptin, an adipocyte derived hormone, seems to indirectly regulate bone metabolism since it increases osteoblast proliferation and bone matrix synthesis, resulting in increased bone formation, and inhibits RANKL production, decreasing bone resorption. Szalay et al[71] observed decreased leptin levels in PBC patients and also demonstrated a positive correlation between leptin, BMI, and BMD[71].
Although sarcopenia is a fascinating new topic in hepatology and has been recently studied in ACLD patients, very few data are available on its relationship with PBC in non-cirrhotic patients. The only article available in the literature at the time of writing this review has been previously cited and dates back to May 2020[48]. Saeki et al[48] reported the prevalence of sarcopenia, diagnosed according to the Japan Society of Hepatology guidelines[48,72], between PBC patients: 23.1% for all patients and 25% for female patients, greater than in other non-cirrhotic liver conditions, where the prevalence was reported to be approximately 15%[73,74]. These findings suggest that patients with PBC are more susceptible to sarcopenia, compared to those with other chronic liver diseases. This study also proved that sarcopenia is strongly correlated with osteoporosis and increased fracture risk (especially VFs) and vice versa, proving that osteosarcopenia as a unified clinical entity is an important complication of PBC, occurring in up to 15.4% of patients, requiring careful monitoring in all patients, especially post-menopausal women, who represent the majority of PBC patients. The clinical relevance of osteosarcopenia has been proven in studies focused on geriatric patients, in which the osteosarcopenic group had greater impairment of physical performance and balance than the non-osteosarcopenic and sarcopenia/ osteoporosis alone groups. Consequently, osteosarcopenia conferred an increased rate of falls and fractures and a consequent higher mortality rate[75-77]. Despite the clinical relevance, recommendations on follow-up and treatment cannot yet be made, as these aspects are still in the early stages of definition[78].
PSC is a chronic, cholestatic liver disease characterized by immune-mediated inflammation and fibrosis of both intrahepatic and extrahepatic bile ducts, leading to the formation of multifocal bile duct strictures and to the development of biliary cirrhosis[79]. Although it is a clinical entity of considerable interest, both for its hepatological implications and for the set of clinical conditions with which it is associated, there are still very few studies that focus on the link between PSC and bone disease, and almost none that investigate a possible relationship of PSC with sarcopenia.
As with other chronic liver diseases, the prevalence of osteoporosis in PSC is higher than that of the general population, accounting for 15%–30% of patients with PSC[80,81]. General risk factors include female gender, age, and low BMI. Studies are still needed to evaluate if duration of the disease is a risk factor and, curiously, osteoporosis does not appear to be related to the severity of the underlying PSC[27]. Although previous studies reported a possible association between osteoporosis and the stage of disease[82,83], more recent studies observed otherwise and failed to prove a significance correlation between osteoporosis and the severity of liver disease. It is possible that this difference is based on population cohorts analyzed, since in previous studies the patients were less heterogeneous and predominantly post-menopausal women[84]. Moreover, the close association between PSC and IBD[85], the consequent malabsorption, and the possible use of steroid therapy at high doses for long periods of time can certainly influence bone metabolism and represent an important risk factor for the development of osteoporosis. In addition, patients with IBD presents themselves with a lower bone mass at the diagnosis[86], likely due to the systemic inflammatory state of IBD.
No conclusive data are yet available regarding the possible relationship between PSC and sarcopenia but, considering the cholestasis-associated malabsorption, which may be worsened by the eventual concomitant IBD related to PSC, and the increased prevalence of sarcopenia in patients with other liver diseases including PBC, it is reasonable to assume that these patients also have an increased prevalence of sarcopenia compared to the general population that should be investigated at the time of diagnosis and during the follow-up. Interestingly, Shteyer et al[87] failed to prove a significant difference between pediatric patients with PSC and the control group; also, children and young adults with concomitant PSC and IBD appeared to have lesser degree of sarcopenia in comparison to patients with PSC alone[87]. Although interesting, larger studies are required to confirm these curious findings.
Management of osteoporosis in cholestatic diseases cannot be evidence-based and no guidelines have been developed for diagnosis and treatment of osteoporosis in this specific clinical setting. However, based on our personal experience and the few studies so far published on the topic, some recommendations could be provided (Table 2). DXA measurement of BMD should be performed at the initial diagnosis of PBC to identify subjects with low BMD at higher risk of fractures.
| Risk for osteoporosis should be considered in all patients with cholestatic liver diseases | |
| DXA should be considered to assess BMD at presentation and at follow-up where indicated | T-score > -1.5 - > repeat in 2-3 yr |
| Osteopenia, T-score ≤ -1.5 but > -2.5, or presence of risk factors - > repeat in 1-2 yr | |
| Osteoporosis, T score ≤ -2.5, or pathological fractures with normal BMD - > repeat in one year | |
| VFs should be investigated at presentation with lateral spine X-rays radiograph in all patients with cholestatic liver diseases | |
| Alcohol and smoking cessation in addition to increasing aerobic exercise and practicing routine weight-bearing exercises are highly recommended in all patients with cholestatic liver diseases | |
| Consider including supplements of 25-(OH)-vitamin D (800 IU daily) and calcium (1000–1500 mg daily) in patients with cholestatic liver disease and osteopenia or osteoporosis | |
| Consider utilizing bisphosphonates in patients with osteoporosis and patients with VFs, regardless of underlying disease and BMD values | |
| For patients with PBC, denosumab might have a beneficial role both for osteoporosis treatment and for PBC but data are scarce, and recommendation cannot be made yet | |
| Consider evaluating sarcopenia by cross-sectional imaging when strong clinical suspicion is present in all patients with cholestatic liver diseases | |
| Consider exercise programs and adequate nutritional and caloric intake in all patients with sarcopenia and cholestatic liver diseases |
The optimal timing of monitoring PBC patients is yet to be defined, but in clinical practice bone densitometry with DXA should be performed, depending on the presence of risk factors for osteoporosis and fractures, in 1 to 3 years if initial results are normal. A more stringent follow-up is indicated in the presence of altered BMD or risk factors such as severe cholestasis, menopause before the age of 45 years old, family history of osteoporosis or fragility fractures, BMI less than 19 kg/m2, tobacco use, heavy alcohol abuse, and glucocorticoid use greater than 3 mo and or > 5 mg daily. In patients who are already on osteoporosis treatment, DXA should be performed annually to assess treatment response. In addition to imaging, routine monitoring of vitamin D, calcium, phosphorus, and PTH should be performed every 1 to 2 years based on the current risk of developing bone disease[58,61].
Over the last decade, several algorithms (e.g., FRAX) have been proposed to improve the value of DXA results in predicting fracture risk[88] but their use in secondary osteoporosis in general and in CLD in particular has not been validated. Noteworthy, the traditional risk factors of osteoporosis and fractures included in the algorithm FRAX seem to have a role also in influencing the occurrence of fragility fractures in subjects with CLD.
The new generation DXA machines can also provide information on bone quality. For instance, the trabecular bone score (TBS) is a texture parameter obtained directly from DXA images through the evaluation of the average pixel gray-scale variation. A low TBS value correlates with a weaker microarchitecture with reduced and scarcely interconnected trabeculae, resulting in lower bone strength and mechanical resistance[89]. Measuring this parameter, the clinicians may have another reliable information on risk of fractures even in individuals with either normal or only slightly decreased BMD[90].
As in other forms of secondary osteoporosis, the search of VFs is indicated in all subjects at diagnosis of CLD since they may occur even in the context of normal BMD. In more than 55% of the cases, VFs occur without specific clinical symptoms and the radiological and morphometric approach has emerged as the method of choice for evaluating the true prevalence and incidence of these fractures in the clinical practice. VFs are identified by marking the vertebral body with six points to describe the vertebral shape and heights. According to the quantitative morphometric approach, VFs are defined mild, moderate, and severe based on a height ratio decrease of 20%-25%, 25%-40%, and more than 40%, respectively[91]. VFs are routinely assessed by examining lateral projection images of conventional spine X-ray radiographs, although other approaches using DXA and the low-dose biplane X-ray imaging system (EOS imaging, Paris, France) have been proposed as alternative tools to limit radiation exposure in clinical practice[92,93]. The current guidelines indicate that finding of non-traumatic VFs, regardless of underlying disease and BMD values, is sufficient to establish the diagnosis of osteoporosis and to consider pharmacologic treatment as secondary prophylaxis[94].
Bone active agents used to treat osteoporosis are classified as anti-resorptive and anabolic drug[95]. Bisphosphonates inhibit bone resorption and are the most prescribed drugs for the treatment of osteoporosis. Denosumab is a human monoclonal antibody (IgG2 immunoglobulin isotype) binding RANKL with high affinity and specificity and inducing a reversible inhibition of osteoclastogenesis and bone resorption. Teriparatide is the 1-34 active fragment of PTH with stimulating effects on osteoblastogenesis and bone formation when intermittently administered once daily. Teriparatide is currently the only anabolic drug approved for treatment of osteoporosis at high risk of fractures and for glucocorticoid-induced osteoporosis.
Data on the efficacy and safety of bone-active drugs in CLD are scant and their use in this clinical setting can be guided by evidence extrapolated from the literature on the treatment of postmenopausal osteoporosis[96]. A comprehensive Cochrane systematic review published in 2011 concluded that there are no conclusive data showing the benefits of bisphosphonate use on BMD, mortality, or reduced fracture risk in this specific clinical context[97]. There is only one randomized clinical trial that compares newer generation bisphosphonates (in this case alendronate) to placebo in PBC[98]: It proved that after 1 year, there was a significant improvement in lumbar spine BMD in patients treated with bisphosphonates (10.4% vs –0.12% in patients in the placebo arm, P < 0.005) but failed to prove a significant reduction in fractures (0% vs 7.1% in patients in the placebo arm, P = 0.3)[98]. Particular attention must be given to patients with ACLD with a high risk of oesophageal varices since esophagitis and oesophageal ulcers are side-effects of oral bisphosphonates. In these patients, parenteral bisphosphonates can be proposed[99,100].
Preliminary data on denosumab in patients with PBC indicate that lumbar spine T-score significantly improved after 1 and 3 years of treatment, along with levels of markers of bone formation, despite that the prevalent mechanism determining osteoporosis is osteoblast dysfunction[101,102]. As the studies are extremely small, no recommendations can be made yet, but certainly the use of denosumab in these patients is promising. Importantly, recent evidence strengthens a critical role of RANK/RANKL signaling in autoimmunity besides bone density, with the immune and skeletal systems being closely interconnected. It has been demonstrated that cholangiocytes from PBC patients express high levels of RANK; most importantly, the immune infiltrates within the portal areas around bile ducts in PBC are highly RANKL positive and the hepatic level of RANKL was associated with disease severity[25]. Lleo et al[25] hypothesized that damaged cholangiocytes in PBC, which show high levels of RANK, determine the recruitment of RANKL positive cells and consequently portal tract infiltrates. Taken all together, a number of recent and old evidence point out that denosumab might have a beneficial role in PBC therapy, besides osteoporosis, but data are scarce and more studies are needed to make recommendation[25].
No data are available on the efficacy and safety of teriparatide and PTH-analogues but, whereas the main pathogenetic mechanism that determines the development of osteoporosis in patients with PBC is reduced osteoblasts activity, osteoanabolic agents could play a crucial role in the treatment of the condition in this setting.
Inactivity, alcohol consumption, tobacco use, and reduced dietary calcium intake can all lead to reduced bone density. For this reason, alcohol and smoking cessation in addition to increasing aerobic exercise and practicing routine weight-bearing exercises is highly recommended. Dietary supplementation of calcium (1000-1500 mg daily) and vitamin D [800 international units (IU) daily] is also recommended in patients who are at particularly high risk of developing osteoporosis, especially in patients with ACLD[58,103]. Patients receiving cholestyramine should be monitored closely since its administration may reduce intestinal absorption of vitamin D[104,105]. Although this is standard clinical practice, data on the effect of calcium and vitamin D supplementation are controversial: As previously stated, some studies prove a significant improvement in BMD[34] while other studies failed to do so[36,37].
It is important to assess the presence of osteoporosis by DXA at the moment of diagnosis of PSC, as well as considering supplementation with calcium and vitamin D in all patients, though this has no clear benefit based on the literature[106,107]. No protocols exist on how to handle the specific follow-up and treatment of these patients, so WHO or ISCD guidelines for the management of osteoporosis are usually used[4] and the recommended diagnostic-therapeutic approach is the same as in PBC, even if the two diseases differ both in pathogenesis and in the phenotype of the population affected.
The diagnosis of sarcopenia should be made by cross-sectional imaging when strong clinical suspicion is present. Treatment can be derived by studies based on ACLD, but even in this setting clear guidelines are not currently available, both because the studies existing so far showed great heterogeneity and were based on a small number of patients, thus limiting the possibility of defining specific and reliable guidelines for the pathology, and no treatment had proven to be particularly effective[57]. Exercise programs finalized on avoiding natural deterioration of muscle mass[108,109] and nutritional supplementation with BCAA[110-112] are no established treatment and the data on these approaches are controversial. It should also be noted that physical activity may be difficult as one of the salient clinical features of this disease is chronic asthenia and fatigue. Considering the molecular mechanisms that lead to sarcopenia, improving protein synthesis and reducing autophagy with molecules like myostatin antagonists, direct mTORC1 activators, antioxidants, and mitochondrial protective agents could have the potential to benefit skeletal muscle protein turnover but have not been adequately evaluated[113]. More studies are needed to define the correct timing of follow-up.
Osteosarcopenia is a common complication of CLD that strongly influences quality of life and leads to severe morbidity. Indeed, cholestasis is directly associated with both bone and lean mass loss and the prevalence of bone damage is demonstrated to be higher in CLD than in the general population. Risk factors and etiopathogenesis of osteoporosis in PBC have been widely investigated; however, evaluation of the efficacy of osteoporosis drugs and preventive measures remain poorly known and data on PSC are scarce. On the other hand, studies on risk factors, etiologic mechanisms, and management of sarcopenia in CLD are lacking.
It is widely accepted that preventing the reduction of bone density is important to decrease the risk of fractures and improve morbidity and mortality. Further, PSC and PBC mostly affect young patients, and therefore prevention and screening are widely recommended. However, the timing is not yet defined, and no clinical guidelines are available for management of osteosarcopenia in CLD. Unfortunately, the overall quality of evidence is low and data on the treatment of CLD-related osteosarcopenia are inadequate.
Table 2 provides a proposed algorithm for the management of osteosarcopenia in CLD. Not pharmacological measures, including alcohol and smoking cessation, and aerobic and weight-bearing exercises, are highly recommended in all patients. The primary medical intervention for the treatment of osteoporosis in CLD is bisphosphonates in association with calcium and vitamin D supplements, though a benefit in terms of fracture reduction has never been shown. The use of further therapies for osteoporosis in CLD are based on the postmenopausal osteoporosis literature and new studies are desperately needed to define the best therapeutic approach to osteosarcopenia in a group of patients with high prevalence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; European Association for the Study of the Liver (EASL).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Filipec Kanizaj T, Croatia; Reshetnyak VI, Russia S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | de Vries E, Beuers U. Management of cholestatic disease in 2017. Liver Int. 2017;37 Suppl 1:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Assis DN. Chronic Complications of Cholestasis: Evaluation and Management. Clin Liver Dis. 2018;22:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173:R131-R151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E, Berger C, Burt L, Chapurlat R, Chevalley T, Ferrari S, Goltzman D, Hanley DA, Hannan MT, Khosla S, Liu CT, Lorentzon M, Mellstrom D, Merle B, Nethander M, Rizzoli R, Sornay-Rendu E, Van Rietbergen B, Sundh D, Wong AKO, Ohlsson C, Demissie S, Kiel DP, Bouxsein ML. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol. 2019;7:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 7. | Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women's Health Initiative. Semin Reprod Med. 2014;32:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, Papadimitropoulos EA, Hopman W, Poliquin S, Prior JC, Hanley DA, Olszynski WP, Anastassiades T, Brown JP, Murray T, Jackson SA, Tenenhouse A; Canadian Multicentre Osteoporosis Study (CaMos) Research Group. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001;12:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Miller PD. Management of severe osteoporosis. Expert Opin Pharmacother. 2016;17:473-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Compston JE, Horton LW, Thompson RP. Treatment of osteomalacia associated with primary biliary cirrhosis with parenteral vitamin D2 or oral 25-hydroxyvitamin D3. Gut. 1979;20:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Guañabens N, Parés A. Liver and bone. Arch Biochem Biophys. 2010;503:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Raszeja-Wyszomirska J, Miazgowski T. Osteoporosis in primary biliary cirrhosis of the liver. Prz Gastroenterol. 2014;9:82-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Danford CJ, Trivedi HD, Bonder A. Bone Health in Patients With Liver Diseases. J Clin Densitom. 2020;23:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] |
| 15. | Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2017;167:ITC17-ITC32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 513] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 16. | Marchioni Beery RM, Vaziri H, Forouhar F. Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis: a Review Featuring a Women's Health Perspective. J Clin Transl Hepatol. 2014;2:266-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Ruiz-Gaspà S, Martinez-Ferrer A, Guañabens N, Dubreuil M, Peris P, Enjuanes A, Martinez de Osaba MJ, Alvarez L, Monegal A, Combalia A, Parés A. Effects of bilirubin and sera from jaundiced patients on osteoblasts: contribution to the development of osteoporosis in liver diseases. Hepatology. 2011;54:2104-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Ruiz-Gaspà S, Guañabens N, Enjuanes A, Peris P, Martinez-Ferrer A, de Osaba MJ, Gonzalez B, Alvarez L, Monegal A, Combalia A, Parés A. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur J Clin Invest. 2010;40:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | de la Garza RG, Morales-Garza LA, Martin-Estal I, Castilla-Cortazar I. Insulin-Like Growth Factor-1 Deficiency and Cirrhosis Establishment. J Clin Med Res. 2017;9:233-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol. 2003;176:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Mazziotti G, Frara S, Giustina A. Pituitary Diseases and Bone. Endocr Rev. 2018;39:440-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Liu Z, Han T, Werner H, Rosen CJ, Schaffler MB, Yakar S. Reduced Serum IGF-1 Associated With Hepatic Osteodystrophy Is a Main Determinant of Low Cortical but Not Trabecular Bone Mass. J Bone Miner Res. 2018;33:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 244] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Szalay F, Hegedus D, Lakatos PL, Tornai I, Bajnok E, Dunkel K, Lakatos P. High serum osteoprotegerin and low RANKL in primary biliary cirrhosis. J Hepatol. 2003;38:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Lleo A, Bian Z, Zhang H, Miao Q, Yang F, Peng Y, Chen X, Tang R, Wang Q, Qiu D, Fang J, Sobacchi C, Villa A, Di Tommaso L, Roncalli M, Gershwin ME, Ma X, Invernizzi P. Quantitation of the Rank-Rankl Axis in Primary Biliary Cholangitis. PLoS One. 2016;11:e0159612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, Mayr A, Jocher J, Xu Q, Pietschmann P, Teitelbaum S, Smolen J, Willeit J. Soluble RANKL and risk of nontraumatic fracture. JAMA. 2004;291:1108-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Bone metabolism in advanced cholestatic liver disease: analysis by bone histomorphometry. Hepatology. 2002;36:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Immunosuppressive and postoperative effects of orthotopic liver transplantation on bone metabolism. Liver Transpl. 2004;10:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Klein GL, Soriano H, Shulman RJ, Levy M, Jones G, Langman CB. Hepatic osteodystrophy in chronic cholestasis: evidence for a multifactorial etiology. Pediatr Transplant. 2002;6:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Pietschmann P, Resch H, Müller C, Woloszczuk W, Willvonseder R. Decreased serum osteocalcin levels in patients with liver cirrhosis. Bone Miner. 1990;8:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Bagur A, Mautalen C, Findor J, Sorda J, Somoza J. Risk factors for the development of vertebral and total skeleton osteoporosis in patients with primary biliary cirrhosis. Calcif Tissue Int. 1998;63:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Cuthbert JA, Pak CY, Zerwekh JE, Glass KD, Combes B. Bone disease in primary biliary cirrhosis: increased bone resorption and turnover in the absence of osteoporosis or osteomalacia. Hepatology. 1984;4:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Seki A, Ikeda F, Miyatake H, Takaguchi K, Hayashi S, Osawa T, Fujioka SI, Tanaka R, Ando M, Seki H, Iwasaki Y, Yamamoto K, Okada H. Risk of secondary osteoporosis due to lobular cholestasis in non-cirrhotic primary biliary cholangitis. J Gastroenterol Hepatol. 2017;32:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Kehayoglou AK, Holdsworth CD, Agnew JE, Whelton MJ, Sherlock S. Bone disease and calcium absorption in primary biliary cirrhosis with special reference to vitamin-D therapy. Lancet. 1968;1:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res. 2008;23:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Crippin JS, Jorgensen RA, Dickson ER, Lindor KD. Hepatic osteodystrophy in primary biliary cirrhosis: effects of medical treatment. Am J Gastroenterol. 1994;89:47-50. [PubMed] |
| 37. | Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology. 1982;83:103-108. [PubMed] |
| 38. | Guichelaar MM, Schmoll J, Malinchoc M, Hay JE. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Bjøro K, Brandsæter B, Wiencke K, Bjøro T, Godang K, Bollerslev J, Schrumpf E. Secondary Osteoporosis in Liver Transplant Recipients: a Longitudinal Study in Patients With and Without Cholestatic Liver Disease. Scand J Gastroenterol. 2003;38:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 42. | Reiss J, Iglseder B, Kreutzer M, Weilbuchner I, Treschnitzer W, Kässmann H, Pirich C, Reiter R. Case finding for sarcopenia in geriatric inpatients: performance of bioimpedance analysis in comparison to dual X-ray absorptiometry. BMC Geriatr. 2016;16:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, Cavalier E, Cerreta F, Cherubini A, Fielding R, Gielen E, Landi F, Petermans J, Reginster JY, Visser M, Kanis J, Cooper C. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 44. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3837] [Article Influence: 274.1] [Reference Citation Analysis (0)] |
| 45. | Kim SE, Kim DJ. Sarcopenia as a prognostic indicator of liver cirrhosis. J Cachexia Sarcopenia Muscle. 2022;13:8-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Lanza E, Masetti C, Messana G, Muglia R, Pugliese N, Ceriani R, Lleo de Nalda A, Rimassa L, Torzilli G, Poretti D, D'Antuono F, Politi LS, Pedicini V, Aghemo A; Humanitas HCC Multidisciplinary Group. Sarcopenia as a predictor of survival in patients undergoing bland transarterial embolization for unresectable hepatocellular carcinoma. PLoS One. 2020;15:e0232371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 47. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1499] [Article Influence: 249.8] [Reference Citation Analysis (0)] |
| 48. | Saeki C, Oikawa T, Kanai T, Nakano M, Torisu Y, Sasaki N, Abo M, Saruta M, Tsubota A. Relationship between osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia in patients with primary biliary cholangitis. Eur J Gastroenterol Hepatol. 2021;33:731-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28:2781-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 50. | Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol Behav. 2017;176:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 866] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 51. | Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 52. | García PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 54. | McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 55. | Wing SS, Lecker SH, Jagoe RT. Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks. Crit Rev Clin Lab Sci. 2011;48:49-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 57. | Pugliese N, Lanza E, Aghemo A. Sarcopenia in chronic liver disease: easy to diagnose but hard to treat. Liver Int. 2020;40:2627-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 908] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 59. | Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018;24:3513-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Glass LM, Su GL. Metabolic Bone Disease in Primary Biliary Cirrhosis. Gastroenterol Clin North Am. 2016;45:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Trivedi HD, Danford CJ, Goyes D, Bonder A. Osteoporosis in Primary Biliary Cholangitis: Prevalence, Impact and Management Challenges. Clin Exp Gastroenterol. 2020;13:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Menon KV, Angulo P, Weston S, Dickson ER, Lindor KD. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001;35:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Schmidt T, Schmidt C, Schmidt FN, Butscheidt S, Mussawy H, Hubert J, Hawellek T, Oehler N, Barvencik F, Lohse AW, Schinke T, Schramm C, Amling M, Rolvien T. Disease Duration and Stage Influence Bone Microstructure in Patients With Primary Biliary Cholangitis. J Bone Miner Res. 2018;33:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, Monegal A, Peris P, Rodés J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Parés A, Guañabens N. Primary biliary cholangitis and bone disease. Best Pract Res Clin Gastroenterol. 2018;34-35:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewood L, Evrovski J, Peltekova VD, Heathcote EJ. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Parés A, Guañabens N, Rodés J. Gene polymorphisms as predictors of decreased bone mineral density and osteoporosis in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Lakatos PL, Bajnok E, Tornai I, Folhoffer A, Horvath A, Lakatos P, Habior A, Szalay F. Insulin-like growth factor I gene microsatellite repeat, collagen type Ialpha1 gene Sp1 polymorphism, and bone disease in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2004;16:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Tang R, Wei Y, Li Z, Chen H, Miao Q, Bian Z, Zhang H, Wang Q, Wang Z, Lian M, Yang F, Jiang X, Yang Y, Li E, Seldin MF, Gershwin ME, Liao W, Shi Y, Ma X. A Common Variant in CLDN14 is Associated with Primary Biliary Cirrhosis and Bone Mineral Density. Sci Rep. 2016;6:19877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Szalay F, Folhoffer A, Horváth A, Csak T, Speer G, Nagy Z, Lakatos P, Horváth C, Habior A, Tornai I, Lakatos PL. Serum leptin, soluble leptin receptor, free leptin index and bone mineral density in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 479] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 73. | Hayashi M, Abe K, Fujita M, Okai K, Takahashi A, Ohira H. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol Res. 2018;48:893-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, Hasegawa K, Ishii N, Yuri Y, Nishimura T, Iijima H, Nishiguchi S. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Sepúlveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, Conzade R, Kirk B, Al Saedi A, Probst V, Duque G. The Joint Occurrence of Osteoporosis and Sarcopenia (Osteosarcopenia): Definitions and Characteristics. J Am Med Dir Assoc. 2020;21:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kässmann H, Kreutzer M, Dovjak P, Reiter R. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z Gerontol Geriatr. 2019;52:688-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 77. | Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R; FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 79. | Fricker ZP, Lichtenstein DR. Primary Sclerosing Cholangitis: A Concise Review of Diagnosis and Management. Dig Dis Sci. 2019;64:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Raszeja-Wyszomirska J, Kucharski R, Zygmunt M, Safranow K, Miazgowski T. The impact of fragility fractures on health-related quality of life in patients with primary sclerosing cholangitis. Hepat Mon. 2015;15:e25539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Williamson KD, Chapman RW. Primary sclerosing cholangitis. Dig Dis. 2014;32:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Angulo P, Therneau TM, Jorgensen A, DeSotel CK, Egan KS, Dickson ER, Hay JE, Lindor KD. Bone disease in patients with primary sclerosing cholangitis: prevalence, severity and prediction of progression. J Hepatol. 1998;29:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Hay JE, Lindor KD, Wiesner RH, Dickson ER, Krom RA, LaRusso NF. The metabolic bone disease of primary sclerosing cholangitis. Hepatology. 1991;14:257-261. [PubMed] |
| 84. | Campbell MS, Lichtenstein GR, Rhim AD, Pazianas M, Faust T. Severity of liver disease does not predict osteopenia or low bone mineral density in primary sclerosing cholangitis. Liver Int. 2005;25:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Annese V. A Review of Extraintestinal Manifestations and Complications of Inflammatory Bowel Disease. Saudi J Med Med Sci. 2019;7:66-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Targownik LE, Bernstein CN, Leslie WD. Inflammatory bowel disease and the risk of osteoporosis and fracture. Maturitas. 2013;76:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Shteyer E, Cytter-Kuint R, L Winberg L. P401 Sarcopenia in children and young adults with primary sclerosing cholangitis and IBD. J Crohns and Colitis. 2020;14:S371-S372. |
| 88. | Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1958-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 89. | Ulivieri FM, Silva BC, Sardanelli F, Hans D, Bilezikian JP, Caudarella R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine. 2014;47:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 90. | McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJM, Fujita Y, Glüer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren Ö, Lorentzon M, Mellström D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J Bone Miner Res. 2016;31:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 497] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 91. | Chesnut C, Majumdar S, Gardner J, Shields A, Newitt DC, Erickson E, Glott M, Kriegman A, Mindeholm L. Assessment of bone quality, quantity, and turnover with multiple methodologies at multiple skeletal sites. Adv Exp Med Biol. 2001;496:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Griffith JF, Genant HK. New advances in imaging osteoporosis and its complications. Endocrine. 2012;42:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, Fiore CE, Iolascon G, Maggi S, Michieli R, Migliaccio S, Minisola S, Rossini M, Sessa G, Tarantino U, Toselli A, Isaia GC. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. 2019;14:85-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 94. | Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 925] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 95. | Mazziotti G, Bilezikian J, Canalis E, Cocchi D, Giustina A. New understanding and treatments for osteoporosis. Endocrine. 2012;41:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Danford CJ, Ezaz G, Trivedi HD, Tapper EB, Bonder A. The Pharmacologic Management of Osteoporosis in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. J Clin Densitom. 2020;23:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Rudic JS, Giljaca V, Krstic MN, Bjelakovic G, Gluud C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst Rev. 2011;CD009144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Zein CO, Jorgensen RA, Clarke B, Wenger DE, Keach JC, Angulo P, Lindor KD. Alendronate improves bone mineral density in primary biliary cirrhosis: a randomized placebo-controlled trial. Hepatology. 2005;42:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Treeprasertsuk S, Silveira MG, Petz JL, Lindor KD. Parenteral bisphosphonates for osteoporosis in patients with primary biliary cirrhosis. Am J Ther. 2011;18:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 100. | Lima TB, Santos LAA, Nunes HRC, Silva GF, Caramori CA, Qi X, Romeiro FG. Safety and efficacy of risedronate for patients with esophageal varices and liver cirrhosis: a non-randomized clinical trial. Sci Rep. 2019;9:18958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 101. | Sugiyama T. Letter to the Editor: Bone Health and Denosumab Treatment in Autoimmune Liver Diseases: A Possible Involvement of Physical Activity. Hepatology. 2020;71:1131-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Arase Y, Tsuruya K, Hirose S, Ogiwara N, Yokota M, Anzai K, Deguchi R, Shiraishi K, Shirai T, Kagawa T. Efficacy and Safety of 3-Year Denosumab Therapy for Osteoporosis in Patients With Autoimmune Liver Diseases. Hepatology. 2020;71:757-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Bjelakovic G, Nikolova D, Bjelakovic M, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Danielsson A, Lorentzon R, Larsson SE. Normal hepatic vitamin-D metabolism in icteric primary biliary cirrhosis associated with pronounced vitamin-D deficiency symptoms. Hepatogastroenterology. 1982;29:6-8. [PubMed] |
| 105. | Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol. 1987;2:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Lundqvist K, Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Lima CA, Lyra AC, Mendes CMC, Lopes MB, Coqueiro FG, Rocha R, Santana GO. Bone mineral density and inflammatory bowel disease severity. Braz J Med Biol Res. 2017;50:e6374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 108. | Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 109. | Román E, García-Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, Alvarado-Tapias E, Malouf J, Nácher L, Serra-Grima R, Guarner C, Cordoba J, Soriano G. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One. 2016;11:e0151652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 110. | Park JG, Tak WY, Park SY, Kweon YO, Chung WJ, Jang BK, Bae SH, Lee HJ, Jang JY, Suk KT, Oh MJ, Heo J, Woo HY, Jang SY, Lee YR, Lee JS, Kim DY, Kim SH, Suh JI, Kim IH, Kang MK, Lee WK. Effects of Branched-Chain Amino Acid (BCAA) Supplementation on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Prospective, Observational, Cohort Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 111. | Kitajima Y, Takahashi H, Akiyama T, Murayama K, Iwane S, Kuwashiro T, Tanaka K, Kawazoe S, Ono N, Eguchi T, Anzai K, Eguchi Y. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol. 2018;53:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 112. | Ooi PH, Gilmour SM, Yap J, Mager DR. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin Nutr ESPEN. 2018;28:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Ponziani FR, Gasbarrini A. Sarcopenia in Patients with Advanced Liver Disease. Curr Protein Pept Sci. 2018;19:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 114. | Guañabens N, Parés A, Navasa M, Martínez de Osaba MJ, Hernández ME, Muñoz J, Rodés J. Cyclosporin A increases the biochemical markers of bone remodeling in primary biliary cirrhosis. J Hepatol. 1994;21:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 115. | Solaymani-Dodaran M, Card TR, Aithal GP, West J. Fracture risk in people with primary biliary cirrhosis: a population-based cohort study. Gastroenterology. 2006;131:1752-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |