Published online Feb 21, 2021. doi: 10.3748/wjg.v27.i7.561
Peer-review started: December 11, 2020
First decision: December 27, 2020
Revised: December 28, 2020
Accepted: January 13, 2021
Article in press: January 13, 2021
Published online: February 21, 2021
Processing time: 71 Days and 0.6 Hours
Although coronavirus (CoV) infection is often characterized by respiratory symptoms, the virus can also result in extrapulmonary symptoms, especially the symptoms related to the digestive system. The outbreak of coronavirus disease 2019 (COVID-19) is currently the world’s most pressing public health threat and has a significant impact on civil societies and the global economy. The occurrence of digestive symptoms in patients with COVID-19 is closely related to the development and prognosis of the disease. Moreover, thus far, there are no specific antiviral drug or vaccine approved for the treatment or prevention of COVID-19. Therefore, we elaborate on the effects of CoVs on the digestive system and the potential underlying mechanisms.
Core Tip: In this review, it is reported that coronavirus infections can cause a series of digestive diseases, and may also be accompanied by digestive manifestations and abnormal digestive function. Furthermore, the potential mechanisms of coronavirus disease 2019 on the digestive system, such as angiotensin-converting enzyme 2, immune injury, gut microbiota, hypoxemia, and psychological stress, are also discussed. This review provides a new perspective for the prevention and treatment of infections with severe acute respiratory syndrome coronavirus 2 and other coronaviruses.
- Citation: Zhan GF, Wang Y, Yang N, Luo AL, Li SY. Digestive system involvement of infections with SARS-CoV-2 and other coronaviruses: Clinical manifestations and potential mechanisms. World J Gastroenterol 2021; 27(7): 561-575
- URL: https://www.wjgnet.com/1007-9327/full/v27/i7/561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i7.561
In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously called 2019-nCoV), initially emerged in Wuhan, China. It has posed a serious threat to human health worldwide. The World Health Organization has declared that COVID-19 is a public health emergency of pandemic proportions[1]. As of December 9, 2020, a total of 67780361 laboratory-confirmed cases including 1551214 deaths have been reported in 220 countries, areas, and territories[1]. The COVID-19 pandemic is currently the world’s most pressing public health threat and has a significant impact on civil societies and the global economy.
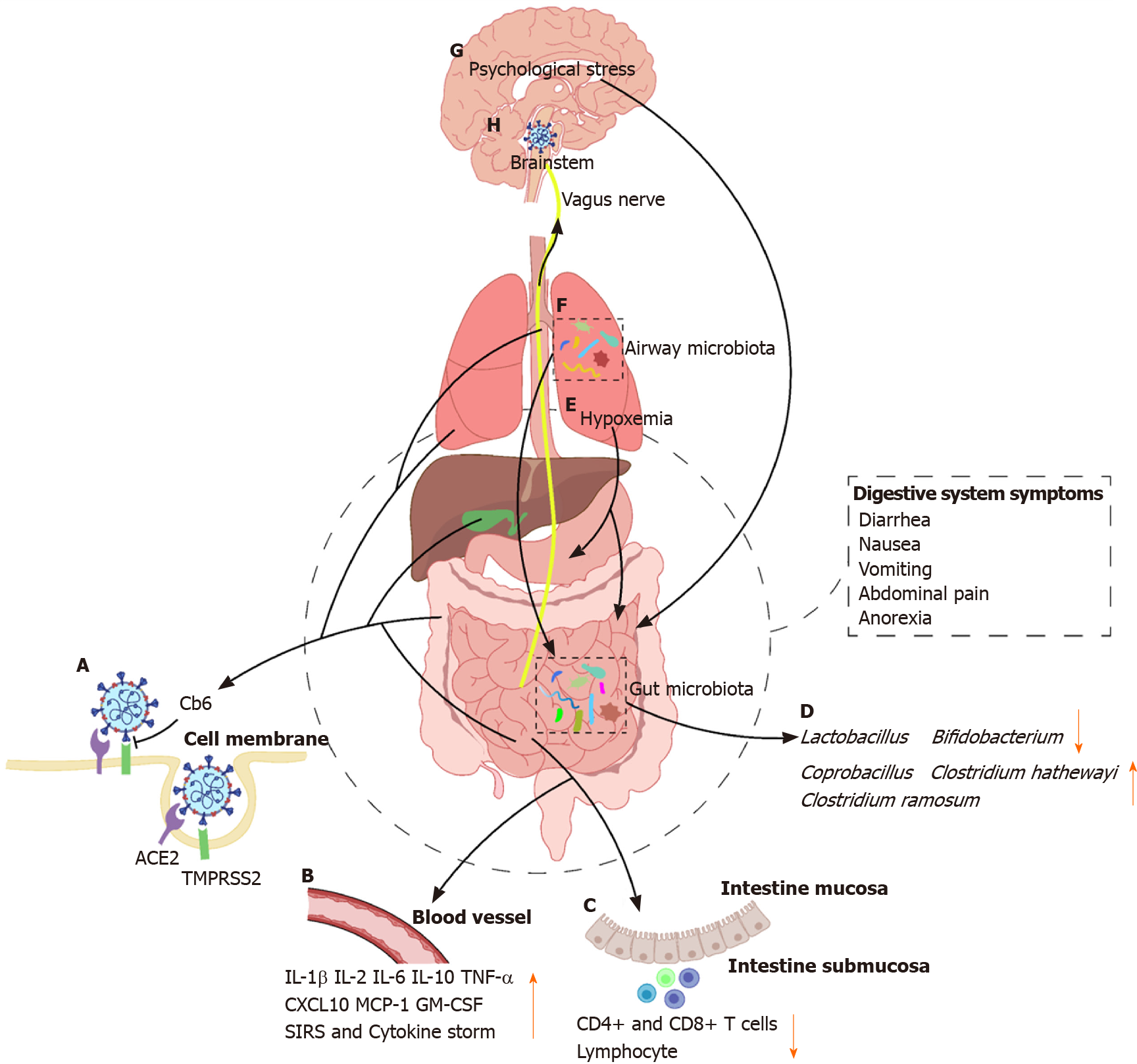
It is well established that most individuals with COVID-19 present with fever and typical respiratory symptoms, such as cough and dyspnea, similar to those of SARS and Middle East respiratory syndrome (MERS)[2-5]. Therefore, nasopharyngeal and oropharyngeal swabs are the suitable samples for reverse transcriptase-polymerase chain reaction detection of SARS-CoV-2. However, concurrent extra-pulmonary symptoms, such as gastrointestinal manifestations, mainly including diarrhea, nausea, vomiting, abdominal pain, and anorexia, have also been reported in recent studies[6,7]. In addition, rectal swabs and stool specimens of patients with COVID-19 have shown to be positive for SARS-CoV-2, and the virus remained detectable even after the clearance of the virus in the respiratory tract[7-10]. Furthermore, Pan et al[11] demon-strated that patients with digestive symptoms have a longer time from onset to admission and a worse prognosis than that of patients without digestive symptoms[11]. Together, these indicate that SARS-CoV-2 can infect and replicate in the gastrointestinal tract, and it may also have potential to cause digestive system damage. With the ongoing COVID-19 pandemic, those studies are of great guiding significance for disease prevention, control, and management. The present review aims to summarize data regarding epidemiology, gastrointestinal characteristics, possible mechanisms, and potential therapeutic strategy in patients infected with coronaviruses (CoVs) from the perspective of the digestive system (Figure 1).
CoVs are a group of enveloped, positive-sense, single-stranded RNA viruses (+ssRNA) with a crown-like appearance, which belongs to the family Coronaviridae of order Nidovirales[12,13]. CoVs harbor the largest genome among those known RNA viruses, with a genome length ranging from 26-32 kilobases (kb) and a diameter in the range of 120-160 nm[14-16]. CoVs are genetically classified into four genera: Alpha-coronavirus, Beta-coronavirus, Gamma-coronavirus, and Delta-coronavirus[12,17]. Alpha-coronavirus and Beta-coronavirus primarily infect mammals, whereas Gamma-coronavirus and Delta-coronavirus usually infect birds[16]. To date, seven coronavirus species under two genera have been identified to cause diseases in human: Alpha-coronavirus (HCoV-NL63 and HCoV-229E) and Beta-coronavirus (HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2)[18].
HCoV-NL63, HCoV-229E, HCoV-OC43, and HCoV-HKU1 cause mild common cold symptoms, whereas the other three highly pathogenic CoVs have caused large-scale pandemic since the beginning of the 21st century: SARS-CoV in 2002 and 2003, MERS-CoV in 2012, and the newly emerged SARS-CoV-2[19]. The three highly contagious pathogens are zoonotic in origin and have crossed the species barrier to human. Furthermore, these three viruses are known to cause digestive symptoms.
SARS-CoV: SARS-CoV has a large RNA genome length of 27.9 kb, emerged in the Guangdong Province of China, and had spread to 29 countries and areas worldwide. By July 2003, there were a total of 8098 laboratory-confirmed cases including 774 deaths (case-fatality rate: 9.6%) of SARS[20]. SARS-CoV has been considered to originate from bats, and market palm civets and racoon dogs have been unequivocally considered the intermediate hosts[21].
SARS is characterized by fever and respiratory complications, and it may also lead to acute respiratory distress syndrome and multiple organ failure in severe cases. Moreover, gastrointestinal manifestations are frequently observed in patients with SARS-CoV infection[22]. The tropism of SARS-CoV in the digestive system is commonly known to occur, and a retrospective study demonstrated that 38.4% of patients with SARS had diarrhea, usually within the first week of the disease course[23]. It was also demonstrated that up to 60% of patients with SARS suffered from liver impairment, and SARS-CoV was detected in liver tissue although viral inclusions were not observed[24]. In addition, the rate of SARS-CoV RNA positivity in collected stool specimens increased progressively and peaked at day 11 of the illness, with viral RNA remaining detectable in a stool sample in a patient even 73 d after symptom onset[23,25]. Furthermore, the presence of SARS-CoV RNA in the gastrointestinal tract may indicate a poor prognosis, and the viral load in the stool is correlated with death[26].
MERS-CoV: MERS-CoV has a large RNA genome length of 30.1 kb, emerged in the Saudi Arabia, and then spread to 27 countries and areas with a total of 2468 confirmed cases and including 851 associated deaths (case-fatality rate: 34.4%) by the end of September 2019[27]. MERS-CoV has originated from bats, but dromedary camels has been considered the intermediate host[28].
Human-to-human transmission of MERS-CoV occurred mainly through nosocomial transmission[29]. Although most patients with MERS present with nonspecific respiratory symptoms, such as fever, cough, and shortness of breath, approximately one third of patients have gastrointestinal symptoms, such as diarrhea, vomiting, and abdominal pain, which were the most commonly demonstrated extrapulmonary clinical features[30]. In addition, MERS-CoV RNA was detected in 14.6% of stool specimens from patients with MERS, but the positive rate of virus detection was lower than that of SARS[31,32]. Moreover, an in vitro study has shown that MERS-CoV can successfully replicate in human primary intestinal epithelial cells[33].
SARS-CoV-2: SARS-CoV-2 has a genome length of 29.9 kb, shares an approximately 79% sequence identity to the SARS-CoV and the similarity of the whole-genome to BatCoV RaTG13 (a bat coronavirus detected in Rhinolophus affinis) is up to 96.2%[34]. Recent studies have suggested that bats may be the natural reservoir of SARS-CoV-2, and that there may be multiple potential intermediate hosts, such as pangolins, minks, and snakes[35-37]. In terms of the current pandemic situation, the case fatality of COVID-19 may not reach as high as that of SARS and MERS.
The majority of patients with COVID-19 exhibit mild to moderate pulmonary symptoms, and SARS-CoV-2 can extend to multiple extra-pulmonary organs including the heart, brain, kidneys, liver, and gut. Based on the data presented in Table 1, 6.7% of the total patients with SARS-CoV-2 infection have diarrhea and there are a great number of patients who had abnormal liver function with a paucity of concurrent or isolated pre-existing digestive system comorbidities. It was reported that COVID-19 patients with diarrhea presented severe symptoms of pneumonia compared to those without diarrhea, and COVID-19 patients with gastrointestinal manifestations or liver injury are more likely to require mechanical ventilation and hospitalization in the intensive care unit than those without gastrointestinal manifestations or liver injury[7,38-40]. Followed by the detection of SARS-CoV-2 RNA in the stool of the first case of COVID-19 in the United States, it was recently reported that viral RNA was detected in 59% of patients with COVID-19 in stool samples and the SARS-CoV-2 lasts significantly longer in stool samples than in respiratory and serum specimens[41,42]. In addition, To and colleagues demonstrated that SARS-CoV-2 was detected in the self-collected saliva specimens in 91.7% patients with COVID-19[43]. Furthermore, autopsy studies have demonstrated varying degrees of degeneration, necrosis, and shedding in the gastrointestinal mucosa and segmental dilatation and stenosis in the small intestine of patients with COVID-19[44,45]. Taken together, it is therefore likely that SARS-CoV-2 poses a serious threat to the digestive system.
| Ref. | Patients with COVID-19 | Gastrointestinal symptoms | Patients with abnormal biochemical indicators of liver function | Patients with pre-existing digestive diseases | Patients testing positive for SARS-CoV-2 in stool or rectal swab specimens | ||
| Diarrhoea | Nausea and vomiting | Abdominal pain | |||||
| Guan et al[7] | 1099 | 42 (3.8%) | 55 (5.0%) | NA | Abnormal AST: 168/757 (22.2%). Abnormal ALT: 158/741 (21.3%). Abnormal TBIL: 76/722 (10.5%) | CHB: 23 (2.1%) | Stool specimens: 4/NA. Rectal swab: 4/NA |
| Wang et al[6] | 138 | 14 (10.1%) | 19 (13.7%) | 3 (2.2%) | Mild elevation of AST and ALT | 4 (2.9%) | NA |
| Huang et al[40] | 41 | 1/38 (2.6%) | NA | NA | Abnormal AST: 15 (31.0%) | Chronic liver disease: 1 (2%) | NA |
| Fan et al[116] | 148 | 6 (4.1%) | 3 (2.0%) | NA | Abnormal AST: 32 (21.6%). Abnormal ALT: 27 (18.2%). Abnormal TBIL: 9 (6.1%) | 9 (6.1%) | NA |
| Cai et al[49] | 298 | 9 (3.0%) | NA | NA | Abnormal AST: 25 (8.4%). Abnormal ALT: 39 (13.1%). Abnormal TBIL: 24 (8.1%) | CHB: 5 (1.7%). NAFLD: 14 (4.7%). ALD: 9 (3.0%) | NA |
| Chen et al[3] | 99 | 2 (2.0%) | 1 (1.0%) | NA | Abnormal AST: 35 (35.0%). Abnormal ALT: 28 (28.0%). Abnormal TBIL: 18 (18.0%). Abnormal albumin: 97 (98.0%) | NA | NA |
| Holshue et al[41] | 1 | 1 | 1 | NA | Abnormal AST: 1. Abnormal ALT: 1. Abnormal TBIL: 1. Abnormal albumin: 1 | NA | Stool specimens: 1/1 |
| Zhang et al[117] | 140 | 18/139 (12.9%) | 31/139 (22.3%) | 8/139 (5.8%) | NA | FLD and abnormal liver function: 8 (5.7%). Chronic gastritis and gastric ulcer: 7 (5.0%). Cholelithiasis: 6 (4.3%) | NA |
| Xiao et al[10] | 73 | 26 (35.6%) | NA | NA | NA | NA | Stool specimens: 39/73 (53.4%) |
| Zhou et al[118] | 191 | 9 (4.7%) | 7 (3.7%) | NA | Abnormal ALT: 59/189 (31.2%) | NA | NA |
| Zhang et al[119] | 82 (Deaths) | 10 (12.2%) | 2 (2.3%) | NA | Abnormal AST: 44/72 (61.1%). Abnormal ALT: 22/72 (30.6%). Abnormal TBIL: 22/72 (30.6%). Abnormal albumin: 56/72 (77.8%) | Liver disease: 2/82 (2.4%) | NA |
| Xu et al[120] | 62 | 3 (4.8%) | NA | NA | Abnormal AST: 10 (16.1%) | Liver disease: 7 (11.3%) | NA |
| Chen et al[121] | 21 | 4/20 (20.0%) | NA | NA | Abnormal AST: 6 (28.6%). Abnormal albumin: 8 (38.1%) | NA | NA |
| Pan et al[11] | 204 | 29 (14.2%) | 8 (3.9%) | 4 (2.0%) | Abnormal AST: NA. Abnormal albumin: NA | CHB: 1 (0.5%). FLD: 1 (0.5%). Gastritis: 1 (0.5%) | NA |
| Luo et al[122] | 1141 | 68 (5.9%) | Nausea: 134 (11.7%). Vomiting: 119 (10.4%) | 45 (3.9%) | Mild elevation of AST and ALT | NA | NA |
| Total | 3738 | 174/2592 (6.7%) | |||||
Digestive diseases include a series of disorders that affect the oropharynx and digestive tract, liver and biliary system, and pancreas, affecting human lives commonly. At the beginning of the COVID-19 outbreak, more medical resources were freed up to control the spread of the virus so that more individuals can be protected. Meanwhile, it is safe for most patients with pre-existing diseases. However, it may also increase the relative risk of complications caused by delayed screening, diagnosis, and treatment, and being afraid of seeking medical attention[46]. Challenges encountered regarding the most common pre-existing digestive diseases during the ongoing COVID-19 pandemic are described below.
Hepatitis refers to inflammation of the liver caused by multiple pathogens (viruses, bacteria, drugs, autoimmune factors, and so on), which can progress to fibrosis, cirrhosis, and even liver cancer, posing a great threat to human health. The most common causes of hepatitis are the five main hepatitis virus types (A, B, C, D, and E)[47]. In a number of studies, hepatitis was noted in patients with SARS and COVID-19[7,11,48,49]. SARS patients with hepatitis B are more likely to develop severe hepatitis than in those without. Similarly, severe cases of COVID-19 were more prone to infect HBV than non-severe cases[48,50]. Collectively, these findings show that CoVs might play a significant role in hepatitis, although detailed studies are needed to confirm this.
Fatty liver disease is a spectrum of disorders characterized predominantly by macrovesicular hepatic steatosis, and it presents a condition of excess fat stored in the liver, including non-alcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (ALD). Patients with hypertension, diabetes, obesity, and old age have a high risk of developing severe COVID-19[51]. These comorbidities also increase the risk of NAFLD, thus leading to liver injury. The ongoing COVID-19 pandemic has several negative effects on individuals, especially those encountering job loss, financial strain, death of a loved one, and so on. Under these stressors, an individual’s alcohol consumption is likely to increase[52]. Therefore, COVID-19 is likely to have a long-lasting impact on the development of ALD.
Given that patients with liver cirrhosis and cancer have a high risk of infection due to the immunocompromised status[53], measures to prevent SARS-CoV-2 infection are needed. Xiao and colleagues designed a study showing that none of the participants who undertook precautionary and protective measures had clinical symptoms suggestive of SARS-CoV-2 infection, whereas 17 of 101 patients with decompensated cirrhosis who did not undertake these measures were diagnosed with COVID-19[54]. This research suggests that more strategies should be implemented to prevent SARS-CoV-2 infection in patients with liver cirrhosis and cancer, such as online health education and medication guidance.
The outbreaks of SARS and MERS might potentially increase the risk of transmission of viral infection from donors to recipients and thus even lead to death[40,55]. Tzedakis et al[56] reported that a local liver transplant center decreased transplant activity by 60% since the COVID-19 pandemic and that it is crucial and necessary to balance costs and benefits while performing liver transplant[56]. During the COVID-19 pandemic, all donors and recipients of liver transplant must be screened for the presence of SARS-CoV-2 to ensure the safety and success of the liver transplantation.
Ulcerative colitis and Crohn's disease are two major idiopathic inflammatory bowel disorders (IBDs) that could cause prolonged inflammation of the gastrointestinal tract[57]. Patients with IBD who use immunosuppressive and biological agents are more susceptible to opportunistic and severe infections than those who do not[58,59]. Therefore, it is reasonable to assume that patients with IBD have more severe COVID-19, although there has been no direct clinical evidence to prove it. An and colleagues showed that none of the 318 patients with IBD (204 with ulcerative colitis and 114 with Crohn‘s disease)developed SARS-CoV-2 infection with early effective warning and protective measures[60]. In addition, patients with cancer also have an increased risk of infection[61]. Furthermore, it was demonstrated that individuals with cancer may have a high risk of severe COVID-19 and a poorer prognosis[62]. Although the risk of SARS-CoV-2 infection in patients with gastrointestinal cancer remains unknown, effective precautionary approaches are necessary to better protect patients.
It has been commonly known that respiratory droplets and direct contact are the two major transmission pathways of SARS-CoV-2. However, a recent study performed by Liu and colleagues has shown a possibility of aerosol transmission[63]. A large number of studies have reported gastrointestinal symptoms and liver dysfunction in patients with SARS-CoV-2 infection. At the same time, SARS-CoV-2 could be detected in rectal swabs and stool specimens in patients with COVID-19[7,10]. Moreover, biopsy and autopsy reports have revealed that COVID-19 caused pathological changes in the digestive system. In addition, high airborne concentration of SARS-CoV-2 was detected in toilet room of Fangcang Hospital where patients with COVID-19 were treated[63]. Taken together, these research findings provide a theoretical basis for the spread of COVID-19 through the faecal-oral route, although there is no direct evidence for the transmission pathway thus far.
The digestive tract communicates with the outside world directly, similar to the respiratory tract. Therefore, SARS-CoV-2 has the opportunity to enter the gastrointestinal tract and cause a direct cytopathic effect by local replication. In addition, systemic responses to excessive immune inflammation induced by SARS-CoV-2 infection may also cause damage to the digestive system indirectly. However, the mechanisms of how COVID-19 affects the digestive system remain poorly known. Furthermore, there is also no specific antiviral drug or vaccine approved to treat or prevent COVID-19 to date. Hence, it is particularly urgent to identify the potential therapeutic strategies for COVID-19.
Angiotensin-converting enzyme 2 (ACE2) is a well-known receptor located on cells of various organs for regulating cardiovascular function through the renin-angiotensin system, playing a major role in regulating hypertension and anti-atherosclerosis mechanisms[64,65]. In recent two decades, the role of ACE2 in SARS-CoV and influenza virus infection as a target functional receptor has attracted widespread attention[66-68]. It has been reported that the entry of SARS-CoV-2 into the host cell depends on the binding of viral spike (S) proteins to the SARS-CoV receptor ACE2 followed by the transmembrane serine protease 2 (TMPRSS2) for the S protein priming[69]. Therefore, human cells co-expressing ACE2 and TMPRSS2 are susceptible to SARS-CoV-2 infection[70]. A recent study performed by Zhang and colleagues has demonstrated that type II alveolar cells have higher expression of ACE2 and TMPRSS2 than type I alveolar cells in the lung. Besides, co-expression of ACE2 and TMPRSS2 has also been found in the glandular cells of upper esophageal and absorptive enterocytes of the ileum and colon[70,71]. Intestinal epithelial cells can act as a barrier and help to coordinate immune responses in microbial infections[72]. A number of studies have reported that absorptive enterocytes can be damaged or developed malabsorption and intestinal secretion abnormalities due to coronavirus or rotavirus infection, thus resulting in diarrhea and vomiting[73,74]. Therefore, gastrointestinal manifestations in patients with COVID-19 might be associated with infected enterocytes co-expressing ACE2 and TMPRSS2. Moreover, co-expression of ACE2 and TMPRSS2 has been observed in cholangiocytes, but not in hepatocytes[75]. Cholangiocytes play a pivotal role in liver regeneration and immune responses, and injury of cholangiocytes may cause a variety of diseases and even lead to liver failure[76]. Therefore, we can preliminarily conclude that the abnormal liver function in patients with COVID-19 may not be directly caused by the damage to hepatocytes, but it may be caused by damage to cholangiocytes. Liver damages may also be caused by other factors, such as drugs used in the treatment or systemic inflammatory response.
Compared with control mice, ACE2 knockout mice are more susceptible to induced colitis upon treatment with chemical irritants[77]. The expression of ACE2 protein is downregulated after virus entry, which may worsen the digestive symptoms. Recently, studies have demonstrated that the fusion protein of human recombinant ACE2 (hrACE2) with the Fc fragment of the human immunoglobulin IgG1 showed high affinity to the receptor-binding domain (RBD) of SARS-CoV-2 and potently neutralized SARS-CoV-2 in vitro[78,79]. A newly online published report in Nature demonstrated that CB6, a neutralizing monoclonal antibody (mAb) isolated from a patient with convalescent COVID-19, can block the binding of soluble SARS-CoV-2 RBD with ACE2 receptor and showed inhibitory effect to SARS-CoV-2 infection in vitro and in rhesus monkeys[80]. Additionally, a recent in vivo study has reported that hrACE2 can block the early stages of SARS-CoV-2 infection at the organoid level in engineered human blood vessels, kidneys, and small intestinal enterocytes[78,81]. Moreover, a serine protease TMPRSS2 inhibitor has been approved for clinical use to block the entry of CoVs[69]. Taken together, it is therefore likely that ACE2 and TMPRSS2 may be the potential targets for prevention and treatment of the COVID-19 patients with digestive symptoms.
Immune injury plays an important role in the occurrence, development, and prognosis of digestive diseases[82-84]. A growing body of evidence suggests that severe SARS-CoV-2 infection can activate innate and adaptive immune responses, increase serum levels of pro-inflammatory cytokines and chemokines including interleukin (IL)-6, IL-1β, IL-2, IL-10, tumor necrosis factor α (TNF-α), interferon-gamma-inducible protein-10 (CXCL10), monocyte chemoattractant protein-1, and granulocyte-macrophage colony stimulating factor, and even induce systemic inflammatory response syndrome (SIRS) and cytokine storm, thus leading to local and systemic tissue damage[40,85,86]. Concentrations of IL-6 were significantly different between patients with mild and severe COVID-19, and elevated IL-6 was found to be a stable indicator of adverse outcomes for severe COVID-19 patients. A clinical trial (ChiCTR2000029765) that explored the potential therapeutic effect of IL-6 receptor-targeted mAb in patients with severe COVID-19, has shown the effectiveness of controlling fever and improving respiratory function quickly[87]. It has been reported that vagus nerve stimulation (VNS) can exert anti-inflammatory effects via activation of the cholinergic anti-inflammatory pathway[88,89]. Based on preliminary observations and available scientific and clinical data, it is speculated that VNS may play a vital role in improving the prognosis of patients with COVID-19[90]. Additionally, patients with severe COVID-19 more frequently have lymphopenia, and drastically decreased numbers of CD4+ and CD8+ T cells than moderate cases. Furthermore, inflammation-mediated gastrointestinal tissue damage in patients with COVID-19 is supported by the histopathological evidence of diffuse endothelial inflammation in the small intestine submucosa[91]. It has also been confirmed by the presence of numerous infiltrating plasma cells and lymphocytes with interstitial edema in the lamina propria of the stomach, duodenum, and rectum of patients with COVID-19[10].
The human intestine harbors nearly 100 trillion microorganisms, which are composed of more than 1000 different bacterial species, including but not limited to bacteria, fungi, and viruses[92,93]. It is known that gut microbiota plays a vital role in a variety of diseases, including digestive, metabolic, respiratory, and even neuropsychiatric diseases[94-96]. Additionally, many studies have reported that chronic respiratory diseases and pneumonia can not only change the airway microbiota, but also alter the gut microbiota indirectly through the circulatory and lymphatic systems[97-100]. SARS-CoV-2 can down-regulate the expression of ACE2 in the intestine, which affects the absorption of tryptophan, resulting in damage to the gut microbiota and possibly influencing intestinal inflammation[101,102]. Xu et al[103] indicated that some patients with COVID-19 showed gut microbial dysbiosis with decreased abundance of Lactobacillus and Bifidobacterium[103]. Zuo et al[104] showed that the increased levels of Coprobacillus, Clostridium hathewayi, and Clostridium ramosum were positively correlated with the susceptibility and severity of COVID-19[104]. Furthermore, studies have shown that short-chain fatty acids, the most critical metabolites of the gut microbiota, play an important role in reducing the intestinal pH, maintaining the integrity of the intestinal epithelium, and enhancing the host systemic immunity[105-107]. Antibiotics are commonly prescribed in patients with COVID-19, however, it can also profoundly perturb the composition of the human gut microbiota[108]. Guidance from China’s National Health Commission and National Administration of Traditional Chinese Medicine suggested that the use of probiotics has a good curative effect in patients with severe SARS-CoV-2 infection. Taken together, improvement of the composition of the gut microbiota and its metabolites may be a potential strategy for the treatment of COVID-19.
After SARS-CoV-2 infects the lungs, it causes inflammation and edema of the pulmonary parenchyma and interstitium, which in turn affect alveolar gas exchange and thereby lead to hypoxemia[109]. Hypoxemia can cause metabolism and normal physiological dysfunctions in various tissues and organs including the digestive system. Long-term hypoxemia can cause cell necrosis, which in turn leads to damage of the gastrointestinal mucosal cells, thus leading to gastrointestinal ulceration and bleeding.
Psychological stress has a profound influence on the digestive system, it can alter the intestinal motility, increase the gastrointestinal permeability, and change the composition of intestinal microbiota[110]. A study on active Weibo users showed that individuals were more concerned about their health and showed more negative emotions, such as anxiety and depression, after the outbreak of the COVID-19[111]. Quarantine is an effective measure to protect individuals from contagious patients and those who are at risk of infection, however, it may also increase negative psychological stress[112]. Taken together, digestive diseases and symptoms in patients with COVID-19 may be partially caused by psychological stress.
Multiple lines of evidence has shown that some patients with COVID-19 have digestive system symptoms, such as nausea, vomiting, and anorexia, which indirectly reflect the impairment of the dorsal vagal complex[6,113,114]. One of the possible mechanisms is that the neurotropic virus retrogrades along the vagus nerve once the virus enters the vagal nerve endings, thus damaging the brainstem[115]. Moreover, the virus may reach the brainstem through the circulatory system with or without crossing the blood–brain barrier[113].
CoV infections can cause a series of digestive diseases, and may also be accompanied by digestive manifestations and abnormal digestive function. Although it is still unknown whether the SARS-CoV-2, which causes abnormalities in the digestive system, enters the digestive system directly or indirectly affects the digestive system, it is necessary to undertake early measures to prevent the spread of the virus through the fecal-oral transmission. In addition to ACE2 and immune injury, gut microbiota, hypoxemia, and psychological stress may also be targets for future intervention and treatment of digestive system damage caused by SARS-CoV-2 infection. Further research is warranted to elucidate the relationship between the COVID-19 and the digestive system.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dogrul AB, Galloro G S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | World Health Organization. WHO Coronavirus Disease (COVID-19) situation Dashboard. 2020 [cited 9 December 2020]. Available from: https://www.who.int/. |
| 2. | Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1365] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 3. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12970] [Article Influence: 2594.0] [Reference Citation Analysis (1)] |
| 4. | Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 781] [Cited by in RCA: 787] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 5. | Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 683] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 6. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14762] [Article Influence: 2952.4] [Reference Citation Analysis (0)] |
| 7. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18867] [Article Influence: 3773.4] [Reference Citation Analysis (7)] |
| 8. | Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 992] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 9. | Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB. Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child, China. Emerg Infect Dis. 2020;26:1337-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 10. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1993] [Article Influence: 398.6] [Reference Citation Analysis (1)] |
| 11. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1204] [Article Influence: 240.8] [Reference Citation Analysis (0)] |
| 12. | Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1184] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 13. | Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 524] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 14. | Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1277] [Cited by in RCA: 1183] [Article Influence: 197.2] [Reference Citation Analysis (0)] |
| 15. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1219] [Cited by in RCA: 1255] [Article Influence: 251.0] [Reference Citation Analysis (0)] |
| 16. | Tang Q, Song Y, Shi M, Cheng Y, Zhang W, Xia XQ. Inferring the hosts of coronavirus using dual statistical models based on nucleotide composition. Sci Rep. 2015;5:17155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3:237-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2002] [Cited by in RCA: 1840] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 18. | Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J, Quan L, Xia Z, Tan W, Cheng G, Jiang T. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27:325-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1413] [Cited by in RCA: 1500] [Article Influence: 300.0] [Reference Citation Analysis (0)] |
| 19. | Singh A, Shaikh A, Singh R, Singh AK. COVID-19: From bench to bed side. Diabetes Metab Syndr. 2020;14:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available from: https://www.who.int/csr/sars/. |
| 21. | Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, Liang W, Zheng H, Wan K, Liu Q, Cui B, Xu Y, Zhang E, Wang H, Ye J, Li G, Li M, Cui Z, Qi X, Chen K, Du L, Gao K, Zhao YT, Zou XZ, Feng YJ, Gao YF, Hai R, Yu D, Guan Y, Xu J. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J Virol. 2005;79:11892-11900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2097] [Cited by in RCA: 2153] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 23. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 24. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 25. | Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF, Kong J, Yam LY, Seto WH, Yuen KY, Peiris JS. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH, Chu CM, Wong MM, Hui WT, Poon LL, Tse DM, Chan KS, Woo PC, Lau SK, Peiris JS, Yuen KY. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10:1550-1557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available from: https://www.who.int/emergencies/mers-cov/. |
| 28. | Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 523] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 29. | Chowell G, Abdirizak F, Lee S, Lee J, Jung E, Nishiura H, Viboud C. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 30. | Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 31. | Poon LL, Guan Y, Nicholls JM, Yuen KY, Peiris JS. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect Dis. 2004;4:663-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, Almasri M, Muth D, Sieberg A, Meyer B, Assiri AM, Binger T, Steinhagen K, Lattwein E, Al-Tawfiq J, Müller MA, Drosten C, Memish ZA. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin Infect Dis. 2016;62:477-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 33. | Zhou J, Li C, Zhao G, Chu H, Wang D, Yan HH, Poon VK, Wen L, Wong BH, Zhao X, Chiu MC, Yang D, Wang Y, Au-Yeung RKH, Chan IH, Sun S, Chan JF, To KK, Memish ZA, Corman VM, Drosten C, Hung IF, Zhou Y, Leung SY, Yuen KY. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3:eaao4966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 34. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14118] [Article Influence: 2823.6] [Reference Citation Analysis (1)] |
| 35. | Ji W, Wang W, Zhao X, Zai J, Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92:433-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 533] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 36. | Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 37. | Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020;94:44-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 699] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 38. | Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, Faden HS, Tang Z, Shi M, Jiao N, Li Y, Cheng S, Huang Y, Wu D, Xu Z, Pan L, Zhu J, Yan G, Zhu R, Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 869] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 40. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30098] [Article Influence: 6019.6] [Reference Citation Analysis (3)] |
| 41. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3820] [Article Influence: 764.0] [Reference Citation Analysis (1)] |
| 42. | Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1052] [Cited by in RCA: 1022] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 43. | To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM, Leung WS, Chik TS, Choi CY, Kandamby DH, Lung DC, Tam AR, Poon RW, Fung AY, Hung IF, Cheng VC, Chan JF, Yuen KY. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020;71:841-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1052] [Cited by in RCA: 1119] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 44. | Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 45. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 46. | Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 47. | World Health Organization. Hepatitis. Available from: https://www.who.int/health-topics/hepatitis. |
| 48. | Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 49. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 50. | Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 51. | Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (1)] |
| 52. | Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl). 2011;218:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 53. | Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 54. | Xiao Y, Pan H, She Q, Wang F, Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:528-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3:977-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Tzedakis S, Jeddou H, Houssel-Debry P, Sulpice L, Boudjema K. COVID-19: Thoughts and comments from a tertiary liver transplant center in France. Am J Transplant. 2020;20:1952-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1354] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 58. | Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 747] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 59. | Kucharzik T, Maaser C. Infections and Chronic Inflammatory Bowel Disease. Viszeralmedizin. 2014;30:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | An P, Ji M, Ren H, Su J, Kang J, Yin A, Zhou Q, Shen L, Zhao L, Jiang X, Xiao Y, Tan W, Lv X, Li J, Liu S, Zhou J, Chen H, Xu Y, Liu J, Chen M, Cao J, Zhou Z, Shen L, Tan S, Yu H, Dong W, Ding Y. Protection of 318 inflammatory bowel disease patients from the outbreak and rapid spread of COVID-19 infection in Wuhan, China. Soc Sci Elec Publishing. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 61. | Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH; Chinese Society of IBD; Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 62. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3332] [Cited by in RCA: 3123] [Article Influence: 624.6] [Reference Citation Analysis (0)] |
| 63. | Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, Liu X, Xu K, Ho KF, Kan H, Fu Q, Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1138] [Article Influence: 227.6] [Reference Citation Analysis (0)] |
| 64. | Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 621] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 65. | Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin Auton Res. 2019;29:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 66. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2710] [Cited by in RCA: 2645] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 67. | Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5792] [Cited by in RCA: 6473] [Article Influence: 1294.6] [Reference Citation Analysis (0)] |
| 68. | Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang L, Duan Y, Zhang S, Chen W, Zhen W, Cai M, Penninger JM, Jiang C, Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 69. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14257] [Article Influence: 2851.4] [Reference Citation Analysis (0)] |
| 70. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;gutjnl-2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 71. | Van Pelt J, Van Kuik JA, Kamerling JP, Vliegenthart JF, Van Diggelen OP, Galjaard H. Storage of sialic acid-containing carbohydrates in the placenta of a human galactosialidosis fetus. Isolation and structural characterization of 16 sialyloligosaccharides. Eur J Biochem. 1988;177:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1429] [Cited by in RCA: 1182] [Article Influence: 147.8] [Reference Citation Analysis (1)] |
| 73. | Desmarets LMB, Theuns S, Roukaerts IDM, Acar DD, Nauwynck HJ. Role of sialic acids in feline enteric coronavirus infections. J Gen Virol. 2014;95:1911-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 427] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 75. | Seow J, Pai R, Mishra A, Shepherdson E, Lim T, Goh B, Chan J, Chow P, Ginhoux F, DasGupta R, Sharma A. scRNA-seq reveals ACE2 and TMPRSS2 expression in TROP2+ Liver Progenitor Cells: Implications in COVID-19 associated Liver Dysfunction. bioRxiv. 2020;. [DOI] [Full Text] |
| 76. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (1)] |
| 77. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 985] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 78. | Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020; 181: 905-913. e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1660] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 79. | Lei C, Qian K, Li T, Zhang S, Fu W, Ding M, Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun. 2020;11:2070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 80. | Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, Gao G, Hu X, Zhang Y, Tong Z, Huang W, Liu WJ, Wu G, Zhang B, Wang L, Qi J, Feng H, Wang FS, Wang Q, Gao GF, Yuan Z, Yan J. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1045] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 81. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1310] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 82. | Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 83. | Zhou JA, Jiang M, Yang X, Liu Y, Guo J, Zheng J, Qu Y, Song Y, Li R, Qin X, Wang X. Unconjugated bilirubin ameliorates the inflammation and digestive protease increase in TNBS-induced colitis. Mol Med Rep. 2017;16:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Lohse AW, Weiler-Normann C, Tiegs G. Immune-mediated liver injury. J Hepatol. 2010;52:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3336] [Article Influence: 667.2] [Reference Citation Analysis (0)] |
| 86. | Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 87. | Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1118] [Article Influence: 223.6] [Reference Citation Analysis (0)] |
| 88. | Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1134] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 89. | Tracey KJ. The inflammatory reflex. Nature. 2002;420:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2484] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 90. | Staats P, Giannakopoulos G, Blake J, Liebler E, Levy RM. The Use of Non-invasive Vagus Nerve Stimulation to Treat Respiratory Symptoms Associated With COVID-19: A Theoretical Hypothesis and Early Clinical Experience. Neuromodulation. 2020;23:784-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 91. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4576] [Article Influence: 915.2] [Reference Citation Analysis (0)] |
| 92. | Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2328] [Cited by in RCA: 2917] [Article Influence: 324.1] [Reference Citation Analysis (0)] |
| 94. | Selber-Hnatiw S, Rukundo B, Ahmadi M, Akoubi H, Al-Bizri H, Aliu AF, Ambeaghen TU, Avetisyan L, Bahar I, Baird A, Begum F, Ben Soussan H, Blondeau-Éthier V, Bordaries R, Bramwell H, Briggs A, Bui R, Carnevale M, Chancharoen M, Chevassus T, Choi JH, Coulombe K, Couvrette F, D'Abreau S, Davies M, Desbiens MP, Di Maulo T, Di Paolo SA, Do Ponte S, Dos Santos Ribeiro P, Dubuc-Kanary LA, Duncan PK, Dupuis F, El-Nounou S, Eyangos CN, Ferguson NK, Flores-Chinchilla NR, Fotakis T, Gado Oumarou H D M, Georgiev M, Ghiassy S, Glibetic N, Grégoire Bouchard J, Hassan T, Huseen I, Ibuna Quilatan MF, Iozzo T, Islam S, Jaunky DB, Jeyasegaram A, Johnston MA, Kahler MR, Kaler K, Kamani C, Karimian Rad H, Konidis E, Konieczny F, Kurianowicz S, Lamothe P, Legros K, Leroux S, Li J, Lozano Rodriguez ME, Luponio-Yoffe S, Maalouf Y, Mantha J, McCormick M, Mondragon P, Narayana T, Neretin E, Nguyen TTT, Niu I, Nkemazem RB, O'Donovan M, Oueis M, Paquette S, Patel N, Pecsi E, Peters J, Pettorelli A, Poirier C, Pompa VR, Rajen H, Ralph RO, Rosales-Vasquez J, Rubinshtein D, Sakr S, Sebai MS, Serravalle L, Sidibe F, Sinnathurai A, Soho D, Sundarakrishnan A, Svistkova V, Ugbeye TE, Vasconcelos MS, Vincelli M, Voitovich O, Vrabel P, Wang L, Wasfi M, Zha CY, Gamberi C. Human Gut Microbiota: Toward an Ecology of Disease. Front Microbiol. 2017;8:1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 95. | Schirmer M, Franzosa EA, Lloyd-Price J, McIver LJ, Schwager R, Poon TW, Ananthakrishnan AN, Andrews E, Barron G, Lake K, Prasad M, Sauk J, Stevens B, Wilson RG, Braun J, Denson LA, Kugathasan S, McGovern DPB, Vlamakis H, Xavier RJ, Huttenhower C. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 367] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 96. | Jiang C, Li G, Huang P, Liu Z, Zhao B. The Gut Microbiota and Alzheimer's Disease. J Alzheimers Dis. 2017;58:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 589] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 97. | Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 98. | Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 365] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 99. | Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome. 2018;6:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 100. | Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 1008] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 101. | Ma C, Cong Y, Zhang H. COVID-19 and the Digestive System. Am J Gastroenterol. 2020;115:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 102. | Perlot T, Penninger JM. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 103. | Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 183] [Reference Citation Analysis (0)] |
| 104. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020; 159: 944-955. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1062] [Article Influence: 212.4] [Reference Citation Analysis (0)] |
| 105. | Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. 2015;9:343-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 106. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1688] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 107. | Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 108. | Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1738] [Article Influence: 102.2] [Reference Citation Analysis (1)] |
| 109. | Duan J, Wu Y, Liu C, Yang C, Yang L. Deleterious effects of viral pneumonia on cardiovascular system. Eur Heart J. 2020;41:1833-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591-599. [PubMed] |
| 111. | Li S, Wang Y, Xue J, Zhao N, Zhu T. The Impact of COVID-19 Epidemic Declaration on Psychological Consequences: A Study on Active Weibo Users. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 824] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 112. | Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9682] [Cited by in RCA: 8081] [Article Influence: 1616.2] [Reference Citation Analysis (1)] |
| 113. | Chigr F, Merzouki M, Najimi M. Autonomic Brain Centers and Pathophysiology of COVID-19. ACS Chem Neurosci. 2020;11:1520-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 114. | Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, Kim D, Hsing A, Ahmed A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159:775-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 115. | Tassorelli C, Mojoli F, Baldanti F, Bruno R, Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur J Neurol. 2020;27:e41-e42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 116. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 117. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2341] [Article Influence: 468.2] [Reference Citation Analysis (0)] |
| 118. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 119. | Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 120. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 121. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3416] [Article Influence: 683.2] [Reference Citation Analysis (0)] |
| 122. | Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |