Published online Feb 14, 2021. doi: 10.3748/wjg.v27.i6.487
Peer-review started: December 6, 2020
First decision: December 17, 2020
Revised: December 21, 2020
Accepted: January 7, 2021
Article in press: January 7, 2021
Published online: February 14, 2021
Processing time: 60 Days and 21.3 Hours
Gastric cancer (GC) is a prevalent malignancy, leading to a high incidence of cancer-associated death. Cisplatin (DDP)-based chemotherapy is the principal therapy for clinical GC treatment, but DDP resistance is a severe clinical challenge and the mechanism remains poorly understood. Circular RNAs (circRNAs) have been identified to play crucial roles in modulating the chemoresistance of gastric cancer cells.
To explore the effect of circVAPA on chemotherapy resistance during GC progression.
The effect of circVAPA on GC progression and chemotherapy resistance was analyzed by MTT assay, colony formation assay, Transwell assay, wound healing assay, and flow cytometry analysis in GC cells and DDP resistant GC cell lines, and tumorigenicity analysis in nude mice in vivo. The mechanism was investigated by luciferase reporter assay, quantitative real-time PCR, and Western blot analysis.
CircVAPA expression was up-regulated in clinical GC tissues compared with normal samples. CircVAPA depletion inhibited proliferation, migration, and invasion and increased apoptosis of GC cells. The expression of circVAPA, STAT3, and STAT3 downstream genes was elevated in DDP resistant SGC7901/DDP cell lines. CircVAPA knockdown attenuated the DDP resistance of GC cells. Mechanically, circVAPA was able to sponge miR-125b-5p, and miR-125b-5p could target STAT3 in the GC cells. MiR-125b-5p inhibitor reversed circVAPA depletion-enhanced inhibitory effect of DDP on GC cells, and STAT3 knockdown blocked circVAPA overexpression-induced proliferation of DDP-treated SGC7901/DDP cells. The depletion of STAT3 and miR-125b-5p inhibitor reversed circVAPA depletion-induced GC cell apoptosis. Functionally, circVAPA contributed to the tumor growth of SGC7901/DDP cells in vivo.
CircVAPA promotes chemotherapy resistance and malignant progression in GC by miR-125b-5p/STAT3 signaling. Our findings present novel insights into the mechanism by which circVAPA regulates chemotherapy resistance of GC cells. CircVAPA and miR-125b-5p may be considered as the potential targets for GC therapy.
Core Tip: We concluded that circVAPA contributed to chemotherapy resistance and malignant progression in gastric cancer (GC) by miR-125b-5p/STAT3 signaling. Our findings present novel insights into the mechanism that circVAPA regulates chemotherapy resistance of GC cells. CircVAPA and miR-125b-5p may be considered as potential targets for GC therapy.
- Citation: Deng P, Sun M, Zhao WY, Hou B, Li K, Zhang T, Gu F. Circular RNA circVAPA promotes chemotherapy drug resistance in gastric cancer progression by regulating miR-125b-5p/STAT3 axis. World J Gastroenterol 2021; 27(6): 487-500
- URL: https://www.wjgnet.com/1007-9327/full/v27/i6/487.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i6.487
Gastric cancer (GC) is a prevalent malignancy and the second cause of tumor-associated mortality in the world[1]. Chemotherapy is a favored strategy toward advanced-stage GC cases, in which oxaliplatin, cisplatin (DDP), and 5-FU are usually admitted as first-line therapies[2-4]. Although the improved chemotherapy effectiveness, the GC patients' survival is still unsatisfactory, and chemotherapy always attains a low response rate[5,6]. Whether intrinsic or acquired, chemoresistance is a significant challenge for GC treatment efficiency in the clinic[7]. The mechanism for GC chemotherapy resistance development is not well-understood, in which microenvironmental and genetic contributors are able to result in chemoresistance[8]. Hence, understanding the molecular mechanism of chemotherapy resistance in GC is urgently needed.
Circular RNAs (circRNAs), as an emerging class of non-coding RNAs, present various functions in the regulation of gene expression at the post-transcriptional and transcriptional levels[9]. It has been identified that circRNAs are able to serve as the competitive endogenous sponge for microRNAs (miRNAs) and thereby inhibit miRNAs, providing a new manner for modulating miRNAs and indicating a promising mechanism of circRNA function[10,11]. Meanwhile, previous studies have reported that circVAPA is elevated in tumors and contributes to cancer progression, such as liver cancer and breast cancer[12,13]. MiRNAs are short non-coding RNAs with a length of about 22nt and can target various genes by pairing with the mRNAs at the post-transcriptional level[14,15]. MiR-125b-5p is a well-investigated tumor suppressor in multiple cancer models, including bladder cancer, laryngeal squamous cell carcinoma, and colon cancer[16-18]. Furthermore, it has been well-identified that circRNAs and miRNAs are involved in the chemotherapy resistance in GC[8,19-21]. However, the role of circVAPA and miR-125b-5p in modulating chemotherapy resistance of GC cells is still unreported.
It has been recognized that STAT3 serves as a critical regulator in tumorigenesis[22]. As an important transcriptional factor, STAT3 is able to mediate multiple gene expression in modulating cell survival response to different stimulations[23]. Furthermore, STAT3 remarkably promotes chemoresistance as a crucial indicator in GC cells by various mechanisms, such as increasing the expression of vacuolar ATPase pump and oncogenes as well as the apoptotic factors[24]. Nevertheless, the impact of circVAPA and miR-125b-5p on STAT3 in the regulation of GC cells chemotherapy resistance is still unclear.
In this study, we were interested in the function of circVAPA in the progression of chemotherapy resistance of GC cells. We found an unreported role of circVAPA in enhancing chemotherapy resistance and promoting the malignant progression of GC by modulating miR-125b-5p/STAT3 signaling.
The clinical GC tissue samples (n = 50) and adjacent normal tissues (n = 50) were obtained from the First Affiliated hospital of China Medical University. The diagnosis of cases was performed by two clinicians based on histopathological analysis. The samples were collected and immediately stored at -80 °C followed by further analysis. The samples were written-approved by the patients. This study conformed to the experimental guidelines of the World Medical Association and the Ethics Committee of the First Affiliated Hospital of China Medical University.
The SGC7901, HGC27, and SGC7901/DDP cell lines were maintained in the laboratory. The cells were incubated in an incubator at 5% CO2 and 37 °C in the Dulbecco's modified Eagle’s medium (DMEM) medium (Hyclone, United States) with fetal bovine serum (10%, Hyclone, United States), streptomycin (0.1 mg/mL, Hyclone, United States), and penicillin (100 units/mL, Hyclone, United States). DDP was obtained from Sigma (United States). The circVAPA shRNA, pcDNA3.1-circVAPA, pcDNA3.1-STAT3, STAT3 siRNA, miR-125b-5p mimic, and miR-125b-5p inhibitor were purchased from GenePharma (China). The transfection in the cells was performed with Liposome 2000 (Invitrogen, United States).
The cell viability was assessed by MTT assays at various time points in 6-well dishes. Briefly, the MTT solution (Solarbio, China) was added into the cells and cultured at the condition of 5% CO2 and 37 °C for 4 h. After that, dimethyl sulfoxide (DMSO; 100 μL, 10 min; Sigma, United States) was used to terminate the reaction. The cell viability was analyzed at the absorbance of 490 nm by applying a microplate reader (Thermo, United States).
About 1 × 103 cells were plated in 6-well dishes and cultured in DMEM at the condition of 5% CO2 and 37 °C. After 2 wk, cells were washed with phosphate buffered saline (PBS) buffer, made in methanol about 30 min, and dyed with 1% crystal violet dye solution, after which the number of colonies was calculated.
Transwell assays were performed to analyze cell invasion and migration using a Transwell plate (Corning, United States) according to the manufacturer's instructions. Briefly, the upper chamber was plated with around 1 × 105 cells. The cells were then fixed with 4% paraformaldehyde and dyed with crystal violet. The invaded and migrated cells were recorded and calculated.
Cells were plated in a 24-well plate at 3 × 105/well and cultured overnight to reach full confluence as a monolayer. A 20 μL pipette tip was applied to slowly cut a straight line across the well. Then the well was washed with PBS three times and changed with the serum-free medium and continued to culture. The wound healing percentage was calculated.
Cell apoptosis was measured by applying the Annexin-V-FITC apoptosis kit (BD, United States) based on flow cytometry analysis using a FACSCalibur flow cytometer, followed by the quantification analysis using FlowJo software.
SGC7901 and HGC27 cells were plated in 24-well dishes followed by treatment with miR-125b-5p mimic. After 48 h of the treatment, the luciferase activities were analyzed by utilizing the luciferase reporter assays (Promega, United States). The relative luciferase activities were analyzed by the Renilla luciferase activities.
RNA isolation was performed by applying TRIzol reagent (Solarbio, China) and the first-strand cDNA was synthesized (Solarbio, China). Quantitative real-time PCR was carried out by applying SYBR-Green (Takara, China). The primer sequences are as follows: circVAPA forward: 5′-TGGATTCCAAATTGAGATGCGTATT-3′, reverse: 5′-CACTTTTCTATCCGATGGATTTCGC-3′; miR-125b-5p forward: 5′-TCCCTGAGACCCTAACTTGTGA-3′, reverse: 5′- AGTCTCAGGGTCCGAGGTATTC-3′; STAT3 forward: 5′-CTGGCCTTTGGTGTTGAAAT-3′, reverse: 5′-AAGGCACCCACAGAAACAAC-3′; GAPDH forward: 5′-AAGAAGGTGGTGAAGCAGGC-3′, reverse: 5′-TCCACCACCCAGTTGCTGTA-3′.
Total proteins were extracted from the cells using radioimmunoprecipitation assay buffer (CST, United States) and quantified using the BCA Protein Quantification Kit (Abbkine, United States). The proteins at the same concentration were subjected to sodium dodecyl sulfate- polyacrylam-ide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, United States), followed by incubation with 5% milk and with the primary antibodies at 4 °C overnight. The corresponding second antibodies (BOSTER, China) were used for incubating the membranes for 1 h at room temperature, followed by visualization using a chemiluminescence detection kit (Beyyotime, China). The primary antibodies applied in this study comprised STAT3 (Abcam, United States), p-STAT3 (Abcam, United States), Survivin (Abcam, United States), Mcl-1 (Abcam, United States), Bcl-xl (Abcam, United States), and β-actin (Abcam, United States).
The tumor growth of GC cells in vivo was tested in nude Balb/c mice (4-week-old, male, n = 5). And about 1 × 107 SGC7901/DDP cells were subcutaneously injected in the mice. Five days after injection, we measured tumor growth every 5 d. We sacrificed the mice 30 d after injection, and tumors were scaled. Tumor volume (V) was observed by estimating the length (L) and width (W) with calipers and calculated as (L × W2) × 0.5. Animal care and method procedure were authorized by the Animal Ethics Committee of the First Affiliated Hospital of China Medical University.
Data are presented as the mean ± SD, and statistical analyses were performed with GraphPad Prism 7. The unpaired Student’s t-test was applied for comparing the difference between two groups, and one-way ANOVA was applied for comparing among multiple groups. aP < 0.05 were considered statistically significant.
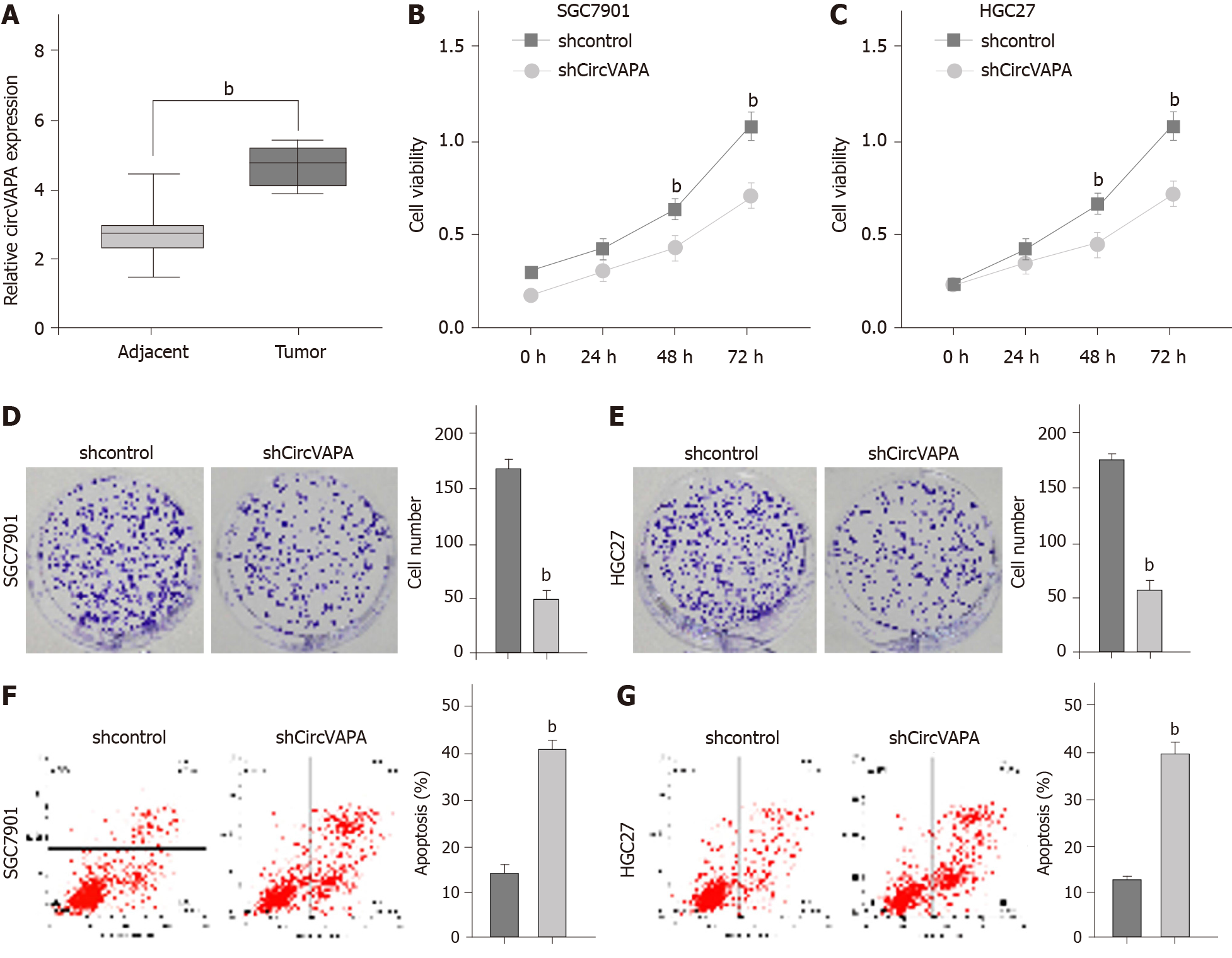
To identify the clinical association of circVAPA with GC, we analyzed the expression of circVAPA in the clinical GC samples. Our data showed that circVAPA expression was elevated in the GC tissue samples (n = 50) relative to that in the adjacent normal tissues (n = 50), implying the potential correlation of circVAPA with GC (Figure 1A). The cell viability was reduced by the depletion of circVAPA in the SGC7901 and HGC27 cell lines (Figure 1B and C). Similarly, colony formation assays revealed that circVAPA shRNA treatment remarkably decreased the colony numbers of SGC7901 and HGC27 cells (Figure 1D and E). Furthermore, apoptosis of SGC7901 and HGC27 cells was induced by circVAPA knockdown (Figure 1F and G). These findings indicated that circVAPA is able to increase proliferation and attenuate apoptosis of GC cells
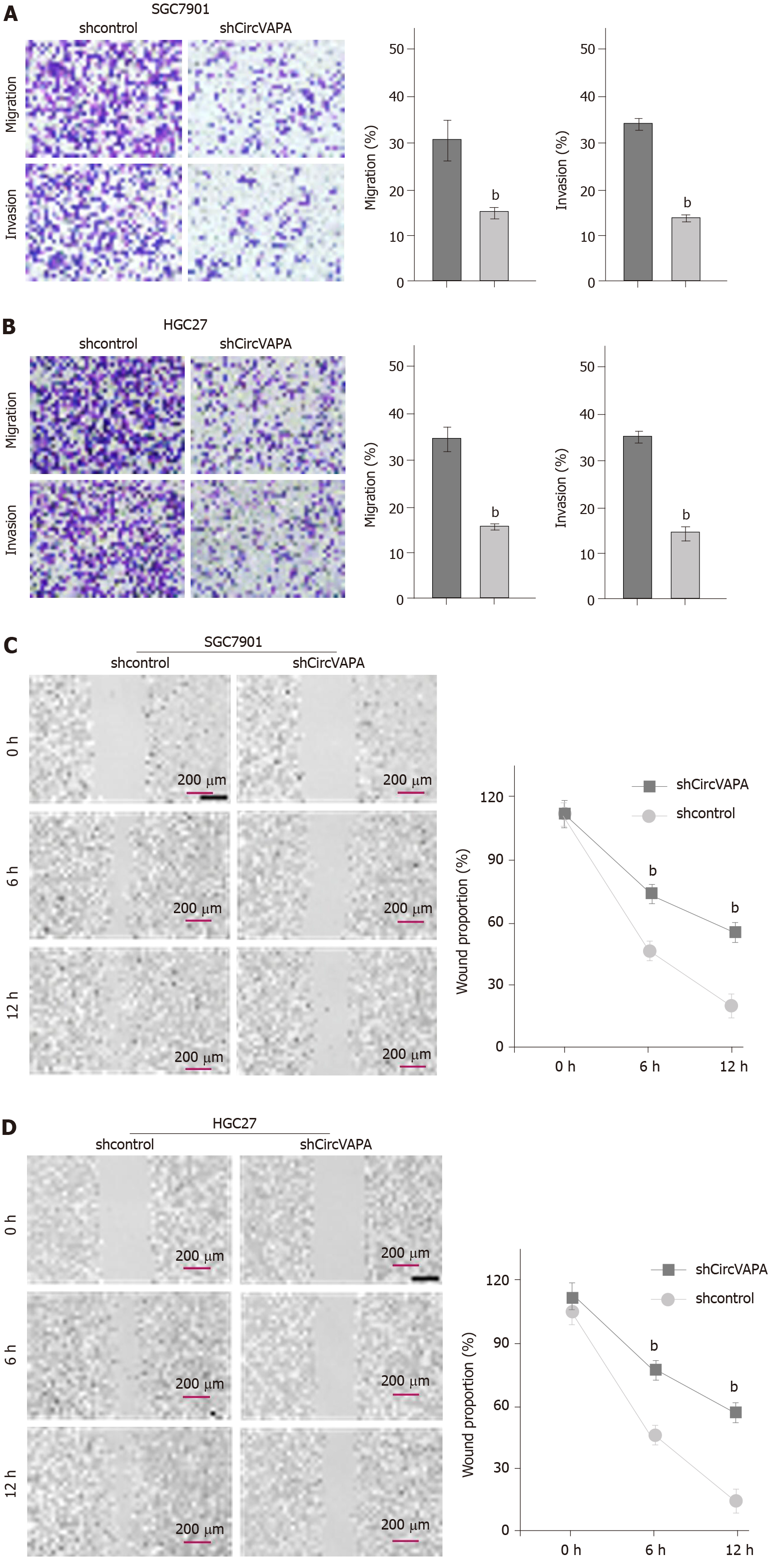
Next, the function of circVAPA in modulating migration and invasion of GC cells was assessed. Transwell assays demonstrated that migration and invasion of SGC7901 and HGC27 cells were alleviated by circVAPA shRNA (Figure 2A and B). Besides, wound healing assays showed that circVAPA depletion remarkably increased wound proportion in SGC7901 and HGC27 cell lines (Figure 2C and D). These data indicated that circVAPA contributes to migration and invasion of GC cells.
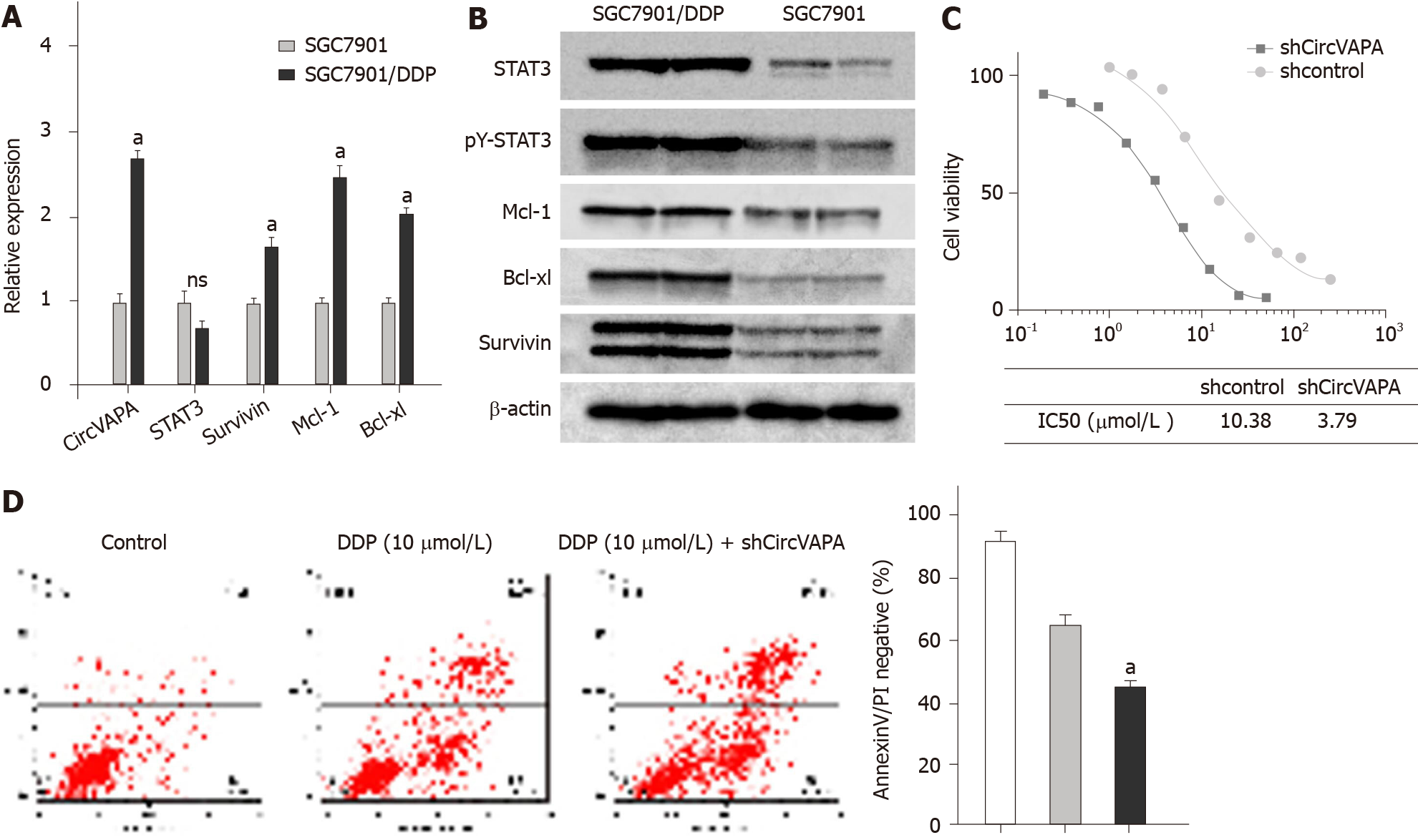
Next, we were interested in the impact of circVAPA on the chemoresistance of GC cells. To this end, we first explored the expression of circVAPA in SGC7901 and DDP resistant SGC7901/DDP cell lines. Significantly, the expression of circVAPA was up-regulated in SGC7901/DDP cells compared with that in SGC7901 cells (Figure 3A). Meanwhile, STAT3 phosphorylation, STAT3 expression, and STAT3 downstream gene (including Survivin, Mcl-1, and Bcl-xl) expression were elevated in the SGC7901/DDP cells relative to those in SGC7901 cells (Figure 3A and B). Importantly, depletion of circVAPA remarkably reduced the IC50 value of DDP for the inhibition of SGC7901/DDP cells (Figure 3C). In addition, DDP was able to induce apoptosis of SGC7901/DDP cells, and circVAPA depletion notably reinforced this effect in the system (Figure 3D). Together, these findings suggested that circVAPA contributes to chemotherapy drug resistance of GC cells.
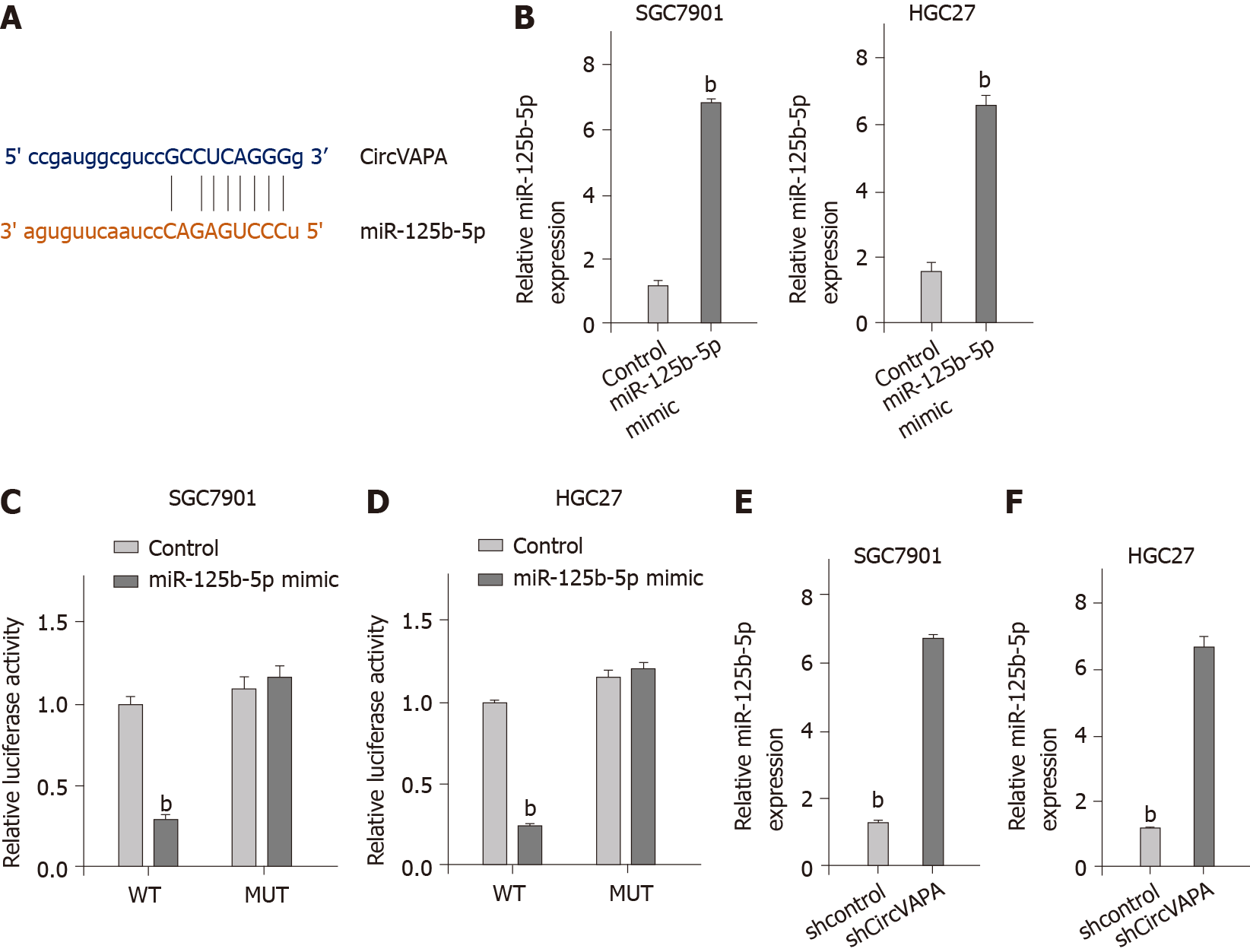
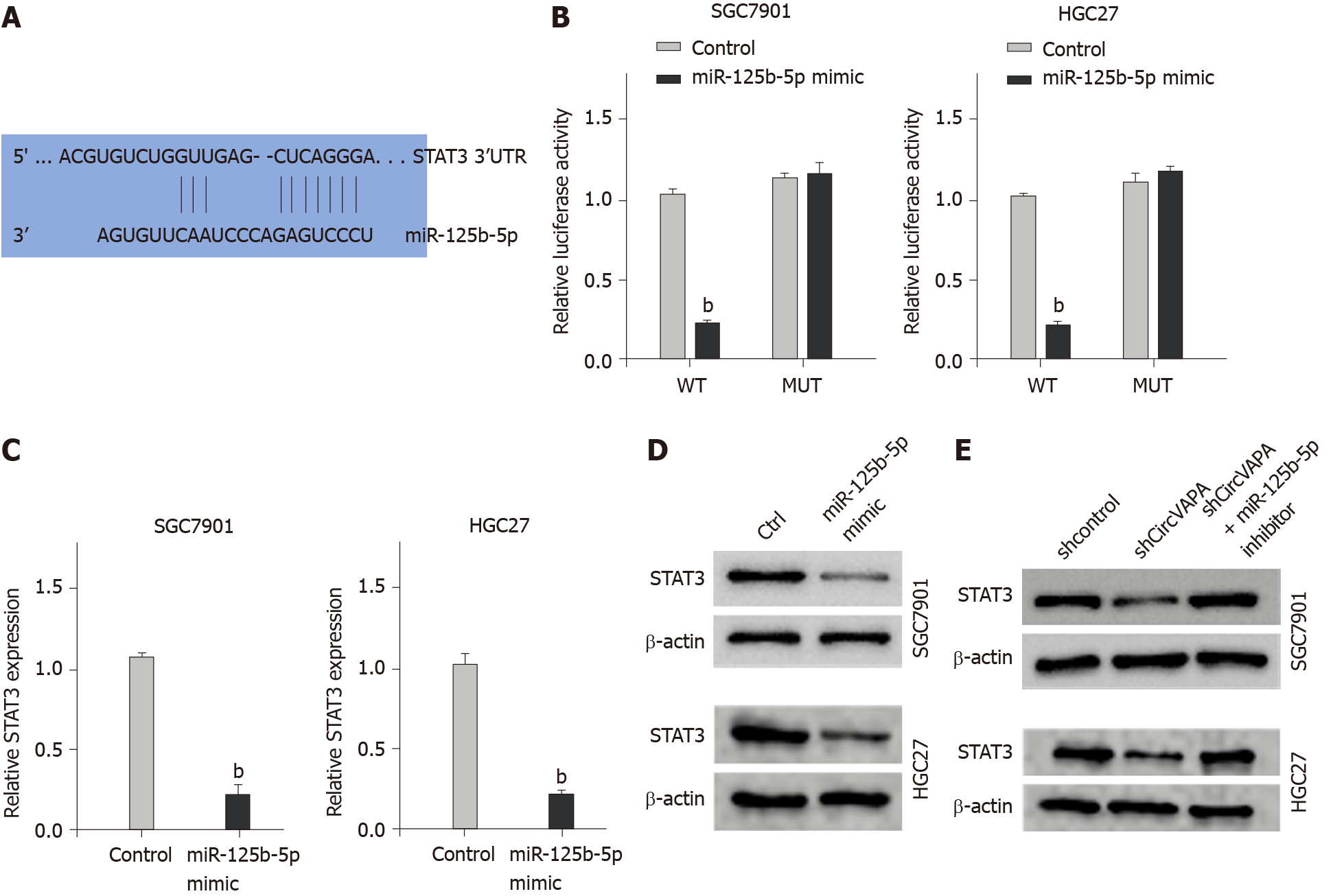
Next, we explored the mechanism of circVAPA-mediated GC progression and chemotherapy resistance. The bioinformatic analysis revealed the potential interaction of circVAPA and miR-125b-5p (Figure 4A). The SGC7901 and HGC27 cells were treated with miR-125b-5p mimic and the enhancement of miR-125b-5p was confirmed in the cells (Figure 4B). Treatment with miR-125b-5p mimic was able to decrease luciferase activities of wild type circVAPA, but not circVAPA mutant in the cells (Figure 4C and D). Moreover, the expression of miR-125b-5p was up-regulated by circVAPA shRNA in the SGC7901 and HGC27 cells. These findings indicated that circVAPA spongs miR-125b-5p in the GC cells (Figure 4E and F).
Furthermore, we identified the binding site of miR-125b-5p within the 3’ untranslated region of the STAT3 gene through bioinformatic analysis (Figure 5A). Treatment with miR-125b-5p mimic repressed luciferase activities of STAT3, but not STAT3 mutant in the SGC7901 and HGC27 cells (Figure 5B). Consistently, the mRNA and protein levels of STAT3 were inhibited by the miR-125b-5p mimic in the cells (Figure 5C and D). Moreover, circVAPA knockdown down-regulated STAT3 in the SGC7901 and HGC27 cells, while miR-125b-5p inhibitor was able to reverse this effect in the cells (Figure 5E).
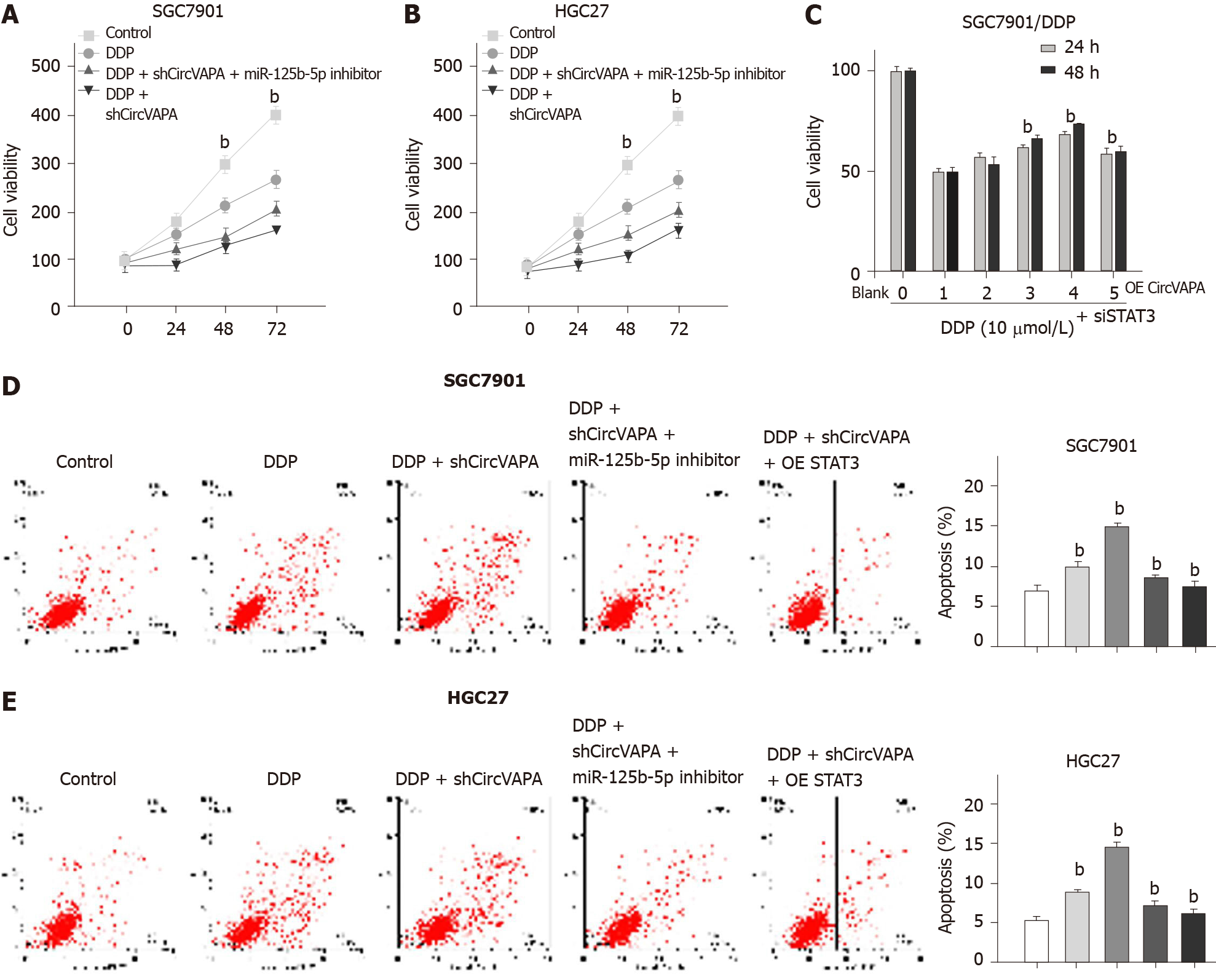
We then investigated whether circVAPA mediates chemotherapy drug resistance of GC cells by modulating the miR-125b-5p/STAT3 axis. Significantly, depletion of circVAPA enhanced the inhibitory effect of DDP on SGC7901 and HGC27 cell viability, while miR-125b-5p inhibitor rescued this phenotype in the cells (Figure 6A and B). In addition, overexpression of circVAPA promoted the proliferation of DDP-treated SGC7901/DDP cells, while STAT3 siRNA could reduce this effect in the system (Figure 6C). Meanwhile, circVAPA knockdown reinforced DDP-induced apoptosis of SGC7901 and HGC27 cells, whereas miR-125b-5p inhibitor or STAT3 overexpression could reverse this effect (Figure 6D). These results suggested that circVAPA promotes chemotherapy resistance of GC cells by modulating the miR-125b-5p/STAT3 axis (Figure 6E).
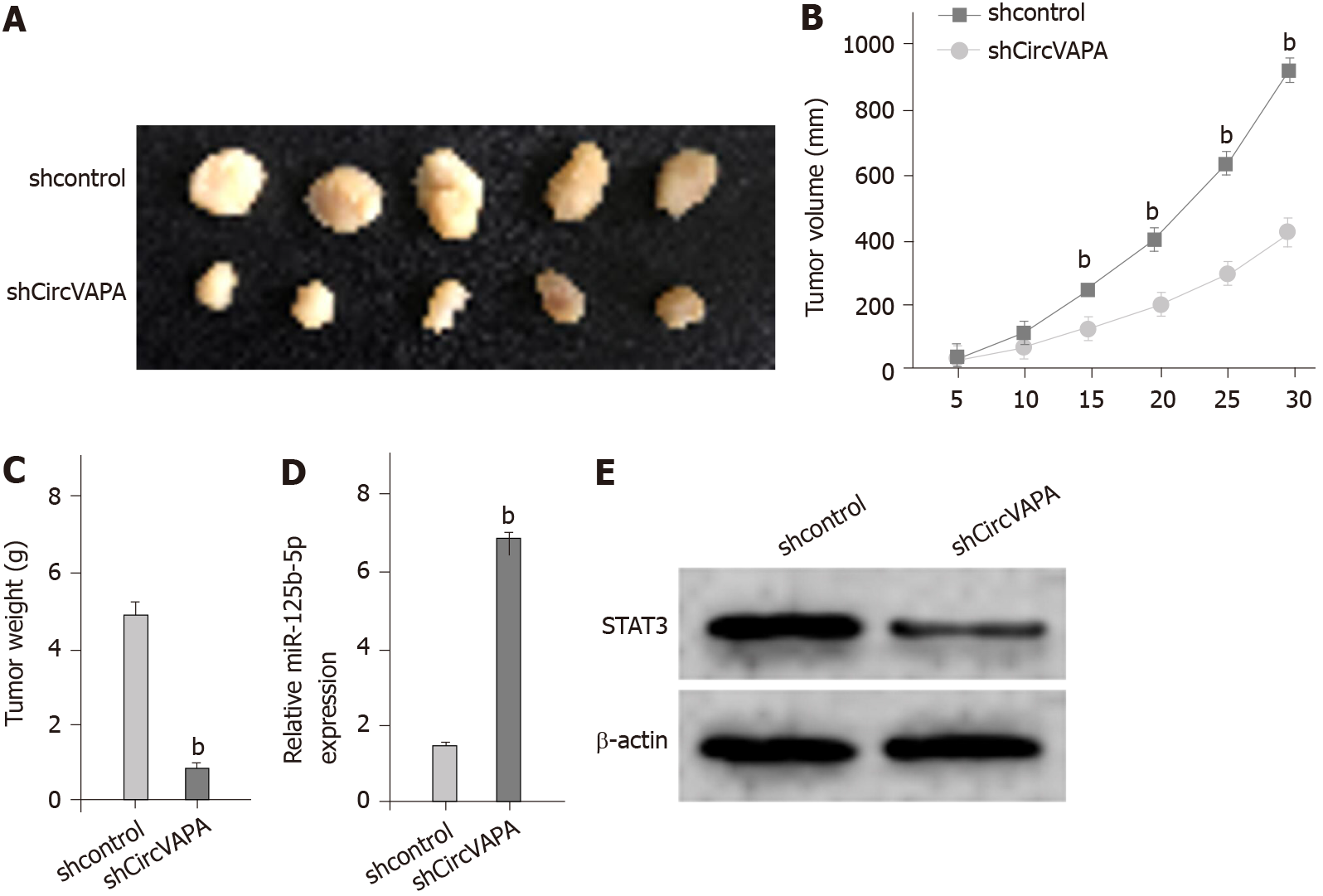
Next, the circVAPA function in GC cell growth was analyzed by tumorigenicity analysis in vivo. The tumor growth of GC cells was inhibited by circVAPA knockdown, as presented by the decreased tumor size, tumor volume, and tumor weight in the nude mice (Figure 7A-C). Meanwhile, we validated that miR-125b-5p expression was up-regulated while STAT3 expression was down-regulated by circVAPA depletion in the tumor tissues (Figure 7D and E). Together, these data indicated that circVAPA contributes to the tumor growth of chemoresistant GC cells in vivo.
The chemotherapy resistance in GC patients is a severe clinical challenge, and the molecular mechanism is still poorly understood[7]. CircRNAs have been identified to participate in the modulation of GC progression[25]. Nevertheless, the specific functions of circVAPA in the modulation of GC development remain obscure. In the present study, we uncovered that circVAPA contributed to chemotherapy resistance and promoted the malignant progression in GC by modulating miR-125b-5p/STAT3 signaling.
As a critical cancer regulator, several circRNAs have been identified in the modulation of chemotherapy resistance during GC progression. It has been reported that circ-PVT1 promotes paclitaxel resistance by regulating miR-124-3p/ZEB1 in GC cells[26]. Circ0026359 increases the DDP resistance of GC cells by modulating the miR-1200/POLD4 axis[27]. CircAKT3 promotes DDP resistance by up-regulating PIK3R1 through targeting miR-198 in GC cells[28]. CircFN1 contributes to DDP resistance of GC cells by targeting miR-182-5p[29]. CircMCTP2 attenuates DDP resistance by miR-99a-5p/MTMR3 signaling in GC cells[30]. Moreover, circVAPA presents oncogenic properties in cancers, including liver cancer and colorectal cancer[13,31]. In this study, circVAPA expression was elevated in the clinical GC samples. CircVAPA depletion inhibited proliferation, migration, and invasion, and increased apoptosis of GC cells. CircVAPA knockdown reduced tumor growth of GC cells in vivo. CircVAPA contributed to chemotherapy drug resistance of GC cells. These findings present a novel function of circVAPA in modulating chemotherapy drug resistance and malignant progression of GC cells, indicating valuable evidence of circRNA function in GC development.
MiRNAs are the essential contributor for regulating chemotherapy drug resistance of GC cells. It has been reported that miR-625 inhibits chemotherapy resistance by regulating ALDH1A1 in GC cells[32]. MiR-21-5p regulates doxorubicin resistance of GC cells via modulating TIMP3 and PTEN expression[33]. MiR-187 affects DDP resistance by targeting transforming growth factor-β signaling in GC cells[34]. Moreover, constitutive activation of STAT3 contributes to chemotherapy resistance of GC cells[35]. STAT3 signaling is involved in fibroblasts-mediated chemoresistance of GC cells[36]. Suppression of STAT3 inhibits drug resistance of GC cells to chemotherapeutic drugs[37]. In the mechanistic study, we found that circVAPA sponged miR-125b-5p and miR-125b-5p targeted STAT3 in the GC cells. MiR-125b-5p inhibitor rescued circVAPA depletion-enhanced inhibitory effect of DDP on GC cells, and STAT3 knockdown blocked circVAPA overexpression-induced proliferation of DDP-treated SGC7901/DDP cells. Depletion of STAT3 and miR-125b-5p inhibitor reversed circVAPA depletion-induced GC cell apoptosis. These data demonstrate an unreported regulation axis associated circVAPA with STAT3 and miR-125b-5p, revealing a new mechanism involving the circVAPA/miR-125b-5p/STAT3 axis in GC development.
CircVAPA promotes chemotherapy resistance and the malignant progression in GC by modulating miR-125b-5p/STAT3 signaling. Our findings present novel insights into the mechanism by which circVAPA regulates chemotherapy resistance of GC cells. CircVAPA and miR-125b-5p may be considered as potential targets for GC therapy.
Gastric cancer (GC) is a common malignant tumor and causes a high incidence of cancer-associated death. Cisplatin (DDP)-based chemotherapy is the main therapy for clinical GC treatment, but the mechanism of chemotherapy resistance is still poorly understood, which is a severe clinical challenge. CircRNAs have been identified to play a vital role in regulating the chemoresistance of gastric cancer cells. The development process of GC chemotherapy resistance is not well-understood. In addition, it is well known that circRNAs and miRNAs are involved in chemotherapy resistance of GC cells. However, the role of circVAPA and miR-125b-5p in regulating the chemotherapy resistance of GC cells has not been reported yet.
To investigate the impact of circVAPA on chemotherapy resistance during the progression of GC.
We collected the clinical GC tissue samples (n = 50) and the adjacent normal tissues (n = 50) and analyzed the effect of circVAPA on GC progression and chemotherapy resistance.
The role of circVAPA in chemotherapy resistance and GC progression was analyzed by transwell assay, MTT assay, colony formation assay, healing assay, and wound flow cytometry analysis in GC cells and DDP resistant GC cell lines, and in vivo tumorigenicity analysis in nude mice. The mechanism was investigated by quantitative real-time PCR, luciferase reporter assay, and Western blot analysis.
CircVAPA depletion inhibited proliferation, migration, and invasion and increased apoptosis of GC cells. CircVAPA knockdown reduced tumor growth of GC cells in vivo. CircVAPA promoted the chemotherapy resistance of GC cells. We found that circVAPA targeted miR-125b-5p and miR-125b-5p to STAT3 in GC cells. MiR-125b-5p inhibitor rescued the depletion of circVAPA and enhanced the inhibitory effect of DDP on GC cells, while STAT3 knockdown prevented the proliferation of DDP-treated SGC7901/DDP cells induced by circVAPA overexpression. Depletion of STAT3 and miR-125b-5p inhibitor reversed circVAPA depletion-induced GC cell apoptosis.
CircVAPA promotes chemotherapy resistance and the malignant progression of GC cells by modulating miR-125b-5p/STAT3 signaling. CircVAPA and miR-125b-5p may be considered as potential targets for GC therapy.
CircRNA circVAPA promotes chemotherapy drug resistance in the progression of gastric cancer by regulating the miR-125b-5p/STAT3 axis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Ying G S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Marin JJ, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales JM, Macias RI. Mechanisms of Resistance to Chemotherapy in Gastric Cancer. Anticancer Agents Med Chem. 2016;16:318-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM, Choi KS, Son SY, Han SU, Brekken RA, Lee D, Hur H. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019;18:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 487] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 5. | Guo W, Deng L, Chen Z, Chen Z, Yu J, Liu H, Li T, Lin T, Chen H, Zhao M, Zhang L, Li G, Hu Y. Vitamin B12-conjugated sericin micelles for targeting CD320-overexpressed gastric cancer and reversing drug resistance. Nanomedicine (Lond). 2019;14:353-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Xu Z, Chen L, Xiao Z, Zhu Y, Jiang H, Jin Y, Gu C, Wu Y, Wang L, Zhang W, Zuo J, Zhou D, Luan J, Shen J. Potentiation of the anticancer effect of doxorubicinin drug-resistant gastric cancer cells by tanshinone IIA. Phytomedicine. 2018;51:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, Lee KH, Kim TY, Oh DY, Bang YJ, Im SA. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 9. | Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 796] [Cited by in RCA: 1090] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 10. | He J, Xie Q, Xu H, Li J, Li Y. Circular RNAs and cancer. Cancer Lett. 2017;396:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Mo Y, Gong Z, Yang X, Yang M, Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, Zhang W, Li X, Li X, Li Y, Li G, Zeng Z, Xiong W. Circular RNAs in human cancer. Mol Cancer. 2017;16:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 12. | Zhou SY, Chen W, Yang SJ, Li J, Zhang JY, Zhang HD, Zhong SL, Tang JH. Circular RNA circVAPA regulates breast cancer cell migration and invasion via sponging miR-130a-5p. Epigenomics. 2020;12:303-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Liu C, Zhong X, Li J, Xu F. Circular RNA circVAPA Promotes Cell Proliferation in Hepatocellular Carcinoma. Hum Gene Ther Clin Dev. 2019;30:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5605] [Article Influence: 295.0] [Reference Citation Analysis (0)] |
| 15. | Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 16. | Liu S, Chen Q, Wang Y. MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Hum Cell. 2020;33:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Hui L, Zhang J, Guo X. MiR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. Biomed Pharmacother. 2018;103:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Shi H, Li K, Feng J, Liu G, Feng Y, Zhang X. LncRNA-DANCR Interferes With miR-125b-5p/HK2 Axis to Desensitize Colon Cancer Cells to Cisplatin vis Activating Anaerobic Glycolysis. Front Oncol. 2020;10:1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Yang W, Ma J, Zhou W, Cao B, Zhou X, Yang Z, Zhang H, Zhao Q, Fan D, Hong L. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Dehghanzadeh R, Jadidi-Niaragh F, Gharibi T, Yousefi M. MicroRNA-induced drug resistance in gastric cancer. Biomed Pharmacother. 2015;74:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y, Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 22. | Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, Shen S, Priceman SJ, Kujawski M, Pal SK, Raubitschek A, Hoon DS, Forman S, Figlin RA, Liu J, Jove R, Yu H. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 449] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 25. | Shan C, Zhang Y, Hao X, Gao J, Chen X, Wang K. Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Mol Cancer. 2019;18:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 26. | Liu YY, Zhang LY, Du WZ. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Yu X, Zhou B, Zhang J, Chang J. Circular RNA circ_0026359 Enhances Cisplatin Resistance in Gastric Cancer via Targeting miR-1200/POLD4 Pathway. Biomed Res Int. 2020;2020:5103272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Huang X, Li Z, Zhang Q, Wang W, Li B, Wang L, Xu Z, Zeng A, Zhang X, Zhang X, He Z, Li Q, Sun G, Wang S, Li Q, Wang L, Zhang L, Xu H, Xu Z. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 29. | Huang XX, Zhang Q, Hu H, Jin Y, Zeng AL, Xia YB, Xu L. A novel circular RNA circFN1 enhances cisplatin resistance in gastric cancer via sponging miR-182-5p. J Cell Biochem. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Sun G, Li Z, He Z, Wang W, Wang S, Zhang X, Cao J, Xu P, Wang H, Huang X, Xia Y, Lv J, Xuan Z, Jiang T, Fang L, Yang J, Zhang D, Xu H, Xu Z. Circular RNA MCTP2 inhibits cisplatin resistance in gastric cancer by miR-99a-5p-mediated induction of MTMR3 expression. J Exp Clin Cancer Res. 2020;39:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Li XN, Wang ZJ, Ye CX, Zhao BC, Huang XX, Yang L. Circular RNA circVAPA is up-regulated and exerts oncogenic properties by sponging miR-101 in colorectal cancer. Biomed Pharmacother. 2019;112:108611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Gong X, Xu B, Zi L, Chen X. miR-625 reverses multidrug resistance in gastric cancer cells by directly targeting ALDH1A1. Cancer Manag Res. 2019;11:6615-6624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Chen J, Zhou C, Li J, Xiang X, Zhang L, Deng J, Xiong J. miR215p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3. Int J Mol Med. 2018;41:1855-1866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Zhu QL, Li Z, Lv CM, Wang W. MiR-187 influences cisplatin-resistance of gastric cancer cells through regulating the TGF-β/Smad signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:9907-9914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 35. | Song S, Min H, Niu M, Wang L, Wu Y, Zhang B, Chen X, Liang Q, Wen Y, Wang Y, Yi L, Wang H, Gao Q. S1PR1 predicts patient survival and promotes chemotherapy drug resistance in gastric cancer cells through STAT3 constitutive activation. EBioMedicine. 2018;37:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Ma J, Song X, Xu X, Mou Y. Cancer-Associated Fibroblasts Promote the Chemo-resistance in Gastric Cancer through Secreting IL-11 Targeting JAK/STAT3/Bcl2 Pathway. Cancer Res Treat. 2019;51:194-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Huang S, Chen M, Shen Y, Shen W, Guo H, Gao Q, Zou X. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett. 2012;315:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |