Published online Dec 21, 2021. doi: 10.3748/wjg.v27.i47.8081
Peer-review started: January 27, 2021
First decision: March 29, 2021
Revised: April 8, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: December 21, 2021
Processing time: 323 Days and 11.9 Hours
Viral hepatitis can result in important morbidity and mortality, with its impact on health conditioned by the specific type of hepatitis, the geographical region of presentation and the development and access to new drugs, among other factors. Most acute presentation forms are self-limiting and may even go unnoticed, with just a small percentage of cases leading to acute liver failure that may necessitate transplantation or even cause the death of the patient. However, when they become chronic, as in the case of hepatitis B virus and C virus, unless they are diagnosed and treated adequately they may have severe consequences, like cirrhosis or hepatocarcinoma. Understanding of the mechanisms of transmission, the pathogenesis, the presence of vaccinations and the development over recent years of new highly-efficient, potent drugs have meant that we are now faced with a new scenario in the management of viral hepatitis, particularly hepatitis B virus and hepatitis C virus. The spectacular advances in hepatitis C virus treatment have led the World Health Organization to propose the objective of its eradication by 2030. The key aspect to achieving this goal is to ensure that these treatments reach all the more vulnerable population groups, in whom the different types of viral hepatitis have a high prevalence and constitute a niche that may perpetuate infection and hinder its eradication. Accordingly, micro-elimination programs assume special relevance at the present time.
Core Tip: The various types of viral hepatitis have resulted in important morbidity and mortality for many years. Greater understanding of the pathogenesis as well as the development of new, highly efficient potent drugs mean that we are now faced with a new scenario in the approach to this disease. The spectacular advances in the treatment of hepatitis C virus suggest that we can now envisage its eradication, as put forward by the World Health Organization in its objectives for 2030. In this review we comment on the current situation, recent advances and future perspectives in the approach to viral hepatitis.
- Citation: González Grande R, Santaella Leiva I, López Ortega S, Jiménez Pérez M. Present and future management of viral hepatitis. World J Gastroenterol 2021; 27(47): 8081-8102
- URL: https://www.wjgnet.com/1007-9327/full/v27/i47/8081.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i47.8081
The different types of viral hepatitis have resulted in great morbidity and mortality for many years, due to their high prevalence and incidence. Over 250 million persons are estimated to be infected with the chronic form of hepatitis B virus (HBV)[1] and more than 70 million with hepatitis C virus (HCV) in the world[2], with over 1.5 million cases of hepatitis A virus (HAV) annually[3], resulting largely from lack of understanding of the pathogenesis and the absence of efficient treatment. Fortunately, recent years have seen important advances, impacting very positively on the management of viral hepatitis, particularly HBV and HCV, though not so much on HAV and hepatitis E virus (HEV). The most significant advances in these latter types have occurred in aspects related to the epidemiology and pathogenesis of the disease rather than in therapy. Studies have shown new pathways of contagion, especially relevant in certain population groups, such as patients with previous liver disease or immunosuppressed patients where the disease can have an important impact on morbidity and mortality.
Advances in understanding the viral pathogenesis of HBV have mainly led to the development of new drugs. The first significant leap occurred in the early 2000s, with the appearance of the first nucleotide/nucleoside analogs (NAs), like lamivudine and adefovir dipivoxil. This enabled oral treatment, with great efficacy, safety and tolerability, though limited by the development of resistance that was overcome later with entecavir (ETV) and tenofovir (TDF), which have a high genetic barrier to the development of resistance[4,5]. Nevertheless, limitations still exist, such as the need to administer the drugs for prolonged periods of time, even indefinitely, and they are unable to inhibit the initial formation of covalently closed circular (ccc)DNA in newly infected hepatocytes[6]. This has resulted in current research aimed at developing drugs to inhibit viral replication, acting on any of the various phases of the virus replication cycle[7]. Indirectly, all these advances can have a positive impact on the management of hepatitis D virus (HDV), which whilst being a defective virus that needs the presence of the B virus for reproduction, can nevertheless cause important morbidity and mortality. The first drug for the specific treatment of HDV, bulevirtide, has recently been commercialized in Europe[8].
It is, though, in HCV where the most significant advances have been made, with a major impact on improving health over recent years. The efficacy, safety, tolerability and ease of use in clinical practice of direct-acting antivirals (DAA) have led to the rarely seen possibility of eradicating HCV by 2030, an objective set by the World Health Organization[9]. This requires establishing such strategies as micro-elimination based on the active search for cases, simplification of the diagnosis and treatment and prevention measures.
The classic transmission of HAV and HEV is the fecal-oral route, more prevalent in underdeveloped countries. However, new pathways of infection are leading to changes in the epidemiology of these infections. This, perhaps, is the main novelty.
HAV is one of the most common infectious etiologies of acute hepatitis worldwide. Transmission is fecal-oral via contaminated food or water, either person-to-person or through consumption of contaminated products. Globally, an estimated 1.5 million cases occur each year[3]. There are areas of high, intermediate, low and very low HAV endemicity. In low- and middle-income countries, where sanitation and hygiene practices are poor, infection is common, and most children (90%) have it before the age of 10, very often without presenting symptoms[10]. Epidemics are rare because older children and adults are usually immunized. In these areas, morbidity rates are low, and epidemic outbreaks rarely occur. In developed countries with good sanitation and hygiene, infection rates are low. This translates into an increase in the number of adults who have never been infected and who lack immunity. This increased vulnerability in older age groups can increase morbidity rates and lead to large epidemic outbreaks. The disease can appear in adolescents and adults from high-risk groups, such as injection drug users, men who have homosexual relationships and people who travel to high-endemic areas[11]. Because of the epidemiological features, vaccination is recommended for persons at increased risk of exposure or those liable to fulminant disease[12].
Infection with HAV has a mild or asymptomatic course, although it may be fulminant in < 1% of cases[13], especially in patients with chronic hepatitis[14]. Other atypical presentations of acute hepatitis A include renal insufficiency and relapsing hepatitis, which are usually present in children. Some individuals experience a prolonged hepatitis (5.8%)[15] or cholestasis (6.8%), especially in the presence of HBV[16]. HAV accounts for 0.35% of cases of acute liver failure. HAV-related acute liver failure has a spontaneous resolution rate of 70%, with the remaining 30% requiring a liver transplant[17]. Several studies have concluded that acute HAV infection superimposed on chronic liver disease is associated with greater disease severity and a higher case fatality rate, though a review of the literature failed to delineate a relationship between nonalcoholic steatohepatitis and HAV-induced liver failure[18].
Regarding the prevention of hepatitis A, in addition to improving hygiene and sanitation measures, the vaccine is a very effective tool, already being included in the vaccination calendar from childhood in many countries. Nonvaccinated persons traveling to HAV endemic regions should receive a single vaccine dose before departure[19]. The Centers for Disease Control and Prevention 2020 Advisory Committee on Immunization Practices recommends vaccination for all children aged 1 year and older, men who have sex with men and people who use injection and noninjection drugs, have occupational risk factors for infection, travel to high-endemicity areas or those who have an increased risk for complications from hepatitis A (e.g., chronic liver disease, HIV infection and pregnancy, if at risk for infection)[12]. There are currently two single-antigen inactivated vaccines: HavrixTM (GlaxoSmithKline Biologicals, Rixenstar, Belgium) and VaqtaTM (Merck and Co. Inc., West Point, PA, United States)[20,21]. A live-attenuated vaccine is licensed in China and has been used extensively there[22]. A combination HAV-HBV vaccine (TwinrixTM; GlaxoSmithKline Biologicals) has been available since 1996[23]. Havrix and Vaqta vaccines are given in a two-dose schedule 6 mo apart and Twinrix requires three doses. The efficacy of both live-attenuated and inactivated vaccines has been well established in a large review[24]. A special population concerns HIV-positive individuals who are susceptible to HAV infection, especially because of low adherence to recommended HAV vaccination. In this group a double dose of HAV vaccination is recommended as an additional dose of the HAV vaccine may improve serological responses and durability of seroprotection. Immunoglobulin is used for both pre-exposure and postexposure prophylaxis of HAV. Children younger than 12-mo-old, adults with chronic liver disease, adults older than 40 years of age and immunocompromised individuals should receive a single dose of intramuscular HAV immunoglobulin (0.1 mL/kg) in addition to the vaccine, unless either is contraindicated.
Infection with HEV, traditionally considered a disease almost exclusive to developing countries, has now become a worldwide health problem and is endemic in most developed countries, behaving largely as a zoonosis[25]. HEV infection has a greater clinical impact in populations that are especially vulnerable, such as immunosuppressed patients, pregnant women and patients with underlying liver disease. Thus, the World Health Organization places it as one of the leading causes of death from acute viral hepatitis worldwide[26].
HEV is divided into four species (A–D). Genotypes 1 and 2 of species A are strains that infect humans, whereas genotypes 3 and 4 are zoonoses transmitted via meat consumption or direct contact with affected animals, mainly pigs or wild boar, or through contaminated blood products[25]. In recent years, particularly in Eastern China, genotype 1 has become much less common, and genotype 4 is now the most common genotype found in human cases[27]. Person-to-person transmission (direct contact) of HEV is very inefficient; study of close contacts of a case with documented HEV infection is not recommended, unless they share exposure to the source of infection. Blood transfusion is another route of transmission of HEV. The European Association for the Study of the Liver (EASL) recommends that blood donor services screen blood donors for HEV, given the results of local risk-assessment and cost-effectiveness studies[25].
In most cases, contact with HEV produces an asymptomatic infection, mainly in women and young people, followed by spontaneous clearance of the virus[28]. HEV infection during pregnancy (particularly during the third trimester) has been associated with a poorer prognosis compared to other types of viral hepatitis[29-31]. Maternal mortality rates of up to 30% have been observed in different outbreaks of hepatitis due to HEV in pregnant women[32]. The mortality associated with HEV infection during pregnancy is usually associated with infections caused by genotypes 1 and 2, though cases caused by other genotypes have been reported[33,34].
Although the development of acute liver failure in the course of HEV infection is rare (0.5%-4.0%)[35], in some series, like that of Crossan et al[36], HEV accounts for 5.0% of all cases of acute liver failure. Those most at risk of developing acute liver failure in the course of HEV are pregnant women and patients with underlying chronic liver disease[37,38]. Because of this, all patients with acute hepatitis, acute liver failure or patients with decompensation of chronic liver disease should be screened for HEV infection. Although isolated cases of chronic HEV infection have been reported in immunocompetent patients[39,40], chronic HEV infection occurs primarily in immunocompromised patients, such as solid organ transplant recipients. In transplant patients, HEV infection may progress to chronicity in up to 2/3 of cases, with rapid progression of fibrosis and development of liver cirrhosis in up to 10% of patients[41,42]. Cases have even been reported of retransplantation in liver transplant recipients due to acute liver failure from HEV[43]. Extrahepatic clinical manifestations have been described in 2%-5% of patients with HEV infection[44]. In most of these the liver manifestations of the infection are mild or absent. The EASL recommends HEV testing, irrespective of liver function test results, in patients presenting with neuralgic amyotrophy or Guillain-Barré syndrome and suggests HEV testing for patients with encephalitis/myelitis. Patients with proteinuria should also be tested[25]. Currently, screening for HEV is advisable in all cases of acute hepatitis, including those with suspected drug-induced liver injury, particularly if patients have higher levels of transaminases, in which case acute HEV must be excluded systematically[45,46].
The diagnosis of HEV is based on a combination of serology and nucleic acid amplification techniques. Serological techniques measure anti-HEV immunoglobulin (Ig)M antibodies, which appear approximately 4 wk after contact and may be detected up to 6 mo later, and IgG antibodies, which appear at about the same time as the IgM antibodies but can last for years. The presence of anti-HEV IgM indicates recent or acute infection while anti-HEV IgG antibodies indicate recent or past infection. HEV-RNA can be detected in blood or stool 3 wk after infection and a short time before the appearance of symptoms and constitute the gold standard for the diagnosis of active infection[47]. The combination of serological studies and the determination of HEV-RNA increases the diagnostic specificity and sensitivity, though it is necessary to bear in mind the immune-competence status of the patient. The World Health Organization recommends first determining the presence of anti-HEV IgM antibodies and then determining the viral RNA. In immunocompromised patients, the antibodies may be negative despite HEV infection, and it is necessary to determine the viral load in all cases[48].
Acute HEV infection does not usually require antiviral therapy. In almost all cases HEV infection clears spontaneously. Nevertheless, early therapy of acute hepatitis E may shorten the course of the disease and reduce overall morbidity. Ribavirin treatment may be considered in cases of severe acute hepatitis E or acute-on-chronic liver failure. Corticosteroids have been used in individual cases of acute liver failure, with improvement of liver function parameters. However, there is currently insufficient evidence to support general corticosteroid treatment in this group of patients[25]. The group of transplant patients deserves special mention. The EASL recommends decreasing immunosuppression at diagnosis of chronic HEV infection in solid organ transplant recipients, if possible.
In patients with persisting HEV replication 3 mo after detection of HEV RNA, the EASL recommends ribavirin monotherapy for a duration of 12 wk. Trials have been attempted with other treatments, such as sofosbuvir in single therapy, which showed antiviral activity in vitro but was unable to inhibit viral replication in a phase II pilot trial[49].
As no efficient therapy exists for HEV and bearing in mind it can cause severe symptoms of liver disease, efforts should be focused on both prevention and research.
Preventive measures should be enhanced in immunosuppressed patients and pregnant women; indeed, screening for HEV should be recommended in pregnant women in endemic areas like Sub-Saharan Africa or South Asia, assessing the risk individually[47].
A few European countries are starting to include HEV-RNA detection in all blood donor samples, although certain aspects remain to be clarified, such as the most cost-effective technique for detection, the viral load considered infectious or the characteristics of the recipient that may influence transmission of the disease, such as the immunological status[50].
Currently only one vaccine against HEV is available (Helicon®). It has only received approval in China, though other vaccines are under development. The availability of an effective vaccine would constitute the main tool for prevention of HEV infection[51].
The discovery of the Australia antigen in 1965 represented a starting point for the identification of the virus of hepatitis B, and for many it was the beginning of the study of viral hepatitis. Infection with HBV is now a public health problem worldwide, with some 257 million persons with chronic infection. HBV is endemic in the western Pacific and Africa, with over 6% of the population infected[1]. In Europe and the United States the prevalence is < 2%, mostly related with immigration[52]. HBV is the leading cause of morbidity and mortality of hepatic origin in the world, despite the availability of an efficient vaccine.
Vaccination programs, control of blood donations, serologic evaluation in pregnant women or persons at risk and activities aimed at limiting invasive procedures in unsafe conditions have all reduced the incidence of acute HBV hepatitis and the prevalence of the disease. Although population screening is not indicated, screening is recommended in risk groups, partners of infected patients and before receiving oncologic or immunosuppressive therapy[1].
The spectrum of the disease varies greatly, from inactive carriers to others with hepatic cirrhosis and hepatocarcinoma. The natural history of hepatitis B is a dynamic process, traditionally differentiated into five phases depending on virus and host factors, like the state of immune competence, age or the duration of the infection[53]. They are classified according to liver inflammation data (alanine aminotransferase), determination of hepatitis B e antigen (HBeAg) and quantification of the HBV-DNA viral load[54] as well as estimation of the degree of hepatic fibrosis, usually by elastography[1] as serologic indices of fibrosis have proven less precise in HBV infection[55]. These phases have traditionally been called: immune-tolerant phase, immune-elimination phase, immune-reactive phase, asymptomatic carrier and hepatitis B surface antigen (HBsAg)-negative phase[56]. However, these phases have recently been grouped into two large spectra of chronic forms of hepatitis B with a new nomenclature: infection, encompassing the phases of immune tolerance and inactive carrier, both HBeAg positive and negative vs hepatitis, which refers to the phases in which there exists liver damage, the immune-reactive and the immune-elimination phases[57].
Awareness of the particular phase in which the patient is and its clinical context is important to identify the need to start treatment and the choice of the most suitable antiviral agent.
Initially it was thought that a depressed immune response was involved in the development of HBV infection. Thus, in 1986 the first clinical trial of interferon (IFN) alpha was published, though the findings showed a poor and transitory response[58]. Currently, in its pegylated form it is still considered a treatment option, with PEGα2a being easier to use and having greater efficacy and tolerance[59]. The duration of treatment with this drug is usually finite and the loss of HBsAg and conversion of HBeAg is relevant, although the drug has to be injected and is contraindicated in patients with decompensated cirrhosis, autoimmune disorders, pregnant women and severe depression or psychosis[59].
Later studies on the lifecycle of HBV found that the virus uses an inverse transcriptase for replication. Accordingly, the use of drugs already available for another virus (HIV), NAs with an inhibitory action on this polymerase, were considered[60]. In 1998 the Food and Drug Administration approved the use of lamivudine for the treatment of HBV, controlling viral replication with oral treatment[61]. Lamivudine was the sole oral treatment available until 2002, when other NAs have become available: adefovir dipivoxil, ETV, telbivudine, TDF in 2008[4] and tenofovir alafenamide in 2016, though the latter is not available in all countries.
All these analogs reduce the pool of cccDNA in infected hepatocytes, inhibiting recycling of the nucleocapsids, although they are unable to inhibit the initial formation of cccDNA in newly infected hepatocytes[5]. The main advantages of NAs are that they can be given orally, they have great efficacy in inhibition of HBV replication (very similar among all of them), their long-term safety and the possibility of being used in any situation, including decompensated cirrhosis and liver transplant or even during pregnancy in the case of TDF. They achieve a virological and biochemical response greater than 95%, for both positive and negative HBeAg[62,63]. The main incon
Lamivudine is the drug that has shown the greatest risk for development of resistance as compared with the other antiviral agents currently available, particularly ETV and TDF[64]. The main clinical practice guidelines therefore recommend treatment with ETV, TDF and tenofovir alafenami given their great antiviral potency and high resistance barrier[65]. The choice of one over the other treatment strategy depends of the stage of liver fibrosis, virological factors and the comorbidity profile of the patient, in addition to personal preferences[14]. Tenofovir alafenami is a prodrug that reaches higher levels of TDF than TDF in the hepatocytes with lower doses. Lower exposure to the drug is related with less worsening of renal function and less loss of bone mineral density[66].
Recent studies have shown that combined NA treatment with Peg-IFN, simultaneously or sequentially, increases the probability of HBsAg loss, but the benefits are restricted to just a small group of patients with low HBsAg levels. Further studies are therefore needed before it can be recommended[67].
Recent studies have examined whether TDF vs ETV could impact the risk of developing hepatocarcinoma. Though this would condition the choice of one over the other, neither has yet been found superior in this respect. Some studies have suggested the benefit of TDF, though the differences were not statistically significant[68]. Retrospective analyses of different series have reported contradictory results, with some showing no difference between the drugs[69] whilst others have found benefits for TDF vs ETV in the prevention of hepatocarcinoma[70], a benefit confirmed in a recent meta-analysis[71]. Randomized trials are needed to establish this association.
Patients who required a liver transplant due to HBV initially had a very high risk of recurrence of the infection in the graft. This was particularly so for patients who received their transplant having a high viral load, which in addition was usually a severe, rapidly progressive hepatitis, so much so that HBV infection became considered a contraindication for liver transplantation[1]. With the advent of anti-hepatitis B hyperimmune gammaglobulin during the 1990s, and particularly of the NAs, the perspective changed. The post-transplant results were similar to those of other etiologies. Now that we have potent NAs and a high resistance barrier, the tendency is to use hepatitis B immune globulin for a short time and at lower doses than before and even regimens without hepatitis B immune globulin with NAs in single therapy maintained indefinitely[67]. These advances have thus allowed for an individualized prophylaxis based on the individual risk profile of each patient[72] as well as the use of anti-hepatitis B core positive donors.
The final aim of antiviral therapy is cure of the infection, eliminating all potential forms of HBV replication[59]. Sustained loss of HBsAg and eradication of the HBV-DNA, including the cccDNA[6], though this is hardly feasible with currently available drugs, is the sterilizing cure. As a result, the American Association of the Study of Liver Diseases and EASL agreed to the definition of functional cure, defined as the sustained loss of HBsAg and HBV-DNA in serum with or without the development of anti-HBs. These two situations are those that allow treatment to be suspended safely and with little risk of relapse. However, they are very rarely achieved. Another concept, partial cure, refers to the persistence of HBsAg but with negativization of HBeAg, with or without seroconversion to anti-HBe, normalization of alanine aminotransferase and a low or undetectable viral load, simulating a phase of inactive carrier and thus susceptible to interruption of treatment[6].
Current guidelines[57,73] recommend that treatment can be suspended with NAs for patients with positive HBsAg but without cirrhosis if there is seroconversion to anti-HBe after 1 year of consolidation. For negative HBeAg, treatment can be suspended for patients whose HBsAg clears and who have had viral suppression for over 2-3 years, requiring strict follow-up[74].
Quantified HBsAg is determined by enzyme immunoassay and reflects the amount and transcriptional activity of the cccDNA. It is very useful in untreated patients with negative HBeAg, with HBV-DNA < 2000 IU/mL, in whom values of quantified HBsAg < 1000 IU/mL are indicative of inactive carrier with a low risk of disease progression and appearance of hepatocarcinoma. Although no cut-off value has yet been established, a level < 100 IU/mL seems to predict this sustained response[1]. Among the new markers is the antigen related with the HBV core that correlates with the transcriptional activity of cccDNA, especially in the negative HBeAg patient, and it could be superior to quantified HBsAg for the identification of inactive carrier patients and to predict viral relapse after suspending treatment[75].
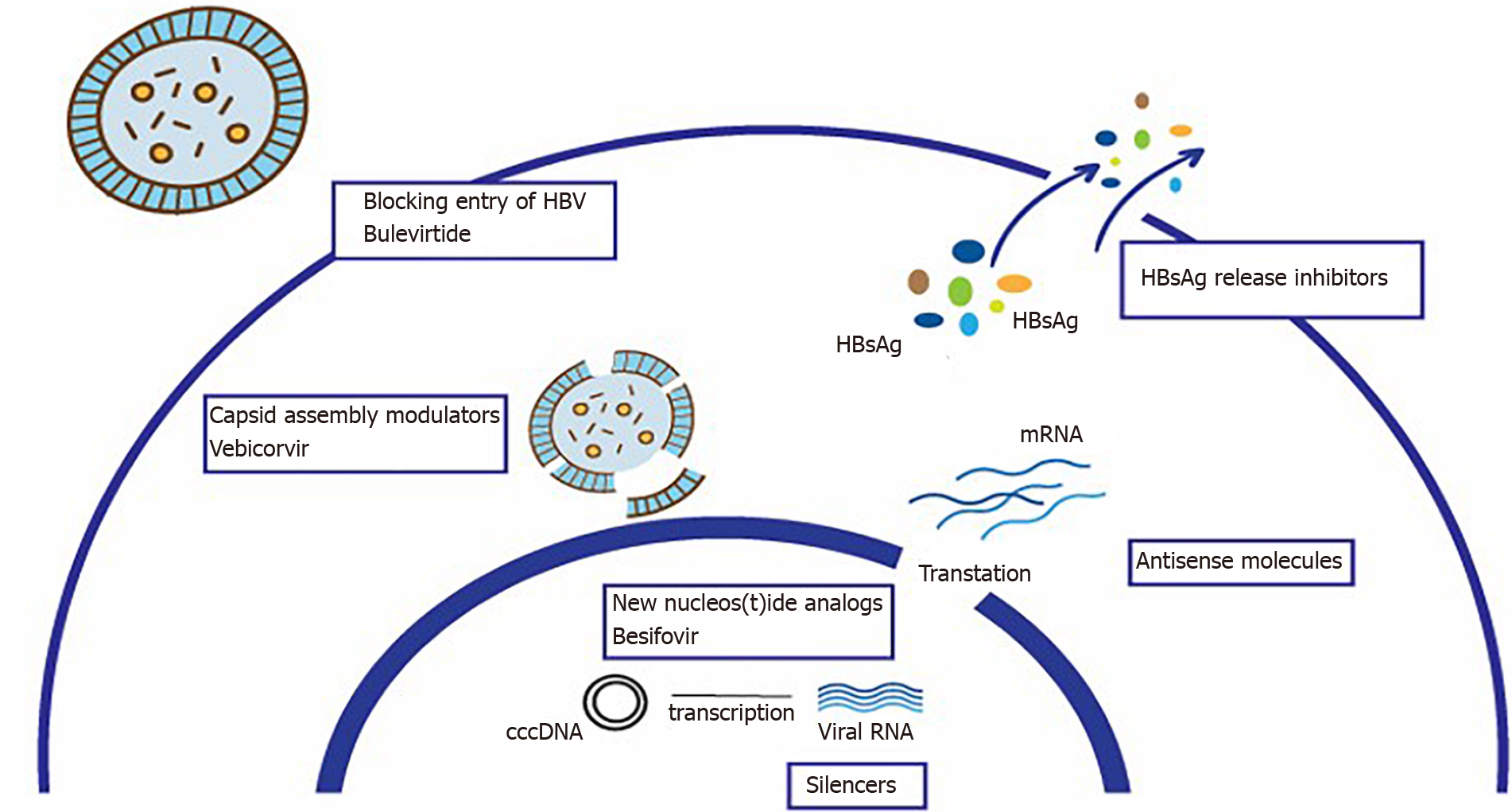
Given that treatment with NAs has little impact on the cccDNA, which in addition can be replaced with no need for the entry of new virus[6], numerous studies are in place to develop new drugs that act at the level of the different stages of the life cycle of HBV, about which more and more is being learnt, or that modulate the host immune response. These drugs are at various stages of clinical trials, with up-to-date information available from: http://www.hepb.org/treatment-and-management/drug-watch[7] (Table 1).
| Treatment | Mechanism of action | Country | Phase |
| Bulevirtide | Inhibitor of HBV entry into the hepatocyte | Germany | Phase II |
| VIR-2218 | Silencers: Interfere and destroy viral RNA | United States | Phase II |
| JNJ-3989 | United States | Phase II | |
| IONIS-HBVRx (GSK3228836) | Antisense molecules: Bind to mRNA to prevent passage by viral protein | United States | Phase II |
| Vebicorvir | Capsid inhibitors | United States | Phase II/III |
| Morphothiadin | China | Phase II | |
| JNJ 56136379 | Ireland | Phase II | |
| ABI-H2158 | United States | Phase II | |
| REP 2139 | HBsAg inhibitors | Canada | Phase II |
| REP 2165 | Canada | Phase II | |
| NASVAC | THERAPEUTIC VACCINES: Use stimulation of the immune system as treatment | Cuba | Phase III |
| GS-4774 | United States | Phase II | |
| HepTcell | United States | Phase II | |
| GS9688 (TLR-8 agonist) | TLR | United States | Phase II |
| GC1102 | Monoclonal antibodies | Korea | Phase II |
| ASC22: Inhibitor PDL1 | Checkpoint inhibitors: Stimulate specific T lymphocytes | China | Phase II |
| IMC-I109V | Other immune modulatorsT-cell receptor | United States | Phase II |
The main drugs under development and their therapeutic targets are described below:
Blocking entry of HBV into the hepatocyte: The sodium taurocholate cotransporting polypeptide receptor is a transmembrane protein that cotransports bile acids and participates in the entry of HBV and HDV into hepatocytes[76]. Bulevirtide couples to this receptor and blocks fixation of the preS1 domain on the coat of HBV, inhibiting entry of the virus. Although this does not directly interfere with the formation of the cccDNA, inhibiting virus entry can block the infection of new hepatocytes. It has also been proposed as an option to prevent reinfection in the transplanted liver[77]. It is currently in phase II[7].
Silencers: These are drugs designed to interfere and destroy viral RNA. The process involves small molecules of RNA producing interference in the transcription of viral RNA. They are short non-encoding sequences present in the cells that regulate the post-transcriptional expression of certain genes, resulting in a reduction in the production of multiple viral antigens[77,78]. There are several studies in various phases: preclinical, I and II.
HBsAg release inhibitors: HBsAg is the most abundant circulating viral antigen. It contributes to T-cell tolerance and attenuation of the host immune response. Its inhibition may enable immune regulators to restore the immune response. There are currently two phase II studies with results that need to be validated.
Capsid assembly modulators: As the nucleocapsid contains the viral DNA necessary for replication, inhibiting its formation is an interesting strategy that can prevent the formation of cccDNA during de novo infection[79]. There are currently several studies in various phases, preclinical, I and II and one with vebicorvir in phase II/III.
Antisense molecules: These bind to messenger RNA inhibiting the formation of viral proteins. There is currently one phase II study in the United States.
New NAs: Besifovir has been approved in Korea, with antiviral efficacy comparable to that of TDF after 48 wk of treatment, with effects lasting 96 wk and a better safety profile than TDF in terms of bone and kidney results[80], though further studies are needed.
Still in the preclinical phase are studies of drugs against HBV-cccDNA. They work via degradation of the lymphotoxin beta receptor using specific antibodies or with cccDNA excision enzymes.
Immunotherapy: Immunological dysfunction due to “exhaustion,” a phenomenon characterized by lack or absence of specific T cells against HBV associated with poor cytotoxic activity, worsening cytokine production and an increased expression of T-cell inhibitor receptors[81], has resulted in various immunotherapeutic strategies aimed at modulating the innate or adaptive immune response, or both, in an attempt to restore a competent immune response against the virus and infected hepatocytes[59]. New modulatory agents of immunity in clinical development include:
Pattern recognition receptor (PRR) agonists. PRR are proteins that detect pathogen-associated molecular patterns and are present in various cell groups, like hepatocytes and cells of the innate immune system. Among the main PRR are the Toll-like receptors[82]. HBV is recognized by the PRR as it provokes a cytokine-mediated response. The aim of therapy with PRR agonists is to enhance the antiviral response of the cytokines to achieve an adaptive immune response with activation of natural killer cells and T lymphocytes[83]. Agonists of Toll-like receptor-7 and Toll-like receptor-8 are under development and appear to stimulate the immune response satisfactorily.
Molecules to revert the exhausted T lymphocytes by blocking immunoinhibitory signals, mainly programmed cell death protein-1, will be the subject of future studies[59]. There is currently one phase II trial in China (ASC22).
Therapeutic vaccines. Stimulation of specific HBV B and T lymphocytes by vaccines is a way of overcoming immune tolerance in patients with chronic HBV. Several categories exist, based on proteins, DNA and vectors. They are all being studied in clinical trials in combination with current antivirals[84] as, so far, on their own they have been unable to control infection.
In summary, interaction at each stage of the life cycle of HBV may be the therapeutic aim to achieve elimination of the cccDNA, though it is also necessary to standardize the method to measure this. As therapeutic research in HBV advances new compounds appear, with data on efficacy, although with complex profiles and multiple pharmacological interactions. Given that, the consensus agrees that cure of HBV means clearance of the HBsAg. Clinical studies should demonstrate that this is possible in a high proportion of treated patients. Future studies should include long-term safety results, interactions with common drugs and the pharmacokinetics in special populations[85]. Additionally, the drugs should be safe and easy to administer, as are current NAs, and moreover the clearance of the HBsAg must be sustained after interrupting treatment.
Figure 1 shows the main therapeutic targets and the drugs under development.
HDV was discovered by Rizzetto et al[86] in 1977 in patients infected by HBV who had severe hepatitis. Despite being an incomplete virus that requires the presence of HBV it can nevertheless cause severe progressive hepatitis[87]. Progression to cirrhosis may occur in 80% of patients at 10 years[88], and HBV-HDV coinfection increases the risk of hepatocarcinoma compared to infection with just HBV alone[89].
HDV has a worldwide distribution, though with great variations in prevalence (higher in Latin America and eastern Europe but lower in western Europe, Japan and North America, where better socioeconomic conditions and HBV vaccination mean it is virtually restricted to injection-drug users). At least 5% of HBV carries in the world are thought to be infected with HDV, though it is probably even higher as there is no homogenous screening protocol and due to the impossibility of performing diagnostic tests in endemic areas.
The clinical course of HDV infection depends on its mode of transmission[87]. Coinfection with HBV can mean a severe clinical course in up to 15% of persons, though limited courses are more common with cure and immunity. Superinfection (HDV infection in a chronic HBV carrier) is more often associated with chronic hepatitis D that can advance to cirrhosis and liver failure[90]. Accordingly, differentiation between coinfection and superinfection is important in the management and prognosis of liver disease.
HDV infection should be investigated in all cases of acute or chronic hepatitis with a positive HBsAg[57], as any patient with HBV infection can be a carrier of HDV, especially in areas of moderate or high prevalence. HDV infection should also be investigated in patients with chronic HBV infection who present a peak of hypertransaminasemia.
No treatment for acute hepatitis due to HDV has proven useful, the disease being managed with support measures and liver transplant in fulminant cases. The only drug approved to date for the treatment of chronic hepatitis due to HDV is IFNα, which produces a modest response in 23%-57% of those treated[91,92]. NAs are not recommended for the treatment of HDV, although sustained suppression of active HBV infection with NAs can reduce the quantification of HBsAg and thus have a beneficial effect on coinfection with HDV[67].
Several new treatments are being tried aimed at blocking the viral cycle at different points: inhibitors of virus entry into the hepatocyte, assembly and other new strategies based on immune stimulation with cytokines and agonists of the receptors (Table 2):
| Bulevirtide | Entry inhibitor | Germany | Approved in Europe |
| Lonafarnib | Prenylation inhibitor | United States | Phase III |
| REP 2139 | HBsAg inhibitor | Canada | Phase II |
| Ezetimibe | NTCP inhibitor | Pakistan | Phase II |
Bulevirtide is the first drug to be approved by the European Commission specifically for hepatitis D. It acts by blocking entry by binding to the receptor that HBV uses to enter the liver cells (sodium taurocholate co-transporting polypeptide), thereby interfering in the life cycle of HBV and, consequently, preventing replication of HDV. Ezetimibe also acts by blocking entry and is being studied in a trial that is currently in phase II.
Inhibitors of HDV assembly: Lonafarnib is an inhibitor of farnesyl transferase, an enzyme that catalyzes prenylation, which is essential for HDV assembly. It significantly reduces the viral load when compared with placebo[93].
Inhibitor of HBsAg: A study in phase II for REP 2139 shows promising results.
As an immune modulator, IFN lambda binds to a single receptor much expressed on hepatocytes and little on hematopoietic and central nervous system cells. It is therefore well tolerated like IFNα, with a similar effect.
In conclusion, HDV, despite being minor, is in fact an important health problem in many areas of the world, being independently associated with long-term complications[94]. Standardization of diagnostic tests to detect HDV-RNA is needed. Although no specific treatment yet exists, bulevirtide has been approved by the European Commission, and other drugs are currently being developed whose efficacy and safety will need to be determined.
HCV, an RNA virus with seven genotypes, was discovered in 1989, before which it was referred to as non-A non-B[95]. HCV infection is the type that has undergone most changes over the last 10 years. From a chronic disease with few treatment options, based on IFN ± ribavirin, it has become a curable disease, even before liver damage is produced, as a result of DAA. This has led to a revolution in HCV, impacting the disease and its complications, liver transplant waiting lists and the incidence of HCV-associated hepatocarcinoma as well as the epidemiological and economic situation. In addition, the availability of an efficient therapy, limited in time and with excellent tolerance, has enabled objectives to be established for worldwide elimination of the disease. Nonetheless, HCV is still considered a prevalent disease, with healthcare repercussions and susceptible to management strategies.
The worldwide prevalence of HCV has fallen from 170 million carriers in 1999[96] to 71 million infected persons according to estimates in 2015[2], corresponding to 1% of the population, mainly due to prevention of nosocomial infection and the progressive access to efficient antiviral agents. Previously, most infections were related with transfusions of blood products, persons born between 1945 and 1965 (the so-called “baby boomer generation”), hemodialysis and hemophilia, and a high percentage of patients had advanced or decompensated disease and failure of prior therapy. More recently, infection has been associated with the use of intravenous drugs, sexual risk-practices like men who have sex with men or chemsex (Party and Play) and certain risk groups such as prisoners or those with severe mental disorders who were not treated during the era of IFN[97], with most being treatment-naïve, having little fibrosis and very often scarce awareness of their condition.
Although hepatitis C is present all over the world, China, Pakistan, India, Egypt, Russia and the United States account for more than 50% of all cases of infection in the world[98], data to be considered with migratory movements.
Despite the reduction in the incidence, approximately 400000 persons die each year from causes related to HCV, mainly cirrhosis and its complications and the development of hepatocarcinoma[2].
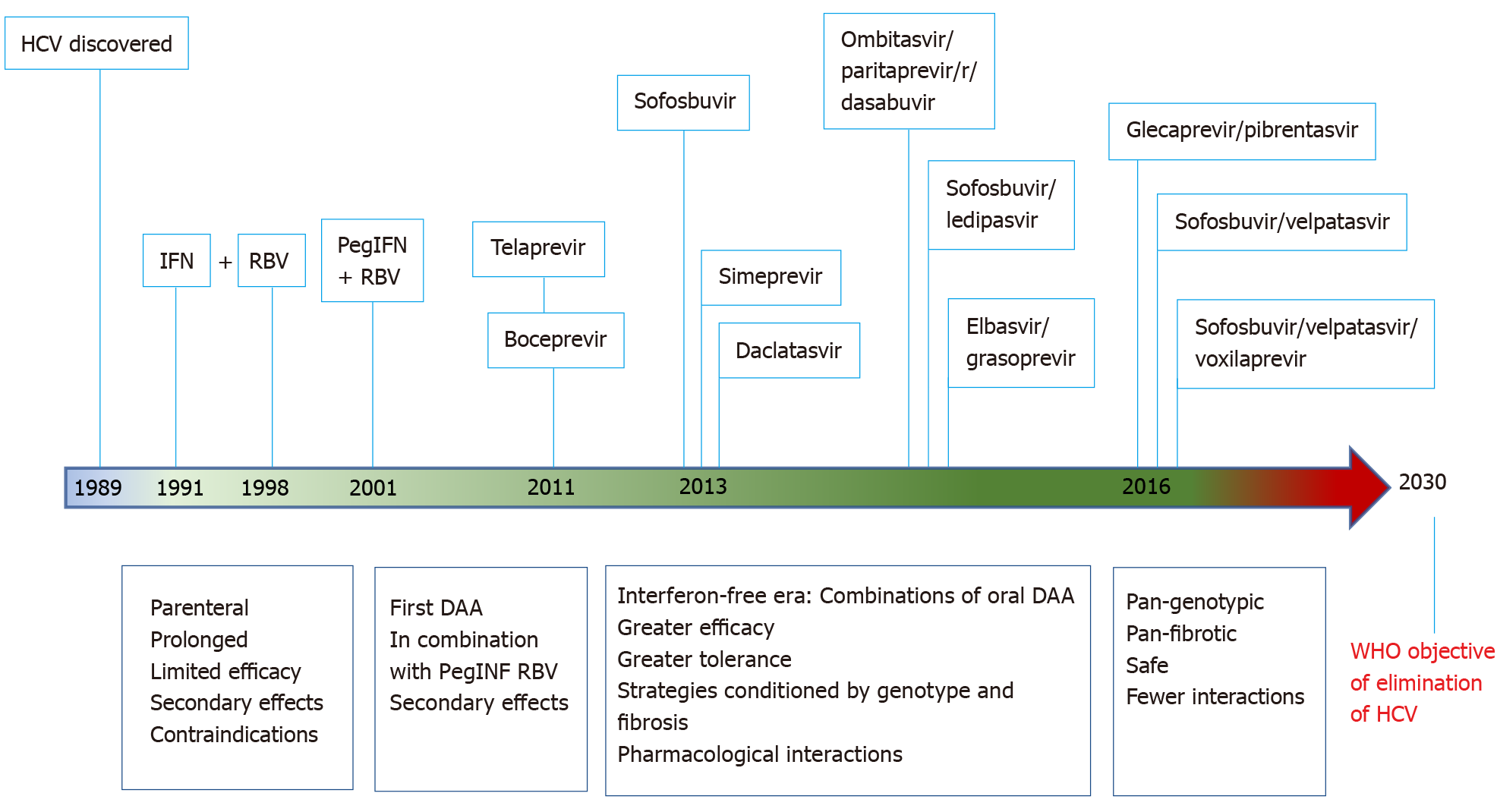
In 1991 the Food and Drug Administration approved treatment with IFN alpha for HCV infection but with a very low response rate, around 16%, a long treatment period, a parenteral route and important side effects[99]. During the 1990s ribavirin was added, slightly increasing the efficacy though again with more side effects[100]. IFN was later modified to its pegylated form, thus reducing the need for injectable doses, slightly improving tolerability. The combination of pegylated IFN and ribavirin, with cure rates up to 41% in genotype 1 and almost 75% in other genotypes[101], was the only treatment available until 2011 but with important limitations due to its multiple contraindications and frequent important secondary effects.
Better understanding of the life cycle and identification of the structural and non-structural proteins of HCV led to the development of antivirals acting directly on certain targets. The first of these were the protease inhibitors, boceprevir and telaprevir, which are oral antivirals approved by the Food and Drug Administration in 2011 for treatment of HCV genotype 1 both in naïve and pretreated patients in combination with the previous dual therapy[102,103]. This triple therapy increased the success of treatment in patients with genotype 1, but at the same time it also increased the toxicity and pharmacological interactions, the costs and the risk of decompensation of the liver disease in more advanced stages[104] as well as still requiring parenteral administration over long periods.
The real revolution in antiviral treatment arose with effect from 2013 with the successive approval of molecules having different mechanisms of action, mainly inhibition of the proteases NS3/NS4 or the polymerase replication complex NS5A. These drugs provided multiple advantages over the earlier drugs, such as oral administration, high efficacy, good tolerance and the possibility of shorter periods of treatment. The different combinations of “the new antivirals” offered multiple treatment lines depending on the clinical setting[105]. Most were IFN-free, which enabled treatment of populations that had previously been considered difficult to treat, such as the psychiatric population, persons with drug addictions or on replacement therapy, patients with kidney failure or solid organ transplant recipients[106].
The main protagonist of this change was sofosbuvir, a polymerase NS5B inhibitor acting on genotypes 1, 2, 3 and 4, though associated with other antivirals or traditional therapy. Soon after came simeprevir, a protease inhibitor with pan-genotype action[107] and daclatasvir, able to inhibit the non-structural protein NS5A and acting on genotypes 1, 3 and 4[108]. Combinations of antivirals were also produced, with different mechanisms of action, like sofosbuvir/ledipasvir[109], ombitasvir paritaprevir/ritonavir and dasabuvir[110] or grazoprevir/elbasvir[111], each with preferences for certain genotypes and different treatment durations. All of these strategies clearly increased cure rates, increasing sustained viral response (SVR) rates globally above 95% in all genotypes and around 85% in patients with advanced cirrhosis[112,113]. However, in these early IFN-free years the clinical management of HCV became increasingly complex. Before choosing the best treatment option in each case it was necessary to identify the genotype as well as the stage of the liver disease and the particular degree of fibrosis, as the drugs did not have a pan-genotype action and their duration and combination were conditioned by these factors. This situation was reflected in the clinical guidelines of the time from the main scientific societies, showing multiple complex tables of recommendations for usual clinical practice[114-117].
The initial high costs also conditioned the slow access to treatment, such that many countries drew up specific protocols for the progressive approach to patients with hepatitis C, with the first patients to be treated being those with more advanced liver disease or in special situations[118]. This, together with the high risk of drug interactions and the outlines for these new therapies necessitated an exhaustive follow-up of the patient during antiviral treatment, with regular measurements of laboratory values and even excessive determinations of the HCV viral load, despite the lack of general rules concerning stopping treatment[119].
A qualitative leap occurred in 2016 with the advent of antiviral therapy with pan-genotypic and pan-fibrotic action plus their universal access. Combinations of sofosbuvir/velpatasvir[120,121] and glecaprevir/pibrentasvir[122] in single tablets have pan-genotypic and pan-fibrotic action in short-duration treatments of just 12 wk or 8 wk with the latter combination[123] and with SVR rates above 97% in practically all clinical contexts. With these two lines, plus the already available association of grazoprevir / elbasvir, also pan-genotypic, the approach is much more simplified for patients who now need treatment. This latter combination is indicated in all cases of active infection, the choice depending on certain determinants or conditioning factors, such as the renal function, which may limit the use of sofosbuvir, decompensated cirrhosis, which discourages protease inhibitors, or individual drug interactions, though these are much less common with the latest DAA compared to the earlier antiviral agents[124].
The pan-genotypic action, the high efficacy and the wide safety margin of currently available strategies have all led to simplification of the requirements to start antiviral therapy. These can be limited to determining the existence of viral replication (presence of RNA or HCV antigens), whether there is cirrhosis, which can be estimated with non-invasive methods like the APRI or FIB-4 indexes and ruling out possible interactions[125]. At the same time, monitoring during treatment has also become simpler, with the general recommendation (but not essential) of just determining the viral load 12 wk after completing treatment to confirm the SVR, with earlier safety controls when necessary due to a particular circumstance, like the cirrhotic patient[125].
An additional advantage of the current panorama is the existence of rescue therapy for the few cases that fail to achieve an SVR. The association of sosfosbuvir / velapatasvir / voxilaprevir is indicated for patients who fail with DAA therapy, with a high SVR rate in all genotypes[126].
This radical change over recent years in the setting of antiviral therapy has enabled a high percentage of the HCV population to be treated, often resulting in the patient being discharged if the degree of fibrosis is only mild and there is no comorbidity. Nevertheless, although eradication of the virus is associated with a reduction in mortality due to hepatic and extrahepatic causes related with HCV and improvement in liver function (and even a reduction in the degree of fibrosis), those patients who already had advanced fibrosis still have a risk for complications of the liver disease or the development of hepatocarcinoma and should therefore undergo regular follow-up, even if they have an SVR[127,128].
DAAs have not only had an impact on the management of chronic hepatitis C, but the approach to cases of acute HCV infection has also changed. Historically, acute hepatitis C, defined as the first 6 mo after contact, was not generally susceptible to treatment, and pegylated IFN was used in some patients if viral replication continued for longer than 12 wk[129]. During the early phase of infection, DAAs have proven effective and beneficial, especially to eliminate the disease and particularly in such risk groups as injection drug users, men who have sex with men, persons who undertake sex practices of risk, patients with HIV or nosocomial infection. In these groups, DAAs reduce morbidity and mortality and the risk of transmission during the period waiting for the criteria of chronicity to be fulfilled before starting treatment[130,131]. Based on the available evidence the EASL recommends sofosbuvir/velpatasvir or glecaprevir/ pibrentasvir for 8 wk for what is now referred to as recently acquired hepatitis C[125]. During the era of DAA post-exposure prophylaxis is still not recommended without documented transmission of HCV[125,132].
Figure 2 shows the timeline of antiviral treatment for HCV.
The efficacy of DAA has impacted two great aspects of liver transplantation. First, it has clearly reduced the indication for liver transplant due to HCV, as shown in different series analyzing the current situation of liver transplant waiting lists[133-135]. Even so, the indication for a liver transplant still exists, mainly due to the development of HCV-associated hepatocarcinoma. Second, DAAs have enabled the majority of patients to reach liver transplantation with no viral replication. This, therefore, eliminates the risk of recurrence of hepatitis C in the graft. Additionally, when necessary, DAA can be used safely after transplantation, with little risk of graft rejection and high efficacy, unlike earlier IFN-based treatments[105,136]. This efficacy and safety have been confirmed in recipients of other solid organ transplants, such as kidney transplant patients with chronic hepatitis C[137], thereby endorsing their use in the transplant population.
Organ donation from patients with active HCV infection remains controversial, especially for seronegative recipients, but the safety and efficacy of DAAs in solid organ recipients nevertheless allows this option to be contemplated. Different series have shown good results in lung, heart[138] and kidney transplantation. Even in liver transplants[139], provided the benefit of the transplant exceeded the risk of death on the waiting list, and early access to DAA treatment is guaranteed[140,141].
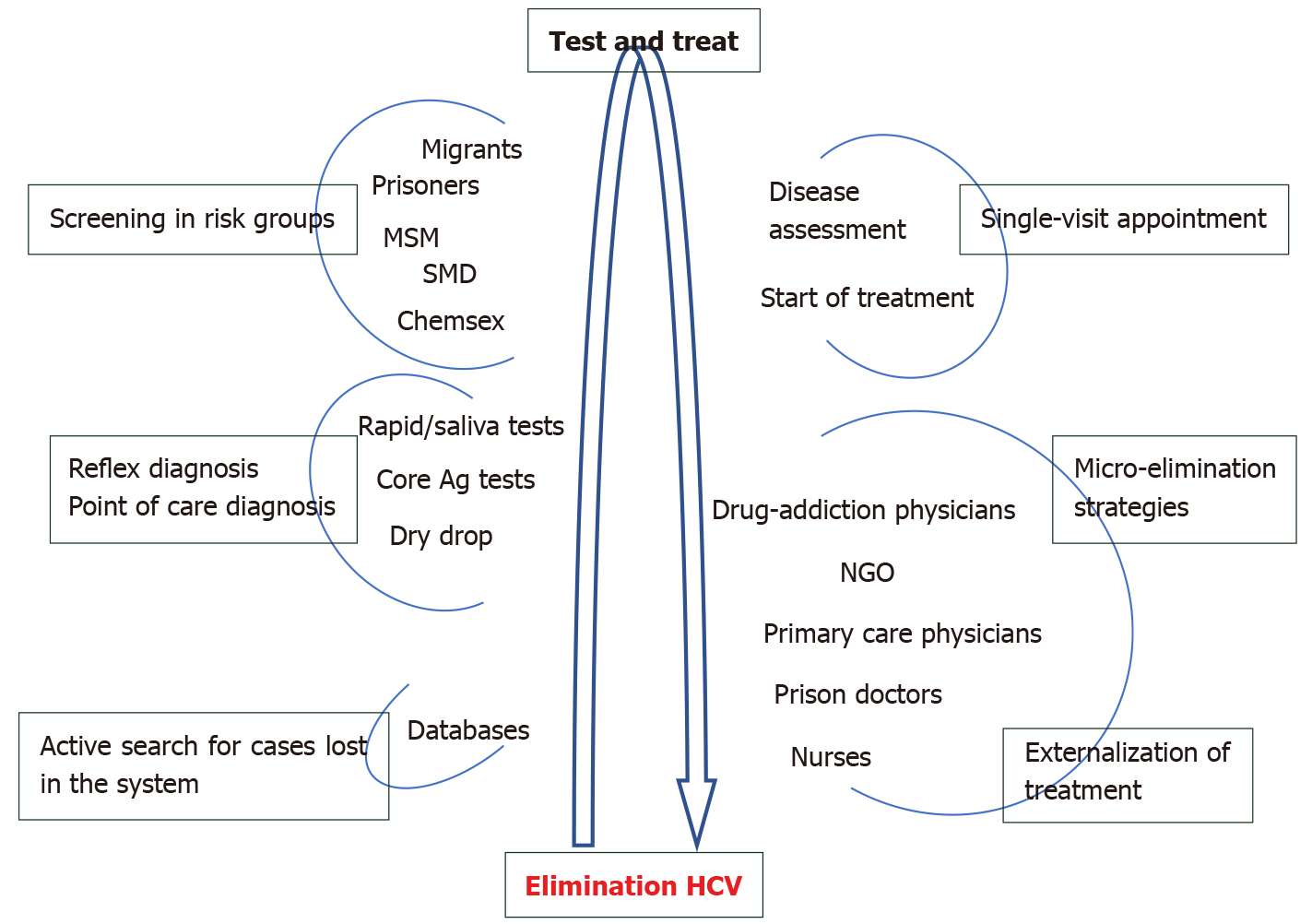
The World Health Organization established the objective of elimination of hepatitis C by 2030, defined as a reduction of 80% in cases of de novo infection and a reduction of 65% in death due to HCV[9]. Besides currently available efficient treatment, this objective also requires first making the population aware by means of information campaigns, second, rescuing patients who have already been diagnosed but not treated and third, simplifying the whole diagnostic process and treatment access when necessary in order to guarantee diagnosis and treatment of the greatest number of cases possible.
The active search for already diagnosed patients is one of the main and most feasible strategies for micro-elimination as it permits cases to be rescued for treatment[142].
Reflex testing[143], as well as points of care, will enable diagnosis to be externalized, reaching risk groups with little contact with the healthcare system, like migrants or prisoners[144]. The diagnosis of hepatitis C should be directly linked to starting treatment (test and treat) so that in certain populations it will also be necessary to externalize treatment in the near future. For this, pilot projects have been designed with treatment administered by non-specialized healthcare personnel, like nurses[145], prison doctors, addiction physicians and primary care physicians[146], which has achieved a cost-effective elimination in the groups attended.
Populations still remain in which treatment needs further study, like pregnant women, in whom it is not recommended given the lack of safety data. It may, though, be the optimal time for screening in this group as it is sometimes the only contact they have with healthcare systems[113].
Notwithstanding the above, these emerging models of care of the hepatitis C patient and micro-elimination must not detract from the importance of preventive measures, which necessitate adequate information for the population, particularly the already-mentioned risk groups.
The elimination of hepatitis C is associated with a significant reduction in complications due to liver disease, a reduction in mortality due to liver disease and the risk of developing hepatocarcinoma, in both patients with mild fibrosis and in those with advanced fibrosis[127]. In addition, suppression of viral replication is associated with a reduction in systemic symptoms, mainly diabetes and vascular events, which in turn is associated with a reduction in mortality due to extrahepatic causes[147].
The impact of SVR on portal hypertension and its complications may result in a discrete reduction in pressure gradients in the hepatic veins, particularly in the year of reaching SVR[148]. However, as not all studies have associated an SVR with regression of portal hypertension or regression of esophageal varices[149], the Baveno VI consensus recommends the same cut points for screening of varices in patients with SVR[150].
Several cohort studies have shown that the risk of hepatocarcinoma is reduced after SVR[127], but it does not disappear. Patients with advanced fibrosis and cirrhosis should continue to be screened for hepatocarcinoma as its annual global incidence is 2.5%-4.5%, even with SVR[151]. In patients with mild fibrosis, continued screening for other risk factors, like diabetes, metabolic liver disease, low albumin levels or thrombocytopenia, for the development of hepatocarcinoma should be contemplated[127].
Figure 3 shows the main recommendations for elimination of hepatitis C.
Although the various types of viral hepatitis still represent a great public health problem, the great advances over recent years are very positively impacting their management and prognosis. Nevertheless, it is still necessary to persist with, and even boost studies designed to find efficient new therapies, though without forgetting the implementation of preventive measures, as a basis of achieving advances in minimizing the impact of this disease on health.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai K, Hercun J S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu M
| 1. | Rodríguez M, Buti M, Esteban R, Lens S, Prieto M, Suárez E, García-Samaniego J. Consensus document of the Spanish Association for Study of the Liver on the treatment of hepatitis B virus infection (2020). Gastroenterol Hepatol. 2020;43:559-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Weekly epidemiological record Relevé épidémiologique hebdomadaire. Wkly Epidemiol Rec. 2012;87:261-76. [PubMed] |
| 4. | Fuentes Olmo J, Uribarrena Amézaga R. [Current treatment of hepatitis B infection: where do the new nucleos(t)ide analogues fit in?]. Gastroenterol Hepatol. 2011;34:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Koumbi L. Current and future antiviral drug therapies of hepatitis B chronic infection. World J Hepatol. 2015;7:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Likhitsup A, Lok AS. Understanding the Natural History of Hepatitis B Virus Infection and the New Definitions of Cure and the Endpoints of Clinical Trials. Clin Liver Dis. 2019;23:401-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Hepatitis B Foundation. Drug Watch. [cited December 10, 2020]. Available from: https://www.hepb.org/treatment-and-management/drug-watch/. |
| 8. | Asselah T, Loureiro D, Tout I, Castelnau C, Boyer N, Marcellin P, Mansouri A. Future treatments for hepatitis delta virus infection. Liver Int. 2020;40 Suppl 1:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. [cited December 10, 2020]. Available from: https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030. |
| 10. | Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653-6657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (3)] |
| 11. | Nelson NP, Murphy TV. Hepatitis A: The Changing Epidemiology of Hepatitis A. Clin Liver Dis (Hoboken). 2013;2:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Desai AN, Kim AY. Management of Hepatitis A in 2020-2021. JAMA. 2020;324:383-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000;14:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lin KY, Chen GJ, Lee YL, Huang YC, Cheng A, Sun HY, Chang SY, Liu CE, Hung CC. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: A review. World J Gastroenterol. 2017;23:3589-3606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 15. | Lee EJ, Kwon SY, Seo TH, Yun HS, Cho HS, Kim BK, Choe WH, Lee CH, Kim JN, Yim HJ. [Clinical features of acute hepatitis A in recent two years]. Korean J Gastroenterol. 2008;52:298-303. [PubMed] |
| 16. | Tekin R, Yolbas I, Dal T, Demirpençe Ö, Kaya S, Bozkurt F, Deveci Ö, Çelen MK, Tekin A. Evaluation of adults with acute viral hepatitis a and review of the literature. Clin Ter. 2013;164:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM; U. S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1462] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 18. | Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ; US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 19. | Abutaleb A, Kottilil S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol Clin North Am. 2020;49:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Werzberger A, Mensch B, Nalin DR, Kuter BJ. Effectiveness of hepatitis A vaccine in a former frequently affected community: 9 years' followup after the Monroe field trial of VAQTA. Vaccine. 2002;20:1699-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Armstrong ME, Giesa PA, Davide JP, Redner F, Waterbury JA, Rhoad AE, Keys RD, Provost PJ, Lewis JA. Development of the formalin-inactivated hepatitis A vaccine, VAQTA from the live attenuated virus strain CR326F. J Hepatol. 1993;18 Suppl 2:S20-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Rao S, Mao JS, Motlekar S, Fangcheng Z, Kadhe G. A review of immunogenicity and tolerability of live attenuated Hepatitis A vaccine in children. Hum Vaccin Immunother. 2016;12:3160-3165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Van Damme P, Van Herck K. A review of the efficacy, immunogenicity and tolerability of a combined hepatitis A and B vaccine. Expert Rev Vaccines. 2004;3:249-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Irving GJ, Holden J, Yang R, Pope D. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev. 2012;CD009051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 26. | World Health Organization. Global Alert and Response (GAR): Hepatitis A. [cited December 10, 2020]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsredc2007/en/index4.html#estimated. |
| 27. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 747] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 28. | Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 29. | Donnelly MC, Scobie L, Crossan CL, Dalton H, Hayes PC, Simpson KJ. Review article: hepatitis E-a concise review of virology, epidemiology, clinical presentation and therapy. Aliment Pharmacol Ther. 2017;46:126-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Gurley ES, Hossain MJ, Paul RC, Sazzad HM, Islam MS, Parveen S, Faruque LI, Husain M, Ara K, Jahan Y, Rahman M, Luby SP. Outbreak of hepatitis E in urban Bangladesh resulting in maternal and perinatal mortality. Clin Infect Dis. 2014;59:658-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol. 2017;27:e1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 33. | Anty R, Ollier L, Péron JM, Nicand E, Cannavo I, Bongain A, Giordanengo V, Tran A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J Clin Virol. 2012;54:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Goel A, Aggarwal R. Advances in hepatitis E - II: Epidemiology, clinical manifestations, treatment and prevention. Expert Rev Gastroenterol Hepatol. 2016;10:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Fontana RJ, Engle RE, Scaglione S, Araya V, Shaikh O, Tillman H, Attar N, Purcell RH, Lee WM; US Acute Liver Failure Study Group. The role of hepatitis E virus infection in adult Americans with acute liver failure. Hepatology. 2016;64:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Crossan CL, Simpson KJ, Craig DG, Bellamy C, Davidson J, Dalton HR, Scobie L. Hepatitis E virus in patients with acute severe liver injury. World J Hepatol. 2014;6:426-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Scobie L, Dalton HR. Hepatitis E: source and route of infection, clinical manifestations and new developments. J Viral Hepat. 2013;20:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Barragué H, Condat B, Petitdidier N, Champagne E, Renou C, Izopet J, Abravanel F. Chronic hepatitis E virus infection in a cirrhotic patient: A case report. Medicine (Baltimore). 2017;96:e7915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | González Tallón AI, Moreira Vicente V, Mateos Lindemann ML, Achécar Justo LM. [Chronic hepatitis E in an immunocompetent patient]. Gastroenterol Hepatol. 2011;34:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 42. | Unzueta A, Rakela J. Hepatitis E infection in liver transplant recipients. Liver Transpl. 2014;20:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Tenorio González E, Robles Díaz M, Sanjuan Jiménez R, González Grande R, Olmedo Martín RV, Rodrigo López JM, Jiménez Pérez M. Retransplant Due to Fulminant Hepatic Failure From Hepatitis E Virus: A Case Report. Transplant Proc. 2018;50:685-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 45. | Sanabria-Cabrera J, Sanjuán-Jiménez R, Clavijo E, Medina-Cáliz I, González-Jiménez A, García-Cortés M, Ortega-Alonso A, Jiménez-Pérez M, González-Grande R, Stephens C, Robles-Díaz M, Lucena MI, Andrade RJ; Spanish DILI Registry. Incidence and prevalence of acute hepatitis E virus infection in patients with suspected Drug-Induced Liver Injury in the Spanish DILI Registry. Liver Int. 2020;47:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Jiménez-Pérez M, González-Grande R, García-Cortés M, Andrade RJ. Drug-Induced Liver Injury After Liver Transplantation. Liver Transpl. 2020;26:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Talapko J, Meštrović T, Pustijanac E, Škrlec I. Towards the Improved Accuracy of Hepatitis E Diagnosis in Vulnerable and Target Groups: A Global Perspective on the Current State of Knowledge and the Implications for Practice. Healthcare (Basel). 2021;9:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Pallerla SR, Harms D, Johne R, Todt D, Steinmann E, Schemmerer M, Wenzel JJ, Hofmann J, Shih JWK, Wedemeyer H, Bock CT, Velavan TP. Hepatitis E Virus Infection: Circulation, Molecular Epidemiology, and Impact on Global Health. Pathogens. 2020;9:856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 49. | Cornberg M, Pischke S, Müller T, Behrendt P, Piecha F, Benckert J, Todt D, Steinmann E, Papkalla A, von Karpowitz M, Koch A, Lohse A, Hardtke S, Manns MP, Wedemeyer H. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E - The HepNet SofE pilot study. J Hepatol. 2020;73:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Boland F, Martinez A, Pomeroy L, O'Flaherty N. Blood Donor Screening for Hepatitis E Virus in the European Union. Transfus Med Hemother. 2019;46:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 51. | Cao YF, Tao H, Hu YM, Shi CB, Wu X, Liang Q, Chi CP, Li L, Liang ZL, Meng JH, Zhu FC, Liu ZH, Wang XP. A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine. 2017;35:5073-5080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 53. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 54. | Buti M, García-Samaniego J, Prieto M, Rodríguez M, Sánchez-Tapias JM, Suárez E, Esteban R. [Consensus document of the Spanish Association for the Study of the Liver on the treatment of hepatitis B infection (2012)]. Gastroenterol Hepatol. 2012;35:512-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Wu J, Mao W. Review of Serum Biomarkers and Models Derived from Them in HBV-Related Liver Diseases. Dis Markers. 2020;2020:2471252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 57. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 58. | Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M, Waggoner JG, Park Y, Jones EA. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 646] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 59. | Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. J Gastroenterol Hepatol. 2011;26 Suppl 1:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Summers J, Mason WS. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1110] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 61. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray DF. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 62. | Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, Manns M, Kaita K, Krastev Z, Lee SS, Cathcart AL, Crans G, Op den Brouw M, Jump B, Gaggar A, Flaherty J, Buti M. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 63. | Luo J, Li X, Wu Y, Lin G, Pang Y, Zhang X, Ao Y, Du Z, Zhao Z, Chong Y. Efficacy of entecavir treatment for up to 5 years in nucleos(t)ide-naïve chronic hepatitis B patients in real life. Int J Med Sci. 2013;10:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Halegoua-De Marzio D, Hann HW. Then and now: the progress in hepatitis B treatment over the past 20 years. World J Gastroenterol. 2014;20:401-413. [PubMed] [DOI] [Full Text] |
| 65. | Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, Kitrinos KM, Marcellin P, Gane EJ. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg- patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 66. | Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 wk treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 67. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 68. | Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of Hepatocellular Carcinoma in Patients Treated With Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019;5:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 69. | Oh H, Yoon EL, Jun DW, Ahn SB, Lee HY, Jeong JY, Kim HS, Jeong SW, Kim SE, Shim JJ, Sohn JH, Cho YK; Long-Term Safety of Entecavir and Tenofovir in Patients With Treatment-Naive Chronic Hepatitis B Virus (CHB) Infection (SAINT) Study. No Difference in Incidence of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B Virus Infection Treated With Entecavir vs Tenofovir. Clin Gastroenterol Hepatol. 2020;18:2793-2802.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology. 2020;158:215-225.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 71. | Choi WM, Choi J, Lim YS. Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:246-258.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 72. | Jiménez-Pérez M, González-Grande R, Mostazo Torres J, González Arjona C, Rando-Muñoz FJ. Management of hepatitis B virus infection after liver transplantation. World J Gastroenterol. 2015;21:12083-12090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1588] [Article Influence: 176.4] [Reference Citation Analysis (2)] |
| 74. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1960] [Article Influence: 217.8] [Reference Citation Analysis (0)] |
| 75. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 76. | Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Fälth M, Stindt J, Königer C, Nassal M, Kubitz R, Sültmann H, Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 623] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 77. | Tao Y, Wu D, Zhou L, Chen E, Liu C, Tang X, Jiang W, Han N, Li H, Tang H. Present and Future Therapies for Chronic Hepatitis B. Adv Exp Med Biol. 2020;1179:137-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, Locarnini S, Zoulim F, Chang KM, Lok AS. Present and future therapies of hepatitis B: From discovery to cure. Hepatology. 2015;62:1893-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 79. | Zoulim F, Yogaratnam JZ, Vandenbossche JJ, Moscalu I, Streinu-Cercel A, Lenz O, Bourgeois S, Buti M, Talloen W, Crespo J, Pascasio JM, Sarrazin C, Vanwolleghem T, Shukla U, Fry J. Safety, Pharmacokinetics and Antiviral Activity of Novel HBV Capsid Assembly Modulator, JNJ-56136379, in Patients with Chronic Hepatitis B. The Liver Meeting; 2018 Nov, San Francisco. AASLD, 2018: 9-13. |
| 80. | Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, Cho YK, Kim YJ, Hong GY, Kim DJ, Um SH, Sohn JH, Lee JW, Park SJ, Lee BS, Kim JH, Kim HS, Yoon SK, Kim MY, Yim HJ, Lee KS, Lim YS, Lee WS, Park NH, Jin SY, Kim KH, Choi W, Han KH. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2019;17:1850-1859.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Rehermann B, Thimme R. Insights From Antiviral Therapy Into Immune Responses to Hepatitis B and C Virus Infection. Gastroenterology. 2019;156:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 82. | Simón Gallo E, Cesar Caraballo C, Mateo Orozco M, Octavio Germán Muñoz. Tratamiento actual y nuevas terapias contra la infección crónica por el virus de la hepatitis B. Revista Colombiana de Gastroenterologia. 2017;32:131-140. [DOI] [Full Text] |
| 83. | Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, Lampertico P, Grossi G, Facchetti F, Brunetto MR, Coco B, Cavallone D, Mangia A, Santoro R, Piazzolla V, Lau A, Gaggar A, Subramanian GM, Ferrari C. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology. 2018;154:1764-1777.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 84. | Dembek C, Protzer U, Roggendorf M. Overcoming immune tolerance in chronic hepatitis B by therapeutic vaccination. Curr Opin Virol. 2018;30:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 85. | Smolders EJ, Burger DM, Feld JJ, Kiser JJ. Review article: clinical pharmacology of current and investigational hepatitis B virus therapies. Aliment Pharmacol Ther. 2020;51:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 642] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 87. | Koh C, Da BL, Glenn JS. HBV/HDV Coinfection: A Challenge for Therapeutics. Clin Liver Dis. 2019;23:557-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Yurdaydın C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat. 2010;17:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Abbas Z, Abbas M, Abbas S, Shazi L. Hepatitis D and hepatocellular carcinoma. World J Hepatol. 2015;7:777-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Niro GA, Smedile A. Current concept in the pathophysiology of hepatitis delta infection. Curr Infect Dis Rep. 2012;14:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R, McBurney R, Schmid P, Albrecht J, Kleiner DE, Ghany MG, Liang TJ, Hoofnagle JH. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014;40:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther. 2014;19:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, Subramanya G, Cooper SL, Pinto P, Wolff EF, Bishop R, Ai Thanda Han M, Cotler SJ, Kleiner DE, Keskin O, Idilman R, Yurdaydin C, Glenn JS, Heller T. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 94. | Yurdaydin C. New treatment options for delta virus: Is a cure in sight? J Viral Hepat. 2019;26:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 95. | Farci P. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome [Science 1989;244:359-362]. J Hepatol. 2002;36:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 273] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 97. | Mayberry J, Lee WM. The Revolution in Treatment of Hepatitis C. Med Clin North Am. 2019;103:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 99. | Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, Goodman Z, Banks SM, Hoofnagle JH. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 863] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 100. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 wk or for 24 wk vs interferon alpha2b plus placebo for 48 wk for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1640] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 101. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 102. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 103. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 104. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M, Alric L, Raabe JJ, Zarski JP, Marcellin P, Riachi G, Bernard PH, Loustaud-Ratti V, Chazouilleres O, Abergel A, Guyader D, Metivier S, Tran A, Di Martino V, Causse X, Dao T, Lucidarme D, Portal I, Cacoub P, Gournay J, Grando-Lemaire V, Hillon P, Attali P, Fontanges T, Rosa I, Petrov-Sanchez V, Barthe Y, Pawlotsky JM, Pol S, Carrat F, Bronowicki JP; CUPIC Study Group. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 105. | González-Grande R, Jiménez-Pérez M, González Arjona C, Mostazo Torres J. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (4)] |
| 106. | Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 107. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Sinha R, Beumont-Mauviel M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 108. | Amano M, Ishikawa H. [Pharmacological properties and clinical efficacy of daclatasvir (Daklinza®) and asunaprevir (Sunvepra®)]. Nihon Yakurigaku Zasshi. 2015;145:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Keating GM. Ledipasvir/Sofosbuvir: a review of its use in chronic hepatitis C. Drugs. 2015;75:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 110. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW, Rodrigues L Jr, Hu YB, Podsadecki T, Bernstein B. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 111. | Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, Robertson MN, Wahl J, Barr E, Butterton JR. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 112. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 113. | Do A, Reau NS. Chronic Viral Hepatitis: Current Management and Future Directions. Hepatol Commun. 2020;4:329-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 992] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 115. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 116. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 117. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1212] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 118. | Crespo-Casal M. Treatment guidelines for Hepatitis C in Spain. Rev Esp Sanid Penit. 2015;17:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Loggi E, Galli S, Vitale G, Di Donato R, Vukotic R, Grandini E, Margotti M, Guarneri V, Furlini G, Galli C, Re MC, Andreone P. Monitoring the treatment of hepatitis C with directly acting antivirals by serological and molecular methods. PLoS One. 2017;12:e0187755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 121. | Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S; ASTRAL-1 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 864] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 122. | Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, Felizarta F, Hassanein T, Hinrichsen H, Rincon D, Morillas R, Zeuzem S, Horsmans Y, Nelson DR, Yu Y, Krishnan P, Lin CW, Kort JJ, Mensa FJ. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 123. | Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, Asselah T, Bourlière M, Ruane PJ, Wedemeyer H, Pol S, Flisiak R, Poordad F, Chuang WL, Stedman CA, Flamm S, Kwo P, Dore GJ, Sepulveda-Arzola G, Roberts SK, Soto-Malave R, Kaita K, Puoti M, Vierling J, Tam E, Vargas HE, Bruck R, Fuster F, Paik SW, Felizarta F, Kort J, Fu B, Liu R, Ng TI, Pilot-Matias T, Lin CW, Trinh R, Mensa FJ. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med. 2018;378:354-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 124. | Falade-Nwulia O, Sulkowski MS. Hepatitis C Virus Treatment: Simplifying the Simple and Optimizing the Difficult. J Infect Dis. 2020;222:S745-S757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 126. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 127. | Masetti C, Lleo A, Colombo M, Aghemo A. Postsustained Virological Response Management in Hepatitis C Patients. Semin Liver Dis. 2020;40:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 128. | Hsu SJ, Yang SS, Kao JH. Risk of hepatocellular carcinoma development after hepatitis C virus eradicated by direct-acting antivirals: Fact or fiction? J Formos Med Assoc. 2020;119:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 130. | Sharma SA, Feld JJ. Acute hepatitis C: management in the rapidly evolving world of HCV. Curr Gastroenterol Rep. 2014;16:371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 131. | Naggie S, Fierer DS, Hughes MD, Kim AY, Luetkemeyer A, Vu V, Roa J, Rwema S, Brainard DM, McHutchison JG, Peters MG, Kiser JJ, Marks KM, Chung RT; Acquired Immunodeficiency Syndrome Clinical Trials Group (ACTG) A5327 Study Team. Ledipasvir/Sofosbuvir for 8 Weeks to Treat Acute Hepatitis C Virus Infections in Men With Human Immunodeficiency Virus Infections: Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals. Clin Infect Dis. 2019;69:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Hughes HY, Henderson DK. Postexposure prophylaxis after hepatitis C occupational exposure in the interferon-free era. Curr Opin Infect Dis. 2016;29:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Crespo G, Trota N, Londoño MC, Mauro E, Baliellas C, Castells L, Castellote J, Tort J, Forns X, Navasa M. The efficacy of direct anti-HCV drugs improves early post-liver transplant survival and induces significant changes in waiting list composition. J Hepatol. 2018;69:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 134. | Trapani S, Rizzato L, Masiero L, Ricci A, Morabito V, Peritore D, Fiaschetti P, Del Sordo E, Cacciotti AR, Montemurro A, Nanni Costa A. Hepatitis C Virus Positive Patients on the Waiting List for Liver Transplantation: Turnover and Characteristics of the Population on the Eve of the Therapeutic Revolution With Direct-Acting Antivirals. Transplant Proc. 2017;49:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 135. | Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, Karam V, Salizzoni M, Andujar RL, Fondevila C, De Simone P, Morelli C, Fabregat-Prous J, Samuel D, Agarwaal K, Moreno Gonzales E, Charco R, Zieniewicz K, De Carlis L, Duvoux C; all the contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 136. | Nogueras López F, López Garrido A, Ortega Suazo EJ, Vadillo Calles F, Valverde López F, Espinosa Aguilar MD. Therapy With Direct-Acting Antiviral Agents for Hepatitis C in Liver Transplant Recipients. Transplant Proc. 2018;50:631-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 137. | Reau N, Kwo PY, Rhee S, Brown RS Jr, Agarwal K, Angus P, Gane E, Kao JH, Mantry PS, Mutimer D, Reddy KR, Tran TT, Hu YB, Gulati A, Krishnan P, Dumas EO, Porcalla A, Shulman NS, Liu W, Samanta S, Trinh R, Forns X. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology. 2018;68:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 138. | Liapakis A, Formica RN, Levitsky J. Solid organ transplantation of viral hepatitis C positive donor organs into viral hepatitis C negative recipients. Curr Opin Organ Transplant. 2018;23:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 139. | Ting PS, Hamilton JP, Gurakar A, Urrunaga NH, Ma M, Glorioso J, King E, Toman LP, Wesson R, Garonzik-Wang J, Ottmann S, Philosophe B, Sulkowski M, Cameron AM, Durand CM, Chen PH. Hepatitis C-positive donor liver transplantation for hepatitis C seronegative recipients. Transpl Infect Dis. 2019;21:e13194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Kapila N, Menon KVN, Al-Khalloufi K, Vanatta JM, Murgas C, Reino D, Ebaid S, Shaw JJ, Agrawal N, Rhazouani S, Navas V, Sheffield C, Rahman AU, Castillo M, Lindenmeyer CC, Miller C, Quintini C, Zervos XB. Hepatitis C Virus NAT-Positive Solid Organ Allografts Transplanted Into Hepatitis C Virus-Negative Recipients: A Real-World Experience. Hepatology. 2020;72:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 141. | Weinfurtner K, Reddy KR. Hepatitis C viraemic organs in solid organ transplantation. J Hepatol. 2021;74:716-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 142. | Kracht PAM, Arends JE, van Erpecum KJ, Thijsen SFT, Vlaminckx BJM, Weersink AJL, Wensing AMJ, Deege MPH, Dimmendaal M, Stadhouders PHGM, Friederich PW, Verhagen MAMT, Boland GJ, Hoepelman AIM. REtrieval And cure of Chronic Hepatitis C (REACH): Results of micro-elimination in the Utrecht province. Liver Int. 2019;39:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 143. | Crespo J, Lázaro P, Blasco AJ, Aguilera A, García-Samaniego J, Eiros JM, Calleja JL, García F. Hepatitis C reflex testing in Spain in 2019: A story of success. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Grebely J, Applegate TL, Cunningham P, Feld JJ. Hepatitis C point-of-care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn. 2017;17:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 145. | Papaluca T, McDonald L, Craigie A, Gibson A, Desmond P, Wong D, Winter R, Scott N, Howell J, Doyle J, Pedrana A, Lloyd A, Stoove M, Hellard M, Iser D, Thompson A. Outcomes of treatment for hepatitis C in prisoners using a nurse-led, statewide model of care. J Hepatol. 2019;70:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 146. | Rattay T, Dumont IP, Heinzow HS, Hutton DW. Cost-Effectiveness of Access Expansion to Treatment of Hepatitis C Virus Infection Through Primary Care Providers. Gastroenterology. 2017;153:1531-1543.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 147. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 148. | Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, Gallego A, Bañares R, Puente A, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, Forns X, García-Pagán JC. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273-1283.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 149. | Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, Cammà C, Grimaudo S, Pipitone RM, Simone F, Peralta S, Arini A, Craxì A. Effects of Eradicating Hepatitis C Virus Infection in Patients With Cirrhosis Differ With Stage of Portal Hypertension. Gastroenterology. 2016;151:130-139.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 150. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 151. | Na SK, Song BC. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C. Clin Mol Hepatol. 2019;25:234-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |