INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic condition, with an immune-mediated pathogenesis, that necessitates continuous medical treatment. It can be divided into three main subtypes: Crohn’s disease (CD), ulcerative colitis (UC), and IBD unclassified (IBD-U). Despite this apparently simple classification, it can manifest with a broad range of clinical phenotypes, which are only partially encompassed by Montreal classification. Medical management of patients with moderately-to-severely active IBD who develop dependence or resistance to corticosteroids is based on immunomodulator drugs (i.e., traditional immunosuppressors and targeted therapies, including biologics and small molecules)[1]. Such therapies are licenced after passing through three phases of randomized controlled trials (RCTs), and are subsequently incorporated in clinical practice. However, there is a number of clinical conditions that fall within the spectrum of IBD but are routinely excluded from RCTs. As previously noted by Ha et al[2], inclusion/exclusion criteria of RCTs prevent the enrolment of a significant proportion of IBD patients. In their 2012 work, they observed that only 31.1% of 206 IBD outpatients evaluated for RCTs would have been eligible for enrolment. It should be taken in consideration that, at that time, previous anti-tumor necrosis factor α (TNFα) exposure was a nearly ubiquitous exclusion criterion, and this might have substantially contributed to determining such a low percentage of eligible patients; however, many other criteria still stand to date – such as chronic refractory pouchitis, isolated proctitis, ileorectal anastomosis, short-bowel syndrome, and stomas in CD. Age ≥ 65 years old was an exclusion criterion in all RCTs with anti-TNFα drugs. The GEMINI program (which investigated the use of vedolizumab in moderately-to-severely active IBD) was the first RCT to include IBD patients up to 80 years of age[3,4]; many – but not all – subsequent trials also extended their upper limit of age for eligibility. However, elderly patients remain an underrepresented population in IBD clinical trials. Some recent progress needs to be recognized, as some RCTs have been conducted for these underrepresented populations (i.e., trials specifically designed for patients with chronic refractory pouchitis). Nevertheless, there is still a significant gap between the real-life IBD population and the population studied to produce evidence: Evidence-based medicine to treat these “orphan patients” is often lacking, and clinicians rely on treatments that have not been studied in these specific forms of IBD.
In this work, we aim to give a comprehensive review on the topic of “orphan IBD patients”, to present evidence available to clinicians and to highlight the main knowledge gaps that need to be fulfilled in the upcoming years.
CHRONIC REFRACTORY POUCHITIS
Acute pouchitis occurs in up to a half of UC patients who undergo total proctocolectomy with creation of an ileal pouch-anal anastomosis[5]. Antibiotic therapy (metronidazole and ciprofloxacin) is used as first-line treatment, but patients can develop dependence or refractoriness to those; in a similar fashion to what happens with corticosteroids in other forms of IBD, in these latter cases advanced therapies are frequently used. In a retrospective study including 594 patients with ileal pouch, the cumulative incidence of pouchitis was 48% during a 2-year follow-up (29% of patients had isolated acute pouchitis and the remaining 19% developed recurrent pouchitis): Of note, 40% of patients with pouchitis received non-antibiotic therapy (21% mesalamine, 7% immunomodulatory, and 7% anti-TNFα)[6].
The evidence supporting the use of immunosuppressive drugs for the treatment of antibiotic-dependent or -resistant pouchitis is quite scarce, and mostly derived from real-life experiences. Only one clinical trial designed for chronic refractory pouchitis has been conducted in the last years[7], while a few other phases 2 and 3 RCTs are congoing, either testing novel treatments [faecal microbiota transplantation (FMT), AST-120 (a spherical carbon adsorbent that binds bile acids and bacterial toxins), alicaforsen (an antisense oligonucleotide that targets the mRNA for the production of human intercellular adhesion molecule 1), AMT-101 (a recombinant biologic protein of human interleukin 10)][8-12], or drugs already approved for CD and UC (such as vedolizumab[13], ustekinumab[14], and tofacitinib[15]).
In a randomized, double-blind, placebo-controlled trial including 13 patients, adalimumab did not show any significant benefit: Among nine patients who completed the 12-wk study period, the primary outcome [reduction in clinical pouchitis activity index (PDAI) ≥ 2] was met by 50% and 43% of patients in the drug and placebo arms, respectively (P > 0.05); no differences in terms of secondary endpoints were recorded between the two groups[7]. Of note, it should be acknowledged that the sample size might have been insufficient to detect statistically significant differences between the two groups. In a large Canadian cohort of 152 patients (29% with CD-like phenotype of the pouch, and the remaining with chronic refractory pouchitis), the outcomes of those who received infliximab (n = 42) were recorded: Post-induction clinical response rate was 74% (48% achieved remission) and 62.6% of patients reported sustained response[16]. In a 2018 meta-analysis, 313 patients who received anti-TNFα treatment (194 infliximab and 119 adalimumab) to treat inflammatory complications of the pouch (i.e., refractory pouchitis and CD-like complications of the pouch) were identified: After induction, rates of clinical remission were significantly higher in CD-like complications of the pouch (0.64, 95%CI: 0.48-0.78) compared to refractory pouchitis (0.10, 95%CI: 0.00-0.35, P = 0.06), while such a difference disappeared at 12 mo (0.57, 95%CI: 0.43-0.71 for CD-like complications; 0.37, 95%CI: 0.14–0.62 for refractory pouchitis, P = 0.57). Remarkably, no difference between infliximab and adalimumab was observed, besides a numerically higher percentage of patients in clinical remission at 12 mo with infliximab compared to adalimumab (59% vs 30%, P = 0.2)[17].
Recently, data on the effectiveness of vedolizumab for the treatment of chronic refractory pouchitis have also been presented. A systematic review summarized the results from case-reports and case-series including 44 patients who received vedolizumab therapy due to antibiotic-dependent or -resistant chronic pouchitis. Only 52.3% of those patients had been previously exposed to anti-TNFα therapy. Clinical improvement at week 12 was reported by 75% of patients, and endoscopic improvement within 6 mo was recorded in 28 out of 38 (73.7%) patients[18]. Recently, Gregory et al[19] reported the results of a multicentric, retrospective study where 83 patients received vedolizumab for the treatment of chronic refractory pouchitis: 81.9% of patients had a previous diagnosis of UC, the remaining having CD (10.8%) or indeterminate colitis (7.2%), and 68.7% of them had been previously exposed to anti-TNFα therapy. Clinical response and remission were reported by 71.1% and 19.3% of patients, respectively, while endoscopic improvement was recorded in 54.1% and mucosal healing in 17.6% of them[19].
In 2019, Verstockt et al[20] reported the results of a cohort of 33 patients who received infliximab (n = 23), adalimumab (n = 13), or vedolizumab (n = 15) for the treatment of refractory pouchitis. Both anti-TNFα and vedolizumab were effective in inducing clinical improvement; interestingly, they observed that patients had a higher risk to discontinue anti-TNFα treatment compared to vedolizumab (HR = 3.0, 95%CI: 1.1-8.8, P = 0.04); this might possibly be attributed to the fact that adverse events accounted for 40.7% of anti-TNFα discontinuations, while no patient withdrew vedolizumab due to safety issues[20].
ISOLATED ULCERATIVE PROCTITIS AND ILEORECTAL ANASTOMOSIS
The mainstay of treatment for ulcerative proctitis is represented by topical aminosalicilates and/or topical steroids. Oral steroids are sometimes used in case of unresponsiveness to topical therapy[21]. Moreover, it has been suggested that oral mesalamine can reduce the risk of disease extension[22]. However, for patients who fail (or do not tolerate) conventional therapies, no evidence-based treatment is available, and clinical management is mainly based on the extrapolation of data from RCTs in UC, which include left-sided colitis and pancolitis – indeed, isolated proctitis is a nearly omnipresent exclusion criterion, as the majority of clinical trials require a disease extension of at least 15 cm from the anal verge.
A 2014 systematic review and meta-analysis concluded that there was not enough evidence to support the use of corticosteroids, thiopurines, and anti-TNFα for the treatment of ulcerative proctitis. Since then, only a few more real-life studies have been published[23]. The effectiveness of thiopurines for the treatment of refractory proctitis has been investigated in a 2017 retrospective study: At the last follow-up evaluation (median time of 46 mo), only 5 out of 25 patients were still on azathioprine treatment, while the remaining 20 were considered treatment failures[24]. Dubois et al[25] published the results of a retrospective study on a large cohort including 118 patients with ulcerative proctitis: 31% of them were refractory to rectal and oral therapy with aminosalicilates and/or steroids, requiring thiopurine monotherapy (19%) and/or biologics (28%, 25 anti-TNFα and 8 vedolizumab). Long-term outcomes pointed at a superiority of biologics over azathioprine for the treatment of refractory ulcerative proctitis, with a rate of clinical response of 70% vs 11% in favour of biologics (P = 0.001)[25]. More recently, a nationwide retrospective study from GETAID investigated the effectiveness of anti-TNFα therapy in 104 patients with refractory proctitis: 50% received infliximab, 39% adalimumab, and 11% golimumab; of note, 45% also received concomitant immunosuppressors. Clinical response was observed in 77% of patients after a median follow-up of 3 mo; the cumulative probability of sustained clinical remission was 86.7%, 74.7%, and 56.4% at 1, 2, and 5 years, respectively. After a median follow-up of 11.7 mo, 60% of the 63 patients with an available endoscopy achieved mucosal healing. Finally, 11 patients with primary nonresponse and 9 with secondary nonresponse to anti-TNFα therapy eventually received vedolizumab (after a second-line therapy with another anti-TNFα) and achieved clinical remission in 82% and 56% of cases, respectively[26].
The evidence becomes even more exiguous when it comes to patients with ileorectal anastomosis who have persistent proctitis. Abdominal colectomy with ileorectal anastomosis represents an alternative to total proctocolectomy with creation of an ileal pouch for the management of refractory UC, to maintain intestinal continuity. To date, ileoanal pouch is considered the gold standard for the surgical management of UC, but ileorectal anastomosis can be proposed to selected patients to postpone the creation of ileal pouch (for instance, in young patients, to postpone pelvic surgery) or when the creation of ileal pouch is not feasible (elderly patients). It has been reported that ileorectal anastomosis seems to be associated with fewer stool movements and night-time evacuations[27,28], and it is usually considered a less complicated procedure, compared to ileo-anal pouch[29]. On the other hand, ileorectal anastomosis comes with two main shortcomings. First, with ileorectal anastomosis, the inflamed rectum is not removed by surgery, so continuation of medical therapy is usually necessary. Indeed, it has been reported, in a retrospective study with 343 patients who received ileorectal anastomosis, that 70% of patients manifested proctitis, 76% of whom developed chronic proctitis[30]. The management of patients with ileorectal anastomosis is similar to those with isolated proctitis, and it is mainly based on topical therapy. In case of refractoriness, proctectomy can be performed to remove the inflamed rectum; otherwise, biologics are sometimes administered, but the experience with their use is extremely limited. The second issue is that ileorectal anastomosis is associated with a non-negligible risk of cancer progression within the rectum[31]. Currently, there are no guidelines for anti-neoplastic surveillance in patients with ileorectal anastomosis, but annual endoscopy is usually recommended.
STOMAS
The presence of an ostomy is an exclusion criterion in RCTs for CD patients. Nevertheless, CD patients with an ostomy are not uncommon: In CD-related surgery, ostomies are usually created in case of urgent procedures (e.g., when the patient has fistulizing disease complicated by abdominal abscess) or to exclude faecal stream for therapeutic purposes (for instance, in case of medical-refractory perianal disease). Therefore, it would appear reasonable that at least some patients with an intestinal stoma might benefit from the administration of advanced therapies. There are three main scenarios where patients with an ostomy might need advanced therapies. First, when not all of the intestinal segments affected by active CD are resected. These patients usually need medical therapy after surgery, to halt disease progression and prevent additional intestinal resections. Second, to prevent postoperative recurrence. Postoperative recurrence has been usually evaluated in patients with ileocolonic-anastomosis, but can occur after each type of resection in CD[32]. In a 2017 retrospective work, out of 83 CD patients with definitive stoma, 42% of them experienced clinical recurrence after a median follow-up of 28 mo, and 38% needed a subsequent intestinal resection after a median time of 29 mo[33]. Similarly, a recent meta-analysis observed that the median cumulative rate of clinical recurrence, in CD patients with permanent ileostomy after total colectomy, was 23.5% and 40% at 5 and 10 years, respectively[34]. Therefore, even for patients with temporary ileostomy, a prophylactic therapy might be indicated, in order to avoid early recurrences which would make it more difficult for surgeons to restore intestinal continuity. Third, advanced therapies might be required when faecal diversion is used for the management of uncontrollable perianal disease; in such cases, patients might need biological therapy for two reasons: As an additional treatment for perianal disease, or to treat active luminal CD.
The appropriateness of biological therapy in patients with ostomies has not been established. In a 2013 Letter, the outcomes of three patients with acute sever CD colitis and perianal disease managed with ileostomy and anti-TNFα therapy were reported: At the end of follow-up, all patients had undergone total colectomy with creation of permanent ileostomy – of note, one patient achieved complete clinical and endoscopic remission while with stoma, but experienced severe clinical relapse just 1 mo after stoma closure, thus requiring colectomy[35]. In a retrospective study including 21 CD patients undergoing faecal diversion due to severe perianal disease, the authors reported that infliximab use was not associated with an increased likelihood of stoma closure[36]. Similarly, Gu et al[37] reported the outcomes of a cohort of 138 CD patients with ostomies created due to perianal CD, among whom 22% managed to achieve stoma closure: Of note, the authors did not observe that biologic therapy was associated with an improved likelihood of restoration of intestinal continuity. Conversely, another retrospective work including 233 patients who had stomas created because of CD colitis reported different outcomes: The incident risk of permanent ostomy was significantly reduced in post-biological era, compared to before the introduction of biologics (19.2% vs 60.8%, P < 0.001), and biologic use was significantly associated with an higher likelihood of rectal preservation, on multivariate analysis (OR = 3.1, 95%CI: 1.0-9.5, P < 0.05)[38]. Finally, a 2017 systematic review with a meta-analysis of 18 studies (including 1438 patients) observed that, in CD patients with permanent ileostomy after total colectomy, there was no difference in the risk of clinical and surgical recurrence between studies published in pre- and post-biological eras[34].
In regard to patients with temporary ostomies, biologics might also have a downside, due to the theoretical risk that pre-operative biologic use might increase the incidence of post-surgical complications, especially infections. However, such a risk has not been unanimously reported. Notably, two recent meta-analyses showed that neither pre-operative anti-TNF[39] nor vedolizumab[40] treatment is associated with an increased risk of postoperative infections. In conclusion, whether and how biologic use before the restoration of intestinal continuity might impact the success rates of permanent stoma closure remains unknown.
SHORT BOWEL
Short bowel and intestinal failure can manifest as rare but severe complications of CD, either in patients with intestinal continuity who had undergone extensive resections, or due to the creation of proximal stomas. Short bowel syndrome represents an exclusion criterion in RCTs; however, it is not uncommon for patients with short bowel to need advanced therapies, as it can be reasonably assumed that these patients have more aggressive diseases. A history of surgical resection is associated with an increased risk of endoscopic recurrence in CD, and biologics – specifically, anti-TNFα – are used to prevent postoperative recurrence.
The effectiveness of biological therapies in patients with short bowel syndrome has not been proven, yet. Limketkai et al[41] analysed the National Impatient Sample (a United States national registry of hospitalizations), and evaluated the trends in hospitalizations and small bowel resections in CD patients with short bowel syndrome and intestinal failure (SBS-IF): When comparing the populations before and after the introduction of biologics, the authors did not observe any reduction in the rate of resections among hospitalized patients (0.7 per 1000 CD hospitalizations per year in the pre-biologic era vs 0.6-0.7 per 1000 CD hospitalizations per year in the post-biologic era). It should be acknowledged that the main limitation of this study is the method for the identification of patients with SBS-IF, as the authors included all patients with a diagnosis code of “post-surgical malabsorption”, which might have led to patient misclassifications in some cases[41]. However, in the same study Limketkai et al[41] observed a significant reduction in rates of overall small bowel resections in CD (from 99.0 to 64.6 resections per 1000 CD hospitalizations per year, P < 0.01): If this could be applied to patients with CD-related SBS-IF as well, a reduced rate of resections would have been observed in patients with the diagnosis code of post-surgical malabsorption. Another issue that should be taken in proper consideration is that patients with short bowel might have a history of multiple therapeutic failures and multiple resections: As they are not eligible in clinical trials, more advanced therapies, such as the combination of biologics, could also be considered to preserve the residual intestine[42].
Recently, growing attention has been paid to the use of teduglutide – a glucagon-like peptide 2 (GLP2) analogue – in CD patients. Teduglutide is currently licenced for the treatment of SBS-IF patients who are dependent on parenteral nutrition. In a post-hoc analysis of the STEPS study, the efficacy of teduglutide (measured in terms of reduction in weekly parenteral support at week 20) was comparable between patients with SBS-IF secondary to IBD and those who did not have IBD[43]. There has been an initial reluctance to use teduglutide in patients with active IBD, due to the concern that its gut-tropic effect might serve as a pro-inflammatory stimulus and cause IBD exacerbation – indeed, the pivotal STEPS-2 trial only allowed CD patients in stable clinical remission for at least 12 wk and biologics use was an exclusion criterion[44]. A 2017 retrospective study reported the outcomes of 13 CD patients who received teduglutide (8 of whom were on concomitant immunosuppressive therapy): Treatment with teduglutide was effective in reducing the need for parenteral support, but data on gut inflammatory activity were not reported[45]. Contrary to the initial concerns, it has been observed in murine models that teduglutide might actually exert anti-inflammatory effects[46-48]. In 2019, two CD patients who achieved control of intestinal inflammation and reduction of parenteral support while receiving the combination of teduglutide and biologics have been reported[49]. Notably, a CD patient treated only with teduglutide, previously unresponsive to multiple biological therapies, whose CD activity improved in parallel with nutritional status, has also been described[50], suggesting a link between teduglutide administration and clinically relevant anti-inflammatory effects[51].
ELDERLY PATIENTS
The elderly IBD population has been constantly expanding in the last decades. Given that IBD is a chronic disease, as life expectancy is prolonging, the prevalence of IBD in the elderly rises as a direct consequence of the ageing of patients combined with an increase of new diagnosis of late-onset IBD (i.e., IBD diagnosed after 65 years of age). The management of elderly IBD patients can be challenging, since this population is characterized by a higher number of comorbidities and an increased risk of adverse events secondary to immunosuppressive treatment. Data from the Veteran’s Health Administration showed that there is an overall reduction of use of both steroids and steroid-sparing agents in elderly IBD patients compared to younger ones[52]. Thiopurine maintenance therapy is usually discouraged in elderly patients, because it correlates with the highest absolute risk of developing lymphomas in patients ≥ 50 years old[53]. Anti-TNFα drugs have not been tested in people older than 65 years old in registration trials; they are generally underused in this population compared to younger patients, and persistence on therapy can be reduced in elderly patients, mostly due to higher rates of serious adverse events.
Vedolizumab was the first IBD drug tested in patients who are 65 to 80 years old; however, only 2-4% of patients enrolled in the GEMINI program were older than 65 years[3,4]. In a post-hoc analysis GEMINI 1 and 2 trials, it was shown that the safety and effectiveness of vedolizumab were comparable among different groups of patients stratified by age (< 35, 35-54, and ≥ 55 years old)[54]. Due to its gut-selective mechanism of action, anti-integrin therapy seems more appealing to clinicians for the treatment of elderly IBD patients, as non-anti-TNFα treatments seemingly have a more favourable safety profile compared to TNFα inhibitors in IBD. Interestingly, a retrospective study comparing the outcomes of anti-TNFα and non-anti-TNFα treatments in elderly patients did not observe any difference in terms of safety between the two groups, thus questioning whether vedolizumab actually represents a safer choice in elderly patients[55]. A 2020 prospective study conducted on patients starting vedolizumab or ustekinumab showed that Charlson comorbidity index (CCI), but not age, correlated with the occurrence of infections in vedolizumab-treated patients, and with hospitalizations in patients treated with either vedolizumab or ustekinumab[56]. We observed similar results in an Italian multicentric prospective study enrolling over 1000 patients, where elderly CD or UC patients (n = 198) were matched 1:2 to younger ones: In this cohort, a CCI > 2 was associated with a higher risk of developing any adverse events[55]. Finally, in a large cohort study including over 10000 patients, an association between pre-treatment frailty and increased risk of infections was observed in IBD patients treated with TNFα antagonists or immunosuppressors[57]. Evidence on biologic use in patients over 80 years of age is extremely scarce. In a 2020 Letter, Ayoub and colleagues reported the outcomes of 32 patients ≥ 80 years old (median age 82.5 years, range: 80-94) who received anti-TNFα agents (53.1%) or vedolizumab (46.9%): Serious infections occurred in about 15% of patients and three deaths due to cardiorespiratory causes were reported[58].
Another major question regarding the management of elderly IBD patients is whether age might impact treatment efficacy. In a 2020 Italian multicentric study, older patients with both CD and UC showed lower persistence on anti-TNFα treatment compared to younger IBD controls. Lobatón et al[59] found reduced short-term effectiveness in patients ≥ 65 years old treated with anti-TNFα therapy, but such a difference disappeared after 6 mo. We recently observed that elderly UC – but not CD – patients had significantly worse outcomes in terms of therapy persistence, steroid-free clinical remission and biochemical remission, when compared to matched younger controls[55]. Conversely, Adar and colleagues reported comparable effectiveness of anti-TNFα and vedolizumab in a retrospective cohort of IBD patients ≥ 60 years old[60].
CONCLUSION
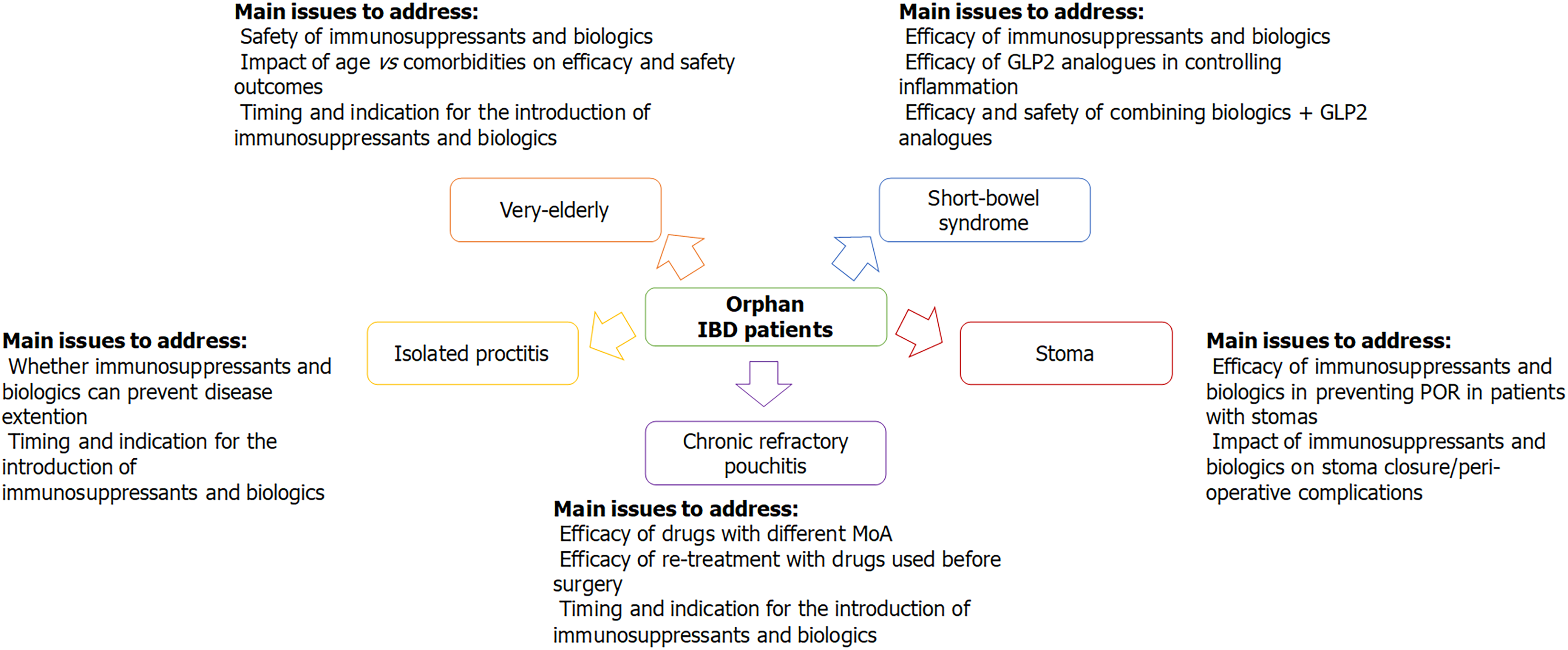
There are several categories of orphan patients in IBD, for whom no (high-)quality evidence is available and whose optimal management has not been established, yet. Accordingly, we can try to identify the main knowledge gaps that need to be filled for these specific populations. Whenever possible, RCTs should be preferred as the optimal source for evidence-based medicine; however, when clinical trials are too difficult to perform due to the relative rarity of the conditions, real-life observational studies become crucial to help clinicians in deciding patients’ management. Figure 1 presents the main conditions with “orphan IBD patients” presented in this review and highlights the main issues that need to be addressed in future research.
Figure 1 Orphan inflammatory bowel disease patients.
IBD: Inflammatory bowel disease; MoA: Mechanism of action; POR: Post-operative recurrence.
Chronic refractory pouchitis
Most data on the effectiveness of immunosuppressants come from real-life observational studies, but several RCTs are ongoing; notably, some of these trials include drugs with mechanisms of action that differ from those already licenced for CD and UC. Another major issue is whether a drug that was not effective on IBD before colectomy is ought to be taken in consideration to treat chronic refractory pouchitis in that patient. Finally, the impact of immunosuppressants on pouch failure, as well as their optimal timing for introduction, is yet to be established.
Ulcerative proctitis and ileorectal anastomosis
There is still a significant uncertainty about the appropriateness of biologic use to treat refractory ulcerative proctitis, as most evidence is derived from retrospective studies. It also needs to be addressed whether systemic immunosuppression can help in preventing disease extension: As it has been previously demonstrated that “extenders” tend to have a worse prognosis, preventing disease extension might theoretically have a positive impact on the natural history of some proctitis. However, to avoid unnecessary overtreatment, predictors to stratify patients at higher risk for colitis extension should be identified.
Stomas
No high-quality data on the effectiveness of biologics in CD patients with ostomies exist. Two main issues need to be addressed: (1) Whether biologic treatment reduces the risk of postoperative recurrence in patients with stomas: Therefore, specific risk factors for precocious recurrence in patients with ostomies should be investigated; and (2) for patients with temporary stomas, the impact of immunosuppressors on the outcomes of surgery for the restoration of intestinal continuity needs to be evaluated to answer the following questions: Do biologics improve the rates of stoma closures? Does pre-operative biologic use worsen the outcomes of intestinal anastomosis?
Short bowel
Data on the effectiveness of biologics in CD patients with short bowel are not available. Crucially, the capability of biologics to prevent further surgery in these patients should be assessed. Encouraging preliminary results on the efficacy of teduglutide in improving the nutritional status of patients who depend on parenteral support have been presented; whether GLP2 analogues might also exert some sort of immunological control over intestinal inflammation is not clear, yet. Finally, more data on safety and effectiveness of combining biologics with teduglutide are required before this strategy can be considered the standard of care for patients with short bowel and active CD.
Elderly patients
Evidence on the use of targeted therapies in the elderly IBD population is scarce. Data on treatment effectiveness suggest that there might be a difference between elderly and younger patients, but more studies are needed before any specific recommendation can be made. The major concern regarding the use of immunomodulators in elderly patients is their safety. Results have not been consistent across different reports on whether age is associated with an increased risk of adverse events, nor on the differences among treatments in regard to safety. The lines of evidence available appear to point out that patient’s functional status, rather than chronological age per se, has a clinically meaningful impact on the efficacy and safety profiles of different treatments.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huguet JM S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ