Published online Dec 14, 2021. doi: 10.3748/wjg.v27.i46.7982
Peer-review started: June 16, 2021
First decision: June 30, 2021
Revised: July 9, 2021
Accepted: November 29, 2021
Article in press: November 29, 2021
Published online: December 14, 2021
Processing time: 176 Days and 23.1 Hours
Inflammatory bowel disease (IBD) affects millions of people worldwide and has emerged as a growing problem in industrialized nations. The lack of therapeutic targets has limited the treatment of IBD. Studies found that parasitic nematode infections can ameliorate clinical and experimental colitis. Our previous study found that rSj16, a 16-kDa secreted protein of Schistosoma japonicum produced by Escherichia coli, has protective effects on dextran sulfate sodium (DSS)-induced colitis in mice. Apoptosis is an important factor in the pathogenesis of colitis. However, it is not clear whether the effect of rSj16 on colitis is related to apoptosis.
To investigate whether the protective effects of rSj16 on colitis is related to apoptosis and its mechanism.
In-vivo, colitis was induced by DSS. The severity of colitis was assessed. WB was used to detect the changes of apoptosis-related genes in colon tissues. Q-PCR was used to detect the changes of miRNA-217-5p and HNF1B. In-vitro, WB was used to detect the changes of apoptosis-related genes in intestinal epithelial cells. TUNNEL staining and flow cytometry were used to detect cell apoptosis.
rSj16 attenuates clinical activity in DSS-induced colitis mice. TUNNEL staining and WB results showed that apoptosis was increased in colon tissue after treatment with DSS, and the apoptosis of colon tissue was significantly reduced after treatment with rSj16. Compared with normal mice, the expression of miR-217-5p was increased in colon tissue of DSS-induced colitis mice. In addition, the miR-217-5p target gene hnf1b was decreased after administration of DSS. After treatment with rSj16, the expression of miR-217-5p was decreased and the expression of HNF1B was increased compared with the DSS-treated group. When Etoposide was used in combination with miR-217-5p mimic on MODE-K cells, the expression of cleaved-Caspase-3 and Bax was increased, and Bcl-2 was decreased compared with only Etoposide treatment, the expression of HNF1B was significantly reduced, suggesting that miR-217-5p acts as a pro-apoptotic in colon epithelial cells and down-regulates the target gene hnf1b. After rSj16 administration in MODE-K cells, miR-217-5p expression was significantly decreased, HNF1B expression was increased, and apoptosis was reduced.
The protective effects of rSj16 on colitis is related to apoptosis and miRNA-217-5p may be a further target for therapeutic intervention against IBD.
Core Tip: The lack of therapeutic targets has limited the treatment of inflammatory bowel disease (IBD). Parasitic nematode infections can ameliorate clinical and experimental colitis. Our previous study found that rSj16, a 16-kDa secreted protein of Schistosoma japonicum produced by Escherichia coli, has protective effects on dextran sulfate sodium (DSS)-induced colitis in mice. We found that rSj16 can inhibit DSS-induced apoptosis in the colons of mice with colitis. In addition, we found that the inhibitory effect of rSj16 on apoptosis was associated with decreased miR-217-5p, and that hepatocyte nuclear factor 1 beta was increased after treatment with rSj16. These results highlight a novel therapeutic target that may be used to treat IBD.
- Citation: Zhang LC, Wu XY, Yang RB, Chen F, Liu JH, Hu YY, Wu ZD, Wang LF, Sun X. Recombinant protein Schistosoma japonicum-derived molecule attenuates dextran sulfate sodium-induced colitis by inhibiting miRNA-217-5p to alleviate apoptosis. World J Gastroenterol 2021; 27(46): 7982-7994
- URL: https://www.wjgnet.com/1007-9327/full/v27/i46/7982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i46.7982
Inflammatory bowel disease (IBD) affects millions of people worldwide and has emerged as a growing problem in industrialized nations[1]. The two distinct forms of IBD, ulcerative colitis and Crohn’s disease, are characterized by intermittent, chronic or progressive inflammation[2]. The etiologies of both forms are multifactorial, including immunoregulatory factors, genetic susceptibility, environmental changes, and abnormalities of gut microbiota. Traditional treatments for IBD include 5-aminosalicylic acid agents, steroids, and antimicrobials. However, as these drugs have limitations and many patients cannot achieve remission, a research focus in this field is to devise biological therapies for the treatment of IBD.
Recent studies have demonstrated that helminth Schistosoma can protect against IBD. In a DSS-induced mouse colitis model, an attenuated inflammatory response was found in those infected with Schistosoma japonicum (S. japonicum)[3]. Schistosoma mansoni (S. mansoni) egg antigen has a beneficial modulatory effect in a DSS-induced mice colitis model[4]. In adult male mice with colitis, S. mansoni infection modulates the colitis mice immune system, suppressing colitis and limiting dysbiosis of intestinal microbiome[5]. Infection with S. mansoni also attenuates disease in rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis[6]. Our previous study confirmed that exosomes derived from dendritic cells treated with S. japonicum soluble egg antigen attenuate DSS-induced colitis in mice[7]. Furthermore, we have also shown that rSj16 has protective effects on DSS-induced mouse colitis[8].
Apoptosis is an important factor in the pathogenesis of colitis. Abnormal apoptosis of intestinal epithelial cells (IECs) is frequently found in IBD[9,10]. IEC apoptosis results in disruption of intestinal barrier integrity, and may allow the infiltration of bacteria, triggering an inflammatory cascade[11]. Aberrant IEC apoptosis stimulates the production of tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ), both of which further induce IEC apoptosis[12]. MicroRNAs are critical post-transcriptional regulators of gene expression and key mediators of pathophysiology of inflammatory bowel disease (IBD)[13]. However, the molecular basis of IEC apoptosis in the pathogenesis of IBD remains unclear.
MicroRNAs (miRNAs) are small non-coding RNAs that are about 22 nucleotides long. MiRNAs negatively regulate gene expression at the post-transcriptional or translational level by complementary binding to the 3′-untranslated region(UTR). MiRNAs control genes involved in cellular processes such as inflammation, cell-cycle regulation, stress response, differentiation, apoptosis, and migration[14,15]. Studies have shown that miRNAs play an important role in IBD. For example, miR-301a promotes intestinal mucosal inflammation by inducing IL-17A and TNF-α in IBD[16]. MiR-31 is increased in colon tissues of patients with IBD, reduces inflammatory signaling and promotes colon regeneration[17]. Myeloid-derived miR-223 Limits intestinal inflammation by constraining the nlrp3 inflammasome[18]. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3[19]. In Shamran’s study, miR-217 may induce Sirt-1 and provide protection against intestinal inflammation[20]. The hepatocyte nuclear factor (HNF) superfamily of transcription factors is essential for the development and maintenance of a variety of humans and mice tissues, and is further classified into four families, HNF1, FOXA, HNF4, and ONECUT, based on their functional domains. In gut, HNFs are expressed in IECs, which regulate a variety of physiological functions, including differentiation, barrier function, and metabolism[21]. Hepatic nuclear factor-4α (HNF4α) mRNA level was also downregulated in mouse model of ileitis (SAMP) compared with control mice[22]. Hepatocyte nuclear factor-1beta (HNF1B) is the most important liver-specific transcription factor, with responsibility for sequence-specific DNA binding. HNF1B is reportedly a target of miR-217, with a role in circ-TTBK2- and miR-217-mediated modulation of malignant glioma progression[23].
In this study, we investigate whether the protective effects of rSj16 on colitis is related to apoptosis and its mechanism. miRNA may function through regulating the expression of encoding genes in IBD[16]. We explore the relationship between rSj16, miR-217-5p and IBD, providing theoretical support for the clinical application of rSj16 in the treatment of IBD.
Male BALB/c mice (aged 6 wk, 18-20 g) were purchased from the Experimental Animal Center of Guangdong. All animal experimental procedures were approved by the Medical Research Ethics Committee of Sun Yat-sen University (SYSU-IACUC-2019-B517) and conformed to the Chinese National Institute of Health Guide for the Care and Use of Laboratory Animals.
Recombinant protein (rSj16) was expressed and purified as described previously[8]. A total of 15 mice were randomly assigned to three groups. Acute colitis was induced by administering water with 3% (wt/vol) DSS (36–50 kDa; MP Biomedicals, Illkirch, France) to mice over a period of 7 d. The control mice (n = 5) received drinking water. Over the same period, rSj16 was administered to the colitis mice (n = 5) via intraperitoneal (i.p.) injection (100 μg per mouse) on each day from 1 to 7. Control groups (n = 5) received the same volume of vehicle (phosphate buffered saline; PBS) over the same time frame. The mice were fed standard mouse chow.
During treatment, mice were observed daily. Changes in body weight, occurrence of diarrhea and bleeding were recorded. Blood in the feces was determined using a Hemoccult assay kit (Nanjing Jiancheng Bio-engineering Institute, China). A clinical disease score (disease activity index, DAI) was evaluated based on weight loss, diarrhea, and bleeding as described previously[8].
Mice were sacrificed on day 7. Colon length was measured, and the macroscopic scores of colons were assessed by an independent observer who was blinded to treatment status[7]. The colons were fixed in 4% formaldehyde and then embedded in paraffin. Colon sections were prepared and stained with hematoxylin and eosin (H & E). Histopathological scores were determined in a blinded fashion, according to the criteria described in our previous study.
Mouse intestinal epithelial cell line, MODE-K cells were purchased from the BeNa Culture Collection (BNCC, China). The cells were cultured with Dulbecco’s modified eagle medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 IU/mL penicillin and 100 mg/mL streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.), incubated in a 5% CO2 environment at 37 °C. The cells were seeded one day prior to transfection in 12-well cell culture plates. Cells were incubated in a serum-free medium for starvation overnight, then stimulated with miRNA mimic (Assay ID: MIMAT0000679) or mimic control (50 nM, Ruibo, Guangzhou, China) using RNAi MAX (Invitrogen, United States). MiRNA mimics are miRNAs that mimic endogenous miRNAs and can be synthesized by chemical synthesis to enhance the function of endogenous miRNAs.
MODE-K cells were seeded in 6-well plates and treated with mimic control, miRNA mimic, miRNA mimic + Etoposide (MedChemExpress, United States, 25 μM)[24], and miRNA mimic + Etoposide + rSj16 (4 μg/mL) for 48 h. Adherent and floating cells were collected and resuspended in 100 μl binding buffer. Each group of cells was stained with 2 μl Annexin-V FITC and propidium iodide (PI, BD Biosciences) at room temperature for 15 min. Samples were analyzed using a CytoFLEX S flow cytometer (Beckman Coulter, United States).
MODE-K cells were homogenized with a protein extraction reagent buffer (RIPA; Beyotime Institute of Biotechnology) containing protease and phosphatase inhibitors. Protein concentration was measured using the bicinchoninic acid assay (Beyotime Institute of Biotechnology, China). Equal amounts of proteins were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride blotting membrane (GE Healthcare Life Sciences, United Kingdom). The membrane was blocked by 10% milk. The membrane was incubated with a primary antibody (proteintech cleaved-Caspase3, Cat No. 19677-1-AP; Bax, Cat No. 50599-2-Ig; Bcl-2, Cat No. 12789-1-AP; HNF1B, Cat No. 12533-1-AP; GAPDH Sigma-Aldrich G9295) diluted 1:1,000 overnight at 4 °C, followed by incubation with the secondary antibody (ProteinTech Group, Inc.; anti-mouse, cat. no. SA00001-1; anti-rabbit, cat. no. SA00001-2) diluted 1:2000 at room temperature for 2 h. Immunodetection was performed using enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.) and visualized using chemiluminescence gel imaging system (Tanon-5200 Multi, Shanghai China). ImageJ (×64) software (National Institutes of Health) was used to quantify the results[25].
MODE-K cells treated with mimic control, miRNA mimic, miRNA mimic + Etoposide, and miRNA mimic + Etoposide + rSj16 were inoculated into 24-well plates for 48 h then fixed with 4% paraformaldehyde for 15 min at room temperature. Diluted TUNEL staining fluid (Beyotime Institute of Biotechnology) was added to cells and colon histological sections according to the manufacturer’s instructions. PBS was used to wash cells, followed by DNA staining with DAPI at room temperature for 10 min, and the staining was observed using a Leica DMI4000B fluorescence microscope (magnification, ×10), positive cells were quantified by Image J software.
Total RNA was harvested using TRIzol according to manufacturer’s instructions, including MODE-K cells and 50 mg mouse colon tissue samples. Complementary DNA (cDNA) was synthesized from 1.0 μg of total RNA with oligo (dT) primers using a cDNA Synthesis Kit (Takara, Japan). The expression of mRNA and miRNA was determined using a SYBR Green Master Mix kit (Takara, Japan), primer sequences are shown in Table 1. GAPDH or U6 were used as an internal control, and the fold change was calculated by the 2-ΔΔCT method.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
| hnf1b | CCCATCCTCAAAGAGCTCCA | AGAGGTGGGATTGGTTCAGG |
| GAPDH(Mouse) | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
| miR-217-5p | UACUGCAUCAGGAACUGACUGGA | mRQ3' Primer (Takara, Kyoto, Japan) |
| U6 | Takara, Kyoto, Japan | Takara, Kyoto, Japan |
HEK 293T cells were transfected using the HNF1B UTR reporter plasmid together with miR-217-5p mimic or control mimic for 48 h using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc). Following this period, cells were lysed using the Dual-Glo® Reagent (Dual-Glo® Luciferase Assay System; Promega Corporation). Renilla luciferase assay substrate and firefly luciferase detection reagent were added and luciferase activities were detected using the Infinite F500 Multimarker Analyser (TECAN, Austria) according to the manufacturer’s instructions. Renilla luciferase was used as an internal reference, with luciferase activity normalized to Renilla luciferase activity[26].
All data are expressed as means ± SD. Results were compared between the two groups using an unpaired two-sample t-test. Multiple comparisons between more than two groups were analysed by one-way ANOVA test or Kruskal–Wallis test (non-parametric). The value of P < 0.05 was considered statistically significant.
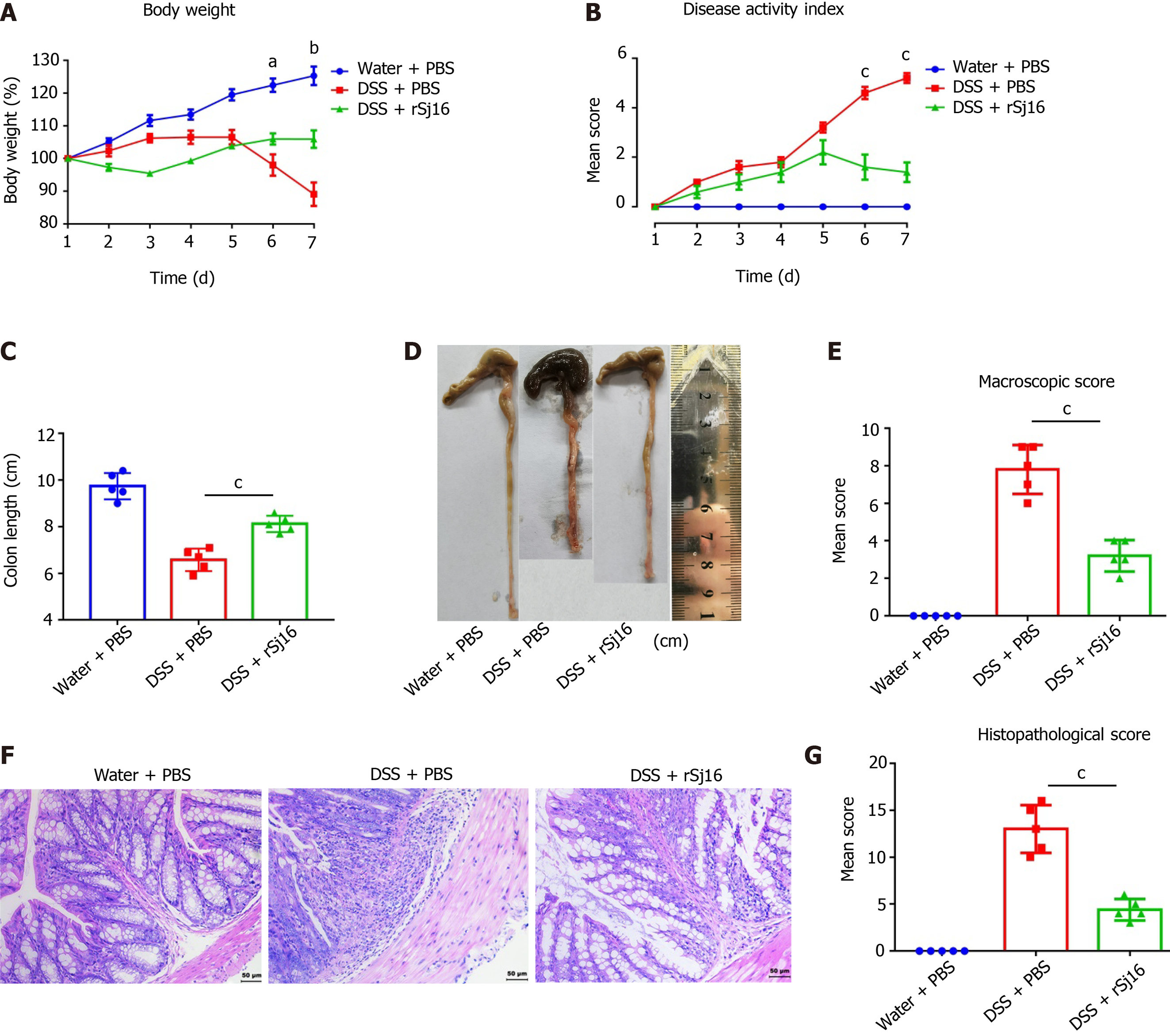
As found in our previous study[8], after DSS administration, the mice lost weight over time. At the same time, the DAI of mice with colitis also increased with time. After treatment with rSj16, body weight loss and DAI were both significantly alleviated in mice with colitis (Figure 1A and B). Colon length was significantly reduced by application of DSS, and was restored after rSj16 treatment (Figure 1C and D). Mean colon macroscopic scores were significantly suppressed in DSS + rSj16 group compared with DSS + PBS group (Figure 1E). Additionally, H&E histopathology results showed that treatment with rSj16 significantly reduced inflammation (Figure 1F). Consistent with this, histopathological scores after treatment with rSj16 + DSS were significantly lower than after treatment with DSS + PBS (Figure 1G and Table 2).
| Body weight loss on day 7 (%) (mean ± SD) | DAI on day 7 (mean ± SD) | Colon length (mean ± SD) | Macroscopic scores (mean ± SD) | Histopathological scores (mean ± SD) | n | |
| Water + PBS | 125.30 ± 6.30 | 0.00 ± 0.00 | 9.70 ± 0.56 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5 |
| DSS + PBS | 89.11 ± 8.02 | 5.20 ± 0.45 | 6.58 ± 0.48 | 7.8 ± 1.30 | 13.00 ± 2.55 | 5 |
| DSS + rSj16 | 106.00 ± 5.97 | 1.40 ± 0.89 | 8.12 ± 0.35 | 3.20 ± 0.84 | 4.4 ± 1.14 | 5 |
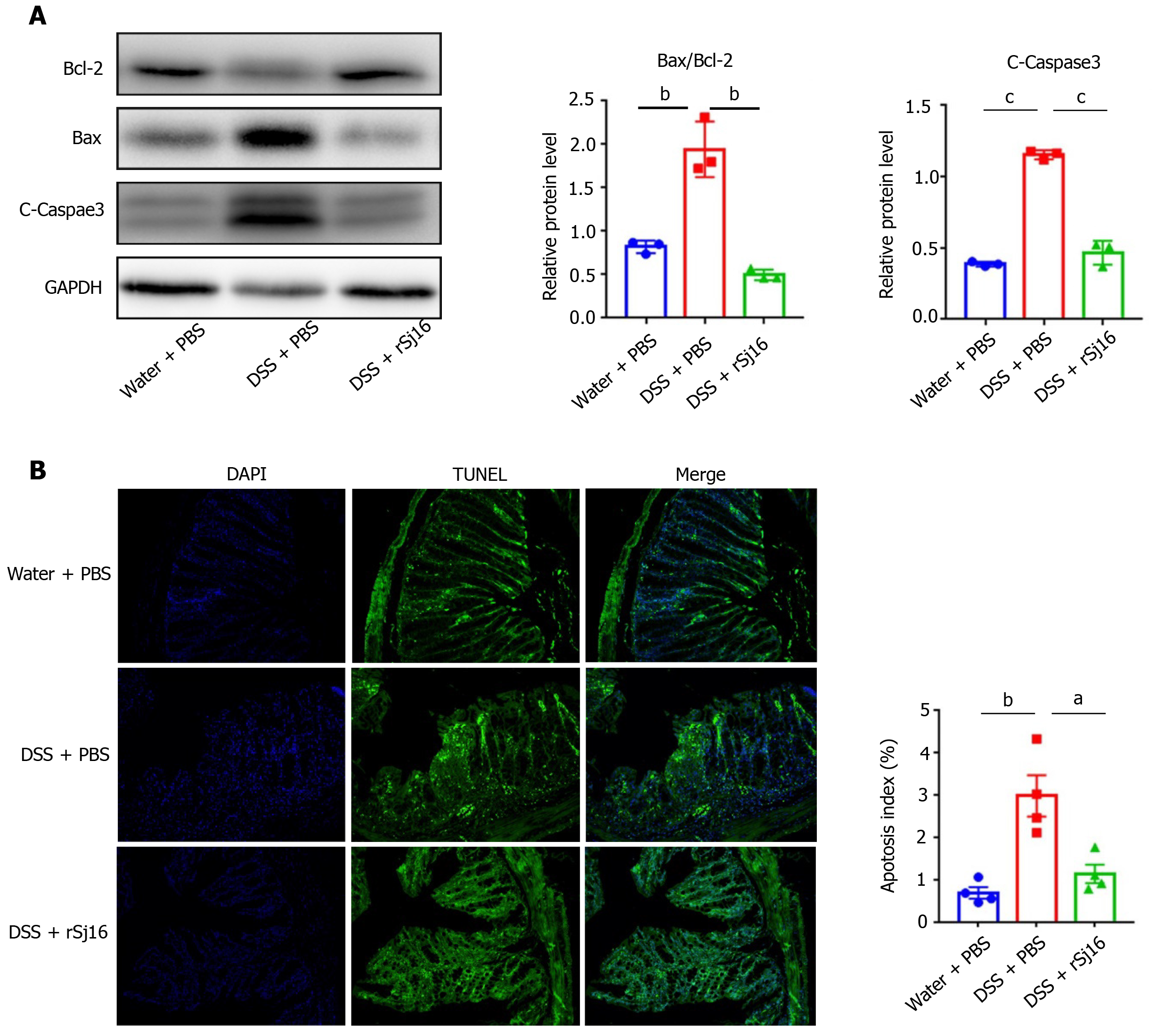
IEC apoptosis is increased in affected areas of IBD[27], leading to the disruption of intestinal barrier integrity that may allow bacteria to penetrate into the intestinal wall from the intestinal cavity and trigger an inflammatory cascade, including the production of pro-inflammatory cytokines, to remove the invading bacteria[28,29]. In the present study, to investigate the mechanism by which rSj16 alleviates DSS-induced acute colitis, 3% DSS was administered to mice daily for 7 d, and western blot was performed to detect the apoptosis of colon epithelial cells in mice. As shown in Figure 2A, the pro-apoptotic protein cleaved-Caspase-3 and Bax were increased, while the anti-apoptotic protein Bcl-2 was decreased after treatment with DSS + PBS compared with the Water + PBS, indicating that DSS can induce apoptosis of colon epithelial cells. In addition, pro-apoptotic Bax was decreased and anti-apoptotic protein Bcl-2 was increased after treatment with rSj16 + DSS compared with DSS + PBS. These results demonstrate that rSj16 may significantly inhibit DSS-induced colon epithelial cells apoptosis (Figure 2A). TUNEL staining, indicating apoptosis, was increased in colon tissue and the number of TUNEL positive cells decreased significantly after administration of rSj16 (Figure 2B).
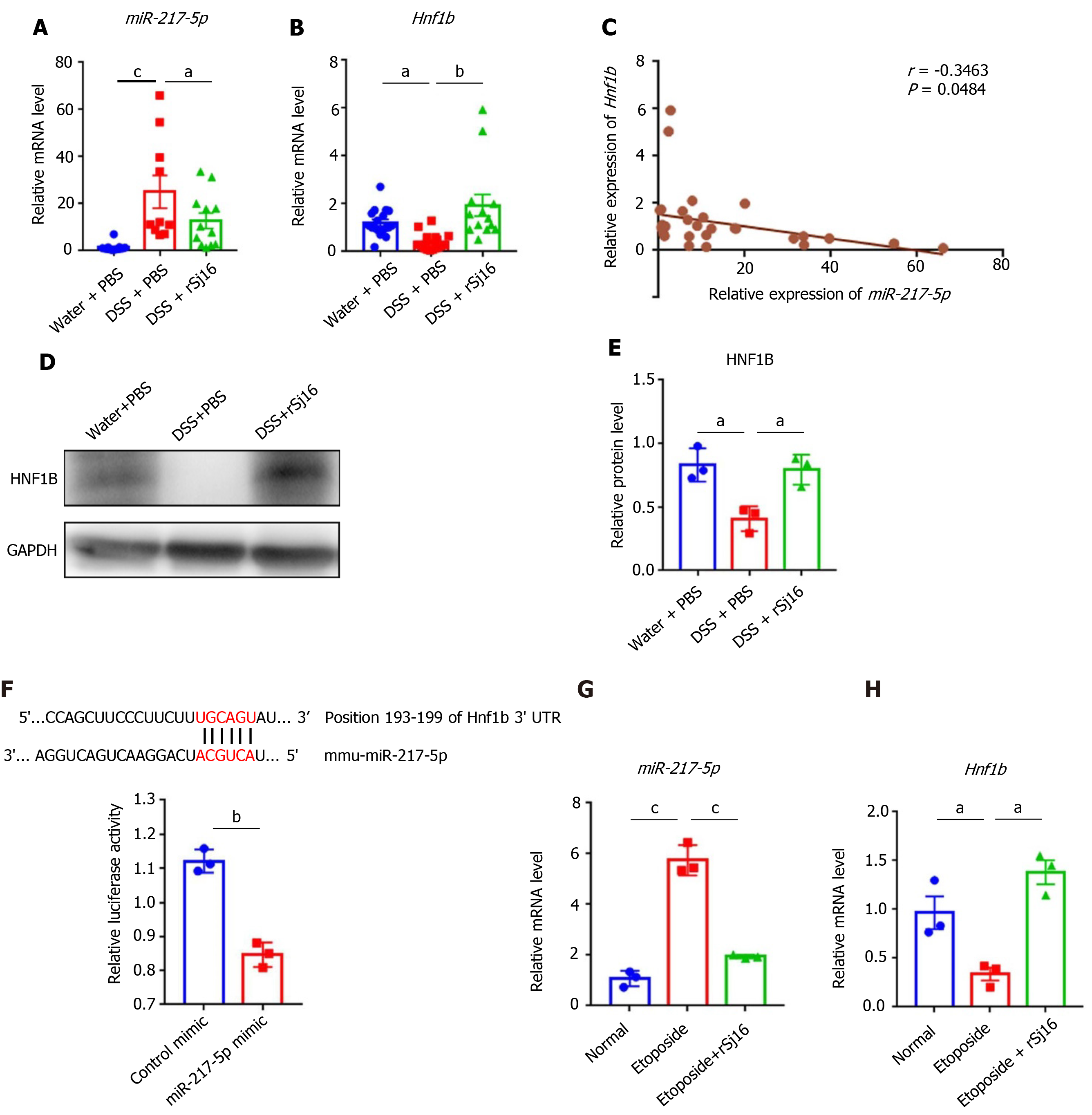
Research has shown that down-regulation of miR-217-5p may reduce the apoptosis of cardiomyocyte derived cell lines[30]. We found that, compared with Water-treated mice, the expression of miR-217-5p was increased in colon tissue of mice with DSS-induced colitis. In addition, the miR-217-5p target gene hnf1b was decreased after administration of DSS. After treatment with rSj16, the expression of miR-217-5p was decreased and the expression of hnf1b was increased compared with the DSS-treated group (Figure 3A and B). Pearson’s correlation coefficient analysis showed a negative correlation between miR-217-5p and HNF1B in in colon tissue of mice (r = -0.3463, P < 0.05) (Figure 3C). Western blot results also indicated that HNF1B was decreased after DSS treatment, and was increased after rSj16 treatment compared with DSS-treated group (Figure 3D and E).
In order to verify whether miR-217-5p regulates the expression of HNF1B, we generated a luciferase reporter plasmid contained 3'- UTR of HNF1B and located on both sides of the binding site of miR-217-5p. Relative luciferase activity of the reporter containing the predicted miR-217-5p binding sites for 3′UTR of HNF1B mRNA transcript was significantly reduced when co-transfected with miR-217-5p mimic compared with a control mimic (Figure 3F). Studies have shown that miRNA-217-5p is closely related to apoptosis[24,31]. Etoposide (an apoptosis inducer) was used to induce the apoptosis of MODE-K, and qPCR results showed that increased miRNA-217-5p expression, and decreased miR-217-5p target gene hnf1b expression in the process of apoptosis. However, rSj16 may inhibit the expression of miR-217-5p, and increase the expression of hnf1b (Figure 3G and H).
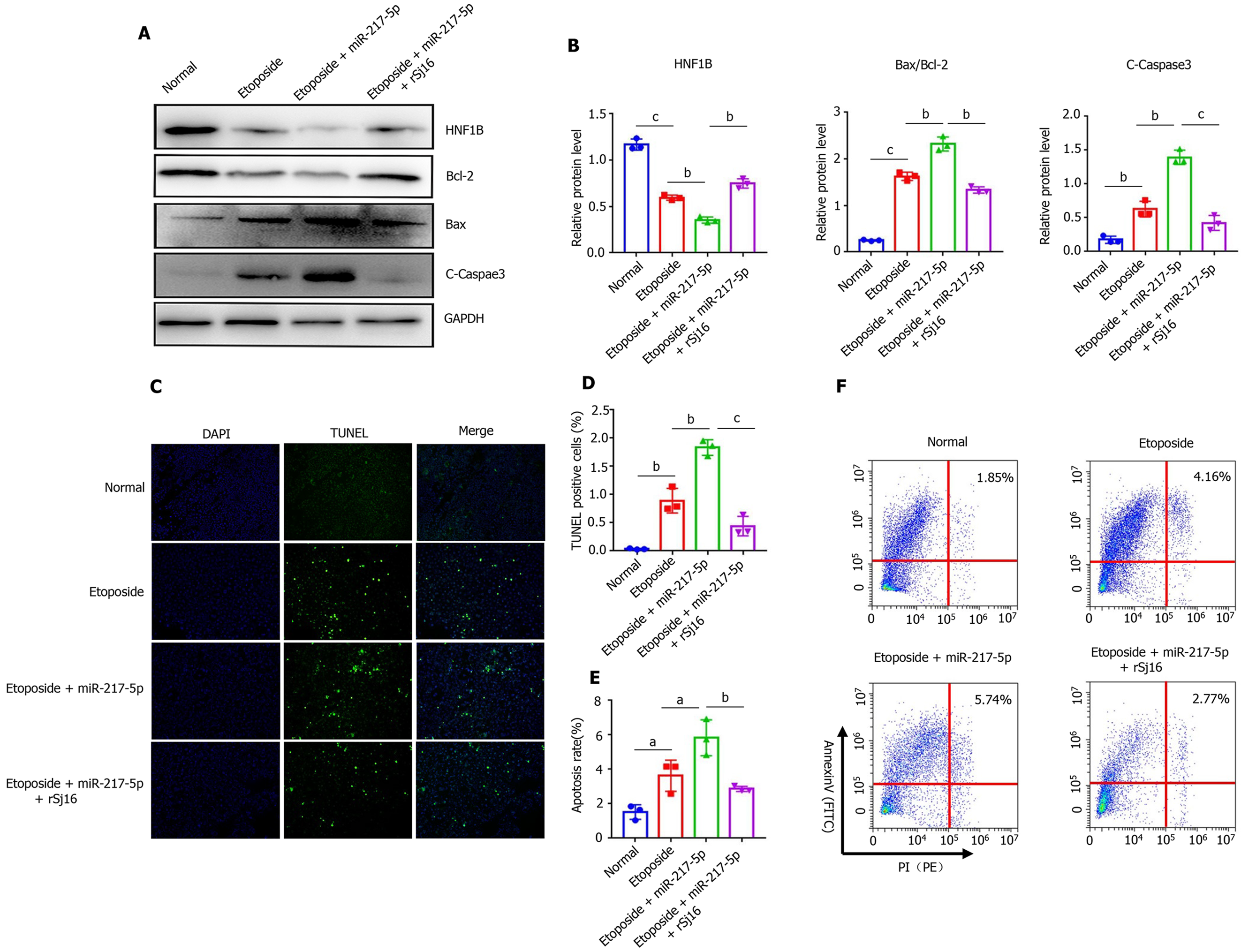
We further verified the role of miR-217-5p in the process of apoptosis, and the mechanism of rSj16 in regulating apoptosis. Western blot showed that when Etoposide was used in combination with miR-217-5p mimic on MODE-K cells, the expression of cleaved-Caspase-3 and Bax was increased, and Bcl-2 was decreased compared with only Etoposide treatment, and the expression of HNF1B was significantly reduced. These results indicate that miR-217-5p acts as a pro-apoptotic in colon epithelial cells and down-regulates the target gene hnf1b. In addition, the expression of cleaved-Caspase-3 and Bax was decreased, while Bcl-2 and HNF1B were increased in mice treated with Etoposide + miR-217-5p + rSj16 compared with Etoposide + miR-217-5p (Figure 4A and B). TUNEL staining of MODE-K after treatment of Etoposide, Etoposide + miR-217-5p, and Etoposide + miR-217-5p + rSj16, showed that the number of TUNEL positive cells increased with Etoposide + miR-217-5p and decreased after treatment with rSj16 (Figure 4C and D). Flow cytometry results also showed that miR-217-5p could obviously promote MODE -K apoptosis. However, rSj16 could signi
IBD encompasses Crohn’s disease, ulcerative colitis and IBD-unclassified. Although newer treatments have increased the chances of remission, most IBD patients cannot maintain remission, and death is not an infrequent outcome of IBD[32,33]. Therefore, it is very important to explore the pathogenesis of IBD and to find effective therapeutic targets. We have found that rSj16 (a 16-kDa secreted protein of Schistosoma japonicum) has protective effects on DSS-induced mouse colitis. Body weight loss was alleviated in mice with colitis after treatment with rSj16. DAI (evaluated based on weight loss, diarrhea, and bleeding) also alleviated in colitis mice after treatment with rSj16. The results of colon length, mean colon macroscopic scores (assessed by hyperemia, wall thickening, ulceration, inflammation extension, and damage), H&E, and histopathological scores (based on extent of inflammation, neutrophil and lympho-histiocyte infiltration, crypt damage, crypt abscess formation, sub-mucosal edema, goblet cell loss, and reactive epithelial hyperplasia displayed) indicate that rSj16 protects against acute DSS-induced colitis.
Apoptosis is an important factor in the pathogenesis of colitis. DSS has been shown to initially cause damage in the colon by inhibition of proliferation and induction of apoptosis[34]. In the present study, we found significant apoptosis of colon epithelial cells after DSS administration in mice, and inhibition of the DSS-induced apoptosis after administration of rSj16. Therefore, we hypothesized that rSj16 alleviates DSS-induced colitis, in part by regulating apoptosis.
In recent years, miRNAs have become the key biomarkers and novel therapeutic targets in IBD[16,35]. MiR-217-5p plays dual roles in regulating cell survival and apoptosis. Flum et al reported that miR-217-5p could induce apoptosis by regulating multiple target genes involved in the ERK-MAPK signaling pathway including PRKCI, BAG3, ITGAV, and MAPK1[24]. Gao et al indicated that upregulation of miR-217-5p significantly inhibited TGF-β1-induced proliferation, migration, extracellular matrix (ECM) deposition, and promoted apoptosis in airway smooth muscle cells[36]. However, Yi et al indicated that upregulation of miR-217-5p improved cell viability and attenuated cell apoptosis in SH-SY5Y cells subjected to oxygen–glucose deprivation/reperfusion[37]. The specific regulatory mechanism between miR-217-5p and apoptosis needs to be further studied. In our study, miRNA miR-217-5p was expressed at a high level in IBD mice colon tissues, and was decreased significantly following treatment with rSj16. After inducing MODE-K apoptosis, miR-217-5p expression was significantly increased, after rSj16 treatment, miR-217-5p expression was significantly reduced. Therefore, we hypothesized that miR-217-5p is involved in the protective effects of rSj16 on colitis. Bcl-2, caspase-3, and Bax play key roles in cell apoptosis[38]. Caspase-3 is a marker of apoptosis because its activity is required for major apoptosis-related morphological and biochemical events, and its activation and function are regulated by the Bcl-2 family of proteins, among other molecules[39]. In the present study, after overexpression of miR-217-5p in MODE-K cells, cleaved-Caspase-3 and Bax expression were increased, but Bcl-2 was reduced, suggesting that miR-217-5p plays a pro-apoptotic role in MODE-K cells. After rSj16 treatment, the miR-217-5p, cleaved- Caspase-3 and Bax expression were decreased, but Bcl-2 was increased, indicating that rSj16 could reduce the apoptosis. Results showed that miR-217-5p aggravated MODE-K cells apoptosis and rSj16 could significantly inhibit the apoptosis by inhibiting miRNA-217-5p expression.
MiRNAs exert pro-apoptotic functions by regulating the expression of target genes[40]. hnf1b acts as an oncogene in various tumors, is overexpressed in human prostate cancer and could promote tumor cell proliferation[41]. Early deletion of HNF1B results in a decrease in the number of pancreatic multipotent progenitor cells due to reduced proliferation and increased apoptosis[42]. In our study, we found that hnf1b is the direct target gene of miR-217-5p. In the present study, we found that DSS may induce apoptosis of colon epithelial cells, with increased expression of miR-217-5p and decreased expression of its target gene hnf1b. We speculated that miR-217-5p/HNF1B was involved in DSS-induced apoptosis of colon epithelial cells. Subsequently, we induced apoptosis and overexpression of miR-217-5p in MODE-K cells. After treatment with rSj16, the expression of miR-217-5p in tissues and MODE-K cells was decreased, the expression of its target gene hnf1b was increased, and the apoptosis of MODE-K cells significantly reduced. The results suggested that miR-217-5p exerted pro-apoptotic functions by regulating expression of the target gene hnf1b.
As for the limitations of the study, because rSj16 affects the progress of the disease through multiple pathways, we only explore one of them, suggesting that miR-217-5p/HNF1B axis could be used as a potential target for the treatment of enteritis. In addition, rSj16 may attenuate IBD through other pathways which we didn’t make a comprehensive exposition, it is still worth exploring. Next, we will conduct a more comprehensive study on the treatment of IBD with rSj16, to provide more possibilities for the development of colitis drugs.
In conclusion, rSj16 attenuates IBD in mice by regulating the miR-217-5p/ HNF1B axis to reduce colon epithelial cell apoptosis. These data indicated that miR-217-5p and HNF1B may be potential biomarkers to improve the accuracy of IBD diagnosis and treatment, and that rSj16 may have potential for clinical drug development.
Inflammatory bowel disease (IBD) encompasses Crohn’s disease, ulcerative colitis and IBD-unclassified. Although newer treatments have increased the chances of remission, most IBD patients cannot maintain remission, and death is not an infrequent outcome of IBD. Therefore, it is very important to explore the pathogenesis of IBD and to find effective therapeutic targets.
Exploring the pathogenesis of IBD and to find effective therapeutic targets.
To apoptosis and its mechanism.
In-vivo, after DSS inducing colitis. The severity of colitis was assessed. WB was used to detect the changes of apoptosis-related genes in colon tissues. In-vitro, WB, Qpcr and tunel was used to detect the changes of apoptosis.
rSj16 attenuates clinical activity in DSS-induced colitis mice. rSj16 could reduce the expression of miR-217-5p in MODE-K cells. rSj16 could regulate the apoptosis of MODE-K cells.
rSj16 attenuates IBD in mice by regulating the miR-217-5p/ HNF1B axis.
miR-217-5p and HNF1B may be potential biomarkers to improve the accuracy of IBD diagnosis and treatment, and that rSj16 may have potential for clinical drug development.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prasetyo EP, Saber A, Yang X S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3526] [Article Influence: 271.2] [Reference Citation Analysis (5)] |
| 2. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1867] [Article Influence: 186.7] [Reference Citation Analysis (1)] |
| 3. | Liu Y, Ye Q, Liu YL, Kang J, Chen Y, Dong WG. Schistosoma japonicum attenuates dextran sodium sulfate-induced colitis in mice via reduction of endoplasmic reticulum stress. World J Gastroenterol. 2017;23:5700-5712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 4. | Hasby EA, Hasby Saad MA, Shohieb Z, El Noby K. FoxP3 + T regulatory cells and immunomodulation after Schistosoma mansoni egg antigen immunization in experimental model of inflammatory bowel disease. Cell Immunol. 2015;295:67-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Floudas A, Aviello G, Schwartz C, Jeffery IB, O'Toole PW, Fallon PG. Schistosoma mansoni Worm Infection Regulates the Intestinal Microbiota and Susceptibility to Colitis. Infect Immun. 2019;87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, Pelckmans PA. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, Lin D, Liu J, Xie H, Sun X, Wu Z. Exosomes Derived from Dendritic Cells Treated with Schistosoma japonicum Soluble Egg Antigen Attenuate DSS-Induced Colitis. Front Pharmacol. 2017;8:651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Wang L, Xie H, Xu L, Liao Q, Wan S, Yu Z, Lin D, Zhang B, Lv Z, Wu Z, Sun X. rSj16 Protects against DSS-Induced Colitis by Inhibiting the PPAR-α Signaling Pathway. Theranostics. 2017;7:3446-3460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 12. | Lin W, Ma C, Su F, Jiang Y, Lai R, Zhang T, Sun K, Fan L, Cai Z, Li Z, Huang H, Li J, Wang X. Raf kinase inhibitor protein mediates intestinal epithelial cell apoptosis and promotes IBDs in humans and mice. Gut. 2017;66:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, Satsangi J. MicroRNAs: new players in IBD. Gut. 2015;64:504-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 14. | Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 15. | Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20:10432-10439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 223] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 16. | He C, Shi Y, Wu R, Sun M, Fang L, Wu W, Liu C, Tang M, Li Z, Wang P, Cong Y, Liu Z. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut. 2016;65:1938-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Tian Y, Xu J, Li Y, Zhao R, Du S, Lv C, Wu W, Liu R, Sheng X, Song Y, Bi X, Li G, Li M, Wu X, Lou P, You H, Cui W, Sun J, Shuai J, Ren F, Zhang B, Guo M, Hou X, Wu K, Xue L, Zhang H, Plikus MV, Cong Y, Lengner CJ, Liu Z, Yu Z. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology. 2019;156:2281-2296.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 18. | Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O'Neill LA, Eltzschig HK, Masters SL, McNamee EN. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214:1737-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 19. | Li M, Zhang S, Qiu Y, He Y, Chen B, Mao R, Cui Y, Zeng Z, Chen M. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017;8:e2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Shamran H, Singh NP, Zumbrun EE, Murphy A, Taub DD, Mishra MK, Price RL, Chatterjee S, Nagarkatti M, Nagarkatti PS, Singh UP. Fatty acid amide hydrolase (FAAH) blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav Immun. 2017;59:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Lussier CR, Brial F, Roy SA, Langlois MJ, Verdu EF, Rivard N, Perreault N, Boudreau F. Loss of hepatocyte-nuclear-factor-1alpha impacts on adult mouse intestinal epithelial cell growth and cell lineages differentiation. PLoS One. 2010;5:e12378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Holton NW, Singhal M, Kumar A, Ticho AL, Manzella CR, Malhotra P, Jarava D, Saksena S, Dudeja PK, Alrefai WA, Gill RK. Hepatocyte nuclear factor-4α regulates expression of the serotonin transporter in intestinal epithelial cells. Am J Physiol Cell Physiol. 2020;318:C1294-C1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Zheng J, Liu X, Xue Y, Gong W, Ma J, Xi Z, Que Z, Liu Y. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J Hematol Oncol. 2017;10:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 24. | Flum M, Kleemann M, Schneider H, Weis B, Fischer S, Handrick R, Otte K. miR-217-5p induces apoptosis by directly targeting PRKCI, BAG3, ITGAV and MAPK1 in colorectal cancer cells. J Cell Commun Signal. 2018;12:451-466. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Zheng M, Karki R, Vogel P, Kanneganti TD. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell. 2020;181:674-687.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 26. | Wang L, Liao Y, Yang R, Yu Z, Zhang L, Zhu Z, Wu X, Shen J, Liu J, Xu L, Wu Z, Sun X. Sja-miR-71a in Schistosome egg-derived extracellular vesicles suppresses liver fibrosis caused by schistosomiasis via targeting semaphorin 4D. J Extracell Vesicles. 2020;9:1785738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn's disease. Dis Colon Rectum. 2003;46:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Xu M, Zhou W, Li D, Zhang H, Chen Y, Ning L, Zhang Y, Li S, Yu M, Zeng H, Cen L, Zhou T, Zhou X, Lu C, Yu C, Li Y, Sun J, Kong X, Shen Z. Deficiency in the anti-apoptotic protein DJ-1 promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via p53. J Biol Chem. 2020;295:4237-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Peng J, Sun T, Li N, Zhang L, Ren J, Yuan H, Kan S, Pan Q, Li X, Ding Y, Jiang M, Cong X, Tan M, Ma Y, Fu D, Cai S, Xiao Y, Wang X, Qin J. Epithelial EZH2 serves as an epigenetic determinant in experimental colitis by inhibiting TNFα-mediated inflammation and apoptosis. Proc Natl Acad Sci U S A. 2017;114:E3796-E3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Qi Y, Zhang K, Li P, Wu Z. Down-regulating miR-217-5p Protects Cardiomyocytes against Ischemia/Reperfusion Injury by Restoring Mitochondrial Function via Targeting SIRT1. Inflammation. 2021;44:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Gong X, Zhu Z. Long Noncoding RNA HOTAIR Contributes to Progression in Hepatocellular Carcinoma by Sponging miR-217-5p. Cancer Biother Radiopharm. 2020;35:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Olén O, Askling J, Sachs MC, Neovius M, Smedby KE, Ekbom A, Ludvigsson JF. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. 2020;69:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4096] [Article Influence: 512.0] [Reference Citation Analysis (110)] |
| 34. | Renes IB, Verburg M, Van Nispen DJ, Taminiau JA, Büller HA, Dekker J, Einerhand AW. Epithelial proliferation, cell death, and gene expression in experimental colitis: alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int J Colorectal Dis. 2002;17:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 36. | Gao Y, Wang B, Luo H, Zhang Q, Xu M. miR-217 represses TGF-β1-induced airway smooth muscle cell proliferation and migration through targeting ZEB1. Biomed Pharmacother. 2018;108:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Yi Z, Shi Y, Zhao P, Xu Y, Pan P. Overexpression of miR-217-5p protects against oxygen-glucose deprivation/reperfusion-induced neuronal injury via inhibition of PTEN. Hum Cell. 2020;33:1026-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Moon DO, Park SY, Heo MS, Kim KC, Park C, Ko WS, Choi YH, Kim GY. Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int Immunopharmacol. 2006;6:1796-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Kuo WT, Shen L, Zuo L, Shashikanth N, Ong MLDM, Wu L, Zha J, Edelblum KL, Wang Y, Nilsen SP, Turner JR. Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology. 2019;157:1323-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 40. | Xu P, Wu Q, Lu D, Yu J, Rao Y, Kou Z, Fang G, Liu W, Han H. A systematic study of critical miRNAs on cells proliferation and apoptosis by the shortest path. BMC Bioinformatics. 2020;21:396. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Wang J, He C, Gao P, Wang S, Lv R, Zhou H, Zhou Q, Zhang K, Sun J, Fan C, Ding G, Lan F. HNF1B-mediated repression of SLUG is suppressed by EZH2 in aggressive prostate cancer. Oncogene. 2020;39:1335-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | De Vas MG, Kopp JL, Heliot C, Sander M, Cereghini S, Haumaitre C. Hnf1b controls pancreas morphogenesis and the generation of Ngn3 + endocrine progenitors. Development. 2015;142:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |