Published online Dec 14, 2021. doi: 10.3748/wjg.v27.i46.7894
Peer-review started: April 19, 2021
First decision: June 23, 2021
Revised: June 23, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: December 14, 2021
Processing time: 235 Days and 1.3 Hours
Hepatic pseudolesion may occur in contrast-enhanced computed tomography and magnetic resonance imaging due to the unique haemodynamic characteristics of the liver. The concept of hepatic arterial buffer response (HABR) has become mainstream for the understanding of the mechanism of the reciprocal effect between hepatic arterial and portal venous flow. And HABR is thought to be significantly related to the occurrence of the abnormal imaging findings on arterial phase of contrast enhanced images, such as hepatic arterial-portal vein shunt and transient hepatic attenuation difference, which mimic hypervascular tumor and may cause clinical problems. Third inflow to the liver also cause hepatic pseudolesion, and some of the cases may show histopathologic change such as focal hyperplasia, focal fatty liver, and focal sparing of fatty liver, and called pseudotumor. To understand these phenomena might be valuable for interpreting the liver imaging findings.
Core Tip: Understanding the characteristics of hepatic blood flow and the patho
- Citation: Kobayashi S. Hepatic pseudolesions caused by alterations in intrahepatic hemodynamics. World J Gastroenterol 2021; 27(46): 7894-7908
- URL: https://www.wjgnet.com/1007-9327/full/v27/i46/7894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i46.7894
Hepatocellular carcinoma (HCC) is among the leading causes of cancer-related death, and colorectal carcinoma is prone to induce hepatic metastasis. Thus, there is a growing need to develop diagnostic imaging techniques that can properly identify localized malignancies in the liver.
Ultrasound is widely available, making it helpful for screening patients with hepatic mass lesions. However, given its lack of objectivity, computed tomography (CT) and magnetic resonance (MR) imaging are mainly utilized for closer examination of potentially malignant lesions.
The liver is an organ with a unique blood supply involving two types of inflow vessels: the hepatic artery and portal vein. The differential diagnosis of hepatic masses is made using imaging findings observed in dynamic contrast-enhanced studies, including hepatic arterial phase, portal venous phase, and equilibrium phase images. However, unique hemodynamic characteristics of the liver may lead to the occurrence of pseudolesions on contrast-enhanced images[1].
Radiologically, a pseudolesion is defined as a focal mass-like finding observed only on diagnostic imaging, without any actual histopathological abnormality[1]. Hepatic pseudolesions represent an important imaging challenge because they sometimes present findings similar to those of hepatic malignancy. In addition, some pseu
Given their importance in the diagnostic imaging of liver lesions, we first introduce the characteristics of hepatic blood flow, following which we describe the mechanisms by which alterations in intrahepatic hemodynamics can lead to pseudolesions. Finally, we review hepatic parenchymal changes that occur in the region containing the intrahepatic hemodynamic abnormality.
The liver is the largest parenchymal organ in the abdomen. It differs from other abdominal parenchymal organs in that there are two types of inflow vessels: the hepatic artery and the portal vein. Total hepatic blood flow is approximately 800-1200 mL/min (approximately 100 mL/min per 100 g liver wet weight). The portal vein supplies 75%-80% of the hepatic blood flow, while the hepatic artery supplies the remaining percentage. Hepatic blood volume is approximately 25-30 mL/100 g liver weight, representing roughly 10%-15% of the total blood volume. The average pressure in the hepatic artery is almost the same as aortic pressure; in contrast, portal vein pressure is approximately 6-10 mmHg in humans, while hepatic venous pressure is approximately 2-4 mmHg[3].
The portal vein collects blood from the splenic, gastric, superior mesenteric, and inferior mesenteric veins and flows into the liver through the hepatic hilum. Portal blood is mostly composed of blood from the gastrointestinal tract, and portal blood flow varies greatly depending on the feeding state. That is, portal blood flow increases after ingestion and decreases during fasting.
Hepatic arterial blood is rich in oxygen, and the peripheral hepatic arterial branches — either directly or after forming a capillary plexus around the bile duct and nourishing the bile duct — flow into sinusoids to supply oxygen to the hepatocytes and other structures.
The ratio of portal to hepatic arterial blood inflow to the liver is approximately 3:1, and the oxygen supply is mainly bestowed by hepatic arteries. Researchers have assumed that there is a complementary interaction between portal blood flow and hepatic arterial blood flow, meaning that hepatic arterial blood flow increases when portal blood flow decreases and that an increase in portal blood flow compensates for a decrease in hepatic arterial blood flow[2].
Several mutual routes of communication connect the hepatic artery and portal vein within the liver, including the trans-sinusoidal route, tumor thrombus-induced transvasal route, transtumoral shunt, transplexal route (peribiliary route), and arterioportal fistula[4,5]. Among these connecting routes, the trans-sinusoidal route may represent the main complementary interaction between portal and hepatic arterial blood flow.
Recent studies have proposed the concept of hepatic arterial buffer response (HABR) for understanding the mechanism underlying the reciprocal effect between hepatic arterial and portal venous flow. As portal blood flow increases or decreases depending on the activity of the gastrointestinal tract, the liver has no control over portal blood flow. Therefore, when portal blood flow is reduced, hepatic arterial blood flow is controlled to maintain hepatic blood flow (i.e., the oxygen supply to the liver)[6].
To elaborate, the space of Mall, which surrounds the terminal branches of the portal vein and hepatic artery before they drain into the hepatic sinusoid, constantly secretes adenosine, a vasoactive substance that serves to dilate the hepatic artery. When the normal portal blood flow is abundant, adenosine in the space of Mall is washed away by the influence of portal blood flow and does not dilate the hepatic arteries. However, when portal blood flow decreases, adenosine remains in the space of Mall, dilates the hepatic artery, and increases hepatic arterial flow to compensate for the decrease in portal blood flow to maintain hepatic sinusoidal blood flow. This is called the adenosine wash-out theory[7-9]. This mechanism of hepatic artery dilatation takes place in the hepatic arteriole, the distal part of the intrahepatic hepatic artery within the portal tract. HABR is thought to be significantly related to abnormal imaging findings observed on contrast-enhanced arterial phase images, such as hepatic arterial-portal vein shunting (AP shunting) and transient hepatic attenuation difference (THAD), as described below (Figure 1).
Choi et al[4] defined AP shunting as an organic or functional communication between the hepatic arterial branch and the portal venous system, resulting in the redistribution of arterial flow into a focal region of the portal venous flow. When blood flow through the portal vein is diminished or absent, the hepatic artery takes over perfusion of the liver through the AP shunt[4]. When portal vein obstruction occurs, increased hepatic arterial blood flow occurs mainly through the peribiliary plexus[10]. Namely, AP shunting is a consequence of the HABR mechanism.
On dynamic contrast-enhanced images, AP shunting is associated with (1) early enhancement of peripheral portal vein branches before the central portal vein is enhanced; and (2) THAD[4] (Figure 1).
THAD refers to transient, peripheral, wedge-shaped hepatic parenchymal enhancement (usually with a straight margin) that occurs during the hepatic arterial phase of contrast-enhanced imaging[10]. This phenomenon arises because increased arterial flow compensates for decreased portal venous flow and because the inflow of contrast material from a high-pressure arterial blood system into a low-pressure portal branch opacifies the focal area of the liver, while contrast material in the adjacent parenchyma is diluted by the unenhanced portal venous flow[4]. On portal venous phase images, the involved site returns to normal or nearly normal attenuation. Normal vessels pass through the area of THAD, and this finding can aid in differentiating THAD from hypervascular liver tumors such as HCC on contrast-enhanced imaging.
The ratio of portal blood flow to hepatic artery blood flow is usually considered to be approximately 3:1 within the liver. Local disruption of this ratio leads to focal changes in blood flow on contrast-enhanced CT and MR images. The causes of intrahepatic hemodynamic changes include increased or decreased hepatic arterial blood flow, increased or decreased portal vein blood flow, and decreased hepatic venous blood flow. Anatomical variations can also cause intrahepatic hemodynamic changes.
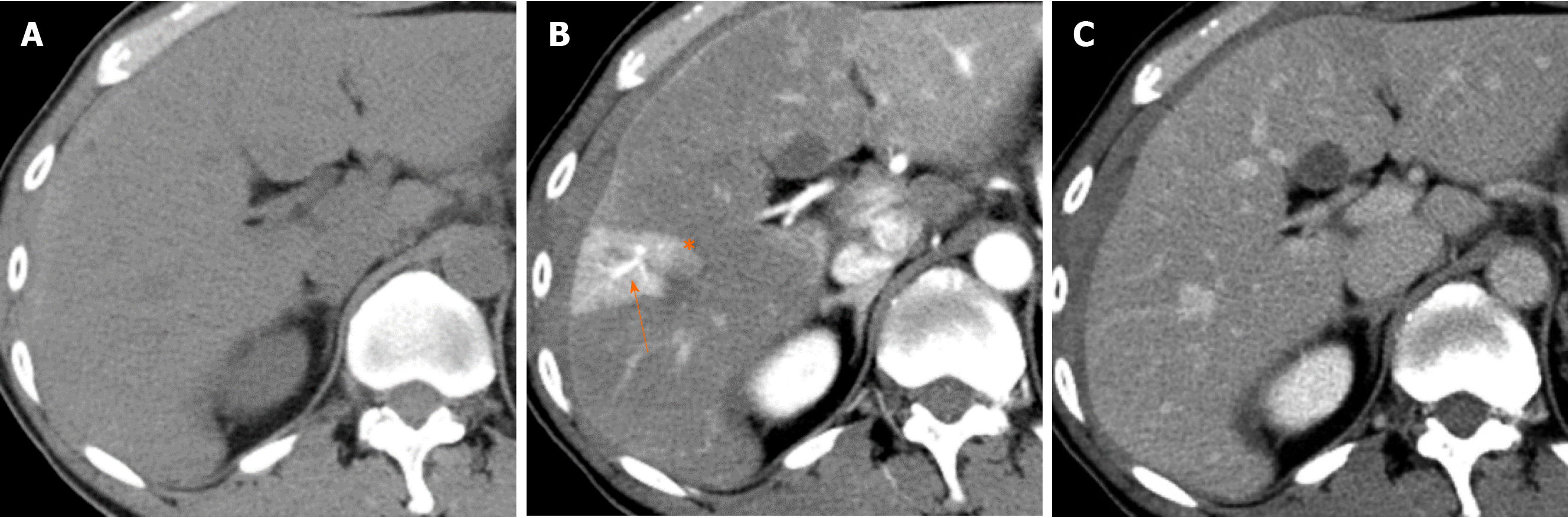
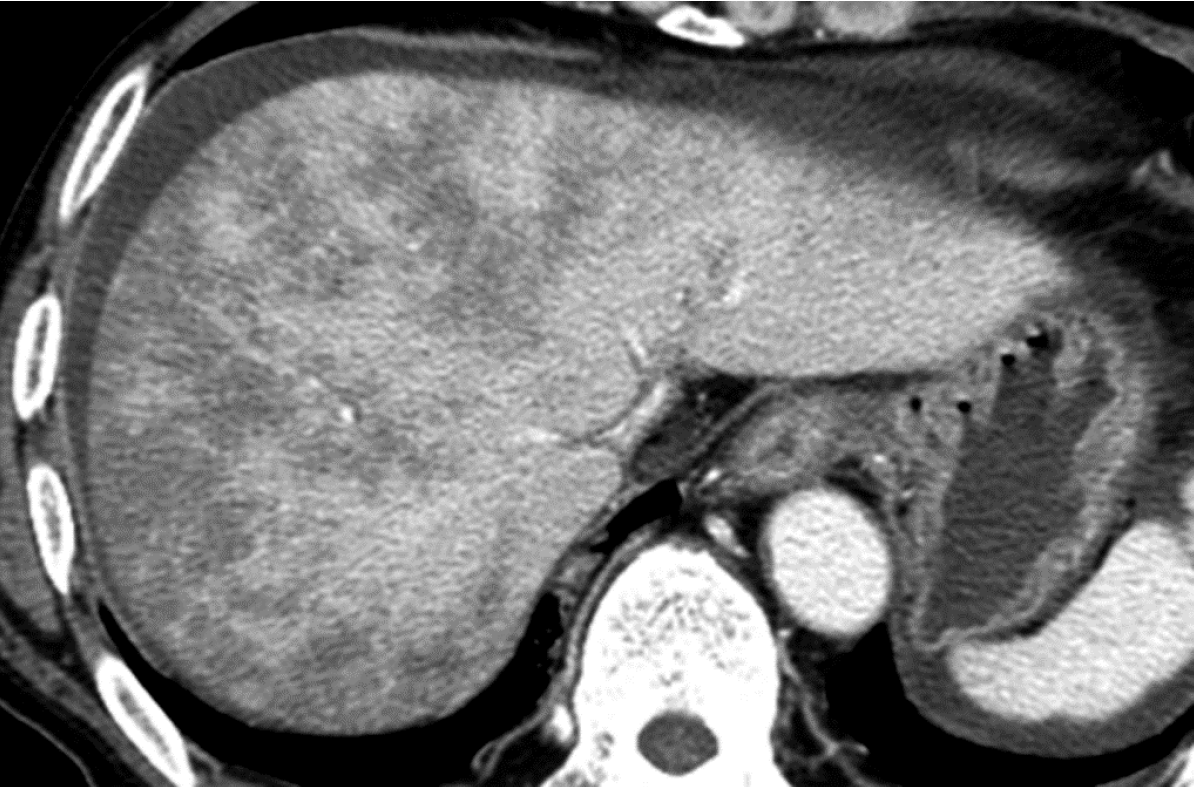
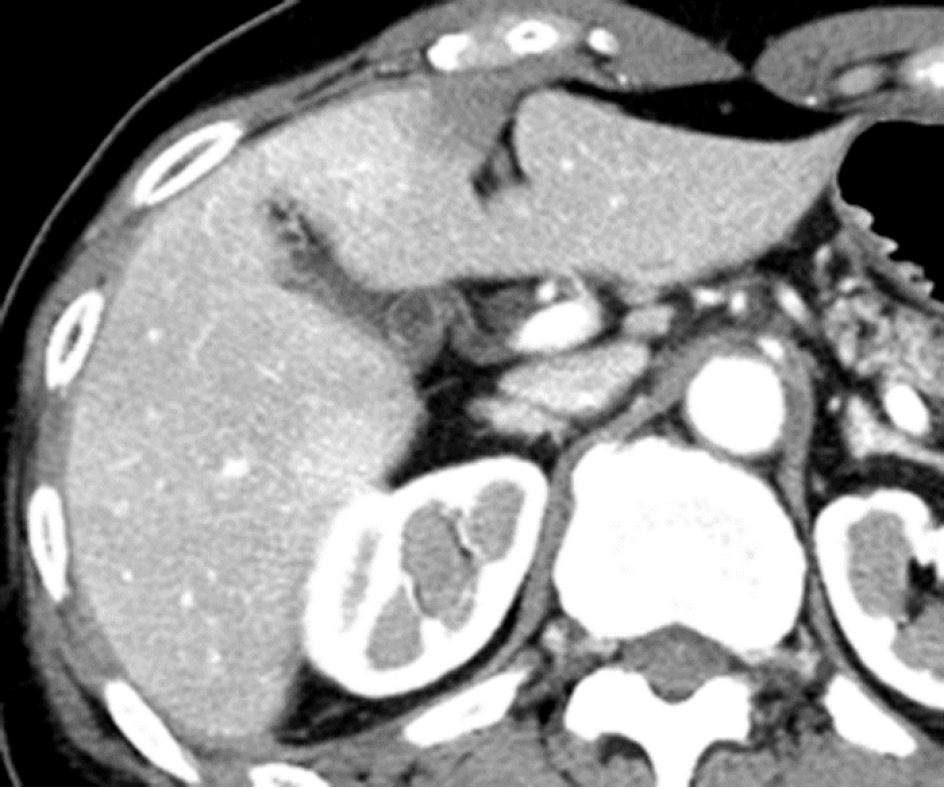
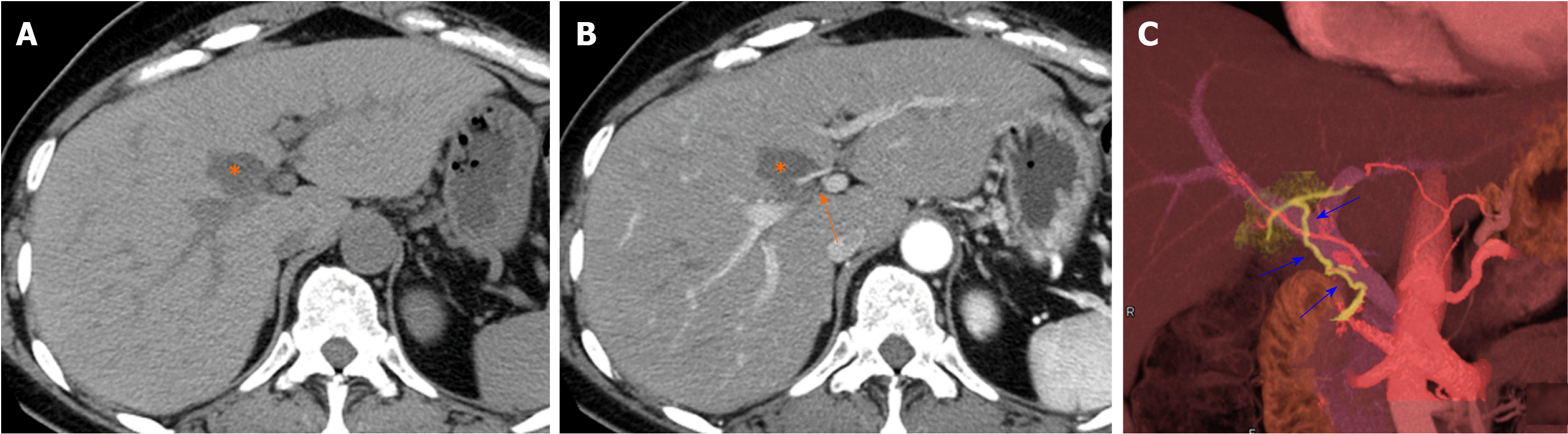
Causes of increased hepatic arterial blood flow include HABR due to decreased portal blood flow and the presence of congenital or acquired shunt pathways [e.g., hepatic AP shunts), hepatic arterial–hepatic venous shunts (AV shunts)], hereditary hemorrhagic telangiectasia (Figure 2), hepatic trauma, and others[11].
Physiological causes of increased portal blood flow include diurnal variations that occur with food intake, while pathological causes include "small-for-size grafts" at liver transplantation.
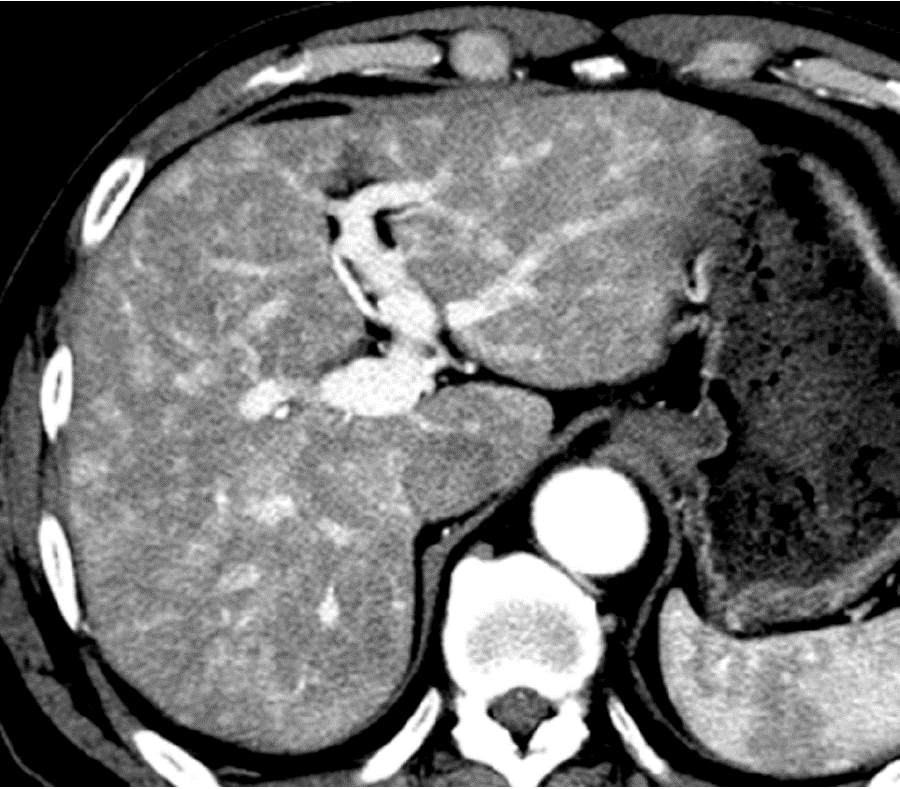
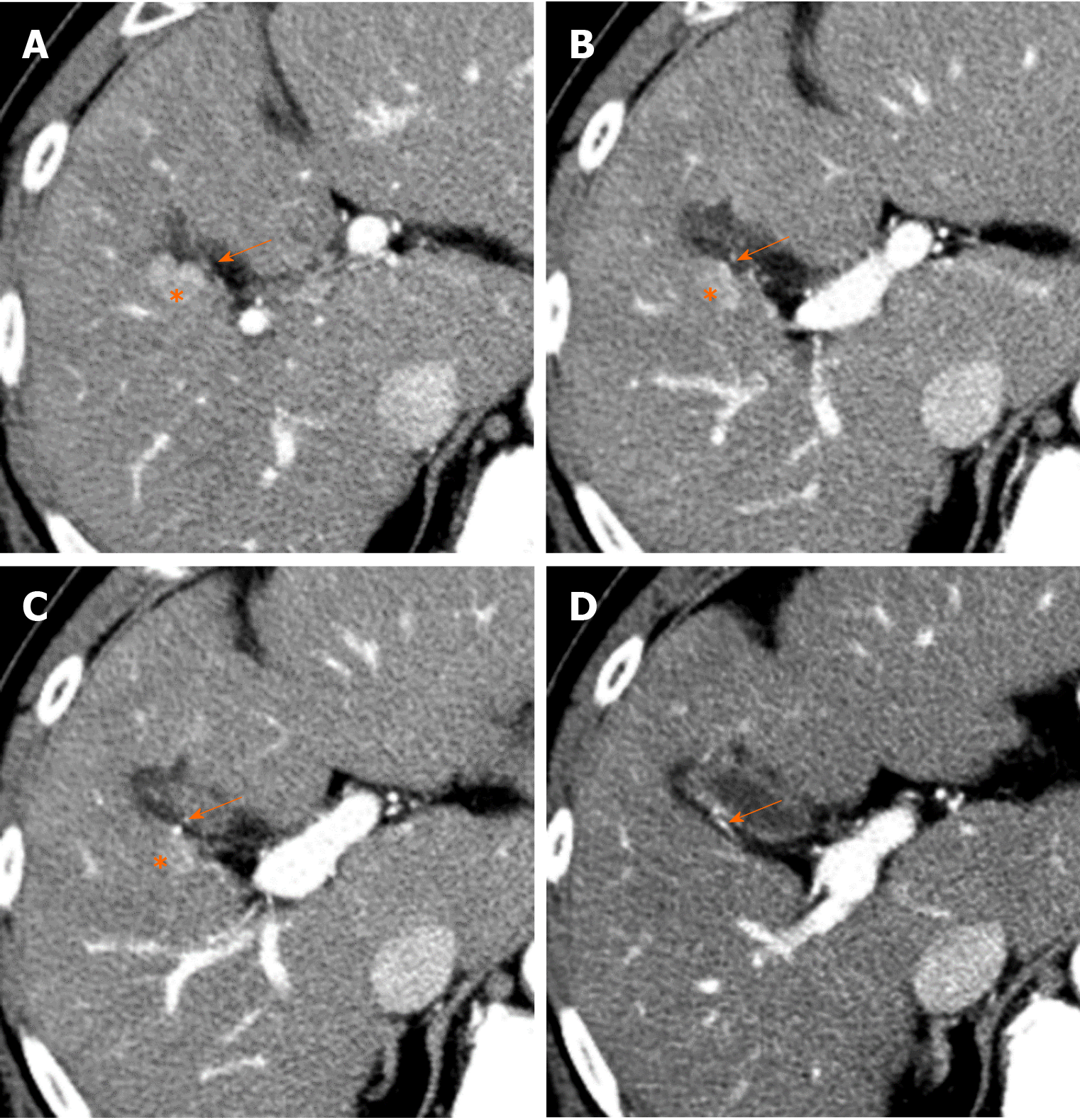
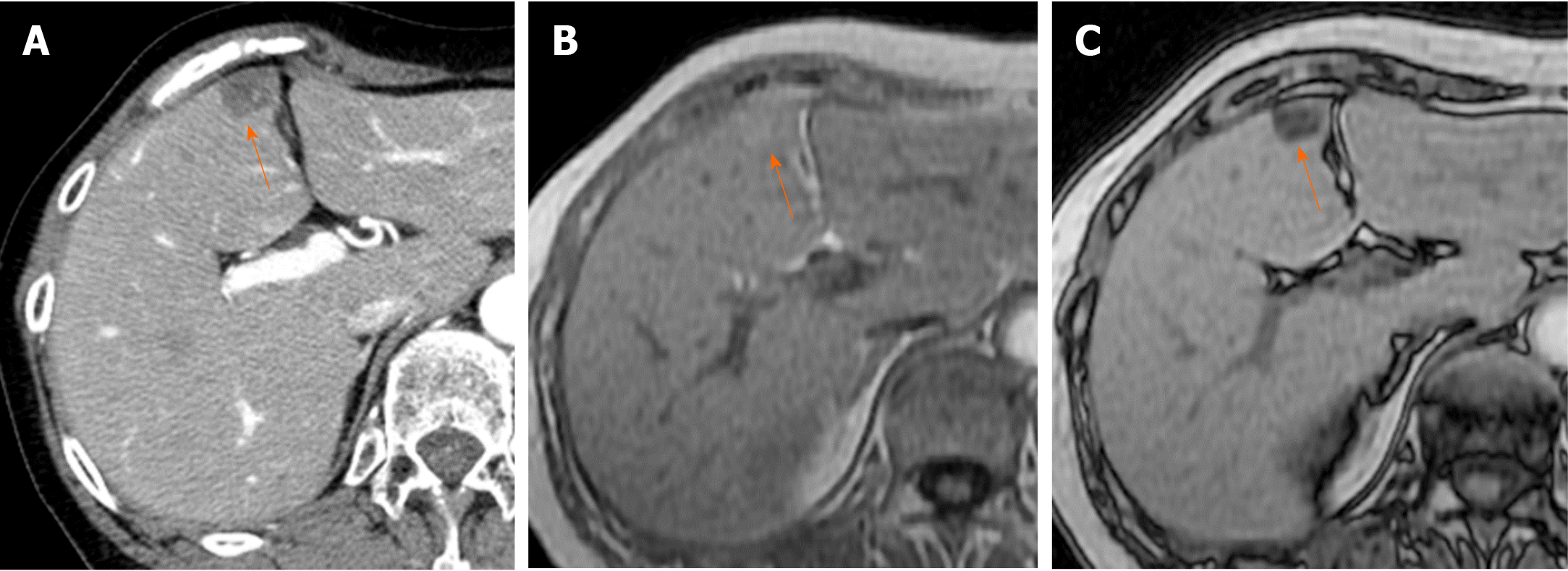
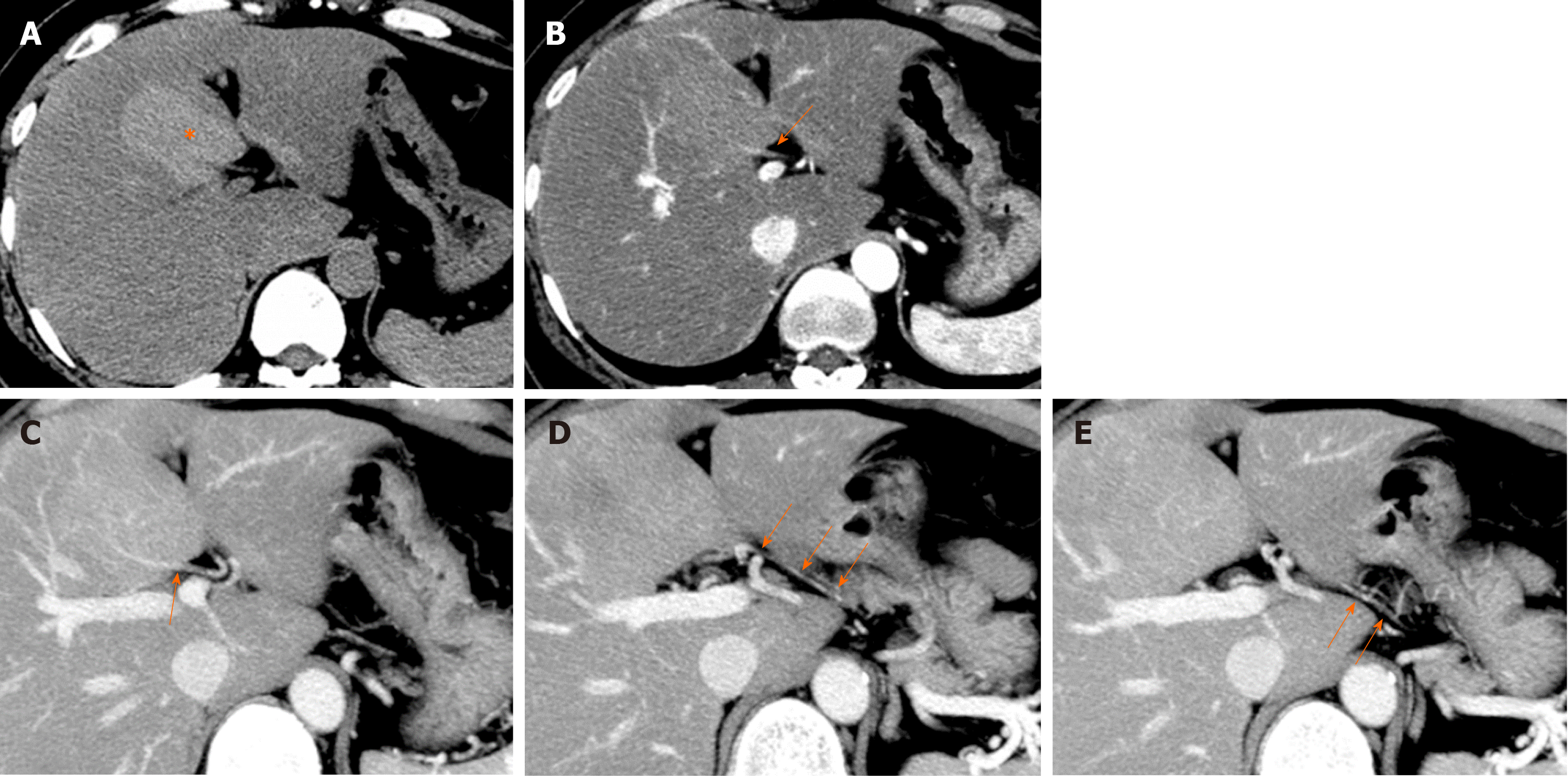
Causes of reduced portal blood flow include extrahepatic portal obstruction (Figure 3), portal vein thrombosis, portal vein tumor thrombus (Figure 4), agenesis of the portal vein (congenital porto-systemic shunt), patent ductus venosus, and porto-sinusoidal vascular disease (formerly known as idiopathic portal hypertension). In addition, blood flow from the portal vein to hepatic sinusoids is reduced in patients with liver cirrhosis exhibiting porto-systemic shunting.
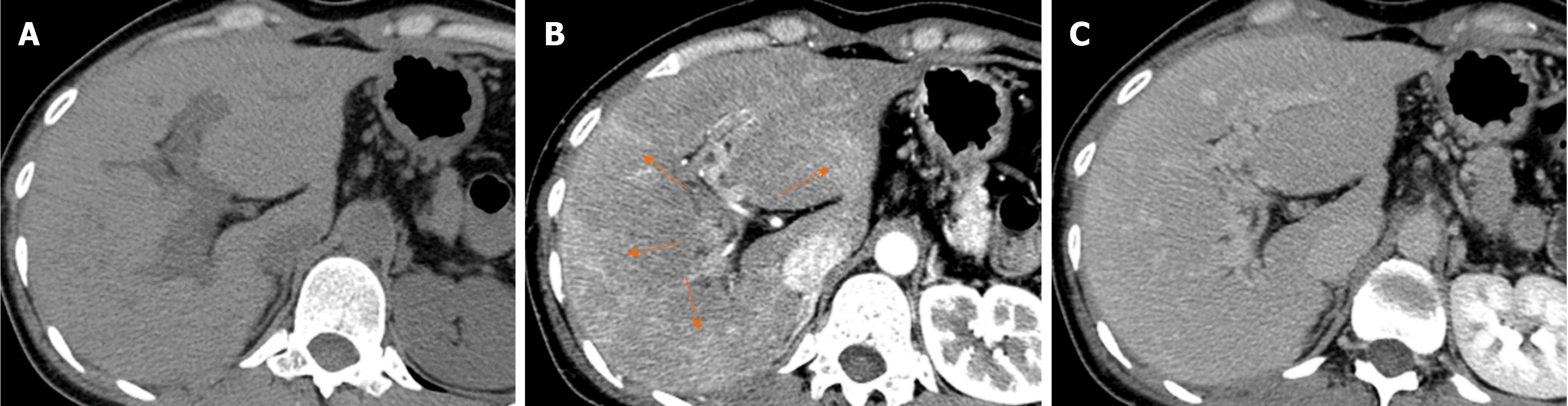
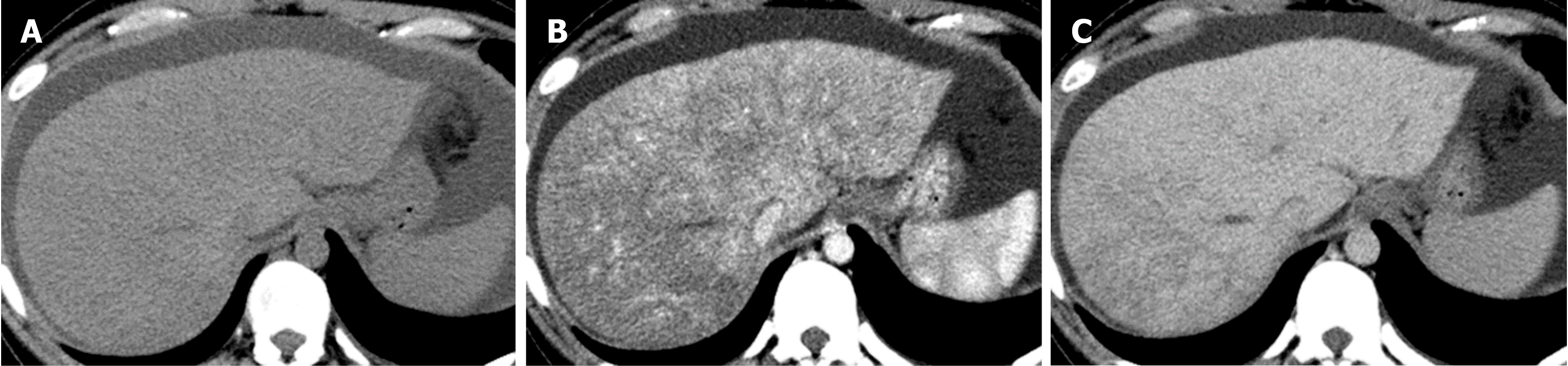
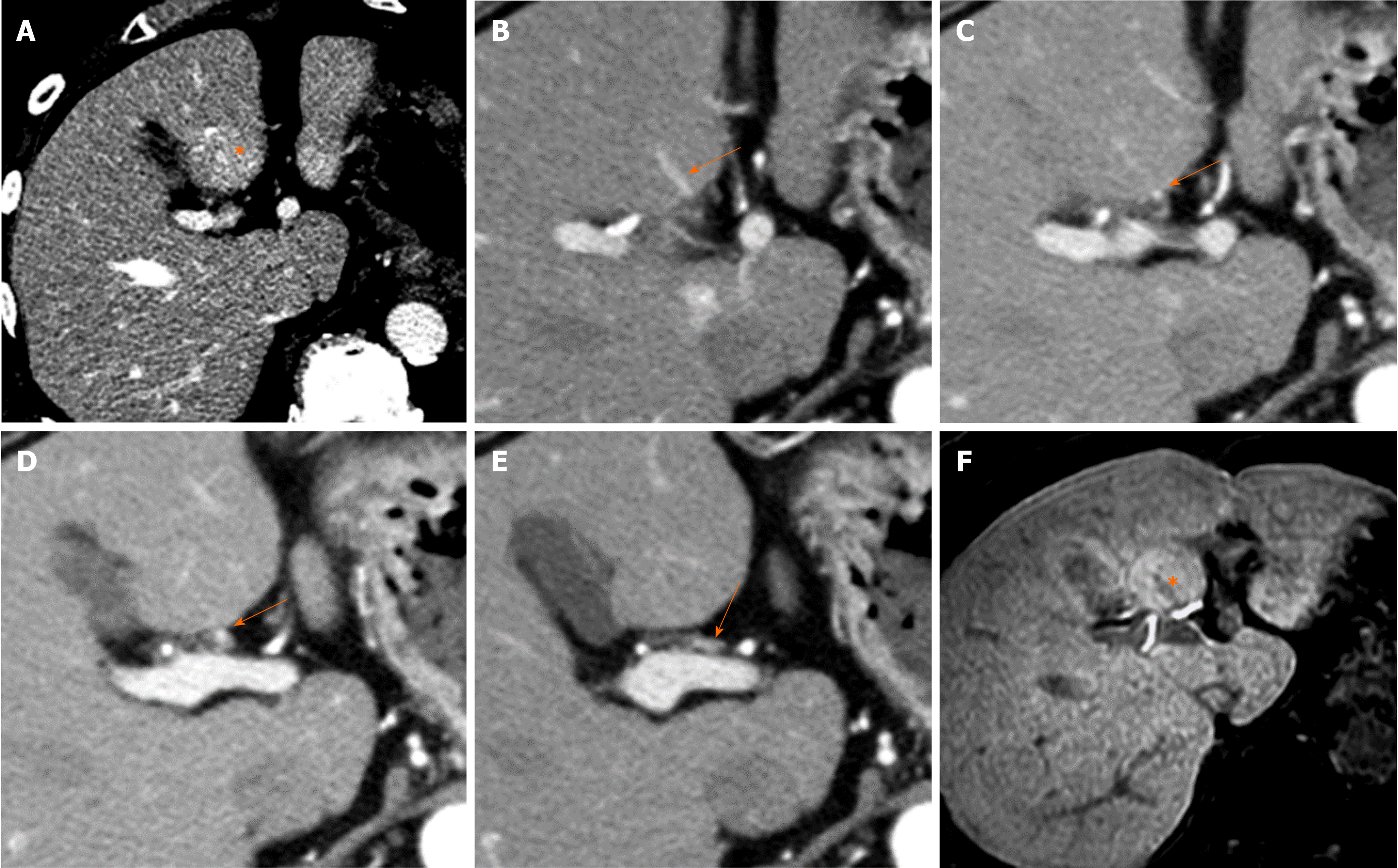
Causes of decreased hepatic venous blood flow include Budd-Chiari syndrome (BCS) (Figure 5), sinusoidal obstruction syndrome (SOS) (Figure 6), hepatic vein thrombosis, and hepatic vein tumor thrombus. Secondary to these lesions, the liver exhibits a state of hepatic congestion. Increased hepatic venous pressure leading to decreased hepatic venous blood flow may also occur in patients with congestive heart failure and those who have undergone the Fontan procedure.
Localized portal hypoperfusion and the associated focal increases in hepatic arterial blood flow due to HABR are associated with THAD and the presence of hyper-vascularized pseudolesions on contrast-enhanced images due to increased inflow of contrast medium into the sinusoids during the arterial phase[12,13] (Figures 1-4). In contrast, when hepatic venous blood flow is decreased due to obstruction of hepatic venous outflow, blood drainage from the sinusoids toward the inferior vena cava is stagnant, and the sinusoidal pressure increases. This results in a decrease in the inflow of low-pressure portal blood into the sinusoids and an increase in the inflow of hepatic arterial blood, which causes reticular heterogeneous staining on arterial and portal phase contrast-enhanced images (Figures 5 and 6). This type of contrast-enhanced imaging finding is usually observed in patients with congestive liver, which occurs secondary to congestive heart failure, BCS, and SOS. Thus, when arterial phase contrast-enhanced images show THAD or reticular staining of the liver parenchyma, the presence of intrahepatic hemodynamic abnormalities can be inferred.
In addition to focal alterations in intrahepatic hemodynamics, anatomical variations in the portal venous system and other characteristic anatomical features of the vessel surrounding the liver can cause focal hemodynamic changes in several specific portions of the liver[14].
The parabiliary venous system, epigastric–paraumbilical venous system, and cholecystic vein directly enter the liver independently of the portal venous system. These vessels are called the “third inflow,” referring to the third hepatofugal flow after the hepatic arterial and portal vein systems[15].
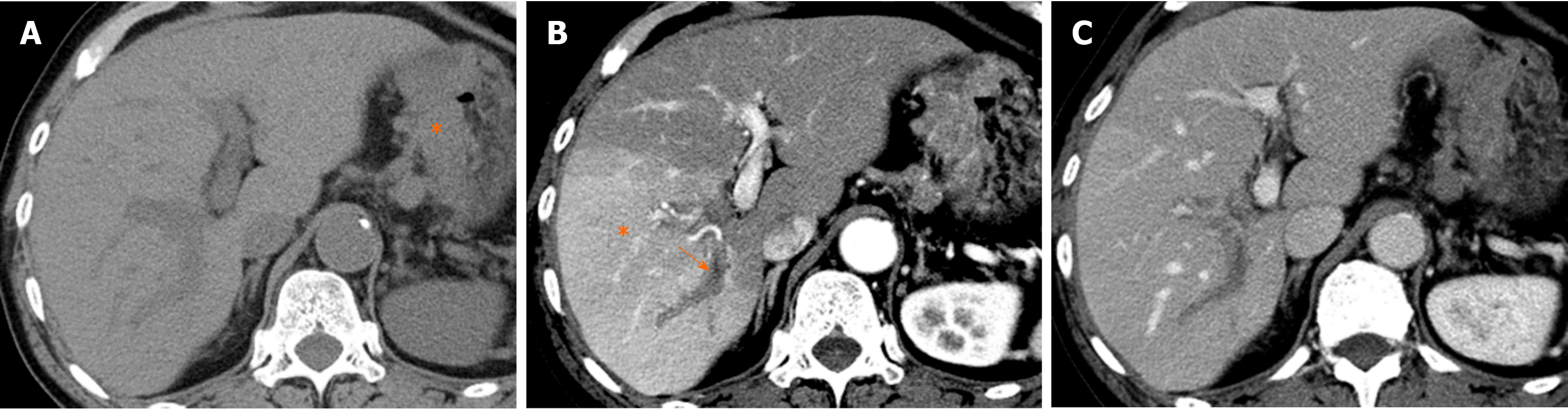
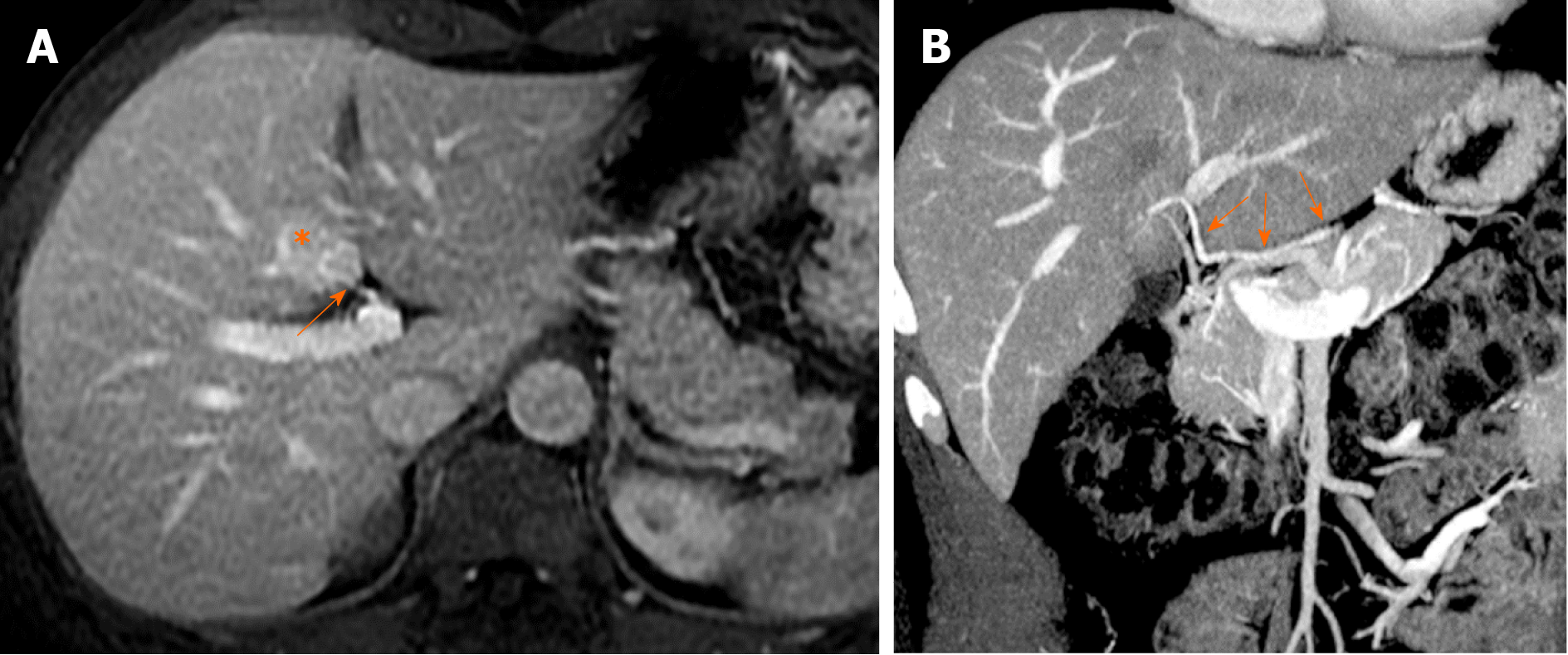
The parabiliary system, termed the pancreatico-pyloro-duodenal vein, collects venous blood from the pancreatic head, stomach, and duodenum and usually joins the main portal vein outside the liver before flowing into the liver. However, in some cases, the pancreatico-pyloro-duodenal vein does not connect to the main portal vein before it enters the liver, instead directly entering the liver and perfusing the hepatic sinusoids at the posterior aspect of segment IV without fusion to the main portal vein. As this venous system includes the right gastric vein, this anatomical variation is sometimes referred to as “aberrant right gastric vein”[17]. The incident of aberrant right gastric vein varies from 1.5% to 49%[16-18], while the incidence of aberrant left gastric vein varies from 0.8% to 4%[18,19].
Although most aberrant gastric veins enter the liver and perfuse the hepatic sinusoids at the posterior aspect of segment IV (Figure 7), some may enter the liver and perfuse the liver parenchyma at the posterior edge of segment II or III[20] (Figure 8).
The paraumbilical vein is divided into the vein of Burow, superior vein of Sappey, and inferior vein of Sappey[21]. Among them, under conditions of portal hypertension, the inferior vein of Sappey is often dilated and forms a porto-systemic collateral pathway connected with the portal system in the anterolateral part of segment IV adjacent to the falciform ligament and epigastric veins.
When superior vena cava obstruction occurs, hyperenhancement of segment IV (i.e., quadrate lobe hot-spot sign) can be observed on contrast-enhanced CT/MR images[22] (Figure 9). This phenomenon is the result of the inferior vein of Sappey acting as the hepatopetal collateral route.
The blood supply and drainage of the gallbladder are also related to the occurrence of focal hepatic hemodynamic changes. Arterial supply of the gallbladder is provided by the hepatic artery (mainly the right hepatic artery[23]), and the cholecystic vein drains into the liver sinusoids surrounding the gallbladder usually after being connected to the peripheral branch of the intrahepatic portal vein. In detail, cholecystic venous blood most frequently enters the peripheral portal branches of liver segments V (96%) and IV (93%)[23]. As the cholecystic venous blood originates from the hepatic artery, the concentration of nutrients and humoral factors such as hormones, which flow into the hepatic sinusoids of the cholecystic venous drainage area, differs from that in other hepatic sinusoids into which the portal venous blood flows. Such differences in the composition of influx blood between the cholecystic venous drainage area and the rest of the liver can cause focal differences in contrast-enhanced imaging and histopathological findings (Figure 10).
Focal alterations in intrahepatic hemodynamics may not only present as pseudolesions on contrast-enhanced images but may also result in histological changes of the liver parenchyma at the site of the blood flow change. There are three major patterns of such histological changes in the liver parenchyma.
Focal hyperplasia of the liver is observed in patients with cirrhotic liver and may be related to the presence of anomalous portal flow such as aberrant gastric venous drainage. Matsui et al[24] reported that in patients with cirrhotic liver with aberrant gastric venous drainage, 22%-50% of cases are associated with focal hyperplastic changes at the posterior aspect of segment IV, where the aberrant gastric venous drainage is present[24] (Figure 11). Focal hyperplastic changes such as anomalous portal venous drainage in the caudate lobe have also been reported in cases of cirrhotic liver, with the authors surmising that the etiology of such hyperplastic changes is intimately related to the anomalous portal flow[25,26]. Similarly, focal hyperplasia with anomalous portal flow in the caudate lobe has also been reported in a patient without cirrhosis[27].
Researchers have examined the etiology of liver hyperplasia occurring after hepatectomy or portal vein embolization. At present, it is believed that blood flow, shear stress, and adenosine are involved in the development of hyperplasia in the liver[28]. Studies on liver regeneration after hepatectomy or portal vein embolization have also suggested that hyperplasia and atrophy of the liver occur when the liver is unable to compensate for changes in blood flow caused by surgery or portal vein embolization. Several studies support the hypothesis that after portal vein embolization, portal blood flow increases in the unembolized liver lobe, which causes acute portal hypertension. This change leads to increased shear stress and nitric oxide production, which in turn triggers liver regeneration[29-31]. In contrast, decreased intrahepatic shear stress is thought to induce liver atrophy[31]. This mechanism is thought to maintain the ratio between liver mass and blood flow, most likely to ensure maintenance of adequate clearance function[31].
In small-for-size syndrome after liver transplantation, HABR reduces hepatic arterial flow due to excessive portal flow, which leads to decreased oxygen delivery to the liver parenchyma. The lack of adequate oxygen for liver regeneration increases the risk of liver failure. However, normalization of portal pressure and portal blood flow is believed to improve liver regeneration[7]. Thus, the degree of hepatic blood flow, especially portal blood flow, plays a major role in liver regeneration and atrophy.
Ethanol consumption is also involved in liver regeneration and atrophy. Gluud et al[32] observed that the frequency of hyperplastic nodules decreased with higher ethanol intake in patients with cirrhosis, indicating that ethanol consumption may inhibit liver regeneration[32]. Histopathologically, ethanol intake causes damage to the hepatic veins, especially perivenular fibrosis[33]. Impaired hepatic veins lead to decreased outflow of sinusoidal blood, resulting in stasis of blood flow and hepatic congestion, thereby inducing liver atrophy.
Focal hyperplastic changes are observed in the aberrant right gastric venous drainage area in patients with alcoholic cirrhotic liver[24]. One possible explanation for this is the difference in blood ethanol concentrations of the main portal and aberrant right gastric vein. In humans, 20% of ingested ethanol is absorbed through the stomach, while 80% is absorbed through the small intestine[34]. This means that the ethanol concentration in the venous blood of the small intestine is higher than that in the venous blood of the stomach. Normally, both the venous blood from the stomach and small intestine join and form the main portal vein before flowing into the liver, so there is no difference in ethanol concentration in the portal venous blood within the liver. However, in the presence of aberrant right gastric venous drainage, hepatic venous injury is less likely to occur at the drainage area because the ethanol concentration is lower in the gastric vein. In contrast, in the area perfused by the portal flow, hepatic venous injury is more likely to occur as the blood flow of the main portal vein collects the venous flow of the small intestine, which contains higher ethanol concentration.
As a result, most of the liver areas that receive blood flow from the main portal vein exhibit congestion due to hepatic vein injury, while the area supplied by the aberrant right gastric venous drainage exhibits less extensive hepatic vein injury and less severe congestion than the rest of the liver. Hepatic atrophy occurs in areas with severe hepatic congestion, while areas with less hepatic congestion are relatively hyperplastic. This may be why focal hyperplasia occurs in the aberrant right gastric venous drainage area in patients with alcoholic cirrhosis.
Similarly, hepatic venous injury is less likely to occur at the cholecystic venous drainage area surrounding the gallbladder in segments IV and V of the liver due to the relatively low amount of ethanol in the venous blood compared to the rest of the liver. Therefore, focal hyperplastic changes may also occur in this area in patients with alcoholic cirrhosis (Figure 12).
The contribution of hepatic venous drainage to liver deformity and atrophy has also been noted in patients with conditions other than alcoholic liver disease. Ozaki et al[35] demonstrated that, because the diameter of the middle hepatic vein is small and venous blood pressure is high compared to that in the other hepatic veins, the middle hepatic venous drainage area tends to exhibit congestive changes. Such changes lead to selective atrophy of the middle hepatic venous drainage area (e.g., segment IV) as well as relative hyperplastic changes in the right and left hepatic venous drainage area[35].
Other factors that may contribute to focal hyperplastic changes in the third inflow area include different concentrations of bile acid in the main portal vein and the third inflow. Previous studies have reported that enterohepatic circulation is involved in liver regeneration[36], and that decrease in bile acid return to the liver triggers hepatocyte proliferation[37]. Because bile acids circulate in the gut–liver axis, the concentration of bile acids in the third inflow is lower than that in the main portal venous flow. Therefore, differences in the concentration of bile acids in the inflow may contribute to the development of focal hyperplasia of the liver parenchyma in the aberrant venous drainage area[38-40].
Localized hepatocellular hyperplastic changes in the normal liver that mimic liver neoplasms on imaging are referred to as focal nodular hyperplasia (FNH). Researchers have proposed that the pathogenesis of FNH is related to a disturbance of sinusoidal blood outflow[41-43] or to the presence of abnormal anomalous vessels[44]. These studies indicate that localized alterations in intrahepatic hemodynamics play a major role in the pathogenesis of FNH.
In summary, the presence of intrahepatic hemodynamic alterations is essential for the development of focal hyperplastic changes in the liver. Such changes are also influenced by concomitant factors such as differences in the blood concentrations of nutrients, ethanol, hormones, and so on at the site. Further research is required to elucidate the mechanism underlying the development of focal hyperplastic changes in the liver.
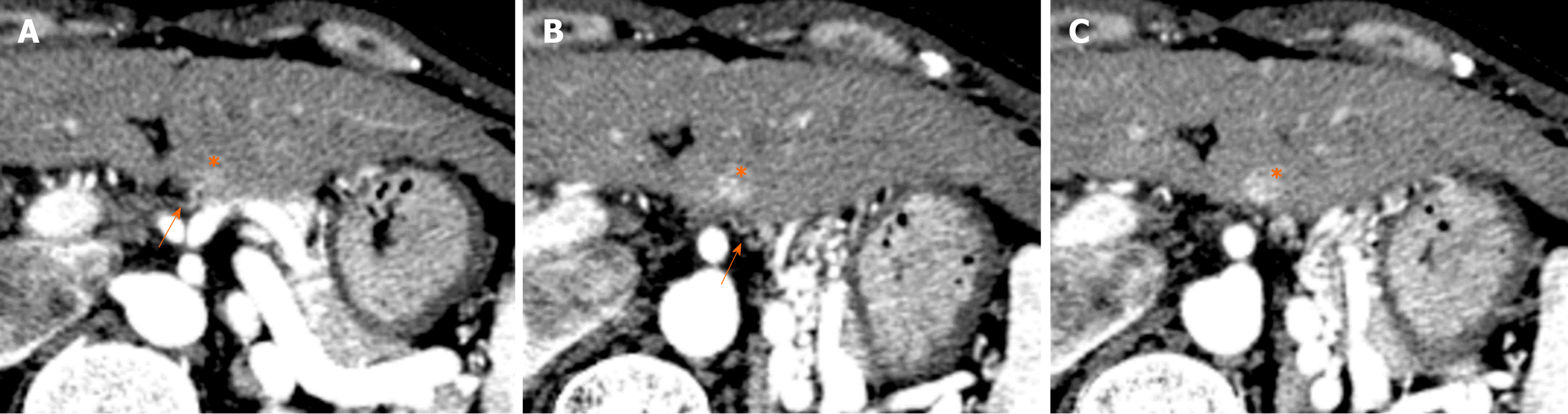
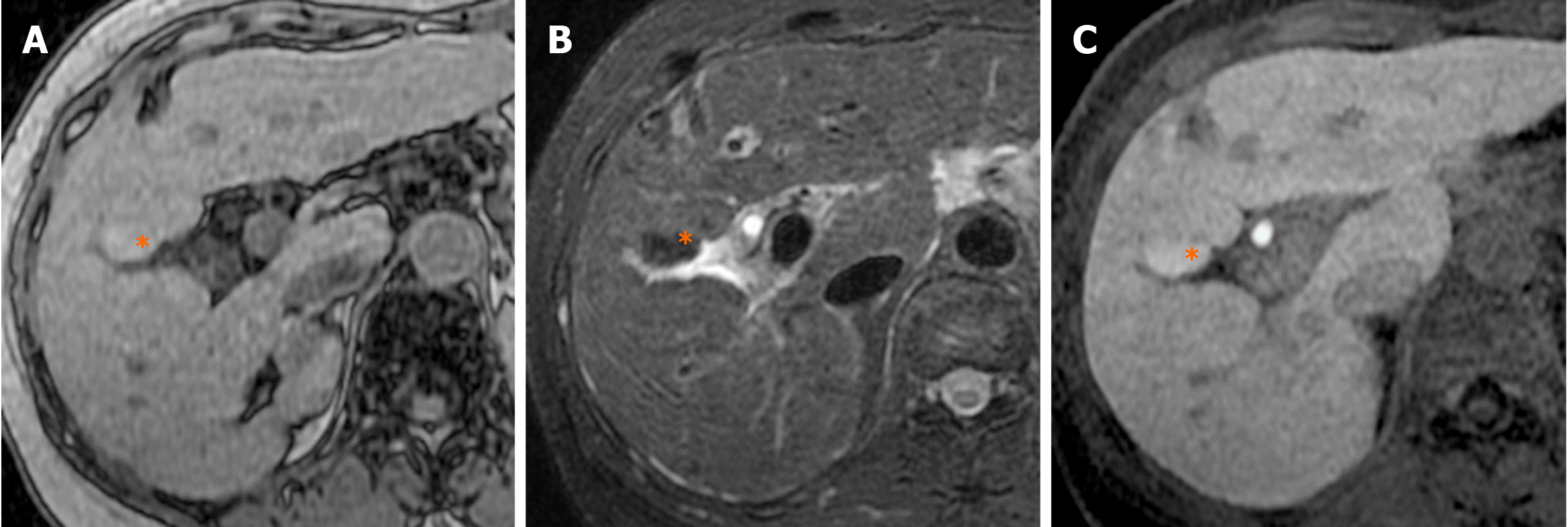
Focal fat deposition in the liver is occasionally observed in the anteromedial portion of segment IV (adjacent to the falciform ligament)[45] and in the posterior aspect of segment IV[46] (Figures 13 and 14). Focal fat deposition at the posterior aspect of segment IV is related to the presence of aberrant right gastric venous drainage[47], while that at the anteromedial portion adjacent to the falciform ligament is related to the presence of inferior vein of Sappey drainage[47,48].
Vilgrain et al[49] suggest that differences in the concentration of insulin in the blood entering the liver contribute to focal fat deposition in the liver. As an aberrant right gastric vein may collect venous blood from the head of the pancreas and flow into the posterior aspect of segment IV, the concentration of insulin may in turn be higher in the inflow area of the aberrant right gastric vein than in other areas. This may lead to focal fat deposition in the posterior aspect of segment IV, where aberrant right gastric venous drainage is present.
Focal fat deposition is also observed in the hepatic parenchyma surrounding the metastasis of pancreas islet cell tumors, which produce insulin. The etiology of focal fat deposition in such cases may be the same as that described above[49,50].
Focal sparing of fatty liver refers to the presence of focal areas exhibiting a relative decrease in the degree of fat deposition in cases of fatty liver. This type of focal sparing represents the opposite of focal fat deposition in terms of steatotic liver changes and is intimately related to alterations in intrahepatic hemodynamics. Focal sparing of fatty liver is sometimes observed in the posterior aspect of segment IV (Figure 15) and in the liver parenchyma surrounding the gallbladder in segments IV and V.
Matsui et al[51] reported a strong correlation between the focally spared area at the posterior edge of segment IV in fatty liver and aberrant gastric venous drainage directed to segment IV.
Fatty liver is an abnormality of the liver caused by overnutrition. However, when aberrant right gastric venous flow with a low level of nutrients compared to the main portal vein enters the posterior aspect of segment IV, focal sparing of fatty liver is assumed to occur in the third inflow area. Vilgrain et al[49] reported that if the insulin concentration of the aberrant gastric venous flow is low in patients with fatty liver, the aberrant venous drainage area will exhibit less fat deposition and focal sparing on liver imaging.
The blood supply of the gallbladder is provided by the cholecystic artery originating from the hepatic arterial branches, in which the blood contains enough oxygen but contains fewer nutrients than the portal venous blood. The cholecystic vein drains into the liver parenchyma surrounding the gallbladder. The venous flow that perfuses the liver area surrounding the gallbladder contains less nutrients than other areas of the liver supplied by the portal vein. For this reason, focal sparing of fatty liver occurs in the liver parenchyma surrounding the gallbladder in segments IV and V. This hypothesis is further supported by the finding that the incidence of focal sparing of fatty liver is significantly lower in patients who have undergone cholecystectomy than in those with intact gallbladders[52].
In the present review, we discussed the characteristics of hepatic blood flow and pathophysiology of pseudolesions that can occur due to alterations in intrahepatic hemodynamics. Understanding HABR, a unique mechanism for regulating hepatic blood flow, might be essential for elucidating the pathogenesis of AP shunting and THAD on dynamic contrast-enhanced imaging of the liver. In addition, some pseudolesions are associated with histopathologic changes such as focal hyperplasia, focal fatty liver, and focal sparing of fatty liver. Understanding these phenomena may aid in interpreting liver imaging findings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Q S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Kobayashi S, Gabata T, Matsui O. Radiologic manifestation of hepatic pseudolesions and pseudotumors in the third inflow area. Imaging Med. 2010;2:519-528. [DOI] [Full Text] |
| 2. | Scriven MW, Shandall A, Fitzgerald EJ, Puntis MC. Hepatic 'pseudotumours': an important diagnostic pitfall. Ann R Coll Surg Engl. 1993;75:43-45. [PubMed] |
| 3. | Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (2)] |
| 4. | Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wang Q, Koniaris LG, Milgrom DP, Patel A, Hu M, Cui E, Deng Y, Akisik F. CT and MRI imaging and interpretation of hepatic arterioportal shunts. Transl Gastroenterol Hepatol. 2019;4:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Jha RC, Khera SS, Kalaria AD. Portal Vein Thrombosis: Imaging the Spectrum of Disease With an Emphasis on MRI Features. AJR Am J Roentgenol. 2018;211:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Richter S, Vollmar B, Mücke I, Post S, Menger MD. Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers. J Physiol. 2001;531:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Lautt WW. Regulatory processes interacting to maintain hepatic blood flow constancy: Vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res. 2007;37:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Rush N, Sun H, Nakanishi Y, Mneimneh W, Kwo PY, Saxena R. Hepatic arterial buffer response: pathologic evidence in non-cirrhotic human liver with extrahepatic portal vein thrombosis. Mod Pathol. 2016;29:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Schmalz MJ, Radhakrishnan K. Vascular anomalies associated with hepatic shunting. World J Gastroenterol. 2020;26:6582-6598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Colagrande S, Centi N, Pradella S, Duranti B, Belli G, Villari N. Transient hepatic attenuation differences and focal liver lesions: sump effect due to primary arterial hyperperfusion. J Comput Assist Tomogr. 2009;33:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Ahn JH, Yu JS, Hwang SH, Chung JJ, Kim JH, Kim KW. Nontumorous arterioportal shunts in the liver: CT and MRI findings considering mechanisms and fate. Eur Radiol. 2010;20:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Hashimoto M, Heianna J, Tate E, Nishii T, Iwama T, Ishiyama K. Small veins entering the liver. Eur Radiol. 2002;12:2000-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yoshimitsu K, Honda H, Kuroiwa T, Irie H, Aibe H, Shinozaki K, Masuda K. Unusual hemodynamics and pseudolesions of the noncirrhotic liver at CT. Radiographics. 2001;21 Spec No:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Deneve E, Caty L, Fontaine C, Guillem P. Simultaneous aberrant left and right gastric veins draining directly into the liver. Ann Anat. 2003;185:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Takayasu K, Aoki K, Ichikawa T, Ohmura T, Sekiguchi R, Terauchi T, Takayama T. Aberrant right gastric vein directly communicating with left portal vein system. Incidence and implications. Acta Radiol. 1990;31:575-577. [PubMed] |
| 18. | Seong NJ, Chung JW, Kim HC, Park JH, Jae HJ, An SB, Cho BH. Right gastric venous drainage: angiographic analysis in 100 patients. Korean J Radiol. 2012;13:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Miyaki T, Yamada M, Kumaki K. Aberrant course of the left gastric vein in the human. Possibility of a persistent left portal vein. Acta Anat (Basel). 1987;130:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Unal E, Ozmen MN, Akata D, Karcaaltincaba M. Imaging of aberrant left gastric vein and associated pseudolesions of segments II and III of the liver and mimickers. Diagn Interv Radiol. 2015;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Martin BF, Tudor RG. The umbilical and paraumbilical veins of man. J Anat. 1980;130:305-322. [PubMed] |
| 22. | Virmani V, Lal A, Ahuja CK, Khandelwal N. The CT Quadrate lobe hot spot sign. Ann Hepatol. 2010;9:296-298. [PubMed] |
| 23. | Yoshimitsu K, Honda H, Kaneko K, Kuroiwa T, Irie H, Chijiiwa K, Takenaka K, Masuda K. Anatomy and clinical importance of cholecystic venous drainage: helical CT observations during injection of contrast medium into the cholecystic artery. AJR Am J Roentgenol. 1997;169:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Matsui O, Kadoya M, Yoshikawa J, Gabata T, Takahashi S, Ueda K, Kawamori Y, Takashima T, Nakanuma Y. Aberrant gastric venous drainage in cirrhotic livers: imaging findings in focal areas of liver parenchyma. Radiology. 1995;197:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Gabata T, Matsui O, Kadoya M, Yoshikawa J, Mitchell DG, Ueda K, Kawamori Y, Takashima T. Giant hyperplasia of the caudate lobe of the cirrhotic liver: correlation with an anomaly of the caudate portal branch. Abdom Imaging. 1999;24:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ohtomo K, Matsuoka Y, Okada M, Amo K, Abe O, Minami M, Kawauchi N, Sasaki Y. Pseudotumorous enlargement of the paracaval portion of the caudate lobe: a report of two cases with CT and MR appearance. Abdom Imaging. 1997;22:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Kwak HS, Lee JM, Lee SY, Han YM, Kim CS, Moon WS, Yu HC. Pseudotumorous hyperplasia of the caudate lobe in the non-cirrhotic liver: MR and CT arterial portography appearance. Hepatogastroenterology. 2000;47:909-911. [PubMed] |
| 28. | Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg. 2012;397:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Gock M, Eipel C, Linnebacher M, Klar E, Vollmar B. Impact of portal branch ligation on tissue regeneration, microcirculatory response and microarchitecture in portal blood-deprived and undeprived liver tissue. Microvasc Res. 2011;81:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Gluud C, Christoffersen P, Eriksen J, Wantzin P, Knudsen BB. Influence of ethanol on development of hyperplastic nodules in alcoholic men with micronodular cirrhosis. Gastroenterology. 1987;93:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Mak KM, Png CYM. The Hepatic Central Vein: Structure, Fibrosis, and Role in Liver Biology. Anat Rec (Hoboken). 2020;303:1747-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Marek E, Kraft WK. Ethanol pharmacokinetics in neonates and infants. Curr Ther Res Clin Exp. 2014;76:90-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Ozaki K, Matsui O, Kobayashi S, Sanada J, Koda W, Minami T, Kawai K, Gabata T. Selective atrophy of the middle hepatic venous drainage area in hepatitis C-related cirrhotic liver: morphometric study by using multidetector CT. Radiology. 2010;257:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Leng L, Ma J, Lv L, Gao D, Li M, Wang Y, Zhu Y. Serum proteome profiling provides a deep understanding of the 'gut-liver axis' in relation to liver injury and regeneration. Acta Biochim Biophys Sin (Shanghai). 2021;53:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Naugler WE. Bile acid flux is necessary for normal liver regeneration. PLoS One. 2014;9:e97426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Milona A, Owen BM, van Mil S, Dormann D, Mataki C, Boudjelal M, Cairns W, Schoonjans K, Milligan S, Parker M, White R, Williamson C. The normal mechanisms of pregnancy-induced liver growth are not maintained in mice lacking the bile acid sensor Fxr. Am J Physiol Gastrointest Liver Physiol. 2010;298:G151-G158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Wu W, Wu Q, Liu X. Chronic activation of FXR-induced liver growth with tissue-specific targeting Cyclin D1. Cell Cycle. 2019;18:1784-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Bioulac-Sage P, Kakar S, Nault JC. Focal nodular hyperplasia of the liver. WHO Classification of Tumours. 5th Edition Digestive System Tumors. Edited by WHO classification of Tumours Editorial Board. Lyon (France), International Agency for Research on Cancer; 2019: 221-223. |
| 42. | Bioulac-Sage P, Laumonier H, Cubel G, Saric J, Balabaud C. Over-expression of glutamine synthase in focal nodular hyperplasia (part 1): early stages in the formation support the hypothesis of a focal hyper-arterialisation with venous (portal and hepatic) and biliary damage. Comp Hepatol. 2008;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Bioulac-Sage P, Laumonier H, Rullier A, Cubel G, Laurent C, Zucman-Rossi J, Balabaud C. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int. 2009;29:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol. 2001;16:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Kawamori Y, Matsui O, Takahashi S, Kadoya M, Takashima T, Miyayama S. Focal hepatic fatty infiltration in the posterior edge of the medial segment associated with aberrant gastric venous drainage: CT, US, and MR findings. J Comput Assist Tomogr. 1996;20:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Fukukura Y, Fujiyoshi F, Inoue H, Sasaki M, Hokotate H, Baba Y, Nakajo M. Focal fatty infiltration in the posterior aspect of hepatic segment IV: relationship to pancreaticoduodenal venous drainage. Am J Gastroenterol. 2000;95:3590-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Kobayashi S, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Kawamori Y, Sanada J, Terayama N. CT arteriographic confirmation of focal hepatic fatty infiltration adjacent to the falciform ligament associated with drainage of inferior vein of Sappey: a case report. Radiat Med. 2001;19:51-54. [PubMed] |
| 48. | Genchellac H, Yilmaz S, Ucar A, Dursun M, Demir MK, Yekeler E. Hepatic pseudolesion around the falciform ligament: prevalence, aberrant venous supply, and fatty infiltration evaluated by multidetector computed tomography and magnetic resonance imaging. J Comput Assist Tomogr. 2007;31:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Vilgrain V, Ronot M, Abdel-Rehim M, Zappa M, d'Assignies G, Bruno O, Vullierme MP. Hepatic steatosis: a major trap in liver imaging. Diagn Interv Imaging. 2013;94:713-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Hoshiba K, Demachi H, Miyata S, Matsui O, Takashima T, Tsuji M, Miwa A. Fatty infiltration of the liver distal to a metastatic liver tumor. Abdom Imaging. 1997;22:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Matsui O, Kadoya M, Takahashi S, Yoshikawa J, Gabata T, Takashima T, Kitagawa K. Focal sparing of segment IV in fatty livers shown by sonography and CT: correlation with aberrant gastric venous drainage. AJR Am J Roentgenol. 1995;164:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Aubin B, Denys A, Lafortune M, Déry R, Breton G. Focal sparing of liver parenchyma in steatosis: role of the gallbladder and its vessels. J Ultrasound Med. 1995;14:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |