Published online Dec 7, 2021. doi: 10.3748/wjg.v27.i45.7844
Peer-review started: August 9, 2021
First decision: August 29, 2021
Revised: September 11, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 7, 2021
Processing time: 115 Days and 16.9 Hours
The incidence of gastric Burkitt lymphoma (BL), presenting as paraplegia and acute pancreatitis, is extremely low. BL is a great masquerader that presents in varied forms and in atypical locations, and it is prone to misdiagnosis and missed diagnosis. The prognosis of BL remains poor because of the difficulty in early diagnosis and the limited advances in chemotherapy.
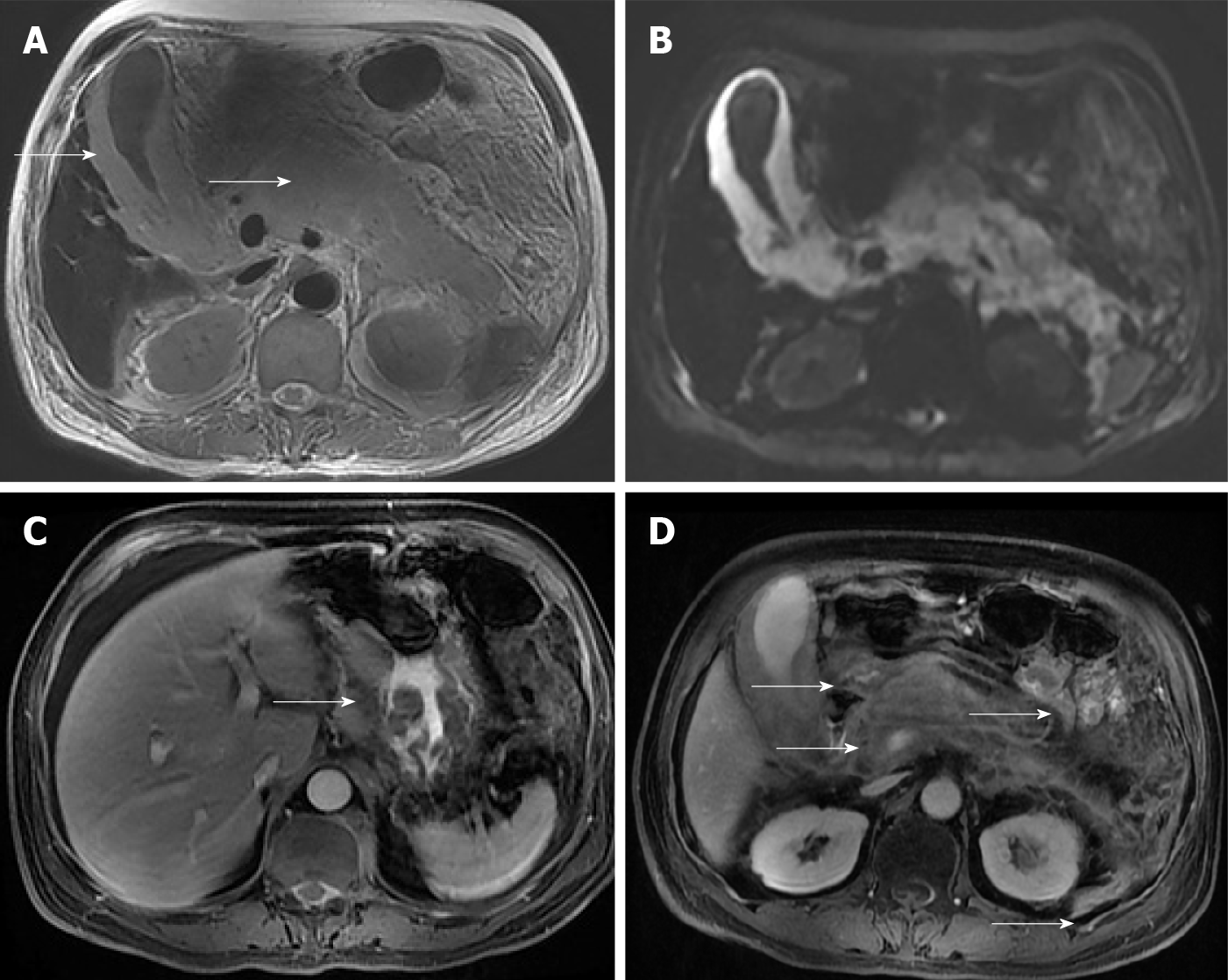
A 53-year-old man was referred to our hospital from the local county hospital due to abdominal pain for two weeks and weakness in the lower extremities for one day. Magnetic resonance imaging of the abdomen and lumbar spine showed a swollen pancreas and gallbladder, with peripancreatic exudation and liquid collection, indicating acute pancreatitis and acute cholecystitis. Additionally, we observed abnormally thickened lesions of the gastric wall, multiple enlarged retroperitoneal lymph nodes and a well-demarcated, posterolateral extradural mass lesion between T9 and T12, with extension through the spinal foramen and definite bony destruction, suggesting metastasis in gastric malignancy. Subse
Clinicians should be aware that BL can be the potential cause of acute pancreatitis or a rapidly progressive spinal tumor with accompanying paraplegia. For gastric BL, gastroscopy biopsies and pathology are necessary for a definite diagnosis.
Core Tip: The incidence of Burkitt lymphoma (BL) is extremely low, and the clinical symptoms are atypical. The misdiagnosis rate is high, and the patient's prognosis is poor. The patient in this case was eventually diagnosed with BL involving the stomach, pancreas and vertebral column presenting with acute pancreatitis and neurological symptoms secondary to compression of the spinal cord. Chemotherapeutic treatment was refused by the patient, and he eventually died after one week due to upper gas
- Citation: Lin Y, Pan YH, Li MK, Zong XD, Pan XM, Tan SY, Guo YW. Clinical presentation of gastric Burkitt lymphoma presenting with paraplegia and acute pancreatitis: A case report. World J Gastroenterol 2021; 27(45): 7844-7854
- URL: https://www.wjgnet.com/1007-9327/full/v27/i45/7844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i45.7844
Burkitt lymphoma (BL) is a subgroup of high-grade non-Hodgkin’s lymphoma (NHL) with an aggressive clinical course that was first described as a clinical entity in children in Central Africa by Denis Burkitt in 1958[1]. Clinically, patients with BL often present with solid tumors or large lymph nodes or symptoms similar to acute leukemia, and bone marrow invasion is present in more than 25% of cases[2]. BL has been classified into three subtypes according to the World Health Organization classification: Sporadic type, endemic type and immunodeficiency-associated type[3]. Endemic BL is most prevalent in children from equatorial Africa and New Guinea. Approximately 50% of endemic BL affects the jaw or kidneys. This endemic subtype could also occur in the distal ileum, cecum, greater omentum, ovaries and breasts. Nearly all cases are associated with Epstein-Barr virus[4]. Sporadic BL most commonly affects children[5] but represents less than 1% of NHL cases among adults[6]. Most sporadic BL occurs in the bowel, respiratory tract-associated lymphoid tissue and gut-associated lymphoid tissue. Immunodeficiency-associated BL is most frequently present in Human Immunodeficiency Virus-positive patients[4]. BL is highly sensitive to chemotherapy. Despite the long-term treatment-related sequelae of patients with BL treated with high-intensity chemotherapy regimens, patients who tolerate highly intensive combination chemotherapy regimens tend to have excellent oncologic outcomes. Currently, most treatment protocols for adult patients are based on pe
A 53-year-old male patient was admitted to the hospital with abdominal pain for two weeks and weakness in the lower extremities for one day.
This patient was admitted to the local hospital because of epigastric pain after alcohol consumption. He described the pain as intermittent, non-radiating and worsening with food consumption. The patient denied nausea, vomiting, constipation, fever or progressive weight loss. Based on abdominal pain, elevated levels of serum amylase, and findings of peripancreatic exudation and effusions by computed tomography (CT), the patient was diagnosed with acute pancreatitis. The patient was treated with antibiotics, proton pump inhibitors, fasting and short-term intravenous feeding and fluid therapy, and the abdominal pain was alleviated slightly. Unfortunately, on the 14th d of hospitalization, this patient developed a sudden onset of aconuresis and paraplegia. He was referred to our hospital for further examination.
The patient reported no remarkable history of past illness.
There was no family history of malignant tumors.
The patient’s vital signs were stable. No superficial lymphadenopathy was palpable. Regarding the pulmonary and cardiac examination, no obvious abnormality was observed. The abdomen was flat and soft. Physical examination revealed epigastric tenderness without rebound tenderness or Murphy’s sign. No jaundice or palpable masses were observed. Neurologic examination revealed no abnormality in his cranial nerves. The muscle strength of the upper limbs was normal, while it was grade I in the lower limbs. Deep tendon reflexes in the affected limbs were diminished or absent. Bilateral Babinski signs were positive. Hypoesthesia beneath the T8 sensory derma
The auxiliary examination at admission showed that the white blood cell count was 14.68 × 109/L (normal range, 3.5 × 109/L - 9.5 × 109/L), RBC count was 3.95 × 109/L (normal range, 4.3 × 109/L - 5.8 × 109/L), HGB was 136.0 g/L (normal range, 130-175 g/L), PLT count was 324 × 109/L (normal range, 100 × 109/L-350 × 109/L), C-reactive protein was 34.64 mg/L (normal range, 0-6 mg/L), procalcitonin was 0.12 ng/mL (normal range, 0-0.05 ng/mL), serum amylase was 266 U/L (normal range, 0-125 U/L), lactic dehydrogenase (LDH) was 526 U/L (normal range, 71-231 U/L), and uric acid was 799 μmol/L (normal range, 71-231 μmol/L). Laboratory tests showed no abnormalities in liver function or electrolytes. His carbohydrate antigen 19-9 was 461.28 U/mL (normal range, 0-35 U/mL), and carbohydrate antigen 12-5 was 126.90 U/mL (normal range, 0-35 U/mL). Other tests revealed normal tumor marker levels, including carcino-embryonic antigen and alpha fetoprotein levels of 0.56 ng/mL (normal range, 0-5 ng/mL) and 2.6 ng/mL (normal range 0-8.1 ng/mL), respectively.
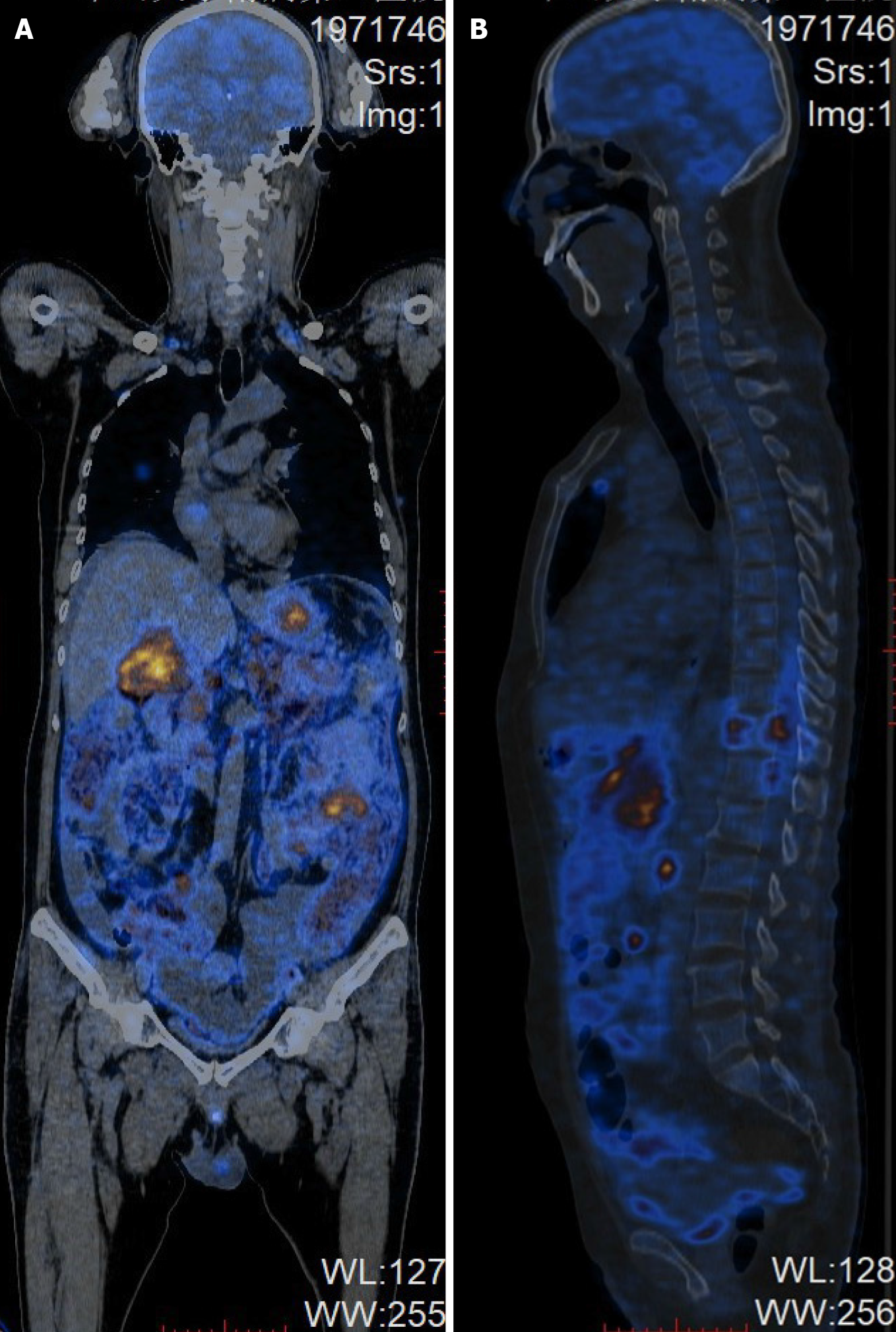
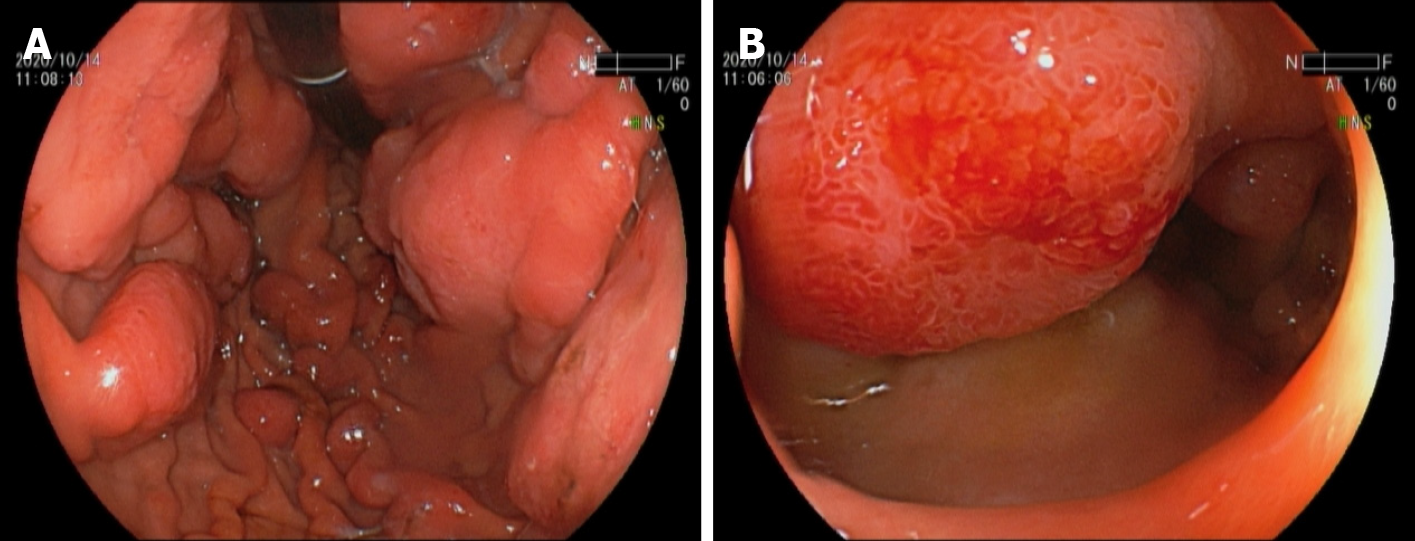
A CT scan at admission showed a swollen pancreas and gallbladder, with peripancreatic exudation and liquid collection, indicating a diagnosis of acute pancreatitis and acute cholecystitis. Magnetic resonance imaging (MRI) of the abdomen and lumbar spine at the 14th d after admission showed a swollen pancreas and gallbladder, with less peripancreatic exudation and liquid collection, indicating the remission of acute pancreatitis and acute cholecystitis. Additionally, MRI showed abnormally thickened lesions of the gastric wall, multiple enlarged retroperitoneal lymph nodes and a well-demarcated, posterolateral extradural mass lesion between T9 and T12, with extension through the spinal foramen and definite bony destruction (Figures 1 and 2). Whole-body positron emission tomography-CT (PET-CT) was then performed and showed multifocal malignant lesions in the stomach, pancreas, gallbladder, bone, bilateral supraclavicular fossa, anterior mediastinum, bilateral axillary and retroperitoneal lymph nodes (Figure 3), indicating multiple metastases of malignant tumors. Gastro
The histological findings, immunophenotype of the biopsies, and radiological findings were consistent with BL involving the stomach, pancreas and vertebral column. However, the primary lesion of BL is unclear. Because the patient had no symptoms of fever or weight loss, it was classified as group A. Due to the lack of bone marrow aspirate and trephine biopsy, we could not confirm the accuracy of the stage classification of BL in this case. Curiously, this patient presented with acute pancreatitis as the initial manifestation. One possible explanation for the presentation of acute pancreatitis is that the main pancreatic duct was obstructed by the substantial mass. Obstru
After admission to our department, this patient received short-term fasting, acid suppression, pancreatic enzyme suppression and fluid replacement for acute pancreatitis. Due to suspicion of necrotic pancreatitis, sulbactam sodium/cefoperazone sodium (3 g/d) was administered IV for one week.
Unfortunately, this patient developed sudden onset of aconuresis and paraplegia. According to the neurology consultation, acute myelitis was suspected. To inhibit the inflammatory response and block the antibodies, high doses of glucocorticoids and gamma globulin were applied for three days. Nevertheless, the efficacy of these treatments appeared poor. Concerning the high cost and potential side effects of these treatments, glucocorticoid and gamma globulin treatment was abandoned. Based on the indication for further imaging tests, this patient was diagnosed with gastric BL via endoscopic biopsy. Accordingly, a chemotherapy combination of cyclophosphamide, adriamycin, vincristine and prednisolone (CHOP) was recommended for the patient, but he refused the chemotherapeutic treatment.
One week after diagnosis and refusal of chemotherapy, the patient died of upper gastro
The incidence of BL is extremely low, and the clinical symptoms are atypical. Thus, we need to raise awareness of BL and reduce the misdiagnosis rates. BL was first described in 1958 by a British surgeon named Denis Burkitt as a sarcoma involving the jaw in African children with characteristic symptoms[1]. There has been some improvement in the understanding of its epidemiological diagnosis and treatment in the ensuing half century. In this article, we report the 11th case of BL involving the stomach, pancreas and spinal cord diagnosed based on the radiological findings and immunophenotype of the biopsies. The clinical features of 10 previous cases of BL involving the stomach, pancreas or spinal cord are summarized in Table 1[8-17].
| Author | Age | Gender | Initial symptom | Affecting area | Biopsy area | Immunohistochemical studies | EB | HIV | Treatment | Prognosis |
| Kim et al[8] | 69 | Female | Low back pain radiating down to the right leg | Spinal cord at the L2 to L4 levels, intestine, live, bone and left supraclavicular lymph node | A posterolate-ral extradural mass lesion between L2 and L3 | CD20 (+), CD79a (+), BCL-6 (+), CD10 (+), BCL-2 (-) | + | NA | NA | NA |
| Seo et al[9] | 40 | Male | Progressive pain and weakness in lower extremities | Spinal cord at the T2 to T4 levels, liver | An intraspinal extramedu-llary mass from T2 to T4, liver | CD20 (+), CD45RO (-) | NA | + | Chemotherapy and radiation therapy with HAART after surgery for intraspinal decompression and mass separation. Radiation | Died by massive pulmonary thromboembolism at 13 wk postoperatively |
| Chieng et al[10] | 9 | Male | Progressive pallor, peripheral oedema and respiratory distress | Stomach | Gastric body mass | CD20 (+), CD10 (+) and CD43 (+) | NA | NA | Induction chemotherapy with COP. Further chemotherapy included two courses of COPADAM followed by two courses of CYM and double intrathecal chemotherapy of methotrexate and hydrocortisone | Remains in clinical remission with complete resolution of the protein-losing enteropathy and no treatment related sequelae 4 yr from initial diagnosis |
| Bolandparvaz et al[11] | 21 | Male | Abdominal pain | Stomach | A huge mass in greater curvature of the stomach | NA | NA | NA | Total gastrectomy and roux-en-y esophagojejunostomy, chemotherapy was given for the patient 1 wk later without any other complication | NA |
| Gurzu et al[12] | 60 | Female | Fulminant hematemesis, recurring melena, epigastric pain, inappetence, and weight loss | Stomach | A huge mass in the antrum and posterior wall of the gastric body | CD20 (+), CD79a (+), BCL-6 (+), CD10 (+), Ki-67 (100%+), CD3 (-), CD5 (-), CD23 (-), TdT (-), bcl-2 (-), and Cyclin D1 (-) | - | NA | Distal gastrectomy | Died ten days after surgical intervention |
| Krugmann et al[13] | 28 | Male | Hematemesis and increasing abdominal pain | Stomach | A huge mass in the middle third of the stomach | CD20 (+), CD10 (+), BCL-6 (+), Ki-67 (95%+), CD3 (-), CD5 (-), CD23 (-), Cyclin D1 (-), BCL-2 (-) and TdT (-) | - | NA | Billroth-II surgical resection | Died due to lymphoma recurrence four months after onset |
| Liao et al[14] | 26 | Male | Fulminant hematemesis, abdominal pain | Stomach | A mass in the body and antrum of the stomach | CD20 (+), CD10 (+), BCL-6 (+), MUM-1 (-), CD30 (-) | NA | NA | Induction chemotherapy with two courses of R-ECHOP. Further chemotherapy included two courses of R-hyper CVAD followed by five courses of intrathecal prophylactic injection of chemotherapy drugs | Lymphoma recurrence six months after onset |
| Sağlam et al[15] | 20 | Male | Weight loss, back pain, mandible numbness, night sweats, and poor exercise tolerance | The body of the pancreas | A mass in the body of the pancreas | NA | NA | NA | Doxorubicin based combination chemotherapy | Died from sepsis during the second month of chemotherapy |
| Nistala et al[16] | 21 | Male | Jaundice, increasing swelling in the epigastric region | The head of the pancreas, cystic duct, portal vein and hepatic artery, duodenum | The first and second parts of duodenum | CD20 (+), CD10 (+), BCL-6 (+), CD5 (-), Mib-1 (99%+) | NA | NA | Two cycles of CHOP followed by hyper CVAD regimen as definitive therapy | NA |
| Konjeti et al[17] | 68 | Female | Belching, abdominal bloating and weight loss | The head of the pancreas, central hepatic duct and portal vein | The pancreatic head mass | CD20 (+), CD10 (+), C-myc (+), BCL-6 (+), CD3 (-), TdT (-), BCL-2 (-), Ki-67 (> 90%+) | NA | NA | Two cycles of chemotherapy regimen consisting of etoposide, prednisone, vincristine (Oncovin), and doxorubicin hydrochloride (Hydroxydaunorubicin hydrochloride) | Die due to the sepsis and bacteremia |
Among the previous cases, nine cases were reported in foreign countries, while only one patient came from China. From our review of the literature, a clear male predominance (70%) can be established. The ages of the patients range from 9 to 69 years, with a median age of 23 years. The initial symptoms, including abdominal distension, abdominal pain, lumbago, weakness in the lower extremities, fulminant hematemesis and progressive weight loss, are atypical. Regarding the detailed treatment protocols, seven patients received chemotherapy. Only one patient received palliative radiation treatment due to severe spinal cord involvement. Among the four patients who underwent surgical intervention, one patient underwent surgery for intraspinal decompression and mass separation, and the other three patients underwent distal or total gastrectomy. The outcome and follow-up of BL were reported in a total of eight cases. Regrettably, only a 9-year-old patient remained in clinical remission with completed chemotherapy, and no treatment-related sequelae 4 years were observed from initial diagnosis. Severe complications, including gastric perforation, sepsis and bacteremia, are always derived from intensive chemotherapy. Due to lymphoma recurrence or severe complications associated with chemotherapy, the other seven patients died within 6 mo of diagnosis.
Three variants of BL have been described worldwide: Endemic, sporadic, and immunodeficiency-associated. Among the three subtypes, sporadic BL is regarded as the most common type[18]. The clinical features of BL are variable. In endemic BL, patients tend to present with jaw and other facial diseases. Cases of sporadic BL with an intraperitoneal mass as the initial manifestations are more common. Additionally, the clinical course of sporadic BL is usually aggressive, with frequent extranodal and central nervous system (CNS) involvement and an overall poor prognosis. BL with extranodal involvement usually occurs in the gastrointestinal tract (50%) and head and neck (25%). According to the statistics, CNS involvement is recognized in 13%-17% of all cases of BL[19]. This kind of cancer cell proliferates rather rapidly, with a doubling time of approximately 24 h, and the Ki-67 proliferation index tends to be 90%-100%. Clinically, a blood test usually reveals markedly elevated LDH and uric acid levels in the early stages, indicating a high tumor burden[20]. Herein, we report a case of gastric BL in an adult patient presenting with paraplegia and acute pancreatitis. Similarly, the auxiliary examination in this case also showed markedly elevated LDH and uric acid levels at admission. During hospitalization, this patient developed acute compression of the spinal cord. Abdominal CT at admission revealed no apparent abnormal findings except for the indication of acute pancreatitis. Unexpectedly, MRI of the abdomen and lumbar spine at the 14th d after admission indicated multisite metastasis in gastric malignancy, including in the pancreas, bone, bilateral supraclavicular fossa, anterior mediastinum, bilateral axillary and retroperitoneal lymph nodes. Finally, gastroduodenal endoscopy revealed massive involvement of the gastric body and duodenum with BL. Dawson’s criteria are used to label primary gastro
Regarding the imaging evaluation of BL, CT scanning and three-dimensional reconstruction are more useful for accurately displaying bone destruction. When spinal cord involvement is suspected for clinical reasons, the preferred choice is MRI since it outperforms CT in depicting associated soft tissues. Additionally, diffusion-weighted MRI is a favorable diagnostic tool in oncologic imaging since it can reflect cellularity and proliferative activity in most malignancies. It is acknowledged that most malignancies are characterized by sustained proliferation, contributing to a high cellular density. More specifically, on diffusion-weighted MRI, BL demonstrates a markedly high signal intensity due to the relative restriction of water associated with high cellular density[22,23]. Since repeated serial imaging is essential for evaluating disease progression, MRI is also superior to CT due to its lack of ionizing radiation. For superior staging and assessment of the treatment response, PET/CT is a better choice since it can evaluate the functional status of abnormally hypermetabolic tissues throughout the whole body[24].
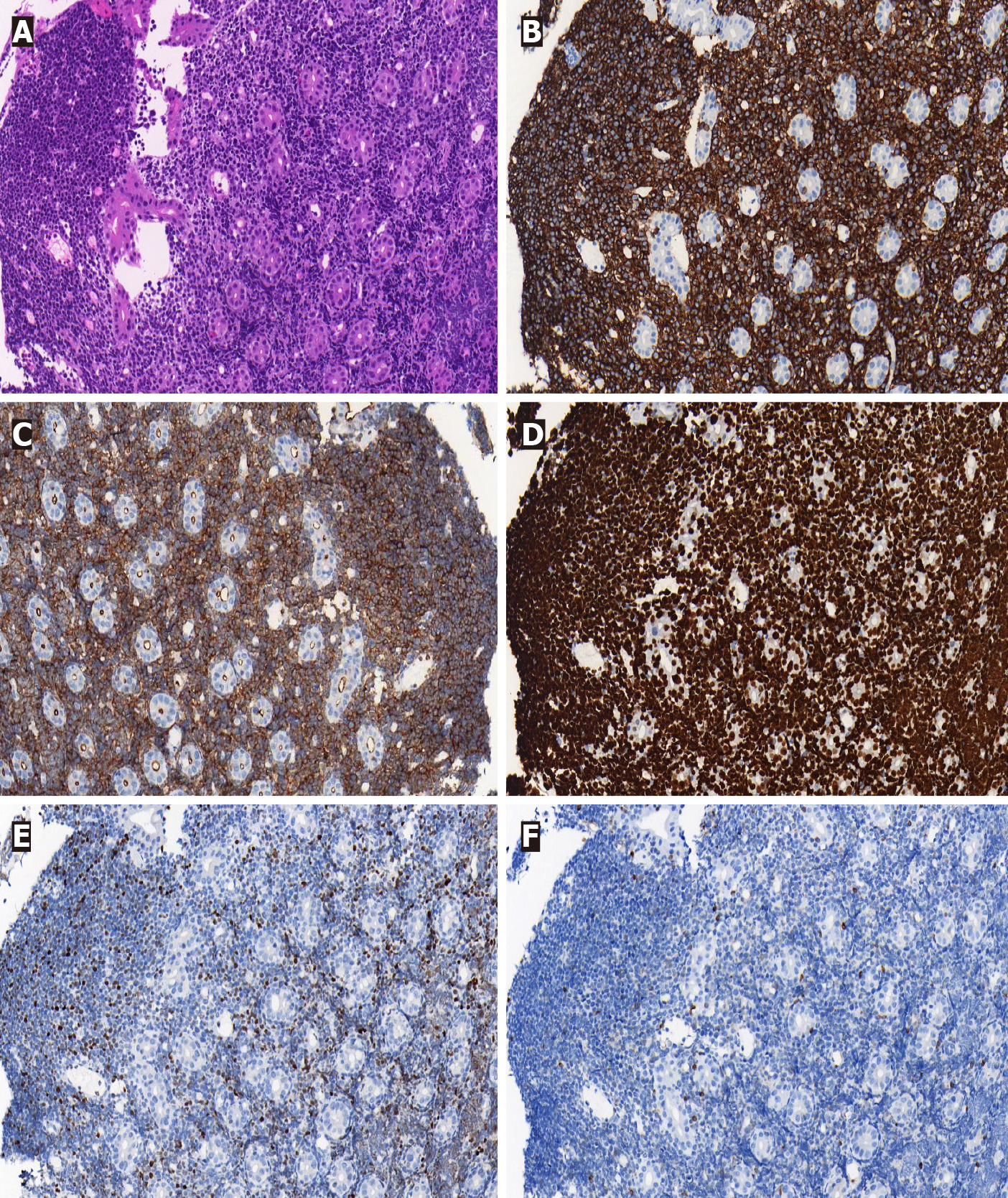
Histologically, the tumor cells of BL are medium-sized with an abundant, basophilic cytoplasm and display the typical “starry sky” pattern. The tumor cells are positive for BCL-6, CD19, CD20, CD22, CD10 and CD79a but negative for CD3, CD5, CD23 and TdT[25]. BL is characterized by the t (8; 14) (q24; q32) translocation of the c-myc and IgH genes, resulting in IgH-myc fusion, which can be detected by molecular analysis via fluorescence in situ hybridization. In our case, the tumor cells were negative for creatine kinase and CD3, indicating that the tumor was not derived from the epi
Systemic chemotherapy is the preferred choice for the treatment of BL. Additionally, conventional radiotherapy, surgery, or a combination of both are recommended as the standard treatment unless severe compression of vital organs by lymphoma is observed[26]. Currently, most treatment protocols for adults are based on pediatric clinical trials. At present, most classical chemotherapy regimens show good efficacy and safety in children and relatively young patients. However, the prognosis of adult patients is poor due to their low response rate and severe treatment-related toxicity. Chemotherapy regimens, including CHOP, hyper-cyclophosphamide, vincristine, epirubicin, dexamethasone, etoposide, prednisone, vincristine, cyclophosphamide, epirubicin and cyclophosphamide, epirubicin, doxorubicin, vincristine, high-dose methotrexate/isophosphamide, cytarabine and etoposide, are still the backbone of therapeutic strategies for BL. Rituximab is an anti-CD20 chimeric antibody that acts by depleting CD20-positive B lymphocytes[27]. It has been reported that common chemo
The incidence of BL is extremely low, and the clinical symptoms are atypical, contri
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dias E S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Burkitt’s Lymphoma: Thorax to Pelvis. Indian J Chest Dis Allied Sci. 2016;58:49-51. [PubMed] |
| 2. | Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Shiramizu B, Barth M, Cairo MS. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children's Oncology Group Report. Br J Haematol. 2014;167:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Dunleavy K, Little RF, Wilson WH. Update on Burkitt Lymphoma. Hematol Oncol Clin North Am. 2016;30:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kalisz K, Alessandrino F, Beck R, Smith D, Kikano E, Ramaiya NH, Tirumani SH. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Kelly JL, Toothaker SR, Ciminello L, Hoelzer D, Holte H, LaCasce AS, Mead G, Thomas D, Van Imhoff GW, Kahl BS, Cheson BD, Magrath IT, Fisher RI, Friedberg JW. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009;9:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Jacobson C, LaCasce A. How I treat Burkitt lymphoma in adults. Blood. 2014;124:2913-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Gastwirt JP, Roschewski M. Management of adults with Burkitt lymphoma. Clin Adv Hematol Oncol. 2018;16:812-822. [PubMed] |
| 8. | Kim YS, Lee JK, Choi KY, Jang JW. Spinal Burkitt's Lymphoma Mimicking Dumbbell Shape Neurogenic Tumor: A Case Report and Review of the Literature. Korean J Spine. 2015;12:221-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Seo JY, Ha KY, Kim MU, Kim YC, Kim YH. Spinal cord compression by B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma in a patient seropositive for human immunodeficiency virus: a case report. J Med Case Rep. 2014;8:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chieng JH, Garrett J, Ding SL, Sullivan M. Clinical presentation and endoscopic features of primary gastric Burkitt lymphoma in childhood, presenting as a protein-losing enteropathy: a case report. J Med Case Rep. 2009;3:7256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Bolandparvaz S, Jelodar S, Heidari Esfahani M, Moslemi S. Chemotherapy-Induced Perforation of Gastric Burkitt Lymphoma; A Case Report and Review of the Literature. Bull Emerg Trauma. 2014;2:133-135. [PubMed] |
| 12. | Gurzu S, Bara T, Bara TJ, Turcu M, Mardare CV, Jung I. Gastric Burkitt lymphoma: A case report and literature review. Medicine (Baltimore). 2017;96:e8954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Krugmann J, Tzankov A, Fiegl M, Dirnhofer S, Siebert R, Erdel M. Burkitt's lymphoma of the stomach: a case report with molecular cytogenetic analysis. Leuk Lymphoma. 2004;45:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Liao LS, Zheng Z, Wei T, Xie Y, Chen B. Vascular embolization for treatment of primary gastric lymphoma combined with acute upper gastrointestinal hemorrhage: Report of two cases and review of literature. Journal of Leukemia and Lymphoma. 2017;26 (9):541-544. |
| 15. | Sağlam M, Yilmaz MI, Mas MR, Taşçi I, Ors F, Sönmez A, Deveci S. A case of pancreatic Burkitt lymphoma: radiological findings. Diagn Interv Radiol. 2009;15:39-42. [PubMed] |
| 16. | Nistala SS, Sawalakhe NR, Thiruvengadam NR, Rathi PM. A rare case of primary pancreatic Burkitt lymphoma in a young Indian male. Case report and review of the literature. JOP. 2009;10:686-689. [PubMed] |
| 17. | Konjeti VR, Hefferman GM, Paluri S, Ganjoo P. Primary Pancreatic Burkitt's Lymphoma: A Case Report and Review of the Literature. Case Rep Gastrointest Med. 2018;2018:5952315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Jang SJ, Yoon DH, Kim S, Yoon S, Kim DY, Park CS, Huh J, Lee SW, Lee DH, Ryu JS, Suh C. A unique pattern of extranodal involvement in Korean adults with sporadic Burkitt lymphoma: a single center experience. Ann Hematol. 2012;91:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, Guarnaccia C, Lewis MS, McKendrick J, Stenning SP, Wright D; UKLG LY06 collaborators. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104:3009-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (3)] |
| 22. | Schob S, Meyer J, Gawlitza M, Frydrychowicz C, Müller W, Preuss M, Bure L, Quäschling U, Hoffmann KT, Surov A. Diffusion-Weighted MRI Reflects Proliferative Activity in Primary CNS Lymphoma. PLoS One. 2016;11:e0161386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, Porpaczy E, Weber M, Knogler T, Sillaber C, Jaeger U, Simonitsch-Klupp I, Ubl P, Müllauer L, Dolak W, Lukas J, Raderer M. Evaluation of Diffusion-Weighted Magnetic Resonance Imaging for Follow-up and Treatment Response Assessment of Lymphoma: Results of an 18F-FDG-PET/CT-Controlled Prospective Study in 64 Patients. Clin Cancer Res. 2015;21:2506-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Cheng G, Servaes S, Zhuang H. Value of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan versus diagnostic contrast computed tomography in initial staging of pediatric patients with lymphoma. Leuk Lymphoma. 2013;54:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Chapman CJ, Zhou JX, Gregory C, Rickinson AB, Stevenson FK. VH and VL gene analysis in sporadic Burkitt's lymphoma shows somatic hypermutation, intraclonal heterogeneity, and a role for antigen selection. Blood. 1996;88:3562-3568. [PubMed] |
| 26. | Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol. 2012;156:730-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Ferry JA. Burkitt's lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Ribrag V, Koscielny S, Bosq J, Leguay T, Casasnovas O, Fornecker LM, Recher C, Ghesquieres H, Morschhauser F, Girault S, Le Gouill S, Ojeda-Uribe M, Mariette C, Cornillon J, Cartron G, Verge V, Chassagne-Clément C, Dombret H, Coiffier B, Lamy T, Tilly H, Salles G. Rituximab and dose-dense chemotherapy for adults with Burkitt's lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:2402-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev. 2006;20:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Bernstein JI, Coleman CN, Strickler JG, Dorfman RF, Rosenberg SA. Combined modality therapy for adults with small noncleaved cell lymphoma (Burkitt's and non-Burkitt's types). J Clin Oncol. 1986;4:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 2.1] [Reference Citation Analysis (0)] |